Abstract
The progressive nature of type 2 diabetes necessitates that treatment is intensified as the disease advances. Several studies have shown that basal insulin and glucagon-like peptide-1 receptor agonists (GLP-1RAs) can be used in combination to successfully improve glycemic control and this combination is increasingly being considered as an alternative to intensification with prandial insulin. Insulin degludec/liraglutide (IDegLira) is the first fixed-ratio combination of a basal insulin and a GLP-1RA in a single formulation. Here we consider the benefits and potential limitations of such a combination, focusing on the unique modes of action of insulin degludec and the once-daily GLP-1RA liraglutide. IDegLira offers an efficacious combination therapy (mean end-of-trial HbA1c was 6.4–6.9% across the five completed Phase 3 trials), which was well-tolerated in clinical trials. The complementary modes of action resulted in a low rate of hypoglycemia and no weight gain in insulin-treated patients. As a once-daily injection with effects on both fasting and post prandial hyperglycemia, IDegLira has the potential to help many patients reach glycemic target (60–81% of patients achieved HbA1c <7% in clinical trials).
KEYWORDS: type 2 diabetes, glucagon-like peptide-1 receptor agonist, IDegLira, insulin
The complex pathophysiology of type 2 diabetes is characterized by declining β-cell function resulting in reduced insulin secretion in response to glucose, hypersecretion of glucagon from pancreatic α-cells and insulin resistance in the muscle and liver. This favors a strategic approach involving combination therapy that can address the full spectrum of underlying abnormalities and maximize the chance of treatment success. Metformin is recommended as first-line therapy in most patients with type 2 diabetes, and it is recommended that an additional therapy be added if a patient is not at target after 3–6 months treatment.[1,2] Options for second-line therapy include sulfonylureas, thiazolidinediones (TZD), dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 receptor agonists (GLP-1RA), basal insulin, acarbose and sodium-glucose transport protein 2 inhibitors. However, despite recent treatment advances and the numerous options available, data from observational studies suggest many patients with diabetes do not have adequate glycemic control.[3,4]
Clinical inertia with respect to intensifying treatment when HbA1c remains above target is also a contributing factor. For example, the Study of Once Daily Levemir (SOLVE) study showed that mean HbA1c in patients from 10 countries was 8.9% prior to insulin initiation.[3] Similarly, a UK-based retrospective cohort study showed that the median time to treatment intensification for those taking one, two or three oral antidiabetes drugs (OADs) when HbA1c was over 7.0% exceeded the maximum follow-up time of 7.2 years.[5] Patients and physicians may be reluctant to intensify treatment as it often leads to increased risk of hypoglycemia and weight gain.[6,7]
Therefore, there is still a need for new and innovative therapies that will enable patients to attain glycemic targets easily with minimal side effects. Furthermore, there is an awareness that the control of diabetes needs to involve a combination of lifestyle measures and pharmacological interventions, as well as addressing comorbidities such as dyslipidemia and hypertension while minimizing weight gain.[2] The idea of combination therapies is not new – combination products containing metformin with a number of oral agents, for example, sulfonylureas, TZDs and DPP-4 inhibitors, are available, offering a less complicated option for patients requiring more than one oral agent.
GLP-1RAs are one of the newer classes of therapies that, along with DPP-4 inhibitors, are classified as incretin-based therapies. GLP-1 is a natural hormone secreted from L-cells in the small intestine and colon in response to caloric intake. GLP-1 stimulates insulin secretion, suppresses glucagon secretion when glucose levels are elevated, delays gastric emptying and reduces appetite.[8] GLP-1RAs activate a G-protein-coupled receptor; in pancreatic β-cells this is coupled to adenylyl cyclase, increasing cAMP levels and leading to the release of insulin.[8] Following secretion, natural GLP-1 is rapidly degraded by DPP-4, resulting in a very short half-life of approximately 1.5 min.
Studies have shown that the pancreatic β-cell response to GLP-1 is impaired in patients with type 2 diabetes.[9] Exogenous GLP-1 can augment the β-cell response to reach similar levels to those of healthy individuals.[10] The first marketed GLP-1RA, exenatide, is based on the structure of exendin-4, a hormone with similar properties to GLP-1. Exenatide has only 53% homology to native human GLP-1 and is available in twice-daily (approved in 2005) and once-weekly (approved in 2012) formulations. Liraglutide is a GLP-1 analog, sharing 97% amino acid homology with native human GLP-1. It was approved in 2009 for once-daily dosing. Since then several other GLP-1RAs have reached the market or are expected to gain approval in the near future.
When added to OAD(s), GLP-1RAs can offer significant HbA1c reductions with a low risk of hypoglycemia and clinically significant weight loss.[11] However, even with HbA1c reductions of ~1–1.5% in clinical studies, many patients may require basal insulin therapy in addition to a GLP-1RA in order to achieve HbA1c target.
The combination of insulin and an incretin therapy is mentioned as a possibility in the European Association for the Study of Diabetes (EASD)/American Diabetes Association (ADA) guidelines on the management of hyperglycemia in type 2 diabetes,[2] and these therapies are increasingly being used together. Insulin degludec/liraglutide (IDegLira; Xultophy) is a novel, fixed-ratio combination of insulin degludec and liraglutide in a single formulation.[12] This review will consider the benefits of this combination, focusing on the unique modes of action of the basal insulin degludec and the once-daily GLP-1RA liraglutide, and discussing the clinical evidence for the efficacy and safety of the combination formulation.
Rationale for combining a GLP-1RA and a basal insulin analog
Incretin and insulin therapies are both efficacious blood glucose-lowering therapies, but with different mechanisms of action. GLP-1RAs increase insulin secretion by β-cells and decrease glucagon secretion by α-cells, both in a glucose-dependent manner. Depending on their duration of action, they can decrease both fasting plasma glucose (FPG) and postprandial glucose (PPG), with longer-acting GLP-1RAs having a greater effect on FPG and shorter acting products having a greater effect on PPG.[13] GLP-1RAs also reduce satiety, delay gastric emptying, can reduce body weight and are associated with a low risk of hypoglycemia. However, GLP-1RAs may not lead to sufficient insulin secretion from β-cells to achieve the desired glycemic control. Basal insulin therapy increases circulating insulin in a non-glucose-dependent manner and has been associated with improved β-cell function. Basal insulin has a role in glucose regulation in the liver and peripheral tissues, and modulates hepatic glucose production.[14] Basal insulin is very effective at lowering HbA1c and FPG, but has less of an effect on PPG. Insulin is associated with an increase in body weight (due in part to increased appetite and food intake) and a risk of hypoglycemia.
Therefore, the two mechanisms of action may complement each other, with the glucose-dependent effect of GLP-1RAs on pancreatic islet function counterbalancing the risk of hypoglycemia observed with increasing doses of insulin. By reducing hunger and food intake, GLP-1RAs can decrease the weight gain associated with insulin. The individual effects of basal insulin and GLP-1RAs suggest a theoretical rationale for combination therapy with clinical benefits to be expected.[15]
Introduction to the compounds liraglutide and insulin degludec: the components of IDegLira
Liraglutide
The structure of liraglutide is based on that of native human GLP-1 (Figure 1).[16,17] The attachment of a C16 side chain allows liraglutide to self-associate into heptamers, delaying absorption from the injection site. In the bloodstream, liraglutide binds reversibly to albumin, providing stability and reducing metabolism by DPP-4, resulting in a half-life of 13 h and making it suitable for once-daily dosing.[18] A study in patients with type 2 diabetes indicated that liraglutide can improve β-cell function, restoring insulin secretion in response to glucose to that of healthy individuals.[19] However, a study in Japanese patients with type 2 diabetes suggests that caution should be taken with switching patients with reduced insulin secretory capacity from insulin to liraglutide.[20]
Figure 1.
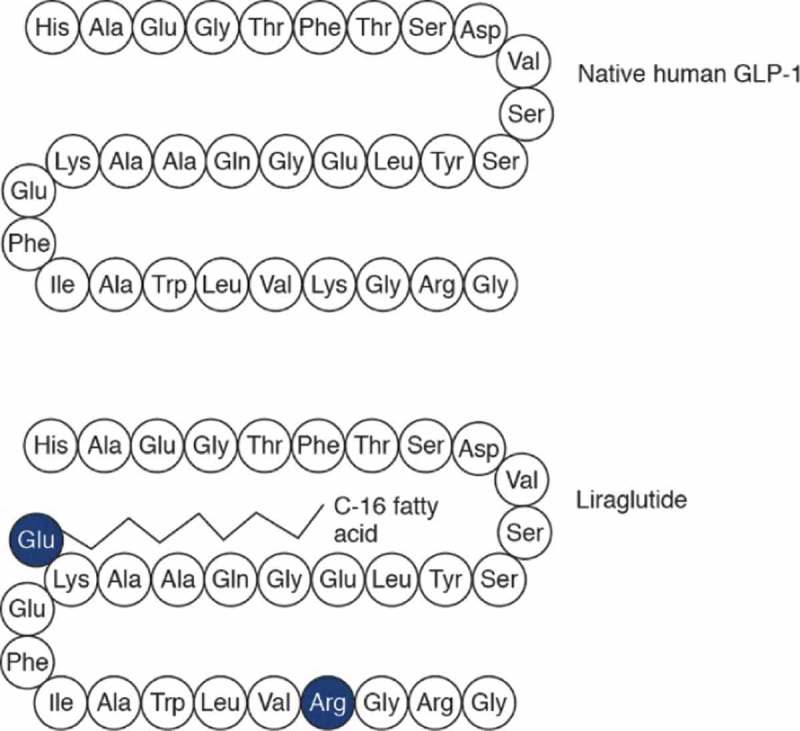
Amino acid structure of native human GLP-1 and liraglutide [16].
Ala: alanine; Asp: aspartic acid; Cys: cysteine; GLP-1: Glucagon-like peptide 1; Gln: glutamine; Glu: glutamic acid; Gly: glycine; Ile: isoleucine; Leu: leucine; Lys: lysine; Phe: phenylalamine; Ser: serine; Thr: threonine; Trp: tryptopan; Tyr: tyrocine; Val: valine.
The liraglutide Phase 3 clinical trial program (LEAD) involved >4000 patients and showed that liraglutide, dosed at 1.2 or 1.8 mg/ day, provided reductions in HbA1c, FPG and PPG.[21] Gastrointestinal side effects are the most commonly reported adverse effects associated with liraglutide, and with other GLP-1RAs; in most cases, they resolve after 4–8 weeks treatment.[21] Head-to-head studies have indicated that liraglutide is superior or comparable to other GLP-1RAs with respect to HbA1c lowering and percentage of patients reaching target.[22–24]
The long-term effects of GLP-1RAs on cardiovascular safety are unknown, and although retrospective analyzes of major adverse cardiovascular events occurring during clinical trials have not raised any issues, several cardiovascular outcomes trials, including the liraglutide LEADER study (NCT01179048), are currently ongoing. There have been safety concerns surrounding the use of incretins and the development of pancreatitis. A post hoc analysis of pooled patient-level data from the liraglutide clinical development program reported eight cases of acute pancreatitis with liraglutide (n = 6345) compared with one case with comparators (n = 1846).[25] The incidence of acute pancreatitis was 1.6 cases/1000 patient-years exposure (PYE) for liraglutide versus 0.7 cases/1000 PYE for total active comparators. The small number of cases observed and confounding variables precluded firm conclusions. Extensive data analyzes by the US FDA and EMA did not reach a final conclusion regarding such a causal relationship, but both agencies agreed that the current knowledge is adequately reflected in the product labeling.[26] Long-term outcome trials have been completed and published for three DPP-4 inhibitors (saxagliptin, alogliiptin and sitaglipin).[27–29] In these placebo-controlled studies, the incidence of acute pancreatitis (0.3–0.4% vs 0.2–0.3%) and pancreatic cancer (0.1% vs 0.1–0.2%) was similar with the DPP-4 inhibitor and placebo.[27–30] In vivo studies showed that long-term exposure to liraglutide in rodents was associated with thyroid C-cell hyperplasia and adenomas;[31] therefore, calcitonin screening was conducted during the LEAD program. These studies showed that the differences in calcitonin levels between liraglutide and comparators after up to 2 years of treatment were extremely small and within the normal ranges.[32]
Insulin degludec
In healthy individuals, basal insulin is secreted by β-cells at an almost constant rate between meals of ~1.3 U/h, thus preventing FPG levels from becoming elevated.[33] Insulin degludec is a long-acting basal insulin that was approved in Europe and Japan in 2013 and the US in 2015. It comprises recombinant desB30 human insulin acylated at the LysB29 residue with a hexadecandioyl-γ-L-Glu side chain (Figure 2A). Insulin degludec has a novel mechanism of protraction, whereby the addition of an acylated side chain promotes self-association beyond the hexameric state following injection, leading to the formation of multi-hexamers (Figure 2B).[34,35] Insulin degludec monomers then slowly and gradually dissociate and are subsequently absorbed into the bloodstream, providing a long duration of action exceeding 42 h.[34,36]
Figure 2.
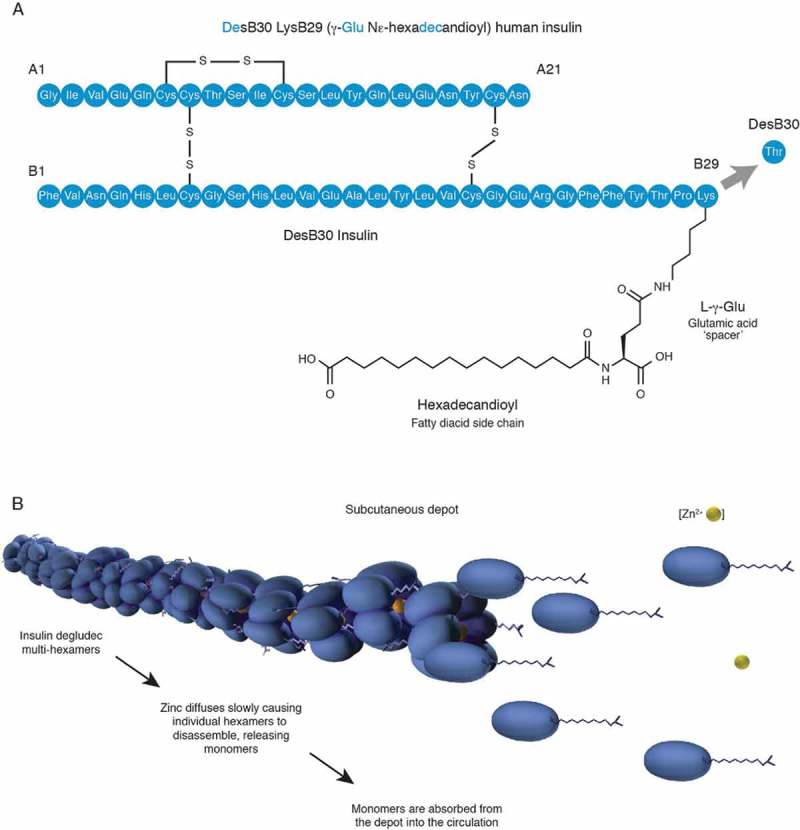
Structure (A) and mechanism of protraction (B) of insulin degludec [34].
Glu: glutamic acid; Lys: lysine.
Insulin degludec has been shown to exhibit a flat and stable steady-state pharmacokinetic and pharmacodynamic profile, with a half-life of >25 h compared with ~12 h for insulin glargine.[37,38] Within-patient variability of glucose-lowering action can influence the effectiveness of an insulin and can increase hypoglycemia.[39] Day-to-day variability in total glucose-lowering effect was four-times lower for insulin degludec than for insulin glargine at 20 versus 82%, respectively.[40] Moreover, once-daily dosing of insulin degludec yields a virtually “peakless” profile, more closely mimicking the profile of physiological basal insulin secretion.
Insulin degludec was assessed in the large-scale BEGIN Phase 3 program, investigating the efficacy and safety across the spectrum of diabetes care. In seven treat-to-target trials with a primary end point of reduction in HbA1c from baseline, insulin degludec was noninferior to insulin glargine.[41] A meta-analysis of the seven trials showed that patients with type 2 diabetes experienced significantly lower rates of overall confirmed [rate ratio (RR): 0.83; 95% CI: 0.74–0.94) and nocturnal confirmed hypoglycemia (RR: 0.68; 95% CI: 0.57–0.82) with insulin degludec versus insulin glargine.[42] In the year 2013, the FDA announced that it required a cardiovascular outcomes trial to be conducted before insulin degludec could be approved in the US. The long-term safety of insulin degludec is currently being investigated in an ongoing, 5-year trial in >7500 patients at high risk of cardiovascular disease.[43]
GLP-1RA and basal insulin in combination: proof of concept
Several trials have investigated the use of GLP-1RAs in combination with basal insulin.[44–48] Together these studies support GLP-1RA/insulin combination therapy as a way to improve glycemic control, with a low risk of hypoglycemia. It was also shown that the weight gain associated with insulin use is mitigated by the addition of a GLP-1RA.[41] Moreover, addition of a GLP-1RA (exenatide) to basal insulin plus metformin was more effective than addition of a DPP-4 inhibitor (sitagliptin) in lowering HbA1c.[49]
Liraglutide and insulin degludec in combination
Two studies have investigated the co-use of insulin degludec and liraglutide, administered separately. VICTOZA ADD-ON compared two treatment strategies in patients who were not at target after 2 years treatment with insulin degludec: addition of once-daily liraglutide or once-daily insulin aspart with the largest meal.[50] After 26 weeks, the addition of liraglutide resulted in a significantly greater mean HbA1c reduction (–0.39%) than insulin aspart (–0.32%; p = 0.0024). There were no significant differences in end-of-trial FPG or 9-point self-measured blood glucose (SMBG) profiles. In the liraglutide add-on arm, there was a mean weight reduction of 2.8 kg compared with a mean weight increase of 0.9 kg in the insulin aspart arm. The rate of overall confirmed hypoglycemia was 87% lower with liraglutide compared with insulin aspart: 1.00 versus 8.15 episodes per (PYE; RR: 0.13; 95% CI: 0.08–0.21; p < 0.0001). Rates of nocturnal confirmed hypoglycemia were low in both groups: 0.17 episodes/PYE versus 1.11 episodes/PYE, translating to an 86% reduction with liraglutide versus insulin aspart (RR: 0.14; 95% CI: 0.05–0.40; p = 0.0002). No severe hypoglycemia occurred in either arm. More gastrointestinal side effects were reported in the liraglutide add-on arm, with one patient withdrawing due to vomiting. However, by week 26 nausea was reported by only 3% of patients in the liraglutide arm.
This study showed that addition of liraglutide was more effective at improving glycemic control than addition of once-daily insulin aspart in patients requiring treatment intensification.[50] It also demonstrated that liraglutide and insulin degludec can be used safely and effectively together, and resulted in an EU label update for expanded use of both products in type 2 diabetes, so that insulin degludec can be prescribed in combination with a GLP-1RA, and liraglutide can be prescribed with a basal insulin.
A second 26-week, double-blind study (add-on to GLP-1) investigated the efficacy of adding insulin degludec versus placebo in patients treated with liraglutide and metformin who required treatment intensification.[51] Insulin degludec was superior versus placebo in improving HbA1c (mean reductions of –1.04 vs –0.16%, respectively; p < 0.0001) and FPG (mean reductions of –2.60 vs –0.28 mmol/l respectively; p < 0.0001). After 26 weeks of treatment, patients in the insulin degludec arm had gained a mean of 2.0 kg, while those on placebo had a mean weight reduction of 1.3 kg. Rates of confirmed hypoglycemia (0.57 vs 0.12 episodes/PYE, respectively; p = 0.0002) were low in both arms but higher with insulin degludec than placebo, and there was no significant difference in the incidence of nocturnal confirmed hypoglycemia (0.05 vs 0.03 episodes/PYE, respectively). No severe hypoglycemia occurred in either arm. This study showed that the addition of insulin degludec to patients uncontrolled on liraglutide could significantly improve glycemic control.[51]
IDegLira: the combination product
IDegLira, the fixed-ratio combination of insulin degludec and liraglutide, is the first combination product of a basal insulin and a GLP-1RA to reach the market. An overview of the ongoing trial program for IDegLira is outlined in Figure 3. Full results from the Phase 1 and Phase 3A trials have been published.[12,52,53] They will be discussed below along with preliminary results from Phase 3B trials, where available.
Figure 3.

The IDegLira clinical trial program: DUAL.
Ext: Extension trial; IAsp: Insulin aspart; IDegLira: Insulin degludec/liraglutide combination; IGlar: Insulin glargine; GLP-1: Glucagon-like peptide-1; OAD: Oral antidiabetic drug; SU: Sulfonylurea. Accessed 26 June 2015. www.clinicaltrials.gov.
Pharmacokinetics and pharmacodynamics
The IDegLira formulation contains 100 U insulin degludec and 3.6 mg liraglutide/ ml. One dose step contains 1 U insulin degludec and 0.036 mg liraglutide, and the maximum dose is 50 dose steps (50 U insulin degludec and 1.8 mg liraglutide). Importantly, the mechanism of protraction of insulin degludec (forming stable dihexamers) and liraglutide (forming heptamers) enables their combination in a co-formulation, while maintaining the distinct pharmacological properties of both monocomponents.[52] A single-dose, randomized, four-period crossover study was conducted in 24 healthy individuals to assess the pharmacokinetics and pharmacodynamics of IDegLira versus its monocomponents. Exposure to insulin degludec was equivalent when dosed as IDegLira or insulin degludec alone. Exposure to liraglutide was lower with IDegLira than liraglutide alone, but still met the criterion for bioequivalence (90% CI within 0.8–1.25) and dose proportionality was confirmed.[52] A population pharmacokinetic analysis of the patient population of DUAL I (see below) was conducted, including 1549 individuals across three treatment groups (IDegLira, insulin degludec and liraglutide). The population pharmacokinetic analysis revealed no relevant deviations from dose proportionality for the components of IDegLira, and the effects of covariates such as body weight were consistent with the findings for insulin degludec and liraglutide. Moreover, the glycemic response to IDegLira was greater than the response to insulin degludec or liraglutide alone throughout the dose/exposure range, indicating that both monocomponents contribute to the glycemic effect.[52] Additionally, administering two therapies using one device also has the ability to simplify treatment for patients.
Clinical efficacy: Phase 3a studies
DUAL I
The DUAL I Phase 3a trial tested the hypothesis that combining the complementary actions of liraglutide and insulin degludec may have a more beneficial effect on glycemic control than either of the therapies used individually.
DUAL I was a 26-week, open-label, treat-to-target trial in 1663 insulin-naive patients with type 2 diabetes, who were uncontrolled on OADs. Patients were randomized 2:1:1 to IDegLira, insulin degludec or liraglutide, all once daily. IDegLira was initiated at 10 dose steps and adjusted twice weekly, based on the mean pre-breakfast SMBG from the three previous days, to a target FPG of 4–5 mmol/l (72–90 mg/dl). Insulin degludec was also initiated at 10 U and titrated to the same target, but there was no dose cap.[12] After 26 weeks, the mean insulin dose was significantly lower (by 28%) with IDegLira compared with insulin degludec (38 vs 53 U; p < 0.0001). Mean liraglutide doses were lower for the IDegLira versus liraglutide group (1.4 vs 1.8 mg, respectively). Overall, 39% of patients received the maximum dose of IDegLira.[12]
Treatment with IDegLira resulted in a significantly greater mean HbA1c reduction of 1.9% (8.3–6.4%) compared with 1.4% (8.3–6.9%) with insulin degludec or 1.3% (8.3–7.0%) with liraglutide alone, both p < 0.0001. Between-treatment differences are shown in Table 1. This enabled significantly more patients to reach the HbA1c targets of 7.0% (81 vs 65 vs 60%) and 6.5% (70 vs 47 vs 41%) with IDegLira versus insulin degludec versus liraglutide. The proportion of patients achieving a composite end point of HbA1c <7% without weight gain and without hypoglycemia was also studied. Significantly more patients achieved this end point with IDegLira versus insulin degludec (36 vs 14%; p < 0.0001) but fewer with IDegLira versus liraglutide (36 vs 52%; p < 0.0001). End-of-trial FPG was significantly lower with IDegLira (5.6 mmol/l) compared with liraglutide (7.3 mmol/l; p < 0.0001), but was similar to that with insulin degludec (5.8 mmol/l; NS, Table 1). The reduction in PPG increment was greater with IDegLira versus insulin degludec, whereas there was no significant difference between IDegLira and liraglutide.[12]
Table 1.
| DUAL I [12] |
DUAL II [53] |
DUAL III [55] |
DUAL IV [56] |
DUAL V [57] |
||
|---|---|---|---|---|---|---|
| IDegLira vs IDeg | IDegLira vs liraglutide | IDegLira vs IDeg | IDegLira vs unchanged GLP-1RA | IDegLira vs placebo | IDegLira vs insulin glargine | |
| HbA1c change†, ETD, %-points | –0.47 (–0.58 to –0.36), p < 0.0001 (noninferior) |
–0.64 (–0.75 to –0.53), p < 0.0001 (superior) | –1.1 (–1.3 to –0.8), p < 0.0001 (superior) | –0.94 (–1.11 to –0.78), p < 0.001 (superior) | –1.02%, p < 0.001 | –0.59 (–0.74 to –0.45), p < 0.001 |
| Fasting plasma glucose change†, ETD, mmol/l | –0.17 (–0.41 to 0.07), NS | –1.76 (–2.00 to –1.53), p < 0.0001 | –0.73 (–0.19 to –0.27), p = 0.0019 | –2.64 (–3.03 to –2.25), p < 0.001 | –2.30, p < 0.001 | –0.01 (–0.35 to –0.33), NS |
| Mean of postprandial plasma glucose increment across all main meals, ETD, mmol/l |
–0.45 (–0.63 to –0.28), p < 0.0001 | 0.06 (–0.11 to 0.23), NS | –0.4 (–0.7 to –0.0) p = 0.0260 |
N/A | N/A | N/A |
| Weight change†, ETD, kg | –2.22 (–2.64 to –1.80), p < 0.0001 | +2.44 (2.02–2.86), p < 0.0001 |
–2.5 (–3.2 to –1.8), p < 0.0001 |
2.89 (2.17–3.62], p < 0.001 |
N/A | –3.2 (–3.77 to –2.64), p < 0.001 |
| Confirmed hypoglycemia‡, rate ratio | 0.68 (0.53 to 0.87), p = 0.0023 |
7.61 (5.17 to 11.21), p < 0.0001 |
0.66 (0.39 to 1.13), NS | 25.4 (10.6 to 60.5), p < 0.001 | 3.74, p < 0.001 |
0.43 (0.30 to 0.61), p < 0.001 |
| Nocturnal confirmed hypoglycemia§, rate ratio | N/A | N/A | 0.81 (0.35 to 1.90), NS | N/A | N/A | 0.17 (0.10 to 0.31), p < 0.001 |
| Daily insulin dose¶, ETD, U | –14.90 (–17.14 to 12.66), p < 0.0001 | N/A | 0.02 NS | N/A | N/A | 41 dose steps vs 66 U p < 0.001 |
Values in square brackets indicate 95% CIs.
† Change from baseline to end of trial (week 26).
‡ Confirmed with plasma glucose <3.1 mmol/l.
§Nocturnal confirmed hypoglycemia, plasma glucose <3.1 mmol/l occurring 00:01–05:59 inclusive.
¶ At week 26.
ETD: Estimated treatment difference; IDegLira: Insulin degludec/liraglutide; N/A: Not applicable/available; NS: Not significant.
After 26 weeks, patients with IDegLira had lost a mean of 0.5 kg, compared with a weight increase of 1.6 kg with insulin degludec, versus 3.0 kg weight loss with liraglutide, showing that the weight gain associated with insulin initiation is avoided by co-administration of liraglutide.
The DUAL I extension trial continued for an additional 26 weeks. The 52-week results were consistent with those at 26 weeks, indicating that IDegLira provides a sustainable improvement in glycemic control with a good tolerability profile.[54]
DUAL II
DUAL II compared once-daily IDegLira with once-daily insulin degludec in a 26-week, double-blind trial of 413 insulin-experienced patients with type 2 diabetes, who were randomized 1:1. The completion rates were similar in both arms: 85% for IDegLira and 83% for insulin degludec, with no withdrawals due to gastrointestinal side effects.[53] To determine the role of the liraglutide component in IDegLira, patients on the insulin degludec arm also had their dose capped at 50 U and in both arms the starting dose was 16 dose steps/U. After 26 weeks, the mean insulin doses were equivalent (45 U).
At end-of-trial, mean HbA1c was reduced by 1.9% (from 8.8 to 6.9%) with IDegLira and by 0.9% (from 8.9 to 8.0%) with insulin degludec, confirming the superiority of IDegLira (p < 0.0001) (for end-of-treatment differences, see Table 1). With IDegLira 60% of patients reached HbA1c <7.0% versus 23% with insulin degludec (p < 0.0001). Significantly more patients also achieved the composite end point of HbA1c <7% with no weight gain and no hypoglycemia with IDegLira versus insulin degludec (40.1 vs 8.5%; p < 0.0001). Significantly greater mean FPG reductions were also apparent with IDegLira (3.5–6.2 mmol/l) versus insulin degludec (2.6–7.0 mmol/l; p < 0.0019, Table 1). Additionally, the mean prandial increment across meals was smaller with IDegLira compared with insulin degludec (2.2 vs 2.4 mmol/l; p = 0.0260, Table 1).[53]
There was a reduction in body weight in the IDegLira arm (mean 2.7 kg), but no change in the insulin degludec arm.
Achieving insulin dose equivalence in this trial enabled the contribution of liraglutide to be assessed and showed that it significantly improved glycemic control and reduced body weight. However, the 50 U dose limit in the insulin degludec arm meant that it was not fully titrated, which may have limited efficacy in this arm.[53]
Post hoc analyzes
The effectiveness of a particular therapy can depend on the patient cohort, with nonresponders to existing therapies limiting their clinical and cost-effectiveness. For example, patients with higher baseline HbA1c or higher BMI are reported to experience greater HbA1c reductions with insulin therapy versus those who are just above target.[58,59] To identify whether IDegLira is likely to be more effective in particular patient cohorts, three post hoc analyzes were performed.
The first examined whether IDegLira was consistently effective across a range of baseline BMI categories (<25, 25–30, 30–35 and >35 kg/m2). In DUAL I, HbA1c reductions were similar across groups; in DUAL II, the greatest HbA1c reduction was seen in the 30–35 kg/m2 BMI category. In DUAL I, IDegLira was more insulin-sparing versus insulin degludec, while providing greater reductions in HbA1c.[60] Across all BMI categories, many patients receiving IDegLira were able to reach a target HbA1c of <7.0% with weight loss and no hypoglycemia (DUAL I: 33.1%; DUAL II: 39.2%).[60]
The second analysis investigated whether HbA1c reduction was dependent on baseline HbA1c. In both DUAL I and II, treatment with IDegLira resulted in substantial HbA1c reductions across all baseline HbA1c categories: ≤7.5, >7.5 to ≤8.5, 8.5 to ≤9.0 and >9.0%. In DUAL I, higher baseline HbA1c was associated with greater HbA1c reductions with IDegLira (p < 0.0001). HbA1c reduction appeared independent of diabetes duration. In DUAL II, pre-trial basal insulin dose (≤30 or >30 U) did not affect HbA1c reduction with IDegLira.[61]
A third analysis investigated the proportion of patients reaching the pre-prandial SMBG target range of ≥3.9 to ≤7.2 mmol/l and the postprandial target of <9 mmol/l in DUAL I and II, which were consistently higher in patients receiving IDegLira versus insulin degludec or liraglutide.[59] Additionally, at end of trial in DUAL I, a greater proportion of patients receiving IDegLira had all nine BG values within the target range of ≥3.9 to <9 mmol/l (39%) compared with insulin degludec (28%) or liraglutide (31%). Similarly, in DUAL II, more patients had all nine BG values at target with IDegLira (32%) than those on insulin degludec (20%).[62] These data reflect that IDegLira combines the effects of liraglutide on postprandial glucose with those of insulin degludec (and liraglutide) on fasting glucose.
Clinical efficacy: Phase 3b studies
Preliminary results have recently been published from three Phase 3b trials in the IDegLira clinical program, which are summarized below.
DUAL III
DUAL III was a 26-week, open-label, treat-to-target trial conducted in 438 insulin-naive patients with type 2 diabetes, who were inadequately controlled on maximum dose (or maximum tolerated dose) GLP-1RA plus OADs (metformin ± pioglitazone ± sulfonylurea).[55] Patients were randomized 2:1 to IDegLira or to continue on their GLP-1RA unchanged, continuing on all pre-trial OADs.
After 26 weeks, mean HbA1c was reduced by 1.3% (from 7.8 to 6.4%) with IDegLira and by 0.3% (from 7.7 to 7.4%) with unchanged GLP-1RA, confirming the superiority of IDegLira to unchanged GLP-1RA, with an estimated treatment difference of –0.94%-points (p < 0.001) (Table 1). A total of 75% of patients receiving IDegLira reached the HbA1c target <7.0% versus 36% on unchanged GLP-1RA. The end-of-trial FPG (Table 1) and 9-point SMBG profiles were also significantly improved with IDegLira compared with unchanged GLP-1RA. There was a significant increase in body weight with IDegLira versus unchanged GLP-1RA, with an estimated treatment difference of 2.89 kg (p < 0.001) (Table 1).[55] Weight gain with IDegLira likely reflects that patients were previously insulin-naive and on maximum dose GLP-1RA therapy and then initiated IDegLira on 16 dose steps (16 U insulin degludec and 0.6 mg liraglutide).
DUAL IV
DUAL IV was a 26-week, double-blind, treat-to-target trial conducted in 435 insulin-treated patients with type 2 diabetes who were randomized 2:1 to IDegLira or placebo as an add-on to treatment with a sulfonylurea ± metformin.[56] The reduction in HbA1c after 26 weeks' treatment was significantly greater with IDegLira (from 7.9 to 6.4%) compared with placebo (from 7.9 to 7.4%) (Table 1). A total of 79.2% of patients treated with IDegLira achieved an HbA1c <7.0% compared with 28.8% of those receiving placebo. The reduction in FPG and 9-point SMBG profile was also significantly greater with IDegLira versus placebo (both p < 0.001). There was a small increase in mean body weight with IDegLira of +0.5 kg versus –1.0 kg with placebo (p < 0.001).
DUAL V
DUAL V compared the efficacy and safety of IDegLira in patients with type 2 diabetes who were inadequately controlled when treated with insulin glargine (at doses of 20–50 U per day). In this 26-week, open-label, treat-to-target trial, patients (n = 557) were randomized 1:1 to treatment with IDegLira (starting at 16 dose steps) or continued treatment with insulin glargine (starting at pre-trial dose with no maximum dose).[57]
Mean HbA1c decreased significantly more with IDegLira by 1.8% (from 8.4 to 6.6%) compared with 1.1% with insulin glargine (from 8.2 to 7.1%) (Table 1). With IDegLira a significantly greater proportion of patients achieved the HbA1c target of <7.0% compared with insulin glargine (71.6 vs 47.0%). Additionally, 38.8% of patients treated with IDegLira achieved the composite end point of HbA1c <7.0%, no confirmed hypoglycemia and no weight gain, compared with 12.2% of those treated with insulin glargine. There was no significant difference in the end-of-trial FPG between treatment in this trial (Table 1). After 26 weeks, IDegLira resulted in a mean decrease in body weight from 88.3 to 86.9 kg compared with an increase from 87.3 to 89.1 kg with insulin glargine (p < 0.001) (Table 1). Treatment with IDegLira was insulin sparing; at end of trial, patients on IDegLira were on a mean dose of 41 dose steps (41 U insulin degludec), whereas those on insulin glargine were on a mean daily dose of 66 U.[57]
Safety and tolerability
Key safety results for DUAL I and II are summarized in Table 2.
Table 2.
| DUAL I [12] |
DUAL II [53] |
||||
|---|---|---|---|---|---|
| IDegLira |
IDeg |
Liraglutide |
IDegLira |
IDeg |
|
| (n = 825) | (n = 412) | (n = 412) | (n = 199) | (n = 199) | |
| Adverse events, n (%) | 521 (63) | 248 (60) | 299 (73) | 115 (57.8) | 122 (61.3) |
| Serious adverse events, n (%) | 19 (2) | 8 (2) | 14 (3) | 7 (3.5) | 11 (5.5) |
| Nausea, n (%) | 73 (9) | 15 (4) | 81 (20) | 13 (6.5) | 7 (3.5) |
Based on the safety analysis sets.
IDegLira: Insulin degludec/liraglutide; N: Number of subjects; %: Percentages of subjects.
DUAL I
In DUAL I, the rates of confirmed hypoglycemia were lower (by 32%) with IDegLira versus insulin degludec (1.8 vs 2.6 episodes/PYE; RR: 0.68; 95% CI: 0.53–0.87; p = 0.0023), but higher with IDegLira versus liraglutide (1.8 vs 0.2 episodes/PYE; RR: 7.61; 95% CI: 5.17–11.21; p < 0.0001) (Table 2). In total, there were five severe hypoglycemic episodes (IDegLira: 3/825 patients; insulin degludec: 2/412 patients). Importantly, the significantly better HbA1c reduction seen with IDegLira did not come with increased hypoglycemia; on the contrary, hypoglycemia was lower with IDegLira than insulin degludec. Similar proportions of patients reported adverse events (Table 2), with headache, nasopharyngitis and gastrointestinal disorders being the most frequent. With IDegLira 9% of patients experienced nausea versus 4 and 20% treated with insulin degludec and liraglutide, respectively.[12] One event of acute pancreatitis occurred in the liraglutide arm (reported as acute pancreatitis in connection to a metastatic pancreatic adenocarcinoma); no events of pancreatitis or pancreatic cancer were positively adjudicated in the IDegLira arm. No medullary thyroid carcinomas were reported, and there were no confirmed thyroid neoplasms. Of the three major adverse cardiovascular events occurring during the trial, only one case (myocardial infarction) was judged as possibly related to study drug, in the liraglutide arm.[12]
DUAL II
In DUAL II, there was no difference in the incidence of overall confirmed hypoglycemia (1.5 vs 2.6 episodes/PYE), which occurred in 24% of patients with IDegLira and 25% with insulin degludec (Table 1). Rates of nocturnal confirmed hypoglycemia were similar and low (Table 1), and only one case of severe hypoglycemia occurred during the trial (in the IDegLira arm). The incidence of adverse events was similar, and nausea was low in both groups (IDegLira 6.5 vs insulin degludec 3.5%) (Table 2).[53] During the trial, one major adverse cardiovascular event occurred in the IDegLira arm (myocardial infarction) and two in the insulin degludec arm (myocardial infarction and stroke). No cases of medullary thyroid carcinoma, thyroid neoplasm or pancreatitis were confirmed, but there was one case of metastatic pancreatic carcinoma in the insulin degludec arm.[53] There were no cases of pancreatic cancer in the IDegLira arm.
DUAL III
Full safety data are not available at this time for DUAL III, but it is noted that the safety profile of IDegLira was consistent with previous trials. The rate of confirmed hypoglycemia was significantly higher with IDegLira compared with unchanged GLP-1RA, with rates of 2.82 and 0.12 episodes/PYE, respectively (Table 1).[55]
DUAL IV
As for DUAL III, there are only limited safety information available on DUAL IV. The rate of confirmed hypoglycemia was higher with IDegLira compared with placebo, with rates of 3.5 versus 1.4 episodes/PYE, and an estimated rate ratio of 3.74 (p < 0.001) (Table 1). The rates of hypoglycemia were higher in this trial than others in the DUAL program, likely due to the concomitant use of a sulfonylurea.[56]
DUAL V
Preliminary safety results for DUAL V showed that the rate of confirmed hypoglycemia was significantly lower with IDegLira compared with insulin glargine, with rates of 2.23 and 5.05 episodes per PYE (p < 0.001) (Table 1). The rate of nocturnal hypoglycemia was also significantly lower with IDegLira (0.22 episodes/PYE) versus insulin glargine (1.23 episodes/PYE). Severe hypoglycemia was reported by one patient in the trial, treated with insulin glargine. The proportion of patients completing the trial was similar: 90% (IDegLira) versus 95% (insulin glargine).[57]
Regulatory affairs
IDegLira was approved in Europe and Switzerland in 2014 [63] and has now launched in several countries, including the UK, Switzerland and Germany. Approval in the US was dependent on the interim analysis of the ongoing insulin degludec cardiovascular outcomes trial, DEVOTE.[43] Insulin degludec was approved in September 2015. A new drug application for IDegLira has been submitted to the FDA.[64]
Conclusions
The combination of basal insulin and GLP-1RA is consistent with the EASD/ADA treatment guidelines for type 2 diabetes, and both addition of a GLP-1RA to basal insulin therapy and adding basal insulin to a GLP-1RA have proven effective treatment strategies. Results from the first two DUAL program clinical trials have demonstrated that the IDegLira combination product provides superior glycemic control and a better tolerability profile than either insulin degludec or liraglutide individually, as evidenced by the lower risk of hypoglycemia and weight gain compared with insulin degludec, and lower risk of nausea compared with liraglutide.
Expert commentary
As described above, the beneficial effects of combining incretin and insulin therapies have now been well documented. When titrated as per the clinical trials and guidelines,[65] IDegLira offers a treatment that is likely to be less complex than adding multiple prandial insulin injections to basal insulin plus OADs, perhaps making therapy adherence less difficult for patients. The end-of-trial HbA1c was particularly impressive in the IDegLira arms of DUAL I–V, with high numbers of patients reaching glycemic target after 26 weeks of treatment, and many patients not requiring the maximum dose in order to do so. In the context of existing therapies, IDegLira provides a novel treatment option that could enable more patients to reach glycemic target, thereby avoiding or delaying future diabetic complications.
It is important to consider where this therapy will fit in the pathway of diabetes care. When patients remain above target on OADs, treatment algorithms recommend therapy intensification. However, the initiation of injectable therapy after oral agents can be problematic and therefore delayed, as is often observed for insulin and therefore potentially IDegLira too. The combination of an effective basal insulin and an effective GLP-1RA in a single co-formulation for once-daily injection, without compromising the properties of either, provides a simple, user-friendly approach to therapy intensification for a broad spectrum of patients. It is recognized that IDegLira may not be the therapy of choice for intensification in all patients; different patients will receive a basal insulin, a GLP-1RA, both basal insulin and a GLP-1RA as two separate injections or the IDegLira fixed ratio combination therapy to improve their glycemic control. The flexibility of dosing the GLP-1RA and basal insulin individually may be advantageous over a fixed ratio product in some patients. For example, in patients with inadequate glycemic control on maximum dose GLP-1RA therapy where weight is also a major issue it may be more appropriate to initiate basal insulin rather than switch to IDegLira initiated at 16 dose steps (equivalent to 16 U IDeg and 0.6 mg liraglutide). Patient ethnicity should also be considered. While there are no IDegLira data stratified by different ethnic groups to date, previous data for GLP-1RAs suggest that their efficacy is greater and risk of hypoglycemia is increased in Asian versus non-Asian populations.[66] Additionally, the price of IDegLira versus other therapies will be an important consideration. The decision as to which intensification option to use will depend on a variety of factors, for example, patients in whom weight is a major issue, a GLP-1RA alone, which can be quickly titrated to maximum dose, may well follow after failure of OADs; this is supported by the composite end point data from DUAL I. On the contrary, patients with a very high HbA1c in whom weight is not a problem may initiate insulin in the first instance.
The mechanism of action and clinical trials support the use of IDegLira throughout the continuum of type 2 diabetes, with particular consideration for patients unable to reach target HbA1c on basal insulin plus OADs. IDegLira is also ideally placed for patients failing to achieve target on metformin or metformin and another OAD. In the latter case, the second oral agent, for example, sulfonylurea, TZD or DPP-4 inhibitor, may be discontinued prior to IDegLira initiation. Post hoc analyzes have suggested that in these patients HbA1c reductions can be expected across all categories of baseline HbA1c, with the greatest reductions being seen in those with higher baseline values. The preliminary results from the Phase 3b trials give us additional information on the efficacy of IDegLira versus insulin glargine and sulfonylureas, which it performed well against. However, it should be acknowledged that there is currently a lack of clinical trials assessing IDegLira in many real-life clinical situations. It will be interesting to see how IDegLira compares to other treatment options such as basal–bolus insulin therapy. The price and availability of IDegLira is likely to vary between countries and economic evaluations of its cost-effectiveness will be necessary. It will be of interest to see in future studies how the cost-effectiveness of IDegLira compares with that of other diabetes treatments.
To summarize, basal insulin and GLP-1RA is an attractive combination for patients and there are clear benefits associated with use of a fixed ratio single injection associated with a low incidence of side effects for many of our patients with type 2 diabetes. Furthermore, IDegLira was granted marketing authorization in the EU and Switzerland in September 2014 and has launched in some countries during 2015;[63,65] therefore, real-world experience will be available in the near future.
Five-year view
According to the International Diabetes Federation, the already high prevalence of diabetes is set to increase considerably over the coming years, to approximately 205 million people by 2035.[67] Therefore, the need for treatment options and the spending on diabetes treatments will also increase. During the next 5 years, the type 2 diabetes market is likely to become increasingly crowded, making a clinician’s decision on which approach to use for treatment intensification for patients with type 2 diabetes increasingly complex. There is likely to be an increased emphasis on the individualization of treatment for a particular patient, as outlined in current treatment guidelines.[2] The full publication of the results of the Phase 3b trials with IDegLira will give clinicians information about the use of IDegLira in patients in need of intensification who are treated with a GLP-1RA and OADs, a sulfonylurea and basal insulin.[55–57] During the next 5 years, real-world data on the use of IDegLira will become available, further aiding clinical decision-making. A second fixed-ratio combination of a basal insulin (insulin glargine) and a GLP-1RA (lixisenatide) is currently in Phase 3 development: insulin glargine/lixisenatide (LixiLan). LixiLan is expected to become available during the next 5 years, providing a competitor to IDegLira. Preliminary results of a Phase 2b study showed that in insulin-naive patients with type 2 diabetes treated with metformin, LixiLan was superior to insulin glargine (least square mean treatment difference: –0.17%; –0.312 to –0.037%; p = 0.0130) and associated with weight loss and no increase in hypoglycemia versus insulin glargine.[68] Head-to-head studies will be needed to compare the efficacy and safety of IDegLira and LixiLan.
Information resources
IDegLira Summary of Product Characteristics [65]
Clinicaltrials.gov—for details on ongoing clinical trials
Financial & competing interests disclosure
S. Gough has received honoraria for lectures and advisory boards from Astra Zeneca, Lilly, Novo Nordisk, Sanofi, GlaxoSmithKline, Merck Sharp & Dohme, Boehringer Ingelheim and Pfizer. S. Gough has received research grants from Eli Lilly, Novo Nordisk and Sanofi. R. Jain has served in advisory boards and/or speaker bureaus for Novo Nordisk, Takeda, Pfizer, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Bristol Myers Squibb and Sanofi. VC. Woo has served on advisory boards and speaker bureaus for Novo Nordisk, Eli Lilly, Merck, Boehringer Ingelheim, Bristol Myers Squibb, Sanofi, AstraZeneca, Johnson & Johnson and Roche and Abbott. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Writing assistance was utilized in the production of this manuscript provided by Adele Norman, Watermeadow Medical, Oxford, UK and editorial assistance was utilized in the production of this manuscript provided by Gabrielle Parker, Watermeadow Medical, Oxford, UK, funded by Novo Nordisk.
Key issues
Several studies have shown that basal insulin and glucagon-like peptide-1 receptor agonists are an efficacious treatment combination for patients with type 2 diabetes.
Insulin degludec/liraglutide (IDegLira) is the first fixed-ratio combination of a basal insulin (insulin degludec) and a glucagon-like peptide-1 receptor agonists (liraglutide) available in a single once-daily injection.
Two Phase 3a trials have demonstrated that IDegLira is superior at improving glycemic control compared with insulin degludec or liraglutide alone.
IDegLira was well tolerated: the rate of hypoglycemia with IDegLira was lower than that with insulin degludec alone and the rate of gastrointestinal side effects was lower than with liraglutide alone.
Several Phase 3b trials were recently completed, with full results expected during 2015 or 2016.
IDegLira has recently received marketing authorization in Europe and Switzerland in 2014 and has launched in several countries during 2015.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
**Phase 3a trial conducted in insulin-naive patients, comparing insulin degludec/liraglutide (IDegLira) to its monocomponents, and demonstrating superior glycemic control with IDegLira.
*A Phase 3 trial showing that addition if liraglutide versus insulin aspart to patients inadequately controlled on insulin degludec resulted in superior glycemic control
*A blinded crossover pharmacokinetic study comparing IDegLira to its monocomponents, showing that the pharmacokinetic properties are preserved in the co-formulation.
**A double-blind, Phase 3a trial conducted in patients inadequately controlled on basal insulin and oral agents, showing that glycemic control was superior with IDegLira compared with insulin degludec.
*One-year results of the DUAL I Phase 3a trial showing the sustainability of the benefits of IDegLira versus its components in glycemic efficacy and safety/tolerability.
- Garber AJ, Abrahamson MJ, Barzilay JI. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19:327–336. doi: 10.4158/endp.19.2.a38267720403k242. [DOI] [PubMed] [Google Scholar]
- Inzucchi SE, Bergenstal RM, Buse JB. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- Khunti K, Damci T, Meneghini L. Study of Once Daily Levemir (SOLVE): insights into the timing of insulin initiation in people with poorly controlled type 2 diabetes in routine clinical practice. Diabetes Obes Metab. 2012;14:654–661. doi: 10.1111/j.1463-1326.2012.01602.x. [DOI] [PubMed] [Google Scholar]
- Valensi P, Benroubi M, Borzi V. The IMPROVE study–a multinational, observational study in type 2 diabetes: baseline characteristics from eight national cohorts. Int J Clin Pract. 2008;62:1809–1819. doi: 10.1111/j.1742-1241.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Khunti K, Wolden ML, Thorsted BL. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411–3417. doi: 10.2337/dc13-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract Suppl. 2009:6–10. doi: 10.1111/j.1742-1241.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- Peyrot M, Barnett AH, Meneghini LF. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in insulin therapy study. Diabet Med. 2012;29:682–689. doi: 10.1111/j.1464-5491.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Hojberg PV, Vilsboll T, Rabol R. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52:199–207. doi: 10.1007/s00125-008-1195-5. [DOI] [PubMed] [Google Scholar]
- Vilsboll T, Krarup T, Madsbad S. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia. 2002;45:1111–1119. doi: 10.1007/s00125-002-0878-6. [DOI] [PubMed] [Google Scholar]
- Eckerle Mize DL, Salehi M. The place of GLP-1-based therapy in diabetes management: differences between DPP-4 inhibitors and GLP-1 receptor agonists. Curr Diab Rep. 2013;13:307–318. doi: 10.1007/s11892-013-0377-9. [DOI] [PubMed] [Google Scholar]
- Gough SC, Bode B, Woo V. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885–932. doi: 10.1016/S2213-8587(14)70174-3. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Buse JB, Taylor K. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- Bethel MA, Feinglos MN. Basal insulin therapy in type 2 diabetes. J Am Board Fam Pract. 2005;18:199–204. doi: 10.3122/jabfm.18.3.199. [DOI] [PubMed] [Google Scholar]
- Balena R, Hensley IE, Miller S. Combination therapy with GLP-1 receptor agonists and basal insulin: a systematic review of the literature. Diabetes Obes Metab. 2013;15:485–502. doi: 10.1111/dom.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöholm A. Liraglutide therapy for type 2 diabetes: overcoming unmet needs. Pharmaceuticals. 2010;3:764–781. doi: 10.3390/ph3030764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen LB. Glucagon-like peptide-1: the basis of a new class of treatment for type 2 diabetes. J Med Chem. 2004;47:4128–4134. doi: 10.1021/jm030630m. [DOI] [PubMed] [Google Scholar]
- Agerso H, Jensen LB, Elbrond B. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45:195–202. doi: 10.1007/s00125-001-0719-z. [DOI] [PubMed] [Google Scholar]
- Chang AM, Jakobsen G, Sturis J. The GLP-1 derivative NN2211 restores beta-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes. 2003;52:1786–1791. doi: 10.2337/diabetes.52.7.1786. [DOI] [PubMed] [Google Scholar]
- Usui R, Yabe D, Kuwata H. Retrospective analysis of safety and efficacy of insulin-to-liraglutide switch in Japanese type 2 diabetes: A caution against inappropriate use in patients with reduced β-cell function. J Diabetes Investig. 2013;4:585–594. doi: 10.1111/jdi.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber AJ. Liraglutide in oral antidiabetic drug combination therapy. Diabetes Obes Metab. 2012;14(Suppl 2):13–19. doi: 10.1111/j.1463-1326.2012.01574.x. [DOI] [PubMed] [Google Scholar]
- Buse JB, Rosenstock J, Sesti G. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- Buse JB, Nauck M, Forst T. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117–124. doi: 10.1016/S0140-6736(12)61267-7. [DOI] [PubMed] [Google Scholar]
- Pratley RE, Nauck MA, Barnett AH. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2:289–297. doi: 10.1016/S2213-8587(13)70214-6. [DOI] [PubMed] [Google Scholar]
- Jensen TM, Saha K, Steinberg WM. Is there a link between liraglutide and pancreatitis? A post hoc review of pooled and patient-level data from completed liraglutide type 2 diabetes clinical trials. Diabetes Care. 2015;38:1058–1066. doi: 10.2337/dc13-1210. [DOI] [PubMed] [Google Scholar]
- Egan AG, Blind E, Dunder K. Pancreatic safety of incretin-based drugs–FDA and EMA assessment. N Engl J Med. 2014;370:794–797. doi: 10.1056/NEJMp1314078. [DOI] [PubMed] [Google Scholar]
- Scirica BM, Bhatt DL, Braunwald E. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- White WB, Cannon CP, Heller SR. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- Green JB, Bethel MA, Armstrong PW. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- Raz I, Bhatt DL, Hirshberg B. Incidence of pancreatitis and pancreatic cancer in a randomized controlled multicenter trial (SAVOR-TIMI 53) of the dipeptidyl peptidase-4 inhibitor saxagliptin. Diabetes Care. 2014;37:2435–2441. doi: 10.2337/dc13-2546. [DOI] [PubMed] [Google Scholar]
- Knudsen LB, Madsen LW, Andersen S. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;131:1473–1486. doi: 10.1210/en.2009-1272. [DOI] [PubMed] [Google Scholar]
- Gough SCL. Liraglutide: from clinical trials to clinical practice. Diabetes Obes Metab. 2012;14(Suppl. 2):33–40. doi: 10.1111/j.1463-1326.2012.01576.x. [DOI] [PubMed] [Google Scholar]
- Kruszynska YT, Home PD, Hanning I. Basal and 24-h C-peptide and insulin secretion rate in normal man. Diabetologia. 1987;30:16–21. doi: 10.1007/BF01788901. [DOI] [PubMed] [Google Scholar]
- Jonassen I, Havelund S, Hoeg-Jensen T. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29:2104–2114. doi: 10.1007/s11095-012-0739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensgaard DB, Schluckebier G, Strauss HM. Ligand-controlled assembly of hexamers, dihexamers, and linear multihexamer structures by the engineered acylated insulin degludec. Biochemistry. 2013;52:295–309. doi: 10.1021/bi3008609. [DOI] [PubMed] [Google Scholar]
- Keating GM. Insulin degludec and insulin degludec/insulin aspart: a review of their use in the management of diabetes mellitus. Drugs. 2013;73:575–593. doi: 10.1007/s40265-013-0051-1. [DOI] [PubMed] [Google Scholar]
- Heise T, Nosek L, Bottcher SG. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab. 2012;14:944–950. doi: 10.1111/j.1463-1326.2012.01638.x. [DOI] [PubMed] [Google Scholar]
- Haahr H, Heise T. A review of the pharmacological properties of insulin degludec and their clinical relevance. Clin Pharmacokinet. 2014;53:787–800. doi: 10.1007/s40262-014-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnert KD, Heinke P, Vogt L. Reduced glucose variability is associated with improved quality of glycemic control in patients with type 2 diabetes: a 12-month observational study. J Endocrinol Metab. 2011;1:64–72. [Google Scholar]
- Heise T, Hermanski L, Nosek L. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859–864. doi: 10.1111/j.1463-1326.2012.01627.x. [DOI] [PubMed] [Google Scholar]
- Vora J, Christensen T, Rana A. Insulin degludec versus insulin glargine in type 1 and type 2 diabetes mellitus: a meta-analysis of endpoints in phase 3a trials. Diabetes Ther. 2014 doi: 10.1007/s13300-014-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner RE, Gough SC, Mathieu C. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175–184. doi: 10.1111/dom.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2015 Jun 30;];ClinicalTrials.gov. A trial comparing cardiovascular safety of insulin degludec versus insulin glargine in subjects with type 2 diabetes at high risk of cardiovascular events (DEVOTE) https://clinicaltrials.gov/ct2/show/NCT01959529 cited. Available from.
- Buse JB, Bergenstal RM, Glass LC. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154:103–112. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- DeVries JH, Bain SC, Rodbard HW. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care. 2012;35:1446–1454. doi: 10.2337/dc11-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle MC, Aronson R, Home P. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L) Diabetes Care. 2013;36:2489–2496. doi: 10.2337/dc12-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle MC, Forst T, Aronson R. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1) Diabetes Care. 2013;36:2497–2503. doi: 10.2337/dc12-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock J, Fonseca VA, Gross JL. Advancing basal insulin replacement in type 2 diabetes Inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37:2317–2325. doi: 10.2337/dc14-0001. [DOI] [PubMed] [Google Scholar]
- Arnolds S, Dellweg S, Clair J. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care. 2010;33:1509–1515. doi: 10.2337/dc09-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu C, Rodbard HW, Cariou B. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON) Diabetes Obes Metab. 2014;16:636–644. doi: 10.1111/dom.12262. [DOI] [PubMed] [Google Scholar]
- Aroda V, Bailey T, Cariou B. The effect of insulin degludec in combination with liraglutide and metformin in patients with type 2 diabetes requiring intensification. Diabetologia. 2014;57(Suppl. 1):OP145. [Google Scholar]
- Kapitza C, Bode BW, Ingwersen SH. Preserved pharmacokinetic exposure and distinct glycemic effects of insulin degludec and liraglutide in IDegLira, a fixed ratio combination therapy. J Clin Pharmacol. 2015 doi: 10.1002/jcph.549. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Buse JB, Vilsboll T, Thurman J. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and Liraglutide (IDegLira) Diabetes Care. 2014;37:2926–2933. doi: 10.2337/dc14-0785. [DOI] [PubMed] [Google Scholar]
- Gough SC, Buse JB, Woo VC. One-year efficacy and safety of a fixed combination of insulin degludec and liraglutide in patients with type 2 diabetes: results of a 26-week extension and a 26-week main trial. Diabetes Obes Metab. 2015 doi: 10.1111/dom.12498. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linjawi S, Bode B, Chaykin L. Efficacy and Safety of IDegLira (Combination of Insulin Degludec + Liraglutide), in Insulin-naïve Patients with T2D Uncontrolled on GLP-1 Receptor Agonist (GLP-1RA) Therapy. Diabetes. 2015;64(Suppl. 1):A255. abstract 1002-P. [Google Scholar]
- Rodbard HW, Bode B, Harris S. IDegLira in insulin-naïve patients with type 2 diabetes (T2D) inadequately controlled on sulfonylureas (SU) alone or in combination with metformin: the DUAL IV study. Diabetes. 2015;64(Suppl. 1):A255–A256. abstract 1003-P. [Google Scholar]
- Buse J, Pérez Manghi FC, García-Hernández PA. Insulin degludec/Liraglutide (IDegLira) is superior to insulin glargine (IG) in A1c reduction, risk of hypoglycemia and weight change: DUAL V Study. Diabetes. 2015;64(Suppl. 1):A43–A44. abstract 166-OR. [Google Scholar]
- Giugliano D, Maiorino M, Bellastella G. Relationship of baseline HbA1c, HbA1c change and HbA1c target of < 7% with insulin analogues in type 2 diabetes: a meta-analysis of randomised controlled trials. Int J Clin Pract. 2011;65:602–612. doi: 10.1111/j.1742-1241.2010.02619.x. [DOI] [PubMed] [Google Scholar]
- Khamseh ME, Prusty V, Latif Z. Type 2 diabetes mellitus management and body mass index: experiences with initiating insulin detemir in the a1chieve study. Diabetes Ther. 2014;5:127–140. doi: 10.1007/s13300-014-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse JB, Rodbard HW, Woo V. Impact of BMI on HbA1c reduction, hypoglycemia rates and insulin requirements in response to IDegLira in patients with type 2 diabetes. Diabetes. 2014;63(Suppl. 1):A18. Abstract 66-OR. [Google Scholar]
- Rodbard HW, Buse JB, Woo VW. Benefits of combination of insulin degludec and liraglutide (IDegLira) are independent of baseline HbA1c and duration of type 2 diabetes. Diabetes Obes Metab. 2015 doi: 10.1111/dom.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AB, Philis-Tsimikas A, Langbakke IH. IDegLira, a combination of insulin degludec and liraglutide, improves both pre- and postprandial plasma glucose in patients with type 2 diabetes. Diabetologia. 2014;57(Suppl. 1) [Google Scholar]
- Novo Nordisk [2015 Sep 14];2014 Sep 18; http://www.novonordisk.com/bin/getPDF.1856972.pdf Press Release 2014. cited. Available from.
- [2015 Sep 25;];Novo Nordisk. Novo Nordisk receives US FDA approval for Tresiba® and Ryzodeg® 70/30. http://www.novonordisk.com/content/Denmark/HQ/www-novonordisk-com/en_gb/home/media/news-details.1954709.html cited. Available from.
- Novo Nordisk [2015 Jun 30;];Xultophy Summary of Product Characteristics. 2014 https://www.medicines.org.uk/emc/medicine/29493 Last updated. cited. Available from.
- Kim YG, Hahn S, Oh TJ. Differences in the HbA1c-lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:900–909. doi: 10.1111/dom.12293. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation [2015 May];Atlas Factsheet. 2014 http://www.idf.org/sites/default/files/Atlas-poster-2014_EN.pdf cited. Available from.
- Rosenstock J, Diamant M, Silvestre L. Benefits of a fixed-ratio formulation of once-daily insulin glargine/lixisenatide (LixiLan) vs. glargine in type 2 diabetes (T2DM) inadequately controlled on metformin. Diabetes. 2014;63(Suppl 1) Abstract 332-OR). [Google Scholar]


