Abstract
Objectives The present study investigated the effect of cements on fracture resistance of monolithic zirconia crowns in relation to their compressive strength.
Materials and methods Four different cements were tested: zinc phosphate cement (ZPC), glass-ionomer cement (GIC), self-adhesive resin-based cement (SRC) and resin-based cement (RC). RC was used in both dual cure mode (RC-D) and chemical cure mode (RC-C). First, the compressive strength of each cement was tested according to a standard (ISO 9917-1:2004). Second, load-to-failure test was performed to analyze the crown fracture resistance. CAD/CAM-produced monolithic zirconia crowns with a minimal thickness of 0.5 mm were prepared and cemented to dies with each cement. The crown–die samples were loaded until fracture.
Results The compressive strength of SRC, RC-D and RC-C was significantly higher than those of ZPC and GIC (p < 0.05). However, there was no significant difference in the fracture load of the crown between the groups.
Conclusion The values achieved in the load-to-failure test suggest that monolithic zirconia crowns with a minimal thickness of 0.5 mm may have good resistance against fracture regardless of types of cements.
Keywords: Monolithic zirconia crowns, fracture resistance, zinc phosphate cement, glass-ionomer cement, resin-based cement
Introduction
Zirconia has become widely used for frameworks of all-ceramic restorations due to its mechanical properties and improved esthetic compared with metal alloys.[1] Still, questions about zirconia-based prostheses have been raised, mostly due to the risk of chipping of the veneering porcelain.[2,3] Newly developed monolithic zirconia restorations without veneering porcelain have overcome this problem. In addition, production of the crowns with computer-aided design (CAD)/computer aided manufacturing (CAM) technique without a veneering process can improve the quality with a high degree of homogeneity and might decrease the cost.
Monolithic zirconia crowns possess sufficient fracture resistance for dental crown restorations due to the high strength of the material. That is attributable to the feature known as stress-induced transformation toughing in yttria stabilized zirconia.[4] A crystalline phase transformation occurs from tetragonal to monoclinic under a stress resulting in a local volume expansion of the crystals. This results in the generation of compressive stress around the crack that impedes further crack propagation. It has been suggested that monolithic zirconia crowns have high fracture resistance enough to be applied in the molar regions even if the crown thickness is thinner than conventional all-ceramic crowns. Nakamura et al. [5,6] showed that monolithic zirconia crowns with a minimal thickness of 0.5 mm displayed a mean fracture load of over 5000 N, which was significantly higher than that of monolithic lithium disilicate crowns with a crown thickness of 1.5 mm. The thin monolithic zirconia crowns are expected to be a new alternative as a less-invasive treatment. Still, there are several issues to be studied before a generalized use of thin monolithic crowns can be recommended.
Fractures of zirconia do not seem to be a common problem, although ceramics, including zirconia, are referred to as brittle materials.[4,7] Even if monolithic zirconia crowns seem to have sufficient fracture resistance, the importance of the cement should not be underestimated.[8,9] It has been demonstrated that the supporting materials, such as abutment material and cement, will influence the fracture resistance of all-ceramic crowns.[10,11] That is, if the abutment material shows increased elastic properties and/or low compressive strength, the fracture resistance of all-ceramic crowns becomes lower. As for type of cement used, it is suggested that the compressive strength is of importance since this factor will influence the support of the reconstruction.[8] Indeed, Bindl et al. [12] demonstrated that the fracture resistance of monolithic all-ceramic crowns made of feldspar ceramic, leucite glass-ceramic and lithium disilicate glass-ceramic increased by using a resin-based cement (RC) with a compressive strength of 320 MPa compared to zinc phosphate cement (ZPC) (121 MPa). In addition to the compressive strength, it is suggested that the crown-cement as well as cement–abutment interface plays an important role in the fracture resistance of all-ceramic crowns.[13,14] The weaker the bond the lower the fracture resistance becomes. It is, however, difficult to treat ceramics based on zirconia for an optimal micromechanical adhesion to RC because of the structure of this oxide ceramic.[9] Even though adhesion between zirconia and RC is not well established, the high compressive strength of the RC may be of importance to give the crown–cement–tooth complex the ability to withstand forces also in the molar region. To author’s knowledge, there is little information about the influence of compressive strength of the cement on the fracture resistance of monolithic zirconia crowns.
The purpose of the present study was, therefore, to investigate the effect of the cements on fracture resistance of monolithic zirconia crowns in relation to their compressive strength. The presented null hypothesis was that the compressive strength of the cement would have no influence on the fracture strength of the monolithic zirconia crowns.
Materials and methods
Cements
Four different types of cements were used; ZPC (De Trey Zinc, Dentsply, York, PA), glass-ionomer cement (GIC; Fuji I, GC, Tokyo, Japan), self-adhesive resin-based cement (SRC; RelyX Unicem2, 3M/ESPE, St. Paul, MN) and adhesive RC (Panavia F2.0, Kuraray Noritake Dental, Tokyo, Japan). RC was tested in both dual cure mode (RC-D) and pure chemical cured mode (RC-C).
When light curing was needed throughout the study, a light curing unit (Bluephase, Ivoclar/Vivadent, Schaan, Lichtenstein, Germany) was used at an irradiance of 1370 ± 50 mW/cm2 controlled using Bluephase meter (Ivoclar/Vivadent) at each occasion.
Compressive strength test of the cement
The compressive strength of cement was tested according to ISO 9917-1:2004.[15] The cement was mixed and set at temperature of 23 ± 2 °C and humidity of 30%. Ten samples from each cement were produced in a mold of polytetra-fluoroethene (PTFE) with the inner dimension of 4 mm in diameter and a height of 6 mm according to the manufacturers’ instructions.
ZPC was mixed on a chilled glass plate. One scoop of powder and six droplets of the liquid were mixed to get sufficient consistency. The cement with slight excess was introduced into the PTFE mold placed on a glass-plate covered with a polyethylene (PE) film (NKV, Umeå, Sweden) using a Jiffy tube (Produits Dentaires SA, Vevey, Switzerland). The upper surface was treated as the lower end by coverage using the same film (PE) with glass plate on top and the cement was left to set. GIC was auto-mixed according to the manufacturer’s instructions using ESPE cap mix (3M/ESPE) and applied in the PTFE mold with slight excess using a Fuji applicator (GC) following the same protocol as for ZPC. For SRC, the PTFE mold was filled with a mix of the two pastes from the mixing syringe. The mold was treated as described above and the cement was light cured through the glass plate from above for 2 s, after which the plate was removed and continued light curing was performed for 40 s. For RC-D, equal amounts of base and catalyst were mixed. A droplet consisting of a mixture of ED primer A and B (Kuraray Noritake Dental) was added to the cement to get proper chemical cure. The cement was applied into the mold using a Jiffy tube following the protocol for ZPC and GIC. The light curing was performed following the protocol for SRC. To test the influence of the chemical curing without light curing on the compressive strength, 10 samples of RC-C were also made following the same protocol except for the light curing.
After curing the end surfaces of each specimen were polished using SiC paper (400 grit) to remove excess cement and to ensure a surface perpendicular to the load direction. The dimensions of the specimens were measured using a digital micrometer (IP 65, Mitutoyo, Tokyo, Japan). After storage in distilled water at 37 ± 1 °C for 24 ± 1 h, the specimens were subjected to a compressive strength test at a cross-head speed of 0.75 mm/min using a universal testing machine (Zwick/Roell, Ulm, Germany). The compressive strength was calculated according to the formula given in the standard.
Preparation of abutments and crowns
Tooth preparation and fabrication of dies were performed according to the protocol from a previous study.[5] Briefly, a plastic tooth model of mandibular right first molar (A5A-500, NISSIN, Kyoto, Japan) was prepared with a 0.5-mm chamfer width, minimal occlusal reduction of 0.6 mm and a total occlusal convergence angle of 10°. The margin was set at 0.5 mm above the cement–enamel junction of the tooth model. Subsequently, the prepared tooth model was scanned and dies were milled from hybrid polymer resin-based blocks (Lava Ultimate, 3M/ESPE) possessing similar mechanical properties to those of dentin.[5]
Manufacturing procedure of the monolithic zirconia crowns tested
Thirty monolithic zirconia crowns were fabricated (n = 6 for each group). The die was scanned using the same dental CAD/CAM scanner. Scanning of a non-prepared tooth model was performed for the outer design of the crown. The cement space was fixed at 70 μm for all samples according to the default setting of the CAD/CAM software (Lava Design 5.50 CAD software, 3M/ESPE). Thus, the minimum thickness of crown at occlusal surface was expected to be >0.5 mm as a result of subtraction of cement space from occlusal reduction. The data of the crown design were transferred to the 3M Lava milling center (Digital Dental Operation, Osaka, Japan) for fabrication of the monolithic zirconia crowns (Lava Plus Zirconia, 3M/ESPE) with the A2 shade. After sintering, margin adjustment was performed manually using a grinding point (CeraPro, Edenta, AU/SG, Switzerland), after which polishing was performed using a series of polishing points (StarGloss, Edenta, Switzerland) and a wheel brush together with polishing agent (Zircon-Brite, Dental Ventures of America, Corona, CA).
Micro-CT analysis of the crown thickness and cement space
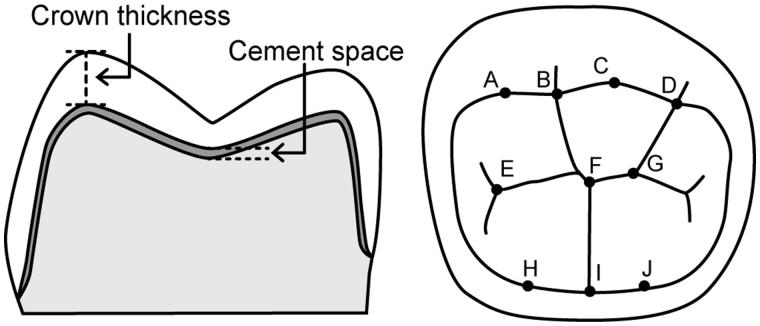
Three crowns and dies were randomly selected, and the crowns seated onto the dies without cement were subjected to the analysis. A cone beam micro-CT (ScanXmate-D2225RSS270, Comscantecno, Kanagawa, Japan) was used with a voltage of 200 kV and current of 200 μA. The number of projections was 1200 with a resolution of 14.9 μm and a 360° rotation. The CT data were reconstructed using a software (coneCTexpress, Comscantecno), and then the reconstructed images were analyzed using an image processing program (ImageJ, NIH, Bethesda, MD). The vertical distance between the inner and outer surface of crowns was regarded as the crown thickness. Similarly, the vertical distance between the inner surface of crowns and the surface of the die was regarded as the cement space. The crown thickness and cement space in the occlusal surface was measured at 10 different points as shown in Figure 1 (mesiobuccal cusp, buccal groove, distobuccal cusp, distal cusp, mesial pit, central fossa, distal pit, mesiolingual cusp, lingual groove and distolingual cusp).
Figure 1.
Schematic representation of measuring points in micro-CT analysis. Crown thickness and cement space were measured as the vertical distance at 10 different points (A–J). The minimal occlusal thickness was obtained at B, F and I.
Load-to-failure test
The protocol followed the recommendations presented by Kelly [16] for in vitro testing of all-ceramic crowns, which was also used in our previous studies.[5,6] Thirty monolithic zirconia crowns and dies were divided into five groups with six samples in each group. Before cementation, the dies were placed in the testing jig and fixed with a silicon impression material (Flexitime Correct flow, Heraeus/Kulzer, Hanau, Germany) to ensure their stabilization during cementation. The inner surface of the crowns and the preparation surface of the dies were degreased with 99% ethanol. The alcohol was left to dry/vaporize for 60 s. The dies used for RC group were additionally treated with the primer. Equal amounts of ED primers A and B were blended and applied to the die surface according to the manufacturer’s instruction.
The cements were prepared and mixed as described above. A thin layer of cement was applied to the internal surfaces of the crowns, after which they were directly positioned on their dies. For ZPC, GIC and SRC, any excess cement was removed with a carver after setting. For RC (both curing modes), the excess was removed with a quick stick (Dentsolve AB, Huddinge, Sweden) and the crown margins were covered with Oxyguard (Kuraray/Noritake). To ensure equal conditions during cementation, the crown seated on the die was placed in a universal testing machine (Zwick/Roell) and a static load of 20 N (load stylus Ø = 10 mm) was applied until the cement had set according to a previous study.[17] A urethane rubber sheet with a thickness of 2 mm and Shore A Hardness of 90 (Kokugo, Tokyo, Japan) was interspersed between the indenter and the occlusal surface to avoid contact damage. For ZPC, GIC and RC-C, the crowns were subjected to the static load for 15 min to ensure proper seating and setting. For the crowns cemented with SRC and RC-D, the pressure was held for 4 min, while the cement was light cured from five directions for 40 s (total: 200 s).
After storage in distilled water at 37 ± 1 °C for 24 ± 1 h, the crown–die samples were mounted in the testing jig using poly di-vinylsiloxane material (Flexitime Bite, Heraeus/Kulzer). The load-to-failure test was performed using the universal testing machine (Zwick/Roell) with a 50 kN load-cell. A custom-made indenter (Ø = 10 mm) of type 304-stainless steel (Zwick/Roell) was placed in the central fossa of the occlusal surface. Caution was taken to place the indenter as equal as possible at each test occasion. To avoid contact damage, the aforementioned urethane rubber sheet was placed between the indenter and occlusal surface. A preload of 20 N was applied vertically to the crown followed by compressive loading at a cross-head speed of 0.5 mm/min until fracture. Load at breakage was recorded and differences were compared between the groups. After the load-to-failure test, fracture analysis was performed with scanning electron microscopy (Carl Zeiss Microscopy, Sigma, Jena, Germany) on two randomly selected samples from each group.
Statistical analysis
Statistical analyses were performed using JMP Pro 11.0.0 software (SAS Institute, Cary, NC). Differences in the compressive strength and fracture load were analyzed with one-way ANOVA followed by the Tukey–Kramer HSD multiple comparison test. The level of significance was set at 5%.
Results
Compressive strength of cement
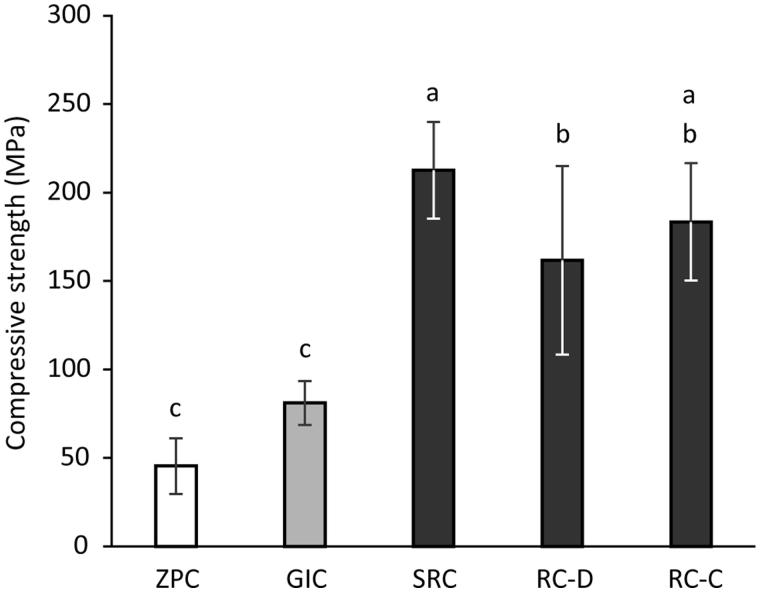
The results of the compressive strength test of the cements are shown in Figure 2. The compressive strengths of ZPC and GIC were significantly lower (p <0.01) than those of SRC, RC-D and RC-C. In addition, SRC showed significantly higher compressive strength (p <0.01) than RC-D.
Figure 2.
Compressive strength of cements tested. Different letters above the columns show significant differences (p < 0.01). ZPC, zinc phosphate cement; GIC, glass-ionomer cement; SRC, self-adhesive resin-based cement; RC-D, resin-based cement (dual cure mode); RC-C, resin-based cement (chemical cure mode).
Micro-CT analysis
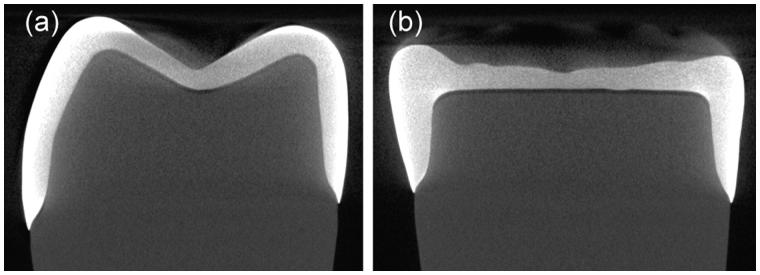
Representative micro-CT images are shown in Figure 3. It was observed that no detectable defects within the crowns existed. The minimal occlusal thickness of the crown recorded at buccal groove, central fossa and lingual groove was 0.5 ± 0.1 mm (Table 1). The cement space in the occlusal surface was in the range of 112–144 μm depending on the measuring points (Table 1), although it was designed to be 70 μm in the CAD/CAM software.
Figure 3.
Representative micro-CT images of (a) bucco-lingual aspect and (b) mesio-distal aspect.
Table 1.
The mean values (SD) of crown thickness and cement space.
| A | B | C | D | E | F | G | H | I | J | |
|---|---|---|---|---|---|---|---|---|---|---|
| Crown thickness (μm) | 1020 | 523 | 1065 | 672 | 666 | 525 | 652 | 1101 | 553 | 1091 |
| (23) | (6) | (3) | (22) | (5) | (20) | (16) | (28) | (16) | (25) | |
| Cement space (μm) | 130 | 125 | 123 | 125 | 139 | 137 | 144 | 119 | 113 | 112 |
| (10) | (4) | (15) | (11) | (2) | (8) | (8) | (22) | (25) | (21) |
A–J correspond to the measuring points shown in Figure 1.
Load-to-fracture test
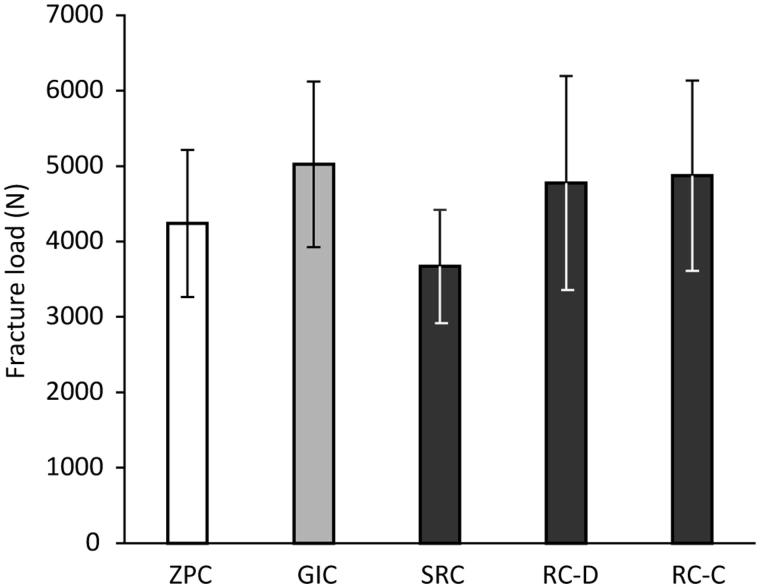
The results of the load-to-failure test of the monolithic zirconia crowns cemented with various types of cements are shown in Figure 4. There were no significant differences in fracture resistance between the different cement groups. Mean values of the fracture load for all groups were greater than 3500 N. No signs of Hertzian cone cracks at the occlusal surface were observed in the SEM images. In all cases, primary fracture origin was located at occlusal surface (Figure 5).
Figure 4.
Fracture resistance of monolithic zirconia crowns cemented to dies using different cements. One-way ANOVA revealed that there was no significant difference between the groups. ZPC, zinc phosphate cement; GIC, glass-ionomer cement; SRC, self-adhesive resin-based cement; RC-D, resin-based cement (dual cure mode); RC-C, resin-based cement (chemical cure mode).
Figure 5.
Representative SEM image of fractured monolithic zirconia crown. The fractographic features (i.e. fracture mirror, mist and hackles) indicated that the fracture origin was located at occlusal surface. The dotted arrows indicate the direction of the fracture wave.
Discussion
The null hypothesis of the present study was confirmed since the cements did not affect the fracture resistance of the monolithic zirconia crowns even though the significant differences in compressive strength between the cements were recorded.
In the present study, standardized crowns and dies were used to eliminate the bias in the evaluation of the effect of cement on the fracture resistance of the monolithic zirconia crowns. The micro-CT analysis revealed that the SD of the crown thickness and cement space was small (<30 μm), suggesting that the influence of difference in dimensions of the crowns and dies was limited. The load-to-failure test was performed basically in compliance with the recommendation for clinically relevant preclinical tests [16] and according to the protocol of recent published studies on thin monolithic zirconia crowns with the same design.[5,6] The dies used were made of hybrid polymer resin-based material that possessed similar flexural strength (196 ± 10 MPa), elastic modulus (10.73 ± 0.28 GPa) and Poisson’s ratio (0.43 ± 0.03) [5] to those of dentin.[18,19] In concordance to previous studies,[5,20] a 2 mm thick urethane rubber sheet was inserted between the crown and steel indenter to avoid contact damage with the steel indenter.
The micro-CT analysis disclosed that the cement space was approximately twice (about 130 μm) as large as that set in the CAD/CAM software (70 μm). As previously reported,[21] designing tool for cement space in a CAD software seems to be semi-quantitative. Since the software tries to compensate errors of milling and/or sintering shrinkage to avoid interference between the internal surface of crown and the abutment, the actual cement space might become thicker than the designed one. Regarding the influence of cement thickness, Scherrer et al. [14] demonstrated that the strength of glass-ceramic plates cemented onto composite resin blocks with ZPC decreased when the cement thickness increased from 30 to 130 μm. However, they also suggested that the effect of cement thickness could be negligible as long as the RC was used with a cement thickness of <300 μm. Thus, it might be considered that the increased cement space observed in the present study would have a stronger influence on the fracture resistance of the crowns cemented with ZPC than those cemented with RCs. Furthermore, ZPC and GIC possess significantly lower compressive strength than RC, which is supposed to be one of the important factors that affect the fracture resistance of all-ceramic crowns.[12] Nonetheless, there was no significant difference in the fracture resistance of the monolithic zirconia crowns between the various cement groups. The mean values of fracture load in each group were recorded at over 3500 N even though the minimal occlusal thickness of the crowns was 0.5 mm, which are much higher than maximum bite force in the molar regions reported in literature.[22,23] This suggests the possibility of the application of thin monolithic zirconia crowns in the molar region regardless of the type of cement used when supported by a sufficient preparation design.
Although the present study was designed and conducted based on the available scientific information on methodology, there is no standardized test method of load-to-failure test for single crowns, especially where molar crowns with anatomical shape are tested. The present study as well as our previous study were preformed based on attempts to simulate clinically relevant failures using an indenter with a diameter of 10 mm.[5,6] According to Kelly,[16] the size of intender (Ø = 10 mm) is the lowest limit where clinically relevant contact pressure can be obtained. Although Oilo et al. [20] showed that using an indenter with a diameter of 30 mm in a load-to-failure test would simulate clinical fractures of all-ceramic crowns with a slight concave occlusal surface (not anatomical shape), we assumed that loading at ridges on the occlusal surface using 10 mm indenter might achieve more clinically relevant load distribution than loading at the top of the cusps using 30 mm indenter. Indeed, several previous studies, in which fracture resistance of molar crowns with anatomical shape was tested, used an indenter or ball with a diameter of around 10 mm.[10,24,25] However, in the case of monolithic zirconia crowns, the crowns seemed to fracture as a result of wedging force generated at the bottom of the central fissure where fracture origin was observed. Since the previous studies testing anatomical all-ceramic crowns other than monolithic zirconia crowns did not report such fracture pattern,[10,24,25] the failure caused by wedging force may be related to the properties of zirconia. One of the possible reasons would be due to the high strength of zirconia by which monolithic zirconia crowns might withstand the load that can fracture other types of all-ceramic crowns in combination with the effect of mechanical and adhesive properties of cement. As a result, the high load might generate wedging force. This should be further studied in the future together with an establishment of optimal test method.
The results of the present study also are in concordance with other results achieved. Cementation with RC does not necessarily result in higher fracture resistance of monolithic zirconia crown. Zesewitz et al.,[26] demonstrated that there was no significant difference in the fracture load between monolithic zirconia crowns cemented onto metal dies with RC and those cemented with GIC. In the case of zirconia-based restorations, it is considered that conventional cementation is acceptable, although RC might be a first choice [1] even if adhesion between zirconia and RC can be difficult to achieve.[9] Indeed, clinical studies in which ZPC and GIC were used for cementation of zirconia-based single crowns reported no increased incidence rate of fracture related to the cementation.[27,28] As shown in a finite element analysis,[29] contribution of cement thickness and cement elastic modulus to maximum principal stress in crowns would be much lower than that of the crown material. Although previous studies have demonstrated that type of cement would significantly affect the fracture resistance of all-ceramic crowns, the tested materials were feldspathic porcelain, leucite glass-ceramic and lithium disilicate glass-ceramic,[10,12] which possessed much lower flexural strength than zirconia.[30] Taking this into consideration, it is suggested that the high strength of zirconia ceramic might prevail against the effect of certain cement properties such as low compressive strength and increased cement film thickness on the fracture resistance of the monolithic zirconia crowns.
Apart from internal stress development due to differences in the properties of the various materials, the preparation has also been found to affect the fracture resistance of all-ceramic crowns. Rekow et al. [31] have convincingly demonstrated that the height of the axial walls of the preparation influenced fracture strength of all-ceramic crowns. Increased height resulted in increased fracture strength. It was also demonstrated that the curvature of the cervical finish line, i.e. the difference in vertical position between the proximal and buccal/lingual surfaces, likewise the preparation design in the present study, would also exert a definitive influence on the location and stress level in all-ceramic crowns,[31] and thus also affect the fracturing mode.[7,20] Therefore, a deeper understanding of the effect of cement will call for a study including various abutment heights, convergence angles and curvatures of the cervical finishing line.
In the fractographic analysis, no signs of Hertzian cone cracks that are not seen in all-ceramic crowns fractured in clinical situations [32,33] was observed, suggesting that the test condition could successfully avoid the contact damage of the steel indenter. With respect to fracture mechanism of all-ceramic crowns, no consensus seems to exist. The mechanism probably can be referred to the existence of many complex and interacting factors. Although in vitro testing will imply a simplification of the in vivo conditions, it will still allow a standardization of certain factors that are difficult to standardize in the clinic. The inherent material properties of the crown–cement–abutment complex will exert a definite influence on the response to loading. The difference in the Poisson’s ratio as well as in the modulus of elasticity between the crown and die material (Poisson’s ratio: 0.33 for zirconia vs. 0.43 for Lava Ultimate, Modulus of elasticity: 220 GPa for zirconia vs. 11 GPa for Lava Ultimate) [5,34] may give rise to stress build-up, eventually leading to material fracture. It is plausible that this can also occur in the clinical situation since the die used in the present study had mechanical properties close to those of wet dentin.[18,19] Oilo et al. [7,20,33] demonstrated that the fractures of all-ceramic crowns seen in the clinical situation and replicated in an in vitro experimental study originated from the cervical parts probably as a result of hoop stress due to volumetric changes of the abutment material at loading. In the present study and contrary to their findings, the fractures in the monolithic zirconia crowns initiated from the occlusal surface, verified by fractographic features displayed in SEM images of the fracture surfaces. The divergence in results may in specific be referred to differences in the crown and abutment materials as well as in the testing methods used. A thicker interspersed sheet and a larger diameter spherical indenter than in the present study would more strongly reduce and level out the loading factor. Lack of information on, e.g. the mechanical properties of the epoxy material used for the abutments and certain loading factors make a direct comparison of results difficult. It may be speculated if not both studies are valid exposing different materials and loading conditions.
It should be noted that there are limitations in terms of clinical relevance of the load-to-failure test with single loading although the results may provide helpful data for comparisons between the groups. It is known that thermal and mechanical cycling procedure simulating mastication in oral cavity affects the fracture resistance of all-ceramic crowns.[35,36] Furthermore, in the case of monolithic zirconia crowns, low-temperature degradation (LTD) also referred to as aging [37] may result in lower fracture resistance after long clinical service.[38] In our previous study,[6] it was, however, demonstrated that, even if the monolithic zirconia crowns with a minimal crown thickness of 0.5 mm cemented to dies using a RC were subjected to cyclic loading with a load of 300 N for 240 000 cycles, the fracture resistance did not decrease. By contrast, LTD experimentally induced by 100 h of autoclaving at 134 °C against the monolithic zirconia crowns before cementation resulted in approximately 30% reduction of the fracture resistance. Although the effect of such aging procedures on the durability of cements should be further studied simulating longer clinical service, there still seems to be a considerable strength safety margin for thin monolithic zirconia crowns, even in situations of high biting forces.
Conclusion
Within the limitations of this study, the following conclusions for the monolithic zirconia crowns tested were drawn: the compressive strength of the cement differed significantly but seemed to be of no importance for the fracture resistance of the crowns tested. The difference in chemical and mechanical properties of the various cements was not reflected in significant differences in fracture resistance, either. Since the relatively high value was achieved in the load-to-failure test, it is suggested that monolithic zirconia crowns with a minimal thickness of 0.5 mm may have good resistance against fractures regardless of types of cements.
Acknowledgements
Special thanks to Dentsply/DeTrey, Kuraray Noritake, GC Corp. and 3M/ESPE for their support.
Declaration of interest
None to declare.
Funding
This study was supported by the grant from Wihelm and Martina Lundgren foundation, Sweden.
References
- 1. Manicone PF, Rossi Iommetti P, Raffaelli L.. An overview of zirconia ceramics: basic properties and clinical applications. J Dent. 2007;35:819–826. [DOI] [PubMed] [Google Scholar]
- 2. Larsson C, Vult von Steyern P.. Five-year follow-up of implant-supported Y-TZP and ZTA fixed dental prostheses. A randomized, prospective clinical trial comparing two different material systems. Int J Prosthodont. 2010;23: 555–561. [PubMed] [Google Scholar]
- 3. Larsson C, Vult von Steyern P, Nilner K.. A prospective study of implant-supported full-arch yttria-stabilized tetragonal zirconia polycrystal mandibular fixed dental prostheses: three-year results. Int J Prosthodont. 2010;23: 364–369. [PubMed] [Google Scholar]
- 4. Denry I, Kelly JR.. State of the art of zirconia for dental applications. Dent Mater. 2008;24:299–307. [DOI] [PubMed] [Google Scholar]
- 5. Nakamura K, Harada A, Inagaki R, et al. Fracture resistance of monolithic zirconia molar crowns with reduced thickness. Acta Odontol Scand. 2015;73:602–608. [DOI] [PubMed] [Google Scholar]
- 6. Nakamura K, Harada A, Kanno T, et al. The influence of low-temperature degradation and cyclic loading on the fracture resistance of monolithic zirconia molar crowns. J Mech Behav Biomed Mater. 2015;47:49–56. [DOI] [PubMed] [Google Scholar]
- 7. Oilo M, Hardang AD, Ulsund AH, Gjerdet NR.. Fractographic features of glass-ceramic and zirconia-based dental restorations fractured during clinical function. Eur J Oral Sci. 2014;122:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanie T, Kadokawa A, Nagata M, Arikawa H.. A comparison of stress relaxation in temporary and permanent luting cements. J Prosthodont Res. 2013;57:46–50. [DOI] [PubMed] [Google Scholar]
- 9. Papia E, Larsson C, du Toit M, Vult von Steyern P.. Bonding between oxide ceramics and adhesive cement systems: a systematic review. J Biomed Mater Res B Appl Biomater. 2014;102:395–413. [DOI] [PubMed] [Google Scholar]
- 10. Mormann WH, Bindl A, Luthy H, Rathke A.. Effects of preparation and luting system on all-ceramic computer-generated crowns. Int J Prosthodont. 1998;11:333–339. [PubMed] [Google Scholar]
- 11. Yucel MT, Yondem I, Aykent F, Eraslan O.. Influence of the supporting die structures on the fracture strength of all-ceramic materials. Clin Oral Investig. 2012;16: 1105–1110. [DOI] [PubMed] [Google Scholar]
- 12. Bindl A, Luthy H, Mormann WH.. Strength and fracture pattern of monolithic CAD/CAM-generated posterior crowns. Dent Mater. 2006;22:29–36. [DOI] [PubMed] [Google Scholar]
- 13. Behr M, Rosentritt M, Mangelkramer M, Handel G.. The influence of different cements on the fracture resistance and marginal adaptation of all-ceramic and fiber-reinforced crowns. Int J Prosthodont. 2003;16:538–542. [PubMed] [Google Scholar]
- 14. Scherrer SS, de Rijk WG, Belser UC, Meyer JM.. Effect of cement film thickness on the fracture resistance of a machinable glass-ceramic. Dent Mater. 1994;10:172–177. [DOI] [PubMed] [Google Scholar]
- 15. ISO 9917-1. Dentistry – water-based cements 2004. [Google Scholar]
- 16. Kelly JR. Clinically relevant approach to failure testing of all-ceramic restorations. J Prosthet Dent. 1999;81: 652–661. [DOI] [PubMed] [Google Scholar]
- 17. Pallis K, Griggs JA, Woody RD, et al. Fracture resistance of three all-ceramic restorative systems for posterior applications. J Prosthet Dent. 2004;91:561–569. [DOI] [PubMed] [Google Scholar]
- 18. Kinney JH, Gladden JR, Marshall GW, et al. Resonant ultrasound spectroscopy measurements of the elastic constants of human dentin. J Biomech. 2004;37:437–441. [DOI] [PubMed] [Google Scholar]
- 19. Kinney JH, Marshall SJ, Marshall GW.. The mechanical properties of human dentin: a critical review and re-evaluation of the dental literature. Crit Rev Oral Biol Med. 2003;14:13–29. [DOI] [PubMed] [Google Scholar]
- 20. Oilo M, Kvam K, Tibballs JE, Gjerdet NR.. Clinically relevant fracture testing of all-ceramic crowns. Dent Mater. 2013;29:815–823. [DOI] [PubMed] [Google Scholar]
- 21. Moldovan O, Luthardt RG, Corcodel N, Rudolph H.. Three-dimensional fit of CAD/CAM-made zirconia copings. Dent Mater. 2011;27:1273–1278. [DOI] [PubMed] [Google Scholar]
- 22. Anusavice KJ. Mechanical properties of dental materials In: Anusavice KJ, Shen C, Rawls HR, editors. Phillips' science of dental materials. 12th ed St. Louis (MO): Saunders; 2013. p. 48–68. [Google Scholar]
- 23. Vallittu PK, Kononen M.. Biomechanical aspects and material properties In: Nilner K, Karlsson S, Dahl BL, editors. A textbook of fixed prosthodontics: the scandinavian approach. 2nd ed Stockholm: Gothia Fortbildning; 2013. p. 1521–1571. [Google Scholar]
- 24. Rosentritt M, Plein T, Kolbeck C, et al. In vitro fracture force and marginal adaptation of ceramic crowns fixed on natural and artificial teeth. Int J Prosthodont. 2000;13: 387–391. [PubMed] [Google Scholar]
- 25. Beuer F, Stimmelmayr M, Gueth JF, et al. In vitro performance of full-contour zirconia single crowns. Dent Mater. 2012;28:449–456. [DOI] [PubMed] [Google Scholar]
- 26. Zesewitz TF, Knauber AW, Northdurft FP.. Fracture resistance of a selection of full-contour all-ceramic crowns: an in vitro study. Int J Prosthodont. 2014;27: 264–266. [DOI] [PubMed] [Google Scholar]
- 27. Ortorp A, Kihl ML, Carlsson GE.. A 5-year retrospective study of survival of zirconia single crowns fitted in a private clinical setting. J Dent. 2012;40:527–530. [DOI] [PubMed] [Google Scholar]
- 28. Tartaglia GM, Sidoti E, Sforza C.. Seven-year prospective clinical study on zirconia-based single crowns and fixed dental prostheses. Clin Oral Investig. 2014;19:1137–1145. [DOI] [PubMed] [Google Scholar]
- 29. Rekow ED, Harsono M, Janal M, et al. Factorial analysis of variables influencing stress in all-ceramic crowns. Dent Mater. 2006;22:125–132. [DOI] [PubMed] [Google Scholar]
- 30. Anusavice KJ. Dental ceramics In: Anusavice KJ, Shen C, Rawls HR, editors. Phillips' science of dental materials. 12th ed St. Louis (MO): Saunders; 2013. p. 418–473. [Google Scholar]
- 31. Rekow ED, Zhang G, Thompson V, et al. Effects of geometry on fracture initiation and propagation in all-ceramic crowns. J Biomed Mater Res B Appl Biomater 2009;88:436–446. [DOI] [PubMed] [Google Scholar]
- 32. Scherrer SS, Quinn GD, Quinn JB.. Fractographic failure analysis of a Procera AllCeram crown using stereo and scanning electron microscopy. Dent Mater. 2008;24: 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oilo M, Gjerdet NR.. Fractographic analyses of all-ceramic crowns: a study of 27 clinically fractured crowns. Dent Mater. 2013;29:e78–e84. [DOI] [PubMed] [Google Scholar]
- 34. Guazzato M, Proos K, Quach L, Swain MV.. Strength, reliability and mode of fracture of bilayered porcelain/zirconia (Y-TZP) dental ceramics. Biomaterials. 2004;25: 5045–5052. [DOI] [PubMed] [Google Scholar]
- 35. Yang R, Arola D, Han Z, Zhang X.. A comparison of the fracture resistance of three machinable ceramics after thermal and mechanical fatigue. J Prosthet Dent. 2014; 112:878–885. [DOI] [PubMed] [Google Scholar]
- 36. Seydler B, Rues S, Muller D, Schmitter M.. In vitro fracture load of monolithic lithium disilicate ceramic molar crowns with different wall thicknesses. Clin Oral Investig. 2014;18:1165–1171. [DOI] [PubMed] [Google Scholar]
- 37. Chevalier J, Gremillard L, Deville S.. Low-temperature degradation of zirconia and implications for biomedical implants. Ann Rev Mater Res. 2007;37:1–32. [Google Scholar]
- 38. Flinn B, deGroot D, Mancl L, Raigrodski AJ.. Accelerated aging characteristics of three yttria-stabilized tetragonal zirconia polycrystalline dental materials. J Prosthet Dent. 2012;108:223–230. [DOI] [PubMed] [Google Scholar]