Abstract
Objective
To compare the effects of esmolol (β-blocker) and dexmedetomidine (α-2-agonist) on patients’ clinical course and cost of application of controlled hypotension during middle-ear surgery.
Methods
Fifty ASA I–II patients scheduled for tympanomastoidectomy were enrolled in the study and were randomized into two groups. Bispectral Index (BIS) and neuromuscular monitoring (TOF GUARD-SX) were applied to all patients. In group E (n=25), 0.5 mg kg−1 min−1 esmolol was infused over 1 min before induction and titrated over a range of 10–200 μg kg−1 min−1; in group D (n=25), 0.5 μg kg−1 dexmedetomidine was infused over 10 minutes before induction, and then titrated over a range of 0.2–0.7 μg kg−1 hr−1 to maintain mean arterial pressure (MAP) between 55 and 65 mmHg and BIS 40–50 after induction. In both groups, 0.25 μg kg−1 min−1 remifentanil infusion was used for anaesthesia maintenance. Maintaining end-tidal CO2 (EtCO2) at 35–40, using 1 MAC sevoflurane in 50% O2-air mixture, mechanical ventilation was started. The effects of both agents on hemodynamic conditions [(heart rate (HR), mean arterial pressure (MAP)], neuromuscular blockage [onset of action (OA), duration of clinical action (DCA), recovery index (RI)], amount of bleeding, surgeon satisfaction, and total dexmedetomidine and esmolol doses used during the intervention were recorded and costs were compared between the groups.
Results
No significant difference was present in hemodynamic conditions, bleeding scores or surgeon satisfaction between groups. Although OA was similar in both groups, DCA and RI were significantly higher in group D. Cost was significantly higher with esmolol than dexmedetomidine.
Conclusion
We conclude that although both agents are feasible in inducing hypotensive anaesthesia, while neuromuscular block time prolonged by using dexmedetomidine, higher costs were observed with esmolol.
Keywords: Anesthesia, esmolol, dexmedetomidine, controlled hypotension, middle ear surgery
Introduction
Controlled hypotensive anaesthesia is deliberate and reversible lowering and maintaining of the arterial blood pressure below 50% of normal values or to a mean blood pressure of 50–65 mmHg (1).
The blood pressure values, which may increase during and after the intervention, can cause bleeding and impairment in the quality of surgical field in middle ear surgery (2).
Controlled hypotensive anaesthesia, by reducing blood loss and providing the surgeon a good field of vision, is frequently used in orthopaedic surgery, neurosurgery, and middle ear and nose surgery (1, 2). Various hypotensive agents such as volatile anaesthetics, sympathetic antagonists, sodium nitroprusside, nitroglycerin, hydralazine, trimethaphan, adenosine, fenoldopam and α2 agonists can be used for this purpose (3).
The agents used to induce controlled hypotensive anaesthesia, can provide better hemodynamic conditions during anaesthesia induction, maintenance and extubation periods by suppressing the sympathoadrenal response (4, 5). This may affect surgeon satisfaction during the intervention, and patient satisfaction regarding the complications that may occur after the intervention.
Another factor affecting patient and surgeon satisfaction is cost. Currently, the high cost of surgical interventions force the physicians to choose drugs and/or consumables with similar or same efficacy and lower costs.
Dexmedetomidine, although does not have a direct effect on neuromuscular block, has been reported to increase plasma rocuronium concentration, and esmolol, by decreasing cardiac output prolongs onset time of rocuronium (6, 7).
In the present study, it was aimed to compare the effects of a selective beta blocker agent, esmolol, and an alpha 2 agonist, dexmedetomidine, with equivalent doses that provide similar surgical conditions on hemodynamic conditions, neuromuscular blockade, bleeding, surgeon and patient satisfaction and cost during controlled hypotensive anaesthesia in middle ear surgery.
Methods
The study was initiated after obtaining approval from the Local Ethics committee of Istanbul Umraniye Education and Research Hospital (Date: 11/01/2011-Number: 16) and informed consents were obtained from the patients. The study included 50 ASA I–II patients between the ages of 18 and 65 years who were scheduled to undergo tympanomastoidectomy surgery.
Patients with unstable angina pectoris, second and third degree heart block, sinus bradycardia, obesity, poorly controlled asthma, history of neuromuscular disease, substance abuse, and allergies to drugs that would be used in the study, pregnant or lactating women, those using antihypertensive and anticoagulant drugs, and those with hepatic and renal insufficiency were excluded from the study.
Patients were randomized into 2 groups. As a standard procedure, all patients were applied ECG (Drager Infinity Delta), non-invasive mean arterial pressure (MAP), heart rate (HR), peripheral oxygen saturation (SpO2), bispectral index (BIS) (Infinity® BISx® SmartPod®) and neuromuscular monitoring (TOF GUARD-SX®) in the operating room.
After premedication with intravenous 0.03 mg midazolam, Group E (n=25) received 0.5 mg kg−1 min−1 esmolol (Brevibloc®; Baxter Healthcare Corporation, Marion, North California, USA) in 1 min before induction and esmolol was titrated over a dose range of 10–200 μg kg−1 min−1 after induction, while Group D (n=25) received 0.5 μg kg−1 dexmedetomidine (Precedex®, Abbott Laboratories, North Chicago USA) in 10 min before induction, and then dexmedetomidine was titrated within a dose range of 0.2–0.7 μg kg−1 hr−1 in order to maintain MAP: 55–65 mmHg and BIS 40–50 after induction.
After preoxygenation for 2 minutes, 2 mg kg−1 propofol, 1 μg kg−1 remifentanil and 0.6 mg kg−1 rocuronium was applied for induction. For anaesthesia maintenance, 0.25 μg kg−1 min−1 remifentanil infusion was started in both groups. Endotracheal intubation was performed after the BIS decreased below 60. Maintaining end-tidal CO2 (EtCO2) at 35–40, using 1 MAC sevoflurane in 50% O2-air mixture, mechanical ventilation was started. After the intervention, atropine (10 μg kg−1) and neostigmine (40 μg kg−1) was used to reverse neuromuscular block, when T1 recovered to 25%. Patients with adequate spontaneous respiration and BIS >80 were extubated.
In case of continued hypertension and tachycardia (MAP and HR 20% above the basal value) an additional dose of 1 μg kg−1 fentanyl application was planned. Mean arterial pressure value below 50 mmHg was considered as hypotension, and HR <45 beats min−1 was considered as bradycardia, and ephedrine (5 mg), atropine (0.5 mg) treatment was planned to be administered.
Drug infusions were decreased to half the dose 10 minutes before the end of the surgery and were stopped at the end of the surgery. Total dexmedetomidine and esmolol doses (mL) used during intervention were recorded. Equivalent drug doses that provided similar hemodynamic effects were calculated (μg kg−1 min−1).
Patients were to be excluded from the study in case of uncontrolled hypotension, intraoperative anaphylaxis and severe bleeding.
MAP, HR, SpO2, BIS, EtCO2 values were recorded before and after induction, at 1, 5, 10 and 15 minutes after intubation and at 15-minute intervals until the end of the surgery.
The effects of both drugs on neuromuscular blockade [onset of action (OA), the time from rocuronium application to maximum block, duration of clinical action (DCA): the time to reversal of T1 from maximum block to 25%, and recovery index (RI): the time to reversal of T1 from 25% to 75%] were recorded.
Severity of bleeding during the intervention was evaluated by the surgical team according to Fromme’s 5 point scale (Table 1) (4).
Table 1.
Frommes’ Bleeding Scale
| 0 | No bleeding, ideal surgical field |
| 1 | Mild bleeding, aspiration not required |
| 2 | Mild bleeding, occasional requirement of aspiration |
| 3 | Significant bleeding, frequent aspiration required, if aspiration is discontinued for 5 seconds surgical field is impaired |
| 4 | Diffuse bleeding, continuous aspiration required |
| 5 | Abundant bleeding, even with continuous aspiration surgical field is obscured and surgery not impossible |
Duration of anaesthesia (the time between the onset of induction and extubation) and duration of surgery were recorded. The effect of esmolol and dexmedetomidine use on total cost was evaluated (Brevibloc Premiks® 10 mg mL−1 250 mL infusion solution, price of the drug 138.89 TL. Price of Precedex® 200 μg, diluted in 50 mL 0.9% physiological saline 40.498 TL).
Consumed rocuronium and fentanyl amount, and total doses were recorded.
Surgeon satisfaction was evaluated using a 4 point scale, as follows, very good (I), good (II), fair (III), and poor (IV) (8).
Statistical analysis
In the power analysis for DCA values, taking delta as 7.5 and a standard deviation of 7.5, a sample size of 24 was fount to be adequate to achieve 80% power with a value of 0.01. For RI values, taking delta as 1.86 and a standard deviation of 2.2 a sample size of 24 was adequate to achieve 80% power with a= 0.05. NCSS (Number Cruncher Statistical System) 2007&PASS 2008 Statistical Software (Utah, USA) program was used to analyze the findings obtained in the study. In the analysis of study data, besides descriptive statistics (mean, standard deviation), Student’s t test was used for quantitative data to compare the parameters with normal distribution between the two groups. Qualitative data was compared with chi-square test. Statistical significance was set at p<0.05.
Results
The study was performed between September-2010 and May-2011 on 50 patients, 25 in Group E and 25 in Group D. No patient was excluded from the study. There was no significant difference between the groups regarding the demographic characteristics and durations of anaesthesia and surgery (Table 2).
Table 2.
General characteristics of the groups (Mean±SD)
| Group E | Group D | p | |
|---|---|---|---|
| Age (years) | 31.72±10.26 | 35.20±12.20 | 0.281 |
| Weight (kg) | 71.52±10.21 | 71.92±15.64 | 0.915 |
| Gender n (%) | |||
| Female | 15 (60%) | 11(44%) | 0.258 |
| Male | 10 (40%) | 14 (56%) | |
| Duration of anaesthesia (min) (min–max) | 110.64±35.53 (59 min-185 min) | 112.32±40.46 (60 min-185 min) | 0.877 |
| Duration of surgery (min) (min-max) | 96.68±33.98 (50 min-170 min) | 99.40±39.66 (50 min-170 min) | 0.796 |
Student t test
In all studied time points, there were no significant differences between the groups in terms of HR, MAP, SpO2, and ETCO2 values, except the significant increase in HR values at 120 and 75 minutes in Group E (Table 3, 4). Comparisons in individual groups showed that MAP and HR values at all time points were significantly lower than the basal values in both groups.
Table 3.
Comparison of HR values between the groups (beats min−1) (Mean±SD)
| HR | Group E | Group D | p |
|---|---|---|---|
| Before induction | 79.84±8.90 | 81.64±12.63 | 0.563 |
| After induction | 71.80±9.51 | 70.48±10.51 | 0.644 |
| After intubation | 74.80±12.04 | 70.88±9.04 | 0.200 |
| At 5 minutes | 69.68±13.14 | 65.96±8.79 | 0.245 |
| At 10 minutes | 65.44±9.92 | 63.52±7.80 | 0.451 |
| At 15 minutes | 62.04±8.61 | 61.00±7.68 | 0.654 |
| At 30 minutes | 62.36±7.91 | 59.12±7.17 | 0.136 |
| At 45 minutes | 61.84±8.04 | 58.92±7.50 | 0.195 |
| At 60 minutes | 62.25±9.05 | 57.68±6.56 | 0.058 |
| At 75 minutes | 61.95±7.80 | 56.94±6.21 | 0.035* |
| At 90 minutes | 63.11±8.39 | 57.93±6.48 | 0.064 |
| At 105 minutes | 63.83±8.80 | 59.91±6.37 | 0.238 |
| At 120 minutes | 67.00±7.71 | 59.30±6.46 | 0.030* |
| At 135 minutes | 68.17±7.41 | 60.17±6.52 | 0.080 |
| At 150 minutes | 66.50±9.39 | 62.83±7.25 | 0.504 |
Student’s t test.
p<0.05 when two groups are compared
Table 4.
Comparison of MAP values between the groups (mmHg) (Mean±SD)
| MAP | Group E | Group D | p |
|---|---|---|---|
| Before induction | 95.64±8.98 | 97.48±12.14 | 0.545 |
| After induction | 77.20±12.76 | 83.92±14.48 | 0.088 |
| After intubation | 82.48±15.25 | 82.84±12.59 | 0.928 |
| At 5 minutes | 69.52±8.96 | 67.28±6.76 | 0.324 |
| At 10 minutes | 65.16±5.05 | 63.40±4.20 | 0.187 |
| At 15 minutes | 62.48±4.37 | 62.56±3.40 | 0.943 |
| At 30 minutes | 60.60±3.27 | 60.68±3.33 | 0.932 |
| At 45 minutes | 59.92±3.73 | 60.33±3.44 | 0.689 |
| At 60 minutes | 61.04±6.64 | 61.18±3.18 | 0.929 |
| At 75 minutes | 59.76±2.82 | 60.94±3.13 | 0.223 |
| At 90 minutes | 60.58±3.40 | 61.07±3.99 | 0.705 |
| At 105 minutes | 60.50±3.42 | 60.64±3.35 | 0.924 |
| At 120 minutes | 61.44±3.77 | 60.80±3.01 | 0.685 |
| At 135 minutes | 63.17±2.13 | 61.57±3.73 | 0.377 |
Student’s t test.
No significant difference was found regarding OA values between the groups (p>0.05). DCA values of Group D were significantly higher than that of Group E (p<0.01). Also, RI values of Group D were significantly higher than that of Group E (p<0.05) (Table 5).
Table 5.
The duration of action of neuromuscular blocker (Mean±SD)
| Group E | Group D | p | |
|---|---|---|---|
| OA (sec) | 118.36±18.56 | 120.60±32.51 | 0.766 |
| DCA (min) | 41.12±7.89 | 48.64±7.20 | 0.001* |
| RI (min) | 6.64±1.25 | 8.50±3.81 | 0.028** |
Student’s t test.
There was no significant difference between the groups regarding the total rocuronium doses used and number of patients requiring additional fentanyl; 2 patients (8%) in Group E, and 5 patients (20%) in Group D required additional fentanyl (p>0.05).
There was no significant difference between the groups regarding the bleeding scores and surgeon satisfaction (p>0.05). Surgeon Satisfaction was “very good” in 80% of patients in Group E and 72% of patients in Group D, “good in 8% of patients in Group E and 20% of patients in Group D, and “fair” in 12% and 8% of patients in Group E and Group D, respectively. Poor surgeon satisfaction was not reported. No bleeding was observed in 60% and 50% of patients in Group E and Group D, respectively. Mild and moderate bleeding was observed in 28% and 12% of patients in Group E and 32% and 12% of patients in Group D, respectively. Severe bleeding was not observed in any of the patients. None of the patients experienced hypotension, bradycardia and resistant hypertension during the surgery and none of them required additional atropine and/or ephedrine. No serious complications was observed during the surgery.
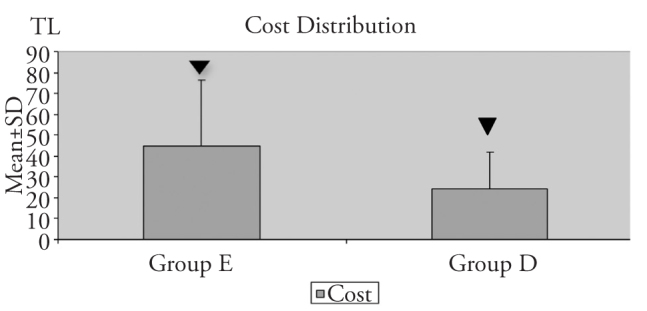
The amounts of total esmolol and dexmedetomidine used and their equivalent doses providing similar hemodynamic effects are shown in Table 6. The cost of esmolol used during the surgery was significantly higher than that of dexmedetomidine (p<0.01) (Figure 1).
Table 6.
Total amounts of esmolol/dexmedetomidine used and the equivalent drug doses providing similar hemodynamic effects (Mean±SD)
| Group E | Group D | |
|---|---|---|
| Total E/D (mL) | 80.34±57.75 | 25.73±8.33 |
| Equivalent drug dose | 91.95±43.62 (μg kg−1 min−1) | 0.39±0.21 (μg kg−1 hrs−1) |
Figure 1.
Cost estimates of the groups
▼ p<0.05 when two groups are compared
Discussion
At the present time, providing bleeding control is substantially important when microsurgical techniques are used during surgeries. As even a mild bleeding can complicate working in the surgical field in middle ear surgery, a bloodless surgical field should be provided. For that purpose, anaesthetists use controlled hypotensive anaesthesia (9, 10).
Controlled hypotension has several benefits such as reducing the blood loss and improving the quality of surgical field, and by decreasing the number of manipulations decrease microtrauma in the tissues and shorten the time of the process (11, 12).
In our study, we aimed to compare the effects of a cardio-selective β blocker, esmolol, and a α2 receptor agonist, dexmedetomidine on hemodynamic conditions, neuromuscular block, bleeding, surgeon and patient satisfaction and cost in hypotensive anaesthesia during middle ear surgery.
Dexmedetomidine, a potent and selective α2 agonist, decrease plasma norepinephrine concentrations in a dose dependent manner, and esmolol, a short-acting β-blocker, which is effective in controlling the perioperative stress response, leads to a decrease in HR and MAP via suppressing sympathetic activity (13–15). These effects are used in suppressing cardiovascular response related to sympathetic activity and reducing the increase in catecholamine levels due to surgical stress during intubation and laryngoscopy (5).
Grant et al. (16), in their study, performed awake fiberoptic intubation using dexmedetomidine and reported that the measured heart rate and blood pressure values showed changes not exceeding 15% of the basal values. Erdil and colleagues (17) evaluated the effects of dexmedetomidine and midazolam on hemodynamic response caused by the local anaesthetic agent containing epinephrine in septoplasty surgeries. They reported that patients in the dexmedetomidine group showed a more stable hemodynamic course in comparison to midazolam group.
Chia et al. (18), aimed to evaluate the intraoperative clinical effects and effects on postoperative pain management of esmolol in 100 patients undergoing hysterectomy; they infused esmolol at a dose of 50 μg kg−1 min−1 during the intervention and compared the hemodynamic effects of esmolol with a control group which received only saline. During anaesthesia, the HR values of patients in the esmolol group were lower than the control group, however there was no significant difference between the two groups regarding MAP values.
Weiskopf et al. (19) compared the effects of fentanyl, esmolol and clonidine in suppressing sympathetic activity during desflurane anaesthesia, and determined that esmolol decreased HR, however did not make a difference in MAP and plasma epinephrine levels. It can be seen that, like in the studies of Chia (18) and Weiskopf (19), an esmolol dose of 50 μg kg−1 min−1 is sufficient to reduce HR, but is not sufficient in decreasing MAP. In our study, it was observed that in order to attain the targeted MAP values (55–65 mmHg), administration of esmolol at a dose of 91.95±43.62 μg kg−1 min−1 was sufficient; and this effect was attributed to the synergistic effect of remifentanil infusion administered at a dose of 0.25 μg kg−1 min−1.
In our study, even in a condition causing severe catecholamine increase like intubation, MAP and HR values did not increase over the basal values measured before induction, and hemodynamic stability was maintained in the preoperative duration in both groups receiving esmolol and dexmedetomidine.
Although the effects of alpha-2 adrenergic agonists on neuromuscular blockers in humans, is not clearly known, there are no large scale studies performed using non-depolarizing drugs (20, 21). Talke and colleagues (6) in their study in patients who received alfentanil/ propofol anaesthesia, found that dexmedetomidine infusion (plasma concentration 0.6 ng mL−1) shortened T1 duration, however as dexmedetomidine had no clinical effect on neuromuscular block, explained the decrease in T1 response with the increase in plasma rocuronium concentration. Although they were not able to completely explain the increase in plasma rocuronium concentration by dexmedetomidine, they observed an increase in plasma rocuronium concentration in all cases after dexmedetomidine infusion was started. They suggested that dexmedetomidine might affect the pharmacokinetics of rocuronium by decreasing renal and hepatic blood flow and by decreasing renal clearance by 6%.
Szmuk et al. (7), in their study evaluating the effects of esmolol and ephedrine, on the OA of rocuronium, reported that esmolol at a dose of 0.5 mg kg−1, decreased the cardiac output by beta adrenergic blockade, and significantly increased OA of rocuronium with minimal hemodynamic changes.
In our study, esmolol and dexmedetomidine groups were similar in terms of OA. We attributed this result to the similar effects of both drugs in decreasing the HR (Table 3, 4). DCA and DI of dexmedetomidine group were significantly higher in comparison to the esmolol group. We suggest that, similar to the study of Talke, this might be associated with the increase in plasma concentrations of rocuronium (6).
Degoute and colleagues (22) performed a study on 40 ASA I children scheduled to undergo middle ear surgery. For the induction of controlled hypotensive anaesthesia, they used sevoflurane and remifentanil 1 μg kg−1 in the first group (n=20) and nitroprusside 0.25 μg kg−1 min−1 and alfentanil 25 μg kg−1 in the second group (n=20), and for anaesthesia maintenance, the first group received 0.2–0.5 μg kg−1 min−1 remifentanil infusion, and the second group received sodium nitroprusside (0.25 μg kg−1 min−1) and alfentanil (0.5 μg kg−1 min−1) infusion. Middle ear blood flow was measured by laser Doppler, and they reported that remifentanil and sevoflurane combination provided a better surgical field by decreasing middle ear blood flow, and these cases required no additional hypotensive agents perioperatively. In the present study, dexmedetomidine and esmolol infusions, added to sevoflurane and remifentanil anaesthesia, were compared. Different from the study of Degoute et al. (22), bleeding and additional analgesic requirement was much more lower in both groups by using a lower remifentanil dose (0.25 μg kg−1 min−1). We suggest that this difference might be associated with remifentanil added to dexmedetomidine and esmolol.
Pilli et al. (23) compared controlled hypotension with normotensive anaesthesia in middle ear surgery, and administered esmolol at a dose range of 50–500 μg kg−1 min−1 in order to achieve a target MAP of 50 mmHg and HR of 50 beats min−1 in the controlled hypotension group. Electrocardiographic evaluation of ischemia in the hypotensive period revealed no pathological finding, and hypotensive anaesthesia induced by esmolol was found to be more effective in providing a bloodless surgical field compared to the control group. The authors reported that esmolol was a safe agent that can be used in inducing controlled hypotension during middle ear microsurgery.
Nasreen and colleagues (24) in 42 adult patients scheduled to undergo middle ear surgery, reported that a better surgical field was provided in the group that received dexmedetomidine (n=21) at a dose of 0.4 μg kg−1 hr−1 compared to the control group (n=21) that received saline. Durmuş et al. (25) evaluated the quality of surgical field and amount of bleeding with dexmedetomidine in patients planned to undergo middle ear surgery and septorhinoplasty. Forty patients were randomized into two groups as dexmedetomidine (a bolus dose of 1 μg kg−1 in 10 min, then 0,5 μg kg−1 hr−1 infusion) and placebo groups. It was observed that isoflurane and fentanyl requirement and the amount of bleeding were lower in the dexmedetomidine group, and additionally dexmedetomidine provided better surgical conditions. Ayoğlu et al. (26), in their study on patients scheduled to undergo septoplasty and tympanoplasty surgery, investigated the effect of dexmedetomidine on the amount of bleeding. They observed that dexmedetomidine group had lower rates of bleeding and lower fentanyl use in septoplasty surgery, compared to the control group. However, in patients undergoing tympanoplasty surgery, although dexmedetomidine (0,7 μg kg−1 hr−1) allowed lower amounts of fentanyl use in comparison to the control group, the decrease in bleeding was not significant. Based on these findings, they reported that dexmedetomidine reduced bleeding and analgesia requirement in septoplasty cases, but despite a decreased analgesic requirement, it was not effective in reducing bleeding in tympanoplasty cases.
In the present study, the effects of dexmedetomidine and esmolol were found to be similar in terms of bleeding, surgeon satisfaction and additional analgesic requirements. The desired level of hypotensive anaesthesia and bleeding control was provided by both drugs, a very good level of surgeon satisfaction was determined in 70–80% of the patients, and as surgeon satisfaction was high, the drugs were considered to provide efficient control of bleeding.
Currently, surgery cost is as important as surgeon and patient satisfaction in the success of surgery. This situation force the surgeon and the anaesthetist to act selectively in providing consumables and drugs, and regarding the drug costs, choosing the cheaper drug between two drugs with similar efficacy. In calculating the costs, the perfusor used, drug and injector used for perioperative hypotension/bradycardia, drug and injector used in antagonization and even human labour and time spent, were taken into consideration. In the present study, the same type of perfusor was used in both groups. No difference was found regarding the demographic data, duration of surgery, additional fentanyl requirements, and rocuronium amounts between the groups. None of the patients required additional atropine/ephedrine.
In this present study, it was calculated that the costs of esmolol and dexmedetomidine doses providing equivalent hemodynamic effect were approximately 44.63 TL and 24.51 TL respectively, and as there was no difference between the groups regarding the other parameters affecting the cost, it may be suggested that dexmedetomidine application is much more cost effective than esmolol application in hypotensive anaesthesia.
Conclusion
Regarding the use of dexmedetomidine and esmolol under sevoflurane and remifentanil anaesthesia in controlled hypotensive anaesthesia during middle ear surgery; although both agents are clinically effective, it should be considered that while the duration of neuromuscular block may be prolonged by using dexmedetomidine, cost may be higher by esmolol use.
Footnotes
Conflict of Interest
No conflict of interest was declared by the authors.
Peer-review: Externally peer-reviewed.
Author Contributions
Concept - N.B., G.K.; Design - N.B., G.K., Z.E.; Supervision - N.B., G.K.; Funding - Z.E., M.A.E.; Materials - Z.E.; Data Collection and/or Processing - Z.E., G.K., M.A.E.; Analysis and/or Interpretation - Z.E., N.B., G.K., Literature Review - Z.E., G.K., Writer - Z.E., N.B., G.K.; Critical Review - N.B., G.K.; Other - Z.E., N.B., G.K., M.A.E.
References
- 1.Kayhan Z. Klinik Anestezi. 2. ed. Logos; 1997. pp. 428–34. [Google Scholar]
- 2.Ülger MH, Demirbilek S, Köroğlu A, Borazan H, Ersoy MÖ. Orta kulak cerrahisinde deksmedetomidine ile kontrollü hipotansiyon. İnönü Üniversitesi Tıp Fakültesi Dergisi. 2004;11:237–41. [Google Scholar]
- 3.Tobias JD. Controlled hypotension in children: a critical review of available agents. Paediatr Drugs. 2002;4:439–53. doi: 10.2165/00128072-200204070-00003. http://dx.doi.org/10.2165/00128072-200204070-00003. [DOI] [PubMed] [Google Scholar]
- 4.Fromme GA, MacKenzie RA, Gould AB, Jr, Lund BA, Offord KP. Controlled hypotension for orthognathic surgery. Anesth Analg. 1986;65:683–6. http://dx.doi.org/10.1213/00000539-198606000-00021. [PubMed] [Google Scholar]
- 5.Karaören G, Adanır T, Atay A, Şencan A, Aksun M, Aran G, et al. Deksmedetomidin ve esmololün ekstubasyona yanıta etkileri. İzmir Atatürk Eğitim HastanesiTıp Dergisi. 2008;46:69–76. [Google Scholar]
- 6.Talke PO, Caldwell JE, Richardson CA, Kirkegaard-Nielsen H, Stafford M. The effects of dexmedetomidine on neuro muscular blockade in human volunteers. Anesth Analg. 1999;88:633–9. doi: 10.1097/00000539-199903000-00031. http://dx.doi.org/10.1213/00000539-199903000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Szmuk P, Ezri T, Chelly JE, Katz J. The onset time of rocuronium is slowed by esmolol and accelerated by ephedrine. Anesth Analg. 2000;90:1217–9. doi: 10.1097/00000539-200005000-00041. http://dx.doi.org/10.1097/00000539-200005000-00041. [DOI] [PubMed] [Google Scholar]
- 8.Doğan Ö, Ünver S, Tuncel Yİ, Keleş S, Süner ZC. Comparison of dexmedetomidine versus midazolam/remifentanil combination for monitorized anaesthesia care. Türk Anest Rean Der Dergisi. 2011;39:292–301. [Google Scholar]
- 9.Simpson P. Preoperative blood loss and its reduction: the role of the anaesthetist. Br J Anaesth. 1992;69:498–507. doi: 10.1093/bja/69.5.498. http://dx.doi.org/10.1093/bja/69.5.498. [DOI] [PubMed] [Google Scholar]
- 10.Dietrich GV, Heesen M, Boldt J, Hempelmann G. Platelet function and adrenoceptors during and after induced hypotension using nitroprusside. Anesthesiology. 1996;85:1334–40. doi: 10.1097/00000542-199612000-00014. http://dx.doi.org/10.1097/00000542-199612000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Newton MC, Chadd GD, O’Donoghue B, Sapsed-Byrne SM, Hall GM. Metabolic and hormonal responses to induced hypotension for middle ear surgery. Br J Anaesth. 1996;76:352–7. doi: 10.1093/bja/76.3.352. http://dx.doi.org/10.1093/bja/76.3.352. [DOI] [PubMed] [Google Scholar]
- 12.Kayhan Z. Kan tasarrufu: cerrahi kan kaybını ve transfüzyon gereksinimini azaltıcı yaklaşımlar. Anestezi Dergisi. 2005;13:149–56. [Google Scholar]
- 13.Wijeysundera DN, Naik JS, Beattie WS. Alpha-2 adrenergic agonists to prevent perioperative cardiyovascular complications: a meta analysis. Am J Med. 2003;114:742–52. doi: 10.1016/s0002-9343(03)00165-7. http://dx.doi.org/10.1016/S0002-9343%2803%2900165-7. [DOI] [PubMed] [Google Scholar]
- 14.Tirelli G, Bigarini S, Russolo M, Lucangelo U, Gullo A. Total intravenous anaesthesia in endoscopic sinus-nasal surgery. Acta Otorhinolaryngol Ital. 2004;24:137–44. [PubMed] [Google Scholar]
- 15.Coloma M, Chiu JW, White PF, Armbruster SC. The use of esmolol as an alternative to remifentanil during desflurane anesthesia for fast-track outpatient gynecologic laparoscopic surgery. Anesth Analg. 2001;92:352–7. doi: 10.1097/00000539-200102000-00014. http://dx.doi.org/10.1097/00000539-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Grant SA, Breslin DS, MacLeod DB, Gleason D, Martin G. Dexmedetomidine infusion for sedation during fiberoptic intubation: a report of three cases. J Clin Anesth. 2004;16:124–6. doi: 10.1016/j.jclinane.2003.05.010. http://dx.doi.org/10.1016/j.jclinane.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Erdil F, But AK, Toprak Hİ, Öztürk E, Ersoy M. Epinefrinin oluşturduğu hemodinamik yanıta deksmedetomidin ve midazolam sedasyonunun etkisi. Anestezi Dergisi. 2007;15:168–72. [Google Scholar]
- 18.Chia YY, Chan MH, Ko NH, Liu K. Role of beta-blockade in anaesthesia and postoperative pain management after hysterectomy. Br J Anaesth. 2004;93:799–805. doi: 10.1093/bja/aeh268. http://dx.doi.org/10.1093/bja/aeh268. [DOI] [PubMed] [Google Scholar]
- 19.Weiskopf RB, Eger EI, 2nd, Noorani M, Daniel M. Fentanyl, esmolol, and clonidine blunt the transient cardiovascular stimulation induced by desflurane in humans. Anesthesiology. 1994;81:1350–5. doi: 10.1097/00000542-199412000-00008. http://dx.doi.org/10.1097/00000542-199412000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Narimatsu E, Niiya T, Kawamata M, Namiki A. Lack in effects of therapeutic concentrations of dexmedetomidine and clonidine on the neuromuscular blocking action of rocuronium in isolated rat diaphragms. Anesth Analg. 2007;104:1116–20. doi: 10.1213/01.ane.0000260317.02748.83. http://dx.doi.org/10.1213/01.ane.0000260317.02748.83. [DOI] [PubMed] [Google Scholar]
- 21.Scholz J, Toner PH. Alpha2 adrenoceptor agonists in anaesthesia: a new paradigm. Curr Opin Anaesthesiol. 2000;13:437–42. doi: 10.1097/00001503-200008000-00007. http://dx.doi.org/10.1097/00001503-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Degoute CS, Ray MJ, Gueugniaud PY, Dubreuil C. Remifentanil induces consistent and sustained controlled hypotension in children during middle ear surgery. Can J Anaesth. 2003;50:270–6. doi: 10.1007/BF03017797. http://dx.doi.org/10.1007/BF03017797. [DOI] [PubMed] [Google Scholar]
- 23.Pilli G, Guzeldemir ME, Bayhan N. Esmolol for hypotensive anesthesia in middle ear surgery. Acta Anaesth Belg. 1996;47:85–91. [PubMed] [Google Scholar]
- 24.Nasreen F, Bano S, Khan RM, Hasan SA. Dexmedetomidine used to provide hypotensive anesthesia during middle ear surgery. Indian J Otolaryngol Head Neck Surg. 2009;61:205–7. doi: 10.1007/s12070-009-0067-8. http://dx.doi.org/10.1007/s12070-009-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durmus M, But AK, Dogan Z, Yucel A, Miman MC, Ersoy MO. Effect of dexmedetomidine on bleeding during tympanoplasty or septorhinoplasty. Eur J Anaesthesi ol. 2007;24:447–53. doi: 10.1017/S0265021506002122. http://dx.doi.org/10.1017/S0265021506002122. [DOI] [PubMed] [Google Scholar]
- 26.Ayoglu H, Yapakci O, Ugur MB, Uzun L, Altunkaya H, Ozer Y, et al. Effectiveness of dexmedetomidine in reducing bleeding during septoplasty and tympanoplasty operations. J Clin Anesth. 2008;20:437–41. doi: 10.1016/j.jclinane.2008.04.008. http://dx.doi.org/10.1016/j.jclinane.2008.04.008. [DOI] [PubMed] [Google Scholar]


