Abstract
Objective
The aim of this study was to compare the effects of a ketamine-propofol mixture (ketofol) and propofol on intraocular pressure (IOP) and haemodynamics in elderly patients during anaesthetic management at each repeated measurement times.
Methods
Forty elderly ASA I and II patients were divided into two random groups and received either propofol (1.5 mg kg−1; group P, n=20) or ketofol (1:1 single syringe mixture of 5 mg mL−1 ketamine and 5 mg mL−1 propofol; group KP, n=20). A proseal laryngeal mask airway (PLMA) was inserted 60 seconds after induction of anaesthesia. IOP, systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) values were recorded at preinduction (t0), immediately following induction (t1), and at 1 (t2), 3 (t3), and 5 (t4) minutes after induction. Haemodynamic complications and the need for ephedrine were also recorded.
Results
Patient characteristics at the beginning of the procedure were similar between the groups. SBP and HR were significantly increased in group KP compared to group P at t1 and t4 (p=0.044). Induction of both anaesthetic agents significantly decreased the IOP values from the t0 (p=0.026). A significant decrease in IOP was found at t1 and t4 in group P compared to group KP (p=0.018). The total dose of ephedrine was statistically different in group P (p=0.034).
Conclusion
Ketofol can be an alternative agent to provide haemodynamic stability with a moderate decrease in IOP during anaesthesia induction in elderly patients.
Keywords: Intraocular pressure, hemodynamic, ketamine, propofol, elderly patient
Introduction
Identification of anaesthetic techniques that provide strict intraocular pressure (IOP) control is of clinical importance during surgery (1). Most anaesthetic agents, except ketamine, reduce IOP (2, 3). Propofol is also reported to reduce IOP, and higher doses may be harmful because of a decrease in blood pressure (BP), leading to cardiorespiratory instability (4). Ketamine, in subanesthetic doses, provides haemodynamic stability in elderly patients (5–7). Side effects, such as hypertension and increased IOP, restrict its use as a single drug (8).
The effects of ketamine and propofol on haemodynamic and respiratory systems are complementary to each other, and their side effects on these systems may be reduced by administering them in combination to lower doses of each drug (9). Thus, a ketamine:propofol combination in one syringe (named “ketofol”) was determined to be a reliable and effective mixture in minor emergency procedures, paediatric cases, and regional anaesthesia (10–12). Ketamine and propofol do not form particles within the same polypropylene injector for 1 hour at 23°C, and can be stored at room temperature with exposure to light (13).
One of the major goals in anaesthetic administration during ocular surgery is to maintain sufficient control of the IOP. Elevated IOP is related to an increased risk for ocular complications. The preferred anaesthetic agents have only a minimal effect on the IOP. The ophthalmic population consists of many elderly patients, who could benefit from ketofol to prevent haemodynamic instability during anaesthesia induction with the maintenance of IOP within a normal range. While there was enthusiasm regarding use of ketofol in clinical practice, data regarding changes in haemodynamics and IOP mediated by this mixture are not available. The aim of this study was to compare the effects of ketofol and propofol induction on IOP and haemodynamic changes in elderly patients at each repeated measurement time.
Methods
This study was approved by the local ethics committee of Inonu University, Medical School (acceptance number 2011/156, Turkey). We selected 50 elderly patients (aged 65 years and above), who were of physical status I-II according to the American Society of Anesthesiologists (ASA), and were scheduled for elective urological procedures under general anaesthesia. Informed written consent was obtained from all patients. The procedures were performed in the early morning to avoid diurnal variations in IOP. Patients who had a history of ocular surgery, ophthalmologic disorder, restricted mouth opening, asthma, psychiatric disease (e.g. schizophrenia), or adverse reactions to ketamine, as well as patients with vascular aneurysms, or who had an anticipated airway were excluded from the study. The computerised randomisation procedure was performed by an independent person who was not involved in the study. A specific study number and group were assigned to each patient, which were then enclosed in envelopes and sealed. Our study design was prospective and double-blind in nature. The patients were randomly divided into two groups: group KP was administered ketofol, whereas group P was administered propofol.
Preoperative evaluation of the patients’ airways was performed using a modified Mallampati test (14). The patients fasted for at least 8 hours on the day of operation. No pre-medications were administered. When a patient was in the operation theatre, intravenous access and standard monitors were applied. Patients were placed in the supine position for examination. IOP was evaluated in all patients using the Perkins hand-held applanation tonometer (Haag-Streit, Clamant Clarka, Essex, England). All IOP measurements were performed by an ophthalmologist who was blinded to the anaesthetic technique, after instillation of 0.5% proparacaine HCl drops and fluorescein dye.
A ketofol solution of a total volume of 20 mL was prepared in the same syringe for group KP using 100 mg ketamine (Ketalar® 50 mg mL−1 Pfizer, Istanbul, Turkey) and 100 mg propofol (1% propofol® Fresenius, Istanbul, Turkey); 2 mL ketamine (100 mg), 8 mL saline, and 10 mL propofol (1% propofol) in the ketofol syringes). Propofol was prepared by an anaesthetist who did not participate in anaesthesia application (9). We used 5 mg mL−1 ketamine and 5 mg mL−1 propofol per mL. Group P was administered 20 mL of 1% propofol (10 mg mL−1). All patients received preoxygenation with 100% oxygen for 3 minutes. Anaesthesia induction was achieved with 1.5 mg kg−1 ketofol or propofol in 20 seconds (15). Consciousness was evaluated on the basis of absence of response to verbal commands and loss of eyelash reflex. If required, further 0.5 mg kg−1 increments of the drugs (ketofol or propofol) were given every 30 sec until loss of consciousness and loss of eyelash reflex was achieved. Additional doses were administered from the drug remaining in the same syringe and recorded.
At 60 seconds after induction, pharyngeal laryngeal mask airway (PLMA; Laryngeal Mask Co, Ltd, Herney-on-Thames, UK) was inserted using the Brain method by a single anaesthesiologist who was blinded to the study, and the insertion was assessed for only the first application (16). Patients were excluded from the study if PLMA insertion was ineffective. After insertion, the cuff was inflated with air to the recommended inflation volume. Effective ventilation was confirmed by observation of chest wall movement and a square wave capnograph trace. After IOP measurements, anaesthesia was maintained with 2–3% sevoflurane in an air/oxygen mixture. The lungs were ventilated mechanically to maintain the end-tidal carbon dioxide concentration (ETCO2) between 35–40 mmHg.
Intraocular pressure, systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) values were recorded at preinduction (t0), immediately following induction (t1), and at 1 (t2), 3 (t3), and 5 (t4) minutes after induction. Hemodynamic complications were also recorded. Complications such as bradycardia, muscle rigidity and excessive secretion were monitored. If SBP or HR decreased below 80 mmHg or 45 beats/minute, respectively, 5 mg ephedrine or 0.5 mg atropine was administered.
Statistical analysis
The sample size was based on a power analysis. At least 13 patients were required for each group in order to detect a maximal IOP difference of 30% between the groups with a type I error of 0.05 and a type II error of 0.20. Data are expressed as mean values standard deviation, median (min-max), or frequency. Within the groups, normality of the variables was measured using the Shapiro-Wilk test. Differences between the two independent groups (KP and P groups) were evaluated using an independent sample t test and Mann-Whitney U test, where appropriate. The Yates-Corrected Chi-square test and Fisher’s exact chi-square test were used for the categorical variables. A p-value of less than 0.05 was considered to be statistically significant. AUC values were estimated for IOP and Hemodynamic data between the KP and P groups.
Results
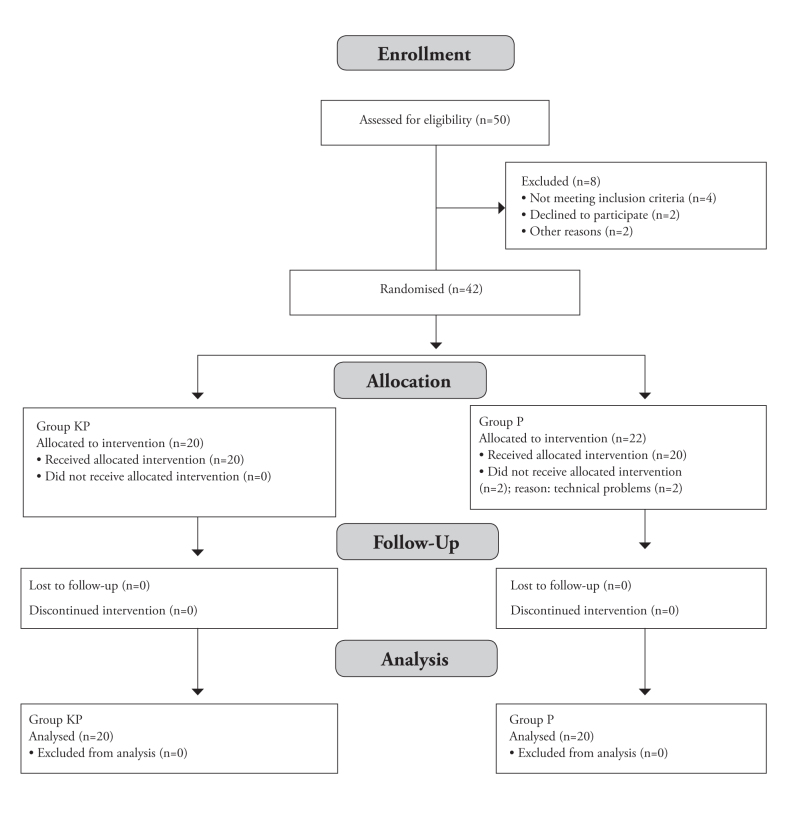
Table 1 shows the demographic and clinical variables of the patients in both groups. Ten patients were excluded from the study for the following reasons: (1) not meeting the inclusion criteria in 4 patients, (2) two patients declined to participate the study, and (3) two patients other reasons. Forty-two elderly patients were enrolled in the present study, of which two patients in group P were excluded due to technical problems (Figure 1).
Table 1.
Patient characteristics
| Variable | Group KP (n=20) | Group P (n=20) | p value |
|---|---|---|---|
| Age (years) | 70 (65–83) | 69 (65–83) | 0.333 |
| Gender ratio (M/F) | 9/11 | 10/10 | 0.999 |
| Height (cm) | 170 (160–186) | 170 (155–180) | 0.217 |
| Weight (kg) | 67 (50–100) | 68 (50–102) | 0.224 |
| ASA Grade (I/II) | 9/11 | 10/10 | 0.999 |
The data are presented as median (min-max). M: Male, F: Female, ASA: American Society of Anesthesiologists, KP: ketofol, P: propofol
Figure 1.
Flowchart of the study
Table 2 shows the changes in haemodynamic variables in each group. SBP and HR were significantly higher in group KP compared to the group P at t1 and t4 (p=0.044). Although the DBP values were higher at all measurement points for group KP, there were no significant differences between the two groups in terms of DBP values during the measurement periods.
Table 2.
Haemodynamic values
| Variable | Group | t0 | t1 | t2 | t3 | t4 |
|---|---|---|---|---|---|---|
| HR (bpm) | KP (n=20) | 69 (58–79) | 85 (60–105)* | 70 (58–98) | 69 (52–92) | 78 (55–91)* |
| P (n=20) | 66 (52–76) | 68 (53–98) | 68 (51–88) | 66 (50–81) | 60 (50–80) | |
|
| ||||||
| SBP (mmHg) | KP (n=20) | 125 (118–153) | 128 (121–138)* | 121 (118–132) | 117 (91–140) | 126 (95–134)* |
| P (n=20) | 124 (116–147) | 112 (100–120) | 115 (110–123) | 114 (77–163) | 105 (75–123) | |
|
| ||||||
| DBP (mmHg) | KP (n=20) | 89 (30–99) | 80 (67–100) | 72 (61–100) | 70 (30–101) | 74 (32–100) |
| P (n=20) | 81 (67–110) | 78 (42–105) | 68 (37–101) | 66 (47–98) | 68 (40–87) | |
The data are presented as median (min-max). HR: heart rate, SBP: systolic blood pressure, DBP: diastolic blood pressure, KP: ketofol, P: propofol.
versus group P, p<0.05
Figure 2 indicates the IOP changes in each group. All IOP supine values were within normal limits. Induction of both anaesthetic agents significantly decreased IOP from the t0 values (p=0.026). However, when comparing the data between the two groups, we found that IOP significantly decreased in group P for the t1 and t4 measurements (p=0.018).
Figure 2.
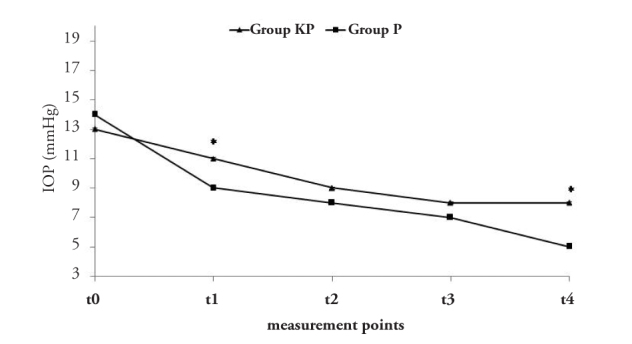
IOP measurements. *vs., group P, p<0.05
The total dose of ephedrine was statistically different in two groups (p=0.034, Table 3). The number of patients in need of ephedrine was not significantly different between the two groups (p=0.114). No adverse effects, such as excessive secretion, bradycardia, or muscular rigidity were observed in any of the patients. Table 4 shows the AUC values estimated for IOP and haemodynamic data between KP and P groups.
Table 3.
The number of patients who received ephedrine and the total dose of ephedrine
| Variable | Group KP (n=20) | Group P (n=20) | p value |
|---|---|---|---|
| The total dose of ephedrine (mg) | 4.0±3.6* | 8.5±4.1 | 0.034 |
| The number of patients in need of ephedrine (n) | 7* | 13 | 0.114 |
The data are presented as mean±standard deviation or frequency of patients. KP: ketofol, P: propofol.
versus group P, p<0.05
Table 4.
IOP and Haemodynamic values for Area Under Curve (AUC) of group P vs group KP
| Variable | Time | AUC | Std. Error | p | Asymptotic 95% | |
|---|---|---|---|---|---|---|
| Confidence Interval | ||||||
| LowerBound | UpperBound | |||||
| t0 | .605 | .094 | .256 | .422 | .788 | |
| t1 | .463 | .096 | .685 | .274 | .651 | |
|
| ||||||
| IOP (mmHg) | t2 | .618 | .093 | .204 | .434 | .801 |
| t3 | .755 | .078 | .006 | .602 | .908 | |
| t4 | .865 | .059 | .000 | .750 | .980 | |
| t0 | .488 | .097 | .892 | .298 | .677 | |
| t1 | .578 | .093 | .402 | .396 | .759 | |
|
| ||||||
| HR (bpm) | t2 | .655 | .092 | .094 | .474 | .836 |
| t3 | .685 | .086 | .045 | .517 | .853 | |
| t4 | .705 | .085 | .027 | .539 | .871 | |
| t0 | .355 | .092 | .117 | .174 | .536 | |
| t1 | .288 | .083 | .021 | .125 | .450 | |
|
| ||||||
| SBP (mmHg) | t2 | .190 | .071 | .001 | .051 | .329 |
| t3 | .505 | .094 | .957 | .321 | .689 | |
| t4 | .520 | .096 | .829 | .332 | .708 | |
| t0 | .289 | .088 | .256 | .026 | .462 | |
| t1 | .306 | .089 | .685 | .041 | .480 | |
|
| ||||||
| DBP (mmHg) | t2 | .378 | .095 | .204 | .198 | .564 |
| t3 | .700 | .087 | .006 | .035 | .871 | |
| t4 | .750 | .084 | .000 | 0.009 | .914 | |
IOP, intraocular pressure; HR, heart rate; SBP; systolic blood pressure, DBP, diastolic blood pressure; KP, ketofol; P, propofol
Discussion
This is the first report on IOP and haemodynamic changes following induction of ketofol in elderly patients. Our main finding was that ketofol caused less IOP reduction and haemodynamic alterations, with fewer patients who needed ephedrine, and total ephedrine consumption in elderly patients for a relatively short period at a dose of 1.5 mg kg−1.
Under normal conditions, IOP is maintained between 12 mmHg and 20 mmHg by balancing the volume of aqueous humour, changes in the choroidal blood volume and vitreous, central venous pressure, and extra ocular muscle tone that presses inward (17). Most anaesthetics reduce IOP via their central depressive effect on the diencephalic control of IOP, relaxing extra ocular muscle tone and improving the aqueous humour outflow (18). A high baseline value of IOP is related to an enhanced risk of ocular complications, with a normal to slightly lower than normal IOP is preferable during the perioperative period. Elevated IOP values may be detrimental in patients with an open eye injury (19). To minimise these risks, the preferred anaesthetic agents should not raise the IOP or have only a minimal effect (20).
Propofol was found to minimise IOP in relation to the depth of anaesthesia, particularly at high doses (21). Many studies demonstrated that administration of propofol alone reduced IOP by 27–31% (22, 23). The results from this study are similar to those of Mirakhur et al. (24), who reported that high dose of propofol (2.2 mg kg−1) decreased IOP up to 53% in elderly patients. Moreover, the present study established that, propofol (1.5 mg kg−1) decreased IOP up to 36% in elderly patients. These results are comparable with the results of the present study, wherein IOP values decreased following propofol induction. However, the degree to which IOP decreases appears to vary in each report, probably because of the different doses of propofol used.
The possible effect of ketamine on IOP is contentious. Yoshikawa et al. (25) reported that ketamine elevated IOP after induction of anaesthesia. In contrast, Peuler et al. (26) indicated that ketamine had no significant effect on IOP at clinical doses. Ausinsch et al. (27) showed that the impact of ketamine on IOP was minimal. Frey et al. (8) compared propofol with a ketamine:propofol combination used at a 3:1 ratio during placement of the retrobulbar nerve block for sedation, and it did not increase IOP in elderly patients. Unlike in our study, Frey et al. (8) prepared a ketofol mixture at a ratio of 3:1 to attenuate hemodynamic stimulation during general anaesthesia in elderly patients. The present study showed that induction of propofol and ketofol decreased IOP, while ketofol, in contrast to propofol, prevented a marked decrease in IOP at t1 and t4.
Propofol administered during induction of anaesthesia caused a significant decrease in blood pressure, which is especially important for the elderly (15). Goh et al. (28) used ketamine, fentanyl, or saline during LMA insertion prior to propofol induction, and observed a higher SBP in the ketamine group in comparison to the fentanyl and saline groups. Even though there were no significant differences in the heart rates, there was a slight tendency to increase in the ketamine group. Gupta et al. (29) performed a study to compare ketamine, fentanyl, and butorphanol before propofol induction in LMA insertion and also observed higher SBP and DBP in the ketamine group. In our study, the SBP and HR were higher in the group KP, however, SBP and HR reached statistically significant levels only at t1 and t4. Some studies have demonstrated that the co-administration of propofol and ketamine is more favourable due to the stabilisation of the hemodynamics, given that the BP and HR effects of the individual agents tend to cancel one another out (30). Ephedrine has both α and β adrenergic properties and is helpful in the treatment of hypotension, but it can cause tachycardia and arrhythmia (31). The vasoconstrictor and hypertensive effects of ephedrine are potential problems. Cardiovascular complications and tachycardia are observed more frequently in elderly patients and could cause serious cardiovascular risks. In our study, group KP needed less ephedrine in comparison to the group P. Thus, the use of ephedrine can be decreased and the negative effects caused by ephedrine may be prevented.
The limitations of the study can be explained as follows: First, a control ketamine group was not included because ketamine is known to increase IOP. Second, the current study was applied to elderly patients without ocular hypertension. While it is possible that these drugs may cause the same effects in children and in patients with glaucoma and/or preoperative high IOP, the results may be different. Finally, the anaesthetic depth could not be measured. Bispectral index (BIS) is widely used to guide the administration of hypnotic drugs. Despite a deepening level of hypnosis, several studies have reported an increase in BIS values when 0.5 mg kg−1 ketamine is administered as a rapid bolus during general anaesthesia (32, 33). Therefore, we did not use BIS for assessment of the level of hypnosis during induction.
Conclusion
We observed the haemodynamic and IOP changes under either ketofol or propofol-based anaesthesia in elderly patients. Our study demonstrated that ketofol induction moderately decreased IOP with minimal hemodynamic changes. The results achieved in this study emphasised that ketofol, used at a dose of 1.5 mg kg−1, may be an alternative method for without haemodynamic instability and lowering IOP during induction of anaesthesia. However, further studies are required to evaluate the effects of different concentrations of ketofol.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Inonu University School of Medicine (2011/156).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.S.A., S.D.; Design - M.S.A., M.A.E.; Supervision - M.S.A., M.D.; Funding - M.S.A.; Materials - S.D., P.F.; Data Collection and/or Processing - M.S.A., S.D., M.A.E., P.F.; Analysis and/or Interpretation - C.C.; Literature Review - M.S.A., M.A.E.; Writer - M.S.A.; Critical Review - M.D., C.C.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.McGoldrick KE. The open globe: is an alternative to succinylcholine necessary? J Clin Anesth. 1993;5:1–4. doi: 10.1016/0952-8180(93)90079-t. http://dx.doi.org/10.1016/0952-8180(93)90079-T. [DOI] [PubMed] [Google Scholar]
- 2.Sızmaz S, Çoban Karataş M, Altan Yaycioğlu R, Canan H, Cantürk S, Akova YA. Fakoemülsifikasyon sonrasında göz içi basınç değişimleri. Turkiye Klinikleri J Med Sci. 2012;32:1512–7. http://dx.doi.org/10.5336/medsci.2011-25614. [Google Scholar]
- 3.Sator S, Wildling E, Schabernig C, Akramian J, Zulus E, Winkler M. Desflurane maintains intraocular pressure at an equivalent level to isoflurane and propofol during unstressed non -ophthalmic surgery. Br J Anaesth. 1998;80:243–4. doi: 10.1093/bja/80.2.243. http://dx.doi.org/10.1093/bja/80.2.243. [DOI] [PubMed] [Google Scholar]
- 4.Murphy DF. Anesthesia and intraocular pressure. Anesth Analg. 1985;64:520–30. http://dx.doi.org/10.1213/00000539-198505000-00013. [PubMed] [Google Scholar]
- 5.Sadove MS, Shulman M, Hatano S, Fevold N. Analgesic effects of ketamine administered in subdissociative doses. Anesth Analg. 1971;50:452–7. http://dx.doi.org/10.1213/00000539-197105000-00037. [PubMed] [Google Scholar]
- 6.Slogoff S, Allen GW, Wessels JV, Cheney DH. Clinical experience with subanesthetic ketamine. Anesth Analg. 1974;53:354–8. http://dx.doi.org/10.1213/00000539-197405000-00009. [PubMed] [Google Scholar]
- 7.Drummond GB. Comparison of sedation with midazolam and ketamine: effects on airway muscle activity. Br J Anaesth. 1996;76:663–7. doi: 10.1093/bja/76.5.663. http://dx.doi.org/10.1093/bja/76.5.663. [DOI] [PubMed] [Google Scholar]
- 8.Frey K, Sukhani R, Pawlowski J, Pappas AL, Mikat-Stevens M, Slogoff S. Propofol versus propofol-ketamine sedation for retrobulbar nerve block: comparison of sedation quality, intraocular pressure changes, and recovery profiles. Anesth Analg. 1999;89:317–21. doi: 10.1097/00000539-199908000-00013. http://dx.doi.org/10.1097/00000539-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Erdogan Kayhan G, Yucel A, Colak YZ, Ozgul U, Yologlu S, Karlıdag R, et al. Ketofol (mixture of ketamine and propofol) administration in electroconvulsive therapy. Anaesth Intensive Care. 2012;40:305–10. doi: 10.1177/0310057X1204000214. [DOI] [PubMed] [Google Scholar]
- 10.Rapeport DA, Martyr JW, Wang LP. The use of “ketofol” (ketamine-propofol admixture) infusion in conjunction with regional anaesthesia. Anaesth Intensive Care. 2009;37:121–3. doi: 10.1177/0310057X0903700108. [DOI] [PubMed] [Google Scholar]
- 11.Andolfatto G, Willman E. A prospective case series of single-syringe ketamine-propofol (Ketofol) for emergency department procedural sedation and analgesia in adults. Acad Emerg Med. 2011;18:237–45. doi: 10.1111/j.1553-2712.2011.01010.x. http://dx.doi.org/10.1111/j.1553-2712.2011.01010.x. [DOI] [PubMed] [Google Scholar]
- 12.Weatherall A, Venclovas R. Experience with a propofol-ketamine mixture for sedation during pediatric orthopedic surgery. Paediatr Anaesth. 2010;20:1009–16. doi: 10.1111/j.1460-9592.2010.03420.x. http://dx.doi.org/10.1111/j.1460-9592.2010.03420.x. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly RF, Willman E, Andolfatto G. Stability of ketamine-propofol mixtures for procedural sedation and analgesia in the emergency department. Can J Hosp Pharm. 2008;61:426–30. [Google Scholar]
- 14.Samsoon GL, Young JR. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;5:487–90. doi: 10.1111/j.1365-2044.1987.tb04039.x. http://dx.doi.org/10.1111/j.1365-2044.1987.tb04039.x. [DOI] [PubMed] [Google Scholar]
- 15.Dundee JW, Robinson FP, McCollum JS, Patterson CC. Sensitivity to propofol in the elderly. Anaesthesia. 1986;41:482–5. doi: 10.1111/j.1365-2044.1986.tb13271.x. http://dx.doi.org/10.1111/j.1365-2044.1986.tb13271.x. [DOI] [PubMed] [Google Scholar]
- 16.Brain AIJ. The larygeal mask airway (LMA) insertion manual. Henley, UK: Intavent Research Ltd; 1995. [Google Scholar]
- 17.Shields MB. Intraocular pressure and tonometry. In: Alingham RR, Damji K, Freedman S, Moroi S, Shafranov G, editors. Shield’s textbook of glaucoma. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2010. pp. 37–55. [Google Scholar]
- 18.Cunningham AJ, Barry P. Intraocular pressure physiology and implications for anaesthetic management. Can Anaesth Soc J. 1986;33:195–208. doi: 10.1007/BF03010831. http://dx.doi.org/10.1007/BF03010831. [DOI] [PubMed] [Google Scholar]
- 19.Sator-Katzenschlager S, Deusch E, Dolezal S, Michalek-Sauberer A, Grubmüller R, Heinze G, et al. Sevoflurane and propofol decrease intraocular pressure equally during non-ophthalmic surgery and recovery. Br J Anaesth. 2002;89:764–6. http://dx.doi.org/10.1093/bja/89.5.764. [PubMed] [Google Scholar]
- 20.McGoldrick KE. The open globe: is an alternative to succinylcholine necessary? J Clin Anesth. 1993;1:1–4. doi: 10.1016/0952-8180(93)90079-t. http://dx.doi.org/10.1016/0952-8180(93)90079-T. [DOI] [PubMed] [Google Scholar]
- 21.Mirakhur RK, Shepherd WF, Darrah WC. Propofol or thiopentone: effects on intraocular pressure associated with induction of anaesthesia and tracheal intubation (facilitated with suxamethonium) Br J Anaesthesia. 1987;4:431–6. doi: 10.1093/bja/59.4.431. http://dx.doi.org/10.1093/bja/59.4.431. [DOI] [PubMed] [Google Scholar]
- 22.Guedes Y, Rakotoseheno JC, Leveque M, Mimouni F, Egreteau JP. Changes in intra-ocular pressure in the elderly during anaesthesia with propofol. Anaesthesia. 1988;43:58–60. doi: 10.1111/j.1365-2044.1988.tb09072.x. http://dx.doi.org/10.1111/j.1365-2044.1988.tb09072.x. [DOI] [PubMed] [Google Scholar]
- 23.Alexander R, Hill R, Lipham WJ, Weatherwax KJ, el-Moalem HE. Remifentanil prevents an increase in intraocular pressure after succinylcholine and tracheal intubation. Br J Anaesth. 1998;81:606–7. doi: 10.1093/bja/81.4.606. http://dx.doi.org/10.1093/bja/81.4.606. [DOI] [PubMed] [Google Scholar]
- 24.Mirakhur RK, Shepherd WF. Intraocular pressure changes with propofol (‘Diprivan’): comparison with thiopentone. Postgrad Med J. 1985;61:41–4. [PubMed] [Google Scholar]
- 25.Yoshikawa K, Murai Y. The effect of ketamine on intraocular pressure in children. Anesth Analg. 1971;2:199–202. [PubMed] [Google Scholar]
- 26.Peuler M, Glass DD, Arens JF. Ketamine and intraocular pressure. Anesthesiology. 1975;5:575–8. doi: 10.1097/00000542-197511000-00018. http://dx.doi.org/10.1097/00000542-197511000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Ausinsch B, Rayburn RL, Munson ES, Levy NS. Ketamine and intraocular pressure in children. Anesth Analg. 1976;55:773–5. doi: 10.1213/00000539-197611000-00005. http://dx.doi.org/10.1213/00000539-197611000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Goh PK, Chiu CL, Wang CY, Chan YK, Loo PL. Randomized double-blind comparison of ketamine-propofol, fentanyl-propofol and propofol-saline on haemodynamics and laryngeal mask airway insertion conditions. Anaesth Intensive Care. 2005;33:223–8. doi: 10.1177/0310057X0503300211. [DOI] [PubMed] [Google Scholar]
- 29.Gupta A, Kaur S, Attri JP, Saini N. Comparative Evaluation of Ketamine-Propofol, Fentanyl-Propofol and Butorphanol-Propofol on Haemodynamics and Laryngeal Mask Airway Insertion Conditions. J Anaesth Clin Pharmacol. 2011;27:74–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Okuyama K, Inomata S, Okubo N, Watanabe I. Pretreatment with small-dose ketamine reduces predicted effect-site concentration of propofol required for loss of consciousness and Laryngeal Mask Airway insertion in women. J Clin Anesth. 2011;23:113–8. doi: 10.1016/j.jclinane.2010.08.004. http://dx.doi.org/10.1016/j.jclinane.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Stoneham MD, Bee SE, Sneyd JR. Facilitation of laryngeal mask insertion after induction. Effects of lignocaine given intravenously before induction with propofol. Anaesthesia. 1995;50:464–6. doi: 10.1111/j.1365-2044.1995.tb06007.x. http://dx.doi.org/10.1111/j.1365-2044.1995.tb06007.x. [DOI] [PubMed] [Google Scholar]
- 32.Hans P, Dewandre PY, Brichant JF, Bonhomme V. Comparative effects of ketamine on Bispectral Index and spectral entropy of the electroencephalogram under sevoflurane anaesthesia. Br J Anaesth. 2005;94:336–40. doi: 10.1093/bja/aei047. http://dx.doi.org/10.1093/bja/aei047. [DOI] [PubMed] [Google Scholar]
- 33.Vereecke HE, Struys MM, Mortier EP. A comparison of bispectral index and ARX- derived auditory evoked potential index in measuring the clinical interaction between ketamine and propofol anaesthesia. Anaesthesia. 2003;58:957–61. doi: 10.1046/j.1365-2044.2003.03403.x. http://dx.doi.org/10.1046/j.1365-2044.2003.03403.x. [DOI] [PubMed] [Google Scholar]