Abstract
Objective
The aim of this study was to evaluate the effects of esmolol infusion on the prevention of haemodynamic responses to tracheal extubation in patients undergoing elective craniotomy.
Methods
With approval from the Medical School Ethics Committee at Marmara University and the patients’ written consent, 30 patients between 20–65 years of age undergoing elective craniotomy were randomly placed in either the Group Esmolol (n=15) or the Group Control (n=15). Anaesthesia was induced with 5–7 mg kg−1 thiopental sodium, 1 μg kg−1 remifentanil, and 0.1 mg kg−1 vecuronium bromide iv, and was maintained with 1 MAC sevoflurane in oxygen-air mixture (50:50) and 0.25 μg kg−1 min−1 remifentanil infusion. At the end of the operation, patients inhaled 100% oxygen after the discontinuation of the anaesthetic agents. For Group Esmolol, 5 min before extubation 2 mg kg−1 esmolol in 50 mL was infused over 10 min (0.2 μg kg−1 min−1), while for Group Control, 50 mL saline was infused over 10 min. The quality of extubation was evaluated with a 5 point scale, recording heat rate, systolic, diastolic, and mean arterial pressures before infusion, 1 min after infusion, during extubation, and at 1, 3, 5, and 10 min after extubation.
Results
In the esmolol group, systolic, diastolic, and mean arterial pressures, as well as heart rate, decreased significantly after esmolol infusion and were significantly lower than in the control group after extubation (p<0.05). The ratio of patients with an extubation score of one was significantly higher in the esmolol group than in the control group (p<0.05).
Conclusion
We concluded that 2 mg kg−1 esmolol infusion before extubation can prevent hypertension and tachycardia caused by extubation in patients undergoing elective craniotomy.
Keywords: Craniotomy, esmolol, extubation
Introduction
Extubation of the trachea should be devoid of significant changes in haemodynamic parameters and adverse events, such as straining at aspiration, coughing, breath holding, or laryngospasm. The receptors, particularly in the larynx, trachea and bronchi, are stimulated by mechanical and chemical factors during extubation as in laryngoscopy and intubation (1, 2). Stimulation of the respiratory tract at the supraglottic and subglottic levels produces respiratory and cardiovascular reflex responses (3, 4). During laryngoscopy, intubation, and extubation, the plasma concentrations of noradrenaline and adrenaline causing a significant increase in blood pressure and heart rate, which may result in severe and even life-threatening complications in patients with coronary heart disease, hypertension, and increased intracranial pressure (5–10). In order to control haemodynamic changes during tracheal intubation and extubation, local anaesthetics, opioids, beta-blocking agents, and calcium channel blockers have been used with varying success rates (11, 12).
Esmolol is a short-acting cardioselective beta-blocker (β1) agent, which is used to prevent or treat hypertension and tachycardia during intraoperative and postoperative periods and has been reported to decrease plasma catecholamine levels (13). It seems to be a proper agent in preventing haemodynamic responses during intubation and extubation. In this study, our goal was to determine the effects of esmolol infusion on haemodynamic responses during endotracheal extubation in patients undergoing craniotomy operations.
Methods
Thirty patients, between 20–65 years of age and of American Society of Anesthesiologists (ASA) physical status class 1–2, scheduled for elective craniotomy were included in this prospective, randomised, and double-blind study after Institutional Ethics Committee approval (No: 09.2011.0033, Date: 03.03.2011) and written consent from the patients. The patients who had significant cardiac, pulmonary, renal, hepatic, and neuropsychiatric disease, chronic alcohol or drug use, a family history of allergy to the drugs used, a heart rate below 50 beats min−1 or over 100 beats min−1, arterial blood pressure below 90/60 mmHg or over 180/100 mmHg, or were using β-blockers, sympathomimetic agents, calcium channel blockers, or monoamine oxidase inhibitors were excluded from the study. Patients were randomly divided into two groups, Group Esmolol (n=15) and Group Control (n=15).
For all patients, heart rate (HR), invasive systolic arterial pressure (SAP), diastolic arterial pressure (DAP), mean arterial pressure (MAP), peripheral oxygen saturation (SpO2), and end-tidal CO2 pressure (ETCO2) were monitored. Anaesthesia was induced with 5–7 mg kg−1 thiopental sodium, 1 μg kg−1 remifentanil, and 0.1 mg kg−1 vecuronium bromide iv, and after endotracheal intubation, it was maintained with 50% air in O2, 1 MAC sevoflurane, and remifentanil infusion at a rate of 0.25 μg kg−1 min−1. Controlled mechanical ventilation was adjusted to maintain ETCO2 pressure between 27–30 mmHg.
At the end of surgery, all anaesthetic agents were discontinued and the patients were ventilated with 100% oxygen. Neuromuscular block was antagonised with iv 0.03 mg kg−1 neostigmine and 0.01 mg kg−1 atropine sulphate. As previously determined, the patients received either esmolol (Brevibloc premixed, 10 mg mL−1, Baxter Healthcare Corporation, USA) or saline infusions. The solutions were prepared and administered in a double-blind manner. For the patients in Group Esmolol, 5 min before extubation, 2 mg kg−1 esmolol diluted in 50 mL was infused over 10 min (0.2 μg kg−1 mi−1), and for those in Group Control, 50 mL 0.9% saline infused over 10 min. When spontaneous breathing began, the patients were extubated after aspiration of oropharyngeal secretions. The quality of extubation was assessed with a 5-point rating scale, where 1: no cough and normal breathing, 2: mild cough, 3: moderate cough, 4: severe cough and difficulty in breathing, 5: laryngospasm with severe cough and forced breathing (14). After extubation, the patients inhaled 100% oxygen with an oxygen mask for 5 min. Heart rate, SAP, DAP, and MAP were recorded 1 min before infusion, at first min of infusion, during extubation, and at 1, 3, 5, and 10 min after extubation.
The following treatments were provided: 0.5 mg atropine sulphate was administered for bradycardia (HR <50 beat min−1), 5 mg ephedrine was administered for hypotension (systolic arterial pressure <100 mmHg), and 0.02 mg nitroglycerine iv was given for hypertension (systolic arterial pressure >180 mmHg).
Statistical analysis
Statistical analysis of the data was performed using Predictive Analytics SoftWare/Statistical Package for the Social Sciences (PASW/SPSS) 19.0 package program. The categorical measurements were summarised as the number and percentage, continuous measurements as the mean and standard deviation (if necessary as the median of the minimum–maximum). The distribution of the data was analysed by the Kologrov-Smirnov test. The T-test was used for the analysis of discrete data, and two-way ANOVA for the analysis of repeated measures. The chi-square and Fisher tests were used for proportional analysis. A p value <0.05 was considered statistically significant.
Results
No statistically significant differences were found between the control and esmolol groups in demographic data, duration of anaesthesia, and surgery (Table 1).
Table 1.
Demographic data, duration of anaesthesia and surgery.
| Control Group | Esmolol Group | |
|---|---|---|
| Age (years) | 45.07±13.27 | 39.40±10.76 |
| Sex (F/M) | 5/10 | 6/9 |
| Anaesthesia duration (min) | 220.93±101.82 | 213.47±78.16 |
| Duration of surgery (min) | 211.13±102.42 | 203.67±78.52 |
Values are expressed as mean±SD, F/M: female/male
The SAP was significantly lower in the esmolol group as compared to the control group during extubation and at 1, 3, and 5 min after extubation (Figure 1).
Figure 1.
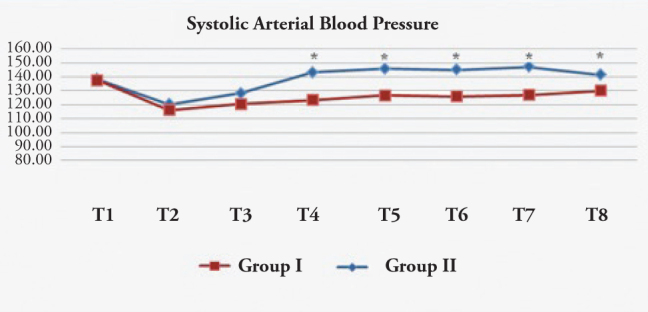
Systolic arterial blood pressure (mmHg)
T1: Preoperative, T2: Before medication, T3: 1 min after medication, T4: During extubation, T5: 1 min after extubation, T6: 3 min after extubation, T7: 5 min after extubation, T8: 10 min after extubation. *: p<0.05 Group I:Esmolol Group, Group II: Control Group.
The DAP was significantly lower in the esmolol group when compared to the control group at extubation, and 3 and 5 min after extubation (Figure 2).
Figure 2.
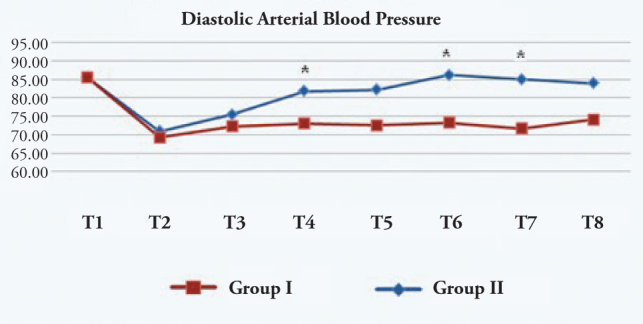
Diastolic arterial blood pressure (mmHg)
T1: Preoperative, T2: Before medication, T3: 1 min after medication, T4: During extubation, T5: 1 min after extubation, T6: 3 min after extubation, T7: 5 min after extubation, T8: 10 min after extubation. *: p<0.05 Group I:Esmolol Group, Group II: Control Group
The MAP in the esmolol group was found to be significantly lower than those of the control group during extubation and at 3 and 5 min after extubation (Figure 3).
Figure 3.
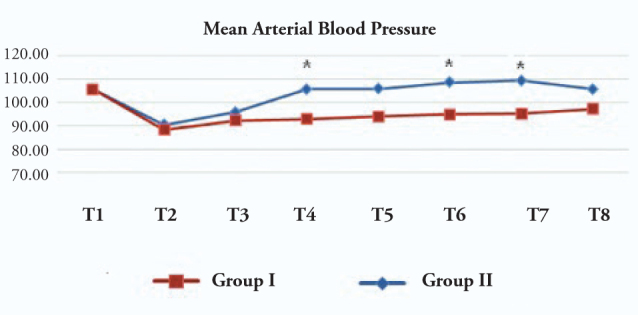
Mean arterial blood pressure (mmHg)
T1: Preoperative, T2: Before medication, T3: 1 min after medication, T4: During extubation, T5: 1 min after extubation, T6: 3 min after extubation, T7: 5 min after extubation, T8: 10 min after extubation. Group I:Esmolol Group, Group II: Control Group
The HR in the esmolol group was significantly lower than in the control group at extubation and 1, 3, 5, and 10 min after extubation (Figure 4).
Figure 4.
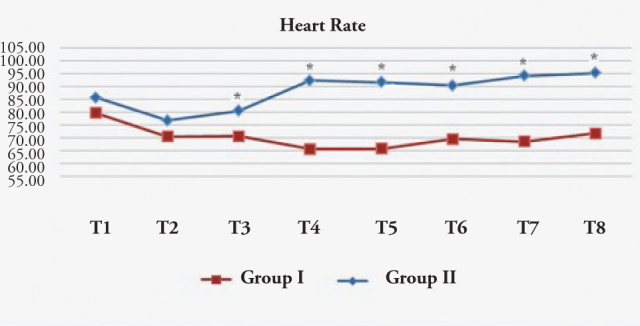
Heart rate (beat/min)
T1: Preoperative, T2: Before medication, T3: 1 min after medication, T4: During extubation, T5: 1 min after extubation, T6: 3 min after extubation, T7: 5 min after extubation, T8: 10 min after extubation. Group I: Esmolol Group, Group II: Control Group
The ratio of patients with an extubation score 1 was significantly higher in the esmolol group than in the control group (Figure 5).
Figure 5.
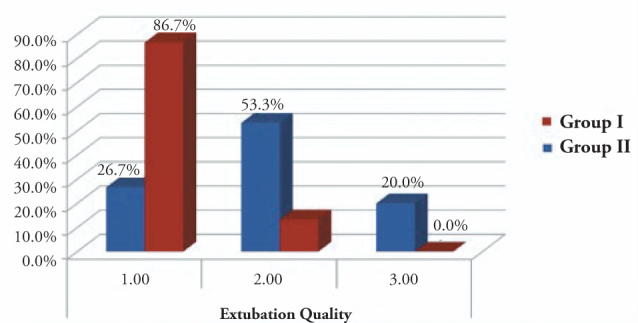
Extubation quality scale
Group I: Esmolol Group, Group II: Control Group
The incidence of hypertension and hypotension did not differ between the groups. No significant difference was noted between the groups with respect to the need for atropine.
Discussion
Tracheal intubation and extubation are associated with marked elevations in heart rate and arterial pressure. Tachycardia and hypertension occurring during tracheal extubation may result in complications such as cardiac failure, pulmonary oedema, and cerebrovascular haemorrhage (15, 16). Although the mechanism of changes occurring in the cardiovascular system during extubation remains to be elucidated, it is associated with increased release of catecholamines as a result of the stress response (5).
Our goal was to investigate the effect of 2 mg kg−1 10 min−1 esmolol infusion on the control of the haemodynamic response due to extubation. Different pharmacological agents were used in several studies to control haemodynamic changes during tracheal intubation and extubation. In a study involving hypertensive patients, it was reported that 1 mg kg−1 lidocaine and 0.2 mg kg−1 diltiazem did not prevent the increase in heart rate and mean arterial pressure at extubation (17).
Used together, PGE1 (0.1 μg kg−1 min−1) and lidocaine (1 mg kg−1) were reported to be more effective for treatment of increased blood pressure as compared to when used individually (18). Although increased heart rate could be controlled in the lidocaine group and in the PGE1+lidocaine group, it could not be achieved in the group using PGE1 alone. In a study comparing lidocaine to verapamil, it was concluded that lidocaine infusion during intubation suppressed tachycardia and hypertension, but it was insufficient to suppress the overall sympathetic response and to eliminate the effects of increased plasma catecholamine concentrations at extubation (19).
Keskin et al. (20) found that both esmolol and lidocaine suppressed haemodynamic responses at intubation, but not during extubation. Wang et al. (21) studied five groups of patients to determine the effects of different doses of esmolol on the haemodynamic response due to extubation. They reported that, when compared to values before premedication, 1.5 mg kg−1 and 2.0 mg kg−1 doses of esmolol decreased the heart rate and systolic blood pressure after extubation.
Esmolol 1.0 mg kg−1, 1.5 mg kg−1, and 2.0 mg kg−1 were also used in patients before extubation in a study by Dyson et al. (22), which showed that the increase in systolic blood pressure could be prevented with 1.5 mg kg−1 and 2.0 mg kg−1 esmolol, but 1 mg kg−1 esmolol was found to be ineffective. Since distinct hypotension was observed with 2.0 mg kg−1 esmolol, 1.5 mg kg−1 esmolol was reported as the optimal dose for the prevention of haemodynamic response due to tracheal extubation. Fuhrman et al. (23) compared the effects of esmolol with alfentanil on heart rate and systolic blood pressure after extubation. The heart rate and systolic blood pressure during extubation did not change significantly in the esmolol group. However, extubation time in this group was significantly prolonged. Therefore, they recommended esmolol to control the haemodynamic responses associated with extubation. We compared 2 mg kg−1 esmolol to the saline group, without the use of any other drug, and found that the haemodynamic changes, particularly in heart rate occurring at extubation, could be prevented with esmolol. Grillo et al. (7) concluded that cerebral hyperaemia following brain surgery was associated with sympathetic hyperactivity, and that the activity could be suppressed by using esmolol.
Lim et al. (24) sought to find the optimal prophylactic esmolol dose for controlling hemodynamic responses in patients undergoing intracranial surgery. Systolic blood pressure and heart rate increased in all groups after extubation. There was no statistically significant difference between infusion doses and, although 0.2 mg kg−1 min−1 was observed to be more effective, 100 mg kg−1 min−1 was considered to be safe. In our study, 2 mg kg−1 10 min−1 esmolol attenuated the heart rate and blood pressure responses during extubation.
The above mentioned and other similar studies had some differences, such as the type of the selected pharmaceutical agent, the doses, and administration times for esmolol. At the planning stage of this study, we assumed that it would be convenient to block sympathetic stimulation using esmolol, a β-blocker agent, in order to prevent exaggerated haemodynamic stimulation during extubation. In previous studies comparing the effectiveness of esmolol with other agents, it was concluded that the prolonged sedative effect of an opioid agent, even if short-acting, may cause delayed extubation (23, 24). Also, as the intracranial pathology and trauma caused by the operation may affect central nervous system functions, we did not want to use an opioid agent to prevent the haemodynamic response at extubation after craniotomy. The reason we did not prefer the method of infusion used in various studies or did not use bolus esmolol after the extubation, was that we wanted to ensure the overlap of the onset of action of esmolol with the time of extubation. We started esmolol infusion 5 min before the predicted extubation time and continued it for 10 min, including the period during, before, and after extubation. Thus, we concluded that the effect of esmolol was achieved when sympathetic impulses were at their highest level. For this reason, no patient had bradycardia or hypotension requiring treatment.
Conclusion
Our findings suggest that, in patients undergoing craniotomy, 2 mg kg−1 10 min−1 esmolol infusion beginning 5 min before extubation provides haemodynamic stability by preventing hypertension and tachycardia without any serious side effects during and after extubation.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Marmara University School of Medicine (03.03.2011, 09.2011.0033).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.A.A., F.Y.G.; Design - M.A.A., F.Y.G.; Supervision - F.Y.G., Z.E.; Funding - M.A.A.; Materials - M.A.A.; Data Collection and/or Processing - M.A.A., G.P., K.T.S.; Analysis and/or Interpretation - M.A.A.; Literature Review - M.A.A., K.T.S.; Writer - M.A.A., K.T.S.; Critical Review - Z.E., F.Y.G.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS, Ravullapalli H, Gandham R. The effects of dexmedetomidine on attenuation of stress response to endotracheal intubation in patients undergoing elective off-pump coronary artery bypass grafting. Ann Card Anaesth. 2012;15:39–43. doi: 10.4103/0971-9784.91480. [DOI] [PubMed] [Google Scholar]
- 2.Akhlagh SH, Vaziri MT, Masoumi T, Anbardan SJ. Hemodynamic response to tracheal intubation via direct laryngoscopy and intubating laryngeal mask airway (ILMA) in patients undergoing coronary artery bypass graft (CABG) Middle East J Anesthesiol. 2011;21:99–103. [PubMed] [Google Scholar]
- 3.Marek W, Muckenhoff K, Prabhakar NR. Significance of pulmonary vagal afferents for respiratory muscle activity in the cat. J Physiol Pharmacol. 2008;59:407–20. [PubMed] [Google Scholar]
- 4.Siddiqui N, Katznelson R, Friedman Z. Heart rate/blood pressure response and airway morbidity following tracheal intubation with direct laryngoscopy, GlideScope and Trachlight: a randomized control trial. Eur J Anaesthesiol. 2009;26:740–5. doi: 10.1097/EJA.0b013e32832b138d. [DOI] [PubMed] [Google Scholar]
- 5.Kayhan Z, Aldemir D, Mutlu H, Oğüş E. Which is responsible for the haemodynamic response due to laryngoscopy and endotracheal intubation? Catecholamines, vasopressin or angiotensin? Eur J Anaesthesiol. 2005;22:780–5. doi: 10.1017/s0265021505001298. [DOI] [PubMed] [Google Scholar]
- 6.Park BY, Jeong CW, Jang EA, Kim SJ, Jeong ST, Shin MH, et al. Dose-related attenuation of cardiovascular responses to tracheal intubation by intravenous remifentanil bolus in severe pre-eclamptic patients undergoing Caesarean delivery. Br J Anaesth. 2011;106:82–7. doi: 10.1093/bja/aeq275. [DOI] [PubMed] [Google Scholar]
- 7.Grillo P, Bruder N, Auquier P. Esmolol blunts the cerebral blood flow velocity increase during emergence from anesthesia In neurosurgical patients. Anesth Analg. 2003;96:1145–9. doi: 10.1213/01.ANE.0000055647.54957.77. [DOI] [PubMed] [Google Scholar]
- 8.Yoo KY, Jeong CW, Kim SJ, Jeong ST, Kwak SH, Shin MH, et al. Altered cardiovascular responses to tracheal intubation in patients with complete spinal cord injury: relation to time course and affected level. Br J Anaesth. 2010;105:753–9. doi: 10.1093/bja/aeq267. [DOI] [PubMed] [Google Scholar]
- 9.Bansal S, Pawar M. Hemodynamic responses to laryngoscopy and intubation in patients with pregnancy-induced hypertension: Effect of intravenous esmolol with or without lidocaine. Int J Anesth. 2002;11:4–8. doi: 10.1054/ijoa.2001.0918. [DOI] [PubMed] [Google Scholar]
- 10.Yoo KY, Jeong CW, Kim SJ, Jeong ST, Shin MH, Lee J. Cardiovascular and arousal responses to laryngoscopy and tracheal intubation in patients with complete spinal cord injury. Br J Anaesth. 2009;102:69–75. doi: 10.1093/bja/aen317. [DOI] [PubMed] [Google Scholar]
- 11.Nho JS, Lee SY, Kang JM, Kim MC, Choi YK, Shin OY, et al. Effects of maintaining a remifentanil infusion on the recovery profiles during emergence from anaesthesia and tracheal extubation. Br J Anaesth. 2009;103:817–21. doi: 10.1093/bja/aep307. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki M, Kadoi Y, Saito S. Effects of landiolol, a short-acting beta-1 blocker, on hemodynamic variables during emergence from anesthesia and tracheal extubation in elderly patients with and without hypertension. J Anesth. 2009;23:483–8. doi: 10.1007/s00540-009-0805-9. [DOI] [PubMed] [Google Scholar]
- 13.Goyagi T, Nishikawa T, Tobe Y. Neuroprotective effects and suppression of ischemia-induced glutamate elevation by β1-adrenoreceptor antagonists administered before transient focal ischemia in rats. J Neurosurg Anesthesiol. 2011;23:131–7. doi: 10.1097/ANA.0b013e31820369c1. [DOI] [PubMed] [Google Scholar]
- 14.Turan G, Ozgultekin A, Turan C, Dincer E, Yuksel G. Advantageous effects of dexmedetomidine on haemodynamic and recovery responses during extubation for intracranial surgery. Eur J Anaesthesiol. 2008;25:816–20. doi: 10.1017/S0265021508004201. [DOI] [PubMed] [Google Scholar]
- 15.Krodel DJ, Bittner EA, Abdulnour RE, Brown RH, Eikermann M. Negative pressure pulmonary edema following bronchospasm. Chest. 2011;140:1351–4. doi: 10.1378/chest.11-0529. [DOI] [PubMed] [Google Scholar]
- 16.Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med. 2011;39:2612–8. doi: 10.1097/CCM.0b013e3182282a5a. [DOI] [PubMed] [Google Scholar]
- 17.Fujii Y, Saitoh Y, Takahashis S. Combined diltiazem and lidocaine reduces cardiovascular responses to tracheal extubation and anesthesia emergence in hypertensive patients. Can J Anesth. 1999;46:952–6. doi: 10.1007/BF03013130. [DOI] [PubMed] [Google Scholar]
- 18.Nishina K, Mikawa K, Takao Y, Shiga M, Maekawa N, Obara H. Prostaglandin E1, lidocaine and prostaglandin E1-lidocaine combination for attenuating cardiovascular responses to extubation. Can J Anaesth. 1997;44:1211–4. doi: 10.1007/BF03013348. [DOI] [PubMed] [Google Scholar]
- 19.Mikawa K, Nishina K, Takao Y, Shiga M, Maekawa N, Obara H. Attenuation of cardiovascular responses to tracheal extubation: Comparison of verapamil, lidocaine and verapamil-lidocaine combination. Anesth Analg. 1997;85:1005–10. doi: 10.1097/00000539-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Keskin HE, Bilgin H. Comparing the effects of lidocaine and esmolol for the control of hemodynamic responses during laryngoscopy, ıntubation and extubation. Türk Anest Rean Der Derg. 2005;33:463–70. [Google Scholar]
- 21.Wang YQ, Guo QL, Xie O. Effects of different doses at esmolol on cardiovascular responses to tracheal extubation. Human Yi Ke Da Bao. 2003;28:259–62. [PubMed] [Google Scholar]
- 22.Dyson A, Isaac PA, Pennant JH, Giesecke AH, Lipton JM. Esmolol attenuates cardiovascular responses to extubation. Anesth Analg. 1990;71:675–8. doi: 10.1213/00000539-199012000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Fuhrman TM, Ewel CL, Pippin WD. Comparison of the efficacy of esmolol and alfentanyl to attenuate the hemodynamic responses to emergence and extubation. J Clin Anesth. 1992;4:444–7. doi: 10.1016/0952-8180(92)90216-n. [DOI] [PubMed] [Google Scholar]
- 24.Lim SH, Chin NM, Tai HY, Wong M, Lin TK. Prophylactic esmolol infusion for the control of cardiovascular responses to extubation after intracranial surgery. Ann Acad Med Singapore. 2000;29:447–51. [PubMed] [Google Scholar]


