Abstract
Objective
Pseudocholinesterase (PChE) is an enzyme responsible for the hydrolysis of succinylcholine. In case of its deficiency, the effect of succinylcholine that is approximately 5–10 min is prolonged up to few hours. The use of succinylcholine has been declined recently. However, it is still actively used in some special conditions and in developing countries. In this study, incidence of PChE enzyme deficiency around Adiyaman city was investigated and presented with the literature review.
Methods
After obtaining an approval from the investigational board of our hospital (Adiyaman University Medical School, Biomedical Research Ethics Board, 30.12.2012, Nr: B.30.2.ADY.0.20.00-600/51), patients undergoing any elective operation under general anaesthesia in the Adiyaman University Medical School Hospital between March and December 2013 were recruited for the study. After obtaining the patients’ written consents, blood PChE, alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea, creatinine, international normalisation ratio (INR) and activated partial thromboplastin time (aPTT) values of the patients were analysed. Possible association of the PChE deficiency with other values was also investigated. The normal value of PChE was taken as 4260–11250 for females aged 16–40 years and 5320–12920 U L−1 for other patients.
Results
The study was completed with 964 patients, 702 (72.8%) of whom were females. PChE enzyme levels were under the normal in 7.2% of the patients. There were no correlation between patient group, ALT, INR, aPTT and creatinine elevation with PChE deficiency (p>0.05), whereas AST and urea level elevation was significantly associated with PChE deficiency (p<0.05). The risk of PChE deficiency was 4.5 and 9 times higher in the patients with the elevation of AST and urea levels, respectively.
Conclusion
Pathological elevations of AST and urea that are a part of normal pre-operative biochemical analysis of blood will indicate the possible deficiency of PChE enzyme.
Keywords: Pseudocholinesterase, butyrylcholinesterase, serum cholinesterase, false cholinesterase, prolonged apnoea
Introduction
Pseudocholinesterase PChE is a complex molecular enzyme that is immediately released in the plasma after being synthesised in the liver (1, 2). Butyrylcholinesterase is also known as serum cholinesterase, plasma cholinesterase, and PChE (3). Except erythrocytes, it can be found in several tissues (4). Although its physiological role is not completely understood, it enables the hydrolysis of ester-structured drugs, such as mivacurium, procaine, chlorprocaine, tetracaine, cocaine, heroin and succinylcholine (5, 6). The most discussed topic on this enzyme’s clinical practice is succinylcholine metabolism. Succinylcholine is a depolarising neuromuscular blocker that is used in anaesthesia practice. PChE terminates the effect of succinylcholine by metabolising it first to succinylmonocholine and subsequently to succinic acid (7). Thus, in enzyme deficiency, the effect of succinylcholine, which normally takes approximately 5–10 min, extends and can take up to several hours.
PChE deficiency may be genetic or acquired. PChE deficiency that genetically occurs is an autosomal recessive disease resulting from a damage in the butyrylcholinesterase gene located in the 3q26.1-26.2 region in the long arm of the third chromosome (4). Depending on whether the chromosomal damage is homo- or heterozygous, the effect of succinylcholine extends. While the effect of succinylcholine takes 5 min to 1 h in heterozygotes, it exceeds 1 h in homozygotes (7). Although the incidence differs in various societies, it is estimated that generally, the incidence of the heterozygous form is 1 in 25–50 individuals, whereas the incidence of the homozygous form is 1 in 3000–5000 individuals (6, 8). It is rarely observed among Asian, African and European descendants; however, it is more common in Eskimos, Indian Vysyas, Persians and Jewish communities (6, 9, 10). Although there are 65 genetic variants, the most common and clinically important variants are atypical (dibucaine resistant), fluoride resistant, the silent and Kalow types (4). Patients with genetic PChE deficiency can live without any problems throughout their lives and may not be diagnosed, unless they are exposed to neuromuscular blockers, such as succinylcholine or mivacurium (4, 11).
Among the causes of acquired PChE deficiency, liver and kidney diseases, malnutrition, pregnancy, cancer, burns, myocardial infarction, myxoedema, chronic infection, cardiopulmonary bypass and leprosy can be considered (4, 7, 12). Several drugs have been associated with a reduction in PChE activity. Echothiophate eye drops, organophosphate insecticides, cyclophosphamide, tacrine, oral contraceptives, phenelzine, pancuronium, bambuterol and metoclopramide are among the causes of PChE deficiency resulting from drug effects (13–25).
Although genetic, acquired and drug effect-induced PChE deficiency alone is not always enough to form a clinical picture, the coexistence of these conditions may create problems (4). When faced with a clinical scenario in a patient with PChE deficiency, in other words, when the effect of neuromuscular blockers extends, fresh whole blood, fresh frozen plasma and human serum cholinesterase can be used (26–29). However, considering that these methods may cause possible complications that are associated with transfusion, the most reliable therapy is to wait for the return of patient’s spontaneous respiration. Even in homozygous silent-type PChE deficiency, in which neuromuscular block is the longest, the duration of the block rarely exceeds 4 h. Of course, appropriate intensive care conditions and mechanical ventilation facilities should be provided during this period. Considering that the patient would be conscious, it is unnecessary to emphasise the importance of adequate sedation and communication with the patient (4).
PChE deficiency can be demonstrated by biochemical and molecular analyses of the blood sample. The amount of enzyme in the plasma can be quantitatively demonstrated by biochemical examination. While PChE levels in the blood vary according to different laboratory standards, 3200–7500 IU L−1 is generally considered as the normal value (4, 7). With this analysis, dibucaine, fluoride, chloride, urea and succinylcholine inhibition degrees of the enzyme can also be determined. In contrast, molecular analysis may reveal the defect in the gene encoding the enzyme (30, 31). Although molecular tests are valuable in determining the genotype, they cannot provide information regarding the acquired enzyme deficiency.
In recent years, with the increase in the use of fast acting non-depolarising neuromuscular blockers, the use of succinylcholine has decreased. However, it is still actively used for patients with full stomach and who require urgent intervention and in anaesthesia for electroconvulsive therapy. In some developing countries, it is also frequently used in elective surgeries. This study aimed to assess the frequency of PChE deficiency in the province of Adiyaman, to examine the relationships among parameters in routine blood biochemical screening and to present the data, along with a literature review.
Methods
The ethics committees’ approval from the Adiyaman University Faculty of Medicine Biomedical Research Ethics Committee (30 December 2012, No: B.30.2.ADY.0.20.00-600/51) was obtained; patients undergoing any elective surgery under general anaesthesia at the Adiyaman University Faculty of Medicine hospital between March and December 2013 were included. Written consents were obtained from the patients. Age was not considered, but children under 3 years were more selectively treated because of the difficulty in establishing vascular access.
At our hospital, a standard pre-operative evaluation guide is available, and of the blood laboratory parameters, complete blood count, haemostasis panel [prothrombin time (PT) and activated partial thromboplastin time (aPTT)], general blood chemistry, including liver alanine transaminase (ALT), aspartate aminotransferase (AST) and renal (urea and creatinine) enzymes, and human immunodeficiency virus (HIV) and hepatitis antigens are routinely examined. As part of pre-operative laboratory examinations, serum PChE levels of the patients were also examined. PChE and other biochemical tests were performed via a Cobas Integra 600® (Roche Diagnostics, USA) device. PT was recorded as the international normalised ratio (INR). The reference ranges of the parameters mentioned above are shown in Table 1.
Table 1.
The reference ranges of parameters in blood biochemistry screening
| Lower limit | Upper limit | |
|---|---|---|
| ALT (U L−1) | 5 | 55 |
| AST (U L−1) | 5 | 34 |
| Urea (mg dL−1) | 10 | 50 |
| Creatinine (mg dL−1) | 0.57 | 1.11 |
| INR | 0.8 | 1.2 |
| aPTT (s) | 24 | 38 |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; INR: international normalised ratio; aPTT: activated partial thromboplastin time
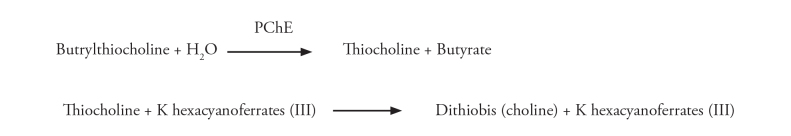
Serum PChE level was assessed by a colorimetric test. Cholinesterase catalyses the hydrolysis of butrylthiocholine to thiokol and butyrate. Thiokol, on the other hand, converts the colour from yellow to colourless by reducing potassium hexacyanoferrate (Figure 1). This reduction in colour can be photometrically measured. Normal reference range of PChE levels for women between 16–40 years is 4260–11250 U L−1, whereas for the other patients, it was considered as 5320–12920 U L−1 (32–34).
Figure 1.
PChE determination test principle (34th reference was used)
PChE: pseudocholinestarese; K: potassium
Blood samples were collected in gelled blood tubes and stored at room temperature after being centrifuged. Because the samples can retain stability for 6 h at 15–25°C, it was ensured that the samples were examined within 5 h (35, 36).
The relationship between PChE levels with demographic and aforementioned laboratory data was examined.
Statistical analysis
Data analysis was performed via Statistical Package for the Social Sciences 15.0 (SPSS Inc., Chicago, Illinois, USA) program. Data were presented as the number of patients. To evaluate the risk factors of PChE deficiency, the univariate logistic regression analysis was used. The evaluation of the factors (p≤0.10) that were associated with PChE deficiency with the univariate logistic regression analysis was performed using the multivariate logistic regression analysis. The goodness of fit of the multivariate logistic regression was assessed with the Hosmer–Lemeshow test. Odds ratio and nominal 95% confidence intervals were presented. For all analyses, two-sided p value of <0.05 was considered significant. In power analysis, previous studies and the population of Adiyaman province were considered and statistical significance between data were aimed to be found.
The study was planned to include at least 900 patients to have 90% strength, with the α value taken 0.05. On taking the possible data loss into consideration, 1000 patients were planned to be included in the study.
Results
Because the blood PChE level data of 36 patients were lost, the study was completed with the data of 964 patients. A total of 702 of these patients (72.8%) were female. Demographic data is provided in Table 2.
Table 2.
Demographic data
| Gender | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Male (n=262) | Female (n=702) | Total (n=964) | ||||
|
|
|
|
||||
| Age (year) | n | % | n | % | n | % |
| ≤15 | 34 | 13,0 | 14 | 2 | 48 | 5 |
|
| ||||||
| 16−40 | 123 | 46.9 | 568 | 80.9 | 691 | 71.7 |
|
| ||||||
| 41−64 | 70 | 26.7 | 91 | 13 | 161 | 16.7 |
|
| ||||||
| ≥65 | 35 | 13.4 | 29 | 4.1 | 64 | 6.6 |
PChE levels of 69 (7.2%) of 964 patients were observed to be below normal. In 7.2% of women between 16 and 40 years and in 7.1% of other patients, PChE levels were below normal. PChE deficiency frequency in terms of sex is shown in Table 3.
Table 3.
PChE deficiency by gender
| PChE level | ||||
|---|---|---|---|---|
| Normal | Low | |||
|
|
|
|||
| Gender | n | % | n | % |
| Male (n=262) | 249 | 95.0 | 13 | 5.0 |
| Female (n=702)* | 646 | 92.0 | 56 | 8.0 |
Different reference range was used for women between 16 and 40 years (refer to materials and methods).
PChE: pseudocholinestarese
The incidences of PChE enzyme level deficiency in patients with normal and above the normal range of ALT, AST, INR, aPTT, urea and creatinine are shown in Table 4.
Table 4.
Univariate logistic regression analysis results
| PChE | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | Below normal | ||||||||
|
|
|
||||||||
| Risk Factors | n | % | n | % | n | % | OR | 95% GA | p |
| Patient group | |||||||||
|
| |||||||||
| Other patients* | 396 | (41.1) | 368 | (92.9) | 28 | (7.1) | 1 | ||
|
| |||||||||
| 16–40 years, female | 568 | (58.9) | 527 | (92.8) | 41 | (7.2) | 1.022 | (0.621–1.683) | 0.930 |
|
| |||||||||
| ALT | |||||||||
|
| |||||||||
| Normal (reference) | 949 | (98.4) | 883 | (93.0) | 66 | (7.0) | 1 | ||
|
| |||||||||
| High | 15 | (1.6) | 12 | (80.0) | 3 | (20.0) | 3.345 | (0.921–12.146) | 0.067† |
|
| |||||||||
| AST | |||||||||
|
| |||||||||
| Normal (reference) | 926 | (96.1) | 866 | (93.5) | 60 | (6.5) | 1 | ||
|
| |||||||||
| High | 38 | (3.9) | 29 | (76.3) | 9 | (23.7) | 4.479 | (2.028–9.893) | <0.001‡ |
|
| |||||||||
| INR | |||||||||
|
| |||||||||
| Normal (reference) | 834 | (86.5) | 775 | (92.9) | 59 | (7.1) | 1 | ||
|
| |||||||||
| High | 130 | (13.5) | 120 | (92.3) | 10 | (7.7) | 1.095 | (0.545–2.198) | 0.799 |
|
| |||||||||
| aPTT | |||||||||
|
| |||||||||
| Normal (reference) | 958 | (99.4) | 890 | (92.9) | 68 | (7.1) | 1 | ||
|
| |||||||||
| High | 6 | (0.6) | 5 | (83.3) | 1 | (16.7) | 2.618 | (0.302–22.724) | 0.383 |
|
| |||||||||
| Urea | |||||||||
|
| |||||||||
| Normal (reference) | 949 | (98.4) | 886 | (93.4) | 63 | (6.6) | 1 | ||
|
| |||||||||
| High | 15 | (1.6) | 9 | (60.0) | 6 | (40.0) | 9.376 | (3.235–27.174) | <0.001‡ |
|
| |||||||||
| Kreatinin | |||||||||
|
| |||||||||
| Normal (reference) | 938 | (97.3) | 871 | (92.9) | 67 | (7.1) | 1 | ||
|
| |||||||||
| High | 26 | (2.7) | 24 | (92.3) | 2 | (7.7) | 1.083 | (0.251–4.682) | 0.915 |
Numerical data indicates the number of patients. Values in parentheses represent percentages. PChE: pseudocholinestarese; ALT: alanine aminotransferase; AST: aspartate aminotransferase; INR: international normalised ratio; aPTT: activated partial thromboplastin time.
Other patients represented except female patients between 16 and 40 years.
p<0.10,
p<0.05
The variables that were believed to be related to PChE deficiency levels (patient group, ALT, AST, INR, aPTT, urea and creatinine) were first examined via the univariate logistic regression analysis. While the relationship of ALT, INR, aPTT and creatinine groups with PChE enzyme level deficiency was not statistically significant; the relationship of AST and urea groups with PChE enzyme level deficiency was statistically significant (p<0.05). According to the results found, the risk of PChE enzyme level deficiency was 4.479 in patients with high levels of AST compared with normal; and 9.376 in patients with high levels of urea compared with normal (Table 4).
AST and urea variables that posed a risk to PChE deficiency level with univariate logistic regression model, and ALT variables below 0.20 significance level were analysed using multivariate logistic regression model. Multivariate logistic regression analysis revealed that AST and urea variables were risk factors for PChE enzyme level deficiency (p<0.05), while ALT was not observed to be a risk factor. It was detected that in terms of PChE enzyme level deficiency incidence, patients with high AST levels had 3.54 times higher and those with high urea levels had 7.06 times higher risk compared with normal values (Table 5).
Table 5.
Multivariate logistic regression analysis results
| B | Wald | P | OR | %95 | GA | |
|---|---|---|---|---|---|---|
| Stable | −2.721 | 396.525 | <0.001 | 0.066 | ||
| ALT (normal/high) | 0.054 | 0.005 | 0.944 | 1.056 | 0.231 | 4.826 |
| AST (normal/high) | 1.264 | 7.208 | 0.007* | 3.540 | 1.407 | 8.910 |
| Urea (normal/high) | 1.954 | 11.682 | 0.001* | 7.058 | 2.301 | 21.643 |
ALT: alanine aminotransferase, AST: aspartate aminotransferase.
p<0.05
Discussion
The reason for female patients of reproductive age forming the large portion of the sampling (approximately 60%) is that we obtained approximately half of the data from the obstetrics and gynaecology department of our hospital. Our obstetrics and gynaecology clinic is the only obstetrics hospital in our city and daily performs an average of 13 caesarean sections. In this hospital, 3 years before we initiated our study, we had experienced prolonged apnoea in six patients after succinylcholine use. The possibility of PChE deficiency being endemic in this region pioneered the initiation of our study.
The main result of our study was that 7.2% patients admitted to the hospital for surgery had PChE deficiency. Because there was a decrease in PChE levels among women of reproductive age, different reference ranges were used in this group. Therefore, it was revealed that both in this group and in other patients, there was 7.2% of PChE deficiency. In our country, the only study on PChE deficiency was conducted by Yildirim et al. (37) in 2009 in which PChE deficiency had been searched in Sivas province. In that study, enzyme deficiency was detected in 3.77 % patients, and in our study, the same reference range was used for all patients. Because the major part of our study involved pregnant women, taking into account that the possible decrease in enzyme levels was due to physiological changes, we used different reference intervals for this patient population (32, 33).
PChE deficiencies may be genetic and acquired. Among the acquired causes, liver and kidney failures, malnutrition, pregnancy, hypoproteinemia, cancer, radiotherapy, chemotherapy, hyperpyrexia, burns, heart failure and myxoedema can be considered. Cyclophosphamide, procainamide, quinidine, phenothiazine, ketamine, pancuronium, propanidid, echothiophate eye drops and organophosphorus poisoning can also result in enzyme deficiency (37, 38). In our study, we examined the potential relationship between enzyme deficiency and other laboratory values. Although we could not identify any relationship with ALT, INR, aPTT and creatinine levels, we identified a significant relationship between AST and urea levels with PChE. While the probability of enzyme deficiency incidence was 4.5 times higher in patients with high AST levels, among patients with high urea levels, this ratio was 9-fold higher. According to the multivariate logistic regression model, these rates were found as 3.5 for AST and 7 for urea. Yildirim et al. concluded that a decrease in PChE levels is related to the male gender and older age and AST, ALT, urea, creatinine, PT and aPTT elevation. In particular, the incidence of enzyme deficiency was observed to be three times higher in AST elevation, and 5 times higher in urea elevation states (37). Our findings regarding AST and urea levels are consistent with these findings.
The most important result of PChE deficiency among patients is prolonged apnoea occurring because of the use of succinylcholine neuromuscular blocking agents. The use of succinylcholine has been decreasing with the introduction of new rapid and short-acting non-depolarising neuromuscular blockers in clinical practice, but still in many centres, in cases such as difficult airway, full stomach and electroconvulsive therapy, succinylcholine continues to be the first choice for clinicians. In cases of indication, after the patient’s kidney and liver enzyme levels are evaluated, PChE deficiency should be considered and a final decision on whether to use or not should be given considering the cost–benefit ratio.
Limitations of our study were that sampling composed of only patients admitted to the hospital and the obtained numeric data that was related to enzyme deficiency cannot be generalised to Adiyaman province. Another limitation was that for the cases with enzyme deficiencies, we could not perform genetic analysis because of limited means. Some of these patients might have a genetic enzyme defect.
Conclusion
In blood laboratory values that we routinely examine in pre-operative evaluation, pathological elevation in AST and urea levels might be associated with PChE deficiency. When considering the use of succinylcholine, the possibility of prolonged apnoea should be considered and necessary measures should be taken.
Acknowledgements
The authors want to thank Adiyaman University Research and Educational Hospital Biochemistry Laboratory staff for their labor.
Footnotes
This study was presented orally in 48th National Symposium of Turkish Anesthesiology and Reanimation Society (25-29.10.2014, Ankara, Turkey).
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Adıyaman University Faculty of Medicine Biomedical Research Ethics Committee (30.12.2012, Number: B.30.2.ADY.0.20.00-600/51).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - R.A., Ö.B.K., R.K., B.Ç., H.K., M.D., Ö.U., M.Ö., A.B., Ü.S., A.A.; Design - R.A., Ö.B.K., R.K., B.Ç., H.K., M.D., Ö.U., M.Ö., A.B., Ü.S., A.A.; Supervision - R.A., Ö.B.K., R.K., B.Ç., H.K., M.D., Ö.U., M.Ö., A.B., Ü.S., A.A.; Funding - R.A., Ö.B.K., R.K., A.A.; Materials - R.A., Ö.B.K., R.K., B.Ç., H.K., M.D., A.A.; Data Collection and/or Processing - R.A., Ö.B.K., R.K., B.Ç., H.K., M.D., Ö.U., M.Ö., A.B., Ü.S., A.A.; Analysis and/or Interpretation - R.A., Ö.B.K., R.K., B.Ç., A.A.; Literature Review - R.A., Ö.B.K., R.K., A.A.; Writer - R.A., Ö.B.K., R.K., B.Ç., A.A.; Critical Review - H.K., M.D., Ö.U., M.Ö., A.B., Ü.S., A.A.; Other - R.A.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Lockridge O, Bartels CF, Vaughan TA, Wong CK, Norton SE, Johnson LJ. Complete amino acid sequence of human serum cholinesterase. J Biol Chem. 1987;262:549–57. [PubMed] [Google Scholar]
- 2.Pedersen NA, Jensen FS. Clinical importance of plasma cholinesterase for the anaesthetist. Ann Acad Med Singapore. 1994;23:120–4. [PubMed] [Google Scholar]
- 3.Manoharan I, Wieseler S, Layer PG, Lockridge O, Boopathy R. Naturally occurring mutation Leu307Pro of human butyrylcholinesterase in the Vysya community of India. Pharmacogenet Genomics. 2006;16:461–8. doi: 10.1097/01.fpc.0000197464.37211.77. [DOI] [PubMed] [Google Scholar]
- 4.Soliday FK, Conley YP, Henker R. Pseudocholinesterase deficiency: a comprehensive review of genetic, acquired, and drug influences. AANA J. 2010;78:313–20. [PubMed] [Google Scholar]
- 5.Van Beck J. Pseudocholinesterase. In: Faust R, editor. Anesthesiology Review. 2nd ed. New York, NY: Churchill Livingstone; 1994. p. 139Y140. [Google Scholar]
- 6.Pantuck E. Plasma cholinesterase: gene and variations. Anesth Analg. 1993;77:380–6. doi: 10.1213/00000539-199377020-00027. http://dx.doi.org/10.1213/00000539-199377020-00027. [DOI] [PubMed] [Google Scholar]
- 7.Williams J, Rosenquist P, Arias L, McCall WV. Pseudocholinesterase Deficiency and Electroconvulsive Therapy. J ECT. 2007;23:198–200. doi: 10.1097/YCT.0b013e318070c686. http://dx.doi.org/10.1097/YCT.0b013e318070c686. [DOI] [PubMed] [Google Scholar]
- 8.Morgan GE, Mikhail MS, Murray MJ. Clinical Anesthesiology. 4th ed. New York, NY: McGraw-Hill; 2006. pp. 205–26. [Google Scholar]
- 9.Jaideep JP, Satish G, Jason A. A hypothesis to explain the high prevalence of pseudo-cholinesterase deficiency in specific population groups. Eur J Anaesthesiol. 2011;28:550–2. doi: 10.1097/EJA.0b013e3283457cfb. http://dx.doi.org/10.1097/EJA.0b013e3283457cfb. [DOI] [PubMed] [Google Scholar]
- 10.Kaback M, Lopatequi J, Portuges AR, Quindipan C, Pariani M, Salimpour-Davidov N, et al. Genetic screening in the Persian Jewish community: A pilot study. Genet Med. 2010;12:628–33. doi: 10.1097/GIM.0b013e3181edef5b. http://dx.doi.org/10.1097/GIM.0b013e3181edef5b. [DOI] [PubMed] [Google Scholar]
- 11.Manoharan I, Boopathy R, Darvesh S, Lockridge O. A medical health report on individuals with silent butyrylcholinesterase in the Vysya community of India. Clin Chim Acta. 2007;378:128–35. doi: 10.1016/j.cca.2006.11.005. http://dx.doi.org/10.1016/j.cca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Niazi A, Leonard IE, O’Kelly B. Prolonged neuromuscular blockade as a result of malnutrition-induced pseudocholinesterase deficiency. J Clin Anesth. 2004;16:40–2. doi: 10.1016/j.jclinane.2003.02.010. http://dx.doi.org/10.1016/j.jclinane.2003.02.010. [DOI] [PubMed] [Google Scholar]
- 13.McGavi DD. Depressed levels of serum-pseu do cho lin es ter ase with ecothiophate-iodide eyedrops. Lancet. 1965;2:272–3. doi: 10.1016/s0140-6736(65)92391-3. http://dx.doi.org/10.1016/S0140-6736(65)92391-3. [DOI] [PubMed] [Google Scholar]
- 14.Kamha AA, Al Omary IY, Zalabany HA, Hanssens Y, Adheir FS. Organophosphate poisoning in pregnancy: a case report. Basic Clin Pharmacol Toxicol. 2005;96:397–8. doi: 10.1111/j.1742-7843.2005.pto_09.x. http://dx.doi.org/10.1111/j.1742-7843.2005.pto_09.x. [DOI] [PubMed] [Google Scholar]
- 15.Nelson TC, Burritt MF. Pesticide poisoning, succinylcholine-induced apnea, and pseu do cho lin es ter ase. Mayo Clin Proc. 1986;61:750–2. doi: 10.1016/s0025-6196(12)62776-1. http://dx.doi.org/10.1016/S0025-6196(12)62776-1. [DOI] [PubMed] [Google Scholar]
- 16.Purdham RS, Gutierrez DS. Pseudocholinesterase deficiency and organophosphorous insecticide use. AANA J. 1986;54:240–4. [Google Scholar]
- 17.Sener EB, Ustun E, Kocamanoglu S, Tur A. Prolonged apnea following succinylcholine administration in undiagnosed acute organophosphate poisoning. Acta Anaesthesiol Scand. 2002;46:1046–8. doi: 10.1034/j.1399-6576.2002.460821.x. http://dx.doi.org/10.1034/j.1399-6576.2002.460821.x. [DOI] [PubMed] [Google Scholar]
- 18.Koseoglu V, Chiang J, Chan KW. Acquired pseudocholinesterase deficiency after high-dose cyclophosphamide. Bone Marrow Transplant. 1999;24:1367–8. doi: 10.1038/sj.bmt.1702097. http://dx.doi.org/10.1038/sj.bmt.1702097. [DOI] [PubMed] [Google Scholar]
- 19.McCaul K, Robinson GD. Suxamethonium “extension” by tetrahydroaminacrine. Br J Anaesth. 1962;34:536–42. doi: 10.1093/bja/34.8.536. http://dx.doi.org/10.1093/bja/34.8.536. [DOI] [PubMed] [Google Scholar]
- 20.Robertson GS. Serum protein and cholinesterase changes in association with contraceptive pills. Lancet. 1967;1:232–5. doi: 10.1016/s0140-6736(67)91298-6. http://dx.doi.org/10.1016/S0140-6736(67)91298-6. [DOI] [PubMed] [Google Scholar]
- 21.Bodley PO, Halwax K, Potts L. Low serum pseudocholinesterase levels complicating treatment with phenelzine. Br Med J. 1969;3:510–2. doi: 10.1136/bmj.3.5669.510. http://dx.doi.org/10.1136/bmj.3.5669.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stovner J, Oftedal N, Holmboe J. The inhibition of cholinesterases by pancuronium. Br J Anaesth. 1975;47:949–54. doi: 10.1093/bja/47.9.949. http://dx.doi.org/10.1093/bja/47.9.949. [DOI] [PubMed] [Google Scholar]
- 23.Motamed C, Fanen P, Feiss P, Kirov K, Duvaldestin P. Dose-response effect of serum butyrylcholinesterase activity after clinical doses of pancuronium. Eur J Clin Pharmacol. 2008;64:1043–5. doi: 10.1007/s00228-008-0548-9. http://dx.doi.org/10.1007/s00228-008-0548-9. [DOI] [PubMed] [Google Scholar]
- 24.Ostergaard D, Rasmussen SN, Viby-Mogensen J, Pedersen NA, Boysen R. The influence of drug-induced low plasma cholinesterase activity on the pharmacokinetics and pharmacodynamics of mivacurium. Anesthesiology. 2000;92:1581–7. doi: 10.1097/00000542-200006000-00014. http://dx.doi.org/10.1097/00000542-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Kao YJ, Turner DR. Prolongation of succinylcholine block by metoclopramide. Anesthesiology. 1989;70:905–8. doi: 10.1097/00000542-198906000-00004. http://dx.doi.org/10.1097/00000542-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Lovely MJ, Patteson SK, Beuerlein FJ, Chesney JT. Perioperative blood transfusion may conceal atypical pseu do cho lin es ter ase. Anesth Analg. 1990;70:326–7. doi: 10.1213/00000539-199003000-00017. http://dx.doi.org/10.1213/00000539-199003000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Smith DC, Ridley SA, Donaldson KF. Fresh frozen plasma and edrophonium in a patient with a plasma cholinesterase deficiency. Anaesthesia. 1993;48:511–3. doi: 10.1111/j.1365-2044.1993.tb07073.x. http://dx.doi.org/10.1111/j.1365-2044.1993.tb07073.x. [DOI] [PubMed] [Google Scholar]
- 28.Gill RS, O’Connell N, Scott RP. Reversal of prolonged suxamethonium apnoea with fresh frozen plasma in a 6-week-old infant. Anaesthesia. 1991;46:1036–8. doi: 10.1111/j.1365-2044.1991.tb09917.x. http://dx.doi.org/10.1111/j.1365-2044.1991.tb09917.x. [DOI] [PubMed] [Google Scholar]
- 29.Ostergaard D, Jensen FS, Viby-Mogensen J. Reversal of intense mivacurium block with human plasma cholinesterase in patients with atypical plasma cholinesterase. Anesthesiology. 1995;82:1295–8. doi: 10.1097/00000542-199505000-00027. http://dx.doi.org/10.1097/00000542-199505000-00027. [DOI] [PubMed] [Google Scholar]
- 30.Jensen FS, Schwartz M, Viby-Mogensen J. Identification of human plasma cholinesterase variants using molecular biological techniques. Acta Anaesthesiol Scand. 1995;39:142–9. doi: 10.1111/j.1399-6576.1995.tb04033.x. http://dx.doi.org/10.1111/j.1399-6576.1995.tb04033.x. [DOI] [PubMed] [Google Scholar]
- 31.Iohom G, Fitzgerald D, Cunningham AJ. Principles of pharmacogenetics: implications for the anaesthetist. Br J Anaesth. 2004;93:440–50. doi: 10.1093/bja/aeh200. http://dx.doi.org/10.1093/bja/aeh200. [DOI] [PubMed] [Google Scholar]
- 32.Tietz NW. Fundamentals of Clinical Chemistry. 3rd ed. Pa: WB Saunders Co; 1987. pp. 405–7. [Google Scholar]
- 33.Kaplan LA, Pesce AJ. Clinical Chemistry Theory. In: Ladig D, Kasper R, editors. Analysis and Correlation. St Louis: CV Mosby Co; 1984. pp. 1108–9. [Google Scholar]
- 34.Schmidt E, Henkel E, Klauke R, Lorentz K, Sonntag O, Stein W, et al. Proposal for standard methods for the determination of enzyme catalytic concentrations in serum and plasma at 37 degrees C. J Clin Chem Clin Biochem. 1990;28:805–8. [PubMed] [Google Scholar]
- 35.Tietz NW, editor. Clinical Guide to Laboratory Tests. 3rd ed. Philadelphia, PA: WB Saunders; 1995. pp. 132–3. [Google Scholar]
- 36.Huizenga JR, van der Belt K, Gips CH. The effect of storage at different temperatures on cholinesterase activity in human serum. J Clin Chem Clin Biochem. 1985;23:283–5. doi: 10.1515/cclm.1985.23.5.283. http://dx.doi.org/10.1515/cclm.1985.23.5.283. [DOI] [PubMed] [Google Scholar]
- 37.Yıldırım S, Şahin AF, Döngel İ, Erşan İ, Özkan F. Sivas ilinde psödokolinesteraz eksikliği görülme sıklığı ve ilişkili klinik parametreler. Turkiye Klinikleri J Anest Reanim. 2012;10:84–8. [Google Scholar]
- 38.Kayhan Z. Klinik Anestezi. 2. Baskı. Ankara: Logos Yayıncılık; 1997. Sinir kas iletimi ve kas gevşeticiler; pp. 135–50. [Google Scholar]