Abstract
Objective
This prospective randomised study was designed to compare the Laryngeal Mask Airway (LMA) Classic, LMA Fastrach and LMA Supreme regarding ease of insertion and insertion time as primary outcomes and reposition, success rate of trials, effects on haemodynamic parameters, provision of an adequate and safe airway, amount of leakage and oropharyngeal and systemic complications as secondary outcomes.
Methods
In this clinical trial, 90 patients aged 18–70 years of American Society of Anesthesiologists (ASA) group I–II were randomised into three groups as providing airway via LMA Classic, LMA Fastrach or LMA Supreme instead of tracheal intubation. No muscle relaxant was used. The allocated LMA was inserted by the same anaesthetist; bispectral index (BIS) was between 40% and 60%.
Results
There was no statistical difference among the groups regarding the ease of insertion and insertion time as primary outcomes; the incidence of repositioning during placement was significantly higher in the LMA Classic group than that in other groups (p<0.05) and the rates of bloodstain on the device as well as oropharyngeal mucosal oedema were higher in the LMA Fastrach group than those in other groups (p<0.05) as secondary outcomes.
Conclusion
We suggest that LMA Classic, LMA Supreme and LMA Fastrach had similar effectiveness regarding efficiency and airway safety. However, LMA Supreme seems to be more advantageous as it is more appropriate for fewer oropharyngeal complications and there was no repositioning.
Keywords: Laryngeal masks, airway management, efficacy
Introduction
As a result of studies regarding the provision of an airway that is less invasive than intubation but safer than mask to maintain the patency of airway after anaesthesia induction in brief surgical interventions, supraglottic airway devices have been introduced into practice (1). They are inserted into glottic entry via the oral route and can be used in emergency conditions when tracheal intubation and mask anaesthesia are challenging (1).
Classic Laryngeal Mask Airway (LMA) was first introduced by Archie Brain, MD, in 1988; it consists of a mask with a surrounding inflatable bag compatible with the shape of the hypopharynx and a tube that has a 30° angle with a mask (2, 3).
Fastrach LMA (LMA Fastrach: LMA; North America. Inc., San Diego, CA) was first introduced in 1997 and has features similar to the LMA Classic, but it is designed to provide a secure upper airway during intubation via blind intubation or fiberoptic assistance (4, 5). It has a rigid handle that allows one-handed insertion, removal or adjustment (Figure 1).
Figure 1.
LMA Classic (# 5), LMA Fastrach (# 4) and LMA Supreme (# 5)
Supreme LMA, first introduced in April 2007, is a novel, sterile, single use, new generation supraglottic airway device that provides more rapid and higher volume gas passage through the airway and can be inserted in a rapid and safe manner because of the advanced cuff and airway tube design (Figure 1). The integrated gastric canal facilitates gastric aspiration (6, 7).
Supraglottic airway devices seem to have more advantages than other devices in different aspects owing to their distinct features. In the literature, there are studies comparing various types of LMA devices for different features. However, to the best of our knowledge, there is no study comparing the three above-mentioned types of LMA regarding these parameters (7, 8). This is the main difference of our study from the studies in the literature.
This study aimed to compare the advantages and disadvantages of LMA Supreme, LMA Classic and LMA Fastrach regarding ease of insertion, repositioning, insertion time, effects on haemodynamic parameters, provision of adequate and safe airway and oropharyngeal and systemic complications.
Methods
This was a single centre, randomised and prospective study conducted at the Anaesthesia Clinic of the Ümraniye Training and Research Hospital in Istanbul. Ninety patients aged 18–70 years with ASA physical status I–II who were scheduled for brief elective surgical interventions, were recruited into the study after obtaining the approval of the local Ethics Committee of the Ümraniye Training and Research Hospital and after obtaining informed consent from the patients.
Normotensive patients with mouth opening of >3 cm, thyromental distance of >6 cm, sternomental distance of >12.5 cm and body mass index of <35 kg m−2 were included in the study.
Patients with ASA III–IV; those with a history of gastroesophageal reflux, pregnancy, cardiovascular and central nervous system diseases; those with difficulty in cooperation and those undergoing intracranial, intra-abdominal and ear-nose-throat surgeries were excluded from the study. None of the methods changed after the commencement of the study.
Randomisation was performed by simple randomisation using computer-generated random numbers. Patients were assigned to one of the three groups according to the LMA type used with 1:1:1 allocation using a group size of 30 patients. Preparation of random numbers list, assigning random number list to groups, insertion of LMA and evaluation of complications were undertaken by different persons.
The patients arriving in the operating room were placed in the supine position and monitored by electrocardiography (ECG; standard DII lead by Datex Ohmeda), mean arterial pressure (MAP), heart rate (HR), oxygen saturation (SpO2) and bispectral index (BIS; vista medical systems) by an electrode placed on the forehead.
Premedication (0.03 mg kg−1 IV midazolam) was administered to all patients at 5–10 min before induction, which was achieved using 1.5 μg kg−1 IV fentanyl and 1–2 mg kg−1 IV propofol. Anaesthesia was maintained using 1%–2% sevoflurane and 50%–50% mixture of O2 air with a fresh gas flow of 5 L min−1. No muscle relaxant was used.
The appropriate deflated LMA was placed when values between 40% and 60% were seen on the BIS monitor. The same anaesthetist performed LMA placement. The manufacturer’s recommendations were followed based on the body weight of the patients for the selection of the tool size.
After achieving adequate airway, cuffs were inflated to pressure levels that allowed minimum air leakage. Ease of insertion was rated using a 4-point scale (4: success at the first attempt without tactile resistance, 3: success at the first attempt with tactile resistance, 2: success at the second attempt, 1: failure at the second attempt). Time to successful insertion and effective airway was defined as the time to observation of three consecutive correct end-tidal CO2 (EtCO2) waves after insertion and was recorded.
If the inserted device failed to provide adequate ventilation, the device position was changed within the oral cavity; this was then defined as repositioning. If adequate ventilation was not provided despite repositioning, the device was removed from the mouth and changed with the different sizes of the device. This was defined as the success rate of trials and was recorded. If the second attempt to place the device failed (i.e. removal of the device from the mouth), these patients were intubated and excluded from the study. Other parameters assessed included demographic data, EtCO2, amount of leakage [difference between inspiration tidal volume (VTI) and expiration tidal volume (VTE)] and oropharyngeal and systemic complications.
Measurements were taken at the following time points: T1=before premedication, T2=before induction, T3=after induction, T4=insertion, T5=1 min after insertion, T6=5 min after insertion, T7=10 min after insertion, T8=20 min after insertion, T9=before extubation, T10=1 min after extubation, T11=5 min after extubation. An otolaryngologist who was blinded to the group assignment examined the patients preoperatively (baseline) and at 12 h postoperatively for complications.
After the completion of surgery, devices were removed when the patients achieved adequate spontaneous ventilation and were able to open his/her mouth on verbal command. No intraoral aspiration was performed during extubation; the presence of blood spots on the cuff was recorded. Complications were recorded during perioperative and postoperative periods.
Different persons performed the insertion of LMA and evaluation of complications. However, as the device can be seen in the mouth, it has no importance whether or not the same clinician who inserted the device performed perioperative measurements.
Statistical analysis
A pilot study was conducted to determine the sample size. In the power analysis, according to the results of this pilot study assessing insertion times, a sample size of 30 patients in each group was calculated to be sufficient to achieve 80% power and an α value of 0.05 by taking a delta value of 3.5 and a standard deviation of 4.8 into consideration. Number Cruncher Statistical System 2007, (NCSS; Utah, USA) software was used for statistical analysis. Descriptive statistics (mean, standard deviation and frequency) were used in the study. One-way ANOVA was used to compare normally distributed parameters among groups. Tukey HDS test was used to identify the source of the difference. To compare quantitative data, chi-square test was used. The results were expressed in 95% confidence interval. A value of p<0.05 was considered to be statistically significant.
Results
A total of 90 patients were evaluated in the study. There were no exclusions from the study. The demographic characteristics were similar among the groups (Table 1).
Table 1.
Patient demographics
| (n=90) | Classic Mean±SD | Fastrach Mean±SD | Supreme Mean±SD | p | |
|---|---|---|---|---|---|
| Age (year) | 44.60±13.79 | 46.17±14.81 | 49.30±17.28 | 0.486 | |
| Height (cm) | 171.43±8.45 | 169.47±8.93 | 169.63±10.10 | 0.657 | |
| Weight (kg) | 75.77±8.05 | 75.10±7.20 | 7700±7.70 | 0.623 | |
| BMI (kg m−2) | 26.00±0.00 | 26.25±1.50 | 28.50±4.95 | 0.647 | |
| n (%) | n (%) | n (%) | |||
| aSex | Female | 9 (30) | 8 (26.7) | 8 (26.7) | 0.946 |
| Male | 21 (70) | 22 (73.3) | 22 (73.3) | ||
| aASA | l | 19 (63.3) | 18 (60.0) | 16 (53.3) | 0.725 |
| ll | 11 (36.7) | 12 (40.0) | 14 (46.7) | ||
One-way ANOVA; achi-square test; n: patient number; ASA: American Society of Anesthesiologists; SD: standard deviation; BMI: body mass index
Heart rate values after induction during and after 5 min of insertion were found to be significantly higher in the LMA Fastrach group than those in the LMA Classic group (p<0.05), whereas HR values after 10 and 20 min of insertion and before and after 1 min of extubation were significantly lower in the LMA Classic group than those in the Fastrach and Supreme groups (p<0.01; Figure 2).
Figure 2.
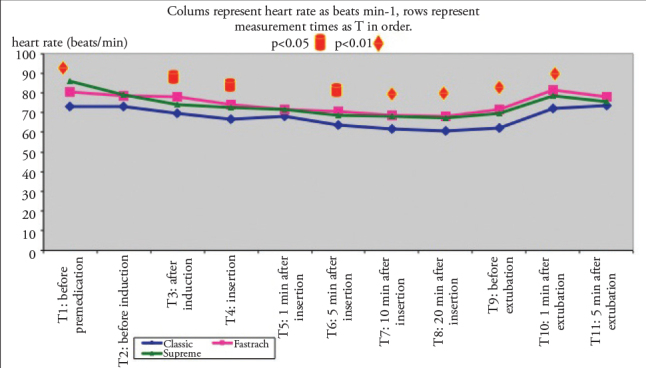
Heart rate variations according to groups (mean)
Heart rate variations according to groups (mean). Columns represent heart rate as beats min–1 and rows represent measurement times as T in order.
Men arterial pressure, SpO2 and EtCO2 values were within the normal range in all patients, although significant differences were observed among the groups in some measurements of SpO2 (only after extubation; p<0.01) and EtCO2 values (after 1, 5, 10 and 20 min of insertion; p<0.05).
No statistical difference was observed among the LMA Classic, LMA Fastrach and LMA Supreme groups regarding the number of trials, ease of insertion (there was no failure during the second attempt in all the three groups, thereby there was no scoring of 1 point; p=0.294), insertion time (p=0.206) and number of times a particular device size was used (#4 and #5 were more common for all devices; p>0.05; Table 2, 3).
Table 2.
Insertion time
| Insertion time (s) | p | ||
|---|---|---|---|
| Min-Max | Mean±SD | ||
| Classic | 18–44 | 27.10±7.09 | 0.206 |
| Fastrach | 20–47 | 28.83±7.10 | |
| Supreme | 18–46 | 25.83±5.11 | |
One-way ANOVA; Min: minimum; Max: maximum; SD: standard deviation
Table 3.
Results of assessment of the success rate of trials, LMA numbers and ease of insertion
| Classic n (%) | Fastrach n (%) | Supreme n (%) | p | ||
|---|---|---|---|---|---|
| Success rate of trials | 1st attempt | 27 (90) | 26 (86.7) | 29 (96.7) | 0.383 |
| 2nd attempt | 3 (10) | 4 (13.3) | 1 (3.3) | ||
| LMA number | 4 | 11 (36.7) | 12 (40) | 15 (50) | 0.553 |
| 5 | 19 (63.3) | 18 (60) | 15 (50) | ||
| Ease of insertion | 2 | 3 (10) | 4 (13.3) | 1 (3.3) | |
| 3 | 4 (13.3) | 8 (26.7) | 4 (13.3) | 0.294 | |
| 4 | 23 (76.7) | 18 (60) | 25 (83.3) | ||
| Reposition | 6 (75) | 2 (25) | 0 (0) | 0.021* |
Chi-square, p<0.05; n: patient number
The incidence of repositioning was significantly higher in the LMA Classic group than that in the LMA Fastrach and LMA Supreme groups (Table 3).
There was no significant difference among the groups regarding the amount of leakage. The minimum and maximum values can be seen in Table 4.
Table 4.
Amount of leakage
| Time (min) | Classic (mL) Mean±SD | Fastrach (mL) Mean±SD | Supreme (mL) Mean±SD | p |
|---|---|---|---|---|
| 1 min after placement | 76.67±17.51 | 74.30±12.58 | 79.47±15.20 | 0.425 |
| 5 min after placement | 78.20±18.01 | 74.90±13.11 | 81.30±15.94 | 0.302 |
| 10 min after placement | 78.57±18.67 | 73.57±14.96 | 82.57±16.29 | 0.119 |
| 20 min after placement | 77.87±19.99 | 75.37±13.78 | 80.37±15.34 | 0.508 |
| Before extubation | 77.10±18.37 | 76.73±14.00 | 81.43±15.74 | 0.458 |
One-way ANOVA. SD: standard deviation
Among the oropharyngeal complications, there was a significant difference among the groups regarding the presence of bloodstain on the device. The rate of bloodstain on the device was 63.6% (n=7) and the rate of mucosal oedema was 60% (n=9) in the LMA Fastrach group that were higher than those in the other groups (p<0.05; Table 5).
Table 5.
Assessment of complications
| Complications | Classic n (%) | Fastrach n (%) | Supreme n (%) | p |
|---|---|---|---|---|
| Oropharyngeal complications | ||||
| Redness of the tip of the tongue | 2 (22.2) | 6 (66.7) | 1 (11.1) | 0.075 |
| Mucosal erosion on the soft palate | 1 (11.1) | 6 (66.7) | 2 (22.2) | 0.081 |
| Bloodstain on the device | 4 (36.4) | 7 (63.6) | 0 (0) | 0.022* |
| Oropharyngeal mucosal oedema | 4 (26.7) | 9 (60) | 2 (13.3) | 0.044* |
| Mucosal redness | 5 (25) | 10 (50) | 5 (25) | 0.200 |
| Hypopharyngeal complications | ||||
| Sore throat | ||||
| 1.No pain | 5 (29.4) | 7 (41.2) | 5 (29.4) | 0.748 |
| 2.Pain less than that experienced in common cold | 1 (100) | 0 (0) | 0 (0) | 0.364 |
| 3.Pain similar to common cold | 6 (40) | 7 (46.7) | 2 (13.3) | 0.186 |
| 4.Very severe pain | 0 (0) | 1 (100) | 0 (0) | 0.364 |
| Dysphagia | 3 (50) | 3 (50) | 0 (0) | 0.200 |
| Systemic complications | ||||
| Mild desaturation (90%–95%) | 2 (66.7) | 1 (33.3) | 0 (0) | 0.355 |
| Cough | 1 (50) | 1 (50) | 0 (0) | 0.600 |
| Hiccup | 2 (40) | 2 (40) | 1 (20) | 0.809 |
Chi-square,
p<0.05; comparison among three groups
There was no significant difference between the groups regarding sore throat, dysphagia, hoarseness and voice alteration (Table 5).
When systemic complications were considered, no significant difference was seen in slight desaturation, cough and hiccup among the groups. No systemic complications such as laryngospasm, bronchospasm, hypercapnia, vomiting or aspiration were observed (Table 5).
Discussion
In the present study, in which the clinical effects of three different types of LMA were compared, it was found that LMA Classic and LMA Supreme were more advantageous than LMA Fastrach because of the presence of blood spots on the cuff and lack of mucosal oedema.
In this study, the more common use of LMA number #5 in all the groups was attributed to demographic characteristics (Table 1).
One of the most important components of haemodynamic response to intubation is elevation in blood pressure and HR, which is considered to be an undesirable event (9).
In previous studies, it has been reported that Classical, Fastrach and Supreme LMAs do not cause marked variation in MAP and HR when compared against each other or with endotracheal intubation (7, 8, 10–14).
At some measurement points, HR values were significantly lower in the LMA Classic group than in other groups of this study. The smaller extent of elevation in HR in LMA Classic can be explained by a lesser degree of stimulation in the receptors at the base of the tongue and pharynx resulting from the more elastic structure of the LMA Classic. All measured values were statistically significant, but these values that were in the normal range, and had no clinical significance (min: 60.53±8.21; max: 86.10±2.22).
In previous studies, SpO2 and EtCO2 values were found to be within the normal range in patients for whom LMA Classic was used (14, 15).
In this study, when EtCO2 distribution graphic was assessed, it was seen that baseline values remained within normal ranges by decreasing during surgery and became closer to baseline values at the end of surgery by increasing at the same rate. The effects of Classic Supreme and Fastrach LMAs on oxygenation and EtCO2 were found to be similar; hence, this result facilitated the interpretation that all the three devices are safe and effective in providing an airway.
The airway is considered safe and effective when a tidal volume (TV) of 6 mL kg−1 and peak airway pressure of 15 cm H2O are provided as well as when normal square-shaped EtCO2 traces on the capnogram are observed (16). The LMA manufacturers recommend a cuff pressure of <60 cm H2O. When the cuff is inflated to a volume higher than the recommended maximum volume, there is a risk of mucosal ischaemia because of compression because pressure applied to the pharynx mucosa by the cuff is higher than the capillary perfusion pressure (17).
In this study, the amount of leakage allowed to maintain a cuff pressure of <60 cm H2O, airway pressure of <20 mmHg and SpO2 of >95%. The amount of leakage was recorded within normal SpO2 and airway pressure after the observation of square-shaped EtCO2 traces by setting TV to 7 mL kg−1. It was measured as 70–80 mL in all patients. This mean value was considered safe because it provided TV within the normal range.
All three airway devices were found to be similar in terms of providing an effective airway and amount of leakage (Table 3).
In this study, there was a significant difference between groups in repositioning rates at the time of insertion (Table 3). The repositioning rate was significantly higher in the LMA Classic group than that in the other groups. The higher repositioning rates in the LMA Classic group were linked to the elastic structure of the tube that can be easily rotated on itself and to the fact that a guide was not used in this group. Lack of repositioning in the LMA Supreme was attributed to the thicker and more rigid structure of the tube that does not allow rotation.
No statistical difference was detected between the study groups when primary outcome measures including ease of insertion and insertion times were assessed (Table 2, 3). However, the shortest insertion time was detected in the LMA Supreme group (25.83±5.11 s) and the rate of success at the first attempt was also highest in the LMA Supreme group, although neither of these was statistically significant (Table 3). It can be suggested that insertion was easier in the LMA Supreme group than that in the other groups because of the structural features of LMA Supreme. This could be elucidated by future studies with larger series. The following are the insertion time reported in some studies: 20±11 s and 23±2 s for LMA Classic; 26 s, 25±22 s and 34±12 s for LMA Supreme and 100 s (min-max: 74–121 s) for LMA Fastrach (11, 14, 18–20). This variation in insertion times can be considered to result from demographic differences in study populations.
In the intraoral complications of the current study, there was a significant difference with respect to the presence of blood spots on the cuff. The rate of blood spots on the device was significantly higher in the LMA Fastrach group (63.6%; n=7) than that in the other groups. The rate of mucosal oedema was also found to be significantly higher in the LMA Fastrach group (60%) than that in the other groups. These findings were attributed to the more rigid structure of LMA Fastrach (Table 5).
No significant difference was found among the groups regarding hypopharyngeal and systemic complications.
In this study, LMA Classic, LMA Supreme and LMA Fastrach were found to be similarly effective in terms of efficiency and airway safety in selected patients undergoing brief surgical interventions at equivalent anaesthesia depths based on BIS values. In conclusion, LMA Supreme may be preferred to a greater extent because of the repositioning risk in LMA Classic and higher rates of oropharyngeal complications in LMA Fastrach. LMA Supreme seems to be more advantageous regarding the ability of aspiration and the number, ease and times of insertion, although there was no statistical difference. Further studies with larger series are required for greater clarification on this topic.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Ümraniye Training and Research Hospital.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - N.B., Ş.G.T., G.K.; Design - N.B., Ş.G.T., G.K.; Supervision - N.B., Ş.G.T., G.K.; Funding - N.B., Ş.G.T., G.K., E.K.; Materials - N.B., Ş.G.T., G.K.; Data Collection and/or Processing - Ş.G.T., G.K., E.K.; Analysis and/or Interpretation - N.B., Ş.G.T., G.K.; Literature Review - Ş.G.T., E.K., Z.T.D.; Writer - N.B., Ş.G.T., G.K., E.K.; Critical Review - Ş.G.T.; Other - Ş.G.T.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Robert A, Jonathan LB, Frederic AB, Casey DB, Robert HB, Frederick WC, et al. The American Society of Anesthesiologists Task Force on Management of the Difficult Airways. Practice guidelines for management of the difficult airway. Anesthesiology. 2003;98:1269–77. doi: 10.1097/00000542-200305000-00032. http://dx.doi.org/10.1097/00000542-200305000-00032. [DOI] [PubMed] [Google Scholar]
- 2.Brain A. The laryngeal mask-A new consept in airway management. Br J Anaesth. 1983;55:801–5. doi: 10.1093/bja/55.8.801. http://dx.doi.org/10.1093/bja/55.8.801. [DOI] [PubMed] [Google Scholar]
- 3.Brain A. The development of the laryngeal mask-a brief history of the invention, early clinical studies and experimental work from which the laryngeal mask evolved. Eur J Anesthesiol. 1991;4:5–17. [PubMed] [Google Scholar]
- 4.Tomas JG. Airway Management. In: Miller RD, editor. Anesthesia. 7th edition. Philadelphia: Churchill Livingstone; 2010. pp. 1573–610. [Google Scholar]
- 5.David ZF, William HR. Use of the Intubating LMA-Fastrach™ in 254 patients with difficult to manage airways. Anesthesiology. 2001;95:1175–81. doi: 10.1097/00000542-200111000-00022. http://dx.doi.org/10.1097/00000542-200111000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Timmermann A, Cremer S, Heuer J, Braun U, Graf BM, Russo SG. Laryngeal mask LMA Supreme. Application by medical personnel inexperienced in airway management. Anaesthesist. 2008;57:970–5. doi: 10.1007/s00101-008-1425-8. http://dx.doi.org/10.1007/s00101-008-1425-8. [DOI] [PubMed] [Google Scholar]
- 7.Seet E, Rajeev S, Firoz T, Yousaf F, Wong J, Wong DT. Safety and efficacy of laryngeal mask airway Supreme versus laryngeal mask airway ProSeal: a randomized controlled trial. Eur J Anesthesiol. 2010;27:602–7. doi: 10.1097/eja.0b013e32833679e3. http://dx.doi.org/10.1097/EJA.0b013e32833679e3. [DOI] [PubMed] [Google Scholar]
- 8.Ahmet A, Türkmen A, Kaya M, Cantürk S, Turgut N, Altan A. Comparison of supreme LMA, proseal LMA and Cobra PLA in adult patients undergoing minor surgery. Turk J Anaesth Reanim. 2013;41:70–4. [Google Scholar]
- 9.Klinik Anestezi: Kayhan Z. 3. Baskı. İstanbul, Logos;: 2004. Endotrakeal entübasyon; pp. 262–73. [Google Scholar]
- 10.Ali A, Canturk S, Turkmen A, Turgut N, Altan A. Comparison of the laryngeal mask airway Supreme and laryngeal mask airway Classic in adults. Eur J Anaesthesiol. 2009;26:1010–4. doi: 10.1097/EJA.0b013e3283313fdd. http://dx.doi.org/10.1097/EJA.0b013e3283313fdd. [DOI] [PubMed] [Google Scholar]
- 11.Verghese C, Ramaswamy B. LMA Supreme-a new single-use LMA with gastric Access: a report on its clinical efficacy. Br J Anaesth. 2008;101:405–10. doi: 10.1093/bja/aen174. http://dx.doi.org/10.1093/bja/aen174. [DOI] [PubMed] [Google Scholar]
- 12.Chang CH, Bai SJ, Kim MK, Nam SB. The usefulness of the laryngeal mask airway Fastrach for laryngeal surgery. Eur J Anaesthesiol. 2010;27:20–3. doi: 10.1097/EJA.0b013e3283317dac. http://dx.doi.org/10.1097/EJA.0b013e3283317dac. [DOI] [PubMed] [Google Scholar]
- 13.Kihara S, Brimacombe J, Yaguchi Y, Watanabe S, Taguchi N, Komatsuzaki T. Hemodynamic responses among three tracheal intubation devices in normotensive and hypertensive patients. Anesth Analg. 2003;96:890–5. doi: 10.1213/01.ANE.0000048706.15720.C9. http://dx.doi.org/10.1213/01.ANE.0000048706.15720.C9. [DOI] [PubMed] [Google Scholar]
- 14.Karabıyık L, Oncül S, Emez G. The Effects of the Cobra perilaryngeal airway on intraocular pressure. Turk J Med Sci. 2012;42:667–73. [Google Scholar]
- 15.Voyagis GS, Dimitriou V, Brimacombe J. The intubating laryngeal mask airway Fastrach for emergence after carotid endarterectomy. Middle East J Anesthesiol. 2005;18:551–7. [PubMed] [Google Scholar]
- 16.Turan A, Kaya G, Koyuncu O, Karamanlioglu B, Pamukcu Z. Comparison of the laryngeal mask (LMA) and laryngeal tube (LT) with the new perilaryngeal airway (CobraPLA) in short surgical procedures. Eur J Anaesthesiol. 2006;23:234–8. doi: 10.1017/S0265021505002243. http://dx.doi.org/10.1017/S0265021505002243. [DOI] [PubMed] [Google Scholar]
- 17.Marjot R. Pressure exerted by the laryngeal mask airway cuff upon the pharyngeal mucosa. Br J Anaesth. 1993;70:25–9. doi: 10.1093/bja/70.1.25. http://dx.doi.org/10.1093/bja/70.1.25. [DOI] [PubMed] [Google Scholar]
- 18.Luis AG, Mostafa JS, Karam K, Boris Y, Sonia VA. Comparison of the Laryngeal Mask Airway Unique™, Pharyngeal Airway X press™ and Perilaryngeal Airway Cobra ™ in Paralyzed Anesthetized Adult Patients. Anesthesiology. 2003;99:1495. [Google Scholar]
- 19.Lee AK, Tey JB, Lim Y, Sia AT. Comparison of the single-use LMA Supreme with the reusable Proseal LMA for anaesthesia in gynaecological laparoscopic surgery. Anaesth Intensive Care. 2009;37:815–9. doi: 10.1177/0310057X0903700537. [DOI] [PubMed] [Google Scholar]
- 20.Liu EH, Goy RW, Lim Y, Chen FG. Success of tracheal intubation with intubating laryngeal mask airways: a randomized trial of the LMA Fastrach and LMA CTrach. Anaesthesiology. 2008;108:621–6. doi: 10.1097/ALN.0b013e318167af61. http://dx.doi.org/10.1097/ALN.0b013e318167af61. [DOI] [PubMed] [Google Scholar]


