Abstract
Objective
In this study, we aimed to compare the effects of different intraoperative end-tidal desflurane concentrations on bispectral index (BIS) values in normal children and children with cerebral palsy.
Methods
Twenty normal children (Group N) and 20 children with non-communicative/nonverbal cerebral palsy (Group CP), between 2 and 15 years of age, undergoing elective orthopaedic surgery were included in the study. Following premedication with midazolam, anaesthesia was induced by infusing 1% propofol at a rate of 200 mL hr−1 until BIS reached 50. Heart rate, blood pressure and BIS values were recorded before and after the induction of anaesthesia, at steady-state end-tidal concentrations of 4% and 6% desflurane, and after emergence from anaesthesia. A p value <0.05 was considered significant in the statistical analyses, including Kolmogorov-Smirnov, t-test, paired samples t-test and chi-square test.
Results
The time to extubation and eye opening after discontinuation of anaesthesia was longer in Group CP. BIS values before the induction of anaesthesia, at a steady-state end-tidal desflurane concentration of 4% and after emergence from the anaesthetic were significantly lower in Group CP. At a steady-state end-tidal desflurane concentration of 6%, BIS values were slightly lower in Group CP but this difference was not statistically significant.
Conclusion
Based on the data obtained, we concluded that BIS monitoring in children with cerebral palsy can be useful in terms of decreasing adverse effects and drug interactions due to multiple drug usage by reducing the use of anaesthetic agents and improving emergence from the anaesthetic.
Keywords: Cerebral palsy, bispectral index, desflurane, paediatric anaesthesia
Introduction
Cerebral palsy is a general term, which defines permanent disorders of posture and movement caused by an injury to the developing brain (1). It is the leading cause of motor disability in childhood with a prevalence of 2 per 1000 live births (2, 3).
Anaesthesia is required for a wide variety of surgical procedures performed in children with cerebral palsy. These include; orthopaedic interventions such as multiple release, tenotomy and osteotomy, tooth extractions and reflux surgery (4). Anaesthetic approach in these patients deserves great attention and care because of risk of aspiration due to gastro-oesophageal reflux, malnutrition related anaemia and electrolyte imbalance, frequent lung infections, scoliosis, the possibility of difficult intubation, epilepsy, contractures and multiple drug use. Moreover, as these children have communication difficulties, and visual and hearing impairment and as they suffer from inability to express their pain, the pre-anaesthetic and post-anaesthetic course become more important.
The Bispectral index (BIS), which is derived from the electroencephalogram (EEG), is being used to guide the depth of sedation with values ranging from 0 (isoelectric EEG) and 100 (fully awake) (5). Although there is increasing evidence that the BIS monitoring, which has been developed in adults, can safely be used in children, there is not enough data to suggest its use in small children and infants because of the on-going development of their neurologic system (6). Non-progressive encephalopathy in children with cerebral palsy may affect BIS values and their correlation with anaesthetic use (7). Although whether BIS values of awake children with cerebral palsy significantly differ from that of normal children is controversial (7, 8), previous studies on BIS, including an earlier work performed at our institution, demonstrated that using the same drugs for both induction and maintenance in these patients produced a deeper anaesthesia and caused delayed emergence (1, 9–11).
It will be important to demonstrate that the anaesthetic requirement is lower in children with cerebral palsy by virtue of reducing the multiple drug-related side effects and drug interactions, hastening emergence from anaesthesia and reducing costs. Therefore, we aimed to compare the effects of different intraoperative end-tidal desflurane concentrations on BIS values in normal children and children with cerebral palsy.
Methods
The study was approved with permission No B.30.2. HAC.0.20.05.04/2276, by the Local Ethics Committee for Medical Research, Hacettepe University Medical Faculty on 28.08.2009. After written informed consents were obtained from the parents of the children, 20 ASA I–II normal children (Group N) aged between 2 and 15 years, with no neurological disorders that affect the mental functions, and 20 children with nonverbal cerebral palsy that could not communicate (Group CP), aged between 2–15 years undergoing elective orthopaedic surgery were included in the study.
All patients received oral premedication with midazolam (0.5 mg kg−1, maximum dose, 15 mg) 15 minutes before entering the operating room. In the operating room, standard monitoring including electrocardiography, non-invasive blood pressure (NIBP), pulse oximetry, capnography, temperature and BIS monitoring, were performed. The Emla cream (2.5% lidocaine and 2.5% prilocaine) was applied to the dorsum of the hand 45 minutes before the induction and a 22 gauge intravenous cannula was placed immediately before the induction of anaesthesia. During the operation, body temperature was kept at 35.0°C and above and the end tidal CO2 between 30–40 mmHg.
Anaesthesia was induced using 1% propofol at an infusion rate of 200 mL/hour with a target BIS of 50. The patients received 1 μg kg−1 of fentanyl for intraoperative analgesia. Neuromuscular blockade was induced with 0.6 mg kg−1 of rocuronium and the patients were intubated using an appropriate size endotracheal tube, based on the child’s age and weight. No additional opioids or analgesics were used during the operation. Maintenance of anaesthesia was performed using an end-tidal desflurane concentration between 4%–6% in 50% oxygen/NO mixture.
BIS monitoring was performed with Datex Ohmeda BIS monitor (Datex-Ohmeda Division, Instrumentarium Corp. Helsinki, Finland), digital signal converter (Aspect Medical Systems model DSC-XP, Newton, MA, USA) and disposable (age-appropriate) paediatric or adult BIS electrodes. The BIS values were recorded; before the induction of anaesthesia (T1), immediately after the induction of anaesthesia (T2), at a steady-state end-tidal desflurane concentration of 4% (T3), at a steady-state end-tidal desflurane concentration of 6% (T4) and immediately after emergence from anaesthesia (T5).
The BIS measurements at steady-state end-tidal desflurane concentrations were started to be recorded 20 minutes after the induction of anaesthesia and the recordings were made when the end-tidal desflurane concentration was kept at least for 15 minutes at the targeted level. As the BIS values were expected to show variability, maximum and minimum BIS values and their arithmetic means were recorded at each steady-state concentration.
Emergence from anaesthesia was accepted as spontaneous eye-opening after extubation, crying, moaning or making volitional movements. All of the measurements, except BIS values obtained after emergence from anaesthesia, were performed without giving any opioids or analgesics in addition to the initial dose of fentanyl. However, for postoperative analgesia, the patients received 15 mg kg−1 of paracetamol and 0.1 mg kg−1 of intravenous morphine 20–25 minutes before the surgeon began closing the skin. In all patients, the vaporizer and NO were turned off when the skin closure began and ventilation was continued manually with 100% oxygen. In order to reverse neuromuscular block, 0.05 mg kg−1 of neostigmine and 0.01 mg kg−1 of atropine were given intravenously. The patients were extubated after the criteria for extubation were met and were transferred to the recovery room.
In addition to the bispectral index values, patient demographics, duration of anaesthesia and surgery, the operation performed, time to extubation (i.e. from cessation of desflurane to extubation), eye-opening time (i.e. from cessation of desflurane to eye opening), the amount of propofol used, the total amount of desflurane used and heart rate and blood pressure at each BIS measurement were recorded.
Statistical analysis
The data were analyzed using SPSS version 16.0 (Statistical Package for the Social Sciences). The chi-square test was used for comparison of categorical variables between the groups. The Kolmogorov-Smirnov test was used to determine whether the variables were normally distributed. All variables that were compared between the two groups were normally distributed. The normally distributed numerical variables were compared using independent samples t-test. The normally distributed parametric variables were presented as mean±standard deviation (mean±SD). Paired sample t test was used for intragroup variation over time. A p value of less than 0.05 was considered to be statistically significant.
Results
The two groups were similar in terms of gender, age, height, weight, and duration of surgery and anaesthesia. As all the patients in Group CP were non-communicative and nonverbal individuals although they have no other diseases, they were classified as ASA II and thus, we found a significant difference between the groups in regard to ASA scores (p<0.001) (Table 1). The most common concomitant diseases in group CP were mental retardation, epilepsy, gastro-oesophageal reflux and hearing disorders. There were no concomitant diseases in group N except hearing disorder in one patient, epilepsy in one, and meningomyelocele in two patients. Multiple surgical procedures including osteotomy, tendon transfer and release were performed in the same session in 5 patients from group CP and in 3 patients from group N. The most common surgical procedures in patients with cerebral palsy were multiple release and extension osteotomies.
Table 1.
Comparison of patients’ demographics, durations of surgery, anaesthesia, time to extubation and eye opening and doses of propofol and desflurane (mean±SD)
| Group CP (n=20) | Group N (n=20) | p value | |
|---|---|---|---|
| Gender (F/M) (n) | 9/11 | 9/11 | 0.624 |
| Age (years) | 9.7±3.5 | 7.5±4.5 | 0.086 |
| Height (cm) | 132.6±18.2 | 120.2±26.6 | 0.102 |
| Weight (kg) | 28.7±11.5 | 24.6±15.3 | 0.349 |
| ASA (I/II) | 0/20 | 11/9 | <0.001 |
| Duration of surgery (min) | 126.9±49.7 | 150.5±81.8 | 0.278 |
| Duration of anaesthesia (min) | 151.8±52.2 | 170.6±83.6 | 0.399 |
| Time to extubation (min) | 4.8±2.6 | 3.3±1.4 | 0.026 |
| Time to eye opening (min) | 7.8±2.7 | 5.4±1.9 | 0.002 |
| Propofol (mg) | 78.0±41.8 | 74.2±50.4 | 0.800 |
| Desflurane (mL) | 162.0±72.6 | 192.7±105.8 | 0.291 |
Chi-square test and Fisher’s exact test were used for the analysis of variables.
When the time to extubation and eye-opening were compared, it was found that there was a significant delay in group CP (p values for the time to extubation and the time to eye-opening were 0.026 and 0.002, respectively) compared to group N. There were no significant differences between the two groups in regard to total amount of propofol (mg) used during the induction of anaesthesia and total amount of desflurane used (mL) during the operation (Table 1).
We found no significant difference between the two groups in terms of heart rate, mean arterial blood pressure and systolic blood pressure in different time periods. There was no significant difference between the two groups in regard to the diastolic blood pressure values recorded in T1, T3 and T4 periods whereas, diastolic blood pressure recorded after the induction of anaesthesia and after the emergence from anaesthesia were found significantly lower in group N than that in group CP (p=0.043 and p=0.010, respectively).
In group CP, heart rate was found to be significantly higher in T4 (at a steady-state end-tidal desflurane concentration of 6%) than that in T3 (at a steady-state end-tidal desflurane concentration of 4%) (p=0.017), whereas heart rate values recorded in T3 and T4 periods were not significantly different in group N. Comparisons within both groups, showed no significant differences in systolic, diastolic and mean arterial pressure values that were recorded at steady-state end-tidal desflurane concentrations of 4% and 6% (Figure 1).
Figure 1.

Comparison of heart rate and mean arterial pressures (mmHg) at T3 and T4 stages.
The highest BIS values, which were recorded before the induction of anaesthesia (T1), at a steady-state end-tidal desflurane concentrations of 4% and 6% (T3, T4) and after emergence from anaesthesia (T5) were significantly lower in Group CP than Group N (p<0.001, p=0.029, p=0.048, p=0.015, respectively). There were no significant differences between the two groups in regard to the highest BIS values recorded after the induction of anaesthesia.
The lowest BIS values which were recorded at T1, T3 and T5 periods were also found to be significantly lower in group CP than that in group N (p<0.001, p=0.037, p=0.003, respectively). However, there were no significant differences between the two groups in regard to the lowest BIS values recorded after the induction of anaesthesia (T2) and at a steady-state end-tidal desflurane concentration of 6% (T4).
Mean BIS values, which were recorded before the induction of anaesthesia (T1), at a steady-state end-tidal desflurane concentration of 4% (T3) and immediately after emergence from anaesthesia (T5) were significantly lower in Group CP than that in Group N (p<.001, p=0.026, p=0.004, respectively). There were no significant differences between the two groups in regard to the mean BS values recorded after the induction of anaesthesia (T2) and at a steady-state end-tidal desflurane concentration of 6% (T4) (Table 2).
Table 2.
Comparison of BIS values according to different stages (mean±SD)
| mean BIS value | Group CP (n=20) | Group N (n=20) | p |
|---|---|---|---|
| T1 | 86.1±7.8 | 95.8±4.6 | <0.001 |
| T2 | 40.6±14.9 | 42.9±10.5 | 0.576 |
| T3 | 44.6±11.9 | 52.1±8.1 | 0.026 |
| T4 | 35.7±10.4 | 42.3±10.4 | 0.056 |
| T5 | 82.7±10.0 | 90.6±5.6 | 0.004 |
T test was used for analysis of variables
In both groups, all the BIS values that were recorded at a steady-state end-tidal desflurane concentration of 6% (T4) were significantly lower than that of those recorded at a steady-state end-tidal desflurane concentration of 4% (T3) (p<0.001, p=0.001, p≤0.001 respectively) (Figure 2).
Figure 2.
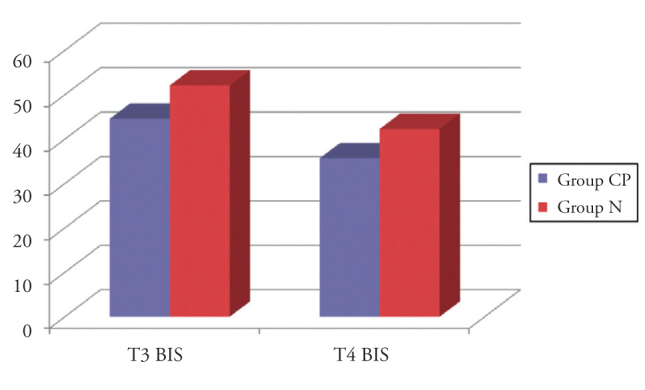
Comparison of the mean BIS values at T3 and T4 stages.
Discussion
In the present study, in which we investigated the association between end-tidal desflurane concentrations and BIS values in normal children and in children with cerebral palsy, we found that the BIS values, which we recorded at a steady-state end-tidal desflurane concentration of 6% were similar in two groups whereas the mean BIS values recorded at a steady-state end-tidal desflurane concentration of 4% were significantly lower in children with cerebral palsy than in normal children.
As the development of central nervous system is mostly completed until two years of age, there are many studies suggesting that BIS monitoring may safely be used in children aged over two years (5, 12–14). Therefore, based on the information in the literature, we included the children who were between 2 and 15 years of age.
Minimum alveolar concentration and MAC-awake values were found to be lower in children with cerebral palsy than in normal children. The use of anticonvulsant drugs further decreases the MAC value. Therefore, prolongation of the effects of volatile anaesthetic agents may delay early postoperative recovery after surgery (1). Susceptibility to hypothermia also contributes to the prolongation of the time to awakening and the time to extubation (1). In one study where sevoflurane anaesthesia was used, the increase in BIS values at 5, 15 and 30 minutes after the cessation of sevoflurane were slower in children with cerebral palsy compared to normal children (11). Similar to data in the literature, we found that the eye-opening time and the time to extubation after anaesthesia was discontinued were significantly longer in children with cerebral palsy.
There are studies suggesting that use of BIS monitoring in the assessment of the depth of anaesthesia decrease the need for propofol during anaesthesia induction. Saricaoglu et al. (9), in their study on 20 children with CP that were scheduled for elective orthopaedic surgery, found that the amount of propofol required for achieving BIS values of 35 to 45 during induction was significantly lower in patients with CP than in normal controls. However, the patients in that study received total intravenous anaesthesia with propofol, and the total amount of propofol that was used throughout the operation was higher in children with CP than in controls. Same study demonstrated that the total amount of propofol required during the induction of anaesthesia was not different between CP children on anticonvulsants who had a history of seizures and those who did not have a history of seizures. There were also no significant differences between children on different anticonvulsants (diphenylhydantoin and phenobarbital) in regard to the amount of propofol required. Despite its small sample size, this study provides information about the importance of BIS monitoring in patients with CP. In this present study, for the induction of anaesthesia, we used 200 ml/hour infusion of propofol until a BIS value of 50 was achieved. However, contrary to the study of Saricaoglu et al. (9), we found no significant difference between the patients with CP and the normal controls in regard to the total amount of propofol used during anaesthesia induction. Although, Saricaoglu et al.(9) avoided preoperative sedation which may affect BIS values, we performed premedication in all patients with oral midazolam 15 minutes before the induction of anaesthesia. The initial BIS values, which were recorded after premedication in the operating room, were lower in children with SP compared to that in normal children. The difference between the two studies in regard to the total amount of propofol used during induction may be associated with the effects of preoperative sedation. Similar to the study of Saricaoglu et al., there are other studies suggesting that awake BIS values in patients with CP are similar to that of patients without CP. The fact that BIS values were not obtained before premedication is among the limitations of our study.
Costa et al. (15) found that BIS values were lower after premedication with oral midazolam in children with CP than in normal children, however, contrary to our findings, this difference was not statistically significant. This controversy may be due to a variety of reasons including; the inclusion of only CP patients who were unable to speak and communicate and had severe mental retardation, whereas Costa et al. (15) included children with all clinical types of CP and also, we recorded the BIS values 15 minutes after premedication whereas Costa et al. (15) recorded the values 40 minutes after premedication. In both studies where patients received similar doses of midazolam (0.06 mg kg−1 and 0.05 mg kg−1) the BIS values of children are at levels that may be suggestive of mild sedation. As a conclusion, midazolam can be considered as a safe medication that may be used to relieve anxiety in children with CP before the operation.
Choudhry et al. (7) investigated the relationship between different sevoflurane concentrations and BIS values in normal children and in children with CP who were mentally retarded and also quadriplegic. They recorded the BIS values after midazolam premedication, after induction with 8% sevoflurane, at 1%, 3%, and again 1% end-tidal sevoflurane concentrations and after awakening from anaesthesia. The BIS values after premedication, at 1% sevoflurane concentrations and after awakening were found significantly lower in patients with cerebral palsy. However, no difference was found between the groups in regard to the values recorded after induction with 8% sevoflurane and at 3% sevoflurane concentrations.
By using short-latency somatosensory evoked potentials and BIS monitoring, Mello et al. (10) supported the fact that anaesthetics had more profound effects in patients with CP. However, in our study, the mean BIS values after premedication, at a steady-state end-tidal desflurane concentration of 4% and after awakening were significantly lower in CP group. The mean BIS values measured at a steady-state end-tidal desflurane concentration of 6% were also lower, however this difference was not of statistical significance (p=0.056). The BIS values after the induction of anaesthesia were similar in the two groups.
In both groups, all BIS values recorded at a steady-state end-tidal desflurane concentration of 6% were significantly lower than that recorded at a steady-state end-tidal desflurane concentration of 4%. During the operation, BIS values were kept below 60, which was being accepted as the safety limit against the risk of awareness during general anaesthesia. However, the marked increase in BIS values at desflurane concentrations <4% in the control group and the substantial decrease to hypnotic levels at 6% desflurane concentrations in the CP group is remarkable.
Except the studies which revealed a perplexing association when the sevoflurane concentrations was raised over 3%, all studies investigating the relationship between end-tidal sevoflurane concentrations and BIS values in children demonstrated that these two parameters were inversely proportional in children as they were in adults (5, 13, 16). We could not identify any studies investigating the relationship between BIS and desflurane in children. In our study, in which children between 2 and 15 years of age were included, we found in both groups that the BIS values recorded at a steady-state end-tidal desflurane concentration of 6% were significantly lower than that recorded at a steady-state end-tidal desflurane concentration of 4%. Bispectral index monitoring seems to be favourable in determining the requirement of anaesthetics in children over 2 years old.
Saricaoglu et al. (9) found no difference between children with CP and normal children in regard to heart rate and systolic blood pressure values, which were obtained before and after propofol induction. In our study, we also found no significant difference between the CP group and the control group in regard to heart rate and mean arterial blood pressure values obtained before and after the induction of anaesthesia, at steady-state end-tidal desflurane concentrations of 4% and 6% and after awakening from anaesthesia. After awakening, the heart rate and the mean arterial blood pressure were higher than initial values in patients with cerebral palsy, whereas such difference was not observed in controls. We think that the significant increase in heart rate and mean arterial pressure in children with cerebral palsy after awakening may be due to a variety of factors including; the higher level of pain due to contractures and spasticity or higher level of anxiety due to communication difficulties and mental retardation in CP children. Most of the time, it is difficult to assess pain in these children because of communication problems. The pain assessment scales may not always be reliable in children with mental problems. For this reason, postoperative pain management may not be sufficient. In our study, all children received 15 mg kg−1 of intravenous paracetamol and 0.1 mg/kg of intravenous morphine 20–25 minutes before skin closure. However, the fact that pain scores were not compared postoperatively between the two groups in the postoperative period may be considered as another limitation of our study.
A correlation may not always exist between the BIS values and the response to certain stimuli such as surgical intervention. There are studies suggesting that hemodynamic responses are not correlated with BIS values during intubation and laryngoscopy, where the BIS values indicating deep anaesthesia (17–19). In our study, although there were no significant difference between the groups in terms of heart rate and mean arterial pressure values that were recorded at all stages, the BIS values recorded before the induction of anaesthesia, at a steady-state end-tidal desflurane concentration of 4% and immediately after awakening from the anaesthesia were significantly lower in patients with CP.
The main limitations of our study were the limited number of patients and not recording the BIS values before premedication. We preferred to start recording the BIS values after midazolam premedication especially because cooperation difficulties in the children with CP, and also because their anxiety might get worse if they were taken to the operating room without premedication and as EMG signals might interfere with BIS values. In the present study, we only included the children with severe CP, who were mentally retarded and had difficulty in communication. However, CP may cover a wide range of clinical situations in which anaesthesia characteristics and BIS values may differ.
Conclusion
The bispectral index had been developed in mentally normal adults. Therefore, our opinion is that further studies are needed on the effectiveness of BIS monitoring in patients with mental disorders, especially children. As we did not encounter any previous studies investigating the relationship between end-tidal desflurane concentrations and BIS values in children, we think that this present study is suggestive on this topic. We conclude that BIS monitoring in children with CP may allow the use of lower concentration of anaesthetics and this fact may be favourable in terms of reducing the multiple drug-related side effects and drug interactions and hastening emergence from anaesthesia, as well.
Footnotes
Conflict of Interest
No conflict of interest was declared by the authors.
Financial Disclosure: N/A.
Peer-review: Externally peer-reviewed.
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Hacettepe University Faculty of Medicine.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Author Contributions
Concept - F.S.; Design - F.S., A.A.Y.; Supervision - F.S., S.B.A., Ü.A.; Funding - B.A., A.A.Y.; Materials - A.A.Y., F.S., S.B.A.; Data Collection and/or Processing - A.A.Y., B.A.; Analysis and/or Interpretation - F.S., S.B.A., B.A., Ü.A., A.A.Y.; Literature Review - B.A., A.A.Y.; Writer - A.A.Y., B.A., F.S.; Critical Review - F.S., S.B.A., B.A., Ü.A.; Other - A.A.Y., F.S., Ü.A.
References
- 1.Nolan J, Chalkiadis GA, Low J, Olesch CA, Brown TC. Anaesthesia and pain management in cerebral palsy. Anaesthesia. 2000;55:32–41. doi: 10.1046/j.1365-2044.2000.01065.x. http://dx.doi.org/10.1046/j.1365-2044.2000.01065.x. [DOI] [PubMed] [Google Scholar]
- 2.Nelson KB, Emery ES., 3rd Birth asphyxia and the neonatal brain: what do we know and when do we know it? Clin Perinatol. 1993;20:327–44. [PubMed] [Google Scholar]
- 3.Dolk H, Parkes J, Hill N. Trends in the prevalence of cerebral palsy in Northern Ireland, 1981–1997. Dev Med Child Neurol. 2006;48:406–12. doi: 10.1017/S0012162206000909. http://dx.doi.org/10.1111/j.1469-8749.2006.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 4.Theroux MC, Akins RE. Surgery and anesthesia for children who have cerebral palsy. Anesthesiol Clin North America. 2005;23:733–43. doi: 10.1016/j.atc.2005.08.001. http://dx.doi.org/10.1016/j.atc.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Denman WT, Swanson EL, Rosow D, Ezbicki K, Connors PD, Rosow CE. Pediatric evaluation of the bispectral index (BIS) monitor and correlation of BIS with end-tidal sevoflurane concentration in infants and children. Anesth Analg. 2000;90:872–7. doi: 10.1097/00000539-200004000-00018. http://dx.doi.org/10.1213/00000539-200004000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Davidson AJ. Monitoring the anaesthetic depth in children - an update. Curr Opin Anaesthesiol. 2007;20:236–43. doi: 10.1097/ACO.0b013e3280c60c66. http://dx.doi.org/10.1097/ACO.0b013e3280c60c66. [DOI] [PubMed] [Google Scholar]
- 7.Choudhry DK, Brenn BR. Bispectral index monitoring: a comparison between normal children and children with quadriplegic cerebral palsy. Anesth A nalg. 2002;95:1582–5. doi: 10.1097/00000539-200212000-00020. http://dx.doi.org/10.1097/00000539-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Costa VV, Torres RV, Arci EC, Saraiva RA. [Comparison of the bispectral index in awake patients with cerebral palsy]. Rev Bras Anestesiol. 2007;57:382–90. doi: 10.1590/s0034-70942007000400005. http://dx.doi.org/10.1590/S0034-70942007000400005. [DOI] [PubMed] [Google Scholar]
- 9.Saricaoglu F, Celebi N, Celik M, Aypar U. The evaluation of propofol dosage for anesthesia induction in children with cerebral palsy with bispectral index (BIS) monitoring. Paediatr Anaesth. 2005;15:1048–52. doi: 10.1111/j.1460-9592.2005.01658.x. [DOI] [PubMed] [Google Scholar]
- 10.Mello SS, Saraiva RA. [Electroneourophysiological changes in anesthesia with sevoflurane: comparative study between healthy and cerebral palsy patients]. Rev Bras Anestesiol. 2003;53:150–9. doi: 10.1590/s0034-70942003000200002. http://dx.doi.org/10.1590/S0034-70942003000200002. [DOI] [PubMed] [Google Scholar]
- 11.Costa VV, Saraiva RA, Duarte LT. [Regression of general anesthesia in patients with cerebral palsy: a comparative study using the bispectral index]. Rev Bras Anest esiol. 2006;56:431–42. doi: 10.1590/s0034-70942006000500001. http://dx.doi.org/10.1590/S0034-70942006000500001. [DOI] [PubMed] [Google Scholar]
- 12.Davidson AJ, McCann ME, Devavaram P, Auble SA, Sullivan LJ, Gillis JM, et al. The differences in the bispectral index between infants and children during emergence from anesthesia after circumcision surgery. Anesth Analg. 2001;93:326–30. doi: 10.1213/00000539-200108000-00017. http://dx.doi.org/10.1213/00000539-200108000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Kim HS, Oh AY, Kim CS, Kim SD, Seo KS, Kim JH. Correlation of bispectral index with end-tidal sevoflurane concentration and age in infants and children. Br J Anaesth. 2005;95:362–6. doi: 10.1093/bja/aei196. http://dx.doi.org/10.1093/bja/aei196. [DOI] [PubMed] [Google Scholar]
- 14.Bannister CF, Brosius KK, Sigl JC, Meyer BJ, Sebel PS. The effect of bispectral index monitoring on anesthetic use and recovery in children anesthetized with sevoflurane in nitrous oxide. Anesth Analg. 2001;92:877–81. doi: 10.1097/00000539-200104000-00015. http://dx.doi.org/10.1097/00000539-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 15.da Costa VV, Torres RV, Arci EC, Saraiva RA. Oral midazolam as pre-anesthetic medication in children and teenagers with cerebral palsy. A comparative study on the variations of the bispectral index. Rev Bras Anestesiol. 2009;59:28–36. doi: 10.1590/s0034-70942009000100005. [DOI] [PubMed] [Google Scholar]
- 16.Katoh T, Suzuki A, Ikeda K. Electroencephalographic derivatives as a tool for predicting the depth of sedation and anesthesia induced by sevoflurane. Anesthesiology. 1998;88:642–50. doi: 10.1097/00000542-199803000-00014. http://dx.doi.org/10.1097/00000542-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Driessen JJ, Harbers JB, van Egmond J, Booij LH. Evaluation of the electroencephalographic bispectral index during fentanyl-midazolam anaesthesia for cardiac surgery. Does it predict haemodynamic responses during endotracheal intubation and sternotomy? Eur J Anaesthesiol. 1999;16:622–7. doi: 10.1046/j.1365-2346.1999.00551.x. http://dx.doi.org/10.1046/j.1365-2346.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- 18.Mi WD, Sakai T, Takahashi S, Matsuki A. Haemodynamic and electroencephalograph responses to intubation during induction with propofol or propofol/fentanyl. Can J Anaesth. 1998;45:19–22. doi: 10.1007/BF03011986. http://dx.doi.org/10.1007/BF03011986. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama M, Kanaya N, Edanaga M, Namiki A. Hemodynamic and bispectral index responses to tracheal intubation during isoflurane or sevoflurane anesthesia. J Anesth. 2003;17:223–6. doi: 10.1007/s00540-003-0186-4. http://dx.doi.org/10.1007/s00540-003-0186-4. [DOI] [PubMed] [Google Scholar]


