Abstract
Objective
In this study, we compared the effects of remifentanil and dexmedetomidine on hemodynamic parameters, inhalation agent consumption and thyroid hormone levels at the late postoperative period.
Methods
Forty-five euthyroid ASA I-II patients between 20 and 75 years of age were randomly assigned into three groups:
During induction,
Group R received 1.0 mcg kg−1 remifentanil as slow bolus in two minutes,
Group D received 1.0 mcg kg−1 dexmedetomidine in 10 minutes as infusion,
Group C received 1.0 mcg kg−1 fentanyl as bolus. Afterwards, all patients received 2.0 mg kg−1 propofol and 0.2 mg kg−1 cisatracurium for induction.
For anaesthesia maintenance during and up to 15 minutes before the end of the surgery;
Group R received 0.05 mcg kg−1 min−1 remifentanil,
Group D received 0.5 mcg kg−1 h−1 dexmedetomidine infusion.
During the surgery, heart rate, mean arterial pressure and end-tidal sevoflurane concentrations were recorded for every patient. Venous blood samples were taken after the operation, at postoperative 24th hour and postoperative 5th day and the variations in fT3, fT4, TSH levels were analyzed.
Results
Mean arterial pressure values and sevoflurane consumption were lower in Group R and Group D in comparison with the control group. In comparison between groups, the decrease in fT3 values at postoperative 24th hour was more significant in the control group than the other two groups.
Conclusion
We suggest that, both agents suppress the hemodynamic response, decrease the consumption of inhalation agents and cause less change in the levels of thyroid hormones, which can be considered as one of the indicators of endocrine response.
Keywords: Thyroid hormones, cholecystectomy, sevoflurane, dexmedetomidine, remifentanil
Introduction
The autonomic, endocrine, metabolic and immune responses directed against harmful stimuli to provide and maintain body homeostasis are known as stress responses. These responses may occur in conditions like major traumas, surgery, sepsis and hunger. One of the aims of modern general anaesthesia is to maintain the functions of vital organs and systems at physiological levels in patients undergoing surgical interventions.
Anaesthesia, anaesthetic agents and surgical intervention, are all stress factors for the body, and lead to the stimulation of the hypothalamic-pituitary-adrenal axis and enhance sympathoadrenal activity.
Pituitary gland is stimulated by the secretion of thyrotropic hormones from the hypothalamus. In response, pituitary gland secretes adrenocorticotropic hormone, thyroid stimulating hormone, growth hormone, prolactin, follicle stimulating hormone, luteinizing hormone and antidiuretic hormone. While the levels of catabolic hormones such as cortisol, glucagon and catecholamines increase, anabolic hormones such as insulin and testosterone decrease by the stimulation of pituitary hormones. These changes support life by supplying the substances required for gluconeogenesis, glycogenolysis and protein synthesis (1–4).
At the present time, the opinion that endocrine response is a requisite for surgical stress is debatable. The benefit of catabolic endocrine response to the patient is controversial. Additionally, if metabolic, endocrine response is prolonged and be in excessive amounts, it may end up with some negative effects. Some complications such as thromboembolism, stress ulcers, cardiac failure due to the increase in cardiac oxygen requirement and load, infarct and respiratory failure may occur (5, 6).
The functions of the thyroid gland, like all endocrine system, change under surgical stress, and the body creates the proper environment for itself. Thyroid hormones adapt to surgical stress by changes in the enzymes both in the thyroid gland and organs other than the thyroid gland (6).
In our study; euthyroid patients confirmed with anamnesis, physical examination and clinical findings, undergoing laparoscopic cholecystectomy with general anaesthesia were treated with remifentanil, a short acting opioid, and dexmedetomidine, a specific alpha-2 agonist. The aim was to compare the hemodynamic parameters, sevoflurane consumption and changes in thyroid hormone levels in the late postoperative period.
Methods
After approval was obtained from the ethics committee of the hospital (approval date and number: 26/5/2008, 145), the present study was performed in a total of 45 patients (American Society of Anaesthesiologist [ASA] physical status classification I-II), aged between 20 and 75 years of age, scheduled for elective laparoscopic cholecystectomy surgery.
Before the study, informed consents were obtained from each patient.
ASA I–II patients, who were determined to be euthyroid according to thyroid function tests, history, physical examination and clinical findings, without any endocrine problems and not using drugs affecting thyroid binding globulin levels, were included in the study.
Oral intake was prohibited 6 hours before surgery. The study was conducted on patients operated between 08:00 AM-12:00 AM. The patients who required blood transfusion were not included in the study.
The patients were randomly divided into three groups. Group R: Remifentanil group, Group D: Dexmedetomidine group and Group C: Control group.
None of the patients were premedicated. When the patient was placed on the operating table, a 20 G venous cannula was placed in one of the superficial veins at the dorsum of the hand, and balanced electrolyte solution infusion was started. Datex-Ohmeda S500 monitor was used for standard DII derivation electrocardiography, end tidal CO2, pulse-oximeter and non-invasive blood pressure [systolic arterial pressure (SAP), diastolic arterial pressure (DAP), mean arterial pressure (MAP)] monitoring.
A-2000 Aspect Bispectral index (BIS) monitor was used to determine the depth of anaesthesia.
At the induction of anaesthesia,
Group D patients received dexmedetomidine at a dose of 1.0 mcg kg−1 by 10 minutes infusion 10 minutes before induction,
Group R patients received remifentanil at a dose of 1.0 mcg kg−1 in 2 minutes as slow bolus,
Group C patients received bolus fentanyl at a dose of 1.0 mcg kg−1.
Thereafter, all patients were given 2.0 mg kg−1 propofol and 0.2 mg kg−1 cisatracurium. After induction, orotracheal intubation was performed.
Keeping the BIS value between 40 and 60, anaesthesia was maintained with sevoflurane in 50% O2 and 50% air mixture.
For maintenance of anaesthesia, during and up to 15 minutes before the end of the surgery, Group R patients received remifentanil at a dose of 0.05 mcg kg−1 min−1, and Group D patients were given dexmedetomidine infusion at a rate of 0.5 mcg kg−1 hr−1.
None of the patients received blood transfusion or colloids during surgery.
Heart rate (HR), MAP and end tidal concentrations of sevoflurane used for each patient were recorded at 5 and 10 minutes of intubation, at 10-minute intervals in the first one hour and at 20 minute-intervals until the end of the surgery.
Venous blood samples were obtained in the preoperative period, at postoperative 24 hours and 5 days.
Free T3 (fT3), fT4 and TSH were analyzed by radioimmunoassay method (Beckman-Coulter Unicel DXI 800 Access Immunoassay System) in the hormone laboratory.
The following ranges were considered normal;
fT3: 0.2–6.5 pg mL−1
fT4: 0.7–2.1 ng dL−1
TSH: 0.35–5.0 mIU mL−1
Statistical analysis
SPSS 16.0 package program was used in the statistical analysis of data. Data was tested for normal distribution. Demographic variables (age, weight, gender) were presented as mean and standard deviation. Kruskal Wallis and One Way ANOVA tests were used in repeated measures analysis. Paired comparisons within groups were performed using the Tukey test. The results were evaluated within a 95% confidence interval and a p value <0.05 was considered to be significant.
Results
The distribution of the patients according to demographic characteristics is presented in Table 1. The groups were similar regarding age, weight and gender.
Table 1.
Comparison of patient characteristics between the study groups
| Remifentanil group (Group R) mean±SD | Dexmedetomidine group (Group D) mean±SD | Control (Group C) mean±SD | p | |
|---|---|---|---|---|
| Age (years) | 54.73±11.09 | 50.06±10.53 | 55.93±7.70 | 0.387 |
| Weight (kg) | 71.06±6.45 | 70.86±10.14 | 73.13±5.56 | 0.713 |
| Gender (F/M) | 10/5 | 8/7 | 7/8 | 0.301 |
There was no significant difference between the groups regarding HR. In comparison of mean arterial pressure values, there was a significant difference between the groups in the values measured at 20, 30, 40, 50 and 60 minutes of surgery (p<0.001) (Figure 1).
Figure 1.
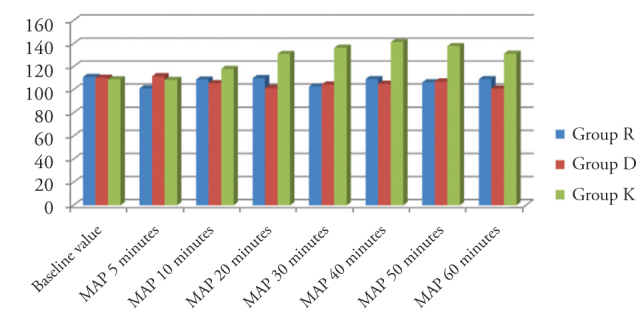
Comparison of mean arterial pressure values measured at different time points between the groups
There was a significant difference between the groups in terms of end-tidal sevoflurane concentrations at 40, 50 and 60 minutes of surgery (p<0.05) (Figure 2).
Figure 2.
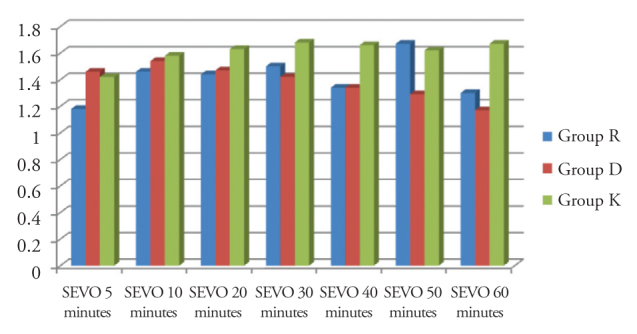
Comparison of end-tidal sevoflurane concentrations between the groups
In paired comparisons, there was a significant difference between the fT3 values measured before the surgery and at postoperative 24 hours, and the values at postoperative 24 hours and at 5 days (p<0.01). The fT3 values at the postoperative 24th hour were significantly lower in the control group than that of the other groups (Figure 3).
Figure 3.
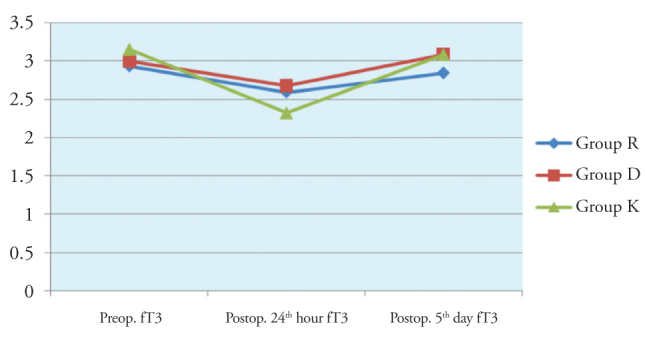
Inter-group comparison of fT3 values
There was no significant difference in time-dependent changes of Free T4 and TSH values between the groups (Figures 4 and 5).
Figure 4.
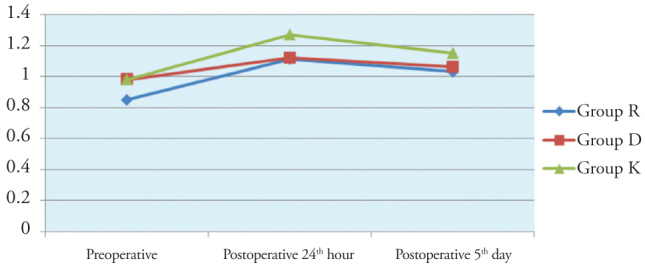
Inter-group comparison of fT4 values
Figure 5.
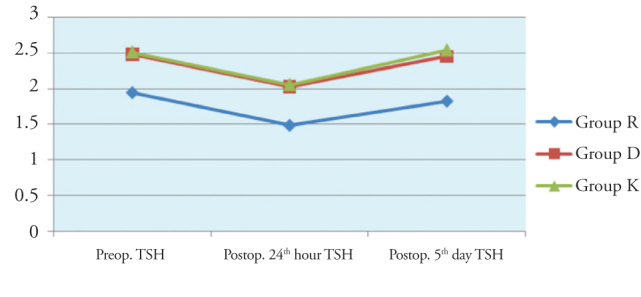
Inter-group comparison of TSH values
Discussion
All functions of the human body are regulated by the nervous and endocrine systems. There are numerous interactions between the hormone system and the nervous system, and hypothalamus receives stimuli from almost all parts of the nervous system. A painful stimulus, even the presence of a fearful or an exciting thought may be sufficient to activate the hypothalamus.
General anaesthesia induces an unconscious state, in which functions of the central and peripheral nervous system are temporarily ceased. In an adequate depth of general anaesthesia, it is certain that painful stimuli are not perceived and no response is generated. However, our study along with the studies of many researchers show that under general anaesthesia, surgical stimuli, by activating the hormones secreted from the anterior lobe of the pituitary gland, lead to some endocrine and metabolic changes (7–12).
The appropriate use of hypnotic and analgesic agents is important in the regulation of the depth of anaesthesia and hemodynamic stability during surgery. Suppression of stress response and the related hemodynamic response is one of the aims of anaesthesia. Stress response increases surgery-related morbidity and mortality (13, 14).
Although the hypnotic effect of anaesthetic agents is at a sufficient level, patients may respond to surgical stimuli by an increase in arterial blood pressure and tachycardia. The reason for that is the increase in cardiac output and systemic vascular resistance by increased catecholamine secretion.
In balanced anaesthesia, opioids provide the required analgesia for blocking autonomic nervous system and suppressing somatic responses against surgical stimuli. Therefore, opioids, by decreasing surgical stress, help to maintain hemodynamic stability during surgery. Remifentanil, a short and rapid-acting opioid, can be titrated according to patient needs and can be used along with new methods and combinations in high-dose opioid anaesthesia (15–17).
In a multicenter study on 2438 patients, the effects of remifentanil and fentanyl on hemodynamic responses against surgical stress were evaluated and lower blood pressure and heart rate values during induction and after intubation were achieved with remifentanil (18). Remifentanil provides a good hemodynamic stability during anaesthesia and surgery (19–21). There are reports stating that remifentanil induces bradycardia and hypotension, and although the mean arterial pressure values of the group treated with remifentanil infusion were significantly lower than the control group in our study, none of the patients experienced hypotension requiring discontinuation of infusion (17). In addition, although heart rates of remifentanil group were lower in comparison to the control group, the difference between two groups was not significant.
Dexmedetomidine, a potent and selective alpha-2 agonist, leads to a dose dependent decrease in plasma norepinephrine concentrations, heart rate and blood pressure (22). Fragen et al. (23) in their study reported that while causing a dose dependent decrease in blood pressure and heart rate, dexmedetomidine provides hemodynamic stability by suppressing sympathetic nervous system activity and reduces anaesthetic agent requirement. However, it is known from many previous studies that dexmedetomidine decreases heart rate by postsynaptic alpha-2 adrenoceptor activity (22–27). Similarly, the mean arterial pressure values of dexmedetomidine group were significantly lower when compared with the control group in our study.
Özköse et al. (28), in their study on patients operated in prone position gave dexmedetomidine infusion after the loading dose, and compared the hemodynamic changes, anaesthetic agent consumption and the duration of recovery with the placebo group. They concluded that continuous infusion of dexmedetomidine after the loading dose results in lower anaesthetic agent consumption, and provide a more rapid recovery. Yagmur et al. (29), in one of their studies, evaluated the effects of dexmedetomidine given intravenously on the minimum alveolar concentration (MAC) of desflurane by BIS monitor; at a BIS value of 40–50 they determined that dexmedetomidine excessively decreased the MAC value of desflurane. We also kept the BIS values between 40 and 60 in our study, and found that end-tidal sevoflurane concentrations of the group treated with dexmedetomidine infusion were significantly lower when compared to that of the control group.
A study on euthyroid sick syndrome is intriguing; Michalaki et al (30), in their study performed in 2001, evaluated the early changes in tT3, tT4, fT3, fT4, TSH, rT3, Interleukin-6 and cortisol values before, during and after surgery in relation with euthyroid sick syndrome in 19 patients undergoing elective abdominal surgery (open cholecystectomy and morbid obesity surgery). In that study, values measured 24 hours before the surgery were considered as baseline values and the difference between baseline values and the values measured at −1, 0, 0.5, 1, 2, 3, 4, 5, and 6 hours of skin incision were evaluated. That study, although was performed in a limited number of patients, is significant as it shows the changes in thyroid hormone levels during extrathyroid surgery. With regard to free T4; when all patients are considered, values started to increase at 30 minutes of surgery and reached to a statistically significant value at the first hour. When the changes in free T3 levels were considered, it was found that a significant decrease was found in the second hour of surgery. With regards to thyroid stimulating hormone (TSH) changes, a statistically significant increase was observed at 30 minutes of surgery. Among these hormones, the elevation in TSH and fT4 levels is considered to be associated with surgical stress.
In our study; a better hemodynamic stability was provided in patients who received remifentanil and dexmedetomidine during surgery compared to the control group. Blood pressure values and sevoflurane consumption during surgery were lower in both groups in comparison to the control group. In comparison to baseline values, some changes were observed in fT3, fT4 and TSH values measured at postoperative Day 1 and Day 5 in all three groups. While TSH and free T3 values measured at postoperative Day 1 were lower than the baseline values in all groups, postoperative Day 5 values approached the baseline values. fT3 changes at postoperative 24 hours in patients treated with remifentanil and dexmedetomidine, was less than the changes observed in the control group, however, there was no difference between Group R and Group D. In all three groups, fT4 values at postoperative day 1 was found to be higher than that of baseline values, and postoperative day 1 values were close to the baseline levels. Although fT4 levels at postoperative 24 hours increased to levels higher than that in the control group, no significant difference could be detected. The changes in TSH levels were similar in all groups.
Conclusion
Both agents, even with low dose infusion, suppress the hemodynamic response caused by surgical stress, decrease the consumption of inhalation agents and lead to small changes in the levels of thyroid hormones, especially fT3, which is one of the indicators of endocrine response.
Footnotes
Conflict of Interest
No conflict of interest was declared by the authors.
Financial Disclosure: N/A.
Peer-review: Externally peer-reviewed.
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Ministry Health Okmeydanı Trainin and Research Hospital.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Author Contributions
Concept - B.Ö., A.E.; Design - B.Ö., A.E.; Supervision - A.E. Materials - A.A.; Data Collection and/or Processing - B.Ö., M.L.U.; Literature Review - B.Ö., A.E.; Writer - B.Ö.; Critical Review - A.A
References
- 1.Derbyshire DR, Smith G. Sympathoadrenal responses to anaesthesia and surgery. Br J Anaesth. 1984;56:725–39. doi: 10.1093/bja/56.7.725. http://dx.doi.org/10.1093/bja/56.7.725. [DOI] [PubMed] [Google Scholar]
- 2.Hall GM. The anaesthetic modification of the endocrine and metabolic response to surgery. Ann R Coll Surg Engl. 1985;67:25–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Longnecker DE. Stress free: To be or not to be? Anesthesiology. 1984;61:643–4. http://dx.doi.org/10.1097/00000542-198412000-00001. [PubMed] [Google Scholar]
- 4.Brandt MR, Fernandes A, Mordhorst R, Kehlet H. Epidural analgesia improves postoperative nitrogen balange. Br Med J. 1978;1:1106–8. doi: 10.1136/bmj.1.6120.1106. http://dx.doi.org/10.1136/bmj.1.6120.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehlet H. Stress free anaesthesia and surgery. Acta Anaesth Scand. 1979;23:503–4. doi: 10.1111/j.1399-6576.1979.tb01479.x. http://dx.doi.org/10.1111/j.1399-6576.1979.tb01479.x. [DOI] [PubMed] [Google Scholar]
- 6.Kayhan Z. Klinik anestezi II. Baskı, Logos yayıncılık; Ankara: 1997. [Google Scholar]
- 7.Derbyshire DR, Smith G. Sympathoadrenal responses to anaesthesia and surgery. Br J Anaesth. 1984;56:725–39. doi: 10.1093/bja/56.7.725. http://dx.doi.org/10.1093/bja/56.7.725. [DOI] [PubMed] [Google Scholar]
- 8.Hall GM. The anaesthetic modification of the endocrine and metabolic response to surgery. Ann R Coll Surg Engl. 1985;67:25–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Halter JB, Pflug E, Porte D. Mechanism of plasma catecholamine increases during surgical stress in man. J Clin Endocrinol Metab. 1977;45:936–44. doi: 10.1210/jcem-45-5-936. http://dx.doi.org/10.1210/jcem-45-5-936. [DOI] [PubMed] [Google Scholar]
- 10.Davis FM, Laurenson VG, Lewis J, Wells JE, Gillespie WJ. Metabolic response to total hip artroplasty under hypobaric subarachnoid or general anaesthesia. Br JAnaesth. 1987;59:725–9. doi: 10.1093/bja/59.6.725. http://dx.doi.org/10.1093/bja/59.6.725. [DOI] [PubMed] [Google Scholar]
- 11.Lacoumenta S, Paterson JL, Burrin J, Causon RC, Brown MJ, Hall GM. Effects of two differing halotane consantrations on the metabolic and endocrine responses to surgery. Br J Anaesth. 1986;58:844–50. doi: 10.1093/bja/58.8.844. http://dx.doi.org/10.1093/bja/58.8.844. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz SI. Principles of surgery. Third edition. Mc-Graw Hill Inc.; USA: 1979. Endocrine and metabolic responses to injury; pp. 1–65. [Google Scholar]
- 13.Ivani G. Protective efficasy of 3 anesthetic methods with reference to surgical stress in children. Anesthesiology. 1996;44:297–302. [PubMed] [Google Scholar]
- 14.Nicholson G, Bryant AE, MacDonald IA, Hall GM. Osteocalcine and the hormonal, inflammatory and metabolic response to major orthopedic surgery. Anaesthesia. 2002;57:319–25. doi: 10.1046/j.1365-2044.2002.02450.x. http://dx.doi.org/10.1046/j.1365-2044.2002.02450.x. [DOI] [PubMed] [Google Scholar]
- 15.Kehlet H. Surgical stress: The role of pain and analgesia. Br J Anaesth. 1989;63:189–95. doi: 10.1093/bja/63.2.189. http://dx.doi.org/10.1093/bja/63.2.189. [DOI] [PubMed] [Google Scholar]
- 16.Camu F, Royston D. Inpatient experience with remifentanil. Anesth Analg. 1999;89:15–21. doi: 10.1097/00000539-199910001-00004. http://dx.doi.org/10.1097/00000539-199910001-00004. [DOI] [PubMed] [Google Scholar]
- 17.Kallar SK, Hurt TW, Wetcher BV, Shaw DL, Jamerson BA. A single-blind comparison of the safety and efficacy of remifentanil and alfentanil for outpatient anesthesia. Anesthsiology. 1994;81:32. http://dx.doi.org/10.1097/00000542-199409001-00032. [Google Scholar]
- 18.Twresky RS, Jamerson B, Warner DS, Fleısher LA, Houg ES. Hemodinamics and emergence profile of remifentanil versus fentanyl prospectively compared in a large population of surgical patients. J Clin Anesth. 2001;13:407–16. doi: 10.1016/s0952-8180(01)00292-6. http://dx.doi.org/10.1016/S0952-8180%2801%2900292-6. [DOI] [PubMed] [Google Scholar]
- 19.Schüttler J, Albrecht S, Breivik H, Osnes S, Prys-Roberts C, Haloder K. A comparison of remifentanil and alfentanil in patients undergoing major abdominal surgery. Anaesthesia. 1997;52:307–17. doi: 10.1111/j.1365-2044.1997.24-az0051.x. http://dx.doi.org/10.1111/j.1365-2044.1997.24-az0051.x. [DOI] [PubMed] [Google Scholar]
- 20.Philip BK, Scuderi PE, Chung F, Conahan TJ, Maurer W, Angel JJ, et al. Remifentanil compared with alfentanil for ambulatory surgery using total intravenous anesthesia. The Remifentanil/Alfentanil Outpatient TIVA Group. Anesth Analg. 1997;84:515–21. doi: 10.1097/00000539-199703000-00009. http://dx.doi.org/10.1213/00000539-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Cartwright DP, Kvalsvik O, Cassuto J, Jansen JP, Wall C, Remy B, et al. A randomized, blind comparison of remifentanil and alfentanil during anesthesia for outpatient surgery. Anesth Analg. 1997;85:1014–9. doi: 10.1097/00000539-199711000-00011. http://dx.doi.org/10.1213/00000539-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. http://dx.doi.org/10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Fragen RJ, Fitzgerald PC. Effect of dexmedetomidine on the minimum alveolar concentration (MAC) of sevoflurane in adult age 55 to 70 years. J Clin Anesth. 1999;11:466–70. doi: 10.1016/s0952-8180(99)00081-1. http://dx.doi.org/10.1016/S0952-8180%2899%2900081-1. [DOI] [PubMed] [Google Scholar]
- 24.Aho M, Scheinin M, Lehtinen AM, Erkola O, Vuorinen J, Korttila K. Intramuscularly administered dexmedetomidine attenuates hemodynamic and stress hormone responses to gynecologic laparoscopy. Anesth Analg. 1992;75:932–9. http://dx.doi.org/10.1213/00000539-199212000-00011. [PubMed] [Google Scholar]
- 25.Duke P, Maze M, Morrison P. Dexmedetomidine: a general overview. International Congress and Symposium Series. 1998;221:11–22. [Google Scholar]
- 26.Tosun Z, Akin A, Guler G, Esmaoglu A, Boyaci A. Dexmedetomidine-ketamine and propofol-ketamine combinations for anesthesia in spontaneously breathing pediatric patients undergoing cardiac catheterization. J Cardiothorac Vasc Anest h. 2006;20:515–9. doi: 10.1053/j.jvca.2005.07.018. http://dx.doi.org/10.1053/j.jvca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Talke P, Chen R, Thomas B, Aggarwall A, Gottlieb A, Thorborg P, et al. The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg. 2000;90:834–9. doi: 10.1097/00000539-200004000-00011. http://dx.doi.org/10.1097/00000539-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Ozkose Z, Demir FS, Pampal K, Yardim S. Hemodynamic and anesthetic advantages of dexmedetomidine, an alpha 2-agonist, for surgery in prone position. Tohoku J Exp Med. 2006;210:153–60. doi: 10.1620/tjem.210.153. http://dx.doi.org/10.1620/tjem.210.153. [DOI] [PubMed] [Google Scholar]
- 29.Yağmur C, Tekin M, Katı İ, Coşkuner İ, Elçiçek K. BIS Monitörizasyonu ile deksmedetomidinin desfluranın MAC değeri üzerine etkilerinin araştırılması. Türk Anest Rean Der Dergisi. 2008;36:19–24. [Google Scholar]
- 30.Michalaki M, Apostolos GV, Makri M, Kyriazopoulou V. Dissociation of the early decline in serum T3 concentration and Serum IL-6 Rise and TNF alpha in nonthyroidal illness syndrome induced by abdominal surgery. J Clin Endocrinol Metab. 2001;86:4198–205. doi: 10.1210/jcem.86.9.7795. http://dx.doi.org/10.1210/jc.86.9.4198. [DOI] [PubMed] [Google Scholar]


