Abstract
BACKGROUND AND OBJECTIVE:
Little is known about in-hospital morbidities and neurodevelopmental outcomes among extremely preterm infants born to women with insulin-dependent diabetes mellitus (IDDM). We examined risks of mortality, in-hospital morbidities, and neurodevelopmental outcomes at 18 to 22 months’ corrected age between extremely preterm infants of women with insulin use before pregnancy (IBP), with insulin use started during pregnancy (IDP), and without IDDM.
METHODS:
Infants 22 to 28 weeks’ gestation born or cared for at a Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network center (2006–2011) were studied. Regression models compared the association between maternal IDDM and timing of insulin use and the outcomes of the 3 groups.
RESULTS:
Of 10 781 infants, 536 (5%) were born to women with IDDM; 58% had IBP, and 36% had IDP. Infants of mothers with IBP had higher risks of necrotizing enterocolitis (adjusted relative risk [RR] = 1.55 [95% confidence interval (CI) 1.17–2.05]) and late-onset sepsis (adjusted RR = 1.26 [95% CI 1.07–1.48]) than infants of mothers without IDDM. There was some indication of higher in-hospital mortality risk among infants of mothers with IBP compared with those with IDP (adjusted RR = 1.33 [95% CI 1.00–1.79]). Among survivors evaluated at 18 to 22 months’ corrected age, average head circumference z score was lower for infants of mothers with IBP compared with those without IDDM, but there were no differences in risk of neurodevelopmental impairment.
CONCLUSIONS:
In this cohort of extremely preterm infants, infants of mothers with IBP had higher risks of necrotizing enterocolitis, sepsis, and small head circumference.
What’s Known on This Subject:
Infants born extremely preterm and infants born to women with insulin-dependent diabetes are at risk for adverse outcomes. Little is known about in-hospital morbidities and neurodevelopmental outcomes among extremely preterm infants born to women with insulin-dependent diabetes.
What This Study Adds:
Extremely preterm infants of mothers with insulin use before pregnancy had higher risks of necrotizing enterocolitis, sepsis, and small head circumference but not of neurodevelopmental impairment. Infants of mothers with insulin use started during pregnancy had no increased risks.
Diabetes mellitus is one of the most common pregnancy complications. In the United States, gestational diabetes mellitus affects ∼7% of pregnancies, and preexisting diabetes complicates ∼1.3% of pregnancies.1,2 Diabetic pregnancies, particularly those with poor glycemic control and more advanced diabetes, are at high risk of both indicated and spontaneous preterm delivery.3–6 Few studies have examined the resulting complications and neurodevelopmental outcomes among extremely preterm infants born to diabetic mothers, and none has examined the impact of glycemic control mode on infant outcomes.7–9 With the increasing incidence of both gestational and pregestational diabetes10 and the high incidence of preterm birth associated with diabetes, it is imperative to examine outcomes in this high-risk group of infants.
We used data from a high-risk infant registry maintained by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NRN) to examine mortality, morbidity, and neurodevelopmental outcomes among extremely preterm infants whose mothers used insulin before pregnancy, those whose mothers first began insulin use during pregnancy, and those whose mothers had no insulin-dependent diabetes mellitus (IDDM).
Methods
Infants born at 1 of the NRN centers from January 1, 2006, through December 31, 2011, with gestational age (GA) of 22 to 28 weeks and enrolled in the registry were studied. In 2006 and 2007, all very low birth weight (VLBW) infants (401–1500 g) born at or admitted to a study center within 14 days of birth were included in the registry. Beginning 2008, eligibility criteria changed to include inborn infants with GA 22 to 28 weeks or birth weight 401 to 1000 g. To maintain consistency over the study period, we restricted our cohort to inborn infants with GA of 22 to 28 weeks. The institutional review board at each center approved participation in the registry.
Trained research nurses prospectively collected maternal demographic, pregnancy, and delivery information and infant data from birth to discharge, transfer, death, or 120 days. Maternal IDDM was recorded if the mother required insulin for diabetes control before or during pregnancy. Except for insulin requirement, data on diabetes onset and type and milder diabetes forms managed by diet or oral hypoglycemic agents were not recorded. Maternal hypertension before or during pregnancy was recorded if noted in the mother’s chart. Preeclampsia and eclampsia were not collected. Infant characteristics, delivery room interventions, and final status were recorded for all infants.
In-Hospital Outcomes
Morbidities diagnosed in-hospital were recorded for infants surviving >12 hours. These included respiratory distress syndrome (RDS); patent ductus arteriosus (PDA); modified Bell’s stage ≥IIA necrotizing enterocolitis (NEC)11,12; early-onset sepsis (EOS; ≤72 h of age) and late-onset sepsis (LOS; >72 h), defined by a positive blood culture and intent to treat with antibiotics for ≥5 days; severe intraventricular hemorrhage (IVH) grade 3 or 413; cystic periventricular leukomalacia (PVL); retinopathy of prematurity (ROP); and bronchopulmonary dysplasia (BPD). Standard scores (z scores) and percentiles for infant weight, length, and head circumference measured at birth and at 36 weeks’ postmenstrual age (PMA) were calculated by using Olsen norms,14 available for males and females born at 23 to 41 weeks’ GA. Ponderal index was calculated at birth and 36 weeks’ PMA as follows: [weight g / (length cm)3] × 100. Microcephaly was defined as head circumference z score < −2.
Follow-up Outcomes
Surviving infants were eligible for assessment at 18 to 22 months’ corrected age (CA) if they weighed 401 to 1000 g at birth (those 18 months’ CA before January 1, 2008), were born at ≤26 weeks’ GA, or were enrolled in an NRN study with follow-up (those 18 months’ CA on or after January 1, 2008). The visit included a neurologic examination and a developmental assessment by using the Bayley Scales of Infant Development III,15 both by certified examiners, as well as caregiver interview and measurement of infant weight, length, and head circumference. Neurodevelopmental impairment (NDI; 2006 definition) was defined as ≥1 of the following: bilateral blindness, hearing impairment (permanent hearing loss), moderate to severe cerebral palsy, gross motor function level ≥2, or Bayley III cognitive composite score <70. Beginning January 1, 2010, the Bayley III motor score was collected and a motor composite score <70 was added to the NDI definition. Growth at follow-up was assessed by weight, length, and head circumference-for-age z scores determined by World Health Organization Child Health Standards16,17 and by the ponderal index.
Data Analysis
Characteristics and outcomes were compared between infants in the following groups defined by maternal IDDM status and timing of insulin use: insulin use before pregnancy (IBP), insulin use started during pregnancy (IDP), and no IDDM. Fisher’s exact, χ2, or t test was used to determine statistical significance for unadjusted comparisons. Poisson regression models with robust variance estimators18 were used to assess risk of mortality and other binary outcomes and included an insulin use and timing indicator (IBP, IDP, no IDDM) to allow for pairwise contrasts adjusting for study center, infant gender, GA, antenatal steroid use, maternal age, and maternal race/ethnicity. Maternal education was added to models assessing 18- to 22-month outcomes. Adjusted relative risks (RRs), 95% confidence intervals (CIs), and P values by the Score or Wald χ2 test from these models are reported. Linear regression models compared mean growth measures in the insulin groups adjusting for the covariates noted earlier, with P values for pairwise contrasts by the t test. Changes in z scores for weight, height, and head circumference at birth, 36 weeks’, and 18 to 22 months’ CA were evaluated in the subset of infants with measurements at 18- to 22-month follow-up in a longitudinal model using generalized estimating equations with an exchangeable correlation structure.
The primary binary outcomes were conditioned on the infant surviving to the risk period. Composite outcomes of morbidity or death were also examined and defined as “yes” if an infant had the outcome or died before the outcome was evaluated (for RDS, PDA, NEC, and EOS, death within 12 hours; for LOS, death within 3 days; for IVH and PVL, death before sonography; for ROP, death within 28 days; for BPD, death before 36 weeks’ PMA) and “no” if the infant survived until evaluation and did not have the morbidity. Composite death or 18- to 22-month outcomes were defined similarly, with deaths before 18 to 22 months included. Supplemental Tables 5 and 7 show results of comparisons on composite outcomes.
As a sensitivity analysis, all models assessing mortality, in-hospital morbidity, growth at 36 weeks’ PMA, neurodevelopmental outcomes at 18 to 22 months’ CA, and growth between birth and 18 to 22 months were rerun including additional adjustment for chronic maternal hypertension. Inference related to comparisons between insulin groups was unchanged, and results after adjusting for chronic maternal hypertension are not shown. P values were not adjusted for multiple comparisons. Analyses were performed by using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Study Population
Between 2006 and 2011, 10 781 infants were born in 24 NRN study centers. Maternal IDDM was reported for 536 (5%): 312 (58%) with IBP, 195 (36%) with IDP, and 29 (5%) missing timing of insulin use who were excluded from subsequent analyses. Because newborn anthropometrics differed between infants of mothers with IBP and infants of mothers with IDP, we did not pool these 2 groups. Mothers with IBP or IDP were older and more likely to have hypertension compared with mothers without IDDM, and the proportion with chronic and pregnancy-induced hypertension was higher among those with IBP compared with those with IDP. Infants of mothers with IBP had lower average length and head circumference z scores at birth than infants of mothers in the other 2 groups (Table 1). Average GA (weeks) was higher for infants with maternal IBP (26.0) or IDP (26.3) than for infants of mothers without IDDM (25.7).
TABLE 1.
Characteristics of Infants Born at 22 to 28 Weeks’ GA in NRN Centers 2006–2011 by Maternal IDDM and Timing of Maternal Insulin Use
| Characteristica | IBP, n = 312 | IDP, n = 195 | No IDDM, n = 1 0245 |
|---|---|---|---|
| Maternal age, y | |||
| Mean (SD) | 30 (5.8)*IDP,**No IDDM | 31 (6.0)**No IDDM | 27 (6.5) |
| Category, n (%) | |||
| <25 | 66 (21)*IDP,**No IDDM | 29 (15)**No IDDM | 3908 (38) |
| 25–29 | 80 (26) | 52 (27) | 2641 (26) |
| 30–34 | 96 (31) | 58 (30) | 2265 (22) |
| 35–39 | 56 (18) | 33 (17) | 1115 (11) |
| ≥40 | 14 (4) | 23 (12) | 315 (3) |
| Maternal education, n (%) | n = 190 | n = 138 | n = 7035 |
| Less than high school degree | 42 (22)***IDP,***No IDDM | 34 (25) | 1826 (26) |
| High school degree | 63 (33) | 32 (23) | 2022 (29) |
| Partial college | 55 (29) | 31 (22) | 1508 (21) |
| College degree or more | 30 (16) | 41 (30) | 1679 (24) |
| Maternal race/ethnicity, n (%) | |||
| Black | 147 (47)**IDP,***No IDDM | 59 (30)**No IDDM | 3821 (37) |
| White | 101 (32) | 79 (41) | 4077 (40) |
| Hispanic | 50 (16) | 33 (17) | 1824 (18) |
| Other | 13 (4) | 24 (12) | 491 (5) |
| ≥1 prenatal visit, n (%) | 306 (98)***No IDDM | 194 (99)**No IDDM | 9679 (95) |
| Maternal HT, n (%) | 183 (59)**IDP,**No IDDM | 76 (39)**No IDDM | 2340 (23) |
| Maternal HT existing before pregnancy or chronic, n (%) | 127 (41)***IDP,**No IDDM | 53 (27)**No IDDM | 937 (9) |
| Maternal HT not existing before pregnancy, n (%) | 53 (17)*No IDDM | 23 (12) | 1302 (13) |
| Maternal antenatal steroids, n (%) | 246 (79)*IDP | 171 (88)*No IDDM | 8444 (83) |
| Cesarean delivery, n (%) | 218 (70)***No IDDM | 131 (67) | 6226 (61) |
| Multiple births, n (%) | 67 (21)*IDP | 60 (31) | 2615 (26) |
| GA, wk | |||
| Mean (SD) | 26.0 (1.6)***No IDDM | 26.3 (1.7)**No IDDM | 25.7 (1.8) |
| Category, n (%) | |||
| 22 | 7 (2)*IDP,*No IDDM | 5 (3)**No IDDM | 450 (4) |
| 23 | 14 (4) | 9 (5) | 950 (9) |
| 24 | 46 (15) | 22 (11) | 1420 (14) |
| 25 | 52 (17) | 27 (14) | 1594 (16) |
| 26 | 47 (15) | 26 (13) | 1696 (17) |
| 27 | 77 (25) | 42 (22) | 2001 (20) |
| 28 | 69 (22) | 64 (33) | 2134 (21) |
| Birth wt, g | |||
| Mean (SD) | 841 (240)***IDP | 903 (262)**No IDDM | 838 (247) |
| Category, n (%) | |||
| <401 | 4 (1)*IDP | 2 (1)**No IDDM | 114 (1) |
| 401–1000 | 234 (75) | 120 (62) | 7458 (73) |
| 1001–1500 | 74 (24) | 73 (37) | 2620 (26) |
| >1500 | — | — | 38 (<1) |
| Length at birth, cm | n = 291 | n = 180 | n = 9424 |
| Mean (SD) | 33.4 (3.1)**IDP | 34.6 (3.3)**No IDDM | 33.6 (3.4) |
| Head circumference at birth, cm | n = 285 | n = 182 | n = 9276 |
| Mean (SD) | 23.7 (2.1)***IDP | 24.2 (2.1)***No IDDM | 23.7 (2.1) |
| Ponderal index (g/cm3) | n = 285 | n = 178 | n = 9236 |
| Mean (SD) | 2.26 (0.30) | 2.22 (0.28) | 2.22 (0.29) |
| Birth measures (23–28 wk)b | |||
| z score, mean (SD)c | |||
| Weight | −0.20 (1.17) | −0.01 (1.16) | −0.10 (1.10) |
| Length | −0.31 (1.08)***IDP,***No IDDM | −0.01 (1.09) | −0.12 (1.10) |
| Head circumference | −0.33 (1.13)*IDP,**No IDDM | −0.08 (1.04) | −0.10 (1.08) |
| Head circumference z score, n (%) | |||
| Less than –2 | 21 (7)**No IDDM | 7 (4) | 388 (4) |
| Less than –2 to less than –1 | 52 (18) | 27 (15) | 1287 (14) |
| Greater than or equal to –1 | 211 (74) | 148 (81) | 7477 (82) |
| Percentiles, n (%) | |||
| Wt | |||
| Small for GA | 54 (18)*IDP | 23 (12) | 1361 (14) |
| Appropriate for GA | 222 (73) | 141 (74) | 7504 (77) |
| Large for GA | 29 (10) | 26 (14) | 917 (9) |
| Length | |||
| Small for GA | 50 (17)*IDP | 18 (10) | 1268 (14) |
| Appropriate for GA | 219 (76) | 143 (80) | 7159 (78) |
| Large for GA | 20 (7) | 18 (10) | 798 (9) |
| Head circumference | |||
| Small for GA | 47 (17)*No IDDM | 21 (12) | 1064 (12) |
| Appropriate for GA | 218 (77) | 144 (79) | 7318 (80) |
| Large for GA | 19 (7) | 17 (9) | 770 (8) |
| Male, n (%) | 147 (47) | 90 (46) | 5372 (52) |
| Apgar at 1 min ≤3, n (%) | 132 (42) | 79 (41) | 4642 (46) |
| Apgar at 5 min ≤3, n (%) | 47 (15) | 24 (12)*No IDDM | 1841 (18) |
| Delivery room interventions, n (%) | |||
| Oxygen | 294 (94) | 182 (93) | 9368 (92) |
| Bagging and mask | 218 (70) | 136 (70) | 7366 (72) |
| CPAP | 109 (35) | 81 (42) | 3646 (36) |
| Intubation | 199 (64) | 119 (61) | 6601 (64) |
| Chest compression | 23 (7) | 11 (6) | 852 (8) |
| Epinephrine | 18 (6) | 8 (4) | 475 (5) |
| Ventilator support, n (%)d | 263 (84) | 153 (78) | 8279 (81) |
| Surfactant therapy, n (%) | 252 (81)*No IDDM | 148 (76) | 7641 (75) |
| Major birth defect, n (%) | 15 (5) | 4 (2) | 373 (4) |
CPAP, continuous positive airway pressure; HT, hypertension.
Information was missing in the groups shown for maternal age, 1 infant; maternal education, 3389 infants; maternal race/ethnicity, 33 infants; prenatal visits, 5 infants; maternal HT, 2 infants; maternal HT before or not before pregnancy, 106 infants; maternal antenatal steroids, 30 infants; birth weight, 15 infants; length at birth, 857 infants; head circumference at birth, 1009 infants; ponderal index, 1053 infants; Apgar at 1 min, 92 infants; Apgar at 5 min, 87 infants; mode of delivery, 5 infants; delivery room oxygen, CPAP, intubation, chest compression, 7 infants; delivery room bagging and mask, 9 infants; delivery room epinephrine, 8 infants; ventilator support, 4 infants; surfactant therapy, 2 infants.
z scores and percentiles from Olsen.14 Small for GA was defined as weight, length, or head circumference <10th percentile and large for GA >90th percentile. The Olsen measures are available for male and female infants born at GA 23–41 wk. Therefore, 462 infants born at 22 wk GA could not be included. In the group of 10 290 infants born at GA 23–28 wk, birth weight was missing/excluded for 13 infants, length for 597 infants, and head circumference for 672.
After adjustment for study center, GA, male gender, antenatal steroid use, maternal age, and maternal race/ethnicity, results for comparisons of z score means were similar to unadjusted results with 1 exception. The mean z score for head circumference was not significantly different for infants whose mothers used insulin before pregnancy versus those whose mothers started insulin during pregnancy, adjusted P = .054 (unadjusted P = .017).
Ventilator support was defined as use of high frequency or conventional ventilation for infants who survived >12 h. For infants born in 2006–2010 who died ≤12 h, all forms of support with a ventilator after initial resuscitation were included. Beginning in 2011, respiratory support recorded for infants who died ≤12 h was defined as any ventilator pressure support and/or supplemental oxygen delivery by any method. In the subset of 9585 infants who survived >12 h, the percent who received mechanical ventilation did not vary significantly between groups (IBP: 88%, IDP: 83%, no IDDM: 88%; P = .17).
P ≤ .05,
P ≤ .001 by t test (means for maternal age, GA, birth weight, length, head circumference, ponderal index, z score), the row mean score χ2 test with modified ridit scores specified (categorical maternal age, GA, birth weight, head circumference z score, and percentiles), the general association χ2 test, or Fisher’s exact test. Pairwise comparisons were performed between infants with maternal IBP versus infants with maternal IDP and infants with no maternal IDDM, and between infants with maternal IDP versus infants with no maternal IDDM. For each significant result, the comparison group is indicated.
P ≤ .01,
In-Hospital Mortality, Morbidities, and Growth at 36 Weeks’ PMA
Overall, 7905 (74%) infants survived to discharge and 2847 (26%) died. Mortality risk was slightly greater for infants with maternal IBP compared with infants of mothers with IDP (27% vs 18%, RR = 1.33, 95% CI: 1.00–1.79; P = .054) but not compared with infants whose mothers had no IDDM (Table 2). Morbidities were recorded for 9585 infants (89%) surviving >12 hours. Infants of mothers with IBP were at increased risk of NEC (RR = 1.55, 95% CI: 1.17–2.05) and of LOS (RR = 1.26, 95% CI: 1.07–1.48) compared with those of mothers without IDDM. The percentages of infants with NEC and LOS were also greater for infants of mothers with IBP compared with IDP, but differences did not reach statistical significance. However, risk of the combined outcomes death within 12 hours or NEC (RR = 1.45, 95% CI: 1.02–2.07) and death within 3 days or LOS (RR = 1.35, 95% CI: 1.07–1.71) were increased for infants of mothers with IBP compared with infants of mothers with IDP and also increased compared with infants without maternal IDDM (Supplemental Table 5). Among infants still hospitalized at 28 days, ROP was diagnosed in 57% of infants with maternal IBP and 49% with maternal IDP (RR = 1.23, 95% CI: 1.04–1.45). RRs were similar when deaths within 28 days were added to this outcome. There were no significant differences among the 3 groups in the risk of PDA, EOS, IVH, PVL, or BPD (Table 2) or the composite risk of each morbidity or death (Supplemental Table 5).
TABLE 2.
In-Hospital Mortality and Morbidities Among Infants Born at 22 to 28 Weeks’ GA by Maternal IDDM and Timing of Maternal Insulin Use
| Outcome, n (%)a | IBP, n = 312 | IDP, n = 195 | No IDDM, n = 10 245 | Adjusted RR (95% CI) for Outcomeb | ||
|---|---|---|---|---|---|---|
| IBP vs IDP | IBP vs No IDDM | IDP vs No IDDM | ||||
| Died before discharge | 85 (27) | 35 (18) | 2727 (27) | 1.33 (1.00–1.79) | 1.17 (0.99–1.38) | 0.87 (0.68–1.12) |
| Died ≤12 h | 26 (8) | 13 (7) | 1128 (11) | 1.15 (0.74–1.79) | 1.08 (0.79–1.48) | 0.94 (0.68–1.30) |
| Infants who survived >12 h | n = 286 | n = 182 | n = 9117 | |||
| RDS | 282 (99) | 182 (100) | 8934 (98) | — | 1.01 (1.00–1.03) | — |
| PDA | 130 (46) | 73 (40) | 4198 (46) | 1.20 (0.98–1.47) | 1.07 (0.94–1.21) | 0.89 (0.75–1.04) |
| NEC | 44 (15) | 16 (9) | 977 (11) | 1.67 (0.96–2.91) | 1.55 (1.17–2.05)c | 0.93 (0.57–1.52) |
| EOS | 6 (2) | 4 (2) | 187 (2) | 0.89 (0.25–3.10) | 1.09 (0.49–2.45) | 1.23 (0.47–3.24) |
| Infants in hospital >3 d | n = 275 | n = 179 | n = 8800 | |||
| LOS | 95 (35) | 36 (20) | 2458 (28) | 1.34 (0.97–1.85) | 1.26 (1.07–1.48)c | 0.94 (0.71–1.24) |
| Infants with cranial sonogram within 28 dd | n = 277 | n = 178 | n = 8892 | |||
| Severe IVH | 43 (16) | 24 (13) | 1382 (16) | 0.91 (0.58–1.44) | 1.02 (0.78–1.33) | 1.11 (0.77–1.61) |
| Infants with cranial sonogram within 28 d and/or closest to 36 wk PMA and after 28 de | n = 279 | n = 179 | n = 8914 | |||
| PVL | 8 (3) | 8 (4) | 410 (5) | 0.56 (0.21–1.47) | 0.64 (0.32–1.28) | 1.14 (0.57–2.27) |
| Infants with sufficient information to include in analysisf | n = 277 | n = 178 | n = 8886 | |||
| Severe IVH or PVL | 46 (17) | 24 (13) | 1550 (17) | 1.01 (0.65–1.57) | 0.97 (0.75–1.26) | 0.96 (0.67–1.39) |
| Infants in hospital at 28 d with ROP examinationg | n = 230 | n = 160 | n = 7569 | |||
| ROP | 130 (57) | 78 (49) | 4345 (57) | 1.23 (1.04–1.45)c | 1.09 (0.99–1.20) | 0.88 (0.77–1.01) |
| ROP stage ≥3 | 34 (15) | 16 (10) | 1018 (13) | 1.32 (0.78–2.24) | 1.27 (0.94–1.71) | 0.96 (0.62–1.49) |
| Infants alive at 36 wk PMA evaluated for BPDh | n = 233 | n = 164 | n = 7637 | |||
| BPD | 84 (36) | 58 (35) | 3355 (44) | 1.04 (0.81–1.33) | 0.93 (0.79–1.09) | 0.90 (0.74–1.08) |
Among survivors >12 h, information was missing for RDS, 1 infant; PDA, 10 infants; NEC, 2 infants; EOS, 3 infants. There were 1496 infants who died within 3 d of birth and 2 infants who stayed in the birth hospital <3 d (both were transferred) who were excluded from the number of infants in the hospital >3 d. LOS was missing for 1 infant who survived >3 d. The numbers of infants with missing values of other outcomes are noted in the following footnotes.
RRs and CIs from modified Poisson regression models fit to each outcome that in addition to the maternal insulin timing indicator (IBP, IDP, no IDDM) included study center, GA (categorical), male gender, antenatal steroid use, maternal race/ethnicity (black, white, Hispanic, other), and maternal age. For the outcome RDS, RRs involving infants whose mothers started insulin during pregnancy were not estimated because all infants in this group had RDS.
RRs significantly different from 1.0.
Severe IVH was defined as grade 3 or 4 and was diagnosed on the basis of the cranial sonogram taken within 28 d of birth with the most severe findings. Of 9585 infants who survived >12 h, 9347 (97.5%) were evaluated by cranial sonogram. Of those who had a cranial sonogram, IVH was missing for 4 infants.
PVL was determined on the basis of the cranial sonogram taken within 28 d of birth with the most severe findings and/or a cranial imaging study taken closest to 36 wk PMA and after 28 d of birth. Of the 9585 infants who survived >12 h, 9372 (97.8%) were evaluated for PVL. Among those with cranial imaging performed, PVL was missing for 4 infants.
Presence of IVH and/or PVL was determined for infants with nonmissing IVH and PVL outcomes, except that a diagnosis of either condition was sufficient to set the outcome.
ROP was defined for infants still hospitalized at 28 d who had a ROP examination. By 28 d, 2331 infants had died, 5 had been discharged, and status was unknown for 10. Of the 8406 infants still in the hospital, 7959 (94.7%) had a ROP examination. Among those who had an examination, ROP was missing for 1 infant.
BPD was defined for infants born <36 wk GA as the need for supplemental oxygen use at 36 wk PMA. For infants discharged or transferred before 36 wk PMA, BPD was defined based on oxygen use at 36 wk if known or oxygen use at the time of discharge or transfer. BPD could not be evaluated for 87 (1%) of the 8121 infants alive at 36 wk PMA. Of the 8034 infants evaluated, 82% were still in the hospital at 36 wk PMA, 12% had been discharged from the hospital, and 5% had been transferred to another hospital.
Among infants still hospitalized at 36 weeks’ PMA, those with maternal IBP had smaller average head circumference z scores (mean [SD] –1.31 [1.01]) than infants of mothers with IDP (–0.99 [1.07]) and infants without maternal IDDM (–1.07 [1.01]) and a larger proportion had microcephaly compared with infants without maternal IDDM (25% vs 18%, P = .003; Table 3). There were no significant differences in weight and length.
TABLE 3.
Growth at 36 Weeks’ PMA and at 18 to 22 Months’ CA by Maternal IDDM and Timing of Maternal Insulin Use
| Outcome | IBP | IDP | No IDDM |
|---|---|---|---|
| Measures at 36 wk PMAa | |||
| Weight measured | n = 221 | n = 146 | n = 6955 |
| Length measured | n = 210 | n = 134 | n = 6460 |
| Head circumference measured | n = 216 | n = 139 | n = 6652 |
| Measurement, mean (SD) | |||
| Weight, g | 2088 (396) | 2132 (436) | 2111 (402) |
| Length, cm | 42.5 (2.8) | 43.0 (3.0) | 42.7 (2.9) |
| Head circumference, cm | 30.5 (1.8)*IDP,**No IDDM | 31.1 (1.8) | 31.0 (1.8) |
| Ponderal Index, g/cm3 | n = 207 | n = 128 | n = 6341 |
| Mean (SD) | 2.73 (0.38) | 2.71 (0.38) | 2.70 (0.37) |
| z score, mean (SD) | |||
| Weight | −1.25 (0.86) | −1.16 (0.95) | −1.21 (0.87) |
| Length | −1.73 (0.94) | −1.55 (1.05) | −1.66 (0.97) |
| Head circumference | −1.31 (1.01)*IDP,**No IDDM | −0.99 (1.07) | −1.07 (1.01) |
| Head circumference z score, n (%) | |||
| Less than –2 | 53 (25)***No IDDM | 25 (18) | 1169 (18) |
| Less than –2 to less than –1 | 72 (33) | 33 (24) | 1991 (30) |
| Greater than or equal to –1 | 91 (42) | 81 (58) | 3492 (52) |
| Small for GA, n (%) | |||
| Weight | 104 (47) | 63 (43) | 3099 (45) |
| Length | 135 (64) | 90 (67) | 4105 (64) |
| Head circumference | 98 (45)**No IDDM | 49 (35) | 2360 (35) |
| Measures at 18–22 mo CAb | |||
| Weight measured | n = 111 | n = 65 | n = 3696 |
| Length measured | n = 111 | n = 65 | n = 3675 |
| Head circumference measured | n = 110 | n = 65 | n = 3648 |
| Measurement, mean (SD) | |||
| Weight, kg | 10.51 (1.60)*No IDDM | 10.71 (1.50) | 10.80 (1.58) |
| Length, cm | 81.05 (4.51) | 81.35 (4.23) | 81.62 (4.54) |
| Head circumference, cm | 46.47 (1.81)*No IDDM | 46.59 (1.93) | 46.89 (1.90) |
| Ponderal Index, g/cm3 | n = 107 | n = 65 | n = 3609 |
| Mean (SD) | 1.97 (0.20) | 1.99 (0.22) | 1.98 (0.21) |
| z score, mean (SD) | |||
| Weight | −0.51 (1.27)*No IDDM | −0.29 (1.16) | −0.31 (1.15) |
| Length | −0.89 (1.35)*No IDDM | −0.72 (1.31) | −0.73 (1.28) |
| Head circumference | −0.46 (1.28)*No IDDM | −0.34 (1.31) | −0.21 (1.31) |
| Head circumference z score, n (%) | |||
| Less than –2 | 12 (11) | 5 (8) | 288 (8) |
| Less than –2 to less than –1 | 25 (23) | 14 (22) | 638 (17) |
| Greater than or equal to –1 | 73 (66) | 46 (71) | 2722 (75) |
Measurements taken between 35 and 37 wk PMA were included. z scores and percentiles based on Olsen.14
z scores were determined based on the World Health Organization’s Child Health Standards.
P ≤ .05,
P ≤ .001 by t test from linear regression models (means) or by Wald χ2 from modified Poisson regression models (categorical outcomes) with adjustment for study center, GA (categorical), male gender, antenatal steroid use, maternal race/ethnicity (black, white, Hispanic, other), and maternal age. Models fit to follow-up outcomes also included maternal education (less than high school degree, high school degree, partial college or more, missing). For categorical head circumference z score, tests were conducted for the binary outcome less than –2 versus Greater than or equal to –2, and study center was not included in the model because of small numbers. Pairwise comparisons were performed between infants with maternal IBP versus infants with maternal IDP and infants with no maternal IDDM and between infants with maternal IDP versus infants with no maternal IDDM. For each significant result, the comparison group is indicated.
P ≤ .01,
Growth at 18 to 22 Months’ Follow-up and Changes From Birth to 18 to 22 Months
Among the 4292 children surviving to 18 to 22 months’ CA and eligible for follow-up, 3883 (90%) completed the follow-up visit between September 2007 and April 2014 with similar compliance with and without maternal IDDM (93% vs 90%). There were no differences in GA or birth weight among infants in the 3 groups who attended follow-up (Supplemental Table 6). Mothers with IBP showed higher rates of chronic hypertension than mothers in the other 2 groups. The rate of birth defects was higher among infants of mothers with IBP (7%) compared with those with IDP (0%) and those without IDDM (2%).
At 18 to 22 months, mean weight, length, and head circumference z scores were significantly lower for infants of mothers with IBP compared with infants of mothers without IDDM. Although also lower compared with z scores for infants of mothers with IDP, differences did not reach statistical significance (Table 3).
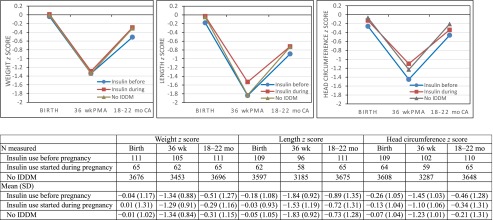
In the subset of children with at least 1 of weight, length, or head circumference measured at 18 to 22 months (n = 3877), z score trends were examined between birth, 36 weeks’ PMA, and 18 to 22 months. Differences between the groups were consistent over time (nonsignificant interaction between insulin group and time for weight, P = .51; length, P = .29; head circumference, P = .48). Mean head circumference z scores were significantly lower at birth for infants with maternal IBP than for infants without maternal IDDM and remained lower through 18 to 22 months (P = .004), but comparisons with infants whose mothers had IDP were not statistically significant (Fig 1). Mean z scores for weight and length did not differ significantly among the 3 groups over time in this subset (P = .53 and P = .18, respectively). Results were nearly identical when analyses were repeated by using the subset of infants with measures at all 3 time points. Excluding infants with birth defects did not change the results.
FIGURE 1.
Unadjusted mean growth measure z scores for age and gender at birth, 36 weeks’ PMA, and 18 to 22 months’ CA compared between infants with maternal IBP, IDP, and no IDDM in the subset of 3877 infants with at least 1 of weight, length, or head circumference measured at the 18- to 22-month visit.
Mortality and Neurodevelopmental Outcomes of Children at 18 to 22 Months’ CA
For survivors seen at follow-up, NDI risk was not significantly increased for children of mothers with IBP or IDP compared with those without maternal IDDM (Table 4), nor was the risk of the combined death or NDI outcome (Supplemental Table 7). Similarly, there were no significant differences in the individual components of NDI among the 3 groups (Table 4).
TABLE 4.
Outcomes for Children at 18 to 22 Months’ CA by Maternal IDDM and Timing of Maternal Insulin Use
| Outcome, n (%) | IBP | IDP | No IDDM | Adjusted RR (95% CI) for Outcomea | ||
|---|---|---|---|---|---|---|
| IBP vs IDP | IBP vs No IDDM | IDP vs No IDDM | ||||
| Children eligible for 18–22 mo follow-up | n = 189 | n = 101 | n = 6598 | |||
| Died before discharge | 66 (35) | 31 (31) | 2423 (37) | 1.10 (0.82–1.47) | 1.06 (0.89–1.26) | 0.97 (0.76–1.23) |
| Total deaths between birth and 18–22 mo CA | 69 (37) | 31 (31) | 2496 (38) | 1.13 (0.85–1.51) | 1.07 (0.90–1.26) | 0.94 (0.74–1.20) |
| Children seen at follow-up for whom NDI was determined | n = 109 | n = 64 | n = 3608 | |||
| NDI | 21 (19) | 8 (13) | 586 (16) | 1.20 (0.58–2.50) | 1.22 (0.83–1.78) | 1.01 (0.53–1.91) |
| Evaluated before 2010, n/N (%) (2006 NDI definition)b | 7/43 (16) | 0/26 (0) | 157/1337 (12) | — | 1.50 (0.74–3.04) | — |
| Evaluated 2010 or later, n/N (%) (2010 NDI definition)b | 14/66 (21) | 8/38 (21) | 429/2271 (19) | 0.86 (0.41–1.83) | 1.09 (0.70–1.70) | 1.26 (0.68–2.33) |
| NDI (2006 definition for all) | 16 (15) | 6 (9) | 521 (14) | 1.23 (0.52–2.93) | 1.05 (0.67–1.64) | 0.85 (0.40–1.80) |
| Children seen at follow-up with key formc | n = 111 | n = 65 | n = 3707 | |||
| Bilateral blindness | 1 (<1) | 1 (2) | 44 (1) | 0.56 (0.04–8.87) | 0.76 (0.10–5.71) | 1.35 (0.18–9.92) |
| Hearing impairment | 3 (3) | 0 (0) | 111 (3) | — | 1.00 (0.32–3.13) | — |
| CP, any grade | 10 (9) | 6 (9) | 428 (12) | 0.84 (0.33–2.12) | 0.78 (0.43–1.40) | 0.93 (0.45–1.92) |
| CP, moderate-severe | 4 (4) | 1 (2) | 217 (6) | 1.71 (0.19–15.05) | 0.63 (0.24–1.68) | 0.37 (0.05–2.59) |
| Cognitive CS <70 | 14 (13) | 5 (8) | 337 (9) | 1.30 (0.50–3.40) | 1.41 (0.86–2.32) | 1.09 (0.47–2.53) |
| Language CS <70 | 21 (19) | 10 (16) | 619 (17) | 0.99 (0.51–1.95) | 1.08 (0.74–1.60) | 1.09 (0.62–1.92) |
| Children seen at follow-up January 2010 or later with Bayley III motor score | n = 66 | n = 38 | n = 2248 | |||
| Motor CS <70 | 12 (18) | 6 (16) | 286 (13) | 0.91 (0.37–2.24) | 1.34 (0.81–2.23) | 1.48 (0.68–3.19) |
CP, cerebral palsy; CS, composite score.
RRs and CIs from modified Poisson regression models fit to each outcome that in addition to the maternal insulin timing indicator (IBP, IDP, no IDDM) included study center, GA (categorical), male gender, antenatal steroid use, maternal race/ethnicity (white, black, Hispanic, other), maternal age, and maternal education (less than high school degree, high school degree, partial college or more, missing). Study center was not included in the models fit to blindness or hearing impairment due to small numbers. None of the infants whose mothers started IDP had NDI in the subset seen at follow-up before 2010, and none had hearing impairment; RRs involving this group were not estimated for these outcomes.
The 2006 definition of NDI was ≥1 of bilateral blindness, hearing impairment, moderate to severe CP, gross motor function level ≥2, or Bayley III cognitive CS <70. Bayley III motor CS <70 was added to the 2010 definition of NDI.
In the group of children seen at follow-up with ≥1 key form, information was missing for the following children: blindness 7; hearing impairment 5; CP 5; Bayley III cognitive CS 83; Bayley III language CS 158.
Discussion
In this large cohort of extremely preterm infants, infants whose mothers used insulin before pregnancy had significantly lower mean z scores for length and head circumference at birth compared with infants whose mothers started insulin during pregnancy and those whose mothers had no IDDM. While hospitalized, infants with maternal IBP were at higher risk of NEC and LOS compared with those born to mothers without IDDM, and those still hospitalized at 36 weeks’ PMA had lower average head circumference z scores than either infants of mothers with IDP or infants without maternal IDDM. Among infants surviving and evaluated at 18 to 22 months, average head circumference z scores were significantly lower at birth, 36 weeks’ PMA, and 18 to 22 months’ CA for infants of mothers with IBP compared with infants of mothers without IDDM. Importantly, however, no differences in neurodevelopmental outcomes at 18 to 22 months were observed between the groups.
The etiology of diabetes might differ based on the timing of insulin use. The IBP group likely comprised women with type 1 and type 2 diabetes, whereas IDP likely included women with gestational diabetes and type 2 diabetes. Increasing fetal growth restriction has been associated with worsening of vascular status among women with type 1 diabetes.19 At birth, the proportion of infants small for GA on weight, length, and head circumference was largest among infants with maternal IBP. Of the 3 anthropometric measurements among infants of women with IBP, head circumference seems to be the most affected.
A study examining microcephaly incidence, defined as head circumference z score ≤−1.88, among liveborn infants (mean GA = 37 weeks) of women with IBP reported that the observed microcephaly incidence was less than expected and attributed this to the tendency of infants of diabetic mothers toward macrosomia and better glycemic control as measured by HbA1c.20 In our study, microcephaly (z score ≤−2) at birth (7% vs 4%) and at 36 weeks (25% vs 18%) was significantly higher among infants of mothers with IBP compared with infants of mothers without IDDM. At 18 to 22 months’ CA, however, microcephaly was not significantly increased among surviving infants of mothers with IBP compared with the other groups (11% vs 8%, respectively). The clinical significance of a smaller head circumference is debatable. Brain volume is a determinant of head size; however, microcephaly at 2 years, but not at birth, is predictive of severe motor and cognitive impairments.21,22
Both poor glycemic control and hypertension have previously been associated with preterm delivery among women with type 1 diabetes.23–26 Nearly 60% of the women with IBP had hypertension, a majority of whom had chronic hypertension. The increased malformations in this group in the subset of infants with follow-up data may be reflective of poor glycemic control.27
One of our main study findings is that infants of mothers with IBP did not have a significantly increased rate of neurodevelopmental delays. Neurodevelopmental delays have been reported at increased rates among infants of diabetic mothers.28,29 Potential mechanisms that have been postulated relate to in utero hypoxia, brain iron deficiency, or hypoglycemia at birth.28,30,31 Whether these same mechanisms are implicated in the smaller head circumference among infants of diabetic mothers with IBP is unknown.
To our knowledge, only 3 studies have examined the impact of maternal diabetes on morbidity and mortality of VLBW infants.7–9 One study in Israel combined VLBW infants of mothers with gestational (n = 825) and pregestational diabetes (n = 120) into 1 group after finding no differences in demographic and delivery characteristics and reported no increased risks in hospital mortality or morbidities compared with VLBW infants of nondiabetic mothers (n = 12 209).7 Another study in Latin America with no information on diabetes type examined in-hospital morbidities between VLBW infants of mothers with any type of diabetes (n = 304) and VLBW infants of mothers without diabetes (n = 10 563). The authors reported increased risk only for NEC (RR = 1.65, 95% CI: 1.21–2.27).8 A third descriptive study of 43 VLBW infants of diabetic women (gestational and pregestational) showed no differences in hospital morbidities or neurodevelopmental outcomes at 18 months compared with controls.9 None of these studies examined glycemic control method. Additionally, given the different database eligibility criteria, the mean GA in these studies was ∼29 weeks, considerably higher than 26 weeks in our study population.
The increased NEC risk in our study (RR = 1.55, 95% CI: 1.17–2.05) for infants of mothers with IBP compared with infants of mothers without IDDM and in the Latin American study might be due to higher polycythemia rates among these infants. Maternal hyperglycemia causes chronic fetal hyperglycemia and hyperinsulinemia, both of which increase fetal metabolic rate and oxygen requirements.30 As the placenta is incapable of upregulating oxygen delivery to meet this higher demand, chronic fetal hypoxia ensues resulting in increased erythropoietin levels and subsequent polycythemia.32,33 Polycythemia has been previously shown to be a risk factor for NEC, although more commonly among term infants.34–38 In addition to the potential role of diabetes in NEC, other maternal risk factors that reduce fetal gut blood flow, such as hypertensive disorders, have also been associated with NEC among VLBW infants.39 In our study, maternal hypertension among mothers with IBP was 2.5 times higher than among mothers without IDDM (59% vs 23%). However, the RR of NEC for infants with maternal IBP compared with infants without maternal IDDM was unchanged after adjusting for maternal chronic hypertension, indicating that chronic hypertension does not account for all of this association. The NRN previously reported a 2.7-fold higher LOS risk in VLBW infants with NEC.40 The increased risk of NEC may have contributed to the increased risk of LOS in this study for infants of mothers with IBP compared with infants of mothers without IDDM (RR = 1.26, 95% CI: 1.07–1.48).
Using a large prospective database of extremely preterm infants, we examined several important outcomes including neurodevelopmental outcomes with a high follow-up rate. We also had data on glycemic control method, which was lacking in previous studies. However, we had no data on diabetes onset and type, diabetes duration, insulin type and dosage, level of glycemic control, usage of antihypertensive medications, or the prevalence of diabetic nephropathy and microalbuminuria. We also had no data on when insulin use started during pregnancy or on the presence of diabetes controlled by diet or oral hypoglycemic agents.
Conclusions
We found that extremely preterm infants of diabetic mothers with IBP have smaller average head circumference and are at higher risk of NEC and LOS but are not at higher risk of adverse neurodevelopmental outcomes at 18 to 22 months’ CA compared with infants born to women without IDDM. Additional study to elucidate factors contributing to the increased risk of NEC and LOS may improve clinical care of extremely preterm infants born to diabetic mothers with IBP.
Acknowledgments
Data collected at participating sites of the NICHD NRN were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed, and analyzed the data for this study. On behalf of the NRN, Ms Nellie Hansen (DCC Statistician) and Dr Abhik Das (DCC Principal Investigator) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chair: Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine.
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904)—Abbott R. Laptook, MD; Martin Keszler, MD; Betty R. Vohr, MD; Angelita M. Hensman, MS, RNC-NIC; Barbara Alksninis, PNP; Kristin M. Basso, BSN MaT; Robert Burke, MD; Melinda Caskey, MD; Katharine Johnson, MD; Mary Lenore Keszler, MD; Theresa M. Leach, MEd CAES; Elisa Vieira, RN BSN; Victoria E. Watson, MS, CAS.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01, RR80)—Michele C. Walsh, MD, MS; Avroy A. Fanaroff, MD; Deanne E. Wilson-Costello, MD; Nancy S. Newman, BA, RN; Bonnie S. Siner, RN; Monika Bhola, MD; Gulgun Yalcinkaya, MD; Harriet G. Friedman, MA.
Children’s Mercy Hospital, University of Missouri Kansas City School of Medicine (U10HD68284)—William E. Truog, MD; Eugenia K. Pallotto, MD, MSCE; Howard W. Kilbride, MD; Cheri Gauldin, RN, BS, CCRC; Anne Holmes RN, MSN, MBA-HCM, CCRC; Kathy Johnson, RN, CCRC; Allison Knutson, BSN, RNC-NIC.
Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084)—Kurt Schibler, MD; Edward F. Donovan, MD; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN, CCRC; Holly L. Mincey, RN, BSN; Jody Hessling, RN; Teresa L. Gratton, PA; Kimberly Yolton, PhD.
Duke University School of Medicine, University Hospital, University of North Carolina, and Duke Regional Hospital (U10 HD40492, M01 RR30)—Ronald N. Goldberg, MD; C. Michael Cotten, MD, MHS; Ricki F. Goldstein, MD; Patricia L. Ashley, MD, PhD; William F. Malcolm, MD; Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD, FNP-BC, IBCLC; Sandra Grimes, RN, BSN; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN, MSN; Joanne Finkle, RN, JD; Matthew M. Laughon, MD, MPH; Carl L. Bose, MD; Janice Bernhardt, MS, RN; Gennie Bose, RN; Cindy Clark, RN; Linda Manor, RPh; Diane Warner, MD, MPH; Janice Wereszczak, NNP.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, M01 RR39)—David P. Carlton, MD; Ira Adams-Chapman, MD; Ellen C. Hale, RN, BS, CCRC; Yvonne Loggins, RN, BSN.
Eunice Kennedy Shriver National Institute of Child Health and Human Development—Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750)—Gregory M. Sokol, MD; Brenda B. Poindexter, MD, MS; Anna M. Dusick, MD (deceased); Leslie Dawn Wilson, BSN, CCRC; Faithe Hamer, BS; Carolyn Lytle, MD, MPH; Heike M. Minnich, PsyD, HSPP; Abbey C. Hines, PsyD.
Nationwide Children’s Hospital and the Ohio State University Medical Center (U10 HD68278)—Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Pablo J. Sánchez, MD; Patricia Luzader, RN; Christine A. Fortney, PhD, RN; Gail E. Besner; Nehal A. Parikh, MD.
RTI International (U10 HD36790)—W. Kenneth Poole, PhD (deceased); Dennis Wallace, PhD; Jamie E. Newman, PhD, MPH; Jeanette O’Donnell Auman, BS; Margaret M. Crawford, BS, CCRP; Carolyn M. Petrie Huitema, MS, CCRP; Kristin M. Zaterka-Baxter, RN, BSN, CCRP.
Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital (U10 HD27880, M01 RR70)—Krisa P. Van Meurs, MD; David K. Stevenson, MD; Susan R. Hintz, MD, MS Epi; Alexis S. Davis, MD, MS Epi; M. Bethany Ball, BS, CCRC; Andrew W. Palmquist, RN; Melinda S. Proud, RCP; Barbara Bentley, PsychD, MSEd; Elizabeth Bruno, PhD; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN, PNP; Lynne C. Huffman, MD; Jean G. Kohn, MD, MPH; Casey Krueger, PhD; Hali E. Weiss, MD.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54)—Ivan D. Frantz III, MD; John M. Fiascone, MD; Brenda L. MacKinnon, RNC; Anne Furey, MPH; Ellen Nylen, RN, BSN; Elisabeth C. McGowan, MD.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10HD34216, M01 RR32)—Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD, MPH; Monica V. Collins, RN, BSN, MaEd; Shirley S. Cosby, RN, BSN; Fred J. Biasini, PhD; Kristen C. Johnston, MSN, CRNP; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Vivien A. Phillips, RN, BSN; Sally Whitley, MA, OTR-L, FAOTA.
University of California–Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (U10 HD68270—Uday Devaskar, MD; Meena Garg, MD; Isabell B. Purdy, PhD, CPNP; Teresa Chanlaw, MPH; Rachel Geller, RN, BSN.
University of California–San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461)—Neil N. Finer, MD; Yvonne E. Vaucher, MD, MPH; David Kaegi, MD; Maynard R. Rasmussen, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Martha G. Fuller, RN, MSN; Wade Rich, BSHS, RRT.
University of Iowa and Mercy Medical Center (U10 HD53109, M01 RR59)—Michael J. Acarregui, MD; Dan L. Ellsbury, MD; Karen J. Johnson, RN, BSN; Donia B. Campbell, RNC-NC; Diane L. Eastman, RN, CPNP, MA; Jacky R. Walker, RN.
University of Miami, Holtz Children’s Hospital (U10 HD21397, M01 RR16587)—Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett-Thomas, RN, MSN; Sylvia Fajardo-Hiriart, MD; Arielle Rigaud, MD; Maria Calejo, MS; Silvia M. Frade Eguaras, MA; Michelle Harwood Berkowits, PhD; Andrea Garcia, MS; Helina Pierre, BA; Alexandra Stoerger, BA.
University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997)—Kristi L. Watterberg, MD; Jean R. Lowe, PhD; Janell F. Fuller, MD; Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Andrea Freeman Duncan, MD, MScr; Rebecca Montman, BSN.
University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (U10 HD68244)—Barbara Schmidt, MD, MSc; Haresh Kirpalani, MB, MSc; Sara B. DeMauro, MD, MSCE; Aasma S. Chaudhary, BS, RRT; Soraya Abbasi, MD; Toni Mancini, RN, BSN, CCRC; Dara M. Cucinotta, RN; Judy C. Bernbaum, MD; Marsha Gerdes, PhD; Hallam Hurt, MD.
University of Rochester Medical Center, Golisano Children’s Hospital and the University of Buffalo Women’s and Children’s Hospital of Buffalo (U10 HD68263, U10 HD40521, M01 RR44, UL1 TR42)—Carl T. D’Angio, MD; Dale L. Phelps, MD; Ronnie Guillet, MD, PhD; Satyan Lakshminrusimha, MD; Linda J. Reubens, RN, CCRC; Holly I.M. Wadkins; Julie Babish Johnson, MSW; Cassandra A. Horihan, MS; Diane Hust, MS, RN, CS; Rosemary L. Jensen; Emily Kushner, MA; Joan Merzbach, LMSW; Gary J. Myers, MD; Mary Rowan, RN; Ashley Williams, MSEd; Kelley Yost, PhD; Lauren Zwetsch, RN, MS, PNP; William Zorn, MD; Anne Marie Reynolds, MD, MPH; Deanna Maffett, RN; Karen Wynn, RN; Farooq Osman, MD.
University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373)—Kathleen A. Kennedy, MD, MPH; Jon E. Tyson, MD, MPH; Georgia E. McDavid, RN; Nora I. Alaniz, BS; Katrina Burson, RN, BSN; Patricia W. Evans, MD; Charles Green, PhD; Beverly Foley Harris, RN, BSN; Margarita Jiminez, MD, MPH; Anna E. Lis, RN, BSN; Sarah Martin, RN, BSN; Brenda H. Morris, MD; M. Layne Poundstone, RN, BSN; Peggy Robichaux, RN, BSN; Saba Siddiki, MD; Maegan C. Simmons, RN; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT(ASCP).
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633)—Pablo J. Sánchez, MD; Luc P. Brion, MD; Roy J. Heyne, MD; Walid A. Salhab, MD; Charles R. Rosenfeld, MD; Lijun Chen, PhD, RN; Alicia Guzman; Gaynelle Hensley, RN; Melissa H. Leps, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Diana M. Vasil, RNC-NIC; Sally S. Adams, MS, RN, CPNP; Catherine Twell Boatman, MS, CIMI; Elizabeth T. Heyne, MS, MA, PA-C PsyD; Linda A. Madden, RN, CPNP; Lizette E. Torres, RN.
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64, UL1 RR25764)—Roger G. Faix, MD; Bradley A. Yoder, MD; Karen A. Osborne, RN, BSN, CCRC; Cynthia Spencer, RNC, BSN; Kimberlee Weaver-Lewis, RN, MS; Shawna Baker, RN; Karie Bird, RN, BSN; Jill Burnett, RNC, BSN; Michael Steffen, MS, CPM; Jennifer J. Jensen, RN, BSN; Sarah Winter, MD; Karen Zanetti, RN.
Wake Forest University, Baptist Medical Center, Forsyth Medical Center, and Brenner Children’s Hospital (U10 HD40498, M01 RR7122)—T. Michael O’Shea, MD, MPH; Robert G. Dillard, MD; Lisa K. Washburn, MD; Barbara G. Jackson, RN, BSN; Nancy Peters, RN; Korinne Chiu, MA; Deborah Evans Allred, MA, LPA; Donald J. Goldstein, PhD; Raquel Halfond, MA; Carroll Peterson, MA; Ellen L. Waldrep, MS; Cherrie D. Welch, MD, MPH; Melissa Whalen Morris, MA; Gail Wiley Hounshell, PhD.
Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10HD21385)—Seetha Shankaran, MD; Athina Pappas, MD; John Barks, MD; Rebecca Bara, RN, BSN; Laura A. Goldston, MA; Girija Natarajan, MD; Mary Christensen, RT; Stephanie A. Wiggins, MS.
Yale University, Yale–New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, ULTR142, M01 RR125)—Richard A. Ehrenkranz, MD; Harris Jacobs, MD; Christine G. Butler, MD; Patricia Cervone, RN; Sheila Greisman, RN; Monica Konstantino, RN, BSN; JoAnn Poulsen, RN; Janet Taft, RN, BSN; Joanne Williams, RN, BSN; Elaine Romano, MSN.
Glossary
- BPD
bronchopulmonary dysplasia
- CA
corrected age
- EOS
early-onset sepsis
- GA
gestational age
- IBP
insulin use before pregnancy
- IDDM
insulin-dependent diabetes mellitus
- IDP
insulin use started during pregnancy
- IVH
intraventricular hemorrhage
- LOS
late-onset sepsis
- NDI
neurodevelopmental impairment
- NEC
necrotizing enterocolitis
- NRN
Neonatal Research Network
- PDA
patent ductus arteriosus
- PMA
postmenstrual age
- PVL
periventricular leukomalacia
- RDS
respiratory distress syndrome
- ROP
retinopathy of prematurity
- RR
relative risk
- VLBW
very low birth weight
Footnotes
Dr Boghossian participated in the conception and design of the study including the analysis plan, participated in the interpretation of the data, wrote the first and subsequent drafts of the manuscript, and helped to revise it critically for important intellectual content; Ms Hansen helped design the analysis plan and was responsible for the data management and analysis; performed the analysis with guidance from Drs Boghossian, Bell, and Das; and helped revise the manuscript critically for important intellectual content; Dr Bell participated in the conception and design of the study including the analysis plan, participated in the interpretation of the data, and revised the manuscript critically for important intellectual content; Drs Brumbaugh, Laptook, Shankaran, Wyckoff, and Colaizy participated in the conception of the study, participated in the interpretation of the data, and reviewed the manuscript critically for important intellectual content; Dr Stoll participated in the conception of the study, chaired the committee responsible for designing and managing the study from which the data were drawn, participated in the interpretation of the data, and helped revise the manuscript critically for important intellectual content; Dr Das participated in the design of the study and the plan for data analysis, was responsible for the data management and participated in the analysis, and helped to revise the manuscript critically for important intellectual content; Dr Higgins participated in the design of the study, participated in the interpretation of the data, and helped revise the manuscript critically for important intellectual content; and all authors approved the final manuscript as submitted.
Although Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) staff members had input into the study design, conduct, analysis, and manuscript drafting, the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This trial has been registered at www.clinicaltrials.gov (identifiers NCT00063063 [Generic Database] and NCT00009633 [Follow-Up Study]).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The Neonatal Research Network is funded by the National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Rosemary D. Higgins is employed by the NICHD. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.American Diabetes Association . Gestational diabetes mellitus. Diabetes Care. 2004;27(suppl 1):S88–S90 [DOI] [PubMed] [Google Scholar]
- 2.Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31(5):899–904 [DOI] [PubMed] [Google Scholar]
- 3.Sibai BM, Caritis S, Hauth J, et al. ; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units . Risks of preeclampsia and adverse neonatal outcomes among women with pregestational diabetes mellitus. Am J Obstet Gynecol. 2000;182(2):364–369 [DOI] [PubMed] [Google Scholar]
- 4.Greene MF, Hare JW, Krache M, et al. Prematurity among insulin-requiring diabetic gravid women. Am J Obstet Gynecol. 1989;161(1):106–111 [DOI] [PubMed] [Google Scholar]
- 5.Evers IM, de Valk HW, Visser GH. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ. 2004;328(7445):915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekbom P, Damm P, Feldt-Rasmussen B, Feldt-Rasmussen U, Mølvig J, Mathiesen ER. Pregnancy outcome in type 1 diabetic women with microalbuminuria. Diabetes Care. 2001;24(10):1739–1744 [DOI] [PubMed] [Google Scholar]
- 7.Bental Y, Reichman B, Shiff Y, et al. ; Israel Neonatal Network . Impact of maternal diabetes mellitus on mortality and morbidity of preterm infants (24–33 weeks’ gestation). Pediatrics. 2011;128(4). Available at: www.pediatrics.org/cgi/content/full/128/4/e848 [DOI] [PubMed] [Google Scholar]
- 8.Grandi C, Tapia JL, Cardoso VC. Impact of maternal diabetes mellitus on mortality and morbidity of very low birth weight infants: a multicenter Latin America study. J Pediatr (Rio J). 2015;91(3):234–241 [DOI] [PubMed] [Google Scholar]
- 9.Rehan VK, Moddemann D, Casiro OG. Outcome of very-low-birth-weight (< 1,500 grams) infants born to mothers with diabetes. Clin Pediatr (Phila). 2002;41(7):481–491 [DOI] [PubMed] [Google Scholar]
- 10.Fong A, Serra A, Herrero T, Pan D, Ogunyemi D. Pre-gestational versus gestational diabetes: a population based study on clinical and demographic differences. J Diabetes Complications. 2014;28(1):29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534 [DOI] [PubMed] [Google Scholar]
- 14.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2). Available at: www.pediatrics.org/cgi/content/full/125/2/e214 [DOI] [PubMed] [Google Scholar]
- 15.Bayley N. Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio, TX: Harcourt Assessment; 2006 [Google Scholar]
- 16.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards: Methods and Development: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age. Geneva, Switzerland: World Health Organization; 2006 [Google Scholar]
- 17.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards: Methods and Development: Head Circumference-for-Age, Arm Circumference-for-Age, Triceps Skinfold-for-Age and Subscapular Skinfold-for-Age. Geneva, Switzerland: World Health Organization; 2007 [Google Scholar]
- 18.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706 [DOI] [PubMed] [Google Scholar]
- 19.Haeri S, Khoury J, Kovilam O, Miodovnik M. The association of intrauterine growth abnormalities in women with type 1 diabetes mellitus complicated by vasculopathy. Am J Obstet Gynecol. 2008;199(3):278.e1–278.e5 [DOI] [PubMed] [Google Scholar]
- 20.Greene MF, Allred EN, Leviton A. Maternal metabolic control and risk of microcephaly among infants of diabetic mothers. Diabetes Care. 1995;18(2):166–169 [DOI] [PubMed] [Google Scholar]
- 21.Cheong JL, Hunt RW, Anderson PJ, et al. Head growth in preterm infants: correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics 2008;121(6). Available at: www.pediatrics.org/cgi/content/full/121/6/e1534 [DOI] [PubMed]
- 22.Kuban KC, Allred EN, O’Shea TM, et al. Developmental correlates of head circumference at birth and two years in a cohort of extremely low gestational age newborns. J Pediatr. 2009;155(3):344–349.e1, e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen DM, Damm P, Ovesen P, et al. Microalbuminuria, preeclampsia, and preterm delivery in pregnant women with type 1 diabetes: results from a nationwide Danish study. Diabetes Care. 2010;33(1):90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovilam O, Khoury J, Miodovnik M, Chames M, Spinnoto J, Sibai B. Spontaneous preterm delivery in the type 1 diabetic pregnancy: the role of glycemic control. J Matern Fetal Neonatal Med. 2002;11(4):245–248 [DOI] [PubMed] [Google Scholar]
- 25.Lepercq J, Coste J, Theau A, Dubois-Laforgue D, Timsit J. Factors associated with preterm delivery in women with type 1 diabetes: a cohort study. Diabetes Care. 2004;27(12):2824–2828 [DOI] [PubMed] [Google Scholar]
- 26.Mimouni F, Miodovnik M, Siddiqi TA, Berk MA, Wittekind C, Tsang RC. High spontaneous premature labor rate in insulin-dependent diabetic pregnant women: an association with poor glycemic control and urogenital infection. Obstet Gynecol. 1988;72(2):175–180 [PubMed] [Google Scholar]
- 27.Hanson U, Persson B, Thunell S. Relationship between haemoglobin A1C in early type 1 (insulin-dependent) diabetic pregnancy and the occurrence of spontaneous abortion and fetal malformation in Sweden. Diabetologia. 1990;33(2):100–104 [DOI] [PubMed] [Google Scholar]
- 28.Georgieff MK. The effect of maternal diabetes during pregnancy on the neurodevelopment of offspring. Minn Med. 2006;89(3):44–47 [PubMed] [Google Scholar]
- 29.Ornoy A. Growth and neurodevelopmental outcome of children born to mothers with pregestational and gestational diabetes. Pediatr Endocrinol Rev. 2005;3(2):104–113 [PubMed] [Google Scholar]
- 30.Georgieff MK, Landon MB, Mills MM, et al. Abnormal iron distribution in infants of diabetic mothers: spectrum and maternal antecedents. J Pediatr. 1990;117(3):455–461 [DOI] [PubMed] [Google Scholar]
- 31.Nelson CA, Wewerka S, Thomas KM, Tribby-Walbridge S, deRegnier R, Georgieff M. Neurocognitive sequelae of infants of diabetic mothers. Behav Neurosci. 2000;114(5):950–956 [PubMed] [Google Scholar]
- 32.Widness JA, Susa JB, Garcia JF, et al. Increased erythropoiesis and elevated erythropoietin in infants born to diabetic mothers and in hyperinsulinemic rhesus fetuses. J Clin Invest. 1981;67(3):637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mimouni F, Miodovnik M, Siddiqi TA, Butler JB, Holroyde J, Tsang RC. Neonatal polycythemia in infants of insulin-dependent diabetic mothers. Obstet Gynecol. 1986;68(3):370–372 [DOI] [PubMed] [Google Scholar]
- 34.Wiswell TE, Robertson CF, Jones TA, Tuttle DJ. Necrotizing enterocolitis in full-term infants. A case-control study. Am J Dis Child. 1988;142(5):532–535 [DOI] [PubMed] [Google Scholar]
- 35.Maayan-Metzger A, Itzchak A, Mazkereth R, Kuint J. Necrotizing enterocolitis in full-term infants: case-control study and review of the literature. J Perinatol. 2004;24(8):494–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson R, del Portillo M, Schmidt E, Feldman RA, Kanto WP Jr. Risk factors for necrotizing enterocolitis in infants weighing more than 2,000 grams at birth: a case-control study. Pediatrics. 1983;71(1):19–22 [PubMed] [Google Scholar]
- 37.Lambert DK, Christensen RD, Henry E, et al. Necrotizing enterocolitis in term neonates: data from a multihospital health-care system. J Perinatol. 2007;27(7):437–443 [DOI] [PubMed] [Google Scholar]
- 38.Thilo EH, Lazarte RA, Hernandez JA. Necrotizing enterocolitis in the first 24 hours of life. Pediatrics. 1984;73(4):476–480 [PubMed] [Google Scholar]
- 39.Bashiri A, Zmora E, Sheiner E, Hershkovitz R, Shoham-Vardi I, Mazor M. Maternal hypertensive disorders are an independent risk factor for the development of necrotizing enterocolitis in very low birth weight infants. Fetal Diagn Ther. 2003;18(6):404–407 [DOI] [PubMed] [Google Scholar]
- 40.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 pt 1):285–291 [DOI] [PubMed] [Google Scholar]