Abstract
OBJECTIVE:
To identify patterns of outpatient care associated with diabetic ketoacidosis (DKA) among pediatric patients with type 1 diabetes (T1D).
METHODS:
Retrospective cohort study using Medicaid claims data from 2009 to 2012 for children with T1D enrolled ≥365 consecutive days in California Children’s Services, a Title V program for low-income children with chronic disease. Outcome was DKA hospitalization >30 days after enrollment. Outpatient visits to primary care, endocrinology, pharmacies, and emergency departments (EDs) were assessed during the 6 months before an index date: either date of first DKA hospitalization or end of enrollment for those without DKA. Univariate and multivariate analysis was used to evaluate independent associations between DKA and outpatient care at clinically meaningful intervals preceding the index date.
RESULTS:
Among 5263 children with T1D, 16.7% experienced DKA during the study period. Patients with DKA were more likely to have had an ED visit (adjusted odds ratio [aOR] 3.99, 95% confidence interval [CI]: 2.60–6.13) or a nonpreventive primary care visit (aOR 1.35, 95% CI: 1.01–1.79) within 14 days before the index date, and less likely to have visited an endocrinologist (aOR 0.76, 95% CI: 0.65–0.89) within the preceding 120 days. Preventive visits and pharmacy claims were not associated with DKA.
CONCLUSIONS:
For children with T1D, recent ED visits and long intervals without subspecialty care are important signals of impending DKA. Combined with other known risk factors, these health-use indicators could be used to inform clinical and case management interventions that aim to prevent DKA hospitalizations.
What’s Known on This Subject:
Diabetic ketoacidosis (DKA) is a dangerous, expensive, and preventable complication of type 1 diabetes. Associations between DKA and outpatient care for pediatric patients with type 1 diabetes are unestablished.
What This Study Adds:
Emergency and acute care visits within a 14-day window are associated with higher odds of subsequent DKA for children with type 1 diabetes. These encounters should serve as time-sensitive signals for case management and prevention efforts.
Type 1 diabetes (T1D) is the third most common chronic disease of childhood.1 More than 15 000 US children are diagnosed with T1D annually, and the incidence is rising.2 Patients with T1D require daily insulin, and lapses in therapy lead to diabetic ketoacidosis (DKA). DKA is the primary cause of morbidity and mortality among children with T1D.3 The standardized cost of medical care for a pediatric DKA hospitalization ranges from $4125 to 11 916,4 not including the additional familial and societal costs associated with missed school and lost days of work. Cumulative costs for the >150 000 annual DKA episodes in the United States make up 25% of the $14.9 billion spent annually on patients with T1D.2,5 This dangerous, costly condition is also largely preventable, with 50% to 75% of DKA after diagnosis attributed to insulin omission and the remainder to suboptimal management of illness.3,5 Among adults with T1D, the Agency for Healthcare Research and Quality considers DKA a prevention quality indicator.6 However, pediatric research has not consistently demonstrated an association between receipt of recommended outpatient services and fewer DKA hospitalizations.7,8 Determining which types and frequencies of ambulatory care are associated with DKA after diagnosis is an essential step toward improved management of pediatric T1D.
Clinical care guidelines for diabetes recommend quarterly subspecialty visits that include ongoing education about diabetes management,9,10 allowing providers to address barriers to insulin adherence and review appropriate sick-day management. Case management interventions that improve adherence to these quarterly visits among adolescents and adults also reduce DKA hospitalizations.11–13 Given these data, as well as literature that children with chronic disease are hospitalized less if they have access to a medical home14 and fewer missed well-child visits,15 we hypothesized that less frequent visits to endocrinology and preventive care would be associated with DKA. We also sought to examine associations between DKA and nonpreventive primary care, emergency department (ED), and pharmacy encounters because these visits represent access to time-sensitive care that might prevent hospitalization.
Methods
Study Design
We conducted a retrospective cohort study of children with T1D enrolled in California Children’s Services (CCS) between July 1, 2009, and June 30, 2012. CCS is a Title V program serving children with chronic disease and low family income in the state of California.
Data Source
We obtained paid claims data for children enrolled in CCS for fiscal years 2009 to 2012 from the California Department of Health Care Services. These data include enrollment dates, eligibility diagnoses, demographics, and claim-specific diagnoses for every eligible child. Institutional review board approval with a consent waiver was received from Stanford University and the California Department of Health Care Services.
Study Population
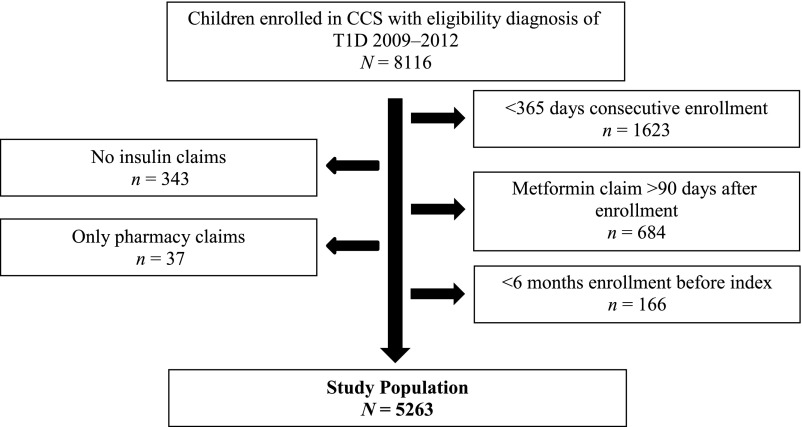
Initial inclusion criterion was enrollment in CCS between July 1, 2009, and June 30, 2012 with an eligibility diagnosis of T1D, encompassing International Classification of Diseases, Ninth Revision (ICD-9) codes 250.x1 and 250.x3 with x = 0 to 9. We then excluded children who had (1) enrollment in CCS for <365 consecutive days during the study period; (2) no pharmacy claim for insulin during the study period; or (3) a pharmacy claim for metformin >90 days after enrollment, suggesting an inaccurate diagnosis of T1D16; (4) only pharmacy claims, suggesting that outpatient visits were billed to another insurer; or (5) no identifiable index date. Index date was designated as the first DKA hospitalization with ≥6 months of DKA-free preceding enrollment or as the date of study completion for those without any DKA hospitalizations. This yielded a study population of 5263 children (see Fig 1) ranging in age from infancy to 21 years, which is the age limit for CCS coverage.
FIGURE 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram depicting selection of the study population.
Outcome
The outcome of interest was any DKA episode that took place >30 days after diagnosis with T1D, which in this study was approximated by the date of enrollment in CCS. Our study measured only DKA episodes severe enough to require hospitalization (inpatient claims with ICD-9 codes 250.10–250.13).
Exposure to Outpatient Care
Outpatient care was assessed in 2 domains: (1) maintenance care, consisting of preventive visits, endocrinology visits, and pharmacy insulin claims, and (2) acute care, consisting of ED visits and nonpreventive visits to primary care. Endocrinology visits were defined as those involving (1) a National Provider Identifier (NPI) taxonomy of 2080P0205X (Pediatric Endocrinology) or 207RE0101X (Endocrinology & Diabetes & Metabolism), (2) a billing clinic with endocrinology or diabetes as part of the clinic name, and/or (3) an individual provider with ≥10 outpatient claims in our data set who indicated a specialty of endocrinology or diabetes on a publicly available Internet source of provider-supplied information.17
Preventive visits were defined as those with Current Procedural Terminology code 99381 to 99385, 99391 to 99395, 99201 to 99215, or ICD-9 code V202, V203, V700, or V705 and a nonendocrinology provider. Nonpreventive visits to primary care were defined as those without the preceding preventive codes and with a primary care NPI taxonomy (see Supplemental Table 5 in online supplement). Among pharmacy encounters, only those involving insulin supplies (identified via Healthcare Common Procedure Coding System and National Drug Codes) were included in the analysis. ED and nonpreventive visits occurring within 1 day before the index date were excluded from the analysis to avoid capturing encounters associated with DKA hospitalization. In addition, we identified the subset of ED visits that were not associated with a DKA diagnostic code (ICD-9 250.10–250.13). Time to event for each type of outpatient care was defined as the number of days between the index date and the most proximal preceding outpatient encounter of that type.
Covariates
Patient-level variables including age, gender, race/ethnicity, insurance status, and mental health comorbidity were extracted from CCS data due to associations with DKA in previous studies.8,18–21 Age was measured at enrollment (or July 1, 2009, for those enrolled before this date). Race/ethnicity was based on parent report at time of enrollment, and only 1 category could be designated per child. Insurance status was categorized based on CCS definitions: MediCal (California’s Medicaid program) with managed care, MediCal without managed care, Healthy Families (California’s State Children’s Health Insurance Program), CCS only (uninsurable by MediCal or Healthy Families), or mixed. Healthy Families income eligibility is up to 250% of federal poverty level (FPL), whereas MediCal’s is 100% to 200% of FPL depending on the child’s age. Mental health comorbidity was defined as ≥1 inpatient or ≥2 outpatient claims associated with ICD-9 codes 290.xx to 319.xx. These codes include major mood disorders, anxiety disorders, eating disorders, and substance disorders, which have all been associated with T1D in pediatric studies.22,23 The characteristics of our study population are displayed in Table 1.
TABLE 1.
Study Population Characteristics
| Covariate | Study Population | Without DKA | With DKA | P | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Population size | 5263 | 100.0 | 4385 | 83.3 | 878 | 16.7 | |
| Age, y | |||||||
| 0–6 | 702 | 13.3 | 630 | 14.4 | 72 | 8.2 | <.001 |
| 7–12 | 1931 | 36.7 | 1628 | 37.1 | 303 | 34.5 | |
| 13–21 | 2630 | 50.0 | 2127 | 48.5 | 503 | 57.3 | |
| Gender | |||||||
| Female | 2609 | 49.6 | 2133 | 48.6 | 476 | 54.2 | .003 |
| Male | 2654 | 50.4 | 2252 | 51.4 | 402 | 45.8 | |
| Race/ethnicity | |||||||
| White | 1676 | 31.8 | 1407 | 32.1 | 269 | 30.6 | <.001 |
| Black | 506 | 9.6 | 376 | 8.6 | 130 | 14.8 | |
| Hispanic | 2530 | 48.1 | 2113 | 48.2 | 417 | 47.5 | |
| American Indian | 56 | 1.1 | 46 | 1.0 | 10 | 1.1 | |
| Asian/Pacific Islander | 154 | 2.9 | 134 | 3.1 | 20 | 2.3 | |
| Other | 228 | 4.3 | 204 | 4.7 | 24 | 2.7 | |
| Unknown | 113 | 2.1 | 105 | 2.4 | 8 | 0.9 | |
| Insurance | |||||||
| MediCal Managed Care | 2469 | 46.9 | 1982 | 45.2 | 487 | 55.5 | <.001 |
| MediCal No Managed Care | 524 | 10.0 | 420 | 9.6 | 104 | 11.8 | |
| Healthy Families | 618 | 11.7 | 559 | 12.7 | 59 | 6.7 | |
| CCS only | 141 | 2.7 | 133 | 3.0 | 8 | 0.9 | |
| Mixed | 1511 | 28.7 | 1291 | 29.4 | 220 | 25.1 | |
| Mental health comorbidity | 1033 | 19.6 | 717 | 16.4 | 316 | 36.0 | <.001 |
Percentages for population size refer to the row. All other percentages refer to the respective column. P values calculated using Pearson’s χ2 test.
Statistical Analysis
We identified outpatient visits to primary care, endocrinology, pharmacies, and EDs during the 6 months before the index date for each child and then assessed the percentages of those with and without DKA who experienced each encounter type during this period. Next we examined exposure to care within shorter, clinically meaningful time frames: nonpreventive visits to primary care or EDs within 14 days before the index, pharmacy claims within 90 days before the index, and endocrinology visits or preventive visits within 120 days before the index date. Multiple logistic regression was used to assess the adjusted odds ratio (aOR) of DKA given exposure to each of these care types within its specified time range, adjusting for covariates that varied significantly between groups in Table 1.
Results
Our study population included 5263 children with T1D, of whom 878 (16.7%) were hospitalized for DKA during the study period (see Table 1). Patients with DKA were more likely to be >12 years old (57.3% vs 48.5%, P < .001), female (54.2% vs 48.6%, P = 0.003), black (14.8% vs 8.6%, P < .001), enrolled in MediCal managed care (55.5% vs 45.2%, P < .001), and to have mental health comorbidity (36.0% vs 16.4%, P < .001). Among these demographic factors, all except black race/ethnicity remained significantly associated with DKA after adjustment via logistic regression (see Table 2). These findings mirror those of previous studies that have associated DKA with age, gender, race/ethnicity, insurance status, and mental health comorbidities.8,18–21
TABLE 2.
Adjusted Odds of DKA With Demographic Risk Factors
| Covariate | Without DKA (n = 4385), % | With DKA (n = 878), % | Unadjusted OR (95% CI) | aOR (95% CI)a |
|---|---|---|---|---|
| Age >12 y | 48.5 | 57.3 | 1.42 (1.23–1.65) | 1.37 (1.18–1.60) |
| Female gender | 48.6 | 54.2 | 1.25 (1.08–1.45) | 1.30 (1.12–1.51) |
| Black race/ethnicity | 8.6 | 14.8 | 0.97 (0.84–1.13) | 1.02 (0.88–1.18) |
| MediCal managed care | 45.2 | 55.5 | 1.51 (1.31–1.75) | 1.41 (1.21–1.64) |
| Mental health comorbidity | 16.4 | 36.0 | 2.88 (2.45–3.37) | 2.73 (2.32–3.21) |
Percentages refer to the percentage of patients in a given column with the specified covariate.
Adjusted by multivariate logistic regression for covariates listed in table.
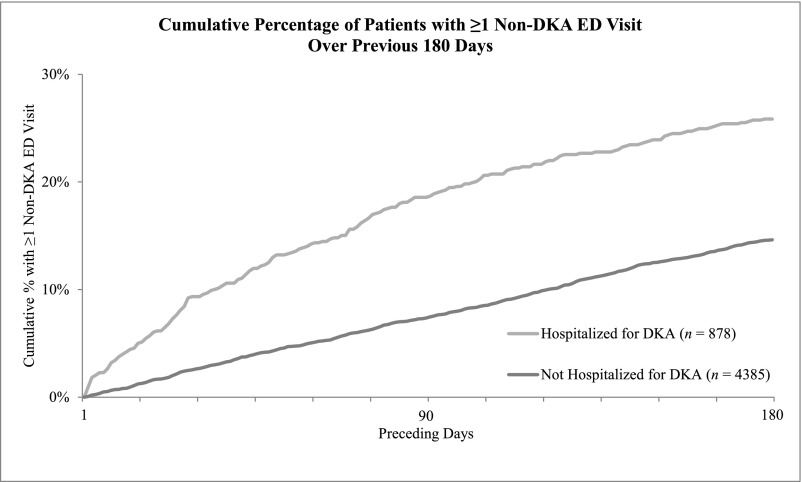
Children with DKA were more likely than those without DKA to have had ED visits as well as preventive and nonpreventive primary care visits during the 6 months before the index date and less likely to have visited endocrinology during that time frame (see Table 3). Among these differences, the disparity in ED use was most dramatic, and children with DKA were twice as likely to have visited the ED compared with their DKA-free counterparts (30.4% vs 14.8%). This difference remained after excluding ED visits associated with DKA and persisted throughout the 6 months (see Fig 2).
TABLE 3.
Prevalence of Outpatient Care Within Previous 6 Months
| Outpatient Care Within Previous 6 Months | Without DKA (4385) | With DKA (878) | P | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Acute care | |||||
| ED visita | 651 | 14.8 | 267 | 30.4 | <.001 |
| Non-DKA EDa,b | 627 | 14.3 | 227 | 25.9 | <.001 |
| Nonpreventive visita | 1701 | 38.8 | 378 | 43.1 | .02 |
| Maintenance care | |||||
| Endocrinology visita | 2789 | 63.6 | 488 | 55.6 | <.001 |
| Preventive visita | 1728 | 39.4 | 419 | 47.7 | <.001 |
| Insulin claim | 4078 | 93.0 | 813 | 92.6 | .69 |
n and % indicate the number and percentage, respectively, of patients in each group who experienced the event. P values calculated using Pearson’s χ2 test.
Visits within 1 day of the index date were excluded to avoid capturing encounters that led directly to hospitalization.
ED visits with DKA diagnostic codes (ICD-9 250.10–250.13) were excluded.
FIGURE 2.
Cumulative percentages of patients with and without DKA hospitalization experiencing an ED visit during the previous 180 days. Visits within 1 day of the index date were excluded to avoid capturing encounters that led directly to hospitalization.
In adjusted analyses, increased odds of DKA was associated with ED visits (aOR 3.99, 95% confidence interval [CI]: 2.60–6.13), including after exclusion of visits for DKA (aOR 3.36, 95% CI: 2.15–5.27), and nonpreventive visits to primary care (aOR 1.35, 95% CI: 1.01–1.79) during the 14 days before the index date. Multivariate analysis also revealed that patients with DKA were less likely than those without to have visited an endocrinologist during the 120 days before the index date (aOR 0.76, 95% CI: 0.65–0.89). Adjusted odds of a preventive visit within the preceding 120 days and of a pharmacy claim within the preceding 90 days did not differ between the 2 groups (see Table 4).
TABLE 4.
Adjusted Odds of DKA With Preceding Outpatient Care
| Outpatient Care | Without DKA (4385) | With DKA (878) | Unadjusted OR (95% CI) | aORa (95% CI) | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Acute care | ||||||
| ED visit within previous 14 db | 48 | 1.1 | 49 | 5.6 | 5.34 (3.56–8.01) | 3.99 (2.60–6.13) |
| Non-DKA ED visit within previous 14 db,c | 48 | 1.1 | 42 | 4.8 | 4.19 (2.73–6.46) | 3.36 (2.15–5.27) |
| Nonpreventive visit within previous 14 db | 246 | 5.6 | 72 | 8.2 | 1.51 (1.15–1.98) | 1.35 (1.01–1.79) |
| Maintenance care | ||||||
| Endocrinology visit within previous 120 d | 2429 | 55.4 | 425 | 48.4 | 0.76 (0.65–0.87) | 0.76 (0.65–0.89) |
| Preventive visit within previous 120 d | 1351 | 30.8 | 332 | 37.8 | 1.36 (1.17–1.59) | 1.16 (0.98–1.37) |
| Insulin claim within previous 90 d | 3745 | 85.4 | 734 | 83.6 | 0.87 (0.72–1.06) | 0.93 (0.75–1.15) |
n and % indicate the number and percentage, respectively, of patients in each group who experienced the event.
Adjusted by multivariate logistic regression for age >12 y, female gender, black race/ethnicity, MediCal managed care, and mental health comorbidity.
Visits within 1 day of index date were excluded to avoid capturing encounters that led directly to hospitalization.
ED visits with DKA diagnostic codes (ICD-9 250.10–250.13) were excluded.
Further analysis of our population in 3 subgroups (ie, those with 0, 1, and ≥2 DKA hospitalizations during the study period) demonstrated the same associations between DKA and patient characteristics listed in Table 1 (see Supplemental Table 6 in the online supplement). In addition, this stratified analysis showed that patients with 1 and those with ≥2 DKA admissions were both more likely to have visited an ED in the 14 days before and less likely to have visited an endocrinologist in the 120 days before the index date, compared with patients without DKA admissions (see Supplemental Table 7 in online supplement).
Discussion
Outpatient care utilization differs between diabetic patients with DKA hospitalizations and those without, particularly during the weeks and months preceding DKA events. ED visits, and, to a lesser degree, nonpreventive primary care visits, signal an increased likelihood of DKA during the subsequent 14 days, while missing a quarterly endocrinology visit is also associated with higher odds of DKA hospitalization, even after adjustment for known risk factors.
Our most important finding is that ED and acute care visits for children with T1D are associated with DKA hospitalization within a 14-day time frame because it represents a novel association in the diabetes literature. Emergency or acute care visits have capacity to prevent DKA via insulin refills, dose review, attention to social issues or care barriers, and communication with primary providers. Thus, it is interesting that they were associated with higher rather than lower odds of DKA hospitalization. Additional research is needed to determine if this is because diabetes care was not addressed at these encounters or rather because the underlying precipitating factors for DKA overwhelmed any prevention efforts.
Our finding that missed endocrinology visits are associated with DKA reinforces a 1997 study by the Joslin Diabetes Center, which showed that irregular outpatient diabetes care was associated with higher rates of DKA among youth with T1D.7 However, it contradicts an analysis by Denver’s Barbara Davis Center in 2002, which found no association between frequency of clinic visits and DKA.8 Differences in patient populations and follow-up practices might explain this disparity (for example, if efforts by the Denver group to see high-risk patients at shorter intervals eliminated this association within their data). This type of analysis should be replicated in a variety of patient populations and practice environments to determine if the same association holds.
In addition, our study suggests that a large proportion of diabetic children are not meeting the recommendation for quarterly subspecialty visits. Even among patients without DKA, only 55.4% had seen an endocrinologist within 120 days and only 63.6% within 6 months before the index date. On the basis of previous publications from Joslin and Barbara Davis, the frequency of diabetes visits may vary widely between patient populations. For example, in the former analysis 20% of patients had <1 endocrinology visit per year,7 and in the latter, only 9.7% to 17.5% of patients had <2 visits per year.8 In our population, the low rate of subspecialty visits suggests that (1) recommended diabetes visits were missed, (2) endocrinologists extended care intervals for well-controlled patients, and/or (3) nonendocrinologists primarily managed a subset of T1D patients. The last scenario is supported by another analysis of CCS data, which indicates that 16.2% of T1D patients in CCS receive their diabetes care from primary care providers and another 10.8% from providers of other or unknown specialties (S.V. Kaiser, V. Sundaram, E. Cohen, R. Shulman, L. Sanders, A. Guttman, unpublished data, 2016).
Another unexpected finding was that MediCal managed care was significantly associated with DKA in our population after adjustment for demographic risk factors (see Table 2). Associations between DKA and female gender, adolescent age, mental health comorbidity, and public insurance are established in the literature,8,21 but to our knowledge, this association with managed care has not been described. In our population, a child was classified as managed care if all claims were listed as such, including before and after DKA events. Therefore, this association cannot reflect children switching to managed care after DKA events as a means to prevent recurrence. However, we cannot rule out that patients deemed high risk for other reasons were enrolled proactively in managed care at time of diagnosis. Further discussion with CCS administrators is needed to explore this and other potential explanations for the association between managed care and DKA hospitalizations.
As with any study using paid claims, our results are limited by the integrity, consistency, and completeness of administrative data. Inaccuracies in diagnostic codes or NPI taxonomies, or omissions of claims for certain encounter types, could significantly affect our results. However, these are likely to result in type II rather than type I errors. Our data are also limited to Medicaid claims, which do not capture care provided for free or billed to alternate insurance. For the CCS population, this is unlikely to represent a significant portion of encounters for DKA hospitalizations, insulin refills, or outpatient care because the program covers these comprehensively. Finally, this analysis cannot incorporate clinical data such as hemoglobin A1C, insulin doses, or disease duration, which are known to associate with DKA incidence.8,21 Our study may also be subject to selection bias in the context of population health because it focuses on a state program for low-income families. As such, our analysis uses an “enriched” sample for DKA risk, which may explain in part the higher rate of DKA in our population (16.7% over a 3-year period) compared with rates of 9.9%21 and 15.7%8 over 1 and 5 years, respectively, in previous US studies. Data from European countries often demonstrate lower rates of DKA24,25 but are difficult to compare with the United States due to differences in health care financing, delivery, and access.
Conclusions
In this vulnerable population of low-income children with T1D, odds of DKA are related not only to demographic and clinical risk factors but also to ambulatory care preceding DKA events. This has implications for clinical care, population health management, and health policy. At a clinical level, ED visits and nonpreventive primary care visits signal an increased likelihood of DKA during the following 2 weeks and should trigger timely outpatient interventions to prevent hospitalization. Similarly, missed visits to endocrinology should activate less urgent case management strategies to prevent DKA, such as renewed education, care coordination, and patient- or family-focused behavioral interventions.26 At a population level, outpatient care utilization should be incorporated into predictive analytic models so that programs like CCS can focus limited human and financial resources on patients during the time when they are at highest risk. In addition, our findings that a large percentage of type 1 patients with diabetes may be receiving diabetes care from nonendocrinologists and that method of care delivery (eg, managed care) is associated with odds of DKA deserve further exploration, given the implications for health policy and system redesign. In our current era of value-based care, this type of analysis is essential to the development of innovative care models that can improve the effectiveness of individual providers caring for children with T1D and the efficiency of the health care systems designed to serve them.
Acknowledgments
We thank the California Department of Health Care Services for providing the claims data for this analysis.
Glossary
- aOR
adjusted odds ratio
- CCS
California Children’s Services
- CI
confidence interval
- DKA
diabetic ketoacidosis
- ED
emergency department
- ICD-9
International Classification of Diseases, Ninth Revision
- NPI
National Provider Identifier
- T1D
type 1 diabetes mellitus
Footnotes
Dr Crossen conceptualized and designed the study, interpreted the data, drafted the initial manuscript, and critically reviewed and revised the manuscript; Dr. Wilson assisted with study conceptualization and design and critically reviewed and revised the manuscript; Ms Saynina contributed to the study design, carried out the data analyses, and critically reviewed the manuscript; Dr. Sanders helped to conceptualize and design the study, facilitated access to the data, aided in analysis and interpretation of results, and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Ramsay Fellowship in Pediatric Translational Medicine; the Lucile Packard Foundation for Children's Health, Stanford NIH-NCATS-CTSA UL1 TR0001085 and Child Health Research Institute of Stanford University; California HealthCare Foundation Grant 17591. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Stanescu DE, Lord K, Lipman TH. The epidemiology of type 1 diabetes in children. Endocrinol Metab Clin North Am. 2012;41(4):679–694 [DOI] [PubMed] [Google Scholar]
- 2.Type 1 Diabetes Facts. Available at: http://jdrf.org/about-jdrf/fact-sheets/type-1-diabetes-facts/. Accessed January 5, 2015
- 3.Dunger DB, Sperling MA, Acerini CL, et al. ; European Society for Paediatric Endocrinology; Lawson Wilkins Pediatric Endocrine Society . European Society for Paediatric Endocrinology/Lawson Wilkins Pediatric Endocrine Society consensus statement on diabetic ketoacidosis in children and adolescents. Pediatrics. 2004;113(2). Available at: www.pediatrics.org/cgi/content/full/113/2/e133 [DOI] [PubMed] [Google Scholar]
- 4.Tieder JS, McLeod L, Keren R, et al. ; Pediatric Research in Inpatient Settings Network . Variation in resource use and readmission for diabetic ketoacidosis in children’s hospitals. Pediatrics. 2013;132(2):229–236 [DOI] [PubMed] [Google Scholar]
- 5.Bismuth E, Laffel L. Can we prevent diabetic ketoacidosis in children? Pediatr Diabetes. 2007;8(suppl 6):24–33 [DOI] [PubMed] [Google Scholar]
- 6.AHRQ Quality Indicators. Guide to Prevention Quality Indicators. Hospital Admission for Ambulatory Care Sensitive Conditions. Rockville, MD: Agency for Healthcare Research and Quality; 2001 [Google Scholar]
- 7.Jacobson AM, Hauser ST, Willett J, Wolfsdorf JI, Herman L. Consequences of irregular versus continuous medical follow-up in children and adolescents with insulin-dependent diabetes mellitus. J Pediatr. 1997;131(5):727–733 [DOI] [PubMed] [Google Scholar]
- 8.Rewers A, Chase HP, Mackenzie T, et al. Predictors of acute complications in children with type 1 diabetes. JAMA. 2002;287(19):2511–2518 [DOI] [PubMed] [Google Scholar]
- 9.Chiang JL, Kirkman MS, Laffel LM, Peters AL; Type 1 Diabetes Sourcebook Authors . Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pihoker C, Forsander G, Wolfsdorf J, Klingensmith GJ. The delivery of ambulatory diabetes care to children and adolescents with diabetes. Pediatr Diabetes. 2009;10(suppl 12):58–70 [DOI] [PubMed] [Google Scholar]
- 11.Holmes-Walker DJ, Llewellyn AC, Farrell K A transition care programme which improves diabetes control and reduces hospital admission rates in young adults with Type 1 diabetes aged 15–25 years. Diabet Med. Jul 2007;24(7):764–769 [DOI] [PubMed]
- 12.Maldonado MR, D’Amico S, Rodriguez L, Iyer D, Balasubramanyam A Improved outcomes in indigent patients with ketosis-prone diabetes: effect of a dedicated diabetes treatment unit. Endocr Pract 2003;9(1):26–32 [DOI] [PubMed]
- 13.Laffel LM, Brackett J, Ho J, Anderson BJ. Changing the process of diabetes care improves metabolic outcomes and reduces hospitalizations. Qual Manag Health Care. 1998;6(4):53–62 [DOI] [PubMed] [Google Scholar]
- 14.Cooley WC, McAllister JW, Sherrieb K, Kuhlthau K. Improved outcomes associated with medical home implementation in pediatric primary care. Pediatrics. 2009;124(1):358–364 [DOI] [PubMed] [Google Scholar]
- 15.Tom JO, Tseng CW, Davis J, Solomon C, Zhou C, Mangione-Smith R. Missed well-child care visits, low continuity of care, and risk of ambulatory care-sensitive hospitalizations in young children. Arch Pediatr Adolesc Med. 2010;164(11):1052–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanderloo SE, Johnson JA, Reimer K, et al. Validation of classification algorithms for childhood diabetes identified from administrative data. Pediatr Diabetes. 2012;13(3):229–234 [DOI] [PubMed] [Google Scholar]
- 17.Health Grades Available at: http://www.healthgrades.com/find-a-doctor. Accessed multiple dates 2014–2015
- 18.Delamater AM, Albrecht DR, Postellon DC, Gutai JP. Racial differences in metabolic control of children and adolescents with type I diabetes mellitus. Diabetes Care. 1991;14(1):20–25 [DOI] [PubMed] [Google Scholar]
- 19.Alvi NS, Davies P, Kirk JM, Shaw NJ. Diabetic ketoacidosis in Asian children. Arch Dis Child. 2001;85(1):60–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith CP, Firth D, Bennett S, Howard C, Chisholm P. Ketoacidosis occurring in newly diagnosed and established diabetic children. Acta Paediatr. 1998;87(5):537–541 [DOI] [PubMed] [Google Scholar]
- 21.Cengiz E, Xing D, Wong JC, et al. ; T1D Exchange Clinic Network . Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D Exchange clinic registry. Pediatr Diabetes. 2013;14(6):447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein CM, Stockwell MS, Gallagher MP, Rosenthal SL, Soren K. Mental health issues in adolescents and young adults with type 1 diabetes: prevalence and impact on glycemic control. Clin Pediatr (Phila). 2013;52(1):10–15 [DOI] [PubMed] [Google Scholar]
- 23.Kakleas K, Kandyla B, Karayianni C, Karavanaki K. Psychosocial problems in adolescents with type 1 diabetes mellitus. Diabetes Metab. 2009;35(5):339–350 [DOI] [PubMed] [Google Scholar]
- 24.de Beaufort CE, Swift PG, Skinner CT, et al. ; Hvidoere Study Group on Childhood Diabetes 2005 . Continuing stability of center differences in pediatric diabetes care: do advances in diabetes treatment improve outcome? The Hvidoere Study Group on Childhood Diabetes. Diabetes Care. 2007;30(9):2245–2250 [DOI] [PubMed] [Google Scholar]
- 25.Fritsch M, Rosenbauer J, Schober E, Neu A, Placzek K, Holl RW; German Competence Network Diabetes Mellitus and the DPV Initiative . Predictors of diabetic ketoacidosis in children and adolescents with type 1 diabetes. Experience from a large multicentre database. Pediatr Diabetes. 2011;12(4 pt 1):307–312 [DOI] [PubMed] [Google Scholar]
- 26.Wagner DV, Stoeckel M, E Tudor M, Harris MA. Treating the most vulnerable and costly in diabetes. Curr Diab Rep. 2015;15(6):606. [DOI] [PubMed] [Google Scholar]