Abstract
In the present study we used a fusion peptide from HIV-1 p24 and Nef as vaccine model and adjuvant activity of Naloxone/alum mixture was evaluated in a peptide vaccine model. HIV-1 p24-Nef fusion peptide was synthesized. Female BALB/c mice were divided into five groups. The first group immunized subcutaneously with the p24-Nef fusion peptide adjuvanted with Naloxone/alum mixture and boosted with same protocol. The second was immunized with fusion peptide adjuvanted in alum. The control groups were injected with NLX (Group 3), Alum (Group 4), or PBS (Groups 5) under the same conditions. To determine the type of induced immune response, sera and splenocytes were analyzed by commercial ELISA method for total IgG and isotypes and cytokine secretion (IL-4 & IFN-γ), respectively. We have also used the ELISPOT assay to monitor changes in the frequency of IFN-γ-producing T cells. The proliferation of T cells was assessed using Brdu method and T-cell cytotoxicity was assessed with CFSE method. Immunization of mice with HIV-1 p24-Nef fusion peptide formulated in Naloxone/alum mixture significantly increased lymphocyte proliferation and shifted cytokine responses toward Th1 profile compared to all other groups. Analysis of humoral immune responses revealed that administration of HIV-1 p24-Nef fusion peptide with Naloxone/alum mixture significantly increased specific IgG responses and also increased IgG1,IgG2a, IgG2b, IgG3, and IgM vs. alum-adjuvanted vaccine groups. Naloxone/alum mixture as an adjuvant could improve cellular and humoral immune response for HIV vaccine model and this adjuvant maybe useful for HIV vaccine model in human clinical trial.
Keywords: Naloxone/alum mixture, Adjuvant, HIV-1, Vaccine
Introduction
In the 21st century, the nature of vaccines is changing and, because of vaccine reactogenicity, new vaccine formulations are rapidly shifting from whole cell-based vaccines to more defined protective antigens. In that condition, vaccines show lower immunogenicity as well as the reduced protectivity effect.1 An adjuvant is able to increase the immunogenicity of such vaccines, and comprises substances that could increase immune responses against vaccines.2 Nowadays, a wide spectrum of materials is used as adjuvants in combination with candidate vaccines. However, because of the toxicity and side effects of the adjuvants, (aluminum-based mineral salt) alum and, more recently, some limited numbers were approved by the US Food and Drug Administration for human vaccination.3 Therefore, it is highly demanded to develop a new adjuvant with lower toxicity and side effects, being able to be reliably used in human vaccine industry. Several studies have recently shown that opioids could modulate immune cells via their receptors present on the immune system cells, such as lymphocytes, macrophages and dendritic cells (DCs). A lot of studies have been conducted on the effect of opioid materials on the operation of immune system against antigens and microbial infections.4 Meanwhile, a great number of studies have demonstrated that endogenous opioids play critical roles in skewing the immune system in a desired direction. Importantly, competitive inhibition of opioids by inhibitors such as naloxone (NLX) medicine leads to skew the immune responses toward the IFN-γ production and cellular immune platform.4 Since the use of naloxone medicine in human is approved by WHO, naloxone is demonstrated to be safe for human use. However, it is widely accepted that naloxone can skew the response profiles from Th2 toward Th1 polarization, considered as an immunologic advantage.5 Our previous study showed that naloxone can stimulate Th1 immune responses, but no Th2 and humoral immune responses.4 By definition, the successful adjuvant should be able to effectively enhance both cellular and humoral immune responses as well as remove or control viral and intracellular pathogen infections.6 To this end, the use of modified naloxone or a mixture of naloxone with a Th2 polarizing adjuvant may be a useful strategy to achieve a suitable adjuvant. Although a well-defined adjuvant for human immunization, being able to trigger humoral immune response (Th2), alum fails to induce the cellular immune response (Th1) which is important for immune responses against a variety of intracellular pathogens.7 Modifying the alum adjuvant formulation to achieve a mixture of Th1/Th2 immune response, can also simultaneously elicit cellular and humoral immune responses against vaccines. Over the last century, the use of vaccines has intensely reduced the mortality rate caused by infectious diseases.2,3 The HIV infection is a major health problem in the developing countries and protective vaccine against this infection remains elusive.8 Therefore, development of an effective HIV vaccine may control the HIV infection worldwide.9 Because adjuvants constitute an important component of vaccines, the discovery of more potent adjuvants may allow the development of prophylactic and therapeutic vaccines against infectious diseases such as HIV-1. As a helper factor, adjuvants are able to increase vaccine efficacy through various known and unknown mechanisms. The development of new adjuvant formulations can optimize a variety of candidate vaccines. Therefore, there is a critical need for the development of novel and improved vaccine adjuvants.10 Herein, the NLX/alum mixture was used as an adjuvant for a multiepitopic HIV-1 vaccine candidate, and assessed cellular and humoral immune responses to evaluate the adjuvanticity of the NLX/alum adjuvant in comparison with alum.
Materials and Methods
Mice
Six- to eight-week-old female BALB/c mice were purchased from Pasteur Institute of Iran (Karaj, Iran). The mice were housed for one week before the experiments, given free access to food and water and maintained in a light/dark cycle with lights on from 6:00 am to 6:00 pm the handling of the mice were carried out with an expert technician and in accordance with the Animal Care and Use Protocol of Pasteur Institute of Iran.
HIV-1p24-Nef fusion peptide
An HIV-1P24-Nef fusion peptide from p24 (aa159–173) and Nef (aa102–117) proteins was synthesized according to the solid-phase method by GL Biochem Company (Shanghai, China). The peptide was purified using HPLC to obtain a purity exceeding 95%.
NLX and vaccine preparation
The candidate vaccine was prepared in alum adjuvant (aluminum hydroxide, Razi vaccine and serum research institute, Iran) with or without NLX (Sigma, Germany) at a concentration of 6 mg/kg in sterile condition. For this purpose, the HIV-1 P24-Nef fusion peptide was dissolved in sterile PBS and then mixed with alum and NLX. According to our experimental setup, the mixture was putted for 60 min at room temperature condition to absorb the peptide on the alum gel particle. Each 200-μl mixture contained 6 mg/kg of NLX (20 gr per mouse) and 20 μg of candidate vaccine.
Experimental groups and immunization
The mice were divided into five groups containing 10–12 mice in each. The first group of mice was subcutaneously immunized on day 0 with 200 μl containing 20 μg of the vaccine candidate formulated in NLX (6 mg/kg) and alum adjuvant (Al-NLX-Vac, n = 12). The fusion peptide formulated in alum adjuvant was injected to second group (Al-Vac, n = 12). As control groups, some mice were injected with NLX (n = 10) or Alum (n = 10) or PBS (n = 10) buffer in the same condition. The mice were boosted on days 21 and 42 with the same condition.
Lymphocyte proliferation assay
Three weeks after third immunization, the spleens of the immunized mice were removed under sterile conditions and suspended in sterile cold PBS. RBCs were lysed with lysis buffer and single-cell suspension was adjusted to 2 × 106 cells per milliliters in RPMI 1640 (Gibco) supplemented with 5% FBS, 4 mM L-glutamine, 1 mM sodium pyrovate, 50 μM 2ME, 100 μg/ml streptomycin and 100 IU/ml penicillin. Then, 100 μl of diluted cell suspensions were dispensed into 96-well flat-bottom culture plates (Nunc) and stimulated with 10 μg/ml of the fusion peptide. Phytohemagglutinin-A (PHA) (5 μg/ml, Gibco), un-stimulated wells and culture medium were used as a positive control, negative control, and a blank, respectively. All experiments were done in triplicate. After 72 h of cell culture, 20 μl of Brdu (Roche, Germany) was added to each well and the plates were further incubated at 37 °C for 18 h. After incubation, the plates were centrifuged at 300 g for 10 min, the supernatant was aspirated carefully, the plates were dried and subsequently 200 μl of Fixation/denaturation buffer was added to each well for 30 min. The plates were aspirated and 100 μl of anti-Brdu was added for 2 h. Afterwards, the plates were washed 5 times with PBS, the TMB substrate was added to wells and incubated for 5 min in the dark at room temperature, and reaction was stopped with adding 100 μl of 2 N H2SO4. The absorbance at A450 was measured for each well. The OD of the blank wells was subtracted from all other wells and then stimulation index (SI) was calculated according to the formula: SI = A450 of the stimulated wells/A450 of the un-stimulated wells.
In vivo CTL assay
To detect the cytotoxicity of the candidate vaccine, the carboxyfluorescein succinimidyl ester (CFSE, Invitrogen, USA) method was used in this study.11 Splenocytes from naive mice were stained 5 μM (high intensity) or 0.5 μM (low intensity) of CFSE. The high-intensity CFSE-labeled cells were loaded with the relevant peptide at a concentration of 20 μg/ml and low-intensity CFSE-stained cells (0.5 μM) were used as a peptide un-pulsed control. They were then transferred intravenously (3 × 106 cells of each one) into the experimental groups of mice. Twenty hours later, lymphocytes were isolated from spleen and resuspended, RBCs were lysed and then flowcytometry analysis carried out to demonstrate the difference in separation pattern based on intensity of CFSE staining. The percent killing of the high- and low-intensity CFSE-labeled cells were calculated and specific killing of HIV-1 P24-Nef-pulsed (CFSEhigh) target cells was calculated as follows:
ELISA of cytokines
Three weeks after the second boosting, a total number of 4 × 106 spleen cells were seeded on each well of a 24-well plate using complete RPMI 1640, stimulated in vitro with 10 μg/ml of the p24-Nef fusion peptide and incubated at 37 °C in 5% CO2 and in the other wells un-stimulated samples were prepared. Seventy-two hours post antigen recall, supernatants were removed and the concentration of IFN-γ and IL-4 was estimated by ELISA Kit (Quantikine, R&D Systems, USA) according to the manufacturer’s instruction. The pg/ml of each sample was calculated according to the standard curve and the absolute cytokine production of each mouse was calculated with subtract of the stimulated well with un-stimulated one.
IFN-γ ELISPOT assay
The frequency of IFN-γ-producing splenocytes was analyzed with the ELISPOT assay (Mabtech, Stockholm, Sweden). Briefly, a total number of 4 × 105 spleen cells were seeded on each well of 96-well and in vitro stimulation was carried out with 10 μg/ml of P24-Nef peptide, as positive control cells stimulated with PHA and wells containing un-stimulated cells and RPMI 1640 were used as negative controls. The plates were incubated at 37 °C in 5% CO2 for 24 h. After in vitro re-stimulation, the plates were washed five times with washing buffer and 100 μl of anti-mouse IFN-γ in PBS containing 0.5% FBS was added to the wells and incubated for 2 h at room temperature. The plates were washed five times with washing buffer and incubated for 1hr at room temperature with 100 μl of 1/1000 diluted streptavidine-conjugated alkaline phosphatase. After final wash with washing buffer, spots were developed by adding 100 μl of BCIP/NBT substrate to wells and incubating 30 min at room temperature in the dark. The plates were then rinsed three times with distilled water and dried at 4 °C. The spots were counted by stereo microscope (Nikon, Japan). The number of specific IFN-γ-producing lymphocytes was calculated by subtracting the spots from stimulated wells with un-stimulated one.
ELISA of antibodies and their isotypes
Specific antibodies were determined by an optimized indirect ELISA method. Briefly, 100 μl of 5 μg/ml of the p24-Nef fusion peptide in 50 mM carbonate–bicarbonate buffer (pH 9.6) was added into 96-well ELISA Maxisorp plates (Nunc, Naperville, IL) and incubated 24 h at 37 °C. The wells were washed with PBS containing 0.05% Tween 20 (washing buffer) and blocked 1 h at 37 °C with 5% skimmed milk in PBS (blocking buffer). Plates were washed with washing buffer and 100 μl of 1/100 diluted sera were added to each wells and incubated at 37 °C for 2 h. The wells washed five times with washing buffer and incubated for 2 h with 100 μl of 1/7000 dilution of anti-mouse conjugated to HRP (Sigma, USA). The wells were washed five times and incubated 30 min with 100 μl of TMB substrate in the dark and reaction was stopped with 2 N H2SO4 and color density was measured at A450 nm with ELISA plate reader. Detection of specific IgG1, IgG2a, IgG2b, IgG3, and IgM subclasses were carried out using goat anti-mouse IgG1, IgG2a, IgG2b, IgG3, and IgM secondary antibodies (Sigma, USA) according to the manufacture’s instruction.
Statistical analysis
The number of mice in each experiment was 6 mice for test groups (Al-NLX-Vac and Al-Vac) and 5 mice for control groups (Alum, NLX and PBS groups). The data expressed as means ± S.D of each experiment. All statistical analyses were carried out by the Mann-Whitney U test. In all of the cases, P values less than 0.05 was considered to be statistically significant.
Results
Lymphocyte proliferation
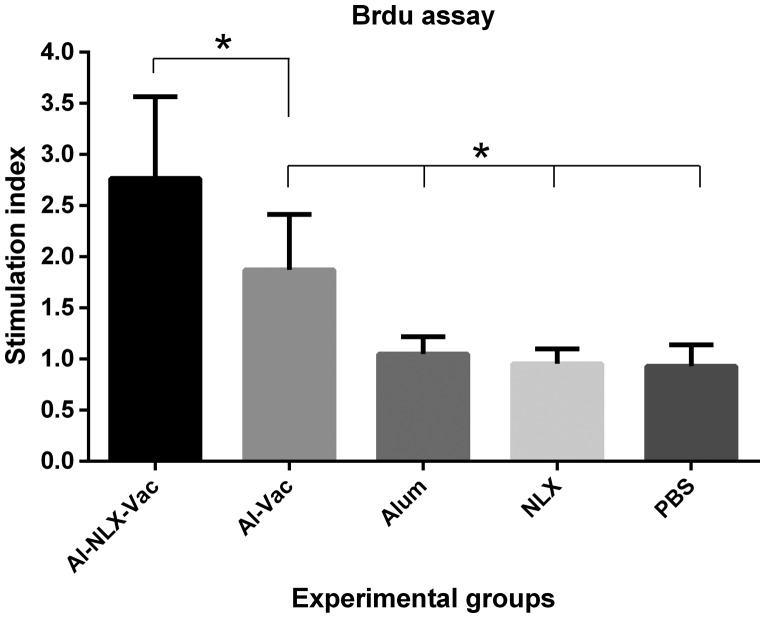
The lymphocyte proliferation of experimental groups was evaluated by the Brdu/ELISA-based method. Antigen recall was carried out three days and then lymphoproliferative activity was reported as a stimulation index of individual mice. As shown in Fig. 1, the mice immunized with Al-NLX-Vac and Al-Vac elicited lymphocyte proliferation, demonstrating a significant difference as compared with control groups (P ≤ 0.004). Interestingly, the mice immunized with Al-NLX-Vac significantly increased lymphocyte proliferation in comparison with the Al-Vac group (p = 0.046). However, there was no significant difference among control groups (p > 0.05).
Figure 1.
Lymphocyte proliferation response of splenic lymphocytes after antigen recall. After immunization course, splenocytes were harvested and re-stimulated in vitro with peptide for 72 h and lymphocyte proliferation was then quantitated using a commercially available Brdu proliferation kit as described in ‘Materials and methods’. Proliferation was presented as stimulation index of individual mice and values were represented as mean ± SD of experimental mice. Asterisks represent the groups which were significantly difference (p < 0.05).
Cytotoxicity assay
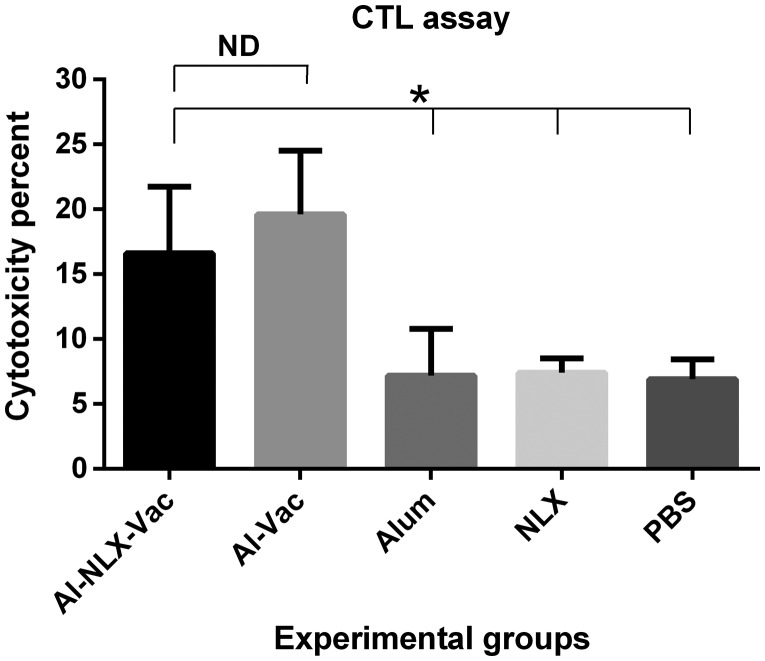
To detect cytotoxicity of lymphocyte, an in vivo CTL assay was performed by the CFSE method. The results (Fig. 2) show that mice immunized with the P24-Nef peptide with or without NLX induced CTL activity when compared with control groups (P ≤ 0.011). The mice immunized with Al-NLX-Vac did not show any significant difference in CTL activity as compared to the Al-Vac group (p = 0.37). There was no significant difference among control groups (p > 0.05).
Figure 2.
Cytotoxicity activity of cytotoxic T lymphocytes in the experimental groups. Results of CFSE assay were presented as mean ± SD of six of mice. Asterisks indicate the groups which were significantly different and ND indicates not detectable differences (p < 0.05).
IFN-γ and IL-4 cytokine assay
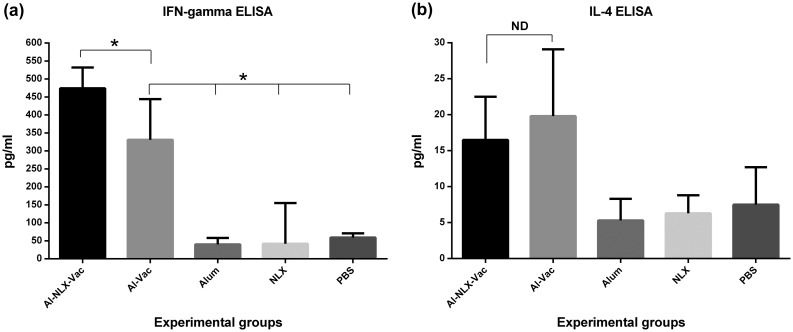
Cytokine analysis revealed that the mice immunized with Al-NLX-Vac and Al-Vac were able to increase an IFN-γ cytokine (Fig. 3(a)) level in compression with control groups (p = 0.004). The data demonstrated that there is a significant difference in the IFN-γ cytokine level between the groups immunized with Al-NLX-Vac and Al-Vac (p = 0.0025). There was no significant difference among control groups (p > 0.05). As shown in Fig. 3(b), the evaluation of IL-4 cytokine in the experimental groups showed that the mice immunized with Al-NLX-Vac and Al-Vac elicited an increased IL-4 cytokine level as compared with control groups (P ≤ 0.016). IL-4 cytokine in the Al-NLX-Vac-immunized group showed a decrease in comparison with the Al-Vac group (p = 0.057). There was no significant difference among control groups (p > 0.05).
Figure 3.
Enzyme-linked immunosorbent assay (ELISA) of IFN-γ (a) and IL-4, (b) cytokines in spleen cell culture supernatant of experimental mice. Results of IFN-γ and IL-4 cytokines were depicted as mean ± SD of experimental mice. Asterisks indicate the groups which were significantly different and ND indicates not detectable differences (p < 0.05).
IFN-γ ELISPOT assay
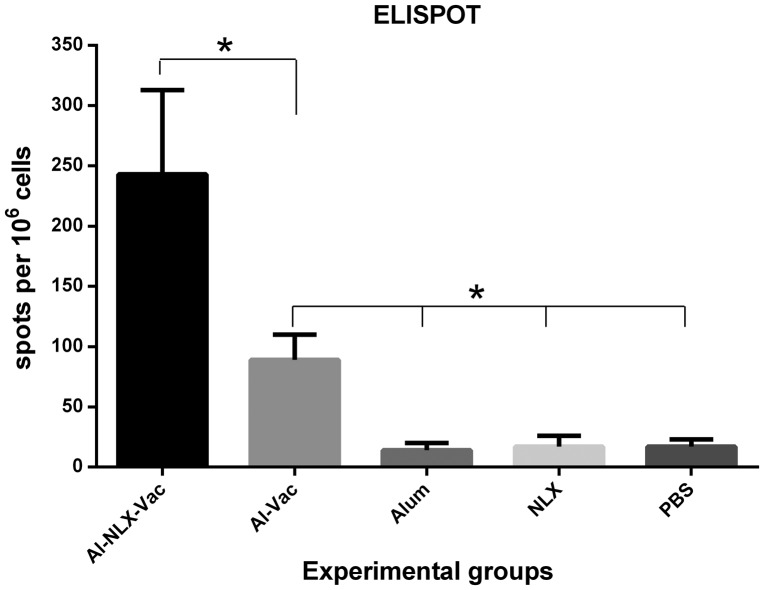
The frequency of IFN-γ secreting lymphocytes were evaluated by the ELISPOT assay. As shown in Fig. 4, the mice immunized with Al-NLX-Vac and Al-Vac significantly increased IFN-γ secreting cells as compared to control groups (P ≤ 0.002). Co-administration of the candidate vaccine with NLX (Al-NLX-Vac) significantly augmented the numbers of IFN-γ secreting lymphocytes as compared with the Al-Vac immunized group (p = 0.002). There was no significant difference among control groups (p > 0.05).
Figure 4.
ELISPOT of IFN-γ cytokine in the experimental groups. Mice were immunized with candidate vaccines and the frequency of IFN-γ producing lymphocytes were analyzed using ELISPOT method by 24 h antigen recall in the in vitro condition. Spots adjusted for 106 spleen cells and Data are shown as the mean ± SD of experimental groups. Asterisks represent the groups which were significantly difference (p < 0.05).
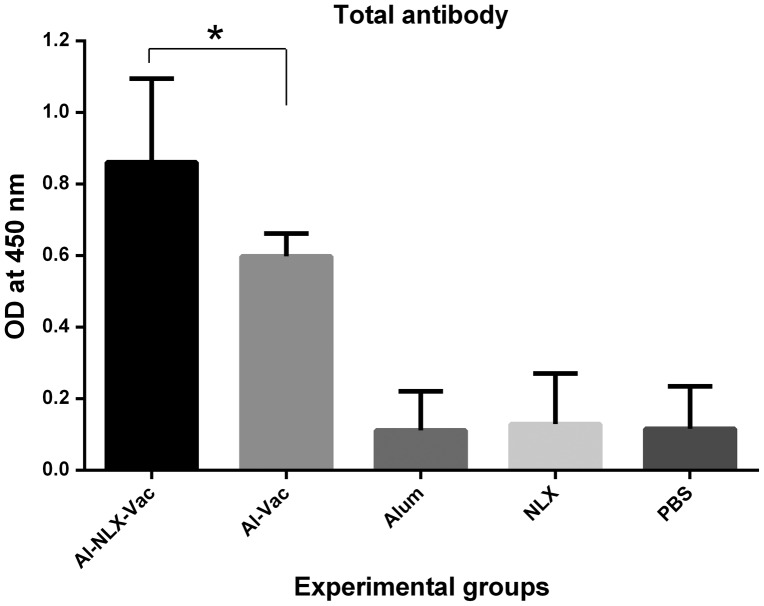
Total antibodies
Total IgG was evaluated by an indirect ELISA method. The results (Fig. 5) show that immunization of mice with candidate vaccine in the presence or absence of NLX induced specific IgG antibody responses, indicating that there was a significant difference compared to control groups (p < 0.003). The level of total specific IgG in the mice immunized with Al-NLX-Vac were significantly higher than the Al-Vac immunized group (p = 0.007). There was no significant difference among control groups (p > 0.05).
Figure 5.
Total antibody responses in experimental groups. Experimental mice were immunized with vaccines with different formulation and total antibody was evaluated with indirect ELISA method. Data are shown as the mean ± SD. Asterisks represent the groups which were significantly difference (p < 0.05).
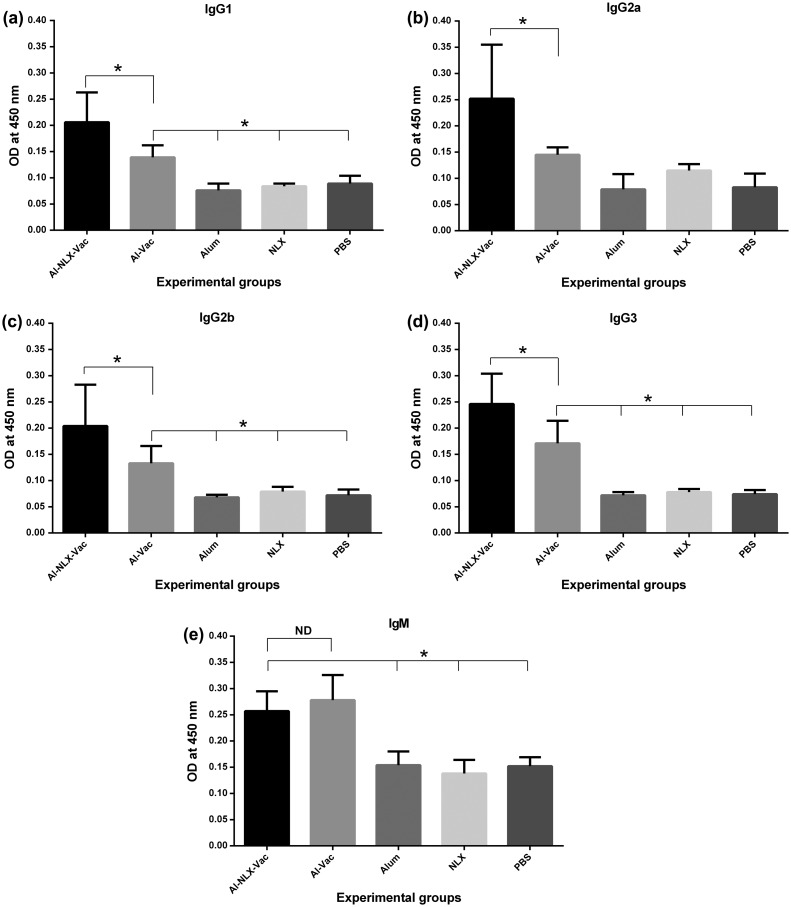
IgG isotyping and IgM
The results of IgG isotyping (Figs. 6(a)–(d)) show that immunization of experimental groups with Al-NLX-Vac and Al-Vac led to an increase in all IgG isotypes compared to control groups (P ≤ 0.028). The immunization of mice with Al-NLX-Vac significantly increased the IgG1 isotype compared to the Al-Vac group (p = 0.018). Monitoring of IgG2a in the Al-NLX-Vac immunized group show a significant increase in comparison to the Al-Vac group (p = 0.028). In addition, immunization of mice with Al-NLX-Vac increased IgG2b and IgG3 isotypes compared to the Al-Vac group (p = 0.068 and p = 0.018, respectively). Overall, the Alum–NLX mixture, as an adjuvant, seems to increase all IgG isotypes when compared to alum alone. Furthermore, evaluation of IgM (Fig. 6(e)) showed that immunization of experimental groups with Al-NLX-Vac and Al-Vac increased specific IgM compared to control groups (P ≤ 0.028). However, no significant differences were observed between Al-NLX-Vac and Al-Vac groups in the induction of specific IgM (p = 0.0808).
Figure 6.
Detection of IgG1 (a), IgG2a (b), IgG2b (c), IgG3 (d) and IgM, (e) specific antibodies. The measurements were made with sera of experimental mice, and the values represent the means of optical density ± standard deviation. Asterisks indicate the groups which were significantly different and ND indicates not detectable differences (p < 0.05).
Discussion
The adjuvant activity of Naloxone in combination with various pathogens is reported previously.2,4 Here, for the first time, the adjuvant activity of the Alum/NLX mixture was evaluated against a multi-epitopic HIV-1 vaccine candidate model. Several substances have exhibited adjuvant effects on immune responses, such as aluminum that can stimulate immune responses, especially Th2-dependent immune responses, which are characterized by the production of IL-4 and IL-5 and the induction of IgE and IgG1. Following immunization, the alum-induced activation of the NALP3 inflammasome results in the induction of Th2-associated inflammation and B cell isotype switching. In addition, alum induces chemokines such as CXCL8 that recruit inflammatory cells to the site of vaccination.12 Recent studies have shown that the Alum/NLX mixture induce potent adjuvant activity for a heat-killed Salmonella Typhimurium vaccine model and it seems that NLX and alum may show synergistic effects on the induction of inflammatory milieu at the site of immunization and also on both cellular and humoral immune responses.3,13 The adjuvants were able to increase the immunogenicity of antigens. It is clear that a successful vaccine requires an adjuvant to elicit a broad spectrum of cellular and humoral immune responses against viral infections.2,14 The results of the present study showed that immunization of mice with Al-NLX-Vac significantly increases lymphocyte proliferation vs. the Al-Vac immunized group. The Study by Jazani et al. shows that NLX/alum mixture with the heat-killed Salmonella typhimurium vaccine increased lymphocyte proliferation vs. an alum-formulated vaccine.13 Lymphocyte proliferation is a criterion of cell-mediated immune responses and the results of our study show that the vaccine mixture in NLX/alum as an adjuvant could increase lymphocyte proliferation, that is stimulate cellular-mediated immune responses.
Evaluation of vaccine candidates should include multiple parameters such as frequency of vaccine-induced CD4+ and CD8+ T cells, T-cell functionality in terms of their lytic activity, secretion of cytokines and the ability to proliferate on antigen re-exposure, and the longevity of vaccine-induced memory T cells and their anatomical distribution.15 It is clear that Th1 responses are typically characterized by the secretion of IFN-γ and the generation of delayed type-hypersensitivity responses. In addition, Th1 cells are thought to mediate the killing of intracellular pathogens. The Th2 cell lines are mainly characterized by the secretion of IL-4 and helps induce the humoral immune responses.10 Recent studies characterize the significant adjuvant activity of small molecule CC chemokine receptor 4 (CCR4) antagonists. The CCR4 was assumed as an adjuvant that express on CD4+ CD25+ regulatory T cells (Tregs), which negatively regulate immune responses induced by DCs. CCR4 antagonists have been described to stimulate cellular and humoral immune responses in experimental models when injected in combination with Ag85A from Mycobacterium tuberculosis (MVA85A) or recombinant HBV surface antigen (HBsAg).16,17 The critical roles of CCR4 in Treg cell recruitment to tumors have been reported with various studies. CCR4+ effector Treg (eTreg) cells abundantly and predominantly infiltrated into esophageal and gastric cancers as observed with melanoma. A different strategy is the use of specific monoclonal antibodies that target surface markers of activated Tregs. The recent studies demonstrate that in vivo administration of anti-CCR4 or anti-CTLA4 mAb markedly reduced the eTreg cell fraction and therefore it evoked and augmented antitumor immunity in cancer patients.18,19 To detect the immune polarization, IFN-γ and IL-4 cytokines were subsequently evaluated and the results show that, when NLX was added to vaccine formula, a significant increase in the amount of secreted IFN-γ will be observed but IL-4 did not show such trend. Jamali et al. investigated the ability of NLX to enhance the protection of a DNA vaccine against HSV-1 infection and found that lymphocyte proliferation and production of IFN-γ cytokine in NLX-formulated vaccine group significantly increased vs. DNA vaccine group.2 For clearing of infected cells and deletion of viral infection, polarization to Th1 immune response and secretion of IFN-γ is obviously critical because the important role of IFN-γ in the induction of CTL immune response is to kill virus-infected cells. The Study by Jamali et al. have suggested that NLX shifts the immune response toward the Th1 pattern, as well as revealed that increasing IFN-γ production through NLX treatment can lead to an increase in virus clearance, confirming our results for polarization into the Th1 pattern.4 Cytotoxic T lymphocytes, as an effector of the cell-mediated immune response, may be of importance in controlling HIV-1 infection.20 In fact, T cells protect the health of an organism by mounting a well-orchestrated immune response consisting of proliferation effector cell differentiation, contraction and induction of cytotoxic T lymphocytes that kill viral infected cells.21 In general, effector CD8+ T cells control infection through cytotoxic activity and the secretion of IFN-γ and TNF-α.22 Moreover, CTL plays a major role in adaptive immunity to viral infections such as HIV-1.23,24 Therefore, it seems that the ability of potential vaccines for HIV to elicit strong CTL responses is very important for eradication of HIV infection.23,25 In this study, the results of CTL activity demonstrated that immunization with vaccine in combination with NLX and alum mixture did not show any significant difference vs. the alum adjuvanted group. However, the study of Molla Hassan et al. showed that NLX significantly increased CTL activity for a cancer vaccine in comparison to the un-adjuvanted vaccine.26 The difference of our result with this study may be related to the type and dose of vaccine as well as dose of NLX which we used 6 mg/kg while in that study NLX were utilized at dose of 1 mg/kg.
Recent evidence suggests that a successful and effective vaccine against HIV should induce a potent memory cell-mediated immune response in addition to neutralizing antibodies against immunodominant antigens.27 To further analyse the cellular immune response, in the next, the frequency of IFN-γ-producing T lymphocytes was analyzed via the ELISPOT method with short-term in vitro antigen recall. Because of short-term exposure to the antigen in the in vitro condition, the response is related to the function of the effector memory T cells.6 The results of the ELISPOT as an effector memory T-cell response show that immunization with Al-NLX-Vac significantly increased the frequency of effector memory T cell vs. Al-Vac group. This is the first report that shows the potency of NLX in the induction of effector memory T cells. Memory T lymphocytes provide guarantee for the long-term protection against infection disease and our study results show that NLX can increase memory formation and thereby increase vaccine potency. Vaccines that effectively induce a strong Th1 cellular response need additional components in the adjuvant, such as MPL (as a TLR4 agonist). MPL (3-O-desacyl-4′-monophosphoryl lipid A) is present in the GSK adjuvants such as Adjuvant System 01, AS01, (liposomal formulation plus QS21; a saponin), and AS02 (combined with a water/oil emulsion plus QS21). AS03 contains α-tocopherol and squalene, two biodegradable polyprenyls, in an oil/water emulsion. AS03 has been considered for the development of prophylactic vaccines, including pandemic influenza and a new generation of seasonal influenza vaccines. It has also been developed to improve upon standard adjuvants such as alum. Administration of AS03 can led to enhanced recruitment of neutrophils, monocytes, and eosinophils at the site of injection, which pick up antigen and traffic to the reticuloendothelial system. AS04 consisting of MPL, a detoxified derivative of the LPS isolated from the Gram-negative bacterium Salmonella minnesota R595 strain, formulated onto a particulate form of aluminum salt is one of these new generation adjuvants now licensed for use in humans. The presence of alum in AS04 is important in stabilizing the MPL and antigen within the vaccine and provides a depot effect. AS04 is currently a component in two licensed vaccines, the HPV (Cervarix) and HBV (Fendrix) vaccines. A third vaccine against herpes simplex two virus is in phase III clinical trials. The MPL stimulate increased numbers of DCs and monocytes in the draining lymph nodes and promote IFN-γ production by specific CD4+ T cells, therefore, shift the immune response toward a Th1 profile.28,29
Antibodies provide the first line of defense against infection by microbial pathogens.30 Our immunogenicity experiments showed that the vaccine formulated in NLX/alum significantly increased total antibodies vs. alum-adjuvanted vaccine group. Previous reports confirmed our results for the potency of NLX/alum mixture in the induction of humoral immune responses.3,13 As mentioned, humoral immune response has critical role in the neutralizing of viruses and clearing of infected cells via ADCC by natural killer cell, lysis by complement or phagocyting by macrophages31 and our results show the superiority of NLX/alum mixture on the induction of humoral immune responses. In addition, analysis of antibody isotypes shows that NLX/alum mixture induced the highest level of IgG1, IgG2a, IgG2b, IgG3, and IgM antibodies vs. other groups. It seems that NLX/alum mixture is a potent adjuvant for induction of poly-isotypic of humoral immune responses. Antibodies react to the surface antigens of pathogens and neutralize them, considering that each isotypes of antibody may have a distinct function in the immune responses, induction of poly-isotypic maybe show more bioactivity of humoral immune response.32 Overall, the results of the present study showed that NLX/alum mixture can strongly induce cellular and humoral immune responses and may be a useful choice for human clinical trial study for HIV vaccine research.
Acknowledgement
We thank Mr Lotfi, Dr Majid Tebianian, and Dr Elly Moraddahandeh for their technical supports.
Funding
This work was supported in part by a grant from Pasteur Institute of Iran, Tehran, Iran.
References
- 1.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–517. 10.1038/nrmicro1681 [DOI] [PubMed] [Google Scholar]
- 2.Jamali A, Mahdavi M, Hassan ZM, Sabahi F, Farsani MJ, Bamdad T, et al. A novel adjuvant, the general opioid antagonist naloxone, elicits a robust cellular immune response for a DNA vaccine. Int Immunol. 2009;21:217–225. 10.1093/intimm/dxn139 [DOI] [PubMed] [Google Scholar]
- 3.Jazani NH, Parsania S, Sohrabpour M, Mazloomi E, Karimzad M, Shahabi S. Naloxone and alum synergistically augment adjuvant activities of each other in a mouse vaccine model of Salmonella typhimurium infection. Immunobiology. 2011;216:744–751. 10.1016/j.imbio.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 4.Jamali A, Mahdavi M, Shahabi S, Hassan ZM, Sabahi F, Javan M, et al. Naloxone, an opioid receptor antagonist, enhances induction of protective immunity against HSV-1 infection in BALB/c mice. Microb Pathog. 2007;43:217–223. 10.1016/j.micpath.2007.06.001 [DOI] [PubMed] [Google Scholar]
- 5.Karaji AG, Hamzavi Y. The opioid antagonist naloxone inhibits Leishmania major infection in BALB/c mice. Exp Parasitol. 2012;130:73–77. 10.1016/j.exppara.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 6.Mahdavi M, Ebtekar M, Khorram Khorshid HR, Azadmanesh K, Hartoonian C, Hassan ZM. ELISPOT analysis of a new CTL based DNA vaccine for HIV-1 using GM-CSF in DNA prime/peptide boost strategy: GM-CSF induced long-lived memory responses. Immunol Lett. 2011;140:14–20. 10.1016/j.imlet.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 7.Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82:488–496. 10.1111/icb.2004.82.issue-5 [DOI] [PubMed] [Google Scholar]
- 8.Mahdavi M, Ebtekar M, Azadmanesh K, Khorramkhorshid HR, Rahbarizadeh F, Yazdi MH, et al. HIV-1 Gag p24-Nef fusion peptide induces cellular and humoral immune response in a mouse model. Acta Virologica. 2010;54:131–136. 10.4149/av_2010_02_131 [DOI] [PubMed] [Google Scholar]
- 9.Ge Q, Hu H, Eisen HN, Chen J. Naive to memory T-cell differentiation during homeostasis-driven proliferation. Microb Infec. 2002;4:555–558. 10.1016/S1286-4579(02)01572-1 [DOI] [PubMed] [Google Scholar]
- 10.O’Hagan DT, MacKichan ML, Singh M. Recent developments in adjuvants for vaccines against infectious diseases. Biomol Eng. 2001;18:69–85. 10.1016/S1389-0344(01)00101-0 [DOI] [PubMed] [Google Scholar]
- 11.Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. CD8+ CTL priming by exact peptide epitopes in incomplete freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol. 2007;179:5033–5040. 10.4049/jimmunol.179.8.5033 [DOI] [PubMed] [Google Scholar]
- 12.Aimanianda V, Haensler J, Lacroix-Desmazes S, Kaveri SV, Bayry J. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol Sci. 2009;30:287–295. 10.1016/j.tips.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 13.Jazani NH, Sohrabpour M, Mazloomi E, Shahabi S. A novel adjuvant, a mixture of alum and the general opioid antagonist naloxone, elicits both humoral and cellular immune responses for heat-killed Salmonella typhimurium vaccine. FEMS Immunol Med Microbiol. 2011;61:54–62. 10.1111/fim.2011.61.issue-1 [DOI] [PubMed] [Google Scholar]
- 14.Blondelle SE, Moya-Castro R, Osawa K, Schroder K, Wilson DB. Immunogenically optimized peptides derived from natural mutants of HIV CTL epitopes and peptide combinatorial libraries. Biopolymers. 2008;90:683–694. 10.1002/bip.21020 [DOI] [PubMed] [Google Scholar]
- 15.Im EJ, Nkolola JP, di Gleria K, McMichael AJ, Hanke T. Induction of long-lasting multi-specific CD8+ T cells by a four-component DNA-MVA/HIVA-RENTA candidate HIV-1 vaccine in rhesus macaques. Europ J Immunol. 2006;36:2574–2584. 10.1002/(ISSN)1521-4141 [DOI] [PubMed] [Google Scholar]
- 16.Bayry J, Tartour E, Tough DF. Targeting CCR4 as an emerging strategy for cancer therapy and vaccines. Trends Pharmacol Sci. 2014;35:163–165. 10.1016/j.tips.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 17.Bayry J, Tchilian EZ, Davies MN, Forbes EK, Draper SJ, Kaveri SV, et al. In silico identified CCR4 antagonists target regulatory T cells and exert adjuvant activity in vaccination. Proc Natl Acad Sci USA. 2008;105:10221–10226. 10.1073/pnas.0803453105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marabelle A, Kohrt H, Levy R. Intratumoral anti-CTLA-4 therapy: enhancing efficacy while avoiding toxicity. Clin Cancer Res. 2013;19:5261–5263. 10.1158/1078-0432.CCR-13-1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA. 2013;110:17945–17950. 10.1073/pnas.1316796110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bojak A, Wild J, Deml L, Wagner R. Impact of codon usage modification on T cell immunogenicity and longevity of HIV-1 gag-specific DNA vaccines. Intervirology. 2002;45:275–286. 10.1159/000067919 [DOI] [PubMed] [Google Scholar]
- 21.van Stipdonk MJ, Sluijter M, Han WG, Offringa R. Development of CTL memory despite arrested clonal expansion. Europ J Immunol. 2008;38:1839–1846. 10.1002/(ISSN)1521-4141 [DOI] [PubMed] [Google Scholar]
- 22.Kaech SM, Wherry EJ. Heterogeneity and Cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. 10.1016/j.immuni.2007.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haglund K, Leiner I, Kerksiek K, Buonocore L, Pamer E, Rose JK. Robust recall and long-term memory t-cell responses induced by prime-boost regimens with heterologous live viral vectors expressing human immunodeficiency virus type 1 gag and env proteins. J Virol. 2002;76:7506–7517. 10.1128/JVI.76.15.7506-7517.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. 10.1038/nri2274 [DOI] [PubMed] [Google Scholar]
- 25.Sasaki S, Takeshita F, Xin KQ, Ishii N, Okuda K. Adjuvant formulations and delivery systems for DNA vaccines. Methods. 2003;31:243–254. 10.1016/S1046-2023(03)00140-3 [DOI] [PubMed] [Google Scholar]
- 26.Molla Hassan AT, Hassan ZM, Moazzeni SM, Mostafaie A, Shahabi S, Ebtekar M, et al. Naloxone can improve the anti-tumor immunity by reducing the CD4+CD25+Foxp3+ regulatory T cells in BALB/c mice. Int Immunopharmacol. 2009;9:1381–1386. 10.1016/j.intimp.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 27.Moore AC, Kong WP, Chakrabarti BK, Nabel GJ. Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice. J virol. 2002;76:243–250. 10.1128/JVI.76.1.243-250.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldwin SL, Bertholet S, Reese VA, Ching LK, Reed SG, Coler RN. The importance of adjuvant formulation in the development of a tuberculosis vaccine. J immunol. 2012;188:2189–2197. 10.4049/jimmunol.1102696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, et al. Adjuvant system AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–2473. 10.1016/j.vaccine.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 30.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. 10.1016/S1074-7613(00)80541-5 [DOI] [PubMed] [Google Scholar]
- 31.McAndrew EG, Dugast AS, Licht AF, Eusebio JR, Alter G, Ackerman ME. Determining the phagocytic activity of clinical antibody samples. J Vis Exp. 2011;30:e3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shajiei A, Rezaei Malal A, Shahabi G, Farhoudi R, Faezi S, Tebianian M, et al. Pseudomonas aeruginosa recombinant flagellin induced poly-isotypic humoral immune responses in the Balb/C mice. Jundishapur J Microbiol. 2013;6:e6760. [Google Scholar]