Abstract
Background
Previous studies have demonstrated that acute poisoning from exposure to organophosphate (OP) pesticides in agricultural workers causes adverse health effects. However, neuropsychological and cognitive effects of chronic occupational exposure to OP pesticides remain controversial.
Objective
To identify, evaluate, and systematize existing evidence regarding chronic exposure to OP pesticides and neuropsychological effects in farmworkers.
Methods
Using the PubMed search engine, a systematic review process was implemented and replicated according to the PRISMA statement. Eligibility criteria included workers over 18 years of age exposed to OP pesticides as well as assessment of neuropsychological and cognitive functioning. Search terms were in English and Spanish languages and included organophosphate and workers.
Results
Of the search results, 33 of 1,256 articles meet eligibility criteria. Twenty-four studies found an association between chronic occupational exposure to OP pesticides and low neuropsychological performance in workers. We classified nine of the studies to have study design limitations. Studies indicated occupational exposure to OP pesticides is linked to difficulties in executive functions, psychomotor speed, verbal, memory, attention, processing speed, visual–spatial functioning, and coordination. Nine studies find no relationship between OP pesticides exposure and neuropsychological performance.
Conclusions
Overall, evidence suggests an association between chronic occupational exposure to OP pesticides and neuropsychological effects. However, there is no consensus about the specific cognitive skills affected.
Keywords: Organophosphate pesticides, Neuropsychological functioning, Occupational exposure, Workers
Introduction
Pesticides have led to increased worldwide agricultural production. However, when not applied safely, they can cause environmental pollution and adverse health effects, which are sometimes irreversible.1,2 Exposure to OPs is usually assessed through analysis of blood and urine biomarkers to determine acetylcholinesterase levels.5–8 Depression of plasma acetylcholinesterase activity in the blood is indicative of OP exposure. Biomarkers that measure concentrations of erythrocyte cholinesterase are used to assess chronic exposure and acute poisoning. The measurement of plasma cholinesterase is used only for the diagnosis of acute poisoning because of the difficulty in determining chronic exposure at low doses.
Currently, urinary biomarkers are the most sensitive measurement of OP exposure. The presence of dialkyl phospate metabolites or specific metabolites of OP pesticides such as chlorpyrifos, methamidophos, malathion, diazinon, and dimethoate are measured in urine. The metabolites analyzed include dimethyl phosphate (DMP), dimethyl tiophosphate (DMTP), dimethyl dithiophosphate (DMDTP), diethyl phosphate (DEP), diethyl tiophosphate (DETP), and diethyl dithiophosphate (DEDTP). Each of these metabolites corresponds to one or more types of OP pesticides. An increasing number of studies have focused on understanding the activity of the enzyme paraoxonase (PON1) and its relationship to genetic polymorphisms PON1 192 and PON155.9 This enzyme is linked to the detoxification of OP pesticides; and its activity is modified by the oxidative stress caused by these pesticides.9
In the United States, Canada, and some European countries, biomonitoring evaluations are conducted every two years with the general population to evaluate exposure to environmental chemicals. These evaluations are used to influence policies on the sale and use of OP pesticides in agriculture and households.5,10–12
Exposure to OP pesticides can occur through multiple pathways, including food contamination, environmental and household pollution, proximity to agricultural fields, and agricultural work. Children may be exposed to pesticides through farm work or as a result of school and/or household exposures.13,14 The health effects of OP pesticides have been reported in numerous international studies. These studies have identified acute and chronic consequences of exposure to OP pesticides among farm workers and their children.15–20 Neurotoxic effects are the most frequently described consequences of exposure.
Studies on agricultural workers including adults and adolescents who apply OP pesticides have shown that acute and moderate poisoning causes irreversible damage to physical and mental health.6,15,21,22 Conversely, the evidence is not entirely conclusive regarding chronic exposure to OP pesticides and neuropsychological performance. Recent studies identified an association between agricultural work and lower performance on memory and coordination tests. Additionally, studies23–25 that measure exposure through biomarkers have found that high concentrations of OP metabolites in urine are associated with lower performance on the aforementioned cognitive variables. However, some of those studies assess diverse specific cognitive functions with variable results.23–25
The assessment of neuropsychological functioning includes global intellectual performance; attention or processing speed; spatiotemporal orientation; visuospatial skills; praxis (voluntary movement planning), coordination, and motor speed; memory; language and communication, reasoning and executive functions; and other functions related to the frontal lobes of the cerebral cortex. Brief scales or cognitive screening tests, neuropsychological batteries, and tests that assess specific cognitive functions are used to measure the neuropsychological functioning listed above.26–28
Previous systematic reviews and meta-analyses have investigated the association between exposure to OP pesticides and neurobehavioral performance or health effects on workers. However, currently available research does not investigate chronic exposure to OP pesticides and the neuropsychological assessment of farmworkers. In this review, we identify, evaluate, and systematize existing evidence regarding chronic exposure to OP pesticides and neuropsychological effects in farmworkers. Results from this study can be used to scrutinize existent evidence and provide information useful for policymakers and researchers.
Methods
A systematic search was performed using PubMed and replicated using Web of Science, EBSCO, SciVerse Scopus, Redalyc, and Lilacs to compare results and to identify items not indexed in PubMed. Search terms were “organophosphate AND pesticides AND workers”; “Pesticides AND workers”; “Organophosphate AND workers AND neuropsychological”; “Organophosphate AND workers AND neurobehavioral”; and “chlorpyrifos AND workers”.
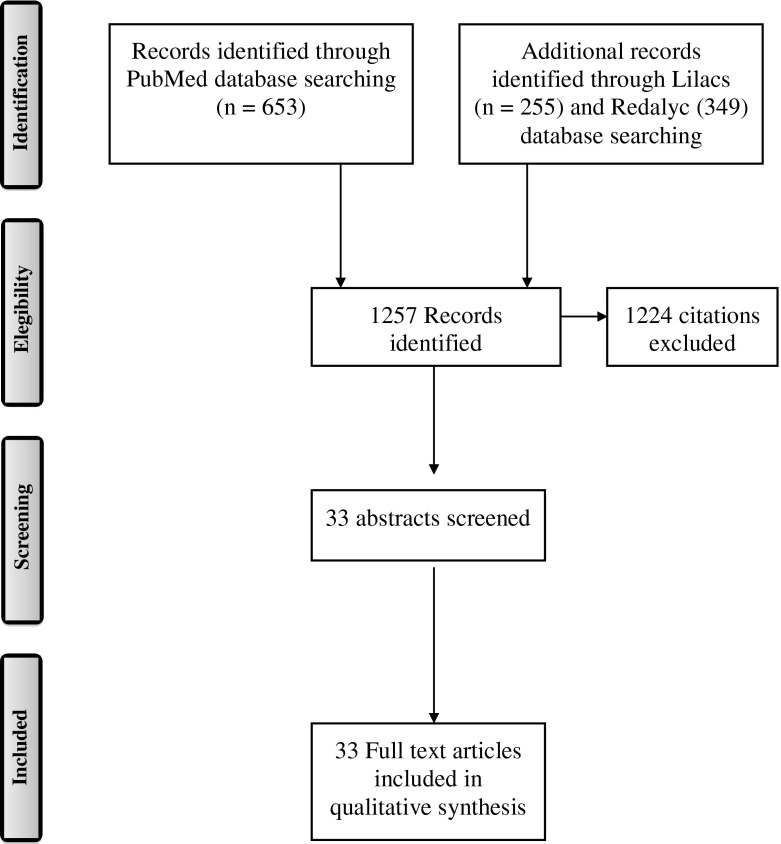
Inclusion criteria for the systematic review were: as follows (a) assessment of chronic exposure to OP pesticides; (b) assessment of neuropsychological functioning (only cognitive functions); (c) agricultural workers exposed and unexposed workers; and (d) study population over 18 years of age. All articles published in 2014 and earlier were considered. A total of 1,257 research articles were identified and 33 met the review eligibility criteria.
For each study, population characteristics, study design, measurement instruments (Table 1), and exposure, results, and control of confounding or bias were reviewed. We reviewed publications in English and Spanish following the PRISMA guidelines (Fig. 1).62
Table 1.
Assessment instruments to study neuropsychological functions in farm workers exposed to organophosphate pesticides
| Instrument | Neuropsychological function |
|---|---|
| Short-term retention of individual verbal ítems of Peterson & Peterson [29] | Memory (short-term memory, working memory, visual spatial memory, auditory memory, verbal memory) |
| Digit span (WAIS, WAIS R, WAIS-III) a,b,d30–47 | |
| Digit Vigilance test34,35,39 | |
| Short-term memory scanning; manipulating numbers I, II, and II37 | |
| Psychological–neurological questionnaire: subtest of concentration and memory48 | |
| Benton Visual Retention test-BVRT30,31,35,37,39,40,43,44,49,50 | |
| Wechsler Memory Scale-III47 | |
| Visual Spatial Memory test33 | |
| Pattern Memory testb32,38,51,52 | |
| Word Span tests 53 | |
| Logical Memory test44 | |
| Match to sample46 | |
| Visual reproduction (WMS-R); Continuous Visual Memory test (CVMT)36 | |
| Story recall parts A and B40 | |
| Delayed matching to sample (DMS); CogniSyst Story Recall test (CSRT)54 | |
| Rey Auditory Verbal Learning test39,43,44,55 | |
| Rey osterrieth figure complex43,44 | |
| Serial digit learningb,d32,45,46 | |
| Wechsler Paired-associates test (WPAT)50 | |
| Adaptation of signal processing as a function of aging (Simon)29 | Processing speed (signal processing time/Information processing speed/Reaction time/Attention /Visual perception) |
| Digit symbol (WAIS, WAIS R, WAIS-III)a30,35–37,39,40,44,56 | |
| Symbol-digit testa,b,d31–33,38,41,42,45,46,51,52 | |
| Syntactic Reasoning test33,42 | |
| Pattern Comparison test; continuous number51 | |
| Reaction Time testa29,31,34,37,54; simple reaction timeb,d32,33,35–39,42,45,46,54; choice reaction time & inspection time37 | |
| Sustained attentionb32 | |
| California computerized assessment package47 | |
| Continuous Performanceb,d36,38,45,46,51,52 | |
| Stroop testa36,44,47,50 | |
| Selective attentiond45,46 | |
| Paced Auditory Serial Addition test (PASAT); Cancellation Letter testa40 | |
| Rapid Visual Information Processing (RVIP); Matching to sample visual (MTS); stockings of Cambridge (SOC)54 | |
| Location recognition42 | |
| Benton Visual Form Discrimination test (BVFT); Poppel Reuter test44 | |
| Trail Making (TMT) A and B a,c30,31,34,36,39,40,44,47,50,57 | |
| Sentence repetition subtest of the multilingual aphasia examination29 | Verbal abilities (long-term memory/Verbal comprehension/semantic access) |
| Category Search; Serial Word Learning Test (ACTS)33,43 | |
| Animal postures I and II; Speaking arrows; Stimulus resistance; Pointing arrows; Echopraxis37 | |
| California Verbal Learning Test (CVLT)36 | |
| Vocabulary Test (WAIS)34,38,39 | |
| Graded Naming Test47 | |
| Information subtest (WAIS)34,36 | |
| Animal Naming Test (Boston Diagnostic Aphasia); Revised Token Test36 | |
| Wechsler Paired-associates Test (WPAT)50 | |
| Armed Forces Qualifying Test (ASQT)51 | |
| Boston Naming Test (BNT)44 | |
| Bender Visual Motor Gestalt Testc57,58 | Motor speed/coordination/visomotor/spatial |
| Santa Ana Peg Boarda30–32,34,35,37,41 | |
| Pursuit Aiminga30,32,34,35,37,39,56 | |
| Grooved Pegboard Test (NES Battery)36,47 | |
| Finger Tappinga,b,c,d32,39,41,45,52,57 | |
| Block Design (WAIS, WAIS R, WAIS-III)34,39,40,44 | |
| Motor Performance Series (MPS)54 | |
| Santana Dexterity39 | |
| Tactual Performance Testc57 | |
| Hand–eye Coordination Taskb51,52 | |
| Manual dexterity test48 | |
| Task proposed by Luria44 | |
| Wechsler Adult Intelligence Scale (WAIS, WAIS R, WAIS-III)43,47,49 | IQ test battery |
| Electroencephalogram57,59 | Other neuropsychological tests |
| The Medical Symptom Validity Test47 | |
| Cambridge neuropsychological test automated battery (CANTAB): Attention and Memory55 | |
| Subjective Neurocognition Inventory-SIN (versión Iraní, evalúa memoria y atención)60 | |
| Mini Mental State Examination, MMSE (Cognitive impairment)35,50,53,59,61 | |
| Neuropsychological exploration(auditive, visual, tactile attention, orientation, memory, language, comprehension, praxes, and gnoses)49 | |
| Wide Range Achievement Test (WRAT-3)36,54 | |
| Battery of psychometric test (RAG)55 |
aSubtest recommended by WHO Neurobehavioral Core Test Battery.
bSubtest of the Neurobehavioral Evaluation System (NES).
cHalstead Neuropsychological Test Battery.
dBehavioral Assessment and Research System (BARS).
Figure 1.
PRISMA diagram of publication selection process.
Studies were assessed individually and rated based on: (a) study design, (b) sample size, (c) exposure assessment, (d) measurement of effect, and (e) control of confounding factors. A score of 0 (lowest) to 2 (highest) for the five parameters was assigned to each study. Six of the coauthors independently rated all the studies according to the scoring criteria. There was a 90% of agreement in the ratings given to each article. Disagreements were resolved through discussion by the reviewing authors until a consensus was reached.
The characteristics of these five parameters and the classification scheme are described in Table 2. Studies were classified into five mutually exclusive categories: low (0–2 points), intermediate low (3–4), intermediate (5 points), intermediate high (6–7 points), and high (8–10 points). This classification criterion was based on previous international systematic reviews, adapted with slight modifications concerning an increased score for cross-sectional studies, the inclusion in the “exposure measures” criteria of biomarkers with no association with effects, and the consideration of five categories of quality instead of three.63,64
Table 2.
Score criteria for qualifying the quality standard of the studies
| Criteria | 2 | 1 | 0 |
|---|---|---|---|
| a. Study design | Longitudinal, exposure before the outcome, experimental | Case-control, cross-sectional | Case study, ecological, exploratory, descriptive |
| b. Sample size | <200 | 50–200 | >50 |
| c. Exposure measure | Specific biomarkers (e.g. blood/urine chlorpyrifos) | General biomarkers (e.g. Dialkyl phosphates[DAP], Serum paraoxonase/arylesterase 1[PON1], Acetylcholinesterase(AchE), butyrylcholinesterase[BuChE]) | Exposure measured without biomarkers(ecological studies, records, live near to fields, occupation, etc.) |
| Used biomarkers but with no association to effects | |||
| Partial use of biomarkers(only for exposed) | |||
| d. Measuring of effects | Use of standardized and validated instruments, laboratory tests, scanner, specific tests | Screening tests, interviews, questionnaires, full scales(but not validated) | Sections of full scales(standardized or not), clinical records |
| e. Control of confounders | Control of important confounders (parents IQ, education level, diseases, exposure to other neurotoxic agents) and standard variables(statistical models analysis) | Control of standard variables with an adequate statistical analysis (e.g. age, sex, occupation) | Neither standard variables nor confounders are considered. Inadequate statistical analysis of confounders was performed |
Categories of quality standard by summing the scores assigned to each parameter: 0–2 low, 3–4 intermediate-low, 5 intermediate, 6–7 intermediate-high, and 8–10 high. OP: organophosphates; IQ: Intelligence quotient.
The main effects observed in workers exposed to OP pesticides were classified into seven categories: (a) Memory includes assessment of short-term memory; working memory; visual, spatial, auditory, and verbal memory; (b) Motor skills is comprised of motor speed, fine coordination, visual motor, and spatial skill; (c) Processing speed includes signal processing time, information processing speed, time of reaction, attention, visual perception; (d) Verbal abilities considers long-term memory, verbal comprehension, semantic access, language test; (e) IQ includes the assessment of global IQ, verbal IQ, performance IQ, and general cognitive functioning; (f) Assessment of cognitive impairment is measured with the Mini Mental State Examination test (MMSE); and (g) Morphology, which includes measures of physical changes in the brain.
Results
Main characteristics of the studies
Table 3 summarizes the characteristics of each study. Publication years ranged from 1975 to 2014. With respect to study design, 24 were cross-sectional; six were cohort, one was retrospective, one was case-control, and one was pre-experimental.
Table 3.
Characteristics of studies published about exposure to OP and effects on the neuropsychological performance of workers aged over 18 years old
| Author(s)/country | Sample size (E/C) | Study design | Exposure assessment E/C | Study rating (score) | Neuropsychological effect(yes/no) | Bias |
|---|---|---|---|---|---|---|
| Rodnitzky et al. 1975/USA29 | 23(12 farmers & 11 applicators)/23 | Cross-sectional | Occupational questionnaire. AChE (E/C) | Intermediate-Low (3) | No | Small sample, no standardized test, unevaluated confounders |
| Korsak et al., 1977/USA*57 | 59 volunteers segregated into two groups of H/L exposure | Cross-sectional | Plasma BuChE, AChE. (H/L). Occupational questionnaire | Intermediate (5) | Yes | The participants were volunteers |
| Delgado 1986/Cuba58 | 36/36 | Cross-sectional | Occupational questionnaire AChE (E/C) | Intermediate-Low (4) | Yes | No control of confounders |
| Maizlish et al. 1987/USA*51 | 46/56 | Pre—experimental | DETP and DMTP measured in pre and post shift urine samples. | Intermediate-High (7) | No | Testing of workers approximately 1 month after initial exposure |
| Rosenstock et al. 1991/Nicaragua30 | 36/36 | Retrospective | Questionnaire of poisoning OP | Intermediate (5) | Yes | No use of biomarkers |
| Daniell et al. 1992/USA52 | 49/40 | Longitudinal | Occupational questionnaire AChE (H/L) | Intermediate-High (7) | No | Lack of bias control |
| Richter et al. 1992/Israel31 | 51/39 | Cross-sectional | Pre-season and in season. ChE activities. Urine DAP | Intermediate (5) | Yes | Use biomarkers but with no association to neuropsychological effects |
| Ames et al. 1995/USA32 | 45/90 | Cross-sectional | Occupational questionnaire, clinical records, records of measures of BuChE from 1985, 1988 y 1988 | Intermediate (5) | No | Sample of exposed smaller than no exposed. Biomarker of effect (BuChe) only on exposed |
| Stephens et al. 1995/England33 | 146/143 | Cross-sectional | Urine analysis (DAP). Occupational questionnaire | Intermediate (5) | Yes | No analysis of association between effects and biomarkers |
| Cole et al., 1997/Ecuador34 | Consumers = 23; Exposed = 28; Applicators = 123; Control = 72 | Cross-sectional | Occupational questionnaire AChE (E/C) | Intermediate-High (7) | Yes | Raw scores of each test were used being converted to Z-score for comparison |
| Fiedler et al., 1997/USA36 | 57/42 | Cross-sectional | Occupational questionnaire (AChE at normal limits for all) | Intermediate-High (6) | Yes | Convenience sampling. Lower educational level on exposed |
| London et al. 1997/South-Africa37 | 163/84 | Cross-sectional | Occupational questionnaire (BuChE only on exposed) | Intermediate (5) | Yes | Biomarker of effect only on farm workers |
| Rodríguez et al. 1997/Venezuela*48 | E = 51; C 1(same area) = 30; C 2 (other area) = 50 | Cross-sectional | Occupational questionnaire. AChE and PChE not considered | Low (2) | Yes | Bivariate analysis. Did not estimate association between biomarkers and effects |
| Gomes et al. 1998/Arab Emirates56 | Farmers = 226; New farmers = 92; Control = 226 | Cross-sectional | Occupational questionnaire. AChE and HAchE E/C | Intermediate-High (6) | Yes | No consideration of alcohol or drugs consumption |
| Bazylewicz et al. 1999/Poland*35 | 26/25 | Longitudinal | Occupational questionnaire | Intermediate (5) | Yes | Biomarkers were not used. None includes predictive models or associations |
| Otero et al. 2000/Mexico49 | WAIS: 30/30; Benton; 27/27; Neuropsychological battery: 21/21 | Cross-sectional | Occupational questionnaire | Intermediate-Low (3) | Yes | Nonprobabilistic sample. No use of biomarker, no control of confounders in statistical analysis |
| Steenland et al. 2000/USA38 | 191/189 | Cross-sectional | Chlorpyrifos (TCPy) in urine (E/C) | Intermediate-High (7) | No | Authors point out that the sample may not be representative |
| Jamal et al. 2002/England55 | 72 sheep livestock farmers: no neuropathy = 15; likely = 34 and with neuropathy = 23 | Case-Control | Occupational questionnaire | Intermediate-Low (4) | No | No matching of control and cases |
| Wesseling et al. 2002/Costa Rica39 | 81/130 | Cross-sectional | Occupational questionnaire; Clinical records; AChE (H/L) | Intermediate-Low (4) | Yes | Sample of poisoned smaller than control group |
| Farahat et al. 2003/Egypt40 | 52/50 | Cross-sectional | AChE (E/C) | Intermediate-High (6) | Yes | Convenience sampling |
| Kamel et al. 2003/USA*41 | 288/51 | Cross-sectional | Occupational questionnaire | Intermediate (5) | Yes | No measuring of biomarkers of exposure |
| Salvi et al. 2003*/Brazil53 | 37/25 | Longitudinal | Occupational questionnaire (ACHe normal for all) | Intermediate-Low (4) | No | Nonrandomized small sample and no consideration of confounders |
| Stephens et al. 2004/England42 | Appliers = 37, pig livestock farmers = 26, builder = 31 | Cross-sectional | Questionnaire DEP/DETP Metabolites (E/C) | Intermediate-High (6) | Yes | Small sample. No controlling selection of workers |
| Orozco et al. 2005/Mexico61 | 17/24 | Cross-sectional | Occupational questionnaire | Low (2) | Yes | Small sample, no control of confounders and no use of biomarkers |
| Roldán et al. 2005/Spain43 | 40/26 | Cross-sectional | Small sample. Bu ChE (E/C) | Intermediate-High (7) | Yes | No consideration of age and education as confounding variables |
| Roldan et al. 2006/Spain44 | Acute = 24; Chronic = 40; control = 26 | Cross-sectional | Occupational questionnaire | Intermediate-High (6) | Yes | No consideration of age, education, and BuChE value |
| Rothlein et al. 2006/USA45 | Exposed workers = 96; Control = 45 | Longitudinal | DAP metabolites (E/C) in fall and spring | High (8) | Yes | Control group with greater educational level than exposed (one more year) |
| Rohlman et al. 2007/USA46 | Farm workers = 119; Control = 56 | Cross-sectional | Occupational questionnaire.Measuring of biomarkers and DNA damage, but not reported | Intermediate-Low (3) | Yes | Different sample size between exposed and no exposed groups. No report of biomarkers results |
| Mackenzie et al., 2010/England47 | 127 workers exposed to sheeps (67 worker and 60 retired); 78 controls (38 workers and 40 retired) | Cross-sectional | Occupational questionnaire. Sample of venous blood for analysis of PON1 (not associated with effect) | Intermediate (5) | Yes | No estimation of relation between effect and biomarker |
| Bayrami et al, 2012*/Iran59 | 40/40 | Cross-sectional | Occupational questionnaire AChE (E/C) | Intermediate (5) | No | No control of confounders. Cognitive functioning measured with a screening test |
| Blanc-Lapierre 2013/France50 | Exposed between 1975 y 1995 = 443; Control = 171 | Longitudinal | Occupational questionnaire, OP Residues in vegetables (environmental arrays) | High (8) | Yes | No use of biomarker to measure OP exposure |
| Malekirad et al. 2013/Iran*60 | 187/187 | Cross-sectional | Occupational questionnaire | Intermediate-High (6) | Yes | No use of biomarkers to measure exposure |
| Berent et al. 2014/USA54 | 53/60 | Longitudinal | CPF (Chlorpyrifos in urine E/C) and activity BuChe y AChE | High (9) | No | Selection of workers from only one workplace |
*This study measured other variables and health effects besides the neuropsychological functioning.
AChE = Acetylcholinesterase. BuChE = Butyrylcholinesterase. HAchE = Hemoglobin adjusted erythrocyte cholinesterase activity. ChE = Cholinesterase. DAP = Dialkyl phosphates. DETP = Diethyl thiophosphate. DMTP = Dimethyl thiophosphate. CPF = Chlorpyrifos. E/C = Exposed/Control. H/L = High/Low.
Measures of exposure and neuropsychological effects
Although 23 studies reported the use of biomarkers, only 15 (45%) used biomarkers to determine the level of exposure in exposed and control workers and its association with neuropsychological performance. Of these, five included biomarkers of exposure, three studies with dialkyl phosphate metabolites42,45,51, and two with urinary chlorpyrifos.38,54 One study measured environmental exposure with vegetable arrays50 and 24 studies (73%) assessed exposure through questionnaires about occupation information relating to pesticide usage. Of these, eight studies determined exposure to OP pesticides and their relation to neuropsychological functioning through survey questions; and three used clinical records of OP poisoning. A synthesis of the neuropsychological effects is shown in Table 4.
Table 4.
Neuropsychological outcomes of organophosphate pesticide exposure studies listed in Table 3
| Studies/country | Memory | Motor speed/coordination/visomotor/spatial | Processing/speed/attention/perception | Verbal abilities | IQ | Cognitive impairment | Brain morphology |
|---|---|---|---|---|---|---|---|
| Rodnitzky et al. 1975/USA29 | No effect | n/a | No effect | No effect | n/a | n/a | n/a |
| Korsak et al. 1977/USA57 | No effect | ↓Bender Visual Motor Gestalt test | ↓Trail Making Test part B. | n/a | n/a | n/a | Affect left frontal hemisphere |
| Delgado et al. 1986/Cuba58 | n/a | ↓ Bender Visual Motor Gestalt Test | n/a | n/a | n/a | n/a | n/a |
| Maizlish et al. 1987/USA51 | No effect | No effect | No effect | No effect | n/a | n/a | n/a |
| Rosenstock et al. 1991/Nicaragua30 | ↓Digit Span and visual memory | ↓Sequencing, problem solving, steadiness y motor dexterity. | ↓Digit Symbol | n/a | n/a | n/a | n/a |
| Daniell et al. 1992/USA52 | No effect | No effect | No effect | n/a | n/a | n/a | n/a |
| Richter et al. 1992/Israel31 | ↓Digit Span and Benton test | No effect | ↓Digit symbol | n/a | n/a | n/a | n/a |
| Ames et al. 1995/USA32 | No effect | No effect | No effect | n/a | n/a | n/a | n/a |
| Stephens et al. 1995/England33 | No effect | n/a | ↑Reaction time, latency in symbol digit, time in syntactic | No effect | n/a | n/a | n/a |
| Cole et al. 1997/Ecuador34 | No effect | ↓Psychomotor, motor functions, spatial tests | Applicators;↓attention tests | No effect | n/a | n/a | n/a |
| Fiedler et al. 1997/USA36 | No effect | No effect | ↓Concentration and answer velocity | No effect | n/a | n/a | n/a |
| London et al. 1997/South Africa37 | No effect | ↓Pursuit Aiming y Santa Ana pegboard nondominant hand | No effect | No effect | n/a | n/a | n/a |
| Rodríguez et al. 1997/Venezuela48 | ↓ Concentration & memory | No effect | n/a | n/a | n/a | n/a | n/a |
| Gomes et al. 1998/Arab Emirates56 | n/a | ↓Aiming Test. | ↓ Digit symbol | n/a | n/a | n/a | n/a |
| Bazylewicz et al. 1999/Poland35 | No effect | ↓ Motor steadiness | ↑Reaction times | No effect | n/a | n/a | n/a |
| Otero et al. 2000/Mexico49 | ↓Digit Span, Test of Benton and recent memory | ↓Premotor & constructive praxia | ↓Incomplete figures, sort of stories, tactile and auditory attention. | ↓Information, comprehension, arithmetic, similarities & vocabulary | 15.3 point <VIQ & 14 point < Full scale IQ | n/a | n/a |
| Steenland et al. 2000/USA38 | No effect | n/a | No effect | No effect | n/a | n/a | n/a |
| Jamal et al. 2002/England55 | No effect | No effect | n/a | n/a | n/a | n/a | n/a |
| Wesseling et al. 2002/Costa Rica39 | ↓ Working memory test | No effect | ↑Reaction time and ↓Processing Speed | No effect | n/a | n/a | n/a |
| Farahat et al. 2003/Egipt40 | ↓Digit Span & Visual Retention | ↓Trail Making A y B | ↓Digit symbol. | ↓Similarities subtest | n/a | n/a | n/a |
| Kamel et al. 2003/USA41 | ↓Digit span | ↓Santa Ana test, Finger Tapping preferred hand and nonpreferred hand | No effect | n/a | n/a | n/a | n/a |
| Salvi et al. 2003/Brazil53 | No effect | n/a | n/a | n/a | n/a | No effect | n/a |
| Stephens et al. 2004/England42 | No effect | n/a | ↓Syntactic reasoning test | No effect | n/a | n/a | n/a |
| Orozco et al. 2005/Mexico61 | n/a | n/a | n/a | n/a | n/a | ↓MMSE | n/a |
| Roldán et al. 2005/Spain43 | ↓Visual memory | ↓ Copy quality ↓Copy time | No effect | ||||
| Roldan et al. 2006/Spain44 | ↓Verbal memory | No effect | ↓Visual perception, Rey test copy & visual recognition | No effect | n/a | n/a | n/a |
| Rothlein et al. 2006/USA45 | No effect | ↑DAP ↓Finger tapping | ↑DAP ↑selective attention latency & Symbol digit latency. | n/a | n/a | n/a | n/a |
| Rohlman et al. 2007/USA46 | ↓Match-Sample, Digit Span forward & reverse | n/a | ↑ Reaction time & latency in Symbol Digit &↓ Continuous performance | n/a | n/a | n/a | n/a |
| Mackenzie et al. 2010/England47 | ↓ Auditory & visual memory; verbal ability; strategy making; digit span; letter-number; immediate & delayed visual; immediate, delayed & recognition auditory | Association between duration of exposure and impairments in these areas. Correlation with fine motor control (rs = 0.20, p < 0.05) | n/a | n/a | ↓ 6 points in WAIS-III full-scale IQ (p < 0.001) | n/a | n/a |
| Bayrami et al. 2012/Iran59 | n/a | n/a | n/a | n/a | n/a | No effect | No effect |
| Blanc-Lapierre 2013/France50 | ↓ BVRT(visual memory) & WPAT (working memory) | n/a | ↓Stroop Test & ↓TMT-A (Visual attention & task switching) | n/a | n/a | ↓ MMSE | n/a |
| Malekirad et al. 2013/Iran60 | ↓ verbal, no verbal & prospective memory | ↓ Psychomotor speed and spatial operation. | ↓Selective & Divided Attention | n/a | n/a | n/a | n/a |
| Berent et al. 2014/USA54 | No effect | No effect | No effect | n/a | n/a | n/a | n/a |
↓ = Low.
↑ = High.
Notes: BVRT = Benton Visual Retention Test. WPAT = Wechsler Paired-associates Test. TMT = Trail Making. rs = Spearman’s correlation. IQ = Intelligence quotient. VIQ = Verbal intelligence quotient. WAIS-III = Wechsler Adults Intelligence Scale, third edition. MMSE = Mini Mental State Examination. N/A = not applicable.
Rating of quality
Three longitudinal studies were high quality, one of which found no effect.54 Eleven studies were rated as intermediate-high quality, ten as intermediate, seven as intermediate-low, and two as low/poor. The latter two found a relationship between workers exposed to pesticides and lower performance in memory and cognitive impairment but had small sample size. These two studies also used screening tools or subsections of neuropsychological tests, did not use biomarkers, and did not control for confounding variables in their statistical analysis.
Nine studies (27%) found no significant association between exposure to OP pesticides and neuropsychological performance in agricultural workers. Of these nine, one was rated as high quality54 and three as intermediate-high quality.38,51,52
Measurement of neuropsychological effects
The neuropsychological tests most commonly administered were all subtests of the Wechsler intelligence scales (Table 2). The digit span subtests (forward and reverse order), which measure auditory working memory, were used in 18 studies.30–47 The digit symbol subtest was used to measure processing speed in eight studies, block design in five studies, vocabulary subtest in three studies,34,38,39 and the information subtest in two studies. However, despite its frequent use, only three studies43,47,49 administered the full Wechsler Adult Intelligence Scale.
The most commonly used tests were instruments that assess memory and processing speed. For memory, the Benton Visual Retention test was administered in 10 studies. With regard to the processing speed and attention, tests assessing reaction time were applied in 14 studies, processing speed of the task with the digit symbol test in 10 studies, and the trail making test A and B in 10 studies. Finally, tests that assess fine motor skills and motor speed were also among the most frequently used, specifically the Santa Ana Pegboard test administered in seven studies and the Pursuit aiming test administered in seven studies.
Association of OP exposure and neuropsychological functioning
Among the fifteen studies (Table 4) that evaluated a dose-response relationship between exposure to OP pesticides using biomarkers and neuropsychological performance in workers and the one study that evaluated environmental exposure,50 nine found an association between increased exposure to OP pesticides and poor performance in some area of neuropsychological functioning. The cognitive functions with poorer performance corresponded to motor speed, motor coordination, and visuospatial coordination; memory, grouped as working memory, short term, verbal, visual or auditory;39,40,43,45,50 and processing speed and attention. With regard to verbal skills, IQ, cognitive impairment, and brain morphology, only one study that utilized biomarkers found an association between low presence of acetylcholinesterase and decreased ability to describe similarities between two concepts.40 Of the two studies that evaluated brain morphology, one found an association between low level of acetylcholinesterase and lesser functioning in the left frontal lobe.57
Of the two high-quality studies that found a neuropsychological effect,45,50 one reported45 that a higher concentration of DAP metabolites in urine resulted in poorer short-term memory performance, visual motor coordination, and increased latency on tests of selective attention and processing speed. A partial correlation (p < 0.05) was observed between metabolites level and selective attention latency = 0.251, symbol digit latency = 0.281; finger tapping (preferred hand) = −0.252; finger tapping (nonpreferred hand) = −0.13; and finger tapping (alternating hand) = −0.208. The second of these high-quality studies assessed exposure to environmental arrays50 and found that higher exposure to OP pesticides was associated with poorer visual and working memory (OR: 3.2, 95% CI 1.5–6.8; OR: 2.3 95% CI 1.2–4.5 respectively), poorer performance on tests of visual attention (OR: 3.0 95% CI 1.3–6.6), poorer processing speed (OR: 2.2 95% CI 1.2–4.0), and greater cognitive impairment (OR: 1.2 95% .01–1.5).
Discussion
This review indicates that although evidence exists regarding the relationship between decreased neuropsychological performance in workers with exposure to OP pesticides, all findings were not consistent. Also, the studies do not all evaluate the same cognitive skills. Many of the reviewed studies have limitations in the study design, including not controlling for bias (e.g. some studies do not make adjustments in the statistical models by age, gender, or education).40,47 Of the 33 studies, nine found no association between exposure to OP pesticides and neuropsychological performance. Only three of the nine studies were classified as intermediate-high38,51,52 or high quality54 with acceptable control of confounders and use of biomarkers to determine exposure. On the other hand, among the studies that find an association between pesticide exposure and neuropsychological performance, only two have suitable control of confounders and include the use of biomarkers of exposure or environmental arrays.45,50 Five have an intermediate-high study quality level with a dose-response analysis, but with some measurement biases.34,40,42,43,56 Therefore, 18 studies (55%) that find an association do not assess biomarkers on neuropsychological performance of workers, and only seven studies of intermediate-high or high quality (21%) found an association between exposure to OP pesticides and poorer cognitive or neuropsychological performance.
However, studies that assessed exposure via questionnaires based on agricultural occupation and years of exposure to OP pesticides or used biomarkers without linking their findings with neuropsychological performance suggest that there are alterations and cognitive difficulties affecting worker health as a result of the conditions of the agricultural labor. Although these studies are less reliable due to their lack of control of confounders or to their lacking of a more specific analysis, they can be useful as a foundation to guide future research. This includes areas and characteristics of the occupational population that must be controlled to reduce bias and confounding that may affect the data. Additionally, a key aspect is the suitable selection of the biomarker type according to the occupational characteristics involved. Biomarkers that measure erythrocyte cholinesterase are biomarkers of effect used to assess acute poisoning or chronic exposure. Plasma cholinesterase measurement is used only for acute poisoning diagnosis. Additionally, neuropathic esterase in human lymphocytes is measured for the diagnosis of retarded neuropathy caused by organophosphate pesticides. On the other hand, urinary biomarkers are useful to measure exposure and are the most sensitive for exposure assessment of organophosphate pesticides. Biomonitoring studies of urinary dialkyl phosphate metabolites may reflect exposure to OP pesticides in farmworkers, and their use is suggested to detect low doses of pesticides.5–8
However, if commonly used pesticides can be identified via questionnaire, specific biomarkers according to the type of pesticide are recommended, similar to studies conducted by Steenland et al.38 and Berent et al.54 While these two studies found no association, they are not sufficient to guide conclusions regarding workers. Chronic exposure to OP pesticide assessment requires consecutive urinary dialkyl phosphates measurements, which was not clearly developed in any of the studies reviewed.
With regard to neuropsychological assessment, the evidence of the studies reviewed shows that there is a trend toward the use of a selection of subtests or nonvalidated brief scales, instead of full scales or full neuropsychological test batteries. Brief scales correspond to tests that require little time and are easy to administer, allowing a first cut point to distinguish those with impairment. However, there are limitations in brief scales related to the low sensitivity of detecting specific cognitive difficulties and the tendency to give false positives.27 Given the costs and time involved in neuropsychological assessment, many researchers prefer to use only some subtests of standardized full scales, reducing the complete assessment of cognitive functions and not allowing for a comparison of the overall performance with more specific cognitive skills. This can make it difficult to rule out specific problems from a broader overview of the neuropsychological performance.26–28 On the other hand, neuropsychological batteries correspond to a set of subtests or tests that measure cognitive functions in a systematic way to detect the presence of neurological disorders. The relevance of these batteries is that they result in a cognitive profile, allow the detection of the characteristics of different brain disorders that could affect an individual, and identify preserved cognitive functions. The Neurobehavioral Core Test Battery (NCTB) was administered in eight of the reviewed studies30,31,34,35,37,39,41,44 and is recommended by the World Health Organization to detect neurotoxicity.65 However, there is evidence that the NCTB may not be reliable for identifying neurotoxic effects among people with less than nine years of education or among diverse cultures (e.g. aboriginal and African cultures).66,67 Among the reviewed studies, only Cole et al.34 report using a translated version of the NCTB procedures manual and field trials permitting adjustments in ordering and instructions prior to application with an Ecuadorean population. These previous procedures could offer an alternative to improve reliability when using the NCTB, especially with populations in developing countries. Notwithstanding, a scientific panel with members from three disciplines (neuropsychology, experimental psychology, neurology) started developing a revised version of the NCTB in 2014.68 Thus, more discussion is needed on evaluating neurotoxicity with this test battery that still represents the international standard for identifying adverse human behavioral effects due to neurotoxic chemical exposure.
Studies evaluating a dose-response relationship between exposure to OP pesticides and neuropsychological performance in farmworkers found decreased performance in processing speed (the ability to perform tasks fluidly linked to nonverbal material and attention, both focused and sustained), poorer performance in motor and visuospatial coordination, and decreased visual and working memory skills compared to unexposed populations. Evidence from 15 studies that assess the association of exposure with OP pesticides using biomarkers and neuropsychological performance through neuropsychological tests highlight difficulties in these three areas, affecting agricultural workers of various nationalities.29,38–40,42,43,45,51,52,54,56–59
With regard to socio-demographic characteristics, years of exposure to agricultural work, male gender, work as a pesticide applicator, and fewer than 12 years of education were most influential on neuropsychological effects. These findings may be related to gender differences in the agricultural work. Women are commonly associated with the cultivation of crops and harvest activities. Meanwhile, a greater proportion of pesticide applicators with direct exposure are men.69 In addition, farmworkers may be more vulnerable than the nonagricultural population due to their fewer years of education, potentially resulting in lower health literacy and therefore knowledge about safe use practices for pesticides.34,35,49 All of the above, in combination with their greater number of years of exposure, may result in a negative impact on their cognitive functioning.
Most studies reviewed do not control for socio-demographic variables and perform comparisons through bivariate statistical analysis. Other weaknesses include the scarcity of longitudinal studies, small sample sizes, difficulties in the selection of cases and controls, biases due to statistical models not adjusted for age, gender, years of education, and lack of control of alcohol or drug consumption as confounders. On the other hand, few studies utilize predictive models to estimate the association between exposure to OP pesticides using biomarkers or environmental arrays and cognitive performance on standardized neuropsychological tests. The evidence suggests that due to recent scientific and technological development in the biochemical analysis of pesticide exposure in humans,7 more research that assesses the effects of OP pesticides on human health with biomarkers of exposure should be generated. The scarcity of studies evaluating exposure to OP pesticides with biomarkers has been observed in other systematic reviews of the association between exposure to OP pesticides and health effects as well as neurobehavioral performance in workers.23,25
Based on our review, we suggest some points for actions regarding health and safety of farmworkers and further research. First, interventions aimed at reducing pesticide exposure in farmworkers should be strengthened. There is some evidence concerning the benefits of the education of agricultural populations. Workplace training of safe pesticide handling practice and application in the agricultural community may reduce pesticide exposure and increase awareness of the harmful effects.70,71 However, other studies have reported that educational interventions with farmworkers are not sufficiently effective in reducing pesticide exposure.72 Therefore, careful monitoring and further research on the results of these interventions is required.
Regarding pathways of exposure, further studies should note the presence of other pathways besides the occupational route. For instance, studies in the general population, specifically in children of Chile,13,63 indicate that pesticide exposure is mainly due to the consumption of vegetables contaminated with residues of chlorpyrifos. Those studies show levels of concentration of OP metabolites in urine greater than those reported in international studies from Europe and USA.
With respect to the neuropsychological assessment, future research should consider new revisions of test batteries, especially the NCTB due to its traditional use in this field of work and specific aim toward the assessment of neurotoxic effects. Currently, there have been interdisciplinary efforts for reconsideration of the NCTB assessment strategy and test selection. Based on that, a new revised version of the NCTB could be expected soon.68
Finally, we suggest that future research and interventions address other variables and issues of interest. These include the risk perception of workers in regard to the use of OP pesticides in the occupational setting; workplace conditions of workers and the use of personal protective equipment in the agricultural workplace and pesticide application; evaluation of the procedures of supervision and control on the part of authorities. Also, it is recommended the development of policies concerning the banning of the use of the most dangerous pesticides (especially in countries with weaker regulations), better medical monitoring of farmworkers, and the providing of pesticides safety education programs for farmworkers and their families.
In conclusion, although a number of studies have determined a relationship between a decreased neuropsychological performance and exposure to OP pesticides among farmworkers, there is no agreement regarding results or cognitive skills measured. Many of these studies have limitations in design or lack of control of bias, and most studies that find an effect do not use biomarkers for exposure measurement. Future studies should more specifically address the use of biomarkers of exposure to assess dose-response, thus improving the control of biases. Furthermore, validated neuropsychological batteries should be administered.
References
- 1.Matthews G. Pesticides: health, safety and the environment. Oxford: Blackwell Publishing; 2006 10.1002/9780470995853 [DOI] [Google Scholar]
- 2.Levine MJ. Pesticides: a toxic time bomb in our midst. Westport, CT: Greenwood Publishing Group; 2007. [Google Scholar]
- 3.Thundiyil JG, Stober J, Besbelli N, Pronczuk J. Acute pesticide poisoning: a proposed classification tool. Bull World Health Organ. 2008;86(3):205–209. 10.2471/BLT.00.000000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.PAHO Health in the Americans 2012 ed Washington, DC: Author; 2012. [Google Scholar]
- 5.CDC Fourth National report on human exposure to environmental chemicals Atlanta, GA: Author; 2009. [PubMed] [Google Scholar]
- 6.Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366(1–2):1–13. 10.1016/j.cca.2005.10.008 [DOI] [PubMed] [Google Scholar]
- 7.Barr DB, Bradman A, Freeman N, Whyatt RM, Wang RY, Naeher L, et al. Studying the relation between pesticide exposure and human development In: DC B, editor. Human developmental neurotoxicology. New York, NY: Taylor & Francis Group; 2006. p. 253–285. [Google Scholar]
- 8.Duggan A, Charnley G, Chen W, Chukwudebe A, Hawk R, Krieger RI, et al. Di-alkyl phosphate biomonitoring data: assessing cumulative exposure to organophosphate pesticides. Regul Toxicol Pharmacol. 2003;37(3):382–395. 10.1016/S0273-2300(03)00031-X [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez V, Huen K, Venkat S, Pratt K, Xiang P, Harley KG, et al. Cholinesterase and paraoxonase (PON1) enzyme activities in Mexican–American mothers and children from an agricultural community. J Expo Sci Environ Epidemiol. 2012;22(6):641–648. 10.1038/jes.2012.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pesticides and pest management. 2016. Available from: http://www.hc-sc.gc.ca/cps-spc/pest/index-eng.php
- 11.CDC Fourth national report on human exposure to environmental chemicals, updated tables 2012 February. 2012. [Google Scholar]
- 12.Health and consumers. Pesticides-EU rules. Available from: http://ec.europa.eu/food/plant/pesticides/index_en.htm
- 13.Muñoz-Quezada MT, Iglesias V, Lucero B, Steenland K, Barr DB, Levy K, et al. Predictors of exposure to organophosphate pesticides in schoolchildren in the Province of Talca, Chile. Environ Int. 2012;47:28–36. 10.1016/j.envint.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coronado GD, Thompson B, Strong L, Griffith WC, Islas I. Agricultural task and exposure to organophosphate pesticides among farmworkers. Environ Health Perspect. 2004;112(2):142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alavanja MC, Hoppin JA, Kamel F. Health effects of chronic pesticide exposure: cancer and neurotoxicity* 3. Annu Rev Public Health. 2004;25:155–197. 10.1146/annurev.publhealth.25.101802.123020 [DOI] [PubMed] [Google Scholar]
- 16.Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, et al. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am J Epidemiol. 2007;165(12):1397–1404. 10.1093/aje/kwm029 [DOI] [PubMed] [Google Scholar]
- 17.Eskenazi B, Rosas LG, Marks AR, Bradman A, Harley K, Holland N, et al. Pesticide toxicity and the developing brain. Basic Clin Pharmacol Toxicol. 2008;102(2):228–236. 10.1111/j.1742-7843.2007.00171.x [DOI] [PubMed] [Google Scholar]
- 18.Rosas LG, Eskenazi B. Pesticides and child neurodevelopment. Curr Opin Pediatr. 2008;20(2):191–197. 10.1097/MOP.0b013e3282f60a7d [DOI] [PubMed] [Google Scholar]
- 19.Handal AJ, Lozoff B, Breilh J, Harlow SD. Effect of community of residence on neurobehavioral development in infants and young children in a flower-growing region of Ecuador. Environ Health Perspect. 2007;115(1):128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurewicz J, Hanke W. Prenatal and childhood exposure to pesticides and neurobehavioral development: review of epidemiological studies. Int J Occup Med Environ Health. 2008;21(2):121–132. [DOI] [PubMed] [Google Scholar]
- 21.Delgado E, McConnell R, Miranda J, Keifer M, Lundberg I, Partanen T, et al. Central nervous system effects of acute organophosphate poisoning in a two-year follow-up. Scand J Work Environ Health. 2004;30(5):362–370. 10.5271/sjweh.824 [DOI] [PubMed] [Google Scholar]
- 22.Steenland K, Jenkins B, Ames RG, O’Malley M, Chrislip D, Russo J. Chronic neurological sequelae to organophosphate pesticide poisoning. Am J Public Health. 1994;84(5):731–736. 10.2105/AJPH.84.5.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohlman DS, Anger WK, Lein PJ. Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. NeuroToxicology. 2011;32(2):268–276. 10.1016/j.neuro.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ismail A, Bodner T, Rohlman D. Neurobehavioral performance among agricultural workers and pesticide applicators: a meta-analytic study. Occup Environ Med. 2012;69(7):457–464. 10.1136/oemed-2011-100204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi N, Hashizume M. A systematic review of the influence of occupational organophosphate pesticides exposure on neurological impairment. BMJ Open. 2014;4(6):e004798. 10.1136/bmjopen-2014-004798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bausela E. Evaluación neuropsicológica en población adulta; instrumentos de evaluación [Neuropsychological assessment in adult population; assessment tools]. CNPs. 2008;2:136–149. [Google Scholar]
- 27.Tirapu Ustárroz J. La evaluación neuropsicológica [Neuropsychological assessment]. Interven Psicosoc. 2007;16:189–211. [Google Scholar]
- 28.Lezak MD. Neuropsychological assessment: Oxford University Press; 2004. [Google Scholar]
- 29.Rodnitzky RL, Levin HS, Mick DL. Occupational exposure to organophosphate pesticides: a neurobehavioral study. Arch Environ Health. 1975;30(2):98–103. 10.1080/00039896.1975.10666651 [DOI] [PubMed] [Google Scholar]
- 30.Rosenstock L, Keifer M, Daniell WE, McConnell R, Claypoole K. Chronic central nervous system effects of acute organophosphate pesticide intoxication. Lancet. 1991;338(8761):223–227. 10.1016/0140-6736(91)90356-T [DOI] [PubMed] [Google Scholar]
- 31.Richter ED, Chuwers P, Levy Y, Gordon M, Grauer F, Marzouk J, et al. Health effects from exposure to organophosphate pesticides in workers and residents in Israel. Isr J Med Sci. 1991;28(8–9):584–598. [PubMed] [Google Scholar]
- 32.Ames RG, Steenland K, Jenkins B, Chrislip D, Russo J. Chronic neurologic sequelae to cholinesterase inhibition among agricultural pesticide applicators. Arch Environ Health. 1995;50(6):440–444. 10.1080/00039896.1995.9935980 [DOI] [PubMed] [Google Scholar]
- 33.Stephens R, Spurgeon A, Calvert I, Beach J, Levy L, Harrington J, et al. Neuropsychological effects of long-term exposure to organophosphates in sheep dip. Lancet. 1995;345(8958):1135–1139. 10.1016/S0140-6736(95)90976-1 [DOI] [PubMed] [Google Scholar]
- 34.Cole DC, Carpio F, Julian J, Leon N, Carbotte R, De Almeida H. Neurobehavioral outcomes among farm and nonfarm rural ecuadorians. Neurotoxicol Teratol. 1997;19(4):277–286. 10.1016/S0892-0362(97)00019-6 [DOI] [PubMed] [Google Scholar]
- 35.Bazylewicz-Walczak B, Majczakowa W, Szymczak M. Behavioral effects of occupational exposure to organophosphorous pesticides in female greenhouse planting workers. Neurotoxicology. 1999;20(5):819–826. [PubMed] [Google Scholar]
- 36.Fiedler N, Kipen H, Kelly-McNeil K, Fenske R. Long-term use of organophosphates and neuropsychological performance. Am J Ind Med. 1997;32(5):487–496. 10.1002/(ISSN)1097-0274 [DOI] [PubMed] [Google Scholar]
- 37.London L, Myers J, Nell V, Taylor T, Thompson M. An investigation into neurologic and neurobehavioral effects of long-term agrichemical use among deciduous fruit farm workers in the Western Cape, South Africa. Environ Res. 1997;73(1):132–145. [DOI] [PubMed] [Google Scholar]
- 38.Steenland K, Dick RB, Howell RJ, Chrislip DW, Hines CJ, Reid TM, et al. Neurologic function among termiticide applicators exposed to chlorpyrifos. Environ Health Perspect. 2000;108(4):293. 10.1289/ehp.00108293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling C, Keifer M, Ahlbom A, McConnell R, Moon J-D, Rosenstock L, Hogstedt C.. Long-term neurobehavioral effects of mild poisonings with organophosphate and n-methyl carbamate pesticides among banana workers. Int J Occup Environ Health. 2002;8(1):27–34. [DOI] [PubMed] [Google Scholar]
- 40.Farahat T, Abdelrasoul G, Amr M, Shebl M, Farahat F, Anger W. Neurobehavioural effects among workers occupationally exposed to organophosphorous pesticides. Occup Environ Med. 2003;60(4):279–286. 10.1136/oem.60.4.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamel F, Rowland AS, Park LP, Anger WK, Baird DD, Gladen BC, et al. Neurobehavioral performance and work experience in florida farmworkers. Environ Health Perspect. 2003;111(14):1765. 10.1289/ehp.6341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephens R, Sreenivasan B. Neuropsychological effects of long-term low-level organophosphate exposure in orchard sprayers in England. Arch Environ Health. 2004;59(11):566–574. 10.1080/00039890409603435 [DOI] [PubMed] [Google Scholar]
- 43.Roldantapia L, Parron T, Sanchezsanted F. Neuropsychological effects of long-term exposure to organophosphate pesticides. Neurotoxicol Teratol. 2005;27(2):259–266. 10.1016/j.ntt.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 44.Roldan-Tapia L, Nieto-Escamez FA, del Aguila EM, Laynez F, Parron T, Sanchez-Santed F. Neuropsychological sequelae from acute poisoning and long-term exposure to carbamate and organophosphate pesticides. Neurotoxicol Teratol. 2006;28(6):694–703. 10.1016/j.ntt.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 45.Rothlein J, Rohlman D, Lasarev M, Phillips J, Muniz J, McCauley L. Organophosphate pesticide exposure and neurobehavioral performance in agricultural and nonagricultural hispanic workers. Environ Health Perspect. 2006;114(5):691–696. 10.1289/ehp.8182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rohlman DS, Lasarev M, Anger WK, Scherer J, Stupfel J, McCauley L. Neurobehavioral performance of adult and adolescent agricultural workers. NeuroToxicology. 2007;28(2):374–380. 10.1016/j.neuro.2006.10.006 [DOI] [PubMed] [Google Scholar]
- 47.Mackenzie Ross SJ, Brewin CR, Curran HV, Furlong CE, Abraham-Smith KM, Harrison V. Neuropsychological and psychiatric functioning in sheep farmers exposed to low levels of organophosphate pesticides. Neurotoxicol Teratol. 2010;32(4):452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodríguez N, López de Andrade S, Arias Rojas F, Romero Vecchione E, Ciarleta E, Vasquez J, et al. Efectos de exposición prolongada a organofosforados en trabajadores agricolas [Effect of long-lasting exposure to OP pesticides in farm workers]. Arch Hosp Vargas. 1997;39(1/2):15–27. [Google Scholar]
- 49.Otero G, Porcayo R, Aguirre DM, Pedraza M. Estudio neuroconductual en sujetos laboralmente expuestos a plaguicidas [Neurobehavioral study in individuals ocupationally exposed to pesticides]. Rev Int Contam Ambient. 2000;16(2):67–74. [Google Scholar]
- 50.Blanc-Lapierre A, Bouvier G, Gruber A, Leffondre K, Lebailly P, Fabrigoule C, et al. Cognitive disorders and occupational exposure to organophosphates: results from the PHYTONER study. Am J Epidemiol. 2013;177(10):1086–1096. 10.1093/aje/kws346 [DOI] [PubMed] [Google Scholar]
- 51.Maizlish N, Schenker M, Weisskopf C, Seiber J, Samuels S. A behavioral evaluation of pest control workers with short-term, low-level exposure to the organophosphate diazinon. Am J Ind Med. 1987;12(2):153–172. 10.1002/(ISSN)1097-0274 [DOI] [PubMed] [Google Scholar]
- 52.Daniell W, Barnhart S, Demers P, Costa LG, Eaton DL, Miller M, et al. Neuropsychological performance among agricultural pesticide applicators. Environ Res. 1992;59(1):217–228. 10.1016/S0013-9351(05)80241-5 [DOI] [PubMed] [Google Scholar]
- 53.Salvi RM, Lara DR, Ghisolfi ES, Portela LV, Dias RD, Souza DO. Neuropsychiatric evaluation in subjects chronically exposed to organophosphate pesticides. Toxicol Sci. 2003;72(2):267–271. 10.1093/toxsci/kfg034 [DOI] [PubMed] [Google Scholar]
- 54.Berent S, Giordani B, Albers JW, Garabrant DH, Cohen SS, Garrison RP, et al. Effects of occupational exposure to chlorpyrifos on neuropsychological function: a prospective longitudinal study. NeuroToxicology. 2014;41:44–53. 10.1016/j.neuro.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 55.Jamal G, Hansen S, Pilkington A, Buchanan D, Gillham R, Abdel-Azis M, et al. A clinical neurological, neurophysiological, and neuropsychological study of sheep farmers and dippers exposed to organophosphate pesticides. Occup Environ Med. 2002;59(7):434–441. 10.1136/oem.59.7.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomes J, Lloyd O, Revitt MD, Basha M. Morbidity among farm workers in a desert country in relation to long-term exposure to pesticides. Scand J Work Environ Health. 1998;24(3):213–219. 10.5271/sjweh.301 [DOI] [PubMed] [Google Scholar]
- 57.Korsak RJ, Sato MM. Effects of chronic organophosphate pesticide exposure on the central nervous system. Clin. Toxicol. 1977;11(1):83–95. 10.3109/15563657708989822 [DOI] [PubMed] [Google Scholar]
- 58.Delgado O. Evaluación de funciones psicomotoras en trabajadores expuestos habitualmente a plaguicidas [Psychomotor function assessment in workers permanently exposed to pesticides]. Rev Cub Hig Epid. 1986;24(1):103–110. [Google Scholar]
- 59.Bayrami M, Hashemi T, Malekirad AA, Ashayeri H, Faraji F, Abdollahi M. Electroencephalogram, cognitive state, psychological disorders, clinical symptom, and oxidative stress in horticulture farmers exposed to organophosphate pesticides. Toxicol Ind Health. 2012;28(1):90–96. 10.1177/0748233711407243 [DOI] [PubMed] [Google Scholar]
- 60.Malekirad AA, Faghih M, Mirabdollahi M, Kiani M, Fathi A, Abdollahi M. Neurocognitive, mental health, and glucose disorders in farmers exposed to organophosphorus pesticides. Arch Ind Hygiene Toxicol. 2013;64(1):1–8. 10.2478/10004-1254-64-2013-2296 [DOI] [PubMed] [Google Scholar]
- 61.Orozco-De Los Ríos IS-V M, González-Ramírez D, García-Piñón J. Neuropatía periférica y deterioro de las funciones cognitivas asociados a exposición crónica a organofosforados [Peripheral Neuropathy and cognitive functiuons impairment related to chronic exposure to organophosphates]. Rev Med Inst Mex Seguro Soc. 2005;43(6):479–486. [PubMed] [Google Scholar]
- 62.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muñoz-Quezada MT, Lucero BA, Barr DB, Steenland K, Levy K, Ryan PB, et al. Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: a systematic review. NeuroToxicology. 2013;39:158–168. 10.1016/j.neuro.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moher D, Liberati A, Tetzlaff J, Altman DG. Reprint—preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–880. [PubMed] [Google Scholar]
- 65.World Health Organization (WHO) Office of Occupational Health Operational Guide for the WHO Neurobehavioral Test Battery. Geneva: WHO; 1986. [Google Scholar]
- 66.Anger WK, Cassitto MG, Liang YX, Amador R, Hooisma J, Chrislip DW, et al. Comparison of performance from three continents on the WHO-recommended neurobehavioral core test battery. Environ Res. 1993;62(1):125–147. 10.1006/enrs.1993.1097 [DOI] [PubMed] [Google Scholar]
- 67.Anger WK, Liang YX, Nell V, Kang SK, Cole D, Bazylewicz-Walczak B, et al. Lessons learned–15 years of the WHO-NCTB: a review. Neurotoxicology. 2000 October;21(5):837–846. [PubMed] [Google Scholar]
- 68.Anger WK. Reconsideration of the WHO NCTB strategy and test selection. NeuroToxicology. 2014;45:224–231. 10.1016/j.neuro.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, et al. The agricultural health study. Environ Health Perspect. 1996;104:362–369. 10.1289/ehp.96104362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orozco FA, Cole DC, Ibrahim S, Wanigaratne S. Health promotion outcomes associated with a community-based program to reduce pesticide-related risks among small farm households. Health Promot Int. 2011;26(4):432–446. 10.1093/heapro/dar006 [DOI] [PubMed] [Google Scholar]
- 71.Salvatore AL, Chevrier J, Bradman A, Camacho J, López J, Kavanagh-Baird G, et al. A community-based participatory worksite intervention to reduce pesticide exposures to farmworkers and their families. Am J Public Health. 2009;99(S3):S578. 10.2105/AJPH.2008.149146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lehtola MM, Rautiainen RH, Day LM, Schonstein E, Suutarinen J, Salminen S, et al. Effectiveness of interventions in preventing injuries in agriculture—a systematic review and meta-analysis. Scand J Work Environ Health. 2008;34(5):327–336. 10.5271/sjweh.1279 [DOI] [PubMed] [Google Scholar]