Abstract
Microbial production of acetone and butanol was one of the first large-scale industrial fermentation processes of global importance. During the first part of the 20th century, it was indeed the second largest fermentation process, superseded in importance only by the ethanol fermentation. After a rapid decline after the 1950s, acetone-butanol-ethanol (ABE) fermentation has recently gained renewed interest in the context of biorefinery approaches for the production of fuels and chemicals from renewable resources. The availability of new methods and knowledge opens many new doors for industrial microbiology, and a comprehensive view on this process is worthwhile due to the new interest. This thematic issue of FEMS Microbiology Letters, dedicated to the 100th anniversary of the first industrial exploitation of Chaim Weizmann's ABE fermentation process, covers the main aspects of old and new developments, thereby outlining a model development in biotechnology. All major aspects of industrial microbiology are exemplified by this single process. This includes new technologies, such as the latest developments in metabolic engineering, the exploitation of biodiversity and discoveries of new regulatory systems such as for microbial stress tolerance, as well as technological aspects, such as bio- and down-stream processing.
Keywords: Biobutanol, ABE fermentation, Weizmann process, biofuels, clostridia, biorefinery
Industrial production of acetone and butanol by fermentation—100 years later.
SOME HISTORY
The first to report about microbial butanol production was Pasteur in 1862 (Pasteur 1862). He observed this alcohol to be a fermentation product of a microbial culture. Pasteur named the culture Vibrion butyrique—most probably though it was a mixed bacterial culture comprising at least one Clostridium strain (Dürre 2007). While scientific interest was documented by some further reports, commercial interest was only triggered in the beginning of the 20th century by the need for synthetic rubber production (Jones and Woods 1986). At this time Chaim Weizmann became involved in the development of this process. He isolated a new bacterial culture, readily fermenting starchy material into acetone and butanol. Clostridium acetobutylicum was the main species in this culture. Starting from there, an industrial solvent production process was developed, based on available plants for ethanol fermentation. Interestingly, Weizmann was a chemist by training and he set about training himself to become a microbiologist as necessary. This underlines the close connection of industrial microbiology with chemistry, which tends to get a bit lost with the advent of genetic engineering and changing study courses. Nevertheless, renewed close collaboration of these two sciences is highly relevant—now more than in the past, as exemplary outlined by the thoughts of Dusselier, Mascal and Sels (2014), who provided a chemist's view of the biorefinery.
In 1915, a patent was issued claiming acetone–butanol fermentation with C. acetobutylicum, and industrial production on large scale commenced in 1916. The outbreak of WWI led to a high demand of acetone for smokeless powder production, shifting the interest from butanol as a product to acetone. During the war, the requirement for acetone resulted in the accumulation of butanol as an unwanted by-product of the fermentation which was stored. After the war, butanol again became a commercially important chemical—this time for the production of quick-drying lacquers, for the rapidly growing automobile industry. Starch was originally the carbon source on which the fermentation was based. However, molasses became cheaply available in large quantities and became the main carbon substrate for solvent production. This became possible only because new bacterial strains, readily fermenting sugars, had been isolated by then. During WWII, the demand reverted to acetone again. Unfortunately, weapons once more were the priority rather than goods. The importance of microbial processes for acetone and butanol production declined rapidly after the war. One reason was that petrochemistry was gaining scale and importance, and the production of solvents from petroleum became very cheap. Another reason was that molasses became increasingly sought after for the feed of cattle, leading to a significant price increase of the substrate.
Today, ∼50 years later, the Weizmann process and modern derivatives of it are gaining interest and importance once more. This time, the driver is the need for sustainability—the desire to shift back from petroleum to readily renewable resources, decreasing the carbon footprint and avoiding toxic chemicals as far as possible.
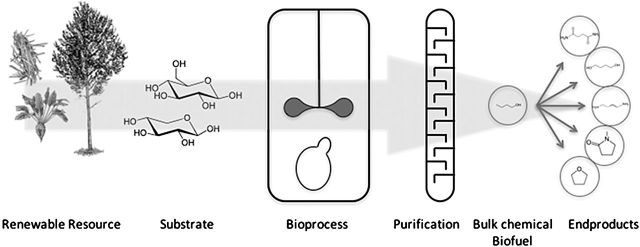
This thematic issue aims to shed some light on recent developments in this context. Microbial butanol fermentation is finding its way into modern biorefineries. Figure 1 depicts very generally the concept of microbial chemical production in biorefineries. A renewable resource, which can be biomass-, or waste-derived is converted into a substrate stream amenable to microbial conversion. The substrate is converted by means of a bioprocess into the product of choice. Importantly, the product must be purified before reaching the market. While clearly the bioprocess is central, and very often of most interest to researchers, the price of the chemical or fuel produced, which decides over failure or success of the approach, is dictated mainly by substrate and purification costs (Porro et al. 2014). This thematic issue will work along those lines, highlighting efforts not only on the microbiology itself, but on all steps of the process chain.
Figure 1.
Schematic representation of microbial chemical production from renewable resources. A renewable resource is converted into a substrate stream, which can be microbially converted into a base chemical. The chemical has to be purified before it can be upgraded to more advanced products.
A general overview about past achievements and pending challenges of (acetone-) butanol fermentation is given by Moon et al. (2016). They set the process into its historic and economic contexts in relation to the newest developments. The current frontiers of the process, with a particular focus on cell and metabolic engineering, are given by Chen and Liao (2016).
THE PROCESS—FROM SUBSTRATE VIA MICROBE TO PURIFICATION
As outlined before, substrate and purification costs are decisive for the commercial success of any chemical (and concomitantly microbial) process. While starch was historically the substrate of choice (and is still a major substrate for bioethanol production outside the countries which can grow sugar cane), a shift to molasses as a cheap waste product kept the profitability of the process high. With the opening of additional markets for molasses, its price increased, significantly contributing to the death of microbiological acetone and butanol fermentation. Nowadays, lignocellulose is seen as a promising carbon source for chemicals (Hasunuma et al. 2013). It is abundant and not in direct competition with food production and a lot of effort is therefore being invested into the development of its use. A relevant example for biobutanol production is the proposition of simultaneous saccharification and fermentation of pretreated corn stover (Dong et al. 2016). An innovative co-culture system of recombinant Escherichia coli for the bioconversion of cellulose hydrolysate has been suggested by Saini et al (2016). Baral et al. (2016), give a general overview about the use of corn stover as carbon source with a focus on economical aspects. Finally, the use of gaseous substrates for the fermentation of solvents is a very different approach (Dürre 2016), which can be based on various sources for carbon-rich gas, opening ways to valorize industrial off gases or gasified carbon-rich waste streams.
Clearly, the core of the fermentation process is microbiology. A major focus of this thematic issue will therefore be to present different views and approaches for the microbial process itself, from its history to the high end of microbial cell design. The history and importance of this process have led to a range of studies and reports, which can be used as a model for developing other microbial production processes. This thematic issue aims therefore to show the reader how modern technologies and new discoveries in all fields of microbiology can be employed for industrial needs.
Clostridia are natural acetone-butanol-ethanol (ABE) fermentation organisms. They are spore forming bacteria—what has medical relevance for some of the species—and which has technical relevance in context of solvent fermentation. Kolek et al. (2016) used flow cytometry to evaluate population heterogeneity with particular focus on spore numbers and quality during a solventogenic fermentation process. Spore formation constitutes a special challenge for the provision of the inoculum for large-scale microbial processes. Sandoval-Espinola, Chinn and Bruno-Barcena (2015) report about an optimized strategy for inoculum preparation.
Optimal production is dependent on optimized growth conditions, including the medium. The influence of calcium and zinc ions on fermentation performance has been characterized by Wu et al. (2016). However, the tolerance of the production organisms to high solvent concentrations remains a major challenge. High concentrations are needed to decrease purification costs, but are detrimental to the survival and productivity of the cultures. Peabody and Kao (2016) give an overview of the recent progress in increasing microbial solvent tolerance. Stress tolerance of microbial cell factories is clearly a major topic in industrial microbiology. It is a complex property of living cells, which is only partially understood and rational engineering approaches have therefore been difficult. An interesting approach by Jones, Venkataramanan and Papoutsakis (2016) shows that an increase of butanol tolerance is achieved by overexpression of two stress-responsive small, non-coding RNAs. The importance of such RNAs in the prokaryotic world has only recently been appreciated. Discovery of these new signalling molecules has brought a completely new level of cellular organisation to light, with implications for process understanding and bioengineering. This indicates once more that the common separation between fundamental and applied research is in most cases not useful. Fundamental research is not only a prerequisite for the development of applications, but engineering cell factories can lead to many fundamental discoveries. Another example is the question of sugar uptake of the bacteria. Essalem and Mitchell (2016) describe the characterization of a clostridial glucose-mannose phosphotransferase system, shedding light on sugar uptake and guiding further metabolic engineering approaches which bring us to the next field of interest. Historically, natural isolates were used for industrial ABE fermentation. These isolates might have been optimized by mutagenesis and selection. New methods for mutagenesis are being developed, such as an inducible transposon system for efficient random mutagenesis (Zhang et al. 2016). Furthermore, genetic engineering and thereby rational strain design are gaining importance also for ABE fermentation. Chen and Liao give a good review of the metabolic engineering of Clostridia. The understanding of the metabolism of the cells is the basis for rational strain design. Metabolic modelling helps to identify genetic targets, which might very well be non-intuitive. Dash, Ng and Maranas (2016) give a concise overview about current developments and applications in the metabolic modelling of Clostridia. Liao, Seo and Lu (2016) shed some light on approaches for systems-level modelling of the entire fermentation process. While ABE processes relied historically on Clostridia as productions hosts, other microorganisms are now attracting attention—the exploitation of biodiversity becomes important. Branduardi and Porro (2016) present a general outline of the requirements and provisions needed for a viable cell factory for butanol production. They conclude that yeasts might very well be alternative production hosts, thereby agreeing with Kuroda and Ueda (2016) who summarize recent engineering approaches using Baker's yeast for advanced biobutanol production. Escherichia coli has also been suggested as an alternative production host (Saini et al. 2016) as has Pseudomonas putida (Cuenca Mdel et al. 2016). Another aspect in this context is that in nature many metabolic tasks are split over different species. An example is the microbial community in the rumen of many grass eating animals. Digestion of cellulose and hemicellulose and conversion of the carbon into volatile organic acids (which are the actual nutritional basis of the ruminant) are achieved by a complex community of microorganisms carrying out different biochemical reactions (Sauer, Marx and Mattanovich 2012). Such concepts are in their infancy in the field of industrial microbiology, but are now being considered for microbial butanol production, as shown by Saini et al. (2016).
The purification of the solvents is the final step of the process. However, it is of utmost importance for the economic success. Friedl (2016) summarizes various aspects of possible downstream processing strategies. In situ recovery of the solvents can relieve the toxicity of the fermentation products. Intelligent bioreactor design can increase productivity from the downstream side of the process by influencing the microbe (Li et al. 2016).
Finally, entirely new approaches are being explored for the establishment of biorefineries. Clostridial fermentations proceed via two very distinct stages—an acidogenic phase, where organic acids such as butyric acid are the main products, followed by the solventogenic phase, when the acids are converted to the corresponding alcohols. Historically, the acids have been of minor industrial importance, while the alcohols were sought after. However, the acids can be converted in many ways to alcohols or other products of interest as outlined by Sjöblom et al. (2016). The message here is to keep the eyes open to find creative solutions to pending problems based on nature's capabilities.
CONCLUDING REMARKS
About 100 years after the first industrial exploitation of the Clostridial acetone–butanol fermentation this process is still of high interest, commercially as well as scientifically. It still poses many questions, holds a lot of secrets, and many aspects have yet to be understood. Future generations of scientists will have enough to do to understand and optimize the underlying metabolic processes. At the same time, it is one of the oldest and best studied microbial chemical production processes, so we can learn a lot from its development. The centennial year is a good point to stop for a moment, look at this development and think about what it has to teach us for many other endeavours. This is of special relevance now, as our societies strive to free ourselves from our dependence on petroleum. I hope that this thematic issue of FEMS Microbiology Letters contributes to this thinking, and the development of new ideas based on past experience.
Acknowledgments
The author is particularly grateful to Giuseppe Rizzo for the wonderful art work for the graphical abstract. Furthermore, the author is indebted to Matthias Steiger for critically reading the manuscript. EQ BOKU VIBT GmbH is acknowledged for providing mass spectrometry and bioreactor instrumentation used in the course of the author's research on microbial chemical production.
FUNDING
The author's research on microbial chemical production is being financed by the Christian Doppler Research Society, by the and by the , the Federal Ministry of Traffic, Innovation and Technology (bmvit), the Styrian Business Promotion Agency SFG, the Standortagentur Tirol, the Government of Lower Austria and ZIT—Technology Agency of the City of Vienna through the COMET-Funding Program managed by the .
Conflict of interest. None declared.
REFERENCES
- Baral NR, Slutzky L, Shah A, et al. Acetone-butanol-ethanol fermentation of corn stover: current production methods, economic viability and commercial use. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw033. [DOI] [PubMed] [Google Scholar]
- Branduardi P, Porro D. n-butanol: challenges and solutions for shifting natural metabolic pathways into a viable microbial production. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw070. [DOI] [PubMed] [Google Scholar]
- Chen CT, Liao JC. Frontiers in microbial 1-butanol and isobutanol production. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw020. [DOI] [PubMed] [Google Scholar]
- Cuenca Mdel S, Molina-Santiago C, Gómez-García MR, et al. A Pseudomonas putida double mutant deficient in butanol assimilation: a promising step for engineering a biological biofuel production platform. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw018. [DOI] [PubMed] [Google Scholar]
- Dash S, Ng CY, Maranas CD. Metabolic modeling of clostridia: current developments and applications. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw004. [DOI] [PubMed] [Google Scholar]
- Dong JJ, Ding JC, Zhang Y, et al. Simultaneous saccharification and fermentation of dilute alkaline-pretreated corn stover for enhanced butanol production by Clostridium saccharobutylicum DSM 13864. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw003. [DOI] [PubMed] [Google Scholar]
- Dürre P. Biobutanol: an attractive biofuel. Biotechnol J. 2007;2:1525–34. doi: 10.1002/biot.200700168. [DOI] [PubMed] [Google Scholar]
- Dürre P. Butanol formation from gaseous substrates. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw040. [DOI] [PubMed] [Google Scholar]
- Dusselier M, Mascal M, Sels BF. Top chemical opportunities from carbohydrate biomass: a chemist's view of the Biorefinery. Top Curr Chem. 2014;353:1–40. doi: 10.1007/128_2014_544. [DOI] [PubMed] [Google Scholar]
- Essalem ME, Mitchell WJ. Identification of a glucose-mannose phosphotransferase system in Clostridium beijerinckii. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw053. [DOI] [PubMed] [Google Scholar]
- Friedl A. Downstream Process Options for the ABE Fermentation. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw073. [DOI] [PubMed] [Google Scholar]
- Hasunuma T, Okazaki F, Okai N, et al. A review of enzymes and microbes for lignocellulosic biorefinery and the possibility of their application to consolidated bioprocessing technology. Bioresource Technol. 2013;135:513–22. doi: 10.1016/j.biortech.2012.10.047. [DOI] [PubMed] [Google Scholar]
- Jones AJ, Venkataramanan KP, Papoutsakis ET. Overexpression of two stress-responsive small, non-coding RNAs, 6S and tmRNA, imparts butanol tolerance in Clostridium acetobutylicum. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw063. [DOI] [PubMed] [Google Scholar]
- Jones DT, Woods DR. Acetone-butanol fermentation revisited. Microbiol Rev. 1986;50:484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolek J, Branska B, Drahokoupil M, et al. Evaluation of viability, metabolic activity and spore quantity in clostridial cultures during ABE fermentation. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw031. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Ueda M. Cellular and molecular engineering of yeast Saccharomyces cerevisiae for advanced biobutanol production. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnv247. [DOI] [PubMed] [Google Scholar]
- Li SY, Chiang CJ, Tseng IT, et al. Bioreactors and in situ product recovery techniques for acetone-butanol-ethanol fermentation. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw107. [DOI] [PubMed] [Google Scholar]
- Liao C, Seo SO, Lu T. System-level modeling of acetone-butanol-ethanol fermentation. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw074. [DOI] [PubMed] [Google Scholar]
- Moon HG, Jang YS, Cho C, et al. One hundred years of clostridial butanol fermentation. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw001. [DOI] [PubMed] [Google Scholar]
- Pasteur L. Quelques résultats nouveaux relatifs aux fermentations acétique et butyrique. Bull Soc Chim Paris. 1862:52–3. [Google Scholar]
- Peabody GL, Kao KC. Recent progress in biobutanol tolerance in microbial systems with an emphasis on Clostridium. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw017. [DOI] [PubMed] [Google Scholar]
- Porro D, Branduardi P, Sauer M, et al. Old obstacles and new horizons for microbial chemical production. Curr Opin Biotech. 2014;30:101–6. doi: 10.1016/j.copbio.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Saini M, Chiang CJ, Li SY, et al. Production of biobutanol from cellulose hydrolysate by the Escherichia coli co-culture system. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw008. [DOI] [PubMed] [Google Scholar]
- Sandoval-Espinola WJ, Chinn M, Bruno-Barcena JM. Inoculum optimization of Clostridium beijerinckii for reproducible growth. FEMS Microbiol Lett. 2015;362 doi: 10.1093/femsle/fnv164. [DOI] [PubMed] [Google Scholar]
- Sauer M, Marx H, Mattanovich D. From rumen to industry. Microb Cell Fact. 2012;11:121. doi: 10.1186/1475-2859-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöblom M, Matsakas L, Christakopoulos P, et al. Catalytic upgrading of butyric acid towards fine chemicals and biofuels. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Xue C, Chen L, et al. Synergistic effect of calcium and zinc on glucose/xylose utilization and butanol tolerance of Clostridium acetobutylicum. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw023. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu S, Chai C, et al. Development of an inducible transposon system for efficient random mutagenesis in Clostridium acetobutylicum. FEMS Microbiol Lett. 2016;363 doi: 10.1093/femsle/fnw065. [DOI] [PMC free article] [PubMed] [Google Scholar]