Abstract
Background
Free tissue transfer is a mainstay in reconstruction of complex head and neck defects. The purpose of this study was to determine if perioperative complications were more common in patients with body mass index (BMI) >30 kg/m2 undergoing free flap reconstruction.
Methods
A multi-institutional retrospective cohort was created. Medical complications, surgical complications, and procedural variables were recorded. Logistic regression was used to investigate univariate and multivariate associations between outcomes and predictors.
Results
Of 582 cases, 128 patients (22%) had BMI >30. Surgical complications occurred in 153 cases (26.3%), with an adjusted odds ratio (OR) for association of surgical complications with BMI >30 of 0.92 (p 5 .71). Medical complications occurred in 178 cases (30.6%), with an adjusted OR of 0.78 (p 5 .26). Age and advanced comorbidity status (Adult Comorbidity Evaluation-27 [ACE-27] 2 or 3) were associated with medical complications (p < .0001).
Conclusion
BMI >30 does not predict medical or surgical complications in patients undergoing head and neck free flap surgery.
Keywords: obesity, free flap, reconstruction, complications, outcomes
INTRODUCTION
Free tissue transfer is the gold standard for reconstruction of complex head and neck defects. The majority of patients who benefit from free tissue transfer require treatment for advanced head and neck cancer. Free flap reconstruction is complex, involves multiple operative sites, and sometimes necessitates postoperative immobilization. Patients with head and neck cancer frequently are malnourished and may have had chemotherapy or local radiotherapy in the past, and procedures are nearly always clean-contaminated. These factors put patients with head and neck free flap at high risk for medical and surgical complications.
The increasing prevalence of obesity in the U.S. population has been well-described. In 2010, the prevalence of obesity was 35.9%, defined in adults as body mass index (BMI; weight divided by square of height) >30 kg/m2.1 The data regarding BMI and its relationship to perioperative complications are sparse and conflicting. Obesity has been found to unfavorably impact perioperative surgical outcomes after other complex procedures, including hip arthroplasty, hepatic resection, and laparoscopic colectomy.2-4 Obese patients experience higher rates of flap loss (5.5%) in postmastectomy abdominal-based free flap reconstruction.5 Increased BMI has also been shown to be a risk factor for perioperative medical complications (such as venous thromboembolism) after head and neck free flaps.6 In contrast, in a large prospective study of head and neck free flap recipients, medical complications were less common for those with a higher BMI.7 The purpose of this study was to determine if perioperative complications were more common in obese patients undergoing head and neck free flap reconstruction.
MATERIALS AND METHODS
After institutional review board approval at each participating site, a retrospective cohort was assembled that included adult patients seen and treated from July 2010 to December 2012 at Midwest Head and Neck Cancer Consortium member departments: the University of Iowa, University of Nebraska, University of Minnesota, University of Missouri, University of Kansas, and Sanford Health, Sioux Falls, SD. All patients who underwent free flap reconstruction of a head and neck site for any indication were included. Patient demographic data and surgery-specific variables were recorded from medical records. Descriptive data including flap indication, donor and recipient flap sites, BMI, previous treatment with radiation, current tobacco use, and American Society of Anesthesiologists (ASA) score were collected. The Adult Comorbidity Evaluation-27 (ACE-27) score, a validated head and neck cancer comorbidity scale, was assigned to each patient.8,9 The evaluated complications are shown in Table 1. Postoperative medical and surgical complications were recorded if they occurred within 4 weeks of surgery.
TABLE 1.
Evaluated complications.
| Medical | Surgical |
|---|---|
| Pneumonia | Total/partial flap loss |
| Cardiac dysrhythmia | Hematoma |
| Myocardial infarction | Wound dehiscence |
| Congestive heart failure | Fistula |
| Venous thromboembolism | Surgical site infection |
| Delirium | Seroma |
| Stroke | |
| Renal failure | |
| Clostridium difficile infection |
Logistic regression was used to investigate univariate associations between predictors and the presence of medical complications. Statistical significance was assessed using the Wald chi-square test. Predictor variables showing association with complications, defined by p < .1, were entered into a multivariable model in order to compute an adjusted odds ratio (OR) of association between obesity and medical complications. A similar analysis was performed to compute an adjusted OR associating obesity and surgical complications.
RESULTS
At the 6 participating institutions, 582 head and neck free tissue transfers occurred during the study period. The distribution of BMI in the study cohort is shown in Figure 1. The BMI criterion for obesity (BMI >30 kg/m2) was present in 22% of the cases (n = 128). Patient characteristics are described in Table 2. Patients who had been previously treated with radiotherapy and were current smokers were less likely to be obese (p < .0001 and p = .025, respectively). Mean age in obese patients was 59.3 years, compared with 61.9 years in nonobese patients (p = .05).
FIGURE 1.
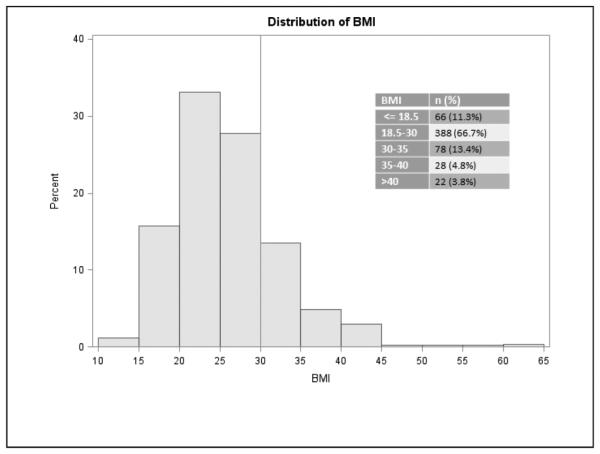
Distribution of body mass index (BMI).
TABLE 2.
Patient characteristics.
| Factor | No. of patients | % obese | p value |
|---|---|---|---|
| Sex | .14 | ||
| Male | 391 | 20.2 | |
| Female | 191 | 25.7 | |
| Prior radiotherapy | < .0001 | ||
| Yes | 210 | 12.4 | |
| No | 372 | 27.4 | |
| Current smoking | .025 | ||
| Yes | 270 | 17.8 | |
| No | 310 | 25.5 | |
| Bone flap | .36 | ||
| Yes | 199 | 24.1 | |
| No | 375 | 20.8 | |
| ACE-27 | .22 | ||
| 0, 1 | 279 | 19.0 | |
| 2, 3 | 163 | 23.9 | |
| ASA classification | .19 | ||
| 1, 2, 3 | 523 | 22.8 | |
| 4, 5 | 59 | 15.3 | |
| Indication for surgery | .16 | ||
| Cancer | 511 | 22.9 | |
| Noncancer | 71 | 15.5 | |
| Institution | .57 | ||
| A | 138 | 20.3 | |
| B | 140 | 25.7 | |
| C | 29 | 31.0 | |
| D | 93 | 20.4 | |
| E | 49 | 16.3 | |
| F | 133 | 21.1 | |
| Flap type | .42 | ||
| Radial forearm | 334 | 22.8 | |
| Anterolateral thigh | 100 | 17.0 | |
| Fibula | 113 | 25.6 | |
| Subscapular | 24 | 12.5 | |
| Other | 11 | 27.3 | |
| Recipient site | .13 | ||
| Oral cavity | 269 | 21.6 | |
| Oropharynx | 51 | 9.8 | |
| Hypopharynx | 40 | 17.5 | |
| Skull base | 26 | 23.1 | |
| Skin | 45 | 31.1 |
Abbreviations: ACE-27, Adult Comorbidity Evaluation Index; ASA, American Society of Anesthesiologists physical classification.
Of the cases studied, 153 (26.3%) were associated with a surgical complication; 32 in patients whose BMI was >30, and 121 in patients with BMI <30. The OR for association of presence of surgical complication and BMI >30 was 1.92 (95% confidence interval [CI] = 0.58–1.44; p 5 .71). Results of univariate analysis are shown in Table 3: there were differences in odds of complication by institution, and the effect of reconstructed site approached significance. Multivariate logistic regression included covariates representing institution, reconstructed site, prior radiotherapy, and current smoking. The resultant adjusted OR for association of surgical complication and BMI >30 was 1.03 (0.59– 1.80; p 5 .91).
TABLE 3.
Univariate logistic regression analyzing surgical complications.
| Factors | OR (95% CI) | p value |
|---|---|---|
| Age, per y | 0.99 (0.98–1.02) | .94 |
| Obesity | .71 | |
| Yes | 0.92 (0.58–1.44) | |
| No | 1 | |
| Sex | .52 | |
| Male | 1.14 (0.77–1.7) | |
| Female | 1 | |
| Prior radiotherapy | .26 | |
| Yes | 1.25 (0.85–1.82) | |
| No | 1 | |
| Current smoking | .60 | |
| Yes | 0.91 (0.62–1.31) | |
| No | 1 | |
| Bone flap | .95 | |
| Yes | 1.01 (0.69–1.49) | |
| No | 1 | |
| ACE-27 | .11 | |
| 2, 3 | 1.41 (0.92–2.16) | |
| 0, 1 | 1 | |
| ASA classification | .44 | |
| 4,5 | 1.26 (0.70–2.27) | |
| 1, 2, 3 | 1 | |
| Indication for surgery | .85 | |
| Cancer | 1.06 (0.60–1.87) | |
| Noncancer | 1 | |
| Institution | .0007 | |
| A | 2.49 (1.45–4.28) | |
| B | 0.98 (0.55–1.76) | |
| C | 0.98 (0.36–2.63) | |
| D | 1.62 (0.88–2.97) | |
| E | 0.63 (0.25–1.54) | |
| F | 1 | |
| Flap type | .95 | |
| Radial forearm | 0.9 (0.23–3.46) | |
| Anterolateral thigh | 1.09 (0.27–4.40) | |
| Fibula | 0.96 (0.24–3.87) | |
| Subscapular | 1.10 (0.22–5.40) | |
| Other | 1 | |
| Recipient site | .10 | |
| Oral cavity | 0.97 (0.47–1.98) | |
| Oropharynx | 0.94 (0.38–2.34) | |
| Hypopharynx | 2.49 (1.01–6.16) | |
| Skull base | 1.22 (0.42–3.54) | |
| Skin | 1 |
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval; ACE-27, Adult Comorbidity Evaluation Index; ASA, American Society of Anesthesiologists physical classification.
Medical complications occurred in 178 cases (30.6%), including 34 in patients with BMI >30 and 144 in patients with BMI <30 (OR 5 0.78; 95% CI 5 0.50–1.21; p = .26). Results of univariate logistic regression analyses are shown in Table 4: age and an ACE-27 score of 2 or 3 (moderate to severely decompensated comorbidity) demonstrated significant associations with presence of medical complication; sex, ASA class 4 or 5, and cancer-related surgery approached significance. These factors, along with prior radiotherapy and current smoking, were included in the multivariate logistic regression. Because the ACE-27 score and the ASA score measure similar traits, multivariate models were fit using one at a time. With ACE-27 in the model, the adjusted OR for association of medical complication and BMI >30 was 0.60 (95% CI = 0.34–1.08; p = .09). With ASA in the model, the adjusted OR was 0.78 (95% CI = 0.49–1.24; p = .29).
TABLE 4.
Univariate logistic regression analyzing medical complications.
| Factors | OR (95% CI) | p value |
|---|---|---|
| Age, per y | 1.04 (1.02–1.05) | < .0001 |
| Obesity | .26 | |
| Yes | 0.80 (0.50–1.21) | |
| No | 1 | |
| Sex | .10 | |
| Male | 0.73 (0.51–1.06) | |
| Female | 1 | |
| Prior radiotherapy | .24 | |
| Yes | 0.80 (0.55–1.16) | |
| No | 1 | |
| Current smoking | .60 | |
| Yes | 1.07 (0.75–1.53) | |
| No | 1 | |
| Bone flap | .76 | |
| Yes | 1.06 (0.73–1.54) | |
| No | 1 | |
| ACE-27 | < .0001 | |
| 2, 3 | 2.78 (1.83–4.22) | |
| 0, 1 | 1 | |
| ASA classification | .08 | |
| 4, 5 | 1.64 (0.95–2.86) | |
| 1, 2, 3 | 1 | |
| Indication for surgery | .07 | |
| Cancer | 1.75 (0.96–3.18) | |
| Noncancer | 1 | |
| Institution | .65 | |
| A | 0.86 (0.51–1.43) | |
| B | 0.84 (0.50–1.40) | |
| C | 1.03 (0.44–2.40) | |
| D | 0.61 (0.33–1.10) | |
| E | 1.04 (0.52–2.07) | |
| F | 1 | |
| Flap type | .52 | |
| Radial forearm | 2.04 (0.43–9.58) | |
| Anterolateral thigh | 1.58 (0.32–7.80) | |
| Fibula | 2.10 (0.43–10.24) | |
| Subscapular | 3.21 (0.57–18.20) | |
| Other | 1 | |
| Recipient site | .53 | |
| Oral cavity | 1.19 (0.58–2.41) | |
| Oropharynx | 1.93 (0.81–4.57) | |
| Hypopharynx | 1.32 (0.52–3.37) | |
| Skull base | 1.01 (0.34–3.01) | |
| Skin | 1 |
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval; ACE-27, Adult Comorbidity Evaluation Index; ASA, American Society of Anesthesiologists physical classification.
We repeated analyses of BMI to predict perioperative complications after defining standard categories of under-weight and obesity. There were no significant associations (data not shown). We therefore elected to represent BMI in regression models as a binary variable.
DISCUSSION
This study offers a large sample of patients undergoing head and neck free flap surgery at 6 academic medical centers. Our main findings were that surgical and medical complications do not occur with increased incidence in patients with an elevated BMI. This finding will prove useful as surgeons counsel patients and assess clinical outcomes.
We identified some of the same factors as described by Clark et al10 as being predictive of perioperative complications after head and neck free flaps, including age and burden of comorbid conditions, along with current smoking and prior radiotherapy. However, in that study, body habitus was included in the form of weight alone and the effect of obesity through BMI was not assessed. Clark et al10 investigated body weight under 60 kg as a risk factor for perioperative complications, and found that there was no correlation on multivariable analysis. A follow-up study from the same institution found that BMI, modeled as a continuous variable, was inversely correlated with the odds of medical complications.7 Our data indicated a trend toward BMI >30 being protective of medical complications, but did not reach statistical significance. The median BMIs in the cohorts described by Patel et al7 and in the present study were similar, but it is not clear if BMI was distributed similarly.
The effects of increased BMI on clinical outcomes in patients with head and neck cancer are multifaceted and complex. Being overweight (BMI >25 kg/m2) has been associated with improved long-term survival for smokers with head and neck cancer11 as well as in a broader cohort of patients with head and neck cancer.12 Another study found recurrence rate and disease-specific survival were better for those eating a healthy diet and for those with BMI >25 and BMI >30.13 However, conflicting data also exist, leaving the relationship between BMI and survival unclear.14
Age and advanced comorbidity status have previously been associated with increased odds of surgical and medical complications.7 In that cohort, age and advanced comorbidity were significant predictors of medical complications, but not surgical complications. We did not find that advanced comorbidity status was associated with surgical complications.
Although inclusion of several high-volume centers allows for a large patient cohort, the study design does impose a few limitations. We attempted to minimize differences in data collection through use of common data collection forms and of standardized measures of comorbidity; however, each study institution carried out its own data abstraction, and differences are possible.
The ACE-27 score was used as an objective measure to assess comorbidity status, which includes obesity-related complications (diabetes mellitus, hypertension, and congestive heart failure), but our data did not allow for a more specific comparison of those with BMI >30 with and without obesity-related complications. BMI does not provide a direct assessment of healthy weight. Still, it is commonly used, is easily computed in any clinical setting, and its use to define obesity in our study is unlikely to result in a systematic misclassification error.
In addition, study patients were managed by a large number of ablative and reconstructive surgeons, leading to variability both in flap selection and in perioperative management. This variability, especially given the large sample size, should be seen to enhance the external validity of the study. However, it does mean that our data do not permit conclusions about the impact of body habitus on the choice of flap donor site.
The purpose of our retrospective study was to measure clinical outcomes after free flap reconstruction. Although we were able to address outcomes of central interest, our study did not permit assessment of functional or aesthetic reconstructive outcomes. The characteristics of the soft tissues of available free flaps are critical in selection of a reconstructive modality because of their relative bulk, compliance, and donor site morbidity. It would be valuable to have a better understanding of the impact of body habitus on functional outcomes, but this type of study would require data not available in medical records, and would be better suited to prospective analysis.
CONCLUSION
In this large multi-institutional study, BMI >30 was not independently associated with medical or surgical complications for head and neck free flap recipients. This data is valuable to surgeons managing patients who might be considered at high risk of adverse outcomes. Our data do not permit conclusions about functional outcomes of reconstruction or survival.
Acknowledgments
This work arose out of the collaborative efforts of the members of the Midwest Head and Neck Cancer Consortium departments: University of Iowa, Iowa City, IA; University of Kansas, Kansas City, KS; University of Minnesota, Minneapolis, MN; University of Missouri, Columbia, MO; University of Nebraska, Omaha, NE; and Sanford Health, Sioux Falls, SD.
Footnotes
This work was presented at the 5th World Congress of the International Federation of Head and Neck Oncologic Societies, New York, NY, July 26–30, 2014.
REFERENCES
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Mathur AK, Ghaferi AA, Osborne NH, et al. Body mass index and adverse perioperative outcomes following hepatic resection. J Gastrointest Surg. 2010;14:1285–1291. doi: 10.1007/s11605-010-1232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mustain WC, Davenport DL, Hourigan JS, Vargas HD. Obesity and laparoscopic colectomy: outcomes from the ACS-NSQIP database. Dis Colon Rectum. 2012;55:429–435. doi: 10.1097/DCR.0b013e31823dfb17. [DOI] [PubMed] [Google Scholar]
- 4.Belmont PJ, Jr, Goodman GP, Hamilton W, Waterman BR, Bader JO, Schoenfeld AJ. Morbidity and mortality in the thirty-day period following total hip arthroplasty: risk factors and incidence. J Arthroplasty. 2014;29:2025–2030. doi: 10.1016/j.arth.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Fischer JP, Nelson JA, Sieber B, et al. Free tissue transfer in the obese patient: an outcome and cost analysis in 1258 consecutive abdominally based reconstructions. Plast Reconstr Surg. 2013;131:681e–692e. doi: 10.1097/PRS.0b013e31828e2159. [DOI] [PubMed] [Google Scholar]
- 6.Thai L, McCarn K, Stott W, et al. Venous thromboembolism in patients with head and neck cancer after surgery. Head Neck. 2013;35:4–9. doi: 10.1002/hed.22920. [DOI] [PubMed] [Google Scholar]
- 7.Patel RS, McCluskey SA, Goldstein DP, et al. Clinicopathologic and therapeutic risk factors for perioperative complications and prolonged hospital stay in free flap reconstruction of the head and neck. Head Neck. 2010;32:1345–1353. doi: 10.1002/hed.21331. [DOI] [PubMed] [Google Scholar]
- 8.Piccirillo JF, Creech C, Zequeira R, Anderson S, Johnston AS. Inclusion of comorbidity into oncology data registries. J Registry Manag. 1999;26:66–70. [Google Scholar]
- 9.Piccirillo JF, Lacy PD, Basu A, Spitznagel EL. Development of a new head and neck cancer-specific comorbidity index. Arch Otolaryngol Head Neck Surg. 2002;128:1172–1179. doi: 10.1001/archotol.128.10.1172. [DOI] [PubMed] [Google Scholar]
- 10.Clark JR, McCluskey SA, Hall F, et al. Predictors of morbidity following free flap reconstruction for cancer of the head and neck. Head Neck. 2007;29:1090–1101. doi: 10.1002/hed.20639. [DOI] [PubMed] [Google Scholar]
- 11.Gaudet MM, Patel AV, Sun J, et al. Prospective studies of body mass index with head and neck cancer incidence and mortality. Cancer Epidemiol Bio-markers Prev. 2012;21:497–503. doi: 10.1158/1055-9965.EPI-11-0935. [DOI] [PubMed] [Google Scholar]
- 12.Karnell LH, Sperry SM, Anderson CM, Pagedar NA. Influence of body composition on survival in patients with head and neck cancer. Head Neck. 2014 doi: 10.1002/hed.23983. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Arthur AE, Peterson KE, Rozek LS, et al. Pretreatment dietary patterns, weight status, and head and neck squamous cell carcinoma prognosis. Am J Clin Nutr. 2013;97:360–368. doi: 10.3945/ajcn.112.044859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyengar NM, Kochhar A, Morris PG, et al. Impact of obesity on the survival of patients with early-stage squamous cell carcinoma of the oral tongue. Cancer. 2014;120:983–991. doi: 10.1002/cncr.28532. [DOI] [PMC free article] [PubMed] [Google Scholar]


