Abstract
In the past few years we have seen the development of several new technologies for the continuous and non-invasive monitoring of physiological glucose, such as the GlucoWatch®, glucose-sensing skin patches and approaches based on a glucose-sensing tattoo. One approach that differs from current thinking is based on the determination and monitoring of tear glucose, which is well known to track blood glucose with an approximate 30 min lag time, using disposable and colorless contact lenses. These contact lenses can be worn by diabetics who can colorimetrically see changes in their contact lens color or other fluorescence-based properties, giving an indication of tear and blood glucose levels.
Introduction
Over the past 150 years, significant attention has been given to the development of physiological glucose-monitoring bio/technologies [1,2,3•,4,5,6•,7–9,10••,11–24]. This is because one important aspect of diabetes management involves the tight control of blood glucose levels, so as to manage food intake and the dosage and timing of insulin injection. Tests for determining serum glucose concentration typically require blood collection by some invasive technique, usually a needle or other device causing arterial or venous puncture. Currently, millions of diabetics are left with very few alternatives, except to invasively draw blood many times a day to determine their blood sugar levels. To this end, many technologies have been developed over the past 20 years in an attempt to provide a technology that promises both non-invasive and continuous physiological glucose monitoring. These have included the use of near-infrared spectroscopy [14,15], optical rotation [16,17], and colorimetric [18,19] and fluorescence detection [20–24], to name but a few.
Recently, we have seen the launch of the new GlucoWatch®, which was approved by the FDA in 2001 and is the first step towards the continuous and non-invasive monitoring of physiological glucose. However, in addition to wearing this wrist watch based glucose sensor, it is also recommended that glucose monitoring by another blood sampling technique be used from time to time, which in our opinion both defeats and undermines the approach and objective, as well as potentially increasing diabetic patient care and supply cost. Other emerging technologies include glucose-monitoring skin patches, implantable glucose sensor coupled insulin pumps, and laser blood drawing, which is deemed less painful than finger pricking with a lancet or needle [25••]. Yet, there is still a need for new technologies that are truly noninvasive and continuous. To this end, we have recently seen the development of fluorescence-based glucose-sensing contact lenses. When worn by diabetics, who often require vision correction in any case, these lenses can potentially monitor tear glucose levels, which are known to track blood glucose levels (Figure 1). These contact lenses incorporate new monosaccharide fluorescent signaling boronic acid containing probes (Figure 2); the unique chemical and photophysical features of these probes overcome lens environmental obstacles, such as pH and polarity. Recent findings show that this approach might indeed be suitable for the continuous non-invasive monitoring of tear glucose in the concentration range 250 µM to 5 mM, which is approximately tenfold lower than the concentration range found in diabetic blood.
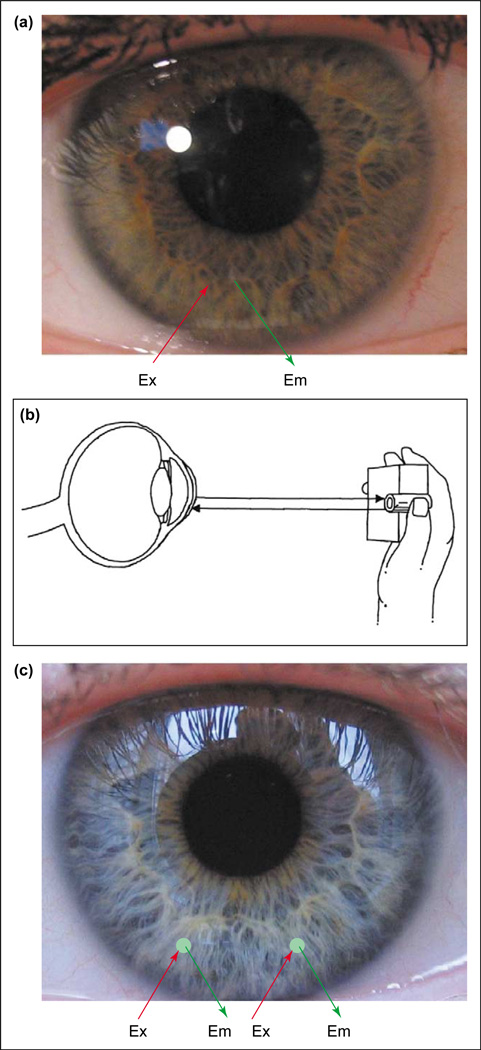
Figure 1.
Potential methods for non-invasive continuous tear glucose monitoring. (a) Boronic acid doped contact lenses. (b) Schematic of a possible tear glucose-sensing device. The hand-held device works by flashing a light into the eye (Ex) and measuring the emission (Em) intensity. (c) Sensor spots on the surface of the lens can be included to monitor other analytes in addition to glucose, such as drugs, biological markers, Ca2+, K+, Na+, O2 and Cl−. Sensor regions could also allow for ratiometric, lifetime or polarization based fluorescence glucose sensing. (Figure modified from [39].)
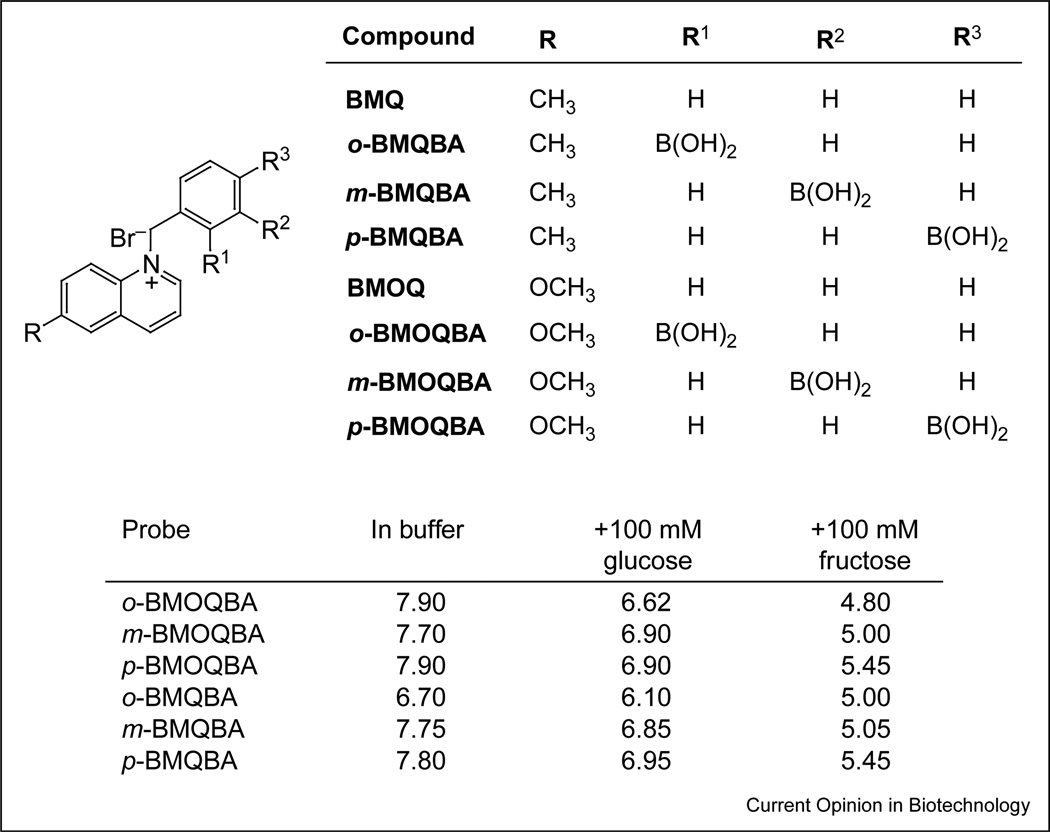
Figure 2.
Molecular structure of the boronic acid probes based on the quinolinium nucleus and the apparent pKa values for the boronic acid probes in buffer. The effect of 100 mM sugars is also shown. Abbreviations: o-, ortho; m-, meta; p-, para.
In this article we review the fabrication of these new glucose-sensing contact lenses and probes and discuss the rationale behind their design. We also provide a perspective on their practical role in blood glucose determination, as compared with more traditional glucose-sensing technologies such as finger pricking. We believe that the broader implications of this technology have still yet to be realized. It is known that tears house a variety of physiological analytes, such as Na+, K+, Ca2+, Mg2+, histamine, urea, lactate and cholesterol, which could also be monitored in the near future through similar intelligent contact lens based sensing platforms.
Lens feasibility and probe design
In early contact lens studies it was deemed necessary to understand the contact lens environment, with regard to pH, polarity and possible interferents, before any fluorescence- or colorimetric-based contact lens sensing system could be developed [26••]. Commercially available lenses were tested using well-characterized boronic acid containing probes, where the boronic acid group is well-known to chelate monosaccharides [27–34] and thus provides a possible glucose-sensing mechanism. Such probes have been used in other technologies developed for monitoring glucose levels. The probes can detect a wide range of sugar moieties and the extent of chelation can be monitored using fluorescence, colorimetric, fluorescence-lifetime or polarization based methods [1–13].
Feasibility studies of ‘doped’ lenses using available boronic acid probes produced poor glucose responses indeed, which was attributed to the mildly acidic pH (~6.2) and methanol-like polarity within the contact lens [26••,35]. This was not too surprising, given the fact that these probes were originally designed for sensing at a physiological pH of ~7.4, the probes typically having pKa values around 9. Hence, to obtain a notable glucose response in the contact lens polymer, it was necessary to design new probes with significantly reduced sugar-bound pKa values (Figure 2). The probes also had to be sensitive to the very low concentrations of tear glucose (~500 µM for a healthy person increasing to several millimolar for diabetics, but recalling that the blood glucose levels for a healthy person are ~tenfold higher than the tear level [36,37•,38,39]).
The pKa of phenyl boronic acid is known to be tunable with the appropriate substituents [40,41]; for example, an electron-withdrawing group reduces the pKa of the sugar-bound form, whereas an electron-donating group increases it. The interaction between the quaternary nitrogen of the 6-methyl- and methoxyquinolinium moieties, and the boronic acid group can therefore be utilized to reduce the pKa of the probe. In this regard, the environmental constraints of the contact lens were addressed by synthesizing two new classes of isomeric boronic acid containing probes (eight probes in total; Figure 2) in which the spacing between the interacting moieties (i.e. the quaternary nitrogen of the 6-methyl- or 6-methoxyquinolinium group and the boronic acid group) were varied. These probes have promoted a greater understanding of the sensing mechanism and enabled the selection of the most suitable isomer based on its glucose-binding affinity [40,41].
Developing a new glucose signaling mechanism
How do these new probes sense glucose and translate this to a signal that can be monitored? To understand this, it is informative to consider Figure 3a. The boronic acid group is an electron-deficient Lewis acid having an sp2-hybridized boron atom with a trigonal planar conformation. The anionic form of the boronic acid, formed in the presence of glucose, is characterized by a more electron-rich sp3-hybridized boron atom with a tetrahedral geometry. The change in the electronic properties and the geometry at the boron atom induces the fluorescence spectral changes of the probes. Upon addition of glucose the electron density on the boron atom is increased, facilitating the partial neutralization of the positively charged quaternary nitrogen of the quinolinium moiety. This interaction has been termed a ‘charge neutralization-stabilization mechanism’ [40,41], and a schematic representation of this mechanism with regard to glucose binding/sensing is illustrated in Figure 3a.
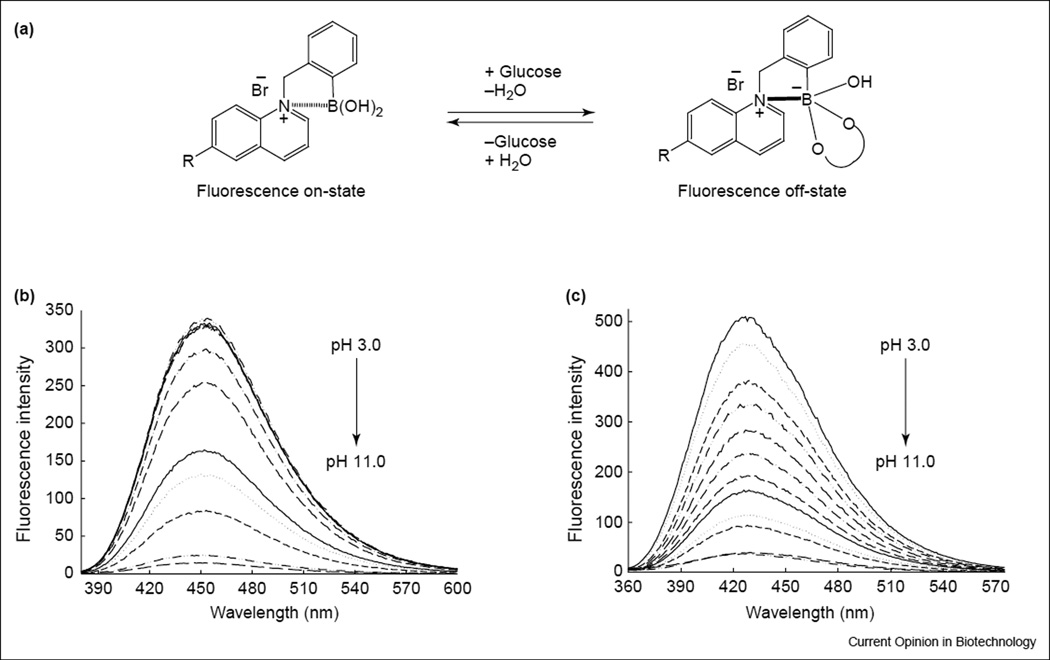
Figure 3.
Signaling mechanism. (a) Schematic representation of the charge neutralization-stabilization mechanism with regard to glucose sensing. The bold-line between the N+ and boron atom in the structure shown on the right-hand side of the equation indicates the increased interaction between them, and is not intended to show covalent bond formation between the two atoms. Emission spectra of (b) o-BMOQBA and (c) o-BMQBA in buffered media. λex for BMOQBA and BMQBA was 345 nm and 320 nm, respectively.
Reduced pKa of the probes: lens environmental compatibility
Figures 3b and c shows the emission spectra of N-(boronobenzyl)-6-methoxyquinolinium bromide (o-BMOQBA) and N-(boronobenzyl)-6-methylquinolinium bromide (o-BMQBA) in buffered media where the pH is increased from 3 to 11. The emission spectra of the boronic acid containing probes typically show a steady decrease in fluorescence intensity with increasing pH. By contrast, the control compounds, (N-benzyl-6-methoxyquinolinium bromide [BMOQ] and N-benzyl-6-methylquinolinium bromide [BMQ]) having no boronic acid group, show no change in fluorescence intensity. The apparent pKa values obtained from the titration curves shown in Figures 3b and c are given in Figure 2. Here we can see considerably reduced pKa values for the new phenylboronic acid containing fluorophores in buffered media, as compared with the typical boronic acid probes reported in the literature [2,4,6•,8,10••], which are typically in the range 8–9. The quaternary nitrogen of the quinolinium nucleus not only reduces the pKa of the probes, but also serves to stabilize the boronatediester formed upon sugar complexation [40,41]. This in turn is thought to increase the affinity of the probes for sugar. Hence, the reduced sugar-bound pKa of the probes, coupled with their increased glucose affinity, is a most attractive notion for glucose-sensing contact lens applications, noting the previous findings of a lens pH around 6.2 [26••].
Contact lens based glucose sensing
Figures 4a and b shows the response of o-BMOQBA- and o-BMQBA-doped contact lenses, respectively, to increasing concentrations of glucose. As with the solution-based measurements reported by Badugu and colleagues [25••,36,37•,38,39], the probes show a decrease in fluorescence intensity. This has been attributed to the complexation of glucose with boronic acid and the subsequent charge neutralization mechanism described earlier [40,41]. The I′/I plots, where I′ and I are the fluorescence intensities in the absence and presence of sugar, respectively (Figures 4c,d), show the normalized response as compared with the response of the lenses in the absence of sugar. As was observed in solution [40,41], fructose had a greater response, reflecting the greater affinity of monophenyl boronic acid derivatives for fructose. However, in the low sugar concentration ranges (<2 mM sugar; Figures 4e,f) the response towards both sugars was comparable [36–39]: the 90% response time (i.e. the time for the fluorescence signal to change by 90% of the initial value) was ~10 min.
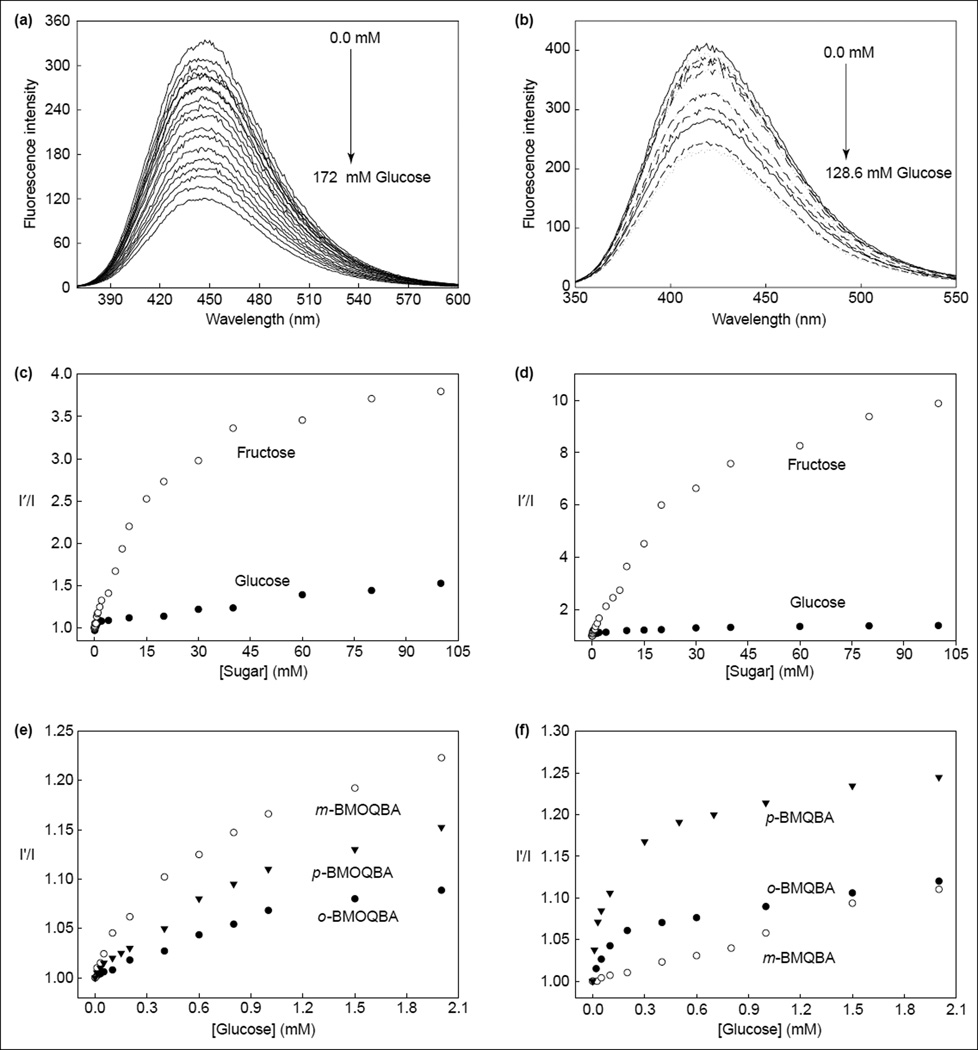
Figure 4.
The response of contact lenses to sugars. The emission spectra of (a) an o-BMOQBA- and (b) an o-BMQBA-doped contact lens in the presence of increasing glucose concentrations. λex was 345 nm for o-BMOQBA and 320 nm for o-BMQBA. The corresponding emission intensity ratio at band maximum for (c) o-BMOQBA- and (d) o-BMQBA-doped contact lenses in the absence (I′) and presence (I) of both glucose and fructose. The response of different isomers of (e) BMOQBA and (f) BMQBA in the contact lens in the tear glucose concentration range. (Figure adapted from [25••].)
Differences in the response towards glucose for the isomers shown in Figure 2 could also be observed in the lenses (Figures 4e,f), where m-BMOQBA was reported to have the greatest response amongst this class of probes. From Figures 4e and f, we can clearly see a notable response in the lens towards sugars, with p-BMQBA showing a greater than 20% fluorescence signal change with as little as 2 mM glucose. This was not unexpected, and is simply explained by the pKa of the probes being <7, the probes being compatible with the mildly acidic lens environment. The dissociation constants (KD) obtained for all six probes with glucose and fructose within the contact lens have also been recently published [36,37•,38,39].
Probe leaching, shelf-life and interferents
Not all changes in fluorescence will result from interactions with sugars, and problems such as leaching could reduce the intensity. It was reported that up to an ~8% change in fluorescence intensity could be observed, attributed to lens probe leaching, for the BMOQBA class of probes, with very little change after about 25 min [42•]. By contrast, the BMQBA probes show a much greater extent of leaching over the same time period and under identical conditions. Given that the BMQBA probes typically show a greater response towards glucose in the lens, this suggests that the BMQBA probes might be more accessible within the lens than the BMOQBA probes [42•]. Similar results were obtained at 38 8C (core temperature), but with a different leaching rate [42•].
In the early contact lens studies reported by Geddes and coworkers, lenses were pre-leached to a steady-state fluorescence intensity before use. After glucose measurements were undertaken, the outer lens fluid volume surrounding the contact lens was found to be non-fluorescent indicating that dye had not leached from the lens during actual glucose-sensing measurements [36,37•,38, 39]. It should be noted that although chemistries are available to covalently attach the probes within the contact lens polymer, which would eliminate any leaching whatsoever, it is an important design consideration of this approach that the lenses remained unmodified, so that their physiological characteristics/biocompatibilities and optical parameters remain unchanged. The approach described here is targeted at reducing future lens redesign costs for industry by using simple probe doping.
As with all sensors it is important to consider the effects of potential interferents and sensor shelf-life on the working response of the device. We commented earlier on the response of the probes towards fructose, primarily because of its well-known greater affinity for the boronic acid moiety [2,4,6•,8,10••]. However, the concentration of fructose in blood is ~10 times lower than glucose [26••,36,37•, 38,39], a relationship that is also thought to occur in tears [26••]. Hence, fructose is not thought to be a major interferent in tears, but is simply shown here to place the binding trends in context. However, tears are a complex mixture of proteins and other analytes, such as sodium (120–170 mM), potassium (6–26 mM) and chloride (100 mM) [42•]. The new contact lens probes have been tested with various aqueous halides, given that the counter cations sodium and potassium are known to be non-quenching metal ions of the common fluorophores. The BMOQBA probes are modestly quenched by chloride, with steady-state Stern–Volmer constants [43] in the range 170–182 M−1; the BMQBA probes have significantly smaller quenching constants in the range17–44 M−1. This result is simply explained by the shorter life time of the BMQBA probes as compared with the BMOQBA probes, and the probability of an excited-state chloride ion encounter [38,43]. Encouragingly, the BMQBA probes typically show a greater response towards glucose, as well as being the least perturbed by aqueous chloride. In any event, simple corrections in the fluorescence signal can easily be made to account for chloride interference on the glucose response (see Figure 1). Sensor spots on the surface of the lens could contain a reference chloride compound or indeed another probe sensitive to both glucose and chloride.
With regard to the glucose-sensing contact lens shelf-life, lenses that had been doped, leached and stored for several months, both wet and dry, gave identical sugar-sensing results, indicating no lens polymer–fluorophore interactions over this time period and no probe degradation.
Future developments based on this technology
We have described here the design rationale and testing of fluorescent probes that are compatible with commercially available, daily use disposable contact lenses, which have already been assessed and optimized with regard to vision correction and oxygen permeability. This research has enabled the first prototype to be realized. With regard to glucose-monitoring by this approach, we speculate below on several future improvements to this technology.
Clear or colored contact lenses?
Many boronic acid containing fluorophores absorb in the visible spectrum [2,4,6•,8,10••], which would introduce color into a doped lens. Although colored lenses are attractive to a few people, as sports or even fashion accessories, the majority of contact lenses worn today are clear. The colorless lenses described here are thus ideal in this regard. One disadvantage of these lenses, however, is the requirement for an excitation and detection device, as shown in Figure 1. One improvement to this technology could be the use of colored contact lenses that change color in response to the concentration of tear and therefore blood glucose. This can be achieved by the ground-state binding of glucose to boronic acid, which would lead to subsequent changes in the fluorophore absorption spectrum. A patient wearing the lenses could simply look into a mirror and compare the color to a precalibrated color strip to assess the extent of hyperglycemia. The color could even be determined by an onlooker, making this technology most attractive to parents of young diabetic children or for carers of the elderly.
Sensor spots
As briefly mentioned earlier and shown in Figure 1, sensor spots on the surface of contact lenses could correct signal responses for interferents such as aqueous chloride. Indeed the spots could either be visible to the wearer (self-readout) or readable by an external monitoring device. In this regard sensor spots could also be used to determine the concentrations of multiple analytes in addition to glucose, such as sodium and potassium.
Detection methods
Although simple colorimetric methods are likely to be the easiest to introduce to the market place, other fluorescence sensing methodologies, such as polarization, lifetime and ratiometric sensing, offer many spectroscopic sensing advantages over the simple intensity measurements described for the lenses to date [35]. For example, fluorescence lifetime and ratiometric measurements are independent of total light intensity or fluctuations in ambient room light.
How do these lenses compare to existing glucose-sensing technologies?
As briefly mentioned in the introduction, there are very few truly non-invasive and continuous physiological glucose monitors for diabetics on the market today. Even the GlucoWatch® is not without its constraints, but does have particular advantages in that glucose level alarms and past history are built-in features. The glucose-sensing contact lens approach, although unlikely to undergo any form of clinical trials for several years yet, is likely to be a significant future competitor to the GlucoWatch® or indeed enzymatic finger-pricking based methods. This is because many diabetics require vision correction and already wear either contact lenses or spectacles, the technology is truly continuous and noninvasive, and patients can simply see their own glucose levels or even be assessed by on-lookers. With the prevalence of diabetes on the increase, this new approach is likely to be a significant alternative to diabetes care and management.
Conclusions
Over the past few years a range of new glucose-sensing contact lenses have been developed by doping strategically designed fluorescent probes into commercially available contact lenses. The probes are completely compatible with the new lenses and can readily detect glucose changes of up to several millimolar, appropriate for the tear glucose concentration range of diabetics (i.e. ~0.5–5 mM) [25,36,37•,38,39,42].
The lenses have a 90% response time of about 10 min, allowing the continuous and noninvasive monitoring of ocular glucose. This is a significant improvement over enzymatic methods based on blood sampling by finger pricking, with many diabetics currently begrudgingly testing between four and six times daily.
With diabetes being widely recognized as one of the leading causes of death and disability in the western world, this new doped contact lens approach represents a notable step towards the continuous and non-invasive monitoring of physiological glucose. In addition, the contact lens approach is likely to be the first of a whole range of contact lens sensing platforms for monitoring ocular fluid analytes. In this regard, new contact lenses for the determination of multiple tear analytes, such as lactate, sodium and potassium, might be realized, and could even be developed for monitoring the core temperature using multiple transduction agents in multiple sensor spots as depicted in Figure 1.
Finally in closing, although the prognosis for diabetes has changed little in 3000 years since its first report in the Ebers Papyrus in 1500 BC [1], the past 20 years has seen significant advances in its clinical diagnosis.
Acknowledgments
The authors would like to thank the NIH for financial support: NCRR (RR-08119). The authors also wish to thank the MBC, UMBI for partial salary support to JRL and CDG.
Abbreviations
- BMQBA
N-(boronobenzyl)-6-methylquinolinium bromide
- BMOQBA
N-(boronobenzyl)-6-methoxyquinolinium bromide
References and recommended reading
• of special interest
•• of outstanding interest
- 1.Badugu R, Geddes CD. Progress in glucose sensing. J Fluores. 2004;14:475. [Google Scholar]
- 2.Fang H, Kaur G, Wang B. Progress in boronic acid-based fluorescent glucose sensors. J Fluores. 2004;14:481–489. doi: 10.1023/b:jofl.0000039336.51399.3b. [DOI] [PubMed] [Google Scholar]
- 3. Scognamiglio V, Staiano M, Rossi M, D’Auria S. Protein-based biosensors for diabetic patients. J Fluores. 2004;14:491–498. doi: 10.1023/b:jofl.0000039337.30726.6d. A review of protein and enzyme-based glucose sensors for diabetics.
- 4.Kawanishi T, Romey MA, Zhu PC, Holody MZ, Shinkai S. A study of boronic acid-based fluorescent glucose sensors. J Fluores. 2004;14:499–512. doi: 10.1023/b:jofl.0000039338.16715.48. [DOI] [PubMed] [Google Scholar]
- 5.Wickramasinghe Y, Yang Y, Spencer SA. Current problems and potential techniques in in vivo glucose monitoring. J Fluores. 2004;14:513–520. doi: 10.1023/b:jofl.0000039339.36839.19. [DOI] [PubMed] [Google Scholar]
- 6. Cappuccio FE, Suri JT, Cordes DB, Wessling RA, Singaram B. Evalution of pyranine derivatives in boronic acid based saccharide sensing: significance of charge interaction between dye and quencher in solution and hydrogel. J Fluores. 2004;14:521–533. doi: 10.1023/b:jofl.0000039340.94188.2a. An evaluation of boronic acid containing probes. Readers are also also recommended to consult reference cited therein.
- 7.Moschou EA, Sharma BV, Deo KS, Daunert S. Fluorescence glucose detection: toward the ideal in vivo biosensor. J Fluores. 2004;14:535–547. doi: 10.1023/b:jofl.0000039341.64999.83. [DOI] [PubMed] [Google Scholar]
- 8.Philips MD, James TD. Boronic acid based modular fluorescent sensors for glucose. J Fluores. 2004;14:549–559. doi: 10.1023/b:jofl.0000039342.10260.21. [DOI] [PubMed] [Google Scholar]
- 9.Schaferling M, Wu M, Wolfbeis OS. Time-resolved fluorescent imaging of glucose. J Fluores. 2004;14:561–568. doi: 10.1023/b:jofl.0000039343.02843.12. [DOI] [PubMed] [Google Scholar]
- 10. Cao H, Heagy M. Fluorescent chemosensors, for carbohydrates: a decade’s worth of bright spies for saccharides in review. J Fluores. 2004;14:569–583. doi: 10.1023/b:jofl.0000039344.34642.4c. An interesting perspective on building in vivo glucose biosensors.
- 11.Chinnayelka S, McShane MJ. Glucose-sensitive nonoassemblies comprising affinity-binding complexes trapped in fuzzy microshells. J Fluores. 2004;14:585–595. doi: 10.1023/b:jofl.0000039345.57924.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fehr M, Lalonde S, Ehrhardt DW, Frommer WB. Live imaging of glucose homeostasis in nuclei of COS-7 cells. J Fluores. 2004;14:603–609. doi: 10.1023/b:jofl.0000039347.94943.99. [DOI] [PubMed] [Google Scholar]
- 13.Rusin O, Alpturk O, He M, Escobedo JO, Jiang S, Dawan F, Lian K, McCarroll ME, Warner IM, Strongin RM. Macrocycle-derived functional xanthenes and progress towards concurrent detection of glucose and fructose. J Fluores. 2004;14:611–615. doi: 10.1023/b:jofl.0000039348.74270.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson MR, Eaton RP, Haaland DM, Koepp GW, Thomas EV, Stallard BR, Robinson PL. Non-invasive glucose monitoring in diabetic patients: a preliminary evaluation. Clin Chem. 1992;38:1618–1622. [PubMed] [Google Scholar]
- 15.Heise HM, Marbach R, Koschinsky TH, Gries FA. Non-invasive blood glucose sensors based on near-infrared spectroscopy. Ann Occup Hyg. 1994;18:439–447. doi: 10.1111/j.1525-1594.1994.tb02230.x. [DOI] [PubMed] [Google Scholar]
- 16.March WF, Rabinovitch B, Adams R, Wise JR, Melton M. Ocular glucose sensor. Trans Am Soc Artif Intern Organs. 1982;28:232–235. [PubMed] [Google Scholar]
- 17.Rabinovitch B, March WF, Adams RL. Non-invasive glucose monitoring of the aqueous humor of the eye. Part 1, measurement of very small optical rotations. Diabetes Care. 1982;5:254–258. doi: 10.2337/diacare.5.3.254. [DOI] [PubMed] [Google Scholar]
- 18.Schier GM, Moses RG, Gan IET, Blair SC. An evaluation and comparison of Reflolux II and Glucometer II, two new portable reflectance meters for capillary blood glucose determination. Diabetes Res Clin Pract. 1988;4:177–181. doi: 10.1016/s0168-8227(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 19.Clarke W, Becker DJ, Cox D, Santiago JV, White NH, Betschart J, Eckenrode K, Levandoski LA, Prusinki EA, Simineiro LM, et al. Evaluation of a new system for self blood glucose monitoring. Diabetes Res Clin Pract. 1988;4:209–214. doi: 10.1016/s0168-8227(88)80020-2. [DOI] [PubMed] [Google Scholar]
- 20.Trettnak W, Wolfbeis OS. Fully reversible fiber-optic glucose biosensor based on the intrinsic fluorescence of glucose-oxidase. Anal Chim Acta. 1989;221:195–203. [Google Scholar]
- 21.Meadows D, Schultz JS. Fiber optic biosensor based on fluorescence energy transfer. Talanta. 1988;35:145–150. doi: 10.1016/0039-9140(88)80053-5. [DOI] [PubMed] [Google Scholar]
- 22.Tolosa L, Malak H, Rao G, Lakowicz JR. Optical assay for glucose based on the luminescence decay time of the long wavelength dye Cy5. Sensors Actuators B. 1997;45:93–99. doi: 10.1016/S0925-4005(97)00275-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tolosa L, Gryczynski I, Eichorn LR, Dattelbaum JD, Castellano FN, Rao G, Lakowicz JR. Glucose sensors for low cost lifetime-based sensing using a genetically engineered protein. Anal Biochem. 1999;267:114–120. doi: 10.1006/abio.1998.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Auria S, Dicesare N, Gryczynski Z, Gryczynski I, Rossi M, Lakowicz JR. A thermophilic apoglucose dehydrogenase as a nonconsuming glucose sensor. Biochem Biophys Res Commun. 2000;274:727–731. doi: 10.1006/bbrc.2000.3172. [DOI] [PubMed] [Google Scholar]
- 25. Badugu R, Lakowicz JR, Geddes CD. Ophthalmic glucose monitoring using disposable contact lenses. In: Geddes CD, Lakowicz JR, editors. Reviews in Fluorescence 2005. New York: Springer; in press. A detailed review of glucose sensing in contact lenses.
- 26. Badugu R, Lakowicz JR, Geddes CD. Non-invasive continuous monitoring of physiological glucose using a monosaccharide-sensing contact lens. Anal Chem. 2004;76:610–618. doi: 10.1021/ac0303721. A feasibility study of fluorescence-based glucose-sensing contact lenses using boronic acid based probes doped within contact lenses.
- 27.James TD, Sandanayake KRAS, Shinkai S. Chiral discrimination of monosaccharides using a fluorescent molecular sensor. Nature. 1995;374:345. [Google Scholar]
- 28.Norrild JC, Eggert H. Evidence for monodentate and bidentate boronate complexes of glucose in the furanose form – application of (1)J(C-C)-coupling-constants as a structural probe. J Am Chem Soc. 1995;117:1479–1484. [Google Scholar]
- 29.Smith BD, Gardiner SJ, Munro TA, Paugam MF, Riggs JA. Facilitated transport of carbohydrates, catecholamines, and amino acids through liquid and plasticized organic membranes. J Incl Phenom Mol Recogn Chem. 1998;32:121–131. [Google Scholar]
- 30.Soundararajan S, Badawi M, Kohlrust CM, Hagerman JH. Boronic acids for affinity chromatography: spectral methods for determinations of ionization and diol-binding constants. Anal Biochem. 1989;178:125–134. doi: 10.1016/0003-2697(89)90367-9. [DOI] [PubMed] [Google Scholar]
- 31.Dicesare N, Lakowicz JR. Evaluation of two synthetic glucose probes for fluorescence-lifetime based sensing. Anal Biochem. 2001;294:154–160. doi: 10.1006/abio.2001.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dicesare N, Lakowicz JR. Wavelength-ratiometric probes for saccharides based on donor-acceptor diphenylpolyenes. J Photochem Photobiol A. Chem. 2001;143:39–47. doi: 10.1016/S1010-6030(01)00471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dicesare N, Lakowicz JR. New color chemosensors for monosaccharides based on Azo dyes. Org Lett. 2001;3:3891–3893. doi: 10.1021/ol016813p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dicesare N, Lakowicz JR. Chalcone-analogue fluorescent probes for saccharide signaling using the boronic acid group. Tet Lett. 2002;43:2615–2618. doi: 10.1016/s0040-4039(02)00312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakowicz JR. Principles of Fluorescence Spectroscopy. 2nd. New York: Kluwer/Academic Plenum Publishers; 1997. [Google Scholar]
- 36.Badugu R, Lakowicz JR, Geddes CD. Ophthalmic glucose sensing: a novel monosaccharide sensing disposable and colorless contact lens. Analyst. 2004;129:516–521. doi: 10.1039/b314463c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Badugu R, Lakowicz JR, Geddes CD. A glucose sensing contact lens: a non-invasive technique for continuous physiological glucose monitoring. J Fluores. 2003;13:371–374. doi: 10.1023/A:1026103804104. First report of a fluorescence-based glucose contact lens sensor.
- 38.Badugu R, Lakowicz JR, Geddes CD. A glucose sensing contact lens: a new approach to non-invasive continuous physiological glucose monitoring. Proc SPIE. 2004;5317:234–245. [Google Scholar]
- 39.Geddes CD, Badugu R, Lakowicz JR. Contact lenses may provide window to blood glucose. Biophotonics Int. 2004 Feb;:50–54. [Google Scholar]
- 40.Badugu R, Lakowicz JR, Geddes CD. Fluorescence sensors for monosaccharides based on the 6-methylquinolinium nucleus and boronic acid moiety: application to ophthalmic diagnostics. Talanta. doi: 10.1016/j.talanta.2004.08.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badugu R, Lakowicz JR, Geddes CD. Boronic acid fluorescent sensors for monosaccharide signaling based on the 6-methoxyquinolinium heterocyclic nucleus: progress towards noninvasive and continuous glucose monitoring. Biomed Chem Lett. doi: 10.1016/j.bmc.2004.09.058. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Badugu R, Lakowicz JR, Geddes CD. Ophthalmic glucose monitoring using disposable contact lenses — a review. J Fluores. 2004;14:617–633. doi: 10.1023/b:jofl.0000039349.89929.da. Review of fluorescence-based glucose sensing in a contact lens.
- 43.Geddes CD. Optical halide sensing using fluorescence quenching: theory, simulations and applications — a review. Meas Sci Tech. 2001;12:R53. [Google Scholar]