Abstract
Background
We have recently demonstrated that treatment with suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor, before a lethal dose of lipopolysaccharide (LPS) improves survival in mice. The purpose of the present study was to determine whether SAHA treatment would attenuate LPS-induced shock and improve survival when given postinsult in a rodent model.
Methods
C57BL/6J mice were intraperitoneally (IP) injected with LPS (30 mg/kg), and 2 hours later randomized into 2 groups: (1) vehicle animals (n = 10) received dimethyl sulfoxide (DMSO) solution only; and (2) SAHA animals (n = 10) were given SAHA (50 mg/kg, IP) in DMSO solution. Survival was monitored over the next 7 days. In a second study, LPS-injected mice were treated with either DMSO or SAHA as described, and normal (sham) animals served as controls. Lungs were harvested at 4, 6, and 8 hours after LPS injection for analysis of gene expression. In addition, RAW264.7 mouse macrophages were cultured to assess the effects of SAHA post-treatment on LPS-induced inflammation using enzyme-linked immunosorbent assay.
Results
All LPS-injected mice that received the vehicle agent alone died within 24 hours, whereas the SAHA-treated animals displayed a significant improvement in 1 week survival (80% vs 0%; P < .001). LPS insult significantly enhanced gene expression of MyD88, tumor necrosis factor (TNF)-α and interleukin (IL)-6, and was associated with an increased protein secretion of TNF-α and IL-6 into the cell culture medium. In contrast, SAHA treatment significantly attenuated all of these LPS-related alterations.
Conclusion
We report for the first time that administration of SAHA (50 mg/kg IP) after a lethal dose of LPS significantly improves long-term survival, and attenuates expression of the proinflammatory mediators TNF-α and IL-6. Furthermore, our data suggest that the anti-inflammatory effects of SAHA may be due to downregulation of the MyD88-dependent pathway, and decreased expression of associated proinflammatory genes.
Sepsis is a systemic inflammatory disorder, and its progression to septic shock is a serious clinical problem associated with a high mortality rate. Despite significant advances in critical care, treatments that can efficiently protect against the consequences of septic shock remain elusive.1 Although antibiotic therapy may effectively treat the inciting infection, this treatment does not reverse the systemic inflammatory response and its consequences.
Lysine acetylation is becoming increasingly appreciated as a key post-translational modification in the endogenous regulation of protein function. Histone acetyl transferase and histone deacetylase (HDAC) regulate chromatin remodeling via histone acetylation/deacetylation,2,3 but these enzymes also modify a large number of nonhistone proteins4 to control multiple cell processes. Small molecule inhibitors of HDAC (HDACI) have been found to alter expression of numerous genes, including inflammatory genes, and interfere with a wide variety of cellular functions.5 We have recently demonstrated that pretreatment of rodents with suberoylanilide hydroxamic acid (SAHA), an HDACI, can improve survival and attenuate acute lung injury in lipopolysaccharide (LPS)-induced septic shock.6 Yet, it is unclear whether SAHA post-treatment could reduce animal mortality and modulate pulmonary inflammation after LPS-induced septic shock.
LPS, the major constituent of the outer membrane of Gram negative bacteria and a ligand for Toll-like receptor (TLR)-4, has been implicated as the bacterial product responsible for inducing septic shock. By binding to host TLR-4, LPS triggers an inflammatory reaction characterized by the release of a large number of inflammatory mediators, such as tumor necrosis factor (TNF)-α and interleukin (IL)-6. LPS–TLR-4 binding initiates these inflammatory cascades by activating signaling pathways that are mediated by key adapter proteins. Myeloid differentiation factor 88 (MyD88) is the central adapter protein for LPS signaling. MyD88 recruits IL-1 receptor-associated kinase (IRAK)-4, which phosphorylates IRAK-1.7 Activated IRAK-1 further recruits TNF receptor-associated factor-6, which activates the transforming growth factor-β activated kinase (TAK)-1/TAK-binding protein-2 complex as well as MAP kinases. Finally, nuclear factor (NF)-κB and activator protein 1 transcription factor are activated, resulting in the transcription of inflammatory genes. Studies on MyD88-deficient mice demonstrate that MyD88 is indispensable for mounting a response to LPS.8 In MyD88-deficient animals, macrophages fail to produce proinflammatory cytokines such as TNF-α and IL-6, and mice are resistant to LPS-induced septic shock.8 However, the effect of SAHA on MyD88 expression and downstream inflammatory responses remains unknown.
The purpose of the present study was to determine whether SAHA post-treatment could attenuate LPS-induced septic shock and improve survival in a murine model, and assess whether SAHA could affect expression of some key mediators in LPS-TLR-4 signaling. SAHA was administrated IP to septic mice 2 hours after LPS challenge. Then, expression of the proinflammatory gene TNF-α and IL-6, and the key mediator MyD88 were examined in lung tissue. Also, secretion of TNF-α and IL-6 proteins from cultured mouse macrophages was analyzed. In addition, the effect of SAHA on mortality after LPS-induced septic shock was determined.
MATERIALS AND METHODS
Materials
LPS (from S typhosa) and dimethyl sulfoxide (DMSO) were purchased from the Sigma Chemical Co (St. Louis, MO). SAHA was purchased from Biomol International (Plymouth Meeting, PA). Primary antibody against MyD88 was purchased from Abcam Inc (Cambridge, MA). Anti-actin antibody was purchased from Sigma. Anti-mouse and anti-rabbit IgG secondary antibodies were purchased from Amersham Biosciences (Piscataway, NJ). Protease Inhibitor Cocktail II was purchased from Calbiochem (San Diego, CA). RPMI 1640 medium, fetal bovine serum (FBS), and phosphate-buffered saline (PBS) were from Gibco-BRL (Grand Island, NY). l-Glutamine, fetal calf serum, and Earle’s balanced salt solution were from Invitrogen (Carlsbad, CA). All other chemicals in this study were of analytical grade and obtained from Sigma if not mentioned otherwise.
Animals
All the research was conducted in compliance with the Animal Welfare Act, and was approved by the Institutional Animal Care and Use Committee. Male C57BL/6J mice (6–8 weeks old) weighing 20–25 g were purchased from Jackson Labs (Bar Harbor, ME). All animals were housed in plastic cages and had access to chow and water throughout the experiment. The animals were kept at room temperature (24 ± 2°C) and exposed to alternating 12 hour cycles of light and darkness. During the experiments the animals were monitored over 7 days, and survival rate was compared between the experimental and control groups.
Experimental protocols
In the first experiment, LPS (30 mg/kg) dissolved in normal saline was injected intraperitoneally (IP). Mice were randomly divided into 2 groups: LPS and LPS + SAHA. Two hours after LPS challenge, the mice were given either DMSO (1 µL/g body weight) or SAHA (50 mg/kg IP) dissolved in DMSO. Survival rate was then recorded over the next 7 days. In the second experiment, mice were divided into 3 groups: LPS, LPS + SAHA, and normal sham (no LPS, no SAHA, and no vehicle) mice. LPS-injected mice were treated with either DMSO or SAHA as described; normal sham animals served as controls. Lungs were harvested at 4, 6, and 8 hours after LPS injections.
Cell culture and treatment
RAW 264.7 cells obtained from American Type Culture Collection were maintained in RPMI 1640 medium supplemented with 10% FBS. These murine macrophages were grown at 37°C in a humidified incubator in 5% CO2 and 95% air. The RAW 264.7 cells were subcultured before 70–80% confluence and seeded at a density of 5 × 104 cells/ mL. The cells were allowed to attach to the bottom of the plates for 24 hours and then serum starved (0.5% FBS) overnight before treatment with LPS (1 µg/mL). Two hours later SAHA (1 µmol/L) was added into the cell culture medium. The cells were harvested at different time points.
Evaluation of LPS-induced TNF-α and IL-6 in RAW264.7 cells
Quantitative determination in the cell culture supernatants was made using the Quantikine ELISA Kit (R&D Systems, Minneapolis, MN) for TNF-α and IL-6 according to manufacturer’s instruction. The concentration of cytokine was measured by optical densitometry at 450 nm in a SpectramaxPlus 384 microplate reader (Molecular Devices, Sunnyvale, CA). All of the analyses were performed in triplicate.
Real-time polymerase chain reaction
Total RNA was prepared from mouse lung tissues in RNAlater solution using the RNeasy Mini kit (Qiagen, Valencia, CA). RNA from each sample was converted into cDNA with High-Capacity cDNA Reverse Transcription kit, following the manufacturer’s protocol (Applied Biosystems, Foster City, CA). Equal amounts of cDNA were submitted to the polymerase chain reaction (PCR) in the presence of SYBR green Master Mix (Roche Applied Science, Indianapolis, IN), forward and reverse primers, and the ABI PRISM 7300 Real-Time PCR detection machine. Primers were designed for specific genes using primer3 software (Table). PCR was performed with 40 cycles of 15 seconds at 95°C, and 1 minute at 60°C. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. Each sample was run in triplicates. Relative mRNA expression was calculated using the parameter threshold cycle (CT) values. ΔCT was the difference in the CT values derived from the specific gene being assayed and the GAPDH mRNA. ΔΔCT represented the difference between the paired samples, as calculated by the formula ΔCT of a sample minus the ΔCT of reference (the average ΔCT of sham samples). The amount of target, normalized to GAPDH and reference, was calculated as 2−ΔΔCT.
Table.
Primers of selected genes for real-time PCR
| Gene name | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| MyD88 | GGA GTC CGA GAA GCC TTT AC | GGA TCA TCT CCT GCA CAA AC |
| IL-6 | CTA CCC CAA TTT CCA ATG CT | ACC ACA GTG AGG AAT GTC CA |
| TNF-α | CCC ACT CTG ACC CCT TTA CT | TTT GAG TCC TTG ATG GTC GT |
| GAPDH | GGA GCG AGA CCC CAC TAA CA | ACA TAC TCA GCA CCG GCC TC |
Western blot analysis
Proteins (about 100 µg per lane) were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis on 12% polyacrylamide gels and transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). The membranes were blocked in 0.05% PBS-Tween (PBST) containing 5% milk (Bio-Rad Laboratories) and then incubated with the primary antibody at 4°C overnight. The primary antibody was detected by incubation with horseradish peroxidase-coupled second antibody (1:3,000 in PBST with 5% milk) at room temperature for 2 hours. The chemiluminescence detection was performed by using Western Lighting Chemiluminescence Reagent Plus (Perkin-Elmer LAS, Inc., Boston, MA). Films were developed using a standard photographic procedure and quantitative analysis of detected bands was carried out by densitometer scanning using the VersaDoc Imaging System (Bio-Rad Laboratories).
Statistical analysis
Statistical differences were determined by Student t tests and ANOVA for 2 group and multiple group comparisons, respectively (SPSS statistical software package; SPSS Inc., Chicago, IL). Kaplan-Meier survival curves were analyzed by using the MedCalc Statistical Software (Mariakerke, Belgium) for the comparison of LPS and LPS + SAHA groups. Differences were considered to be statistically significant when P < .05.
RESULTS
SAHA suppresses LPS-stimulated gene expression of MyD88, TNF-α, and IL-6 in mouse lung tissue
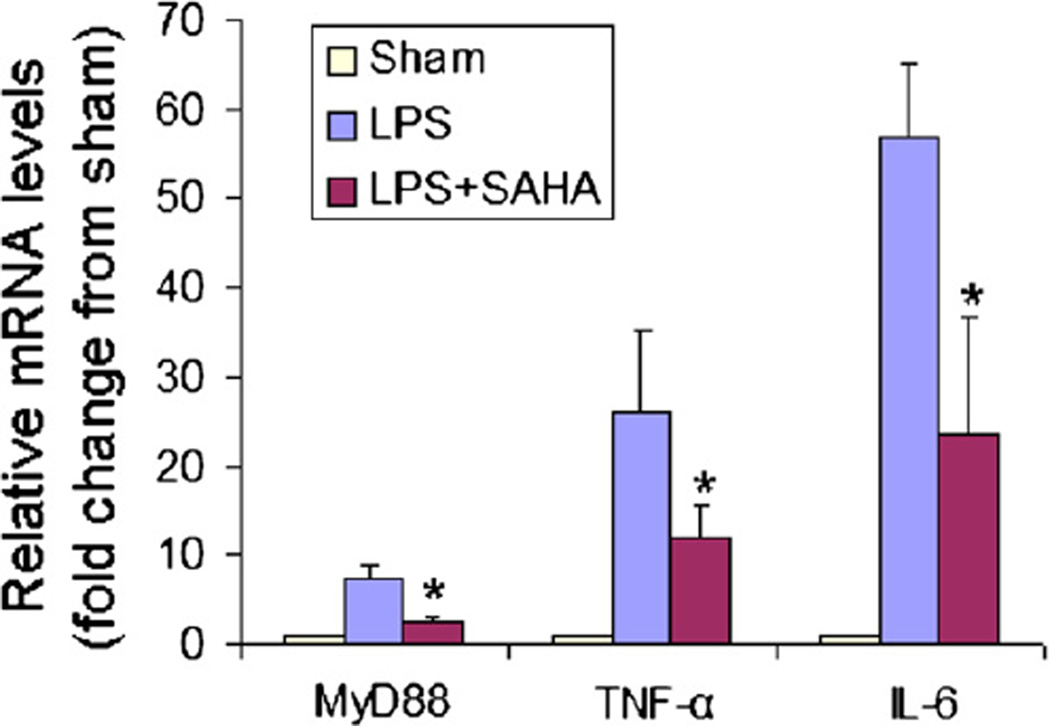
Acute lung injury is a major adverse consequence of septic shock. In our previous studies, we demonstrated that SAHA pre-treatment attenuates LPS-stimulated expression of proinflammatory genes in mouse lung tissues.6 To determine whether SAHA post-treatment would similarly affect inflammatory gene expression, mouse lungs were harvested at 4 hours after LPS challenge (ie, 2 hours after SAHA IP injection), and expression of MyD88, TNF-α, and IL-6 was examined using real-time PCR. In the sham group, expression of these genes was very low. In contrast, LPS dramatically increased the expression of these genes. SAHA treatment given 2 hours after LPS insult significantly decreased the expression of MyD88, TNF-α, and IL-6 genes by 2.95 (P < .001), 2.17 (P < .05), and 2.39 (P < .016) folds, respectively (Fig 1). These results demonstrate that administration of SAHA after LPS insult can attenuate gene expression of MyD88, TNF-α and IL-6.
Fig 1.
SAHA suppresses LPS-stimulated gene expression in mouse lung tissue. Mouse lung tissues were isolated from the LPS and LPS + SAHA groups 4 hours after LPS insult. Untreated, normal mice served as sham controls. MyD88, TNF-α and IL-6 mRNA levels were determined by real-time PCR. Data shown as group mean values ± SD (n = 3); *P < .05 compared with the LPS groups.
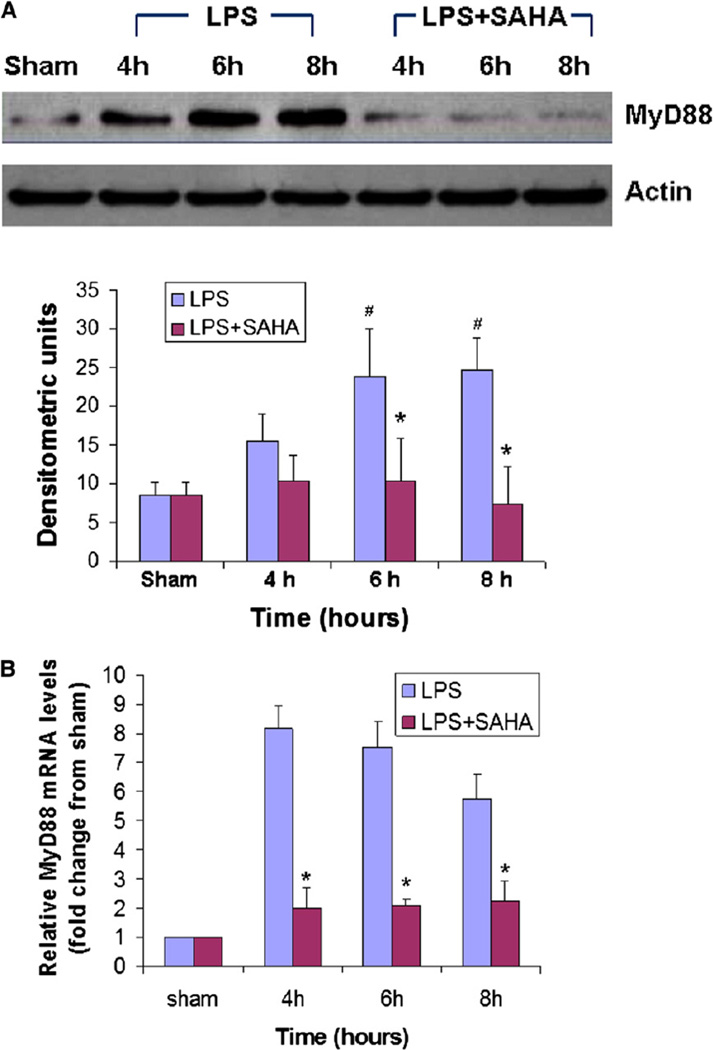
SAHA inhibits LPS-induced expression of the MyD88 gene and protein in mouse lung tissue
MyD88, a cytosolic adaptor protein, is an essential mediator for LPS-TLR signaling.7,9 As such, we investigated whether there was any difference in the expression of MyD88 between LPS-insult and SAHA post-treatment by using real-time PCR and Western blotting. As shown in Fig 2, a low level of MyD88 protein was detected in lung tissues from sham animals. LPS stimulated expression of MyD88 protein at all time points. SAHA treatment significantly decreased LPS-induced MyD88 protein expression at 6 and 8 hours after LPS injection (Fig 2, A; P < .05). Similarly, there was a significant increase in LPS-induced MyD88 gene expression compared with the sham group, and a significant decrease in mRNA levels in the LPS + SAHA group compared with the LPS group (Fig 2, B; P < .05). These results indicate that SAHA post-treatment inhibits LPS-induced MyD88 expression at both the gene and protein levels.
Fig 2.
SAHA decreases LPS-induced MyD88 expression in mouse lung tissue at both the protein (A) and mRNA (B) levels. Whole tissue lysate of mouse lung from sham, LPS, and LPS + SAHA groups at different time points after LPS insult was subjected to western blotting with anti-MyD88 and antiactin antibodies. Specific bands were quantified by densitometry and expressed as mean values ± SD (n = 3). MyD88 mRNA levels were determined by real-time PCR and expressed as mean values ± SD. #Differs significantly (P < .05) from sham groups. *P < .05 versus the LPS group. Note: All time points (hours) in this study use LPS injection as the reference point (time zero).
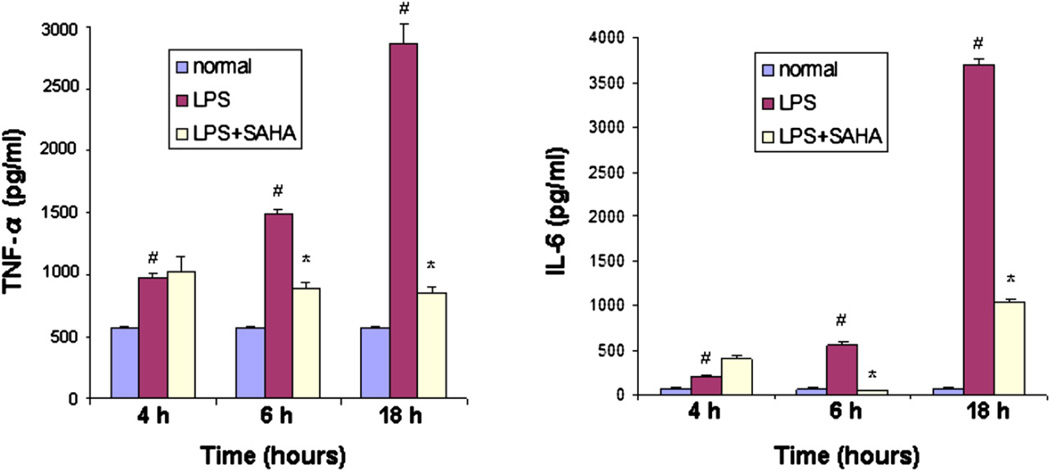
SAHA decreases secretion of the proinflammatory proteins TNF-α and IL-6 from mouse macrophages
Macrophages are one of the major immune cells that produce TNF-α and IL-6 cytokines. To assess the effect of SAHA on LPS-induced secretion of these cytokines, mouse RAW264.7 macrophages were cultured in RPMI medium and treated with LPS (1 µg/mL). SAHA (1 µmol/L) was added into the cell culturemedium2 hours after LPS insult. The cell culture medium was collected at 4, 6, and 18 hours after LPS challenge. Enzyme-linked immunosorbent assay was performed to analyze the secretion of TNF-α and IL-6 from the macrophages. LPS markedly increased protein secretion of TNF-α and IL-6 at all time points. Treatment with SAHA did not decrease the cytokine secretion at 4 hours. However, SAHA significantly suppressed secretion of TNF-α and IL-6 induced by LPS at 6 and 18 hours (Fig 3). These results demonstrate that SAHA attenuates LPS-stimulated secretion of proinflammatory cytokines, suggesting that the inhibitory effect of SAHA on LPS-induced septic shock may, in part, result from a decrease in production of TNF-α and IL-6 proteins.
Fig 3.
SAHA decreases protein secretion of TNF-α and IL-6 from mouse macrophages. RAW264.7 macrophages were cultured as described in Materials and Methods. Concentrations of TNF-α and IL-6 in the supernatant of the cultured cells were determined by ELISA at 0, 4, 6, and 18 hours after LPS treatment in the absence and presence of SAHA. Untreated macrophages served as normal control. The cytokine concentration was expressed as mean values ± SD (n = 3). #Significantly differs from normal control groups. *Significantly differs from the LPS groups.
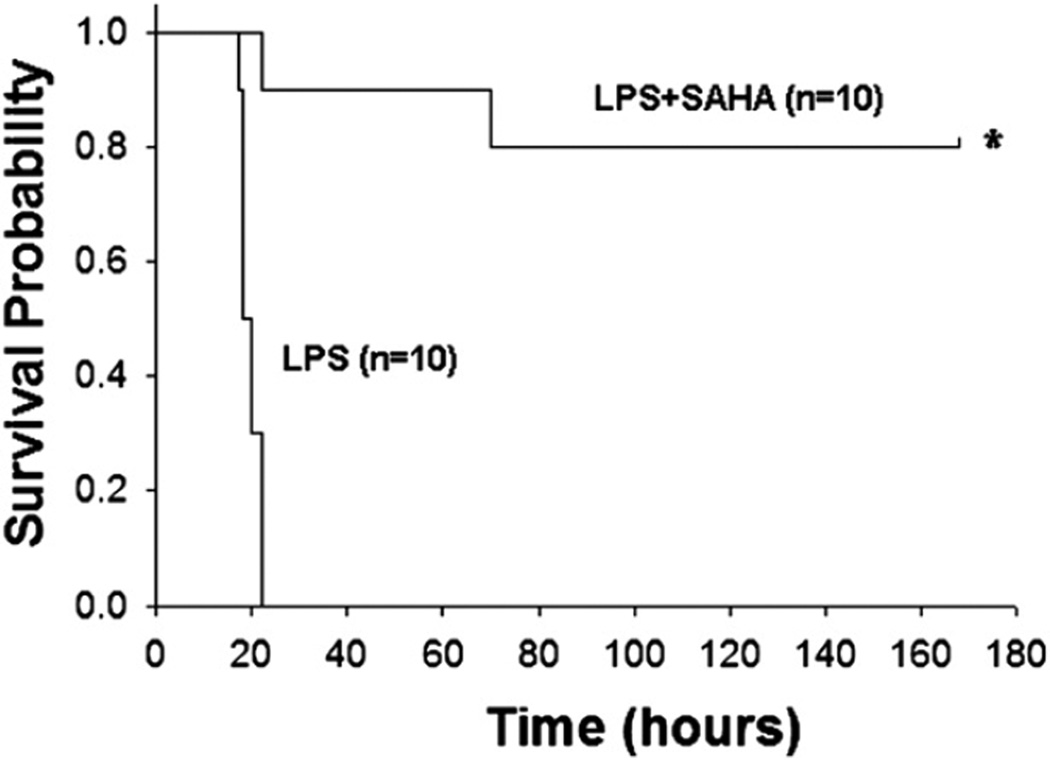
SAHA post-treatment improves survival in a mouse model of LPS-induced septic shock
We have recently reported that treatment of mice with SAHA (50 mg/kg) twice (2 hours before and after LPS insult) can improve survival in a model of LPS-induced septic shock.6 To assess whether SAHA post-treatment could protect the mice from LPS-induced septic shock, we injected SAHA (50 mg/ kg) into mice IP 2 hours after LPS insult and compared the survival rates between LPS and LPS + SAHA–treated animals. First, we established the lethal dose of LPS by performing a dose escalation study, and found that administration of LPS (lot #L6386) at 30 mg/kg (IP) resulted in 100% mortality (data not shown). Mice were administrated this dose of LPS and 2 hours later, the SAHA group was given SAHA (50 mg/kg IP) in DMSO (n = 10), and the LPS group (n = 10) was injected with the same dose of DMSO (1 µL/g body weight).6 All animals were observed to assess survival. As shown in Fig 4, all mice in the LPS group died in <24 hours. However, SAHA-treated animals displayed a significantly higher long term survival rate (80% of mice survived >7 days), compared with the LPS group (0%). The results indicate that SAHA treatment significantly decreases mouse mortality after LPS-induced septic shock.
Fig 4.
SAHA decreases LPS-induced mortality. Mice were injected IP with SAHA (50 mg/kg; LPS + SAHA group; n = 10) or DMSO vehicle (1 µL/g body weight; LPS group; n = 10) 2 hours after LPS (30 mg/kg IP) challenge. Survival rates were recorded for 168 hours (7 days) after LPS insult. *P < .001 versus the LPS group. Kaplan-Meier curves were used for the comparison of survival rates between the LPS control and LPS + SAHA groups.
DISCUSSION
In the present study, we investigated the therapeutic effect of SAHA post-treatment on LPS-induced septic shock. Using the potent HDACI, our in vitro and in vivo experiments revealed some important findings: (1) SAHA attenuates LPS-stimulated expression of proinflammatory cytokine TNF-α and IL-6; (2) SAHA suppresses expression of MyD88 adaptor (at both gene and protein levels), a central player in the LPS-TLR-4 signal transduction pathway; and (3) SAHA improves survival even when administered 2 hours after the LPS insult.
HDAC are important drug targets in cancer and neurodegenerative diseases such as Parkinson’s and Alzheimer’s diseases, with >80 clinical trials currently underway.10,11 Recently HDACI have been found to have potent anti-inflammatory properties. SAHA is a hydroxamic acid that is a pan-HDACI. We have previously shown that hemorrhage is associated with HDAC/histone acetyl transferase imbalance, which influences the acetylation status of cardiac, lung and liver histones, and that the imbalance can be corrected by treatment with pharmacologic HDACI.12–14 In a rat model of lethal hemorrhagic shock, we have shown that SAHA reduces TNF-α level after hemorrhage and LPS “second hit” and improves animal survival.15 In a mouse model of LPS-induced septic shock, we have recently demonstrated that SAHA pretreatment can improve survival after LPS challenge. Furthermore, we have found that the beneficial effects are due to restoration of normal protein acetylation, a decrease in inflammatory cytokines, and attenuation of subsequent organ damage.6 Our current results suggest that benefits of SAHA post-treatment are nearly identical to what has been established in the pretreatment paradigm.
LPS is a well-known immunostimulatory component of bacteria and can induce systemic inflammation and septic shock if excessive stimulatory signaling persists. LPS activation of mammalian cells occurs through TLR-4. Upon LPS recognition, TLR-4 undergoes oligomerization and recruits its downstream adaptors through interactions with the Toll–IL-1 receptor (TIR) domains. TIR domains contain 3 highly conserved regions, which mediate protein–protein interactions between the TLR-4 and signal transduction adaptor proteins in LPS–TLR-4 signaling. So far, 5 TIR domain-containing adaptor proteins have been reported: MyD88, TIRAP (TIR domain-containing adaptor protein, also known as Mal, MyD88-adaptor–like), TRIF (TIR domain-containing adaptor inducing interferon-β), TRAM (TRIF-related adaptor molecule), and SARM (sterile α and HEAT-Armadillo motif-containing protein).
Of the 5 adaptors, MyD88 has been most extensively studied. Based on the MyD88 protein, TLR-4 signaling has been divided into MyD88-dependent and MyD88-independent pathways. The former contains TIRAP and MyD88 adaptors and the later carries TRAM, TRIF and SARM adaptors. Using MyD88-deficient macrophages, the MyD88-dependent pathway has been shown to be responsible for proinflammatory cytokine (eg, TNF-α, IL-6) expression, whereas the MyD88-independent pathway mediates the induction of type I interferons and interferon-inducible genes. The fact that SAHA treatment attenuates LPS-induced proinflammatory gene expression (Fig 1) led us to examine whether the inhibition of HDAC interferes with proteins involved in MyD88-dependent pathway.
Because LPS-TLR-4 signaling can induce potent inflammatory responses including septic shock, inhibition of the pathway is necessary to protect the host from inflammation-induced damage. Many negative regulators are known to target multiple levels in the LPS-TLR-4 signal transduction pathway. In the MyD88-dependent pathway, some molecules such as radioprotective 105, single immunoglobulin IL-1R–related molecule and ST2L are expressed on the cell surface and their inhibitory functions act at the initiation stage of LPS–TLR-4 signaling. Other intracellular negative regulatory proteins such as IRAK-M, IRAK-2c, MyD88s, and A20 act further downstream in the signaling pathway through different mechanisms.16 Our preliminary studies here provide the first evidence that SAHA downregulates the MyD88-dependent pathway through inhibition of MyD88 expression.
It is well known that TLR-4-MyD88 signaling can be generated by binding of LPS to the receptor. Although LPS clearly can stimulate TLR-4, endogenous substances such as heparin sulfate were also found to be effective agonists.17 Recently Brunn and Platt summarized that (1) under quiescent conditions, intact extracellular matrix inhibits TLR-4 activation; (2) on the first step in sepsis, degradation of extracellular matrix relieves inhibition of TLR-4 after LPS insult; and (3) not only microbial substances but also endogenous molecules can trigger TLR-4.18 These new discoveries about the mechanism of action clarify why LPS-induced TLR-4–MyD88 signaling can last for a long period. On the basis of these findings, Campo et al reported that chondroitin-4–sulphate and chondroitin-6–sulphate can inhibit MyD88 protein expression in chondrocytes 4 hours after LPS induction and this inhibition remains for >24 hours.19 In our present study, SAHA post-treatment significantly suppresses MyD88 protein expression, suggesting that the anti-inflammatory effects of SAHA, at least in part, are exerted through the TLR-4-MyD88–dependent pathway.
Currently, our knowledge of whether SAHA can induce acetylation of the MyD88 protein or other proteins along the MyD88 pathway remains scarce. Also, an explanation as to how acetylation affects MyD88 expression is elusive. Accumulating evidence suggests that acetylation of signal transducer and activator of transcription (STAT)-1 could be involved in down-regulation of MyD88 expression through a putative cross-talk between STAT-1 and NF-κB signaling.20,21 It has been known that HDACI can induce acetylation of Stat-1.22 Acetylated STAT-1 is able to interact with NF-κB signaling and inhibit the transcriptional activity of the NF-κB p50/p65 heterodimer.23 As a consequence, NF-κB nuclear localization, DNA binding, and expression of NF-κB target genes are decreased.22 Recently, Wasiluk et al24 reported that NF-κB elements regulate transcriptional activity within the murine MyD88 promoter. It is conceivable that SAHA treatment in our septic shock model could reduce signaling through the MyD88-dependent pathway by inhibition of MyD88 expression through interaction of acetylated STAT-1 and NF-κB. However, we cannot rule out the possibility that SAHA could also inhibit expression of proinflammatory cytokines by interruption of the MyD88-dependent pathway at other distinct levels.
Another possibility is that HDACI could have induced acetylation of some important nonhistone proteins. For instance, tumor suppressor p53 is a key player in cellular signaling and stress responses. It has already been reported that HDAC can deacetylate p53 in vitro and in vivo.25 Unacetylated lysines in p53 are targets for ubiquitination by MDM2 (mouse double minute gene number 2), which ultimately leads to the destruction of p53. The lysine residues acetylated in p53 overlap with those that are ubiquitinated, and p53 acetylation serves to promote p53 stabilization.25 Recently, Liu et al26 reported that culture of LPS-stimulated neutrophils and macrophages with a specific inducer of p53 stabilization (eg, nutlin-3a) attenuates NF-κB DNA-binding activity and production of proinflammatory cytokines. However, the scope of protein acetylation involvement in the complex biological process of septic shock and HDACI treatment remains uncertain. Further analysis of protein acetylation profiling using proteomics technology would help us to better understand how protein acetylation by SAHA protects the animals against LPS-induced septic shock.
We have observed some differences in TNF-α inhibition by SAHA based on the timing of SAHA treatment. In our previous study, cultured RAW264.7 macrophages were cotreated with LPS and SAHA; therefore, SAHA exposure was 2 hours earlier than the present project. Although the concentrations of LPS and SAHA remained constant, the earlier SAHA treatment decreased TNF-α protein secretion at 2 hours,6 whereas SAHA post-treatment does not suppress cytokine production until 6 hours after LPS stimulation (or 4 hours after SAHA treatment; Fig 3). The delayed effect of SAHA on the TNF-α secretion may be due to retarded acetylation of proteins by SAHA.
Like any scientific investigation, this study has certain limitations that must be acknowledged. LPS challenge, although well established, does not completely replicate all aspects of septic shock. We are currently validating these findings in a more realistic model of cecal ligation and puncture. Also, for logistical reasons we could not measure many other cytokines (eg, IL-1β, IL-8, IL-10), regulatory proteins (eg, TIRAP, IRAK-1, NF-κB) and pathways (eg, MyD88-independent pathway, stress-activated protein kinase/c-Jun NH2-terminal kinase pathway, extracellular signal-regulated kinase pathway) that may also be involved. We are currently exploring a number of these pathways using chromatin immunoprecipitation, genomic DNA microarray, and high-throughput proteomic techniques. In the future, we are also planning to carry out an experiment to determine dose–response relationships for SAHA treatment.
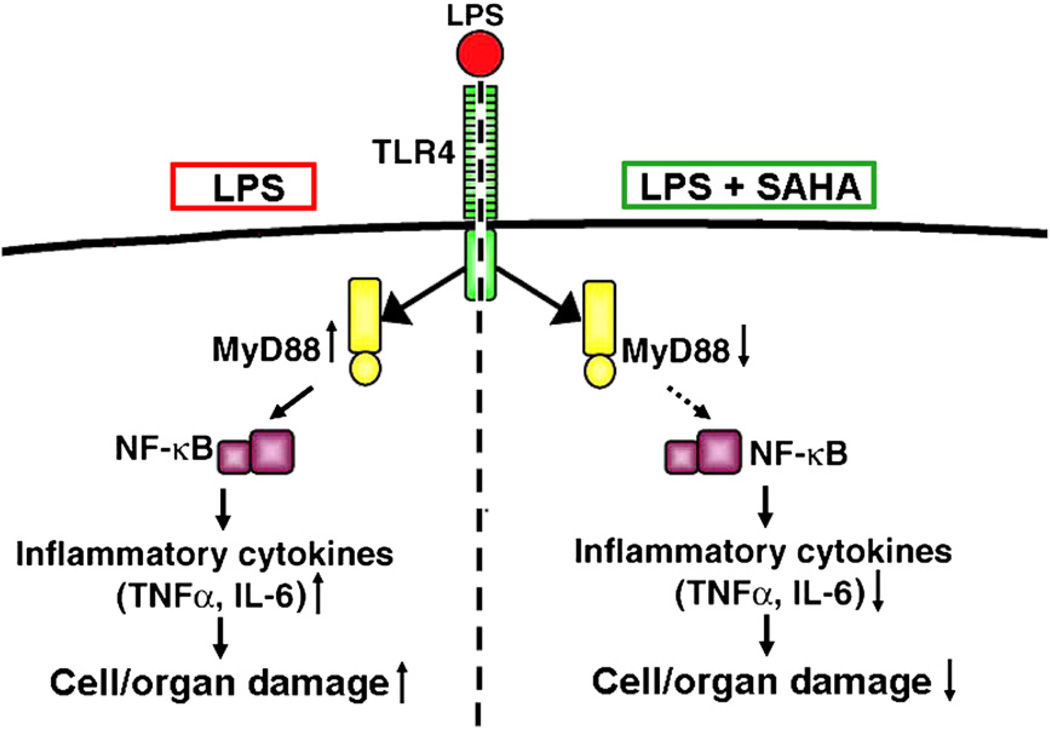
In conclusion, we have demonstrated for the first time that post-treatment with an HDACI can improve survival after a lethal dose of LPS in rodents. Furthermore, our data suggest that the beneficial effects may be in part, related to negative regulation of MyD88 expression and inflammatory cytokine production (Fig 5). In agreement with our findings, HDACI have been studied in various models of inflammation and the anti-inflammatory efficacy has been observed by several independent investigators.27,28 Although the fundamental molecular and cellular signaling events, whereby HDACI suppresses multiple inflammatory cytokines, still requires further investigation, protein acetylation seems to regulate gene expression and protein function, and represents a novel and promising therapeutic strategy for septic shock.
Fig 5.
Possible protective mechanisms of SAHA treatment in LPS-induced septic shock. Treatment with SAHA somehow inhibits expression of the MyD88 gene and protein, reduces LP–TLR-4 signaling, attenuates LPS-stimulated expression of proinflammatory cytokines, and improves survival.
Acknowledgments
Funded by a generous research endowment by the Polsky family and by NIH RO1 GM084127 (H.B.A.).
Footnotes
Data accepted for an oral presentation at the 5th Annual Academic Surgical Congress, San Antonio, Texas, February 3, 2010.
REFERENCES
- 1.Weighardt H, Holzmann B. Role of toll-like receptor responses for sepsis pathogenesis. Immunobiology. 2008;212:715–722. doi: 10.1016/j.imbio.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Gallinari P, Di Marco S, Jones P, Pallaoro M, Steinkuhler C. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res. 2007;17:195–221. doi: 10.1038/sj.cr.7310149. [DOI] [PubMed] [Google Scholar]
- 3.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 4.Yang XL, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halili MA, Andrews MR, Sweet MJ, Fairlie DP. Histone deacetylase inhibitor in inflammatory disease. Curr Top Med Chem. 2009;9:309–319. doi: 10.2174/156802609788085250. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Liu B, Zhao H, Saihamer EA, Fukudome EY, Zhang X, et al. Protective effect of suberoylanilide hydroxamic acid against LPS-induced septic shock in rodents. Shock. 2009;32:517–523. doi: 10.1097/SHK.0b013e3181a44c79. [DOI] [PubMed] [Google Scholar]
- 7.Janssens S, Beyaert R. A universal role for MyD88 in TLR/ IL-1R-mediated signaling. Trends Biochem Sci. 2002;27:474–482. doi: 10.1016/s0968-0004(02)02145-x. [DOI] [PubMed] [Google Scholar]
- 8.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 9.Beutler B. Tlr4: central component of sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 10.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 11.Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7:854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- 12.Lin T, Chen H, Koustova E, Sailhamer EA, Li Y, Shults C, et al. Histone deacetylase as therapeutic target in a rodent model of hemorrhagic shock: effect of different resuscitation strategies on lung and liver. Surgery. 2007;141:784–794. doi: 10.1016/j.surg.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Alam HB, Shults C, Ahuja N, Ayuste EC, Chen H, Koustova E, et al. Impact of resuscitation strategies on the acetylation status of cardiac histones in a swine model of hemorrhage. Resuscitation. 2008;76:299–310. doi: 10.1016/j.resuscitation.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Gonzales E, Chen H, Munuve R, Mehrani T, Britten-Webb J, Nadel A, et al. Valproic acid prevents hemorrhage-associated lethality and affects the acetylation pattern of cardiac histones. Shock. 2006;25:395–401. doi: 10.1097/01.shk.0000209522.28120.c8. [DOI] [PubMed] [Google Scholar]
- 15.Sailhamer EA, Li Y, Smith EJ, Shuja F, Shults C, Liu B, et al. Acetylation: a novel method for modulation of the immune response following trauma/hemorrhage and inflammatory second hit in animals and humans. Surgery. 2008;144:204–216. doi: 10.1016/j.surg.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signaling transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Brunn GJ, Bungum MK, Johnson GB, Platt JL. Conditional signaling by Toll-like receptor 4. FASEB J. 2005;19:872–874. doi: 10.1096/fj.04-3211fje. [DOI] [PubMed] [Google Scholar]
- 18.Brunn G, Platt J. The etiology of sepsis: turned inside out. Trends Mol Med. 2006;12:10–16. doi: 10.1016/j.molmed.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Campo GM, Avenoso A, Campo S, Traina P, D’Ascola A, Calatroni A. Glycosaminoglycans reduced inflammatory response by modulating toll-like receptor-4 in LPS-stimulated chondrocytes. Arch Biochem Biophys. 2009;491:7–15. doi: 10.1016/j.abb.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Shen H, Lentsch AB. Progressive dysregulation of transcription factors NF-κB and STAT1 in prostate cancer cells causes proangiogenic production of CXC chemokines. Am J Physiol Cell Physiol. 2004;286:C840–C847. doi: 10.1152/ajpcell.00335.2003. [DOI] [PubMed] [Google Scholar]
- 21.Kimura A, Naka T, Nakahama T, Masuda K, Nohara K, Fujii-Kuriyama Y, et al. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med. 2009;206:2027–2035. doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer OH, Baus D, Knauer SK, Stein S, Jager E, Stauber RH, et al. Acetylation of Stat1 modulates NF-κB activity. Genes Dev. 2006;20:473–485. doi: 10.1101/gad.364306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchwald M, Kramer OH, Heinzel T. HDACi: targets beyond chromatin. Cancer Letters. 2009;280:160–167. doi: 10.1016/j.canlet.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 24.Wasiluk KR, McCuloch KA, Banton KL, Dunn DL. NF-κB elements regulate transcriptional activity within the murine MyD88 promoter. J Surg Res. 2006;130:332. doi: 10.1089/sur.2006.7.489. [DOI] [PubMed] [Google Scholar]
- 25.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Liu G, Park YJ, Tsuruta Y, Lorne E, Abraham E. p53 attenuates lipopolysaccharide-induced NF-κB activation and acute lung injury. J Immunol. 2009;182:5063–5071. doi: 10.4049/jimmunol.0803526. [DOI] [PubMed] [Google Scholar]
- 27.Glauben R, Batra A, Fedke I, Zeitz M, Lehr HA, Leoni F, et al. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol. 2006;176:5015–5022. doi: 10.4049/jimmunol.176.8.5015. [DOI] [PubMed] [Google Scholar]
- 28.Lin HS, Hu CY, Chan HY, Liew YY, Huang HP, Lepescheux L, et al. Anti-rheumatic activities of histone deacetylase (HDAC) inhibitors in vivo in collagen-induced arthritis in rodents. B J Pharmacol. 2007;150:862–872. doi: 10.1038/sj.bjp.0707165. [DOI] [PMC free article] [PubMed] [Google Scholar]