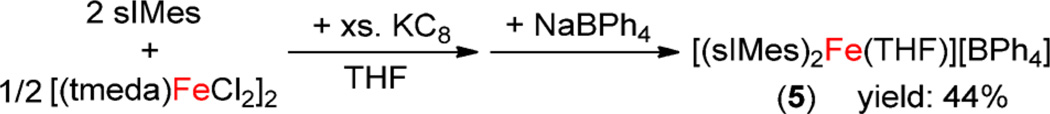Abstract
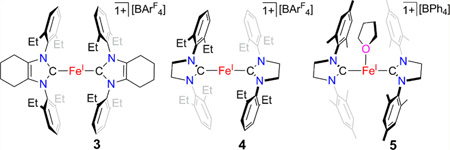
The use of the N-heterocyclic carbene (NHC) ligands 1,3-bis(2′,6′-diethylphenyl)-4,5-(CH2)4-imidazol-2-ylidene (cyIDep), 1,3-bis(2′,6′-diethylphenyl)-imidazolin-2-ylidene (sIDep), and its N-mesityl analogue sIMes enables the preparation of the two-coordinate homoleptic iron(I)-NHC complexes [(cyIDep)2Fe][BArF4] (3, ArF denoted for 3,5-di(trifluoromethyl)phenyl) and [(sIDep)2Fe][BArF4] (4) and the T-shaped iron(I)-NHC complex [(sIMes)2Fe(THF)]-[BPh4] (5, THF = tetrahydrofuran). Complexes 3–5 were prepared via the sequential protocol of control reduction of iron(II) dihalides by KC8 in the presence of the corresponding NHC ligands followed by halide-abstraction with NaBAr4. Spectroscopic characterization, including single-crystal X-ray diffraction studies and 57Fe Mössbauer spectroscopy, in combination with density functional theory calculations, suggest their high-spin nature. Solution property study (absorption spectroscopy and cyclic voltammetry) indicates that 3 and 5 keep their corresponding two- and three-coordinate nature in THF solution, and 4 might reversibly coordinate a THF molecule to form, presumably, the T-shaped species [(sIDep)2Fe(THF)][BArF4]. The isolation of 3 and 4 demonstrates the accessibility of homoleptic two-coordinate iron(I)-NHC complexes.
Graphical Abstract
INTRODUCTION
Low-coordinate iron(I) complexes (e.g., coordination numbers (CNs) of 3 and 2) are distinctive as iron(I) is an uncommon oxidation state and CNs less than 4 are rare for iron.1,2 The coexistence of these unusual structure features renders the synthesis of low-coordinate iron(I) complexes challenging as this type of species could have the tendency not only to undergo disproptionation but also to coordinate with exogenous ligands. Their promising chemical and physical properties, for example, small molecule activation3 and single-molecule magnet behavior,4 however, have stimulated significant synthetic efforts in the recent years.
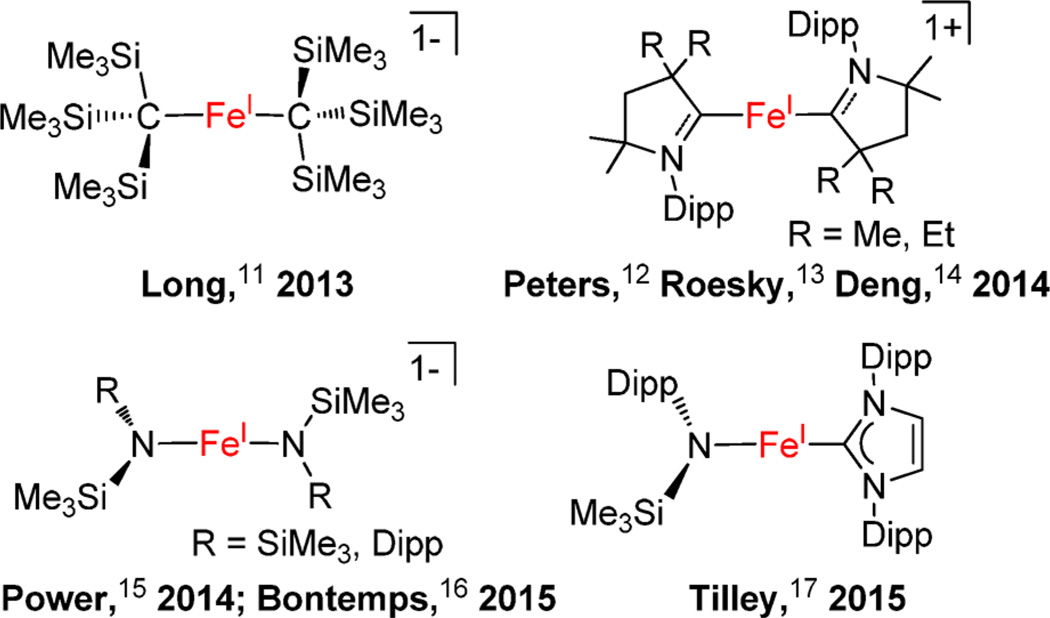
The stabilization of low-coordinate iron(I) complexes necessitates bulky supporting ligands. So far, bidentate β-diketiminate ligands bearing bulky N-aryl substituents proved privileged in stabilizing three-coordinate iron(I) complexes. Holland, as the pioneer in this area, achieved the syntheses of three-coordinate β-diketiminate iron(I) complexes with hydride,5 sulfide,6 alkenes,7 and alkynes7 as coligands. Some of the β-diketiminate iron(I) species can activate N2.3a,8 In addition to the β-diketiminate iron(I) complexes, Caulton reported a unique T-shaped iron(I) complex [((tBu2PCH2SiMe2)2N)Fe],9 and Jones reported a dinuclear iron(I) guanidinate compound [(DippN)2C(cis-2,6-Me2NC5H8)Fe]2 (Dipp = 2,6-diisopropylphenyl) that contains an Fe–Fe bond.10 Two-coordinate iron(I) complexes have remained unknown until Long’s first report on [K(crypt-222)][Fe(C(SiMe3)3)2] in 2013.11 Until now, several two-coordinate iron(I) complexes have been reported (Chart 1).12–17 In these two-coordinate iron(I) complexes, bulky anionic ligands, such as [C(SiMe3)3]1−, [NSiMe3(Dipp)]1−, and [N(SiMe3)2]1−, and neutral carbene ligands, imidazol-2-ylidenes, cyclic alkylaminocarbenes (cAACs),18 are utilized. A notable property of these two-coordinate complexes is slow magnetic relaxation behavior typical of single-molecule magnets.11,13,16
Chart 1.
Reported Two-Coordinate Iron(I) Complexes
Different from cAACs that are good π-accepting ligands, imidazol-2-ylidenes, the more common N-heterocyclic carbene (NHC) ligands, are thought to be good σ-donating ligands with a weak but variable π-accepting ability.18b,19 Recently, we and others found that monodentate imidazol-2-ylidenes can serve as supporting ligands for low-coordinate iron(II)20 and iron(IV)21 complexes. With olefins as coligands, the low-coordinate iron(0)-NHC complex, for example, [(IMes)Fe(η2:η2-dvtms)] (dvtms = divinyltetramethyldisiloxane), is also accessible.21 Regarding these, limited progress has been made on the synthesis of low-coordinate iron(I)-NHC complexes. We found that the one-electron reduction reaction of [(IMes)2FeCl2] (IMes: 1,3-dimesitylimidazol-2-ylidene) by sodium amalgam can furnish a three-coordinate iron(I) complex [(IMes)2FeCl], which readily disproportionates in solution to a mixture of iron(0) and iron(II) species.14 The attempts to prepare the two-coordinate species [(IMes)2Fe]+ from [(IMes)2FeCl] by chloride-abstraction with NaBAr4 were unsuccessful.14 On the other hand, Tilley and his co-workers achieved the synthesis of the first heteroleptic two-coordinate iron(I) complex bearing an imidazol-2-ylidene ligand, [(IPr)Fe(NDippSiMe3)] (IPr: 1,3-bis(2′,6′-diisopropylphenyl)imidazol-2-ylidene) using the two-coordinate iron(II) complex [Fe(NDippSiMe3)2] as the iron precursor.17 The attainment of [(IPr)Fe(NDippSiMe3)], thus, hints the accessibility of the homoleptic two-coordinate iron(I)-imidazol-2-ylidene complexes. Inspired by these, we then targeted NHCs with more steric hindrance and/or less steric flexibility over IMes as supporting ligands. We report herein the preparation and characterization of the first homoleptic two-coordinate iron(I)-NHC complexes and a three-coordinate T-shaped iron(I) complex with the six-membered ring-fused imidazol-2-ylidene and imidazolin-2-ylidenes as supporting ligands (Chart 2).
Chart 2.
Designations for NHC Ligands and Iron(I)-NHC Complexes
RESULTS AND DISCUSSION
Synthesis and Characterization of Three-Coordinate NHC-Iron(I) Bromides
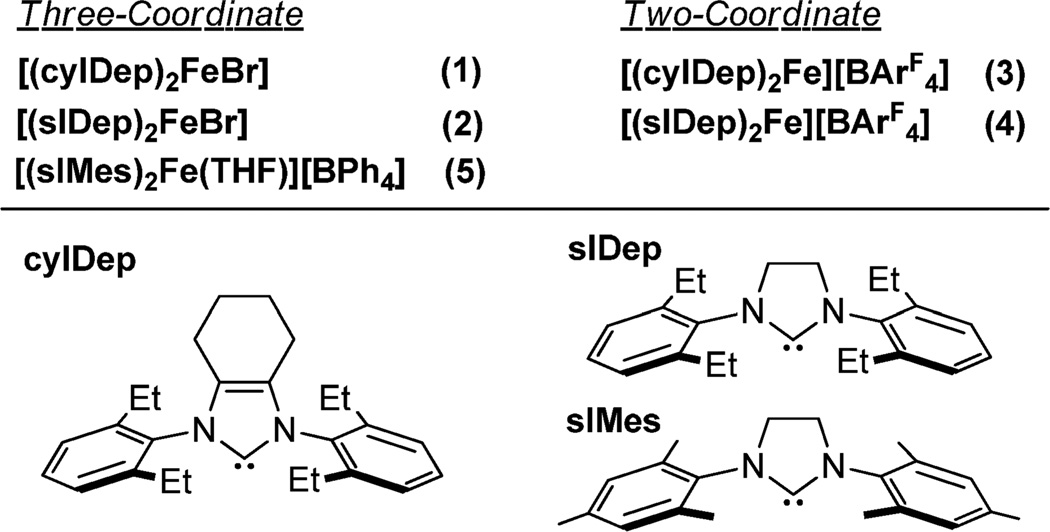
Following the synthetic route for [(IMes)2FeCl],14 we examined the reduction reactions of ferrous halides with KC8 in the presence of a variety of NHCs and successfully prepared two new three-coordinate iron(I)-NHC complexes (1 and 2).
The addition of an excessive amount of KC8 (2.5 equiv) to a preformed mixture of FeBr2 with 1,3-bis(2′,6′-diethylphenyl)-4,5-(CH2)4-imidazol-2-ylidene (cyIDep, 2 equiv) or 1,3-bis-(2′,6′-diethylphenyl)-imidazolin-2-ylidene (sIDep, 2 equiv) in toluene produced a brown or purple suspension, from which the iron(I)-NHC complex [(cyIDep)2FeBr] (1) or [(sIDep)2-FeBr] (2) was isolated as brown or purple solid, respectively, in good yields (Scheme 1). Attempts to access similar iron(I) halide complexes using 1,3-bis(2′,6′-diisopropylphenyl)-imidazol-2-ylidene (IPr), 1,3-diadamantylimidazol-2-ylidene (IAd), and 1,3-di-tert-butylimidazol-2-ylidene (ItBu) as the ancillary ligands were unsuccessful. In these cases, while homogeneous solution could be formed when mixing the ferrous salt with 2 equiv of the NHC ligands, the further interaction of the mixture with KC8 led to the formation of iron black and free NHCs. Noting the similar electronic properties of these NHCs,18b the different outcome indicated the selection of NHCs with appropriate steric properties is crucial for the preparation of low-coordinate iron(I)-NHC halides.
Scheme 1.
Preparation Route for 1 and 2
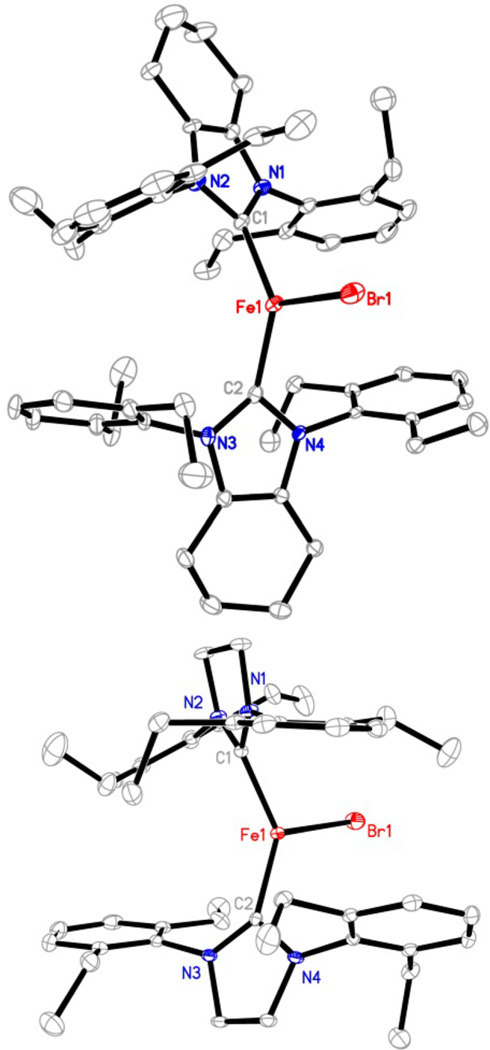
Complexes 1 and 2 are air- and moisture-sensitive. Under N2 atmosphere, they decompose slowly in solution (C6H6 and tetrahydrofuran (THF)) with the formation of black precipitates and free NHCs (as revealed by 1H NMR spectroscopy). In the solid state, they can be kept for weeks at −25 °C without noticeable decomposition. X-ray crystallographic studies established the molecular structures of 1 and 2 as trigonal planar iron(I) complexes (Figure 1). Table 1 lists their key structural parameters in addition to those of [(IMes)2FeCl] for comparison.14 In spite of the difference of the ligands, the Fe–C(carbene) distances in these three-coordinate iron(I)-NHC halide complexes are all ~2.00 Å, and their C(carbene)–Fe–C(carbene) angles span a narrow range (125.6(3) to 133.3(1)°). The angles are much smaller than those (166.4(1) to 168.3(1)°) of the congeners of the T-shaped nickel complexes [(NHC)2NiX] (NHC = IMes, X = Cl, Br, I; NHC = IPr, X = Cl).22 The larger atomic radius of iron over nickel, which renders long M–C(carbene) bonds and less severed steric repulsion between the NHCs in the iron complexes, could be one of the causes leading to the structure difference between the iron(I) and nickel(I) complexes. The quadrupole doublets in the 80 K Mössbauer spectra of 1 and 2 (Figures S1 and S2) have comparable isomer shifts (δ = 0.69 and 0.65 mm/s, respectively) and quadrupole splittings (ΔEQ = 2.36 and 2.27 mm/s), similar to the parameters previously reported for [(IMes)2FeCl] (δ = 0.65 mm/s, ΔEQ = 2.63 mm/s).14 These parameters are in agreement with the parameters expected for high-spin three-coordinate iron(I) complexes.
Figure 1.
Molecular structures of 1 (upper, showing one of the two crystallographically independent molecules in the unit cell) and 2 (lower) with 30% probability ellipsoids. Hydrogen atoms are omitted for clarity.
Table 1.
Selected Bond Lengths and Angles of 1–5, [(IMes)2FeCl] (6), and [(Me2-cAAC)2Fe][BArF4] (7) from X-ray Diffraction Studies, and their 57Fe Mössbauer Spectroscopic Data
| 1a | 2 | 3a | 4 | 5 | 6b | 7b | |
|---|---|---|---|---|---|---|---|
| Fe–C (Å) | 2.023(4) | 2.013(3), 2.020(3) | 1.996(7) | 1.983(5), 1.971(5) | 1.989(5), 1.972(5) | 1.998(9), 2.030(8) | 1.997(3), 1.997(3) |
| Fe–X (Å)c | 2.402(1) | 2.387(1) | 2.145(3) | 2.258(3) | |||
| C–N (Å)d | 1.373(5) | 1.353(3) to 1.362(3) | 1.359(8) | 1.333(6) to 1.351(6) | 1.347(6) to 1.357(6) | 1.349(13) to 1.404(11) | 1.310(3) |
| C–Fe–C (deg) | 133.3(1) | 130.6(1) | 178.4(3) | 175.8(2) | 162.9(1) | 125.6(3) | 180 |
| C–Fe–X (deg)c | 113.3(1) | 109.9(1), 119.5(1) | 95.0(1), 102.1(1) | 116.4(3), 118.0(3) | |||
| δ (mm/s) | 0.69 | 0.65 | 0.48 | 0.55 | 0.65 | 0.65 | 0.49 |
| ΔEQ (mm/s) | 2.36 | 2.27 | 5.75 | 6.82 | 5.99 | 2.63 | 4.58 |
Structure data are the average of two crystallographically independent molecules in the unit cell.
Data from ref 14.
X = Br for 1 and 2, O for 5, Cl for 6.
Range of the C(carbene)–N distances.
Synthesis and Characterization of Two-Coordinate Iron(I)-NHC Complexes
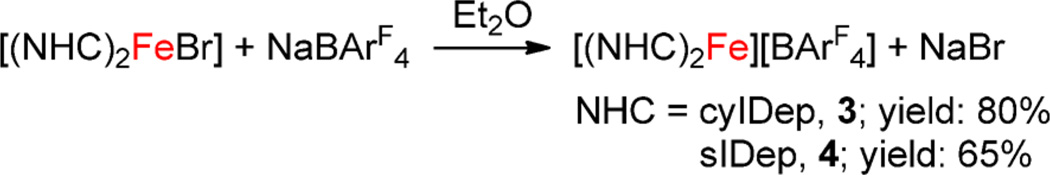
The 2,6-diethylphenyl (Dep)-substituted NHC complexes, 1 and 2, proved to be effective precursors for the synthesis of two-coordinate iron(I)-NHC complexes. The interaction of 1 or 2 with 1 equiv of NaBArF4 in Et2O led to the formation of a red or orange solution, respectively. After further workup and recrystallization, red purple crystals of [(cyIDep)2Fe][BArF4] (3) and orange crystals of [(sIDep)2Fe][BArF4] (4) were isolated in 80% and 65% yields, respectively (Scheme 2). Complexes 3 and 4 were characterized by 1H NMR spectroscopy, solution magnetic susceptibility measurement, UV–vis–NIR spectroscopy, X-ray crystallography, 57Fe Mössbauer spectroscopy, and elemental analysis. They are air- and moisture-sensitive, soluble in Et2O and THF, and insoluble in toluene and n-hexane.
Scheme 2.
Preparation Route for 3 and 4
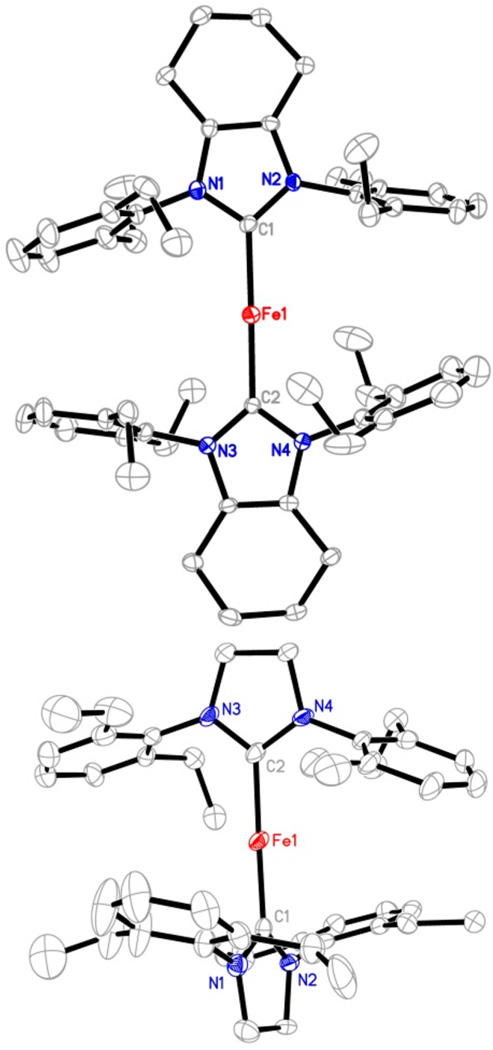
Complexes 3 and 4 represent rare two-coordinate homoleptic iron-NHC complexes. Transition metal imidazol-2-ylidene complexes in the form of [(NHC)2M]n are well-known for groups 10 and 11 metals (Ni,23 Pd,24 Pt,25 Cu,26 Ag,27 Au28). For the earlier transition metals, only the cobalt complex [(IMes)2Co][BPh4] was reported by us.29 Table 1 lists the key structural parameters of 3 and 4 obtained from single-crystal X-ray diffraction studies. The structures of the cations in 3 and 4 are shown in Figure 2. Similar to their group 9–11 metal analogues,23–29 the C–Fe–C cores in 3 and 4 show a linear alignment (178.4(3)° and 175.8(2)°). The Fe–C distances in 3 and 4 (1.996(7) and 1.977(5) Å in average, respectively) are longer than those of their group 9–11 analogues, for example, 1.937(3) Å in [(IMes)2Co]+,29 1.940(3) Å in [(6-Mes)2Ni]+,23 and 1.871(3) Å in [(IMes)2Cu]+,26 consistent with the larger atomic radius of iron versus the other later 3d metals. The two imidazole planes in 3 form a small dihedral angle of 24.2(1)°, whereas a large dihedral angle of 70.4(1)° between the two planes is observed in 4. The different dihedral angles might be due to crystal packing forces, suggesting the free rotation of the NHC ligands along the Fe–C(carbene) axis in [(cyIDep)2Fe]+ and [(sIDep)2Fe]+ in solution. In addition, the eight ethyl groups on the Dep moieties in both complexes align randomly, and no short contact between the N-Dep groups and the iron center was observed.
Figure 2.
Structures of the cationic part of 3 (top, showing one of the two crystallographically independent cations in the unit cell) and 4 (bottom) showing 30% probability ellipsoids. Hydrogen atoms are omitted for clarity.
The 80 K 57Fe Mössbauer spectra of 3 and 4 feature quadrupole doublets (Figure 3). Their isomer shifts (0.48 and 0.55 mm/s for 3 and 4, respectively), being negatively shifted by ca. 0.20 and 0.10 mm/s versus those of the corresponding iron(I) bromide complexes 1 and 2, are comparable to that of the linear iron(I)-cAAC complex [(Me2-cAAC)2Fe][BArF4] (0.49 mm/s).14 The fitting quadrupole splitting values from the spectra of 3 and 4 are very large (5.75 and 6.82 mm/s, respectively). To our knowledge, the latter value is the largest quadrupole splitting ever observed in iron complexes. Precedents of iron complexes with quadrupole splittings larger than 5 mm/s are known for high-valent iron nitride complexes supported by tripodal ligands and low-valent iron–chromium complexes supported by double-decker ligands.30 While the causes leading to these large quadrupole splittings of 3 and 4 need further detailed electronic structure studies, we noted that [(Me2-cAAC)2Fe][BArF4] also has a large quadrupole splitting of 4.58 mm/s, and speculated that the asymmetric electron distribution around the iron centers caused by the diagonal NHC ligand fields might be one of causes.30d,31 Lastly, the broadness observed in the doublet of 4 likely represents small variations in bond distances and angles of this complex in the solid-state powder.
Figure 3.
80 K 57Fe Mössbauer spectra of [(cyIDep)2Fe][BArF4] (3, upper) and [(sIDep)2Fe][BArF4] (4, lower) measured under a 0.07 T applied magnetic field. The data (dots) and fits (solid lines, red for the major species) are shown. For the spectrum of 3: the parameters of the major species (red line, ~95% of iron) are given in the main text (Table 1). A minor species (blue line, ~5% of iron with δ = 0.66 mm/s and ΔEQ = 0.95 mm/s) is also present. Because of the low intensity, no attempts to fit impurities in the spectrum of 4 were made.
The 1H NMR spectra of 3 and 4 measured in THF-d8 exhibited six (20.67, 17.94, 15.53, 4.00, 3.52, −10.83 ppm) and five (21.70, 18.65, 4.41, 3.19, −78.38 ppm) paramagnetically shifted peaks corresponding to the signals of cyIDep and sIDep ligands, respectively. In addition to these, signals of free sIDep were observed in the spectrum of 4, and their intensity increased after standing in solution for days, whereas no resonances responsible for free cyIDep were noticed in the spectrum of 3 after standing in solution at room temperature for one week. While single-point calculations on the cations in 3 and 4 at their doublet and quartet states based on the molecular structures revealed by XRD indicated a quartet state (S = 3/2) as their common ground state (Table s2), the measured solution magnetic moment for 3 (5.5(2) μB) in THF is larger than the spin-only value (3.8 μB) of S = 3/2 ions, likely due to unquenched orbital momentum contribution as observed in [(DippNCtBu)2CH)Fe(η2-HCCPh)] (4.7 μB).32 The proneness of 4 to coordinate with THF prevents solution magnetic susceptibility measurement (vide infra).
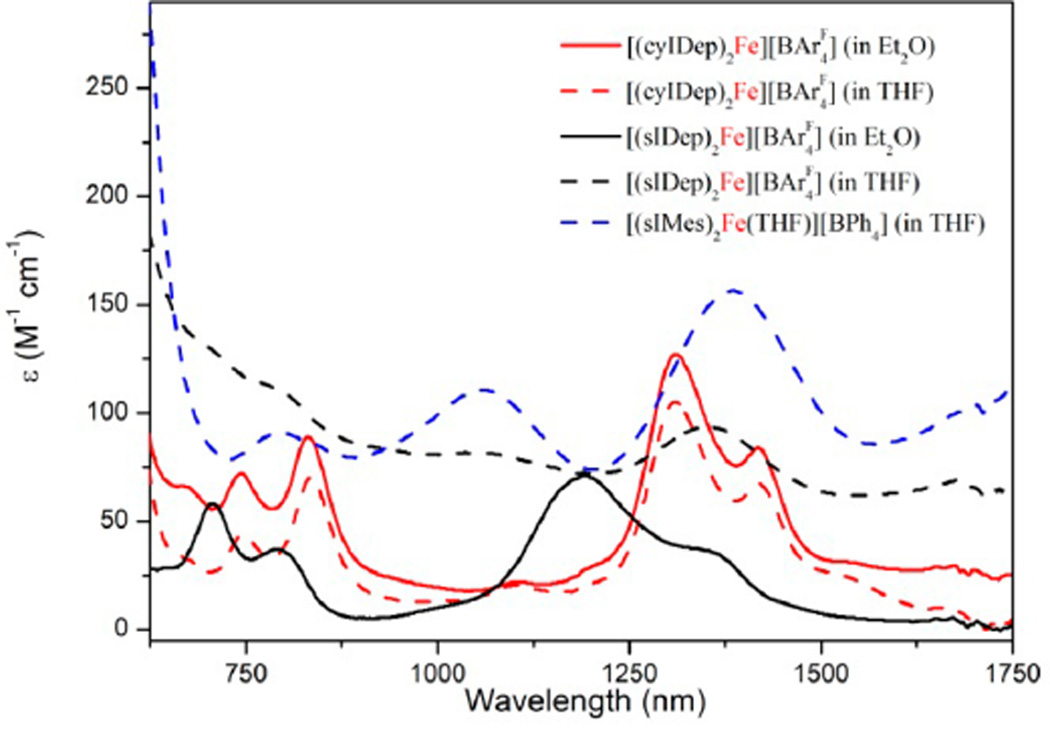
When dissolved in THF, both the solutions of 3 and 4 exhibit red color. However, their absorption spectra in the NIR region are distinct. The spectrum of 3 shows four clear NIR bands around 746, 835, 1310, and 1419 nm with extinction coefficients of ~100 M−1 cm−1 (Figure 4). These bands are tentatively assigned to ligand-field transitions of two-coordinate d7 iron(I) center2d and are consistent with the four-band-pattern observed in the NIR absorption spectrum of the two-coordinate d7 cobalt(II) species [Co(N(SiMe3)2)2].33 When recorded in Et2O, the absorption spectrum of 3 is nearly identical to that measured in THF. In contrast, the spectrum of 4 measured in THF features three heavily broadened NIR absorption bands at 780, 1150, and 1350 nm, and four NIR bands at 706, 791, 1192, and 1352 nm were observed when recorded in Et2O (black lines in Figure 4). These observations suggest that 4 might bind reversibly with THF to form [(sIDep)2Fe(THF)][BArF4] and that 3 is inert toward THF. The difference is probably related to higher steric flexibility of sIDep over cyIDep, which enables the cation [(sIDep)2Fe]+ to coordinate THF upon twisting the arrangement of the N-Dep groups toward the imidazole plane. Accordingly, we reason that the measured 1H NMR spectrum of 4 in THF should be corresponding to the mixture of [(sIDep)2Fe][BArF4] and [(sIDep)2Fe(THF)][BArF4].
Figure 4.
Absorption spectra of [(cyIDep)2Fe][BArF4] (3, red lines), [(sIDep)2Fe][BArF4] (4, black lines), and [(sIMes)2Fe(THF)][BArF4] (5, blue line) in NIR region measured at room temperature.
Synthesis and Characterization of T-Shaped Iron(I)-NHC Complex
The different NIR absorptions of 4 in THF and Et2O prompted attempts to isolate [(sIDep)2Fe(THF)]-[BAr4], which, however, were unsuccessful likely due to the ease of the species to lose THF. Considering this, efforts to access the analogous complex [(sIMes)2Fe(THF)][BAr4], bearing the less sterically demanding NHC ligand sIMes, were pursued. The preparation of (sIMes)2FeCl following a similar protocol to that previously employed for the preparation of [(sIDep)2FeBr] (2) was frustrated by the readily disproportionation reaction of this species in solution. However, the one-pot reaction protocol shown in Scheme 3 eventually enabled the preparation of [(sIMes)2Fe(THF)]-[BPh4] (5) as a red crystalline solid in the form of 5·Et2O in 44% isolated yield.
Scheme 3.
Preparation Route for 5
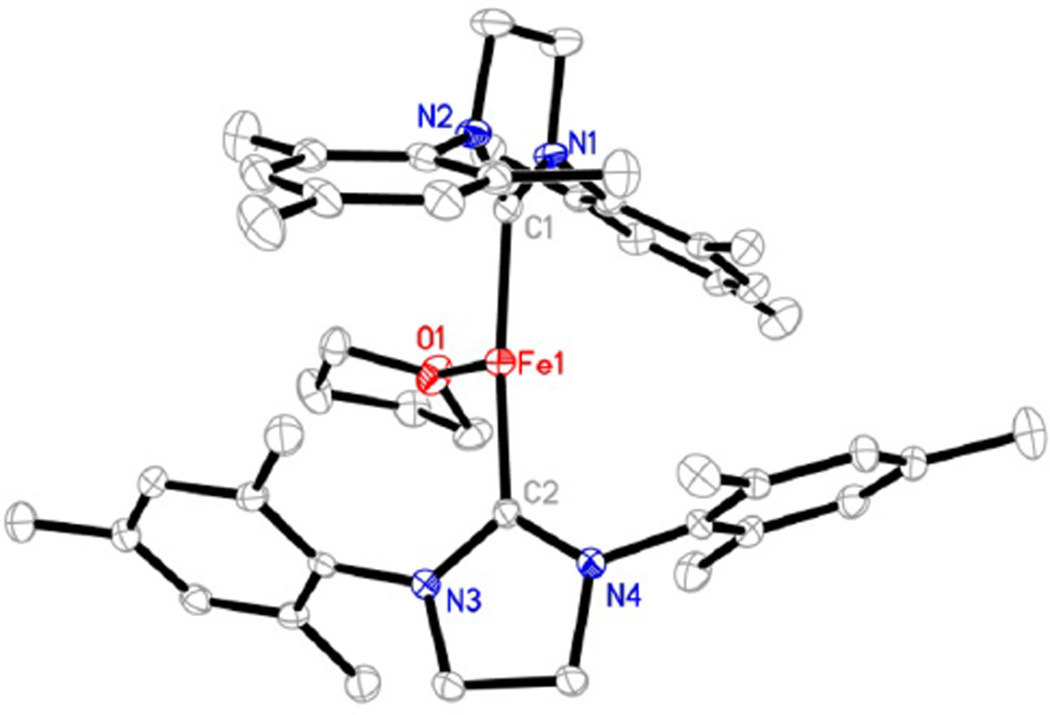
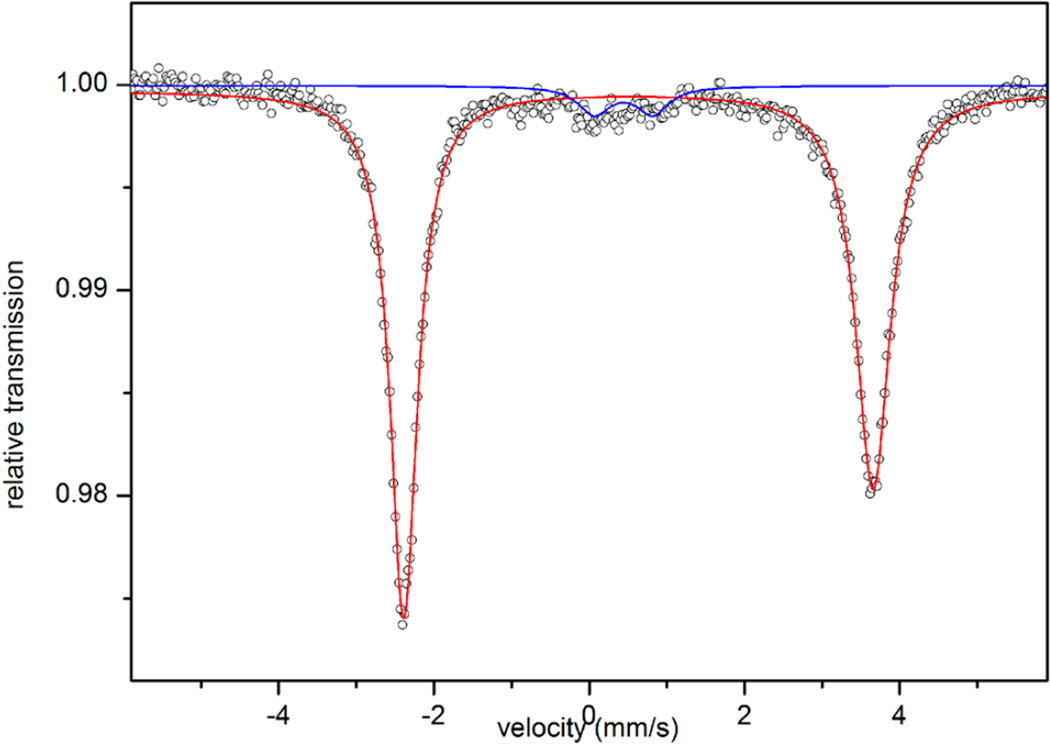
A single-crystal X-ray diffraction study established the structure of the cation in 5 as a T-shaped iron(I) species with two sIMes ligands on the axial positions and a coordinated THF molecule on the equatorial site (Figure 5). The C(carbene)–Fe–C(carbene) angle in [(sIMes)2Fe(THF)]+ (162.9(1)°) is intermediate between those of the two-coordinate species [(sIDep)2Fe]+ (175.8(2)°) and the trigonal planar complex [(sIDep)2FeBr] (130.6(1)°). Its Fe–C-(carbene) bond length (average of 1.980(5) Å) and the dihedral angle between the two imidazole planes (82.9(1)°) are close to the corresponding ones in [(sIDep)2Fe]+. The long Fe–O distance (2.145(3) Å), compared to that in [(nacnac)-Fe(OEt2)][BArF4] (1.978(4) Å),34 indicates the lability of the coordinated THF molecule. The Mössbauer isomer shift (0.65 mm/s) observed from the spectrum of 5 (Figure 6) is comparable to those of trigonal-planar species 1 and 2; meanwhile, the quadrupole splitting (5.99 mm/s) is close to those of the two-coordinate iron(I)-NHC complexes 3 and 4. The broadness observed in the doublet of 5 likely represents small variations in bond distances and angles of this complex in the solid-state powder. The Mössbauer data are suggestive of an iron(I) nature for 5.
Figure 5.
Structure of the cation in 5 showing 30% probability ellipsoids. Hydrogen atoms are omitted for clarity.
Figure 6.
80 K 57Fe Mössbauer spectrum of [(sIMes)2Fe(THF)]-[BPh4] (5) measured under a 0.07 T applied magnetic field. The data (dots) and fits (solid lines, red for the major species) are shown. The parameters of the major species (red line, ~93% of iron) are given in the main text (Table 1). A minor species (blue line, ~7% of iron with δ = 0.44 mm/s and ΔEQ = 0.75 mm/s) is also present.
The solution properties of 5 proved similar to that of 4 in THF. The 1H NMR spectrum of 5 in THF-d8 shows four paramagnetically shifted resonances at 7.97, 2.47, 1.88, and −71.26 ppm. Its absorption spectrum in THF displays three clear NIR bands at 796, 1060, and 1386 nm (Figure 4). The wavelengths of these maximum absorptions resemble those of 4 in THF. Moreover, cyclic voltammetry studies on the solutions of 4 and 5 revealed quasi-reversible redox waves with large peak-to-peak separation (0.58 and 0.20 V, respectively; Figure S6), assignable to the redox processes of [(NHC)2Fe-(THF)]1+/2+. In contrast, the voltammogram of 3 features an irreversible oxidation wave with the peak potential of −0.38 V (Figure S7), suggesting the inability of the bis(cyIDep) ligand field to stabilize iron(II) species. Despite the aforementioned similarities, 5 is found more stable than 4, as the THF solution of 5 can be kept at room temperature for days with negligible decomposition.
CONCLUSIONS
In this study, we have achieved the preparation and characterization of a series of three- and two-coordinate iron(I) complexes with NHCs as supporting ligands. Further studies on the magnetic properties and electronic structures of these low-coordinate iron(I)-NHC complexes are ongoing. The following are the principle findings of this study.
Two NHC ligands, namely, cyIDep and sIDep, were found effective supporting ligands for two-coordinate homoleptic iron(I)-NHC complexes. The one-pot reaction protocol of treating FeBr2 with 2 equiv of cyIDep and sIDep followed by controlled reduction with excess amounts of KC8 enables the preparation of the three-coordinate iron(I)-NHC complexes [(cyIDep)2-FeBr] (1) and [(sIDep)2FeBr] (2). The further salt elimination reactions of 1 and 2 with NaBArF4 in Et2O led to the preparation of the rare two-coordinate homoleptic iron(I)-NHC complexes [(cyIDep)2Fe]-[BArF4] (3) and [(sIDep)2Fe][BArF4] (4), respectively. Complexes 1–4 were characterized by various spectroscopic methods including single-crystal X-ray diffraction and 57Fe Mössbauer spectroscopy, which indicate their high-spin nature. The attainment of the two-coordinate iron(I)-NHC complexes indicates the importance of suitable steric protection conferred by the N-substituents on the NHC ligands for the stabilization of low-coordinate iron(I)-NHC complexes.
The unique T-shaped iron(I)-NHC complex [(sIMes)2Fe(THF)][BPh4] (5) was prepared and fully characterized by various spectroscopic methods. Solution property studies on 3–5 by 1H NMR and UV–vis–NIR spectroscopies indicate that 3 and 5 keep their two-coordinate and T-shaped nature, respectively, in solution phase, and that the cation in 4 reversibly coordinates a THF molecule to form T-shaped species [(sIDep)2Fe-(THF)]+. Cyclic voltammetry studies reveal a quasi-reversible one-electron redox wave for the three-coordinate species [(NHC)2Fe(THF)]1+/2+ (NHC = sIMes and sIDep) in THF. In contrast, the voltammogram of the two-coordinate complex 3 only exhibited an irreversible oxidation wave. The different solution properties of 3–5 might result from the less steric flexibility of cyIDep, which is enforced by the presence of 4,5-subsitutents on the carbene ligand, over sIDep and sIMes.
EXPERIMENTAL SECTION
General Procedures
All experiments referring to NHCs or iron complexes were performed either under an atmosphere of dry dinitrogen with the rigid exclusion of air and moisture using standard Schlenk techniques or in a glovebox. Organic solvents were dried with a solvent purification system (Innovative Technology) and bubbled with dry N2 gas prior to use. 1H NMR, 13C NMR, 11B{1H}, and 19F NMR spectra were recorded on an Agilent 300, 400, or 600 MHz spectrometer. All chemical shifts were reported in units with references to the residual protons of the deuterated solvents for proton and carbon chemical shifts, and to external CF3COOH (0.00 ppm) for fluoro chemical shifts. Elemental analysis was performed by the Analytical Laboratory of Shanghai Institute of Organic Chemistry (CAS). Magnetic moments were measured by the method originally described by Evans with stock and experimental solutions containing a known amount of a (CH3)3SiOSi(CH3)3 standard.35 Absorption spectra were recorded with a Shimadzu UV-3600 UV–vis–NIR spectrophotometer. Cyclic voltammetry measurements were made with a CHI 600D potentiostat in THF solutions using a sweep rate of 100 mV/s, a glassy-carbon working electrode, 0.05 M [Bun4N][BPh4] supporting electrolyte, and a saturated calomel electrode as reference electrode. Under these conditions, E1/2 = 0.55 V for the [Cp2Fe]0/+ couple.
X-ray Structure Determination
Diffraction-quality crystals were obtained as 1–4 and 5·Et2O from recrystallizations in toluene/n-hexane, Et2O, Et2O/n-hexane, and THF/Et2O, respectively, at −25 °C. Crystals were coated with mineral oil and mounted on a Bruker APEX CCD-based diffractometer equipped with an Oxford low-temperature apparatus. Data were collected at 130(2) K. Cell parameters were retrieved with SMART software and refined using SAINT software on all reflections. Data integration was performed with SAINT, which corrects for Lorentz polarization and decay. Absorption corrections were applied using SADABS.36 Space groups were assigned unambiguously by analysis of symmetry and systematic absences determined by XPREP. All structures were solved and refined using SHELXTL.37 Metal and first coordination sphere atoms were located from direct-methods E-maps. Non-hydrogen atoms were found in alternating difference Fourier synthesis and least-squares refinement cycles and during final cycles were refined anisotropically. Table S1 summarizes the crystal data and summary of data collection and refinement for the complexes.
57Fe Mössbauer Spectroscopy
All solid samples for 57Fe Mössbauer spectroscopy were run on nonenriched samples of the as-isolated complexes. Each sample was loaded into a Delrin Mössbauer sample cup for measurements and loaded under liquid nitrogen. Low-temperature 57Fe Mössbauer measurements were performed using a See Co. MS4 Mössbauer spectrometer integrated with a Janis SVT-400T He/N2 cryostat for measurements at 80 K with a 0.07 T applied magnetic field. Isomer shifts were determined relative to α-Fe at 298 K. All Mössbauer spectra were fit using the program WMoss (SeeCo).
Computational Details
Singlet-point calculations on [(cyIDep)2Fe]1+ and [(sIDep)2Fe]1+ were performed with the ORCA 2.8 program38 using the B3LYP39 method. The SVP basis set40 was used for the C, N, and H atoms, and the TZVP basis set41 was used for the Fe atom. The RIJCOSX approximation42 with matching auxiliary basis sets40,43 was employed to accelerate the calculation. TIGHTSCF was used for SCF calculation.44 The single-point calculations at different spin states (S = 1/2 and 3/2) were based on the coordinates obtained from X-ray diffraction study with optimization on hydrogen atoms only. For the xyz files, please see Supporting Information.
Preparation of cyIDep·HBF4
The preparation of the imidazolium salt adopts a modified synthetic procedure described by Glorius for cyIMes·HBr.45 N,N′-bis(2,6-diethylphenyl)formamidine46 (11.6 g, 38 mmol, 1.0 equiv) was suspended in acetonitrile (200 mL). NEt(iPr)2 (5.83 g, 45 mmol, 1.2 equiv) and 2-bromocyclohexanone (13.3 g, 75 mmol, 2.0 equiv) were added successively, and the resulting mixture was stirred at 110 °C for 21 h. Then, the volatiles were removed under reduced pressure. The residue was suspended in toluene (200 mL), to which acetic anhydride (11.5 g, 0.11 mol, 3.0 equiv) and 48% aqueous HBF4 (10.3 g, 56 mmol, 1.5 equiv) were added. The resulting mixture was stirred at 90 °C for 14 h. The mixture was then transferred into a separatory funnel containing CH2Cl2/H2O (400 mL, 1/1 v/v). After separation of the two layers, the aqueous layer was extracted with CH2Cl2 (3 × 100 mL), and then the combined organic layers were washed with distilled water (3 × 100 mL) and dried over anhydrous magnesium sulfate. The volatiles were removed under vacuum to afford oily brown solid. The oily brown solid was washed with Et2O (3 × 20 mL) and cold THF (20 mL) to afford cyIDep·HBF4 as a white solid (10.5 g, 59%). 1H NMR (400 MHz, CD3CN, 22 °C): δ (ppm) 8.72 (s, 1H, NCHN), 7.60 (t, J = 7.6 Hz, 2H, p-Ar-H), 7.43 (d, J = 7.6 Hz, 4H, m-Ar-H), 2.47 (m, 4H, CH3CHH), 2.34 (m, 8H, CH3CHH + CH2(CH2)2CH2), 1.90 (m, 4H, CH2(CH2)2CH2), 1.18 (t, J = 7.6 Hz, 12H, CH3). 13C NMR (101 MHz, CD3CN, 21 °C): δ (ppm) 141.97, 135.65, 133.12, 132.74, 130.65, 128.51, 24.72, 21.98, 20.64, 15.15. 19F NMR (376 MHz, CD3CN, 21 °C): δ (ppm) −150.96 (1F), −151.01(3F). The presence of two 19F NMR signals might be caused by the hydrogen bonding of NCH⋯F–BF3. MS (ESI): m/z [M-BF4−] calcd. for C27H35N2+: 387.2795, found: 387.2810.
Preparation of cyIDep
To a white suspension of cyIDep·HBF4 (22.8 g, 48 mmol, 1.0 equiv) in THF (150 mL) was added KOtBu (10.8 g, 96 mmol, 2.0 equiv) slowly at room temperature. The color of the mixture turned from white to pale yellow within several minutes. The pale yellow suspension was stirred for 11 h and then filtered through diatomaceous earth to afford pale yellow solution. After removal of the volatiles, the residue was extracted with toluene (100 mL) and filtered to afford pale yellow solution. After further removal of the solvent, the residue was washed with cold n-hexane (10 mL) rapidly and dried under vacuum to afford cyIDep as a white solid (17.0 g, 91%). 1H NMR (400 MHz, C6D6, 22 °C): δ (ppm) 7.22 (t, J = 7.6 Hz, 2H, p-Ar-H), 7.12 (d, J = 7.6 Hz, 4H, m-Ar-H), 2.55 (m, 8H, CH2CH3), 2.02 (brs, 4H, CH2(CH2)2CH2), 1.38 (brs, 4H, CH2(CH2)2CH2), 1.22 (t, J = 7.6 Hz, 12H, CH3). 13C NMR (101 MHz, C6D6, 22 °C): δ (ppm) 217.26 (carbene carbon), 142.32, 138.78, 128.44, 127.27, 126.94, 25.30, 23.01, 21.55, 15.35. Anal. Calcd for C27H34N2: C, 83.89; H, 8.87; N, 7.25. Found: C, 83.76; H, 8.92; N, 7.14%.
Preparation of sIDep·HBr.47
The preparation of the imidazolium salt adopts a modified synthetic procedure described by Nechaev48 for the preparation of sIPr·HBr. N,N′-bis(2,6-diethylphenyl)formamidine (34.4 g, 0.11 mol, 1.0 equiv) was suspended in toluene (15 mL). BrCH2CH2Br (31.4 g, 0.17 mol, 1.5 equiv) and NEt(iPr)2 (17.4 g, 0.13 mol, 1.2 equiv) were added successively to the mixture. The resulting mixture was stirred rigorously at 120 °C for 6 h. After it cooled to room temperature, the solid was dissolved in CH2Cl2 (250 mL) and washed with diluted solutions of potassium carbonate (3 × 100 mL). The organic layer was separated, dried over anhydrous magnesium sulfate, and filtered. After removal of the volatiles, the remaining residue was then washed with Et2O and dried under vacuum to afford sIDep·HBr as a pale pink solid (42.5 g, 92%). 1H NMR (300 MHz, CD3CN, 29 °C): δ (ppm) 9.07 (s, 1H, NCHN), 7.48 (t, J = 7.6 Hz, 2H, p-Ar-H), 7.34 (d, J = 7.6 Hz, 4H, m-Ar-H), 4.49 (s, 4H, NCH2), 2.77 (q, J = 7.5 Hz, 8H, CH3CH2), 1.31 (t, J = 7.5 Hz, 12H, CH3CH2). 13C NMR (75 MHz, CD3CN, 29 °C): δ (ppm) 160.70, 142.60, 132.48, 131.58, 128.08, 53.59, 24.71, 15.49.
Preparation of sIDep
To a white suspension of sIDep·HBr (36.0 g, 87 mmol, 1.0 equiv) in THF (150 mL) was added potassium bis(trimethylsilyl)amide (1 M in THF, 87 mL, 87 mmol, 1.0 equiv) at −116 °C. After it was stirred for 30 min, the mixture was warmed to room temperature and further stirred for 3 h. The resulting mixture was filtered through diatomaceous earth to afford pale yellow solution. After removal of the volatiles, the residue was extracted with Et2O (200 mL), filtered through diatomaceous earth to afford a pale yellow solution. Removal of the volatiles gave a yellow residue, which was washed with cold n-hexane (15 mL) rapidly and dried in vacuo to afford sIDep as a white crystalline solid (17.3 g, 60%). 1H NMR (300 MHz, C6D6, 29 °C): δ (ppm) 7.16 (t, J = 7.2 Hz, 2H, p-Ar-H), 7.07 (d, J = 7.6 Hz, 4H, m-Ar-H), 3.31 (s, 4H, CH2CH2), 2.69 (q, J = 7.5 Hz, 4H, CH3CHH), 2.60 (q, J = 7.5 Hz, 4H, CH3CHH), 1.25 (t, J = 7.5 Hz, 12H, CH3CH2). 13C NMR (75 MHz, C6D6, 29 °C): δ (ppm) 243.09 (carbene carbon), 142.87, 140.92, 127.75, 127.10, 52.46, 25.20, 15.93. Anal. Calcd for C23H30N2: C, 82.59; H, 9.04; N, 8.37. Found: C, 82.65; H, 9.09; N, 8.45%.
Preparation of [(cyIDep)2FeBr] (1)
To a pale yellow suspension of FeBr2 (1.12 g, 5.2 mmol, 1.0 equiv) in THF (35 mL) was added cyIDep (4.00 g, 10 mmol, 2.0 equiv). The mixture was stirred rigorously overnight. After removal of the volatiles, toluene (35 mL) was added to afford a pale brown suspension. The addition of potassium graphite (1.78 g, 13 mmol, 2.5 equiv) to the mixture at room temperature led to quick color change to deep brown. The mixture was stirred for 55 min, and then filtered through diatomaceous earth to afford a brown solution. After removal of the volatiles, the residue was washed with n-hexane (50 mL) and dried in vacuo to afford 1 as a brown solid (3.00 g, 64%). Single crystals of 1 suitable for X-ray diffraction studies were grown from its toluene/n-hexane solution at −25 °C. The 1H NMR spectrum of this paramagnetic complex displayed six characteristic peaks in the range from −13.61 to 8.76 ppm. 1H NMR (300 MHz, C6D6, 22 °C): δ (ppm) 8.76, 5.49, 5.09, 0.10, −8.75, −13.61. Anal. Calcd for C54H68BrFeN4: C, 71.36; H, 7.54; N, 6.16. Found: C, 71.09; H, 7.44; N, 6.18%. Because of the instability of the complex in solution, no further characterization data were acquired.
Preparation of [(sIDep)2FeBr] (2)
To a pale yellow suspension of FeBr2 (322 mg, 1.5 mmol, 1.0 equiv) in THF (20 mL) was added sIDep (1.00 g, 3.0 mmol, 2.0 equiv). The reaction was stirred rigorously for 3 h. After removal of the volatiles, toluene (20 mL) was added to the residue to afford a pale brown suspension. One hour later, potassium graphite (504 mg, 3.7 mmol, 2.5 equiv) was added to the pale brown suspension at room temperature. The color of the mixture turned to purple. After it was stirred for 15 min, the mixture was filtered through diatomaceous earth to afford a purple solution. Removal of the volatiles gave a purple residue, which was washed with n-hexane (15 mL) and dried in vacuo to afford 2 as a pale purple solid (980 mg, 82%). Single crystals of 2 suitable for X-ray diffraction studies were grown from its toluene/hexane solution at −25 °C. The 1H NMR spectrum of this paramagnetic complex displayed five characteristic peaks in the range from −34.48 to 11.18 ppm. 1H NMR (400 MHz, C6D6, 22 °C): δ (ppm) 11.18, 4.01, −7.95, −12.11, −34.48. Anal. Calcd for C46H60BrFeN4: C, 68.65; H, 7.51; N, 6.96. Found: C, 68.25; H, 7.59; N, 6.79%. Because of the instability of the complex in solution, no further characterization data were acquired.
Preparation of [(cyIDep)2Fe][BArF4] (3)
To a brown suspension of 1 (1.00 g, 1.1 mmol, 1.0 equiv) in Et2O (15 mL) was added NaBArF4 (sodium tetra(3,5-di(trifluoromethyl)phenyl)borate, 975 mg, 1.1 mmol, 1.0 equiv). The color of the mixture quickly turned to bright red. After it was stirred for 30 min, the mixture was filtered through diatomaceous earth. The solution was concentrated under vacuum to afford a red residue, which was washed with n-hexane (20 mL) and then dissolved in Et2O (25 mL). After the red solution was allowed to stand at −25 °C for two days to facilitate crystallization, 3 was afforded as a red purple crystalline solid (1.48 g, 80%). The 1H NMR spectrum of this paramagnetic complex displayed eight characteristic peaks in the range from −10.83 to 20.67 ppm, which remained almost unchanged for 7 d in THF-d8 at room temperature. 1H NMR (400 MHz, THF-d8, 22 °C): δ (ppm) 20.67, 17.94, 15.53, 7.87, 7.64, 4.00, 3.52, −10.83. Magnetic susceptibility (THF-d8): μeff = 5.5(2) μB. Absorption spectrum (THF): λmax, nm (μ, M−1 cm−1) = 340 (3180), 428 (3710), 491 (3680), 541 (4850), 746 (40), 835 (70), 1310 (110), 1419 (70). Anal. Calcd for C86H80BF24FeN4: C, 61.04; H, 4.77; N, 3.31. Found: C, 60.88; H, 4.79; N, 3.15%.
Preparation of [(sIDep)2Fe][BArF4] (4)
To a purple suspension of 2 (1.00 g, 1.2 mmol, 1.0 equiv) in Et2O (15 mL) was added NaBArF4 (1.10 g, 1.2 mmol, 1.0 equiv). The color of the mixture turned to orange quickly. The mixture was stirred for 30 min and then filtered through diatomaceous earth to afford an orange solution. After removal of the solvent, the residue was washed with Et2O/n-hexane (10 mL, 3/1) and then dissolved in Et2O/n-hexane (25 mL, 10/1). After the orange-red solution was allowed to stand at −25 °C for 3 d to facilitate crystallization, 4 was afforded as an orange crystalline solid (1.28 g, 65%). The 1H NMR spectrum of this paramagnetic complex displayed seven characteristic peaks in the range from −78.38 to 21.70 ppm, which changed slowly after 1 d in THF-d8 at room temperature. 1H NMR (400 MHz, THF-d8, 22 °C): δ (ppm) 21.70, 18.65, 7.87, 7.62, 4.41, 3.19, −78.38. The 1H NMR spectrum should correspond to the mixture of [(sIDep)2Fe][BArF4] and [(sIDep)2Fe(THF)][BArF4]. Absorption spectrum (Et2O): λmax, nm (μ, M−1 cm−1) = 343 (2270), 432 (2820), 470 (4090), 503 (3720), 706 (60), 791 (40), 1192 (70), 1352 (40). Anal. Calcd for C78H72BF24FeN4: C, 58.99; H, 4.57; N, 3.53. Found: C, 59.34; H, 4.98; N, 3.58%.
Preparation of [(sIMes)2Fe(THF)][BPh4]·Et2O (5·Et2O)
To a pale green suspension of [Fe(tmeda)Cl2]2 (595 mg, 1.2 mmol, 1.0 equiv) in THF (20 mL) was added sIMes49 (1.50 g, 4.9 mmol, 4.0 equiv). The mixture was stirred for 2 h. After removal of the volatiles, THF (20 mL) was added to the residue to afford a pale yellow suspension. 0.5 h later, potassium graphite (993 mg, 7.3 mmol, 6.0 equiv) was added to the pale yellow suspension at room temperature. The color of the mixture turned red-purple. The mixture was stirred for 4 min and then quickly filtered through diatomaceous earth to afford a red-purple solution. NaBPh4 (838 mg, 2.5 mmol, 2.0 equiv) was added to the solution immediately. The mixture was stirred for 30 min and then filtered through diatomaceous earth to afford an orange-red solution. After removal of the volatiles, the residue was washed sequentially with n-hexane (20 mL) and Et2O (20 mL) and then dissolved in THF/Et2O (25 mL, 4/1) to afford an orange-red solution. After the solution was allowed to stand at −25 °C for several days to facilitate recrystallization, 5·Et2O was obtained as a red crystalline solid (1.21 g, 44%). The 1H NMR spectrum of this paramagnetic complex displayed six characteristic peaks in the range from −71.26 to 7.97 ppm, which remained almost unchanged for at least 7 d in THF-d8 at room temperature. 1H NMR (400 MHz, THF-d8, 22 °C): δ (ppm) 7.97, 6.88, 6.62, 2.47, 1.88, −71.26. Magnetic susceptibility (THF-d8): μeff = 4.7(2) μB. Absorption spectrum (THF): λmax, nm (μ, M−1 cm−1) = 346 (3620), 489 (6410), 796 (90), 1060 (110), 1386 (160). Anal. Calcd for C74H90BFeN4O2: C, 78.36; H, 8.00; N, 4.94. Found: C, 78.43; H, 7.77; N, 4.94%.
Supplementary Material
Acknowledgments
The work was supported by the National Basic Research Program of China (No. 2011CB808705 to L.D.), the National Natural Science Foundation of China (Nos. 21222208, 21421091, and 21432001 to L.D.), and a grant from the National Institutes of Health (No. R01GM111480 to M.L.N.).
Footnotes
ASSOCIATED CONTENT
Supporting Information
- Table of crystal data and collection parameters, tabulated computed relative free energies, 57Fe Mössbauer spectra, absorption spectra, cyclic voltammograms, photographs of the THF solutions, and NMR spectra. (PDF)
- X-ray crystallographic information for five compounds. (CIF)
- Cartesian coordinates for the computed structures. (XYZ)
The authors declare no competing financial interest.
REFERENCES
- 1.Twigg MV, Burgess J. In: Comprehensive Coordination Chemistry II. McCleverty JA, Meyer TJ, editors. Vol. 5. Amsterdam, The Netherlands: Elsevier; 2005. p. 460. [Google Scholar]
- 2.For reviews on low-coordinate iron(III) and iron(II) compounds, see: Bradley DC, Chisholm MH. Acc. Chem. Res. 1976;9:273. Cummins CC. Progress in Inorganic Chemistry. 1998;47:685. Holland PL. Acc. Chem. Res. 2008;41:905. doi: 10.1021/ar700267b. Power PP. Chem. Rev. 2012;112:3482. doi: 10.1021/cr2004647.
- 3.For examples, see: Rodriguez MM, Bill E, Brennessel WW, Holland PL. Science. 2011;334:780. doi: 10.1126/science.1211906. Anderson JS, Rittle J, Peters JC. Nature. 2013;501:84. doi: 10.1038/nature12435.
- 4.For a recent review, please see: Craig GA, Murrie M. Chem. Soc. Rev. 2015;44:2135. doi: 10.1039/c4cs00439f.
- 5.Chiang KP, Scarborough CC, Horitani M, Lees NS, Ding K, Dugan TR, Brennessel WW, Bill E, Hoffman BM, Holland PL. Angew. Chem. Int. Ed. 2012;51:3658. doi: 10.1002/anie.201109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez MM, Stubbert BD, Scarborough CC, Brennessel WW, Bill E, Holland PL. Angew. Chem. Int. Ed. 2012;51:8247. doi: 10.1002/anie.201202211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Y, Smith JM, Flaschenriem CJ, Holland PL. Inorg. Chem. 2006;45:5742. doi: 10.1021/ic052136+. [DOI] [PubMed] [Google Scholar]
- 8.Smith JM, Sadique AR, Cundari TR, Rodgers KR, Lukat-Rodgers G, Lachicotte RJ, Flaschenriem CJ, Vela J, Holland PL. J. Am. Chem. Soc. 2006;128:756. doi: 10.1021/ja052707x. [DOI] [PubMed] [Google Scholar]
- 9.Ingleson MJ, Fullmer BC, Buschhorn DT, Fan H, Pink M, Huffman JC, Caulton KG. Inorg. Chem. 2008;47:407. doi: 10.1021/ic7023764. [DOI] [PubMed] [Google Scholar]
- 10.Fohlmeister L, Liu S, Schulten C, Moubaraki B, Stasch A, Cashion JD, Murray KS, Gagliardi L, Jones C. Angew. Chem. Int. Ed. 2012;51:8294. doi: 10.1002/anie.201203711. [DOI] [PubMed] [Google Scholar]
- 11.Zadrozny JM, Xiao DJ, Atanasov M, Long GJ, Grandjean F, Neese F, Long JR. Nat. Chem. 2013;5:577. doi: 10.1038/nchem.1630. [DOI] [PubMed] [Google Scholar]
- 12.Ung G, Rittle J, Soleilhavoup M, Bertrand G, Peters JC. Angew. Chem. Int. Ed. 2014;53:8427. doi: 10.1002/anie.201404078. [DOI] [PubMed] [Google Scholar]
- 13.Samuel PP, Mondal KC, Amin Sk N, Roesky HW, Carl E, Neufeld R, Stalke D, Demeshko S, Meyer F, Ungur L, Chibotaru LF, Christian J, Ramachandran V, van Tol J, Dalal NS. J. Am. Chem. Soc. 2014;136:11964. doi: 10.1021/ja5043116. [DOI] [PubMed] [Google Scholar]
- 14.Mo Z, Ouyang Z, Wang L, Fillman KL, Neidig ML, Deng L. Org. Chem. Front. 2014;1:1040. [Google Scholar]
- 15.Lin C-Y, Fettinger JC, Grandjean F, Long GJ, Power PP. Inorg. Chem. 2014;53:9400. doi: 10.1021/ic501534f. [DOI] [PubMed] [Google Scholar]
- 16.Werncke CG, Bunting PC, Duhayon C, Long JR, Bontemps S, Sabo-Etienne S. Angew. Chem. Int. Ed. 2015;54:245. doi: 10.1002/anie.201408802. [DOI] [PubMed] [Google Scholar]
- 17.Lipschutz MI, Chantarojsiri T, Dong Y, Tilley TD. J. Am. Chem. Soc. 2015;137:6366. doi: 10.1021/jacs.5b02504. [DOI] [PubMed] [Google Scholar]
- 18.(a) Lavallo V, Canac Y, Prasang C, Donnadieu B, Bertrand G. Angew. Chem. Int. Ed. 2005;44:5705. doi: 10.1002/anie.200501841. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hahn FE, Jahnke MC. Angew. Chem. Int. Ed. 2008;47:3122. doi: 10.1002/anie.200703883. [DOI] [PubMed] [Google Scholar]
- 19.For reference on the π-acidity of NHC ligands, see: Liske A, Verlinden K, Buhl H, Schaper K, Ganter C. Organometallics. 2013;32:5269. Back O, Henry-Ellinger M, Martin CD, Martin D, Bertrand G. Angew. Chem. Int. Ed. 2013;52:2939. doi: 10.1002/anie.201209109. Jacobsen H, Correa A, Poater A, Costabile C, Cavallo L. Coord. Chem. Rev. 2009;253:687. Fillman KL, Przyojski JA, Al-Afyouni MH, Tonzetich ZJ, Neidig ML. Chem. Sci. 2015;6:1178. doi: 10.1039/c4sc02791d.
- 20.(a) Xiang L, Xiao J, Deng L. Organometallics. 2011;30:2018. [Google Scholar]; (b) Danopoulos AA, Braunstein P, Stylianides N, Wesolek M. Organometallics. 2011;30:6514. [Google Scholar]; (c) Day BM, Pugh T, Hendriks D, Guerra CF, Evans DJ, Bickelhaupt FM, Layfield RA. J. Am. Chem. Soc. 2013;135:13338. doi: 10.1021/ja408589p. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Ouyang Z, Liu Y, Zhang Q, Wang L, Deng L. Angew. Chem. Int. Ed. 2014;53:8432. doi: 10.1002/anie.201404677. [DOI] [PubMed] [Google Scholar]
- 22.(a) Miyazaki S, Koga Y, Matsumoto T, Matsubara K. Chem. Commun. 2010;46:1932. doi: 10.1039/b924716e. [DOI] [PubMed] [Google Scholar]; (b) Lee CH, Cook TR, Nocera DG. Inorg. Chem. 2011;50:714. doi: 10.1021/ic102017t. [DOI] [PubMed] [Google Scholar]; (c) Zhang K, Conda-Sheridan M, Cooke SR, Louie J. Organometallics. 2011;30:2546. doi: 10.1021/om200090d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poulten RC, Page MJ, Algarra AG, Le Roy JJ, López I, Carter E, Llobet A, Macgregor SA, Mahon MF, Murphy DM, Murugesu M, Whittlesey MK. J. Am. Chem. Soc. 2013;135:13640. doi: 10.1021/ja407004y. [DOI] [PubMed] [Google Scholar]
- 24.Altenhoff G, Goddard R, Lehmann CW, Glorius F. Angew. Chem. Int. Ed. 2003;42:3690. doi: 10.1002/anie.200351325. [DOI] [PubMed] [Google Scholar]
- 25.Fortman GC, Scott NM, Linden A, Stevens ED, Dorta R, Nolan SP. Chem. Commun. 2010;46:1050. doi: 10.1039/b920482b. [DOI] [PubMed] [Google Scholar]
- 26.Díez-González S, Stevens ED, Scott NM, Petersen JL, Nolan SP. Chem. - Eur. J. 2008;14:158. doi: 10.1002/chem.200701013. [DOI] [PubMed] [Google Scholar]
- 27.Arduengo AJ, Dias HVR, Calabrese JC, Davidson F. Organometallics. 1993;12:3405. [Google Scholar]
- 28.Gaillard S, Nun P, Slawin AMZ, Nolan SP. Organometallics. 2010;29:5402. [Google Scholar]
- 29.Mo Z, Chen D, Leng X, Deng L. Organometallics. 2012;31:7040. [Google Scholar]
- 30.(a) Hendrich MP, Gunderson W, Behan RK, Green MT, Mehn MP, Betley TA, Lu CC, Peters JC. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17107. doi: 10.1073/pnas.0604402103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Vogel C, Heinemann FW, Sutter J, Anthon C, Meyer K. Angew. Chem. Int. Ed. 2008;47:2681. doi: 10.1002/anie.200800600. [DOI] [PubMed] [Google Scholar]; (c) Scepaniak JJ, Vogel CS, Khusniyarov MM, Heinemann FW, Meyer K, Smith JM. Science. 2011;331:1049. doi: 10.1126/science.1198315. [DOI] [PubMed] [Google Scholar]; (d) Rudd PA, Liu S, Planas N, Bill E, Gagliardi L, Lu CC. Angew. Chem. Int. Ed. 2013;52:4449. doi: 10.1002/anie.201208686. [DOI] [PubMed] [Google Scholar]
- 31.Hohenberger J, Ray K, Meyer K. Nat. Commun. 2012;3:720. doi: 10.1038/ncomms1718. [DOI] [PubMed] [Google Scholar]
- 32.Stoian SA, Yu Y, Smith JM, Holland PL, Bominaar EL, Münck E. Inorg. Chem. 2005;44:4915. doi: 10.1021/ic050321h. [DOI] [PubMed] [Google Scholar]
- 33.Fisher KJ, Bradley DC. J. Am. Chem. Soc. 1971;93:2058. [Google Scholar]
- 34.Gregory EA, Lachicotte RJ, Holland PL. Organometallics. 2005;24:1803. [Google Scholar]
- 35.(a) Evans DF. J. Chem. Soc. 1959 2003. [Google Scholar]; (b) Sur SK. J. Magn. Reson. 1989;82:169. [Google Scholar]
- 36.Sheldrick GM. SADABS, Program for Empirical Absorption Correction of Area Detector Data. Germany: University of Göttingen; 1996. [Google Scholar]
- 37.Sheldrick GM. SHELXTL 5.10 for Windows NT: Structure Determination Software Programs. Madison, WI: Bruker Analytical X-ray systems, Inc.; 1997. [Google Scholar]
- 38.Neese F. ORCA, An Ab Initio, Density Functional, and Semiempirical Program Package (v. 2.8) Germany: Universität Bonn; 2011. [Google Scholar]
- 39.(a) Becke AD. J. Chem. Phys. 1993;98:5648. [Google Scholar]; (b) Lee C, Yang W, Parr RG. Phys. Rev. B: Condens. Matter Mater. Phys. 1988;37:785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 40.Schaefer A, Horn H, Ahlrichs R. J. Chem. Phys. 1992;97:2571. [Google Scholar]
- 41.Schaefer A, Huber C, Ahlrichs R. J. Chem. Phys. 1994;100:5829. [Google Scholar]
- 42.Neese F, Wennmohs F, Hansen A, Becker U. Chem. Phys. 2009;356:98. [Google Scholar]
- 43.Weigend F, Ahlrichs R. Phys. Chem. Chem. Phys. 2005;7:3297. doi: 10.1039/b508541a. [DOI] [PubMed] [Google Scholar]
- 44.Manual for ORCA, Version 2.8. Germany: Universität Bonn; 2010. p. 453. [Google Scholar]
- 45.Hirano K, Urban S, Wang C, Glorius F. Org. Lett. 2009;11:1019. doi: 10.1021/ol8029609. [DOI] [PubMed] [Google Scholar]
- 46.Ueda N, Nikawa H, Takano Y, Ishitsuka MO, Tsuchiya T, Akasaka T. Heteroat. Chem. 2011;22:426. [Google Scholar]
- 47.Hadei N, Kantchev EAB, O’Brien CJ, Organ MG. J. Org. Chem. 2005;70:8503. doi: 10.1021/jo051304c. [DOI] [PubMed] [Google Scholar]
- 48.Kolychev EL, Asachenko AF, Dzhevakov PB, Bush AA, Shuntikov VV, Khrustalev VN, Nechaev MS. Dalton Trans. 2013;42:6859. doi: 10.1039/c3dt32860k. [DOI] [PubMed] [Google Scholar]
- 49.Iglesias M, Beetstra DJ, Knight JC, Ooi L-L, Stasch A, Coles S, Male L, Hursthouse MB, Cavell KJ, Dervisi A, Fallis IA. Organometallics. 2008;27:3279. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.