Abstract
Shock, regardless of etiology, is characterized by decreased tissue perfusion resulting in cell death, organ dysfunction, and poor survival. Current therapies largely focus on restoring tissue perfusion through resuscitation but have failed to address the specific cellular dysfunction caused by shock. Acetylation is rapidly emerging as a key mechanism that regulates the expression of numerous genes (epigenetic modulation through activation of nuclear histone proteins), as well as functions of multiple cytoplasmic proteins involved in key cellular functions such as cell survival, repair/healing, signaling, and proliferation. Cellular acetylation can be increased immediately through the administration of histone deacetylase inhibitors (HDACI).
A series of studies have been performed using: (1) cultured cells; (2) single-organ ischemia-reperfusion injury models; (3) rodent models of lethal septic and hemorrhagic shock; (4) swine models of lethal hemorrhagic shock and multi-organ trauma; and (5) tissues from severely injured trauma patients, to fully characterize the changes in acetylation that occur following lethal insults and in response to treatment with HDACI. These data demonstrate that: (1) shock causes a decrease in acetylation of nuclear and cytoplasmic proteins; (2) hypoacetylation can be rapidly reversed through the administration of HDACI; (3) normalization of acetylation prevents cell death, decreases inflammation, attenuates activation of pro-apoptotic pathways, and augments pro-survival pathways; (4) the effect of HDACI significantly improves survival in lethal models of septic shock, hemorrhagic shock, and complex poly-trauma without need for conventional fluid resuscitation or blood transfusion; and (5) improvement in survival is not due to better resuscitation but due to an enhanced ability of cells to tolerate lethal insults.
As different models of hemorrhagic or septic shock have specific strengths and limitations, this chapter will summarize our attempts to create “pro-survival and anti-inflammatory phenotype” in various models of hemorrhagic shock and septic shock.
Introduction
Shock, such as hemorrhagic shock (HS) and septic shock, remains a major cause of morbidity and mortality among trauma patients and in intensive care units. Generally, HS-induced systemic response shares many features with septic response (Mollen et al. 2008). HS results in an early proinflammatory response, followed by a delayed generalized host immuno-suppression (Xu et al. 1998). Sepsis or septic shock is a complex syndrome that results from the host’s inability to regulate the inflammatory response against infection. In hemorrhagic and septic shock, circulation is sub-optimal and host homeostasis is disturbed. At the molecular level, it has been reported that both hemorrhage and sepsis lead to an imbalance in protein acetylation and that HDACI can restore this balance (Lin et al. 2006; Gonzales et al. 2006; Li et al. 2009).
Lysine Acetylation and Histone Deacetylase Inhibitors
Lysine acetylation or Nε-acetylation, identified initially on core histones in 1968 (Vidali et al. 1968), is mediated by a group of enzymes called histone acetyltransferases (HATs), which transfer acetyl groups from acetyl-coenzyme A to the ε-amino group of lysines. HATs are counterbalanced by the activity of histone deacetylases (HDACs) that catalyze the hydrolytic removal of the acetyl group of lysines. In humans, HDACs are divided into four classes (Table 11.1) based on their homology to yeast HDACs (Marks and Dokmanovic 2005; Carey and La Thangue 2006). Class I HDACs include HDAC1, 2, 3 and 8; these are related to the yeast enzyme Rpd3. Class II HDACs include HDAC4, 5, 6, 7, 9 and 10, and are related to the yeast protein HDA1 (histone deacetylase-A1). Class II HDACs are further divided into two subclasses – IIa (HDAC4, 5, 7 and 9) and IIb (HDAC6 and 10) – according to their structural similarities. Class III HDACs are referred to as sirtuins owing to their homology to the yeast HDAC Sir2. This class includes SIRT1–SIRT7 (Chuang et al. 2009; Suzuli 2009). HDAC11, the most recently identified isoform, is a class IV HDAC due to its distinct structure (Voelter-Mahlknecht et al. 2005). Class I, II, and IV are zinc-dependent enzymes, whereas class III HDACs are nicotinamide adenine dinucleotide (NAD+)-dependent enzymes. Based on their various sub-cellular localizations, intra-tissue variation and non-redundant activities, the different HDACs are implicated in various specific cellular processes, such as proliferation, metabolism and differentiation. For example, class I HDACs are mainly nuclear enzymes, whereas class II HDACs localize either to the cell nucleus or to the cytoplasm, depending on their phosphorylation and subsequent binding of 14-3-3 proteins. Moreover, class I HDACs are ubiquitously expressed (de Ruijter et al. 2003; Hu et al. 2003; Dangond and Gullans 1998; Mai et al. 2003), whereas class II HDACs display a tissue-specific expression.
Table 11.1.
Classification of HDACs and selected HDACI
| HDAC class | HDAC isoforms | Localization of HDAC | Specific HDAC inhibitors | Non-specific HDAC inhibitors |
References |
|---|---|---|---|---|---|
| Class I (Zn++ – dependent) |
HDAC1 | Nucleus | MS-275, FK-228 | TSA | Chuang et al. (2009), Lane and Chamber (2009) |
| HDAC2 | Nucleus | FK-228, apicidin | SAHA | ||
| HDAC3 | Nucleus | MS-275, apicidin | Butyrate | ||
| HDAC8 | Nucleus | Valproic acid | |||
| Class IIa (Zn++-dependent) |
HDAC4 | Nuc/Cyt | TSA | Chuang et al. (2009), Lane and Chamber (2009) | |
| HDAC5 | Nuc/Cyt | SAHA | |||
| HDAC7 | Nuc/Cyt | Butyrate | |||
| HDAC9 | Nuc/Cyt | Valproic acid | |||
| Class IIb (Zn++-dependent) |
HDAC6 | Mainly Cyt | Tubacin | TSA | Lane and Chamber (2009) |
| HDAC10 | Mainly Cyt | SAHA | |||
| Class III (NAD+-dependent) |
SIRT1 | Nuc/Cyt | Suramin | Nicotinamide | Chuang et al. (2009), Suzuli (2009), Scher et al. (2007) |
| SIRT2 | Nuc/Cyt | Suramin, AGK2 | |||
| SIRT3 | Nuc/Mitoch | ||||
| SIRT4 | Mitochondria | ||||
| SIRT5 | Mitochondria | ||||
| SIRT6 | Nucleus | ||||
| SIRT7 | Nucleus | ||||
| Class IV (Zn++-dependent) |
HDAC11 | Nuc/Cyt | TSA | Lane and Chamber (2009) | |
| SAHA |
To date, more than 15 HDACI have been tested in preclinical and early clinical studies for cancer therapy (Lane and Chabner 2009). Many of them are broad-spectrum or pan-HDACI, which inhibit many of the Class I, II and IV isoforms including suberoylanilide hydroxamic acid (SAHA), trichostatin A (TSA) and valproic acid (VPA). Some clinical compounds such as MS-275, FK-228 and apicidin have been termed as “Class I-selective”, since they target several Class I isoforms of HDAC. Tubacin is one of a few HDACI that have been reported as a HDAC6 specific inhibitor (Haggarty et al. 2003) (Table 11.1). Thus far, nearly all of studies are focused on class I and class II HDACs, while very few are focused on class III/sirtuins (Weichert 2009).
Extracellular Signals and Protein Acetylation Balance
Signals that enter the cell nucleus encounter chromatin, and this interaction has a major impact on gene expression. Regulation of gene expression has two components that act in concert: alteration of chromatin structure governed by histone modification and binding by transcription factors (TFs) including activators and repressors. Most importantly, two classes of enzymes control the alteration and binding: HAT and HDAC. HAT modify core histone tails by post-translational acetylation of specific lysine residues and create an appropriate ‘histone code’ for chromatin modification to enhance DNA accessibility of TFs. In general, acetylation of core histones unpacks the condensed chromatin and renders the target DNA accessible to transcriptional machinery, hence contributing to gene expression. In most cases, the TFs can also be acetylated by HAT to facilitate their interactions with DNA and other proteins for transactivation. By contrast, deacetylation of the histones and TFs by HDAC increases chromatin condensation and precludes binding between DNA and TFs leading to transcriptional silencing.
Moreover, HAT and HDAC also target non-histone proteins, which may represent general regulatory mechanisms in biological signaling. In normal conditions, protein concentration and enzyme activity of HAT and HDAC remain in a highly harmonized state of balance. In an attempt to emphasize the importance of regulated acetylation this process is often referred to as ‘acetylation homeostasis’ (Saha and Pahan 2006). For example, recent studies have shown that various neurodegenerative challenges disturb this balance by decreasing HAT activity and the ratio tilts in favor of HDAC. The impaired acetylation homeostasis causes transcriptional dysfunction and facilitates a neurodegenerative cascade, which has been implicated in pathogenesis of several neurodegenerative disorders (Sugars and Rubinsztein 2003; Cha 2000). Indeed, perturbation of acetylation homeostasis is being recognized as a central event in the pathogenesis of neurodegeneration and HDAC inhibitors (HDACI) to be protective in animal models of Huntington’s disease (Steffan et al. 2001; McCampbell et al. 2001; Ferrante et al. 2003; Hockly et al. 2003), amyotrophic lateral sclerosis (Ryu et al. 2005; Petri et al. 2006), experimental autoimmune encephalitis (Camelo et al. 2005), spinal muscular dystrophy (Chang et al. 2001; Avila et al. 2007) and other disorders (Yildirim et al. 2008).
Recently, HDACI have also been identified to be potent pro-survival and anti-inflammatory drugs, offering new lines of therapeutic intervention for hemorrhagic shock and septic shock. Our team and other groups have found that shock causes a global cellular hypoacetylation. Also HDACI, such as VPA, SAHA and TSA prevent hemorrhage-associated lethality in rat and swine models of hemorrhagic shock (Alam et al. 2009; Lin et al. 2006, 2007; Gonzales et al. 2006), suppress expression of proinflammatory cytokines and improve survival in a mouse model of septic shock (Li et al. 2009; Cao et al. 2008; Zhang et al. 2009). We have also demonstrated that inhibition of HDAC can modulate the immune response (trauma/hemorrhage and inflammatory second hit in-vitro) not only in animals, but also in severely injured patients (Sailhamer et al. 2008).
Models for Study of Hemorrhagic Shock and Septic Shock
So far, a broad variety of experimental conditions, including in vivo animal and in vitro cell-based models, have been established to enable investigators to study the effects of the hypovolemic shock. These models have provided researchers with different conditions to assess the potential benefits of a wide spectrum of treatment options.
In essence, there are two types of in vivo experimental models of hemorrhagic shock: controlled hemorrhage and uncontrolled hemorrhage. Uncontrolled-hemorrhage models are clinically realistic as the animals are allowed to bleed regardless of blood pressure or the volume of blood loss (similar to actual trauma patients). This clinical realism, however, introduces a huge degree of animal-to-animal variability. These models are used mostly for translational studies that focus on clinical outcome/survival, and not when the investigators are interested in studying specific mechanisms or pathways. Controlled hemorrhage models are either of fixed-pressure or fixed-volume type (Lomas-Niera et al. 2005). In the former model, animals are bled to a predetermined mean arterial pressure (MAP) and are maintained at that pressure, with periodic bleeding, for a specified period of time based on the degree or outcome of hypotensive shock. In the later model, a controlled volume of blood occuren (as a percentage of total circulating blood volume) is withdrawn. Other important variables must also be controlled in these models such the actual bleeding time, duration of shock, body size, gender, type of anesthesia, and presence or absence of resuscitation, just to name a few. These controlled models, although clinically unrealistic, are suitable for mechanistic studies as they allow the researchers to control most of the confounding variables. They are thus routinely used in the investigation of end organ damage, cardiovascular alterations, subsequent central nervous system and spinal cord injuries, immunological changes and response to fluid resuscitation (Moochhala et al. 2009).
As for the in vivo sepsis models, on the basis of the initiating agent, they can be divided into five broad categories: (1) exogenous administration of a toxin such as liposaccharide (LPS), (2) intravenous infusion of a viable pathogen, (3) administration of fecal material or live organisms into the peritoneal cavity, (4) placement of infected foreign material into the soft tissues of the extremity, and (5) surgical operations that partially destroy the normal barrier of the gastrointestinal tract (e.g., cecal ligation and puncture (CLP) and colon ascendant stent peritonitis (CASP) (Bhatia et al. 2009). Although many animal models of sepsis or septic shock have been developed, none replicate all the aspects of clinical sepsis. The advantages and disadvantages of these animal models are summarized in Table 11.2.
Table 11.2.
Animal models used for study of hemorrhagic shock and septic shock (Lomas-Niera et al. 2005; Bhatia et al. 2009)
| Animal models | Advantage | Disadvantage | |
|---|---|---|---|
| Hemorrhagic shock | Fixed pressure hemorrhage | The extent of hypotension, duration and/or the volume can be controlled/monitored | Animal typically heparinized |
| Fixed volume hemorrhage | Models acute hemorrhage (hypotension) | Degree of hypotension uncertain | |
| Uncontrolled hemorrhage | Considered most clinically relevant | No standardized control of degree/duration of hypotension and extent of blood loss | |
| Septic shock | LPS-induced sepsis |
|
|
| Bacterial infection (intravenous infusion of a viable bacteria, administration of fecal material or live organisms into peritoneal cavity) | Intravenous infusion of live organisms may be appropriate to study the blood clearance kinetics of organisms |
|
|
| CLP-induced Sepsis |
|
|
|
| CASP-induced Sepsis | Induce diffuse peritonitis with persistent systemic infection |
|
Cell and organ-based experiments are now being used as in vitro models and are offering direct molecular and cellular accessibility and micro-environmental controls. Additionally, they provide efficient comparison between many experimental conditions or potential therapeutic compounds. So far, in vitro models such as cultured cells in hypoxic condition or conditional medium (Li et al. 2009) and isolated organ (e.g., heart) perfused with different fluids (Zhao et al. 2007) have been established to analyze mechanisms of HDACI action involved in HS and septic shock.
As the different models of hemorrhagic and septic shock have specific advantages and disadvantages (Table 11.2), it is important to analyze the experimental findings in the context of the selected model. For this manuscript, we will focus on the fixed volume hemorrhage and LPS- induced septic shock (and some in vitro models), to highlight the emerging role of HDACI in the treatment of lethal insults.
HDACI in Models of Hemorrhagic Shock
HS creats a global ischemic insult due to acute blood loss. Current treatment for HS focuses on pathophysiology at the level of organ systems: maintain sufficient tissue perfusion and vital organ function through administration of fluids and blood products, and to surgically control the source of hemorrhage. Unfortunately, this resource intensive protocol remains difficult to administer, particularly in austere environments where advanced surgical interventions are not available (Champion et al. 2003). Moreover, this approach fails to address much of the damage that takes place at the cellular level as a result of hypoperfusion (during hemorrhage) and reperfusion (during resuscitation) (Valko et al. 2007).
In Vivo Rodent and Swine Models of Hemorrhagic Shock
Hemorrhage is responsible for about half of the trauma deaths, and most of these occur during the pre-hospital period. To alter this grim outcome, the first responders must keep the injured alive long enough to be transported to a hospital for definitive care. As the pre-hospital environment is fairly austere, especially in the battlefield, conventional resuscitation strategies that rely on transfusion of blood products are unrealistic. The challenge is for the researchers to develop novel strategies that can maintain life without fluid/blood resuscitation.
Shults and colleagues subjected male Wistar-Kyoto rats to 60% blood volume loss and treated them with or without HDACI such as VPA or SAHA (Shults et al. 2008). They then examined the rats over the next 3 h, using the survival as primary endpoint. The results showed this model was highly lethal as only 25% of the animals survived for 3 h. However, administration of HDACI after hemorrhage (no fluid or blood administration) significantly improved survival (75% and 83% in VPA and SAHA groups, respectively). These results demonstrated that post-shock treatment with HDACI can significantly improve early survival in a highly lethal model of hemorrhagic shock, even in the absence of conventional resuscitation. Following up on this subject, Alam et al. investigated whether VPA treatment would improve survival in a clinical relevant large animal (swine) model of poly-trauma/hemorrhagic shock (femur fracture, 60% blood loss, liver injury, hypothermia, acidosis and coagulopathy). They found that treatment with VPA without blood transfusion improved early survival in this highly lethal model (Alam et al. 2009). How can HDACI maintain organ viability without restoring intra-vascular volume and tissue perfusion? Although still incomplete, recent studies have markedly advanced our appreciation of the underlying mechanisms.
In Vitro Single-Organ Ischemia-Reperfusion Injury Model
Ischemia/reperfusion injury may lead to myocardial infarction, cardiac arrhythmias, and contractile dysfunction. The phenomenon of ischemic preconditioning, in which a period of sublethal injury can protect the cell during a subsequent ischemic insult, has been widely investigated over the last two decades. The preconditioning consists of an early and a late phase. The early phase, termed “early” or “first window,” develops immediately and disappears within 1–2 h of ischemic preconditioning stimulus. Conversely, the late phase also known as the “second window” or “delayed,” manifests after 12–24 h and lasts for 3–4 days (Ping and Murphy 2000). It is known that in the preconditioning stimuli a series of signal transduction pathways carry the signal for protection, and these presumably terminate on one or more end-effectors. In activity the end-effectors cause the protection during the lethal ischemic insult (index ischemia) and/or the subsequent reperfusion period. A memory element exists, somewhere in the signal transduction pathways between the trigger signal and the end-effector, that is set by the preconditioning protocol and keeps the heart in a preconditioned state (Yellon and Downey 2003).
To assess whether HDACI trigger preconditioning-like effects against ischemia/reperfusion injury, Zhao et al. isolated mouse hearts and perfused them with three cycles of 5-min infusion and 5-min washout of 50 nM of TSA, a potent inhibitor of HDAC, to mimic early pharmacologic preconditioning. This was followed by 30 min of ischemia and 30 min of reperfusion. In addition, mice were treated with saline or TSA (0.1 mg/kg, i.p.) to investigate delayed pharmacologic preconditioning. This study found that infusion of TSA itself did not change ventricular function in non-ischemic hearts. However, when the perfused heart was subjected to ischemia/reperfusion in vitro, TSA treatment resulted in an improvement in the recovery of ventricular function and a reduction in infarct size (Zhao et al. 2007).
The survival advantage is due not to improvement in resuscitation but to better tolerance of shock by the cells. The cell protective mechanisms may result from (1) epigenetic regulation through post translation modification of histone proteins, (2) activation of cell survival factors such as the phosphoinositide 3-kinase (PI3-k)/Akt signaling pathway, (3) blockage of gut-liver/lymph-lung axis, and/or (4) breakage of a paracrine loop between leukocytes and endothelial cells. All of these actions directly or indirectly involve restoration of protein acetylation.
Acetylation-Related Epigenetic Regulation
Modulation of histone acetylation to restore and maintain the normal ratio of HAT/HDAC (epigenetic regulation) has been tested as potential treatment for many diseases. A number of preclinical studies have demonstrated that HDACI can improve survival in degenerative diseases, prevent the brain from various insults, attenuate the effects of aging and increase life span (Ryu et al. 2003; Steffan et al. 2001; Chang and Min 2002; Faraco et al. 2006). Our group has reported that administration of HDACI protects organs and cells from hemorrhagic shock-induced injury (Shults et al. 2008; Li et al. 2008; Sailhamer et al. 2008). Several converging lines of inquiry suggest that HDACI protect key organs by minimizing the cellular damage during hemorrhagic shock and resuscitation.
In the heart, ischemia induces deacetylation of histones H3/4 in vitro and in vivo (Granger et al. 2008). Using standard murine model of heart ischemia-reperfusion, Granger et al. demonstrated that treatment with HDACI significantly reduce infarct area, even when delivered 1 h after the ischemic insult. HDACI decrease the response to ischemic injury and lessen the size of myocardial infarction (Granger et al. 2008). In part, this is through prevention of ischemia-induced activation of gene programs that include hypoxia inducible factor-1α, cell death, and by decreasing vascular permeability in vivo and in vitro, which reduces vascular leak and myocardial injury.
In the liver, oxygen deprivation increases HDAC1, 4, and −5 protein levels by twofold and decreases acetylated histone H3 levels to 50–75% of the control values in a turtle model of anoxia (Kochenek et al. 2010). In a rat model of hemorrhagic shock, Gonzales et al. reported that hemorrhage increased serum levels of lactate, lactate dehydrogenase, aspartate aminotransferase, and alanine aminotransferase. Alternatively, treatment with VPA (an HDACI) induced acetylation of histones (H2A, H3, and H4), normalized serum levels of these enzymes and prolonged survival by fivefold. Furthermore, hyperacetylation of the histone proteins indicated the presence of active genes and correlated with improved survival (Gonzales et al. 2008). Gene expression profiling data from our group has shown that VPA treatment up-regulates expression of 17 critical genes at the early stage of HS (Fukudome et al., unpublished data). Two of these genes are peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) and dual specificity protein phosphatase 5 (DUSP5). PGC-1α protects cells from oxidative stress by increasing the expression of various antioxidant defense enzymes including superoxide dismutase and glutathione peroxidase (St-pierre et al. 2006). DUSP5 is an inducible, nuclear, dual-specificity phosphatase, which specifically interacts with and inactivates the extracellular signal-regulated kinase (ERK) 1/2 MAP kinases in mammalian cells (Kucharska et al. 2009). Inactivation of ERK1/2 MAP kinases by DUSP5 could be one of mechanisms responsible for the protective properties of VPA in HS.
In the kidney, it has been discovered that ischemia/reperfusion induces a transient decrease in histone acetylation in proximal tubular cells. This is likely a result of a decrease in histone acetyltransferase activity as suggested by experiments with energy-depleted renal epithelial cells in culture (Marumo et al. 2008). During recovery after transient energy depletion in epithelial cells, the HDAC isozyme HDAC5 is selectively downregulated in parallel with the return of acetylated histone. Knockdown of HDAC5 by RNAi significantly increased histone acetylation and bone morphogenetic protein-7 (BMP7) expression (Marumo et al. 2008). In a rat model of HS, it was found that treatment of animal with VPA or SAHA markedly increases the phosphorylation of Akt and decreases the expression of pro apoptotic BAD (Bcl-xl/Bcl-2 associated death promoter) protein in kidney tissue (Zacharias et al. 2010). Further investigation is needed to find if there is any relationship between HDAC5 inhibition and Akt activation.
In the brain, Faraco et al. found that ischemia (6 h of middle cerebral artery occlusion) drastically decreases histone H3 acetylation levels without evidence of a concomitant change in histone acetyltransferase or deacetylase activities. Treatment with SAHA (50 mg/kg i.p.) increased histone H3 acetylation in the normal brain (approximately eightfold after 6 h) and prevented histone deacetylation in the ischemic brain. These effects were accompanied by increased expression of the neuroprotective Heat-shock protein 70 (Hsp70) and B-cell lymphoma 2 (Bcl-2) protein in the control and ischemic brain 24 h after the insult. At the same time point, mice injected with SAHA at 25 and 50 mg/kg had smaller infarct volumes compared with vehicle-treated animals (28.5% and 29.8% reduction, p < 0.05 versus vehicle). Recently, Li et al. reported that VPA treatment induces acetylation of histone H3, increases expression of β-catenin and Bcl-2 proteins, and prevents neuronal apoptosis in in-vitro hypoxic condition (0.5% O2), as well as in in-vivo model of HS (Li et al. 2008). These findings demonstrate that pharmacological inhibition of HDAC promotes expression of neuroprotective proteins within the ischemic brain, which underscores the therapeutic potential of these drugs.
Activation of Phosphoinositide 3-Kinase (PI3K)-Akt/PKB Pathway
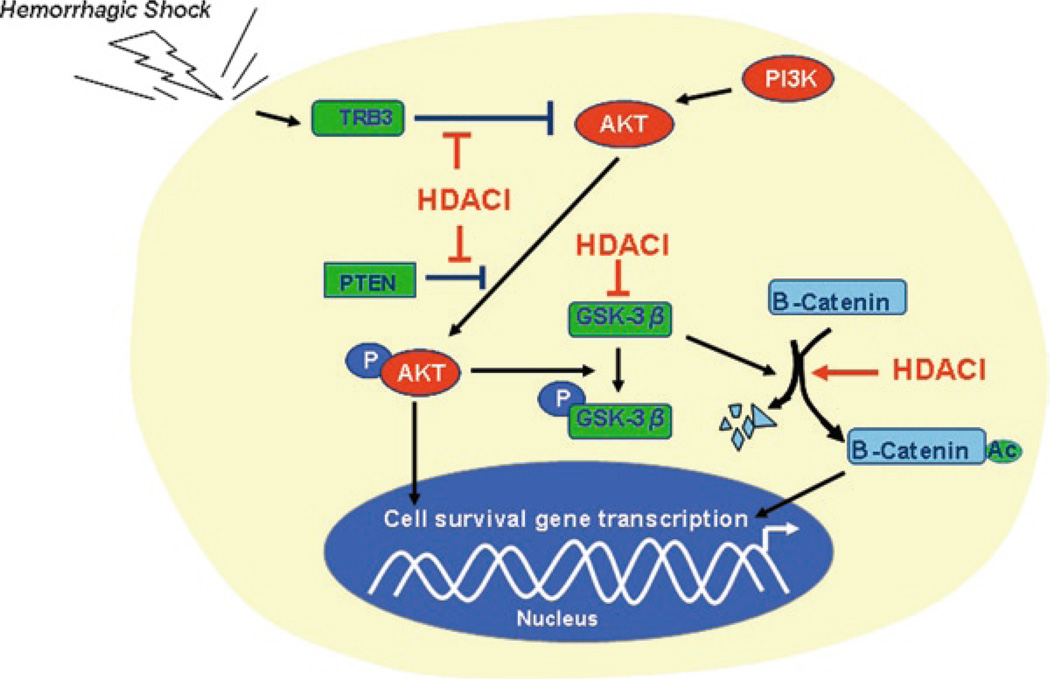
Activation of PI3K enhances cell survival (and decreases apoptosis) via Akt/PKB activity in many cell types including cardiomyocytes, cardiac fibroblast, vascular smooth muscle cells (VSMCs), endothelial cells and hepatocytes (Oudit et al. 2004; Shuja et al. 2009; Alam et al. 2009). Alam et al. recently demonstrated that VPA exerts anti-apoptotic effect through the Akt/PKB signaling pathway to improve survival in a swine model of poly-trauma and massive blood loss (Alam et al. 2009). In this study they compared cell protective effects of the HDAC inhibitor (VPA) to treatment with fresh whole blood (FWB) as well as conventional normal saline resuscitation. VPA treatment increased the levels of activated Akt, deactivated glycogen synthase kinase-3β (GSK-3β), β-catenin and Bcl-2 significantly when compared to FWB and saline control groups (without an alteration in the total Akt and GSK-3β levels) (Alam et al. 2009). VPA has been reported to directly and indirectly inhibit GSK-3β (Kim et al. 2005). However, it is not clear how the HDACI influences the Akt signaling in an animal model of trauma and hemorrhagic shock. There are several other possibilities for the activation of this pathway in addition to the direct inhibition of GSK-3β (Fig. 11.1).
-
An increase in the acetylated tribbles (TRB) 3 and phosphatase and tensin homolog (PTEN) may be associated with PI3K/Akt activity (Yao and Nyomba 2008). Growth factors such as insulin, insulin-like growth factor 1 (IGF-1), erythropoietin, and cytokines that reduce apoptosis rely almost exclusively on the PI3K/Akt pathway, whereas GPCR-induced PI3K/Akt activation and cardioprotection occurs in response to several peptide agonists including urocortin, ghrelin, and adrenomedullin as well as beta2-adrenergic receptor (β2-AR) stimulation (Oudit et al. 2004; Torella et al. 2004; Chesley et al. 2000; Kim et al. 2008). In the heart, overexpression of Akt/PKB causes resistance to apoptosis (Oudit et al. 2004); knockout of Akt2/PKBβ enhances apoptosis in response to myocardial ischemia (DeBosch et al. 2006). Consistent with a critical role for Akt/PKB in cell survival, loss or gain of TRB3 and PTEN activity leads to reduced or enhanced apoptosis, respectively (Avery et al. 2010; Kishimoto et al. 2003). Alternatively. increased expression of TRB3 and PTEN promotes apoptosis in cardiac myocytes (Schwartzbauer and Robbins 2001; Avery et al. 2010).
PTEN is a dual protein/lipid phosphatase whose main substrate is phosphatidyl-inositol,3,4,5 triphosphate (PIP3), the product of PI3K. PTEN degrades PIP3 to an inactive form phosphatidylinositol 4,5-bisphosphate (PIP2) (Lee et al. 1999; Maehama and Dixon 1998; Oudit et al. 2004; Stambolic et al. 1998), inhibiting Akt activation. PTEN is constitutively active and is the major downregulator of PI3K/Akt (Stiles et al. 2004). PTEN also forms signaling complexes with PDZ domain-containing adaptors, such as the MAGUK (membrane-associated guanylate kinase) proteins. These interactions appear to be necessary for the metabolism of localized pools of PIP3 involved in regulating actin cytoskeleton dynamics. Acetylation is major mechanism that regulates PTEN activity (Okumura et al. 2006). Histone acetylase p300/CREB-binding protein-associated factor (PCAF) interacts with PTEN and acetylates lysines 125 and 128 which are located within the catalytic cleft of PTEN and are essential for PIP3 specificity. PCAF functions as a negative regulator of PTEN (Tamguney and Strokoe 2007). TRB3 is an intracellular pseudokinase that modulates the activity of several signal transduction cascades. TRB3 has been reported to inhibit the activity of Akt protein kinases (Du et al. 2003). TRB3 gene expression is highly regulated in many cell types, and hypoxia or endoplasmic reticulum (ER) stress promotes TRB3 expression. TRB3 binds to inactive and unphosphorylated Akt, thus preventing its phosphorylation (Shih et al. 2003). It remains unknown whether and how PCAF regulates TRB3. Recently Yao and Nyomba reported that acetylation status of TRB3 and PTEN is decreased in association with increased HDAC and decreased HAT activities. The hypoacetylated TRB3 and PTEN can inhibit the Akt-activity in a rat model of prenatal alcohol exposure (Yao and Nyomba 2008), which suggests that HDACI treatment could inhibit the activity of TRB3 and PTEN, which in turn would enhance the Akt signaling.
-
Induction of Hsp70 by HDACI may be associated with PI3K/Akt pathway regulation. In a rat model of hemorrhagic shock, Gonzales et al. found that VPA treatment increased the acetylation of nonhistone and histone proteins and expression of Hsp70 in rat myocardium, and significantly prolonged survival (fivefold) compared to the untreated controls (Gonzales et al. 2006). In rat cortical neurons, VPA treatment markedly up-regulated Hsp70 protein levels, and this was accompanied by increased Hsp70 mRNA levels and promoter hyperacetylation and activity (Marinova et al. 2009). Other HDAC inhibitors – sodium butyrate, trichostatin A, and Class I HDAC-specific inhibitors MS-275 and apicidin – all possess the ability to induce HSP70.
Hsp70 is a molecular chaperone, cell-protective and anti-inflammatory agent. Marinova et al. recently reported that HDACI increase Sp1 acetylation, promote the association of Sp1 with the histone acetyltransferases p300 and recruitment of p300 to the Hsp70 promoter. Further, HDAC-Iinduced cell protection can be prevented by blocking Hsp70 induction (Marinova et al. 2009). In addition, Gao and Newton showed that Hsp70 directly binds and stabilizes Akt/PKB as well as protein kinase A and protein kinase C, thus prolonging the signaling lifetime of the kinases (Gao and Newton 2002). Taken together, these findings suggest that the PI3K/Akt pathway and Sp1 are likely involved in Hsp70 induction by HDACI, which in turn can sustain the active state of Akt to attenuate the cellular apoptosis.
Fig. 11.1.
Effect of HDACI on cell survival signaling pathway in hemorrhagic shock. HDACI induce phosphorylation of AKT by inhibition of TRB3 and PTEN. While AKT stimulates transcription of cell survival genes through several other pathways, phosphorylated AKT phosphorylates GSK-3β. Phosphorylated GSK-3β becomes inactivated form, which cannot degrade β-catenin. HDACI can also directly inhibit GSK-3β. Moreover, HDACI induce acetylation and nuclear translocation of β-catenin, leading to downstream survival gene transcription. P phosphorylation, Ac acetylation
In addition to the interaction with the Akt/PKB pathway, Hsp70 also directly interacts with different proteins of the tightly regulated programmed cell death machinery thereby blocking the apoptotic process at distinct key points. For example, Hsp70 can inhibit the apoptotic cascade (Gotoh et al. 2004; Stankiewicz et al. 2005), decrease formation of the functional apoptosome complex (Beere et al. 2000; Saleh et al. 2000), prevent late caspase dependent events such as activation of cytosolic phospholipase A2 and changes in nuclear morphology, and protect cells from forced expression of caspase-3 (Jaattela et al. 1998). Moreover, Hsp70 inhibits c-Jun N-terminal kinase (JNK) mediated cell death by suppressing JNK phosphorylation either directly and/or through the upstream SEK (Stress-activated protein kinase (SAPK)/extracellular signal-regulated kinase (ERK) kinase) kinase (Mosser et al. 2000; Meriin et al. 1999; Volloch et al. 1999), and hampers TNF mediated apoptosis by inhibition of ASK-1 (Park et al. 2002).
Effect of HDACI on Gut and Lung in Hemorrhagic Shock
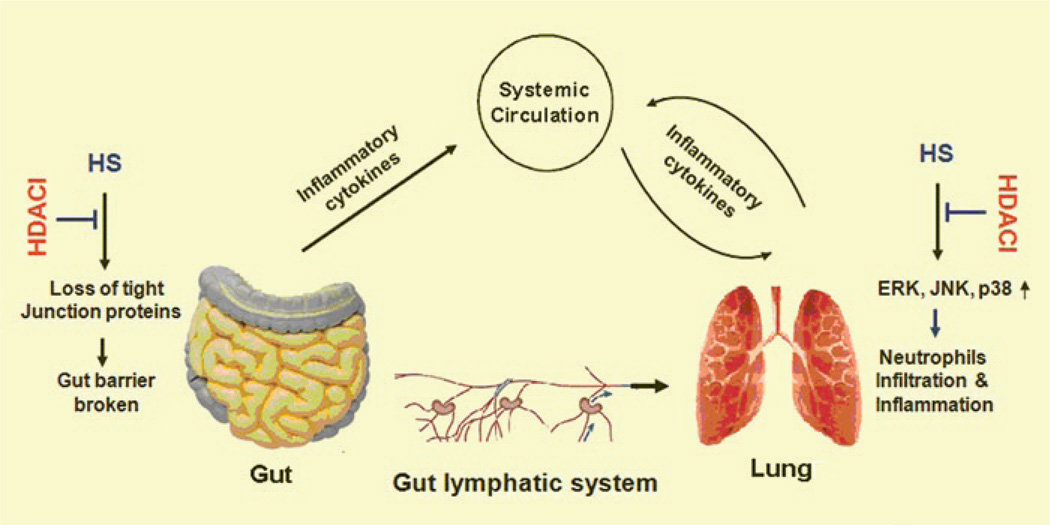
Hemorrhagic shock is characterized by tissue perfusion which is insufficient to meet the oxygen and nutrient demands of cells. Host response to hemorrhagic shock involves a coordinated expression of mediators that act both locally and systemically with profound effects on organ function. The accumulated evidences suggest that gut and lung, especially gut, represent important site(s) of immune mediator production and inflammation. Although two major hypotheses, gut-lymph-lung axis (Deitch 2001; Deitch et al. 2006) and gut-liver-lung axis (Peitzman et al. 1995; Thuijls et al. 2009), have brought much debate based on their different findings, it is clear that hemorrhagic shock is associated with intestinal ischemia which makes it a proinflammatory organ. For the fact that most of these mediators are produced by cells of the immune system, significant immunoregulatory actions occur within the gut-liver and/or lymph-lung axis and in peripheral immune sites (Fig. 11.2). It is well established that the gut plays a pivotal pathogenic role in the pathogenesis of SIRS and multi-organ dysfunction syndrome (MODS) (Deitch 1992; Hassoun et al. 2001).
Fig. 11.2.
Effect of HDACI on gut-lymph-lung axis in response to hemorrhagic shock. Hemorrhagic shock causes destruction of the gut barrier due to tight junction protein (e.g., claudin-3) loss. Bactria, endotoxin, and inflammatory cytokines enter into circulation and lung. In the lung tissue, MAPKs are stimulated and neutrophils infiltrated, resulting in acute lung injury. HDACI block these processing by inhibition of tight junction protein loss in gut and inactivation of MAPKs in lung
Depending on the duration of hemorrhagic shock, the degree of intestinal mucosal disruption which is the result of ischemia, increases and plays the main role in bacterial translocation (Baker et al. 1988). It is also postulated that epithelial disruption occurs, not only due to ischemia, but also due to the effect of protease or free oxygen radicals in the epithelium (Deitch et al. 1990). Hemorrhagic shock appears to promote bacterial translocation by injuring the gut mucosa and impairing its barrier function.
It is clear that gut ischemia can cause lung injury, but how can it be prevented? We proposed that HDACI could protect lung from gut-originated damage. To test this proposal, the superior mesenteric artery (SMA) of rats was clamped for 60 min to induce ischemia and then released for reperfusion. Without any treatment, gut ischemia induced production of pro-inflammatory cytokines or prostaglandin-like compound such as IL-6, cytokine-induced neutrophil chemoattractant (CINC), 8-isoprostane in lung tissues, and increased neutrophil lung infiltration. However, treatment with VPA significantly reduced these mediators in lung tissues and improved survival in a rat model of ischemia and reperfusion (Kim et al., unpublished data). It is not clear how VPA protects gut from ischemic damage and prevents acute lung injury. However, based on our recent findings it is conceivable that VPA maintains gut barrier in part through stabilizing intestinal tight junctions (TJ) (Fig. 11.2).
The function of gut barrier is based on intestinal tight junctions. Encircling epithelial cells, the intestinal TJ is a region where the plasma membrane of epithelial cells forms a series of contacts that appear to completely occlude the extracellular space and create an intercellular barrier and intra-membrane diffusion fence (Wong and Gumbiner 1997). Normally TJ is anchored in the cell via TJ proteins and filamentous actin (F-actin) cytoskeleton. Hypoperfusion, or ischemia, can cause disruption of F-actin cytoskeleton with subsequent TJ loss and barrier failure. Bacterial translocation to mesenteric lymph nodes, liver, and spleen is found at a very early stage of hemorrhagic shock (Thuijls et al. 2009). Loss of gut wall integrity not only leads to paracellular leakage of microbial products (Baker et al. 1988; Fink and Delude 2005), but also contributes to the development of systemic inflammation and distant organ failure (Hierholzer and Billiar 2001).
One of the major TJ proteins is claudin-3. Although the exact function of claudin-3 is not completely clear, it appears to be important in TJ formation and function (Morin 2005). Recent studies from our group (Li et al. 2010) and others (Thuijls et al. 2009) have shown that HS leads to destruction of the gut barrier due to TJ protein loss. Also it was found that the claudin-3 protein is released into circulation very early (30–60 min) after the onset of HS (Li et al. 2010). Alternatively, CINC, a chemokine that promotes neutrophil chemotaxis, is significantly elevated in serum and lung tissue with increased myeloperoxidase (MPO) levels at 4 h after hemorrhage. However, VPA treatment reversed HS-induced claudin-3 loss from the intestine and reduced the levels of CINC and MPO in serum and lung significantly (Fukudome et al., unpublished data). These findings suggest that VPA can stabilize claudin-3 in gut TJ, maintain the intestinal barrier, and prevent harmful gut-derived substances from getting into the systemic circulation.
Furthermore, we recently demonstrated that hemorrhagic shock (40% blood loss) resulted in phosphorylation (activation) of ERK, JNK and p38 mitogen-activated protein kinase (MAPK) in lung tissues at 1 h and 4 h. Post-shock administration of VPA (300 mg/kg, iv) significantly attenuated the MAPK activation without altering the total ERK, JNK and p38 proteins (Kochenek et al. 2010). These kinases are globally expressed and known to be key regulators of stress-mediated cell fate decision. Activation of these proteins has been strongly associated with poor outcomes while inhibition of MAP kinases has been associated with survival in hemorrhage models (Kochenek et al. 2010). VPA can also directly modulate MAPK activation. Cao et al. have studied the effects of HDACI treatment on LPS-induced activation of p38 MAPK and found that HDACI inhibit p38 phosphorylation. In their experiments, HDACI induce acetylation of MAP kinase phosphatase -1 (MKP-1), a protein that dephoaphorylates MAPK and inactivates MAPK pathways. These results demonstrate that HDACI treatment and MKP-1 acetylation increases the interaction between MKP-1 and p38 MAPK, and results in p38 inactivation, reduced inflammation and increased survival among LPS-exposed mice (Cao et al. 2008). Whether similar acetylation-mediated mechanisms exist for the regulation of ERK and JNK is still unknown but is highly plausible.
Breakage of a Paracrine Loop Between Leukocytes and Endothelial Cells
The development of MODS is a critical problem in patients who suffer major blood loss. Although the precise mechanisms and pathways leading to organ injury after HS are still incompletely understood, neutrophils are thought to be a principal mediator of tissue damage. Migration of neutrophils into the tissues during HS leads to significant organ damage through the release of proteases and oxygen-derived radicals (Weiss 1989). Intravital microscopy studies have established a sequence of events involved in leukocyte migration to extravacscular sites: rolling, firm adhesion, and trans-endothelial migration. Under conditions of flow, leukocytes are first observed to roll along the endothelium of postcapillary venules. Subsequently, some of the rolling leukocytes stick firmly, migrate along the endothelial surface, diapedese between endothelial junctions, and then migrate through the sub-endothelial matrix (Harlan and Winn 2002).
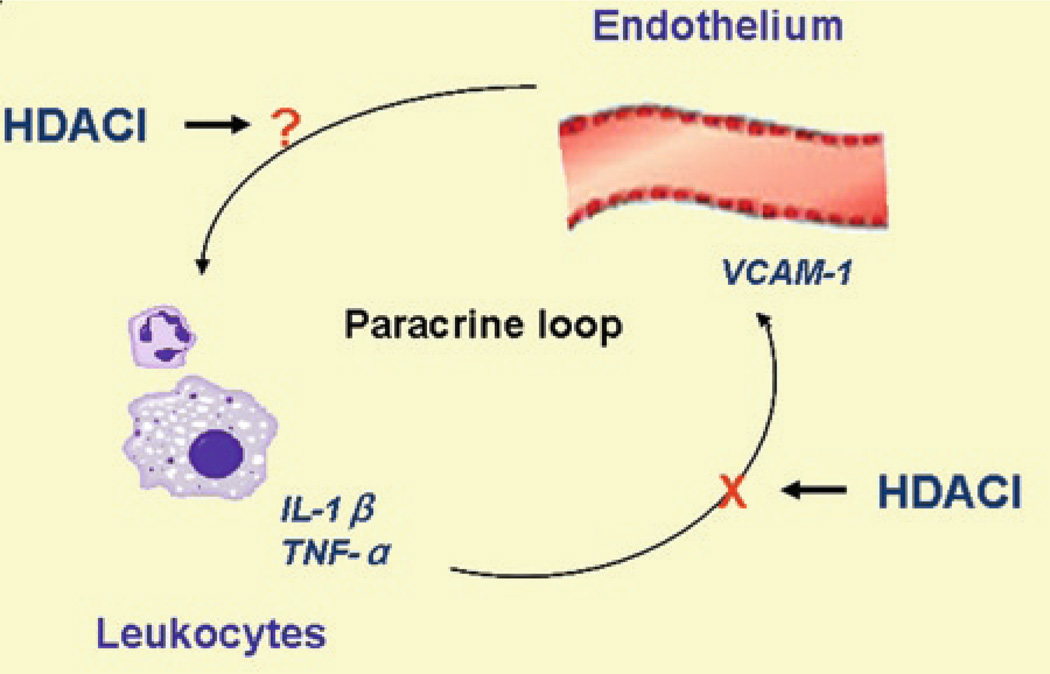
The interaction between leukocytes and endothelial cells seems to form a paracrine loop (Fig. 11.3), and is instrumental in the migration of neutrophils into different tissues. On one hand, leukocyte recruitment to endothelium is a fundamental element of the tissue response to HS. Interleukin (IL)-1 and tumor necrosis factor-a (TNF-a) produced by infiltrating inflammatory cells such as neutrophils and macrophages can induce endothelial cells to express cytokines as well as adhesion molecules (Vadlamani and Lyengar 2004). On the other hand, endothelial cells play an essential role in speeding up this process via their ability to express cell surface adhesion molecules that mediate interactions with leukocytes in the bloodstream. Activated endothelial cells express molecules involved in leukocyte rolling, such as P- and E-selectin, leukocyte adhesion molecules including vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), as well as chemoattractants such as chemokine (c-c motif) ligand 2 (CCL2) and IL-8, which can induce arrest of rolling leukocytes and promote leukocyte emigration from the vasculature (Petri et al. 2008; Zarbock and Ley 2009). Activation of endothelial cells during the response is typically induced by proinflammatory cytokines, such as TNF and IL-1β, released from leukocytes in response to HS (Cheng et al. 2010). Expression of adhesion molecules and additional cytokines from the activated endothelial cells in turn facilitates migration of additional neutrophils from the bloodstream. Anything that breaks this vicious circle could potentially attenuate the neutrophils mediated cellular damage.
Fig. 11.3.
HDACI modulate a paracrine between leukocytes and endothelial cells. The interaction between leukocytes and endothelial cells seems to form a paracrine loop. On one hand, IL-1 and TNF-a produced by infiltrating inflammatory cells such as neutrophils and macrophages can induce endothelial cells to express cytokines and adhesion molecules. On the other hand, endothelial cells play an essential role in speeding up this process via their ability to express cell surface adhesion molecules that mediate interactions with leukocytes in the bloodstream. HDACI break the loop by suppressing TNF-a induced VCAM-1 expression and reducing immune cells adhesion to endothelial cells
HDACI have been shown to modulate the interaction between leukocytes and endothelial cells. Studies of Inous et al. demonstrated that TSA, an inhibitor of HDAC, can suppress TNF-α induced VCAM-1 expression and reduce monocyte adhesion not only to endothelial cells in vitro but also to venules in inflamed mice in vivo (Inoue et al. 2006). These results suggest that HDACI might be useful for the attenuation of the deleterious cross-talk between leukocytes and endothelial cells.
HDACI in Models of Septic Shock
The progression of infection to septic shock begins with the release of inflammatory mediators at the local site of microbial invasion. This induces the migration of white blood cells and platelets to the infection site and contributes to endothelial damage and increased micro-vascular permeability. Blood flow is also reduced which sets the stage for ischemia-reperfusion injury. These physiologic processes are part of the exaggerated SIRS, which can lead to MODS.
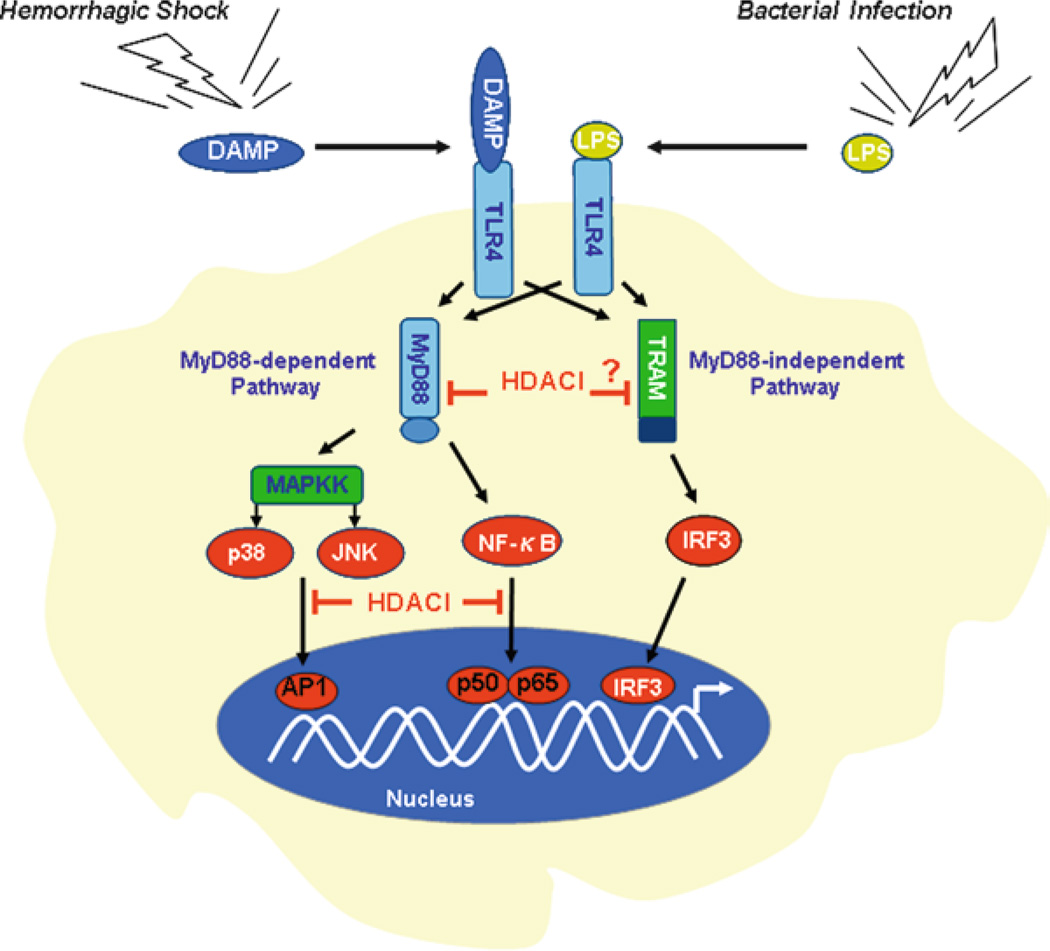
Many of the body’s reactions to infection with gram-negative organisms are due to lipopolysaccharide (LPS), a component of the outer bacterial cell wall membrane, also referred to as endotoxin. It can induce septic shock physiology and has been used extensively to produce shock in laboratory models. LPS exerts the downstream signals through the Toll Like Receptor-4 (TLR4). TLR4 activates two downstream pathways: Myeloid differentiation factor 88 (MyD88)-dependent and MyD88-independent pathways. The former one leads to the production of proinflammatory cytokines such as IL-6, TNF-α and IL-12 with the quick activation of nuclear factor-kappaB (NF-kB) and MAPK. The MyD88-independent pathway is associated with the activation of interferon (IFN) regulatory factor 3 (IRF3), subsequent induction of IFN-β, and maturation of dendritic cells.
It has been shown that HDACI exert anti-inflammatory activities via the suppression of inflammatory cytokines and nitric oxide (Blanchard and Chipoy 2005). In LPS-stimulated human peripheral blood mononuclear cells, ITF2357 (an inhibitor of HDAC) reduces the release of TNF-α, IL-1β and IFN-γ (Leoni et al. 2005). Other HDACI such as TSA and SAHA have been shown to decrease LPS-induced inflammation in mice (Cao et al. 2008; Li et al. 2009; Li et al. 2010). In RAW 264.7 cells, treatment of the macrophages with SAHA significantly suppresses LPS-induced gene expression and protein production of IL-1β, IL-6, and TNF-α (Li et al. 2009; Li et al. 2010). In an in vivo rodent model of septic shock, HDACI attenuate acute lung and liver injury, and improve survival (Zhang et al. 2009; Li et al. 2010; Zhang et al. 2010). Further mechanistic studies have demonstrated that HDACI play an important inhibitory role in TLR-4-MyD88 signaling pathways via NF-kB and MAPKs. Thus, protein acetylation is a potential regulatory mechanism that can modulate the inflammatory response (Fig. 11.4).
Fig. 11.4.
TLR4 signaling – a converged immune response pathway for hemorrhagic shock and septic shock. TLR4 not only serves as a key sensor of pathogen-associated molecular patterns (PAMPs), but also is proposed recently to act as a receptor for some endogenous molecules called “alarmins”. HDACI block TLR4 signaling at multiple steps; therefore they can inhibit immune response for both hemorrhagic shock and septic shock
HDACI Affect NF-kB Activity
NF-kB is an ubiquitiously expressed transcription factor that plays an important role in innate immunity and other critical processes. The NF-kB family consists of p50, p52, p65 (Rel A), c-Rel and Rel B, which form homo- or hetero-dimers. The p50/p65 heterodimer is the most frequently found combination in mammals. Inactive NF-kB complexes are retained in the cytoplasm by the IkB inhibitor. In innate immune signaling, host cells can respond to the threat of bacterial pathogens (e.g., LPS) via extracellular receptor TLRs (e.g., TLR4). TLR4 interacts with MyD88 and recruits interleukin-1 receptor-associated kinase 1 (IRAK1) and IRAK4 to the receptor complex. IRAK phosphorylates TNF receptor associated factor 6 (TRAF6) leading to the activation of the IkB kinase (IKK). The activation of IKK results in IkB phosphorylation, triggering its ubiquitination and proteasomal degradation. Free NF-kB then translocates to the nucleus to regulate the transcription of chemokines, cytokines, and other inflammatory response molecules (Hayden and Ghosh 2008).
In the nucleus, p50 and p60 can be regulated by acetylation. The function of acetylated NF-kB is complicated. Acetylation of p50 at K431, K440 and K441 promotes higher DNA binding affinity towards NF-kB target sequences correlating with increased p300 (histone acetyltransferase) recruitment and transcriptional activation (Deng and Wu 2003; Chen and Greene 2004). P300 can acetylate p65 at multiple lysine residues and result in different consequences. Acetylation of p65 at K221 and K310 is associated with an increased transcription of NF-kB target genes (Chen and Greene 2004) and is required for the full activity of p65 (Chen et al. 2002). In contrast, HDAC1 and HDAC3 deacetylate p65 at either K221 or K310, resulting in the inhibition of NF-kB. Additionally, K122 and K123 acetylation reduces p65 DNA binding affinity accompanied with increased IkB interaction and nuclear export (Kiernan et al. 2003). The p300-mediated acetylation of K314 and K315 in p65 has no obvious effect on NF-kB DNA binding or translocation.
HDACI have been shown to induce hyperacetylation and repress NF-kB signaling and expression of several target genes (Huang et al. 1997; Inan et al. 2000; Kramer et al. 2001). Conversely, other group reported that HDACI enhance NF-kB dependent gene expression in the presence of TNFa (Adam et al. 2003; Ashburner et al. 2001; Quivy et al. 2002; Vanden Berghe et al. 1999). Presumably, inhibitory or enhancive effects of HDACI on NF-kB rely on the cell type and expression of a different set of HDAC isoforms as well as the source of cell stimulation (e.g. LPS, cytokines and high glucose levels) (Blanchard and Chipoy 2005).
HDACI Inhibit MAPK Activity
In mammalian cells, JNK and p38 MAPKs activate mitogen and stress-activated protein kinase 1 (MSK1) such as ribosomal S6 kinase 2 (RSK2). RSK2 has a strong activity towards phosphorylation of histone H3 at Ser10 (Thomson et al. 1999). The phosphorylation of histone H3 occurs on the promoters of the subset on the stimulus-induced cytokine and chemokine genes, recruits NF-kB to the promoters, and stimulates transcription of inflammatory genes such as IL-6, IL-8, IL-12 and macrophage chemoattractant protein 1 (MCP-1) (Saccani et al. 2002).
It has been reported that HDAC inhibitor TSA enhances the activity of mitogen-activated protein kinase phosphotase-1 (MKP-1) (Chi and Flavell 2008; Cao et al. 2008). MKP-1 is a nuclear-localized dual-specificity phosphatase and preferentially dephosphorylates MAPKs such as p38 and JNK. Recently, Cao et al. showed that MKP-1 interacts with HAT, and acetylation of MKP-1 inhibits TLR4 signaling (Cao et al. 2008). They immunoprecipitated the histone acetylase p300 and showed that it was associated with MKP-1. Moreover, MKP-1 was acetylated by p300 on lysine residue K57 within its substrate-binding domain. Acetylation of MKP-1 induced by TSA enhanced the interaction between MKP-1 and p38 MAPK, suggesting that HDACI could increase the phosphatase activity and inactivate p38 MAPK. Indeed, TSA increased MKP-1 acetylation and blocked MAPK signaling in wild-type (WT) cells. However, TSA had no effect in cells lacking MKP-1. Furthermore, TSA reduced inflammation and mortality in WT mice treated with LPS, but failed to protect MKP-1 knockout mice. These findings suggest that acetylation of MKP-1 inhibits innate immune signaling, and targeting the MAPK pathway by HDACI may be an important approach in the treatment of septic shock.
Recently, our group has found that HDAC inhibitor SAHA can reduce the expression of MyD88 gene and protein in vitro and in vivo after LPS insult (Li et al. 2010). Moreover, SAHA acetylates heat shock protein 90 (Hsp90) and de-associates the protein from IRAK1, resulting in IRAK1 degradation (Chong et al., unpublished data). Our new findings have provided evidence that inhibition of HDAC can block, at least in part, activity of NF-kB and MAPKs in the initial steps of the TLR4-MyD88- NF-kB/MAPK pathway (Fig. 11.4).
TLR4 Signaling – A Converged Immune Response Pathway for Hemorrhage and Sepsis
Hemorrhage and sepsis activate several inflammatory and innate immune signaling pathways (Murphy et al. 2004). Systemically, these pathways promote recruitment of neutrophils and release of inflammatory cytokines (Botha et al. 1995). Within the cells, the inflammatory stimuli induce MAPK-dependent phosphorylation or phosphoacetylation of histone proteins and modulate the epigenetic accessibility of DNA (Saccani et al. 2002). Downstream, these signals change the expression profile of the genes in different cells types, altering the competing signals (e.g. pro-survival and pro-death, or anti-inflammation and pro-inflammation) that ultimately determine their fate. Two key pathways in hemorrhage- and sepsis-induced cellular injuries are the mitogen activated protein (MAP) kinase and NF-kB pathways. These proteins, ERK1/2, JNK, p38 protein kinase, and NF-kB, are globally expressed and known to be key regulators of cell fate decisions (Winter-Vann and Johnson 2007; Perkins 2007), which are involved in TLR4 signaling pathway.
It is well known that mammalian TLR4 serves as a key sensor of pathogen-associated molecular patterns (PAMPs) such as LPS. More recently, an additional role for TLR4 has been proposed. A number of reports have emerged to suggest that diverse molecules of host-cell origin may also serve as endogenous ligands of TLR4 (Bianchi 2007; Mollen et al. 2008; Tsan and Gao 2009). These molecules represent members of a recently identified family of molecules including Hsp70, fibrinogen, high mobility group box 1 (HMGB1), nucleolin, and annexins, etc. (Bianchi 2007). They have been found to serve as mediators of inflammation that may be expressed or released in response to tissue damage from trauma including HS. These molecules have been described as “alarmins”, which are the equivalent of PAMPs but are endogenous molecules. They are rapidly released following non-programmed cell death not by apoptotic cells. Immune cells can also be induced to produce and release alarmins without dying. Generally, this is done by using specialized secretion systems or by the endoplasmic reticulum (ER)-Golgi secretion pathway. Endogenous alarmins and exogenous PAMPs can be considered subgroups of a larger family of damage-associated molecular patterns (DAMPs). They convey a similar message and elicit similar responses through TLR4 (Fig. 11.4) leading to activation of MAPKs and NF-kB pathways (Bianchi 2007; Roger and Babensee 2010).
HDACI have been described above for their pro-survival and anti-inflammatory properties. The combined pro-survival and anti-inflammatory effectiveness makes them a highly attractive choice for the treatment of lethal hemorrhagic shock, and its septic complications. In our preliminary studies, we have already discovered that HDACI not only inhibit expression of pro-inflammatory cytokines and chemokines in cells, but also prevent some alarmins from being released from cells in hemorrhagic shock and septic shock (Li et al., unpublished data). Further investigation with different models (e.g., “two-hit” model) are being planned to further clarify the precise mechanisms of action and the role played by protein acetylation.
Future Studies: HDACI in a Model of Hemorrhagic and Subsequent Septic Shock (a Two-Hit Model)
Hemorrhage induces immunosuppression and enhances susceptibility to sepsis. This is evident from depression of lymphocyte functions, production of various lymphokines, macrophage expression of receptors involved in opsonin-mediated phagocytosis, and antigen presentation function of peritoneal, splenic, and Kupffer cells following hemorrhage. Depression in various immune functions is apparent immediately following hemorrhage and persists for a prolonged period of time despite volume resuscitation. It appears that the increased release of systemic mediators, such as IL-1, IL-6, TNF-α, is associated with marked depression in immune responses and increased susceptibility to infection following hemorrhage (Chaudry and Ayala 1993). TNF-α is the trigger cytokine released early after the onset of hemorrhage. This cytokine then activates various populations of macrophages to release IL-1, IL-6, TGF-β and others. Although studies have described additional cytokines following trauma that may also play an important role it appears that TNF-α, IL-1, IL-6, and TGF-β, may play pivotal regulatory roles in the sequence of events leading to protracted immunodepression following hemorrhage. It should be recognized that the cytokine cascade, or “cytokine storm,” may be triggered and amplified in a complex and interdependent manner (Chaudry and Ayala 1993).
The immunosupressive process is now fairly well characterized. The aforementioned imbalance in acetylation of proteins seems to be involved in the setting of both HS and septic shock. However, it remains unclear how to reduce or prevent the subsequent severe sepsis after HS. Aggressive resuscitation, vasopressor therapy, nutritional support and antibiotic prophylaxis alone have been proven to be ineffective when trying to alter the cytokine expression and decrease the infection rate after HS (Esrig et al. 1977; Chaudry and Ayala 1993; Bauhofer et al. 2006).
Focusing on the cellular pathophysiology of hemorrhagic shock and septic shock, we have explored the strategy of using HDACI (with or without resuscitation) as protective agents. HDACI alter the acetylation status of proteins and therefore have the potential to modulate the genomic and proteomic changes induced by hemorrhage and sepsis. We have shown that HDACI can dramatically improve survival in lethal models of hemorrhagic shock in rat (Shults et al. 2008; Fukudome et al. 2010), and swine models (Alam et al. 2009). These inhibitors can protect cells from apoptosis and suppress expression of pro-inflammatory cytokines (Li et al. 2009; Li et al. 2008; Fukudome et al., unpublished data). HDACI can improve survival in a rodent model of septic shock regardless of whether the inhibitors are administrated before or after LPS insult. Moreover, a recent study from our laboratory demonstrated that the HDAC inhibitor SAHA can normalize TNF-α levels following rat hemorrhage in vivo and LPS second hit in vitro (Sailhamer et al. 2008). All of results above indicate that modulation of protein acetylation by HDACI may be an effective therapy for hemorrhage-induced sepsis. However, a critical animal model is still needed to confirm it.
An animal model of the “two-hit” paradigm has been used to target protein molecules involved in hemorrhage-induced septic shock (Fan et al. 2000; Shih et al. 2003; Bauhofer et al. 2006). In this model, animals were subjected to a non-severe resuscitated HS followed by a small dose of intratracheal LPS (Fan et al. 2000), CLP (cecal ligation puncture, Shih et al. 2003), or PCI (peritoneal contamination and infection, Bauhofer et al. 2006). While neither the first (shock) nor the second (infection) insult alone induces severe injury, the combination causes lung neutrophil accumulation and increase albumin transpulmonary flux (Fan et al. 1998), organ injury, colony-forming units of microbes in lung and liver, and mortality. However, so far no in vivo two-hit studies have addressed the effects of HDACI on inflammatory responses caused by HS followed by the development of polymicrobial sepsis. Results from an ongoing experiment in our laboratory should fill this gap in the near future.
Special Consideration
Acetylation is rapidly emerging as a key mechanism that regulates the expression of numerous genes (epigenetic modulation through activation of nuclear histone proteins), functions of multiple non-histone proteins involved in key cellular functions such as cell survival, repair/healing, and inflammation. HDACI hold great promise as a new class of agents for restoration of protein acetylation that can play a therapeutic role in hemorrhagic and septic shock. However, some special consideration should be kept in mind when the mechanism of action for HDACI is analyzed.
Sex hormones significantly impact survival in models of hemorrhagic shock (Lomas-Niera et al., 2005). Findings of Choudhry et al. suggest that the depression of immune functions in trauma hemorrhage is severe in young males, ovariectomized and aged females. In contrast, the immune functions in proestrus females following trauma-hemorrhage are maintained (Choudhry et al. 2007). Studies have also shown that the survival rate in proestrus females following trauma-hemorrhage and the induction of subsequent sepsis is significantly higher than in age-matched males and ovariectomized females. Furthermore, administration of female sex hormone 17β-estradiol in males and ovariectomized females after trauma-hemorrhage prevents the suppression of immune response. Recently, HDACI such as TSA and VPA have been found to induce estrogen response element transactivation. Estradiol treatment in turn can potentiate the HDACI effect (Suuronen et al. 2008). These results indicate that the interaction of sex hormones with HDACI could play a significant role in shaping the host response following trauma and hemorrhagic shock.
Cell type is a big factor when evaluating the impact of HDACI. The response of normal and transformed (e.g. neoplastic) cells to a given HDACI is often completely different. HDACI- induced phenotypes changes in transformed cells include growth arrest, activation of the extrinsic and/or intrinsic apoptotic pathways, autophagic cell death, reactive oxygen species (ROS)-induced cell death, mitotic cell death and senescence. In comparison, normal cells are relatively more resistant to HDACI-induced cell death (Kelly and Marks 2005; Dokmanovic et al. 2007). In many transformed cells, ROS-oxidation-reduction pathways are important mechanisms of HDACI-induced transformed cell death (Ungerstedt et al. 2005). Thioredoxin (Trx) acts as a hydrogen donor required for the activation of many proteins, including ribonucleotide reductase which is essential for DNA synthesis and transcription factors and is an antioxidation scavenger of ROS (Lillig and Holmgren 2007). HDACI upregulate the expression of Trx binding protein 2 (TBP2) (Butler et al. 2002), which binds and inhibits Trx activity and can cause downregulation of Trx in transformed but not normal cells (Butler et al. 2002; Ungerstedt et al. 2005). Trx is an inhibitor of apoptosis signal regulating kinase 1 (ASK1) (Saitoh et al. 1998). Therefore, inhibition of Trx by HDACI in the transformed cells subsequently results in cell apoptosis.
Another potential confounder in animal models is the administration of heparin. The anti-coagulant heparin is frequently used to maintain blood flow through catheters (Rana et al. 1992) and to anti-coagulate shed blood reserved for return during resuscitation. However, high doses of heparin have been associated with effects that can confound results in experimental models of HS (Lomas-Niera et al., 2005). For example, direct addition of heparin to cells has been found to reduce histone acetylation (Buczek-Thomas et al. 2008).
As for the “two-hit” model, the timing of a secondary insult is critical to the ultimate response of the host (Lomas-Neira et al., 2005). Lomas-Neira et al. reported that hemorrhagic shock (priming stimulus) followed 24 h by the induction of sepsis (triggering stimulus) produced significantly higher levels of pro-inflammatory cytokine IL-6, MIP-2a and increased MPO activity in lung tissue. These hemorrhage-induced increases dissipated when sepsis was induced 72 h after hemorrhagic shock. It was found that there is a period of priming during the first 24 h following an initial traumatic inflammatory insult. The priming time keeps the secondary stimulus potential to trigger neutrophil mediated tissue or organ injury (Ayala et al. 2001). Therefore, the timing for drug administration would be a crucial variable in a “two-hit” model.
In summary, the effects of HDACI depend upon the host “cell context” which in turn influences acetylation or the interaction of HDACs with histone and non-histone proteins. Ideally, comprehensive consideration of host gender, cell type, the timing of insult and the nature of host/cell stimulation should be taken into account when effects of the inhibitor are examined in an experimental model. Lack of attention to these details can create an erroneous impression of contradictory results.
Conclusion and Perspectives
Experimental evidence has shown that treatment with HDACI increase endurance of animals subjected to lethal blood loss. The survival benefit is seen even when the drugs are administered post-insult and is reproducible in different species including large animal models of poly-trauma. Protective properties of HDACI are not limited to hemorrhagic shock, as it can also improve survival in LPS models of septic shock. Administration of HDACI modulates the immune system to create a favorable phenotype not only during the acute phase of hemorrhagic shock but also later when the septic complications are likely to occur. Repeated successes of HDACI in well designed animal models of hemorrhagic shock (small and large animals) and septic shock (pre- and post-shock treatments) suggest that modulation of protein acetylation is potentially a very useful strategy for the treatment of these critical diseases.
The future success of shock treatment might come from the implementation of pro-survival and anti-inflammatory strategy in proper animal and cellular models. More studies should further elucidate the function of individual HDAC isoforms in severe hemorrhage and inflammation and assess potential effects of HDACI on sepsis following hemorrhagic shock. Since individual HDAC isoforms have distinctive physiological functions, it is important to develop next generation of HDACI. The new HDACI could potentially target specific HDAC isoforms and presumably result in improved efficacy relative to the first generation pan inhibitors, such as SAHA and TSA, but with little adverse effects. In addition to being used as pro-survival agents for severe trauma hemorrhage or anti-inflammatory compounds for deteriorative sepsis, HDACI could be used as combined pro-survival and anti-inflammatory drugs to prevent hosts from sepsis and even to treat sepsis following hemorrhage. The potential success of HDACI in the two-hit model might provide insight toward the development of pharmacological agents for the treatment of the shock of hemorrhage and sepsis.
Acknowledgments
Dr. Alam acknowledges grant support from the National Institutes of Health (RO1 GM084127), Defense Advanced Research Projects Agency (W911NF-06-1-0220), Office of Naval Research (N000140910378), and the US Army Medical Research Material Command (GRANTT00521959).
Abbreviations
- ASK1
Apoptosis signal regulating kinase 1
- BAD
Bcl-xl/Bcl-2 associated death promoter
- Bcl-2
B-cell lymphoma 2
- β2-AR
Beta2-adrenergic receptor
- BMP7
Bone morphogenetic protein 7
- CASP
Colon ascendant stent peritonitis
- CBP
Cyclic AMP (cAMP) response element binding protein (CREBP) binding protein
- CCL2
Chemokine (C-C motif) ligand 2
- CINC
Cytokine-induced neutrophil chemoattractant
- CLP
Cecal ligation puncture
- DAMPs
Damage-associated molecular patterns
- DNA
Deoxyribonucleic acid
- DUSP5
Dual specificity protein phosphatase 5
- ELISA
Enzyme-linked immunosorbent assay
- ER
Endoplasmic reticulum
- ERK
Extracellular signal regulated kinase
- F-actin
Filamentous actin
- FWB
Fresh whole blood
- GSK-3β
Glycogen synthase kinase-3β
- h
Hour
- H
Histone
- HATs
Histone acetylases
- HDA1
Histone deacetylase A1
- HDACs
Histone deacetylases
- HDACI
Histone deacetylase inhibitors
- HMGB1
High mobility group box 1
- HS
Hemorrhagic shock
- Hsp 70
Heat shock protein 70
- Hsp 90
Heat shock protein 90
- ICAM-1
Intercellular adhesion molecule-1
- IFN
Interferon
- IGF-1
Insulin-like growth factor 1
- IKK
IkB kinase
- IL
Interleukin
- IRAK 1
Interleukin-1 receptor associated kinase 1
- IRF3
Interferon regulatory factor 3
- IV
Intravenous (injection into a vein)
- JNK
c-Jun N-terminal kinase
- LPS
Lipopolysaccharide
- MAGUK
Membrane-associated guanylate kinase
- MAP
Mean arterial pressure
- MAPK
Mitogen-activated protein kinase
- MEF2
Myocyte enhancer factor 2
- MKP-1
MAP kinase phosphatase 1
- MMTV
Mouse mammary tumor virus
- MODS
Multi-organ dysfunction syndrome
- MPO
Myeloperoxidase
- MSK1
Mitogen and stress-activated protein kinase 1
- MyD88
Myeloid differentiation factor 88
- NAD
Nicrotinamide adenine dinucleotide
- NF-kB
Nuclear factor kappa B
- PAMPs
Pathogen-associated molecular patterns
- PCAF
p300/CREB-binding protein-associated factor
- PCI
Peritoneal contamination and infection
- p300
p300 histone acetyl transferase
- PGC-1α
Peroxisome proliferator-activated receptor γ coactivator-1α
- PI3K
Phosphoinositide 3 kinase
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- PIP3
Phosphatidyl-inositol,3,4,5 triphosphate
- PKB
Protein kinase B
- PTEN
Phosphatase and tensin homolog
- ROS
Reactive oxygen species
- RSK2
Ribosomal S6 kinase 2
- SAHA
Suberoylanilide hydroxamic acid
- SEK
Stress-activated protein kinase (SAPK)/extracellular signal-regulated kinase (ERK) kinase
- SIRS
Systemic inflammatory response syndrome
- SIRT
Sirtuins
- SMA
Superior mesenteric artery
- RT-PCR
Reverse transcription polymerase chain reaction
- TBP2
Trx binding protein 2
- TFs
Transcription factors
- TJ
Tight junction
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor α
- TRAF6
TNF receptor associated factor 6
- TRB3
Tribbles 3
- Trx
Thioredoxin
- TSA
Trichostatin A
- VCAM-1
Vascular cell adhesion molecule-1
- VPA
Valproic acid
- VSMCs
Vascular smooth muscle cells
- WT
Wild type
Contributor Information
Yongqing Li, Department of Surgery, Division of Trauma, Emergency Surgery and Surgical Critical Care, Massachusetts General Hospital/Harvard Medical School, Boston, MA, USA.
Hasan B. Alam, Email: hbalam@partners.org, Division of Trauma, Emergency Surgery, and Surgical Critical Care, Massachusetts General Hospital, 165 Cambridge Street, Suite 810, Boston, MA 02114, USA.
References
- Adam E, Quivy V, Bex F, et al. Potentiation of tumor necrosis factor-induced NF-kappa B activation by deacetylase inhibitors is associated with a delayed cytoplasmic reappearance of I kappa B alpha. Mol Cell Biol. 2003;23(17):6200–6209. doi: 10.1128/MCB.23.17.6200-6209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam HB, Shuja F, Butt MU, Duggan M, Li Y, Zacharias N, Fukudome EY, Liu B, Demoya M, Velmahos GC. Surviving blood loss without transfusion in a swine poly-trauma model. Surgery. 2009;146(2):325–333. doi: 10.1016/j.surg.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Andreasen AS, Krabbe KS, Krogh-Madsen R, et al. Huamn endotoxemia as a model of systemic inflammation. Curr Med Chem. 2008;15(17):1697–1705. doi: 10.2174/092986708784872393. [DOI] [PubMed] [Google Scholar]
- Ashburner BP, Westerheide SD, Baldwin AS., Jr The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol. 2001;21(20):7065–7077. doi: 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery J, Etzion S, DeBosch BJ, et al. TRB3 function in cardiac endoplasmic reticulum stress. Circ Res. 2010;106(9):1516–1523. doi: 10.1161/CIRCRESAHA.109.211920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila AM, Burnett BG, Taye AA, et al. Trichostatin A increases SMN expression and survival in amousemodel of spinal muscular atrophy. J Clin Invest. 2007;117:659–671. doi: 10.1172/JCI29562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala A, Chung CS, Lomas J, et al. Shock-induced neutrophil mediated priming for acute lung injury in mice: divergent effects of TLR-4 and TLR-4/FasL deficiency. Am J Pathol. 2002;161(6):2283–2294. doi: 10.1016/S0002-9440(10)64504-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JW, Deitch EA, Li M, et al. Hemorrhagic shock induces bacterial translocation from the gut. J Trauma. 1988;28(7):896–906. doi: 10.1097/00005373-198807000-00002. [DOI] [PubMed] [Google Scholar]
- Bauhofer A, Lorenz W, Kohlert F, et al. Granulocyte colony-stimulating factor prophylaxis improves survival and inflammation in a two-hit model of hemorrhage and sepsis. Crit Care Med. 2006;34(3):778–784. doi: 10.1097/01.ccm.0000201900.01000.6b. [DOI] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Cain K, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2(8):469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Bhatia M, He M, Zhang H, et al. Sepsis as a model of SIRS. Front Biosci. 2009;14:4703–4711. doi: 10.2741/3561. [DOI] [PubMed] [Google Scholar]
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Blanchard F, Chipoy C. Histone deacetylase inhibitors: new drugs for the treatment of inflammatory diseases? Drug Discov Today. 2005;10(3):197–204. doi: 10.1016/S1359-6446(04)03309-4. [DOI] [PubMed] [Google Scholar]
- Botha AJ, Moore FA, Moore EE, et al. Early neutrophil sequestration after injury: a pathogenic mechanism for multiple organ failure. J Trauma. 1995;39(3):411–417. doi: 10.1097/00005373-199509000-00003. [DOI] [PubMed] [Google Scholar]
- Buczek-Thomas JA, Hsia E, Rich CB, et al. Inhibition of histone acetyltransferase by glycosaminoglycans. J Cell Biochem. 2008;105(1):108–120. doi: 10.1002/jcb.21803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler LM, Zhou X, Xu WS, et al. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc Natl Acad Sci USA. 2002;99(18):11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camelo S, Iglesiuas AH, Hwang D, et al. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;164:10–21. doi: 10.1016/j.jneuroim.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Cao W, Bao C, Padalko E, Lowenstein CJ. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J Exp Med. 2008;205(6):1491–1503. doi: 10.1084/jem.20071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey N, La Thangue NB. Histone deacetylase inhibitors: gathering pace. Curr Opin Pharmacol. 2006;6:369–375. doi: 10.1016/j.coph.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Cha JH. Transcriptional dysregulation in Huntington’s disease. Trends Genet. 2000;23(9):387–392. doi: 10.1016/s0166-2236(00)01609-x. [DOI] [PubMed] [Google Scholar]
- Champion HR, Bellamy RF, Roberts CP, Leppaniemi A. A profile of combat injury. J Trauma. 2003;5:S13–S19. doi: 10.1097/01.TA.0000057151.02906.27. [DOI] [PubMed] [Google Scholar]
- Chang KT, Min KT. Regulation of lifespan by histone deacetylase. Ageing Res Rev. 2002;1(3):313–326. doi: 10.1016/s1568-1637(02)00003-x. [DOI] [PubMed] [Google Scholar]
- Chang JG, Hsieh-Li HM, Jong YJ, et al. Treatment of spinal muscular atrophy by sodium butyrate. Proc Natl Acad Sci USA. 2001;98:9808–9813. doi: 10.1073/pnas.171105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudry IH, Ayala A. Mechanism of increased susceptibility to infection following hemorrhage. Am J Surg. 1993;165(2A Suppl):59S–67S. doi: 10.1016/s0002-9610(05)81208-5. [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5(5):392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Chen LF, Wu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kB. EMBO J. 2002;21(23):6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, McKeown SJ, Santos L, et al. Macrophage migration inhibitory factor increases leukocyte-endothelial interactions in human endothelial cells via promotion of expression of adhesion molecules. J Immunol. 2010;185:1238–1247. doi: 10.4049/jimmunol.0904104. [DOI] [PubMed] [Google Scholar]
- Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3’-kinase. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- Chi H, Flavell RA. Acetylation of MKP = 1 and the control of inflammation. Sci Signal. 2008;1(41):pe44. doi: 10.1126/scisignal.141pe44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Park SK, Kim HM, et al. Histone deacetylase inhibitor KBH-A42 inhibits cytokine production in RAW 264.7 macrophage cells and in vivo endotoxemia model. Exp Mol Med. 2008;40(5):574–581. doi: 10.3858/emm.2008.40.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry MA, Bland KI, Chaudry IH. Trauma and immune response-effect of gender differences. Injury. 2007;38(12):1382–1391. doi: 10.1016/j.injury.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32(11):591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangond F, Gullans SR. Differential expression of human histone deacetylase mRNAs in response to immune cell apoptosis induction by trichostatin A and butyrate. Biochem Biophys Res Commun. 1998;247:833–837. doi: 10.1006/bbrc.1998.8891. [DOI] [PubMed] [Google Scholar]
- de Ruijter AJ, van Gennip AH, Caron HN, et al. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370(Pt 3):737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBosch B, Sambandam N, Weinheimer C, Courtois M, Muslin AJ. Akt2 regulates cardiac metabolism and cardiomyocyte survival. J Biol Chem. 2006;281:32841–32851. doi: 10.1074/jbc.M513087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch EA. Multiple organ failure. Pathophysilogy and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch EA. Role of the gut lymphatic system in multiple organ failure. Curr Opin Crit Care. 2001;7(2):92–98. doi: 10.1097/00075198-200104000-00007. [DOI] [PubMed] [Google Scholar]
- Deitch EA, Bridges W, Ma L, et al. Hemorrhagic shock-induced bacterial translocation: the role of neutrophils and hydroxyl radicals. J Trauma. 1990;30:942–952. doi: 10.1097/00005373-199008000-00002. [DOI] [PubMed] [Google Scholar]
- Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci. 2006;11:520–528. doi: 10.2741/1816. [DOI] [PubMed] [Google Scholar]
- Deng WG, Wu KK. Regulation of inducible nitric oxide synthase expression by p300 and p50 acetylation. J Immunol. 2003;171(12):6581–6588. doi: 10.4049/jimmunol.171.12.6581. [DOI] [PubMed] [Google Scholar]
- Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5(10):981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- Du K, Herzig S, Kulkarni RN, et al. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- Esrig BC, Frazee L, Stephenson SF, et al. The predisposition to infection following hemorrhagic shock. Surg Gynecol Obstet. 1977;144(6):915–917. [PubMed] [Google Scholar]
- Fan J. TLR cross-talk mechanism of hemorrhagic shock-primed pulmonary neutrophil infiltration. Open Crit Care Med J. 2010;2:1–8. doi: 10.2174/1874828700902010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Marshall JC, Jimenez M, et al. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J Immunol. 1998;161:440–447. [PubMed] [Google Scholar]
- Fan J, Kapus A, Li YH, et al. Priming for enhanced alveolar fibrin deposition after hemorrhagic shock: role of tumor necrosis factor. Am J Respir Cell Mol Biol. 2000;22(4):412–421. doi: 10.1165/ajrcmb.22.4.3857. [DOI] [PubMed] [Google Scholar]
- Faraco G, Pancani T, Formentini L, et al. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol Pharmacol. 2006;70(6):1876–1884. doi: 10.1124/mol.106.027912. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Kubilus JK, Lee J, et al. Histone deactylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. 2005;21(2):177–196. doi: 10.1016/j.ccc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Fukudome EY, Kochanek AR, Li Y, et al. Pharmacologic resuscitation promotes survival and attenuates hemorrhage-induced activation of extracellular signal-regulated kinase ½. J Surg Res. 2010;163(1):118–126. doi: 10.1016/j.jss.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Newton AC. The turn motif is a phosphorylation switch that regulates the binding of Hsp70 to protein kinase C. J Biol Chem. 2002;277(35):31585–31592. doi: 10.1074/jbc.M204335200. [DOI] [PubMed] [Google Scholar]
- Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: toll-like receptors. Free Radical Biol Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales E, Chen H, Munuve R, Mehrani T, Britten-Webb J, Nadel A, Alam HB, Wherry D, Burris D, Koustova E. Valproic acid prevents hemorrhage-associated lethality and affects the acetylation pattern of cardiac histones. Shock. 2006;25(4):395–401. doi: 10.1097/01.shk.0000209522.28120.c8. [DOI] [PubMed] [Google Scholar]
- Gonzales E, Chen H, Munuve RM, Mehrani T, Nadel A, Koustova E. Hepatoprotection and lethality rescue by histone deacetylase inhibitor valproic acid in fatal hemorrhagic shock. J Trauma. 2008;65(3):554–565. doi: 10.1097/TA.0b013e31818233ef. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Terada K, Oyadomari S, et al. hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004;11(4):390–402. doi: 10.1038/sj.cdd.4401369. [DOI] [PubMed] [Google Scholar]
- Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, Epstein JA, Gruber PJ. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J. 2008;22:3549–3560. doi: 10.1096/fj.08-108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty SJ, Koeller KM, Wong JC, et al. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA. 2003;100(8):4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan JH, Winn RK. Leukocyte-endothelial interactions: clinical trials of anti-adhesion therapy. Crit Care Med. 2002;30(5):S214–S219. doi: 10.1097/00003246-200205001-00007. [DOI] [PubMed] [Google Scholar]
- Hassoun HT, Kone BC, Mercer DW, et al. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15(1):1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clin Chim Acta. 2002;323(1–2):59–72. doi: 10.1016/s0009-8981(02)00180-8. [DOI] [PubMed] [Google Scholar]
- Hierholzer C, Billiar TR. Molecular mechanisms in the early phase of hemorrhagic shock. Langenbecks Arch Surg. 2001;386:302–308. doi: 10.1007/s004230100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockly E, Richon VM, Woodman B, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in amousemodel of Huntington’s disease. Proc Natl Acad Sci USA. 2003;100:2041–2046. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E, Dul E, Sung CM, et al. Identification of novel isoform-selective inhibitors within class I histone deacetylases. J Pharmacol Exp Ther. 2003;307(2):720–728. doi: 10.1124/jpet.103.055541. [DOI] [PubMed] [Google Scholar]
- Huang N, Katz JP, Martin DR, et al. Inhibition of IL-8 gene expression in Caco-2 cells by compounds which induce histone hyperacetylation. Cytokine. 1997;9(1):27–36. doi: 10.1006/cyto.1996.0132. [DOI] [PubMed] [Google Scholar]
- Inan MS, Rasoulpour RJ, Yin L, et al. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology. 2000;118(4):724–734. doi: 10.1016/s0016-5085(00)70142-9. [DOI] [PubMed] [Google Scholar]
- Inoue K, Kobayashi M, Yano K, et al. Histone deacetylase inhibitor reduces monocyte adhesion to endothelium through the suppression of vascular cell adhesion molecule-1 expression. Arterioscler Thromb Vasc Biol. 2006;26(12):2652–2659. doi: 10.1161/01.ATV.0000247247.89787.e7. [DOI] [PubMed] [Google Scholar]
- Jaattela M, Wissing D, Kokholm K, et al. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 1998;17(21):6124–6134. doi: 10.1093/emboj/17.21.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WK, Marks PA. Drug insight: Histone deacetylase inhibitors – development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nal Clin Pract Oncol. 2005;2(3):150–157. doi: 10.1038/ncponc0106. [DOI] [PubMed] [Google Scholar]
- Kiernan R, Bres V, Ng RW, et al. Post-activation turn-off of NF-kappaB-dependent transcription is regulated by acetylation of p65. J Biol Chem. 2003;278(4):2758–2766. doi: 10.1074/jbc.M209572200. [DOI] [PubMed] [Google Scholar]
- Kim AJ, Shi Y, Austin RC, et al. Valproate protects cells from ER stress-induced lipid accumulation and apoptosis by inhibiting glycogen synthase kinase-3. J Cell Sci. 2005;118:89–99. doi: 10.1242/jcs.01562. [DOI] [PubMed] [Google Scholar]
- Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J Pharmacol Exp Ther. 2008;324:160–169. doi: 10.1124/jpet.107.125773. [DOI] [PubMed] [Google Scholar]
- Kishimoto H, Hamada K, Saunders M, Backman S, Sasaki T, Nakano T, et al. Physiological functions of Pten in mouse tissues. Cell Struct Funct. 2003;28:11–21. doi: 10.1247/csf.28.11. [DOI] [PubMed] [Google Scholar]
- Kochenek AR, Fukudome EY, Eleanor JS, et al. Pharmacological resuscitation attenuates MAP kinase pathway activation and pulmonary inflammation following hemorrhagic shock in rodent model. Oral presentation at the annual meeting of the American college of surgeons; October 2010.2010. [Google Scholar]
- Kramer OH, Gottlicher M, Heinzel T. Histone deacetylase as a therapeutic target. Trends Endocrinol Metab. 2001;12(7):294–300. doi: 10.1016/s1043-2760(01)00438-6. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy G, Kelley J, Yerra L, Smith JK, Chi DS. Human endothelium as a source of multifunctional cytokines: molecular regulation and possible role in human disease. J Interferon Cytokine Res. 1999;19(2):91–104. doi: 10.1089/107999099314234. [DOI] [PubMed] [Google Scholar]
- Krivoruchko A, Storey KB. Epigenetics in anoxia tolerance: a role for histone deacetylase. Mol Cell Biochem. 2010;342(1–2):151–161. doi: 10.1007/s11010-010-0479-5. [DOI] [PubMed] [Google Scholar]
- Kucharska A, Rushworth LK, Staples C, et al. Regulation of the inducible nuclear dual-specificity phosphatase DUSP5 by ERK MAPK. Cell Signal. 2009;21(12):1794–1805. doi: 10.1016/j.cellsig.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27(32):5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- Lee JO, Yang H, Georgescu MM, et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- Leoni F, Fossati G, Lewis EC, et al. The histone deacetylase inhibitor ITF2357 reduction of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Mol Med. 2005;11:1–15. doi: 10.2119/2006-00005.Dinarello. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu B, Sailhamer EA, Yuan Z, Shults C, Velmahos GC, deMoya M, Shuja F, Butt MU, Alam HB. Cell protective mechanism of valproic acid in lethal hemorrhagic shock. Surgery. 2008a;144(2):217–224. doi: 10.1016/j.surg.2008.03.037. [DOI] [PubMed] [Google Scholar]
- Li Y, Yuan Z, Liu B, Sailhamer EA, Shults C, Velmahos GC, Demoya M, Alam HB. Prevention of hypoxia-induced neuronal apoptosis through histone deacetylase inhibition. J Trauma. 2008b;64(4):863–870. doi: 10.1097/TA.0b013e318166b822. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu B, Zhao H, Sailhamer EA, Fukudome EY, Zhang X, Kheirbek T, Finkelstein RA, Velmahos GC, deMoya CA, Hales RA, Alam HB. Protective effect of suberoylanilide hydroxamic acid against LPS-induced septic shock in rodents. Shock. 2009;32(5):517–523. doi: 10.1097/SHK.0b013e3181a44c79. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu B, Fukudome EY, Kochanek AR, Finkelstein R, Chong W, Jin G, Lu J, deMoya M, Velmahos GC, Alam HB. Survival lethal septic shock without fluid resuscitation in a rodent model. Surgery. 2010a;148(2):246–254. doi: 10.1016/j.surg.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu B, Dillon S, Liu B, et al. Identification of a novel potential biomarker in a model of hemorrhagic shock and valproic acid treatment. J Surg Res. 2010b;159(1):474–481. doi: 10.1016/j.jss.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Lillig CH, Holmgren A. Thioredoxin and related molecules – from biology to health and disease. Antioxid Redox Signal. 2007;9(1):25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- Lin T, Alam HB, Chen H, Britten-Webb J, Rhee P, Kirkpatrick J, Koustova E. Cardiac histones are substrates of histone deacetylase activity in hemorrhagic shock and resuscitation. Surgery. 2006;139:365–376. doi: 10.1016/j.surg.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Lin T, Chen H, Koustova E, Sailhamer EA, et al. Histone deacetylase as therapeutic target in a rodent model of hemorrhagic shock: effect of different resuscitation strategies on lung and liver. Surgery. 2007;141(6):784–794. doi: 10.1016/j.surg.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Lomas-Niera J, Chung CS, Grutkoski PS, et al. Divergent roles of murine neutrophil chemokines in hemorrhage induced priming for acute lung injury. Cytokine. 2005a;31:169–179. doi: 10.1016/j.cyto.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Lomas-Niera JL, Perl M, Chung C, et al. Shock and hemorrhage: an overview of animal models. Shock. 2005b;24(Suppl 1):33–39. doi: 10.1097/01.shk.0000191411.48719.ab. [DOI] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Mai A, Massa S, Pezzi R, et al. Discovery of (aryloxopropenyl) pyrrolyl hydroxyamides as selective inhibitors of class IIa histone deacetylase homologue HD1-A. J Med Chem. 2003;46(23):4826–4829. doi: 10.1021/jm034167p. [DOI] [PubMed] [Google Scholar]
- Marinova Z, Ren M, Wendland JR, Leng Y, Liang MH, Yasuda S, Leeds P, Chuang DM. Valproic acid induces functional heat-shock protein 70 via Class I histone deacetylase inhibition in cortical neurons: a potential role of Sp1 acetylation. J Neurochem. 2009;111(4):976–987. doi: 10.1111/j.1471-4159.2009.06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks PA, Dokmanovic M. Histone deacetylase inhibitors: discovery and development as anticancer agents. Expert Opin Investig Drugs. 2005;14:1497–1511. doi: 10.1517/13543784.14.12.1497. [DOI] [PubMed] [Google Scholar]
- Marumo T, Hishikawa K, Yoshikawa M, et al. Epigenetic regulation of BMP7 in the regenerative response to ischemia. J Am Soc Nephrol. 2008;19:1311–1320. doi: 10.1681/ASN.2007091040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCampbell A, Taye AA, Whitty L, et al. Histone deacetylase inhibitors reduce polyglutamine toxicity. Proc Natl Acad Sci USA. 2001;98:15179–15184. doi: 10.1073/pnas.261400698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriin AB, Yaglom JA, Gabai VL, et al. Protein-damaging stresses activate c-Jun N-terminal kinase via inhibition of its dephosphorylation: a novel pathway controlled by HSP72. Mol Cell Biol. 1999;19(4):2547–2555. doi: 10.1128/mcb.19.4.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollen KP, Levy RM, Prince JM, et al. Systemic inflammation and end organ damage following trauma involves functional TLR4 signaling in both bone marrow-derived cells and parenchymal cells. J Leukoc Biol. 2008;83(1):80–88. doi: 10.1189/jlb.0407201. [DOI] [PubMed] [Google Scholar]
- Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res. 2005;65(21):9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, et al. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20(19):7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TJ, Paterson HM, Mannick JA, Lederer JA. Injury, sepsis, and the regulation of toll-like receptor responses. J Leukoc Biol. 2004;75(3):400–407. doi: 10.1189/jlb.0503233. [DOI] [PubMed] [Google Scholar]
- Okumura K, Mendoza M, Bachoo RM, et al. PCAF modulates PTEN activity. J Biol Chem. 2006;281:26562–26568. doi: 10.1074/jbc.M605391200. [DOI] [PubMed] [Google Scholar]
- Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Overhaus M, Toegel S, Bauer AJ. Interaction of hemorrhagic shock and subsequent polymicrobial sepsis on gastrointestinal motility. Shock. 2009;31(4):382–389. doi: 10.1097/SHK.0b013e3181862ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Cho SG, Kim CK, et al. Heat shock protein hsp72 is a negative regulator of apoptosis signal-regulating kinase 1. Mol Cell Biol. 2002;22(22):7721–7730. doi: 10.1128/MCB.22.22.7721-7730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitzman AB, Billiar TR, Harbrecht BG, et al. Hemorrhagic shock. Curr Probl Surg. 1995;32(11):925–1002. doi: 10.1016/s0011-3840(05)80008-5. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signaling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8(1):49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Petri S, Kiaei M, Kipiani K, et al. Additive neuroprotective effects of a histone deacetylase inhibitor and a catalytic antioxidant in a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2006;22:40–49. doi: 10.1016/j.nbd.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol. 2008;180:6439–6446. doi: 10.4049/jimmunol.180.10.6439. [DOI] [PubMed] [Google Scholar]
- Ping P, Murphy E. Role of p38 mitogen-activated protein kinases in preconditioning: a detrimental factor or a protective kinase? Circ Res. 2000;86(9):989–997. doi: 10.1161/01.res.86.9.921. [DOI] [PubMed] [Google Scholar]
- Quivy V, Adam E, Collette Y, et al. Synergistic activation of human immunodeficiency virus type 1 promoter activity by NF-kappaB and inhibitors of deacetylases: potential perspectives for the development of therapeutic strategies. J Virol. 2002;76(21):11091–11103. doi: 10.1128/JVI.76.21.11091-11103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana MW, Singh G, Wang P, et al. Protective effects of preheparinization on the microvasculature during and after hemorrhagic shock. J Trauma. 1992;32:420–426. doi: 10.1097/00005373-199204000-00003. [DOI] [PubMed] [Google Scholar]
- Roger TH, Babensee JE. Altered adherent leukocyte profile on biomaterials in toll-like receptor 4 deficient mice. Biomaterials. 2010;31(4):594–601. doi: 10.1016/j.biomaterials.2009.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Lee J, Plpfsson BA, et al. Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via a Sp1-dependent pathway. Proc Nalt Acad Sci USA. 2003;100(7):4281–4286. doi: 10.1073/pnas.0737363100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Smith K, Camelo SI, et al. Sodium phenylbutyrate prolongs survival and regulates expression of antiapoptotic genes in transgenic amyotrophic lateral sclerosis. J Neurochem. 2005;93:1087–1098. doi: 10.1111/j.1471-4159.2005.03077.x. [DOI] [PubMed] [Google Scholar]
- Saccani S, Pantano S, Natoli G. p38-depedent marking of inflammatory genes for increased NF-kB recruitment. Nat Immunol. 2002;3(1):69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- Saha RN, Pahan K. HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell Death Differ. 2006;13:539–550. doi: 10.1038/sj.cdd.4401769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailhamer ES, Li Y, Smith EJ, et al. Acetylation: a novel method for modulation of the immune response following trauma/hemorrhage and inflammatory second hit in animals and humans. Surgery. 2008;144(2):204–216. doi: 10.1016/j.surg.2008.03.034. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–26026. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Srinivasula SM, Balkir L, et al. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- Saltiel AR. Putting the brakes on insulin signaling. N Engl J Med. 2003;349(26):2560–2562. doi: 10.1056/NEJMcibr031668. [DOI] [PubMed] [Google Scholar]
- Schwartzbauer G, Robbins J. The tumor suppressor gene PTEN can regulate cardiac hypertrophy and survival. J Biol Chem. 2001;276:35786–35793. doi: 10.1074/jbc.M102479200. [DOI] [PubMed] [Google Scholar]
- Shih HC, Wei YH, Lee CH. Magnolol alters cytokine response after hemorrhagic shock and increases survival in subsequent intraabdominal sepsis in rats. Shock. 2003;20(3):264–268. doi: 10.1097/00024382-200309000-00011. [DOI] [PubMed] [Google Scholar]
- Shuja F, Tabbara M, Li Y, Liu B, Butt MU, Velmahos GC, deMoya M, Alam H. Profound hypothermia decreases cardiac apoptosis through Akt survival pathway. J Am Coll Surg. 2009;209:89–99. doi: 10.1016/j.jamcollsurg.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Shults C, Sailhamer EA, Li Y, Liu B, Tabbara M, Butt MU, Shuja F, Demoya M, Velmahos G, Alam HB. Surviving blood loss without fluid resuscitation. J Trauma. 2008;64(3):629–638. doi: 10.1097/TA.0b013e3181650ff3. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Stankiewicz AR, Lachapelle G, Foo CP, et al. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280(46):38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- Steffan JS, Bodai L, Pallos J, et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature. 2001;413:739–743. doi: 10.1038/35099568. [DOI] [PubMed] [Google Scholar]
- Stiles B, Groszer M, Wang S, et al. PTENless means more. Dev Biol. 2004;273:175–184. doi: 10.1016/j.ydbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- St-pierre J, Drori S, Uldry M, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Sugars KL, Rubinsztein DC. Transcriptional abnormalities in Huntington disease. Trends Genet. 2003;19(5):233–238. doi: 10.1016/S0168-9525(03)00074-X. [DOI] [PubMed] [Google Scholar]
- Suuronen T, Ojala J, Hyttinen JM, et al. Regulation of ER alpha signaling pathway in neuronal HN10 cells: role of protein acetylation and Hsp90. Neurochem Res. 2008;33(9):1768–1775. doi: 10.1007/s11064-008-9622-z. [DOI] [PubMed] [Google Scholar]
- Suzuli T. Explorative study on isoform-selective histone deacetylase inhibitors. Chem Pharm Bull. 2009;57(9):897–906. doi: 10.1248/cpb.57.897. [DOI] [PubMed] [Google Scholar]
- Tamguney T, Strokoe D. New insight into PTEN. J Cell Sci. 2007;120(Pt23):4071–4079. doi: 10.1242/jcs.015230. [DOI] [PubMed] [Google Scholar]
- Thomson S, Clayton AL, Hazzalin CA, et al. The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. EMBO J. 1999;18(17):4779–4793. doi: 10.1093/emboj/18.17.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuijls G, de Haan JJ, Derikx JP, et al. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock. 2009;31:164–169. doi: 10.1097/SHK.0b013e31817fc310. [DOI] [PubMed] [Google Scholar]
- Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009;85(6):905–910. doi: 10.1189/jlb.0109005. [DOI] [PubMed] [Google Scholar]
- Ungerstedt JS, Sowa Y, Xu WS, et al. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Pro Natl Acad Sci USA. 2005;102(3):673–678. doi: 10.1073/pnas.0408732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamani L, Lyengar S. Tumor necrosis factor alpha polymorphism in heart failure/cardiomyopathy. Congest Heart Fail. 2004;10(6):289–292. doi: 10.1111/j.1527-5299.2004.02020.x. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W, De Bosscher K, Boone E, et al. The nuclear factor-kappaB engages CBP/p300 and histone acetyltransferase activity for transcriptional activation of the interleukin-6 gene promoter. J Biol Chem. 1999;274(45):32091–32098. doi: 10.1074/jbc.274.45.32091. [DOI] [PubMed] [Google Scholar]
- Vidali G, Gershey E, Allfrey VG. Chemical studies of histone acetylation. The distribution of e-N-acetyllysine in calf thymus histones. J Biol Chem. 1968;243:6361–6366. [PubMed] [Google Scholar]
- Voelter-Mahlknecht S, Ho AD, Mahlknecht U. Chromasomal organization and localization of novel class IV human histone deacetylase 11 gene. Int J Mol Med. 2005;16(4):589–598. [PubMed] [Google Scholar]
- Volloch V, Gabai VL, Rits S, et al. ATPase activity of the heat shock protein hsp72 is dispensable for its effects on dephosphorylation of stress kinase JNK and on heat-induced apoptosis. FEBS Lett. 1999;461(1–2):73–76. doi: 10.1016/s0014-5793(99)01428-3. [DOI] [PubMed] [Google Scholar]
- Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009;280(2):168–176. doi: 10.1016/j.canlet.2008.10.047. [DOI] [PubMed] [Google Scholar]
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Winter-Vann AM, Johnson GL. Integrated activation of MAP3Ks balances cell fate in response to stress. J Cell Biochem. 2007;102(4):848–858. doi: 10.1002/jcb.21522. [DOI] [PubMed] [Google Scholar]
- Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occluding perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YX, Ayala A, Chaudry IH. Prolonged immunodepression after trauma and hemorrhagic shock. J Trauma. 1998;44:335–341. doi: 10.1097/00005373-199802000-00018. [DOI] [PubMed] [Google Scholar]
- Yao XH, Nyomba BL. Hepatic resistance induced by prenatal alcohol exposure is associated with reduced PTEN and TRB3 acetylation in adult rat offspring. Am J Physiol Regul Integr Comp Physiol. 2008;294(6):R1797–R1806. doi: 10.1152/ajpregu.00804.2007. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- Yildirim F, Gertz K, Kronenberg G, et al. Inhibition of histone deacetylation protects wildtype but not gelsolin-deficient mice from ischemic brain injury. Exp Neurol. 2008;210(2):531–542. doi: 10.1016/j.expneurol.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Zacharias N, Sailhamer EA, Li Y, Liu B, Butt MU, Shuja F, Velmahos GC, de Moya M, Alam HB, et al. Histone deacetylase inhibitors prevent apoptosis following lethal hemorrhagic shock in rodent kidney cells. [29 October 2010];Resuscitation. 2010 doi: 10.1016/j.resuscitation.2010.09.469. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Ley K. Neutrophil adhesion and activation under flow. Microcirculation. 2009;16:31–42. doi: 10.1080/10739680802350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wan J, Jiang R, et al. Protective effects of trichostatin A on liver injury in septic mice. Hepatol Res. 2009;39:931–938. doi: 10.1111/j.1872-034X.2009.00521.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Jin S, Wang C, et al. Histone deacetylase inhibitors attenuate acute lung injury during cecal ligation and puncture-induced polymicrobial sepsis. World J Surg. 2010;34(7):1676–1683. doi: 10.1007/s00268-010-0493-5. [DOI] [PubMed] [Google Scholar]
- Zhao TC, Cheng G, Zhang LX, et al. Inhibition of histone deacetylases triggers pharmacologic preconditioning effects against myocardial ischemic injury. Cardiovasc Res. 2007;76(3):473–481. doi: 10.1016/j.cardiores.2007.08.010. [DOI] [PubMed] [Google Scholar]