Abstract
Background
Despite global efforts to improve the treatment of sepsis, it remains a leading cause of morbidity and mortality in intensive care units. We have previously shown that suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor, markedly improves survival in a murine model of lipopolysaccharide (LPS)-induced shock. SAHA has anti-inflammatory properties that have not been fully characterized. The liver plays an important role in the production of acute phase reactants involved in the inflammatory cascade and is also one of the major organs that can become dysfunctional in septic shock. The purpose of this study was to assess the effect of SAHA treatment on MAP kinases and associated inflammatory markers in murine liver after LPS-induced injury.
Methods
C57B1/6J mice were randomly divided into three groups: (A) experimental-given intraperitoneal (i.p.) SAHA (50 mg/kg) in dimethyl sulfoxide (DMSO) vehicle solution (n =12); (B) control-given vehicle only (n = 12), and; (C) sham-given no treatment (n = 7). Two hours later, experimental and control mice were injected with LPS (20 mg/kg, i.p.) and experimental mice received a second dose of SAHA. Livers were harvested at 3, 24, and 48 h for analysis of inflammatory markers using Western Blot, Polymerase Chain Reaction (PCR), and Enzyme-Linked Immunosorbent Assay (ELISA) techniques.
Results
After 3 h, the livers of animals treated with SAHA showed significantly (P < 0.05) decreased expression of the pro-inflammatory MAP kinases phosphorylated p38, phosphorylated ERK, myeloperoxidase and interleukin-6, and increased levels of the anti-inflammatory interleukin-10 compared with controls. Phospho-p38 expression remained low in the SAHA treated groups at 24 and 48 h.
Conclusion
Administration of SAHA is associated with attenuation of MAPK activation and alteration of inflammatory and anti-inflammatory markers in murine liver after a lethal LPS insult. The suppression of MAPK activity is rapid (within 3 h), and is sustained for up to 48 h post-treatment. These results may in part account for the improvement in survival shown in this model.
Keywords: septic shock, acetylation, LPS, suberoylanilide hydroxamic acid, histone, MAP kinase, p38, ERK, IL-10, IL-6, liver
INTRODUCTION
Various advances in medical treatment and implementation of protocols have been instrumental in decreasing death from sepsis. However, mortality from sepsis is still about 25% [1]. The progression of infection to septic shock begins with the release of inflammatory mediators at the local site of microbe invasion. This induces the migration of white blood cells and platelets to the infection site, and contributes to platelet aggregation, damage to endothelium, and increased microvascular permeability. Blood flow is also reduced and this can lead to ischemia-reperfusion injury. These physiologic processes constitute what is known as the systemic inflammatory response syndrome (SIRS), which can lead to multiple organ dysfunction syndrome (MODS). Many of the body’s reactions to infection with gram-negative organisms are due to lipopolysaccharide (LPS), a component of the outer bacterial cell wall membrane, also referred to as endotoxin. It can induce septic shock physiology [2] and has been used extensively to produce septic shock in laboratory models. LPS activates Toll-like receptors (TLR) and induces phosphorylation of signaling proteins such as p38 mitogen activated protein kinase (p38 MAPK) and extracellular signal-regulated kinase (ERK), which leads to gene expression and transcription of pro-inflammatory proteins [3–6]. Animal studies have demonstrated that p38 MAPK inhibitors improve survival in sepsis [7–9]. In addition, p38 inhibitors have been shown to decrease TNF-α in human whole blood stimulated by LPS, and also decrease TNF-α stimulation of other inflammatory cytokines such as IL-1β and IL-6 [4].
The basic unit of chromatin is a nucleosome which is made up of DNA wrapped around an octamer of core histone proteins. Histones are acetylated by histone acetyltransferases (HATs), which is one of the steps that allows for transcription of genes [10]. The activity of HATs is balanced by histone deacetylases (HDACs), which remove the acetyl group from the histones and impair gene transcription [11]. Histone deacetylase inhibitors (HDACI) prevent the removal of the acetyl groups and typically up-regulate gene expression. Suberoylanilide hydroxamic acid (SAHA) is a hybrid polar molecule that contains hydroxamic acid and specifically inhibits the activity of histone deacetylase (HDAC), thus enhancing gene transcription.
It has been shown that non-histone proteins such as transcription factors and cell-signaling proteins can also be acetylated and deacetylated, and that their activity is affected by their acetylation status [12,13]. HDACIs not only up-regulate gene expression, but can down-regulate some genes and affect the expression of cytokines due to their effect on non-histone proteins [12, 13]. SAHA has been shown to reduce the production of pro-inflammatory cytokines in vivo and in vitro [14, 15]. We have recently shown that SAHA treatment dramatically enhances survival (87% versus 0%; P < 0.05) in a rodent model of LPS-induced septic shock while restoring acetylation of several histone proteins. It decreases levels of the pro-inflammatory TNF-α and Interleukin-1 in lung tissue and decreases neutrophil infiltration into lung and spleen [14].
Liver plays an important role in the production of acute phase reactants involved in the inflammatory cascade and is also one of the major organs that can become dysfunctional in the setting of septic shock. In addition, several studies have shown that LPS activates p38 in liver [6, 16, 17]. The purpose of this study was to assess the effect of LPS-induced septic shock on molecules in the toll-like receptor (TLR) pathway in murine liver and to determine how SAHA might alter inflammation, thus contributing to the pro-survival phenotype seen in our previous study.
MATERIALS AND METHODS
Materials
LPS from S. typhosa and dimethyl sulfoxide (DMSO) were purchased from the Sigma Chemical Co. (St. Louis, MO). Suberoylanilide hydroxamic acid (SAHA) was purchased from Biomol International (Plymouth Meeting, PA). Primary antibodies against phospho-p38, p38, phospho-ERK, and ERK were purchased from Cell Signaling Technology, Inc., Danvers, MA. Anti-actin antibody was purchased from Sigma Chemical Co. Anti-mouse and anti-rabbit IgG secondary antibodies were purchased from Amersham Biosciences (Piscataway, NJ). Protease Inhibitor Cocktail II was purchased from Calbiochem (San Diego, CA). All other chemicals in this study were of analytical grade and obtained from Sigma Chemical Co. if not mentioned otherwise.
Animals
All the research was conducted in compliance with the Animal Welfare Act, and was approved by the Institutional Animal Care and Use Committee. Male C57B1/6J mice (6–8 wk) weighing 20–25 g were purchased from Jackson Labs (Bar Harbor, ME). All animals were housed in plastic cages and had access to chow and water throughout the experiment. The animals were kept at room temperature (24 ±2 °C) and exposed to alternative cycles of 12 h of light and darkness.
Experimental Protocols
Mice were randomly divided into three groups: (A) experimental, given intraperitoneal (i.p.) SAHA (50 mg/kg) in dimethyl sulfoxide (DMSO) vehicle (n = 12); (B) control (n = 12) given vehicle only; and (C) sham (n = 7) given no injection. Two hours later, experimental and control mice were injected with high dose LPS (20 mg/kg, i.p.), and experimental mice received a second dose of SAHA. Livers were harvested at 3, 24, and 48 h for analysis.
Subcellular Protein Fractionation
Wet weight 50 mg of fresh liver tissue was immersed in extraction buffer and homogenized using the Polytron PT 1200 E Dispersing and Mixing Machine, (Kinematica AG, Switzerland). Subcellular proteome extraction kit (Calbiochem, San Diego, CA) was used according to instructions. Protease inhibitor and phosphatase inhibitor cocktails were added to prevent protein and phosphate degradation, respectively. The supernatants were obtained through different extraction procedures with sequential incubation and centrifugation as follows: fraction I (F1), cytosolic protein extract; fraction II (F2), membrane/organelle protein extract; fraction III (F3), nucleic protein extract; and fraction IV (F4), cytoskeletal matrix protein extract. Total protein concentration in each fraction was determined by Bradford method (Bio-Rad Laboratories, Hercules, CA). For verification, fractionation was performed in quadruplicate and verified by repeat Western blot assays.
Western Blot Analysis
Proteins (about 100 μg per lane) were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12% polyacrylamide gels and transferred onto nitrocellulose membranes (Bio-Rad Laboratories). The membranes were blocked in 0.05% PBS-Tween (PBST) containing 5% milk (Bio-Rad Laboratories) and then incubated with the primary antibody at 4 °C overnight. The primary antibody was detected by incubation with horseradish peroxidase-coupled second antibody (1:3,000 in PBST with 5% milk) at room temperature for 2 h. The chemiluminescence detection was performed by using Western Lighting Chemiluminescence Reagent Plus (PerkinElmer LAS, Inc., Boston, MA). Films were developed and quantitative analysis of bands carried out using VersaDoc Imaging System (Bio-Rad Laboratories). All assays were performed in quadruplicate.
Reverse Transcribed-Polymerase Chain Reaction (RT-PCR)
Total RNA was prepared from the liver of mouse tissue in RNA later solution using Rneasy Mini Kit (Qiagen, Valencia, CA). The mRNA was reversed transcribed into cDNA using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA). Primer sets of IL-10 and IL-6, were used, and 0.15 μg of total RNA was extracted from the liver. Mouse Glyceraldehyde 3 phosphate de-hydrogenase (GAPDH) served as an internal control. The expression levels of IL-10, IL-6, and internal control GAPDH were detected from the same samples using designed primers. Sequences of the primer pair for amplification of each gene were as follows: 5′-CATCACTGCCACCCAGAAGACTGTGGA-3′ and 5′-TACTCCTTGGAGGCCATGTAGGCCATG-3′ (for GAPDH gene); 5′-GTTCTCTGGGAAATCGTGGA-3′ and 5′-TGTACTCCAGGTAGCTATGG-3′ (for IL-6 gene)18 and 5′-CTGCTATGCTGCCTGCTCTTAC-3′ and 5′-GTAGACACCTTGGTCTTGGAGC-3′ (for IL-10 gene). All of the primers were synthesized at Invitrogen (Carlsbad, CA). For the GAPDH and IL-10 genes, amplifications were conducted with 30 cycles (45 s at 94 °C, 45 s at 60 °C, 1 min at 72 °C). For the Il-6 gene, the amplifications were conducted with 35 cycles (15 s at 94 °C, 30 s at 60 °C, 1 min at 72 °C) [18]. PCR products were separated on 2% agarose gel. Band density was quantified using the VersaDoc Imaging System (Bio-Rad Laboratories). All assays were performed in triplicates.
Measurement of Myeloperoxidase (MPO) Activity
To estimate the extent of neutrophil infiltration and macrophage activation in the liver MPO was quantified according to the manufacturer’s instructions. (Mouse MPO ELISA Kit; Cell Sciences, Canton, MA). Mouse liver (50 mg) from sham, LPS, and SAHA+LPS groups was removed and homogenized in 1 mL of a lysis buffer (200 mM NaCl, 5 mM EDTA, 10 mM Tris, 10% glycine [vol/vol], 1 mM phenyl-methylsulfonyl fluoride, 1 μg/mL leupeptide, 28 μg/mL aprotinin). The samples were centrifuged twice at 1500 g at 4 °C for 15 min and supernatants were analyzed for MPO levels by sandwich ELISA. Values represent mean ± SEM (n = 3).
Statistical Analysis
Statistical differences were determined by Student’s t-tests and ANOVA for two groups and multiple groups comparisons, respectively (SPSS statistical software package; SPSS Inc., Chicago, Illinois). Differences were considered to be statistically significant when P values were ≤0.05.
RESULTS
SAHA Decreases the Activation of MAP Kinase In Vivo
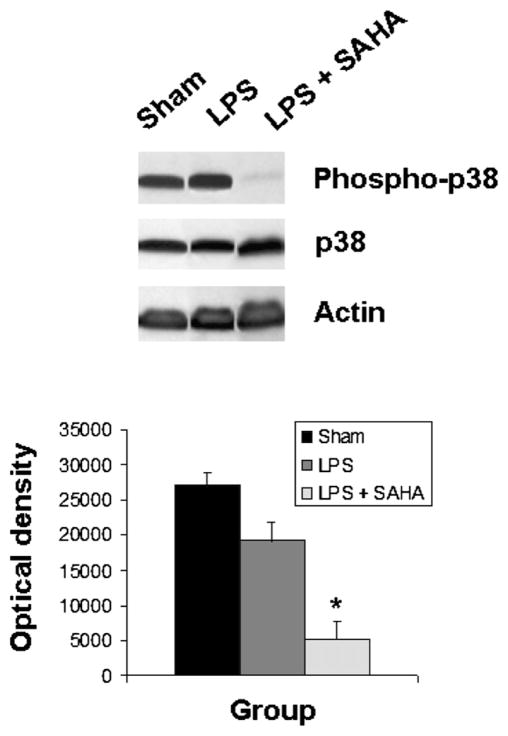
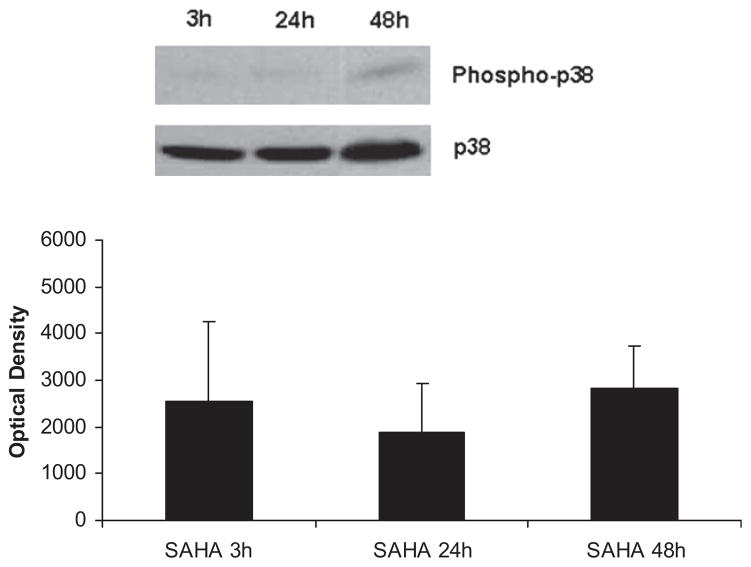
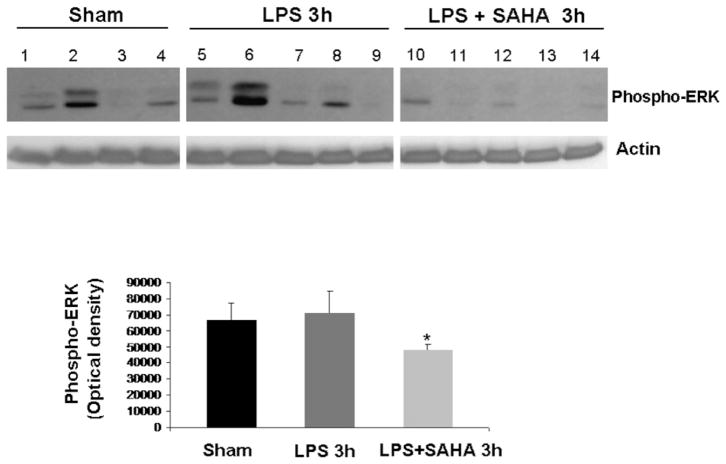
The levels of phospho-p38 were significantly decreased by SAHA treatment at 3 h (Fig. 1), and this effect persisted for up to 48 h post-insult (Fig. 2). We also examined another of the MAP kinase proteins, the extra-cellular signal regulated kinase (ERK) that is known to be activated in the stress response and affect mitosis, meiosis, and apoptosis. Phosphorylation of ERK was also significantly decreased in the SAHA group (Fig. 3). These results indicate that treatment with SAHA attenuated activation of p38 and ERK.
FIG. 1.
SAHA reduces the expression of phospho-p38. The cytosol fraction from the livers of mice treated with or without SAHA at 3 h after LPS insult was subjected to western blot with anti-phospho-p38, p38, and actin antibodies. Upper panel shows a representative western blot. Protein bands of phospho-p38 were quantified by densitometry and expressed as mean ± SD, n = 3. The asterisk indicates that a value significantly differs from the LPS group (P < 0.004). There was no difference between groups in the expression of non-phosphorylated p38.
FIG. 2.
SAHA attenuates p38 activation for up to 48 h. The cytosol fraction from the livers of mice treated with SAHA at 3, 24, and 48 h after LPS insult was subjected to western blot with anti-phospho-p38 and p38 antibodies. Upper panel shows a representative western blot. The levels of phospho-p38 remained low in the SAHA treated group even 48 h after LPS and SAHA injection. There was no significant difference between phospho-p38 expression at 3, 24, or 48 h. Bars represent standard error (SE).
FIG. 3.
SAHA reduces the expression of phospho-ERK. The cytosol fraction from the livers of mice treated with (n = 5) or without SAHA (n = 5) at 3 h after LPS insult was subjected to western blot with anti-phospho-ERK and actin antibodies. Seven sham animals were used as comparison and are represented in the graph, though only four are shown in the Western blot. Protein bands of phospho-ERK were quantified by densitometry and expressed as mean ± SD. The asterisk indicates that the SAHA 3 h group significantly differs from the LPS 3h group (P < 0.006).
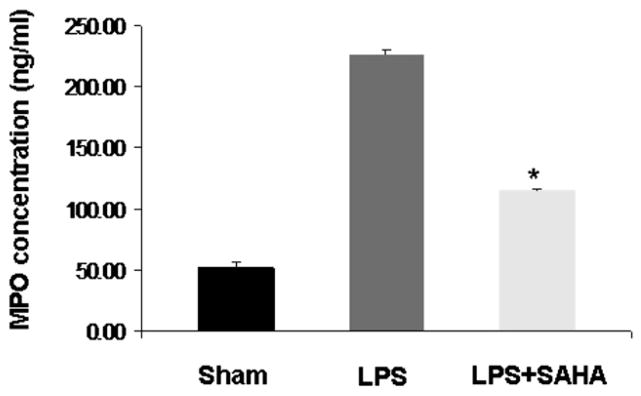
SAHA Decreases Myeloperoxidase in Liver Tissue During Sepsis
We measured MPO levels in liver tissue as a reflection of neutrophil and macrophage activity in the liver. As shown in Fig. 4, MPO concentration was markedly increased in the livers of mice who received LPS compared with sham mice. However, suberoylanilide hydroxamic acid treatment significantly decreased MPO levels after LPS insult (P < 0.05). These results suggest that administration of SAHA attenuates neutrophil and macrophage activity after LPS-induced septic shock.
FIG. 4.
SAHA decreases myeloperoxidase activity. Myeloperoxidase activity in liver tissues of mice treated with or without SAHA at 3 h after LPS injection was analyzed by quantifying MPO concentration. Values represent the mean ± SEM (n = 3). The asterisk indicates that a value significantly differs from the LPS group (P < 0.05).
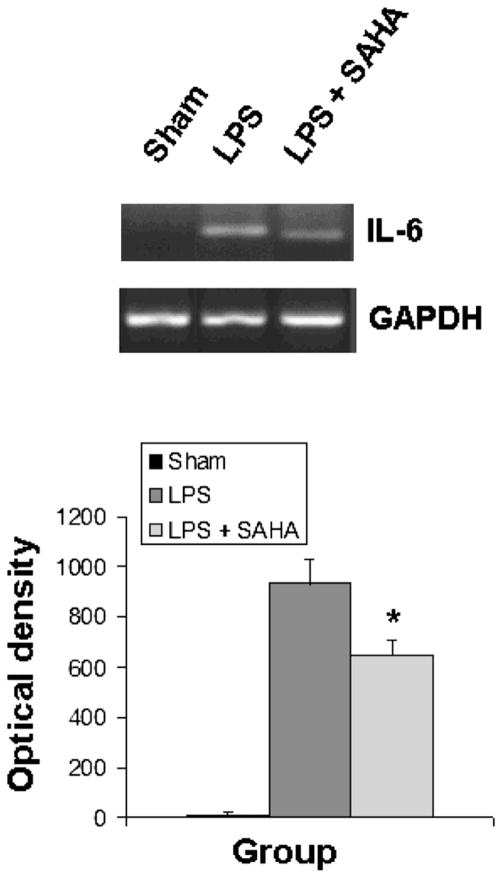
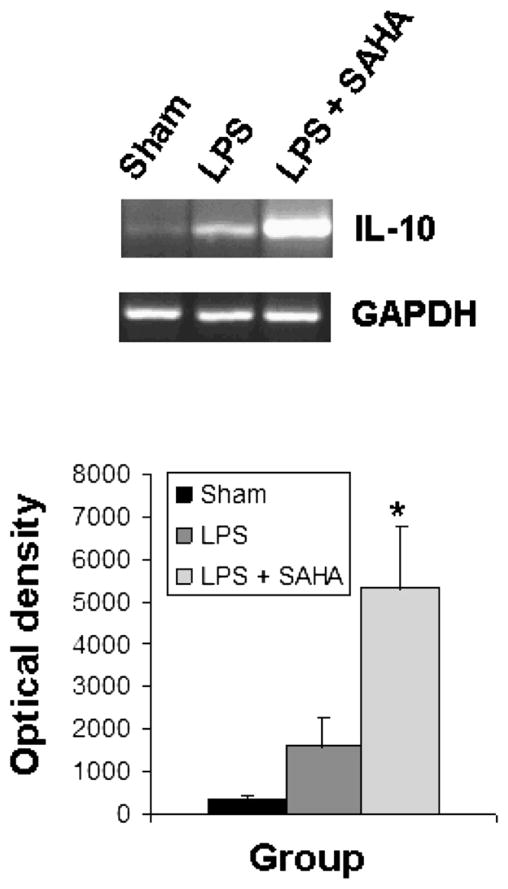
SAHA Inhibits IL-6 Gene Expression and Up-Regulates Il-10 Gene Expression in Liver Tissue In Vivo
The activation of TLRs and MAP kinases by endotoxin stimulates the release of cytokines, leading to sequestration and degranulation of neutrophils. As shown in Figs. 5 and 6, liver tissue from sham mice expressed very little IL-6 and IL-10. SAHA treatment significantly attenuated LPS-induced expression of IL-6 (Fig. 5) and up-regulated expression of the anti-inflammatory IL-10 gene (Fig. 6).
FIG. 5.
SAHA reduces the expression of IL-6. Total RNA from the livers of mice treated with or without SAHA at 3 h after LPS insult was subjected to RT-PCR for analysis of IL-6 gene expression with GAPDH as an internal control. Sham serves as animal control. DNA bands of IL-6 were quantified by densitometry and expressed as mean ±SD (n = 3). The asterisk indicates that a value significantly differs from the LPS group (p < 0.023).
FIG. 6.
SAHA reduces the expression of IL-10. Total RNA from the livers of mice treated with or without SAHA at 3 h after LPS insult was subjected to RT-PCR for analysis of IL-10 gene expression with GAPDH as an internal control. Sham serves as animal control. DNA bands of IL-10 were quantified by densitometry and expressed as mean ±SD (n = 3). The asterisk indicates that a value significantly differs from the LPS group (P < 0.033).
DISCUSSION
In this lethal model of LPS insult, we have shown that treatment with an HDACI improves survival while attenuating the activation of kinase signaling pathways and other inflammatory cascades. P38 belongs to a family of serine/threonine protein kinases with four different isoforms, of which the α isoform is the most studied [19]. It was first discovered in monocytes, where it was noted to be phosphorylated and activated by LPS [4]. In a positive feedback loop, p38 regulates expression of cytokines and is also activated in immune cells by cytokines [20]. It has been shown to be involved in the transcription, translation, and secretion of TNF-α and IL-1β and other macrophage chemokines [4, 5]. It also regulates the levels of IL-6 and IL-10 [21], and p38 knockout mice have diminished production of IL-6 as well as IL-1 [22].
Hsu and Wen have shown that LPS insult induces various kinases to different degrees; phospho-p38 (16-fold), extracellular signaling-regulated kinase (ERK) (12-fold), and c-Jun N-terminal kinases (JNK) (5-fold). In macrophages, the maximum concentration of activated p38 was noted 15 min post-LPS exposure, while the other two MAP kinases reached maximum concentration after 30 min [23]. Thus, p38 response was the earliest and most robust, which is very similar to what has been seen in our study. p38 inhibitors block production of IL-1, TNF-α, and IL-6 in vitro and in vivo [24]. The contribution of p38 activation to immune dysfunction in sepsis had been well studied by Song and colleagues who showed that p38 plays an important role in neutrophil activation and delayed in-vivo inhibition of p38 improved survival in their rodent model of cecal ligation and puncture [7]. Klintman et al. found that inhibiting p38 reduced LPS-stimulated increases in TNF-α, and CXC chemokines in murine liver while decreasing leukocyte infiltration, apoptosis, and preventing increases in serum liver enzymes [6].
C-jun NH2-terminal kinase (JNK) is a stress-activated protein kinase that and binds and phosphorylates C-Jun, a DNA binding protein. This increases transcriptional activity and controls many cytokine genes [20]. One might expect that SAHA would have an effect in suppressing the activation/phosphorylation of JNK in our model. However, we were unable to get adequate expression of either JNK or phospho-JNK. Further experimentation suggests that higher concentration of antibody and longer exposure may improve our ability to assess expression of these proteins. However, our data are not yet developed enough to draw any conclusions at this time. Although SAHA is a non-specific agent, it is possible that it affects p38 substantially more than either ERK or JNK. In one murine endotoxemia model, the histone deacetylase inhibitor KBH-A42 significantly decreased the phosphorylation of p38, but not ERK and JNK, and also inhibited TNF-α, IL-1β, IL-6, and iNOS [25]. An in vitro study of murine Kupffer cells showed that LPS stimulation increased the activation of p38, ERK, and JNK, with the activation of p38 and ERK being the most prominent. In addition, P38 inhibition resulted in decreased TNF-α and IL-10, whereas inhibition of ERK resulted in only decreased TNF-α with no effect on IL-10 [26].
It is interesting that a p38 inhibitor decreased levels of IL-10, whereas in our experiment, SAHA was associated with a decrease in p38, but an increase in IL-10. While specific inhibition of p38 and ERK has been shown to attenuate inflammation, and treatment with SAHA is associated with a reduction in their level of activation, SAHA seems to have some different and possibly broader effects. For instance, subsequent work we have done using this sepsis model has shown that SAHA has an effect on molecules upstream of p38, such as the MyD88 adaptor protein (manuscript in progress). By virtue of its action as a histone deacetylase inhibitor, SAHA can also alter transcription. Specific MAP kinase inhibitors would be less likely to have the same broad effect.
In our study, sham animals showed expression of phospho-p38 that was similar to the controls. This is not surprising because in order to harvest the tissue, sham animals underwent isoflurane anesthesia, laparotomy/sternotomy, cardiac puncture, and organ harvest. All of these insults could up-regulate inflammatory pathways, stimulate p38 MAP kinase kinase (p38 MAPKK), which then phosphorylates p38. However, SAHA pretreatment can hyperacetylate many proteins, including nonhistone proteins such as MAP kinase phosphatase-1 (MKP-1) that can prevent p38 MAPKK activation [14].
In this study, we have demonstrated that SAHA treatment decreases IL-6 gene expression. Interleukin-6 (IL-6) is a pleiotropic cytokine produced by lymphoid and non-lymphoid cells during the course of acute inflammatory reactions. It regulates various processes, including immune reactivity, acute phase response, inflammation, oncogenesis, and hematopoiesis, and it may also be linked to thrombosis in sepsis [27]. IL-6 can be a useful marker of proinflammatory cytokine activation because although its release is stimulated by tumor necrosis factor and interleukin-1 it persists in the plasma for much longer [27–29]. Endotoxin enhances IL-6 production in monocytes and fibroblasts [27], and injection of endotoxin in human volunteers results in peak plasma levels in 2 h [30]. IL-6 appears to be both a marker and a mediator of sepsis. Elevated IL-6 concentrations are found in the serum of patients with bacterial infection and sepsis [27, 31], and higher IL-6 levels have correlated with increased morbidity and mortality in a number of human studies [27, 32, 33]. Indeed, the decreased levels of IL-6 mRNA in the livers of the SAHA treated mice in this experiment are associated with their increased survival.
The other cytokine studied was IL-10, which is produced by macrophages, TH2 lymphocytes and epithelial cells. It has anti-inflammatory and immuno-suppressive properties, inhibiting gene expression, and production of T-cell and macrophage pro-inflammatory cytokines [34]. IL-10 inhibits most inducible inflammatory chemokines suppressing the production of IL-1α, IL-1β, TNF-α, FBK, IL-6, and IL-8 [34, 35]. Specifically, it has been demonstrated that increased expression of IL-10 by adiponectin, an anti-inflammatory adipokine, suppresses TNF-α production in liver Kupffer cells [36, 37]. In addition, it suppresses IL-12, IL-18, and interferon-gamma, thus playing a part in antagonizing the generation of the TH1 T cells [34]. It also reduces multiple granulocyte and macrophage stimulating and inflammatory proteins as well as the synthesis of nitric oxide, gelatinase and collagenase. Typically, sepsis delays neutrophil apoptosis, and it is hypothesized that one of the ways that IL-10 may limit the inflammatory response is by inducing apoptosis in granulocytes [35]. One well accepted model of sepsis is that it is a balance between the systemic inflammatory response syndrome (SIRS) and a compensatory anti-inflammatory response syndrome (CARS), of which IL-10 is thought to be a part.
While IL-10 clearly decreases the inflammatory response, its effect on outcome in sepsis has been variable. Animal septic shock models have demonstrated that endogenous and exogenously administered IL-10 can improve outcome [34]. In a model of cecal ligation and puncture, several authors have shown that IL-10 is protective [38–40]. Pretreatment with exogenous IL-10 improved survival, whereas an antibody to IL-10 decreased it [38, 40]. IL-10 knockout have an increased TNF-α response to LPS and are much more susceptible to LPS than wild-type mice, also suggesting a protective effect [41]. Alternatively, several studies found that survival and rodents’ ability to fight infection was improved when IL-10 was blocked [42–44], and high levels of endogenous Il-10 have been associated with increased mortality in sepsis patients [34, 45]. Oberholzer and colleagues propose that IL-10 given early may attenuate SIRS, but if given during CARS, it may overly suppress the immune system [34]. Thus, timing of IL-10 administration is an important variable in determining what cytokines may be inhibited [35]. For example, in human volunteers, several inflammatory cytokines and pathways were inhibited with pre-treatment with IL-10 [46, 47], but treatment post-septic insult resulted in attenuation of fewer cytokines. It is worth noting that IL-6, (which we found to be reduced in our model), was suppressed even when IL-10 was given post-insult [35]. Inducers of IL-10 synthesis include pro-inflammatory cytokines, such as TNF-α and IL-1 [34]. IL-10, in turn, can activate [48] or suppress [35, 49, 50] the p38 pathway. In our pretreatment model, the decrease in p38 and the altered transcription of the cytokine genes (decreased IL-6 and increased IL-10) may be a direct effect of the SAHA treatment, or a reflection of an overall attenuation of the inflammatory response.
Inflammation and infection have been shown to alter the HAT: HDAC ratio, resulting in histone hypoacetylation, whereas treatment with HDACIs results in normalization of histone acetylation, enhanced transcription of key survival genes, and reduced apoptotic and inflammatory pathways [51, 52]. This may help to explain why sham mice showed MAP kinase activation, but did not demonstrate changes in other markers such as IL-6, or IL-10. MAP kinases could have been activated in sham mice due to the stresses of sacrifice. However, because expression of IL-6 and IL-10 require time for transcription, changes in these levels would not likely be seen in a sham mouse immediately after sacrifice. The insult to the sham mice (i.e., sacrifice and harvest) was a sudden and immediate terminal event that may not have allowed time for transcription, whereas the LPS insult to control mice was not immediately terminal and may have allowed for expression of downstream proteins prior to death. It is well known that administration of SAHA rapidly (within 2 h) increases acetylation of histones [51, 53], and our previous work using this model of rodent sepsis has demonstrated that treatment with SAHA reversed LPS-related histone deacetylation in lung tissue [14]. In addition, we have previously shown that hemorrhage is associated with an imbalance between the enzymes histone acetyltransferase (HAT) and histone deacetylase (HDAC). This imbalance can be corrected by treatment with pharmacologic histone deacetylase inhibitors (HDACI), which increase the level of histone acetylation in heart, lung, and liver [54–56]. SAHA also reduces TNF-α levels after hemorrhagic shock and LPS “second hit,” and improves animal survival [51].
Our study has several limitations, which must be acknowledged. As this was a proof-of-concept study, we selected a well established and widely used model of LPS challenge. However, it does not replicate all the aspects of septic shock. We therefore intend to replicate these findings in a cecal ligation and puncture model in the near future. Another limitation is that the animals were treated with SAHA prior to receiving LPS. This is obviously not clinically realistic. We have previously shown that in models of hemorrhagic shock, post-treatment was equally or more effective than pretreatment as HDACIs are known to preferentially target the injured cells [56, 57]. More importantly, we have reproduced similar survival benefits when a single injection of SAHA was administered 2h following LPS insult (manuscript in preparation). For logistical reasons, we could not measure many other regulatory pathways and cytokines that are involved in septic shock. We are currently exploring a number of these pathways using chromatin immunoprecipitation, genomic DNA microarray, and high-throughput proteomic techniques. One of the inherent restrictions of using a lethal model is that very few untreated control mice survive the entire period of observation, which introduces a survival bias when comparing delayed tissue samples. Therefore, while the effect of SAHA on p38 activation was sustained at 24 and 48 h, we could not properly compare this to the control group of untreated mice, as there were fewer control mice surviving at these time-points. Analysis of these survivors would introduce a selection bias. In order to more extensively investigate the effects of SAHA over time, we intend to develop a sub-lethal model of septic shock, which would allow comparison of SAHA-treated mice and controls at later time-points. Our initial experiments looking at HDAC inhibitors in models of hemorrhagic shock used a lethal model, and we had the same issue of survival bias. However, our follow-up results using a sub-lethal model demonstrated that the effects of HDAC inhibitors on protein and gene expression were sustained over time compared with controls.
In summary, we have demonstrated that treatment with the histone deacetylase inhibitor suberoylanilide hydroxamic acid significantly alters inflammatory and anti-inflammatory markers in murine liver after a lethal LPS insult. SAHA decreases mRNA levels of pro-inflammatory cytokines, which may partially be due to modulation of the p38 and ERK MAP kinase signaling pathways.
Acknowledgments
This research was funded by a generous research endowment by the Polsky family. RAF acknowledges the financial support made possible by Dr. Natan Noviski and Dr. Elliot Melendez of the Department of Pediatric Critical Care Medicine at the MGH. Dr. Alam also acknowledges support by a National Institutes of Heath grant (R01GM084127).
Footnotes
Oral presentation at the 5th Annual Academic Surgical Congress, February 2010, San Antonio, Texas
References
- 1.Rivers EP, Coba V, Whitmill M. Early goal-directed therapy in severe sepsis and septic shock: A contemporary review of the literature. Curr Opin Anaesthesiol. 2008;21:128. doi: 10.1097/ACO.0b013e3282f4db7a. [DOI] [PubMed] [Google Scholar]
- 2.Takemura R, Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984;246(1 Pt 1):C1. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]
- 3.Kishore R, McMullen MR, Nagy LE. Stabilization of tumor necrosis factor alpha mRNA by chronic ethanol: Role of A + U-rich elements and p38 mitogen-activated protein kinase signaling pathway. J Biol Chem. 2001;276:41930. doi: 10.1074/jbc.M107181200. [DOI] [PubMed] [Google Scholar]
- 4.Lee MR, Dominguez C. MAP kinase p38 inhibitors: Clinical results and an intimate look at their interactions with p38alpha protein. Curr Med Chem. 2005;12:2979. doi: 10.2174/092986705774462914. [DOI] [PubMed] [Google Scholar]
- 5.Lee JC, Laydon JT, McDonnell PC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 6.Klintman D, Li X, Santen S, et al. p38 mitogen-activated protein kinase-dependent chemokine production, leukocyte recruitment, and hepatocellular apoptosis in endotoxemic liver injury. Ann Surg. 2005;242:830. doi: 10.1097/01.sla.0000189132.86878.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song GY, Chung CS, Chaudry IH, et al. MAPK p38 antagonism as a novel method of inhibiting lymphoid immune suppression in polymicrobial sepsis. Am J Physiol Cell Physiol. 2001;281:662. doi: 10.1152/ajpcell.2001.281.2.C662. [DOI] [PubMed] [Google Scholar]
- 8.Song GY, Chung CS, Jarrar D, et al. Evolution of an immune suppressive macrophage phenotype as a product of P38 MAPK activation in polymicrobial sepsis. Shock. 2001;15:42. doi: 10.1097/00024382-200115010-00007. [DOI] [PubMed] [Google Scholar]
- 9.Asaduzzaman M, Wang Y, Thorlacius H. Critical role of p38 mitogen-activated protein kinase signaling in septic lung injury. Crit Care Med. 2008;36:482. doi: 10.1097/01.CCM.0B013E31816204FA. [DOI] [PubMed] [Google Scholar]
- 10.Urnov FD, Wolffe AP. Chromatin remodeling and transcriptional activation: The cast (in order of appearance) Oncogene. 2001;20:2991. doi: 10.1038/sj.onc.1204323. [DOI] [PubMed] [Google Scholar]
- 11.Egger G, Liang G, Aparicio A, et al. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Kwon HJ, Lee YM, et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7:437. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- 13.Tong X, Yin L, Giardina C. Butyrate suppresses Cox-2 activation in colon cancer cells through HDAC inhibition. Biochem Biophys Res Commun. 2004;317:463. doi: 10.1016/j.bbrc.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Liu B, Zhao H, et al. Protective effect of suberoylanilide hydroxamic acid against LPS-induced septic shock in rodents. Shock. 2009;32:517. doi: 10.1097/SHK.0b013e3181a44c79. [DOI] [PubMed] [Google Scholar]
- 15.Leoni F, Zaliani A, Bertolini G, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci USA. 2002;99:2995. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahle MK, Overland G, Myhre AE, et al. The phosphatidylinositol 3-kinase/protein kinase B signaling pathway is activated by lipoteichoic acid and plays a role in Kupffer cell production of interleukin-6 (IL-6) and IL-10. Infect Immun. 2004;72:5704. doi: 10.1128/IAI.72.10.5704-5711.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oguro T, Takahashi Y, Ashino T, et al. Involvement of tumor necrosis factor alpha, rather than interleukin-1alpha/β or nitric oxides in the heme oxygenase-1 gene expression by lipopolysaccharide in the mouse liver. FEBS Lett. 2002;516:63. doi: 10.1016/s0014-5793(02)02502-4. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Lim KT. Chemopreventive effect of plant originated glycoprotein on colitis-mediated colorectal cancer in A/J mice. J Biomed Sci. 2008;15:111. doi: 10.1007/s11373-007-9196-9. [DOI] [PubMed] [Google Scholar]
- 19.Toledo-Pereyra LH. Critical role of p38 mitogen protein kinase in sepsis. Crit Care Med. 2008;36:636. doi: 10.1097/01.CCM.0000300543.76997.15. [DOI] [PubMed] [Google Scholar]
- 20.Johnson G. Signal transduction. Scaffolding proteins–more than meets the eye. Science. 2002;295:1249. doi: 10.1126/science.1069828. [DOI] [PubMed] [Google Scholar]
- 21.Foey AD, Parry SL, Williams LM, et al. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: Role of the p38 and p42/44 mitogen-activated protein kinases. J Immunol. 1998;160:920. [PubMed] [Google Scholar]
- 22.Allen M, Svensson L, Roach M, et al. Deficiency of the stress kinase p38alpha results in embryonic lethality: Characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J Exp Med. 2000;191:859. doi: 10.1084/jem.191.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem. 2002;277:22131. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Boehm J, Lee JC. p38 MAP kinases: Key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 25.Choi Y, Park SK, Kim HM, et al. Histone deacetylase inhibitor KBH-A42 inhibits cytokine production in RAW 264. 7 macrophage cells and in vivo endotoxemia model. Exp Mol Med. 2008;40:574. doi: 10.3858/emm.2008.40.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang J, Zhang Y, Ji S, et al. Kinetics of mitogen-activated protein kinase family in lipopolysaccharide-stimulated mouse Kupffer cells and their role in cytokine production. shock. 2002;18:336. doi: 10.1097/00024382-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Song M, Kellum JA. Interleukin-6. Crit Care Med. 2005;33(12 Suppl):S463. doi: 10.1097/01.ccm.0000186784.62662.a1. [DOI] [PubMed] [Google Scholar]
- 28.van der Poll T, van Deventer SJ. Cytokines and anticytokines in the pathogenesis of sepsis. Infect Dis Clin North Am. 1999;13:413. doi: 10.1016/s0891-5520(05)70083-0. [DOI] [PubMed] [Google Scholar]
- 29.Ventetuolo CE, Levy MM. Biomarkers: Diagnosis and risk assessment in sepsis. Clin Chest Med. 2008;29:591. doi: 10.1016/j.ccm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Fong Y, Tracey KJ, Moldawer LL, et al. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1β and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989;170:1627. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helfgott DC, Tatter SB, Santhanam U, et al. Multiple forms of IFN-β 2/IL-6 in serum and body fluids during acute bacterial infection. J Immunol. 1989;142:948. [PubMed] [Google Scholar]
- 32.Pettila V, Hynninen M, Takkunen O, et al. Predictive value of procalcitonin and interleukin 6 in critically ill patients with suspected sepsis. Intensive Care Med. 2002;28:1220. doi: 10.1007/s00134-002-1416-1. [DOI] [PubMed] [Google Scholar]
- 33.Harbarth S, Holeckova K, Froidevaux C, et al. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164:396. doi: 10.1164/ajrccm.164.3.2009052. [DOI] [PubMed] [Google Scholar]
- 34.Oberholzer A, Oberholzer C, Moldawer LL. Interleukin-10: A complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med. 2002;30(1 Suppl):S58. [PubMed] [Google Scholar]
- 35.Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 36.Thakur V, Pritchard MT, McMullen MR, et al. Adiponectin normalizes LPS-stimulated TNF-alpha production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2006;290:G998. doi: 10.1152/ajpgi.00553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H, Park PH, McMullen MR, Nagy LE. Mechanisms for the anti-inflammatory effects of adiponectin in macrophages. J Gastroenterol Hepatol. 2008;23(Suppl 1):S50. doi: 10.1111/j.1440-1746.2007.05284.x. [DOI] [PubMed] [Google Scholar]
- 38.Walley KR, Lukacs NW, Standiford TJ, et al. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Standiford TJ, Strieter RM, Lukacs NW, et al. Neutralization of IL-10 increases lethality in endotoxemia. Cooperative effects of macrophage inflammatory protein-2 and tumor necrosis factor. J Immunol. 1995;155:2222. [PubMed] [Google Scholar]
- 40.van der Poll T, Marchant A, Keogh CV, et al. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J Infect Dis. 1996;174:994. doi: 10.1093/infdis/174.5.994. [DOI] [PubMed] [Google Scholar]
- 41.Berg DJ, Kuhn R, Rajewsky K, et al. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinhauser ML, Hogaboam CM, Kunkel SL, et al. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999;162:392. [PubMed] [Google Scholar]
- 43.Denis M, Ghadirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993;151:5425. [PubMed] [Google Scholar]
- 44.Greenberger MJ, Strieter RM, Kunkel SL, et al. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155:722. [PubMed] [Google Scholar]
- 45.Donnelly SC, Strieter RM, Reid PT, et al. The association between mortality rates and decreased concentrations of interleukin-10 and interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Ann Intern Med. 1996;125:191. doi: 10.7326/0003-4819-125-3-199608010-00005. [DOI] [PubMed] [Google Scholar]
- 46.Pajkrt D, Camoglio L, Tiel-van Buul MC, et al. Attenuation of proinflammatory response by recombinant human IL-10 in human endotoxemia: Effect of timing of recombinant human IL-10 administration. J Immunol. 1997;158:3971. [PubMed] [Google Scholar]
- 47.Olszyna DP, Pajkrt D, Lauw FN, et al. Interleukin 10 inhibits the release of CC chemokines during human endotoxemia. J Infect Dis. 2000;181:613. doi: 10.1086/315275. [DOI] [PubMed] [Google Scholar]
- 48.Song GY, Chung CS, Schwacha MG, et al. Splenic immune suppression in sepsis: A role for IL-10-induced changes in P38 MAPK signaling. J Surg Res. 1999;83:36. doi: 10.1006/jsre.1998.5556. [DOI] [PubMed] [Google Scholar]
- 49.Geng Y, Gulbins E, Altman A, et al. Monocyte deactivation by interleukin 10 via inhibition of tyrosine kinase activity and the Ras signaling pathway. Proc Natl Acad Sci USA. 1994;91:8602. doi: 10.1073/pnas.91.18.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato K, Nagayama H, Tadokoro K, et al. Extracellular signal-regulated kinase, stress-activated protein kinase/c-Jun N-terminal kinase, and p38mapk are involved in IL-10-mediated selective repression of TNF-alpha-induced activation and maturation of human peripheral blood monocyte-derived dendritic cells. J Immunol. 1999;162:3865. [PubMed] [Google Scholar]
- 51.Sailhamer EA, Li Y, Smith EJ, et al. Acetylation: A novel method for modulation of the immune response following trauma/hemorrhage and inflammatory second hit in animals and humans. Surgery. 2008;144:204. doi: 10.1016/j.surg.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Yuan Z, Liu B, et al. Prevention of hypoxia-induced neuronal apoptosis through histone deacetylase inhibition. J Trauma. 2008;64:863. doi: 10.1097/TA.0b013e318166b822. discussion 870. [DOI] [PubMed] [Google Scholar]
- 53.Hockly E, Richon VM, Woodman B, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc Natl Acad Sci USA. 2003;100:2041. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin T, Chen H, Koustova E, et al. Histone deacetylase as therapeutic target in a rodent model of hemorrhagic shock: Effect of different resuscitation strategies on lung and liver. Surgery. 2007;141:784. doi: 10.1016/j.surg.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Alam HB, Shults C, Ahuja N, et al. Impact of resuscitation strategies on the acetylation status of cardiac histones in a swine model of hemorrhage. Resuscitation. 2008;76:299. doi: 10.1016/j.resuscitation.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 56.Gonzales E, Chen H, Munuve R, et al. Valproic acid prevents hemorrhage-associated lethality and affects the acetylation pattern of cardiac histones. Shock. 2006;25:395. doi: 10.1097/01.shk.0000209522.28120.c8. [DOI] [PubMed] [Google Scholar]
- 57.Shults C, Sailhamer EA, Li Y, et al. Surviving blood loss without fluid resuscitation. J Trauma. 2008;64:629. doi: 10.1097/TA.0b013e3181650ff3. discussion 638. [DOI] [PubMed] [Google Scholar]