Abstract
Purpose
This mixed-methods study reports on an outreach clinics program designed to deliver genetic services to medically underserved communities in Wisconsin.
Methodology
We show the geographic distribution, funding patterns, and utilization trends for outreach clinics over a 20-year period. Interviews with program planners and outreach clinic staff show how external and internal constraints limited the program’s capacity. We compare clinic operations to the conceptual models guiding program design.
Findings
Our findings show that state health officials had to scale back financial support for outreach clinic activities while healthcare providers faced increasing pressure from administrators to reduce investments in charity care. These external and internal constraints led to a decline in the overall number of patients served. We also find that redistribution of clinics to the Milwaukee area increased utilization among Hispanics but not among African-Americans. Our interviews suggest that these patterns may be a function of shortcomings embedded in the planning models.
Implications
Planning models have three shortcomings. First, they do not identify the mitigation of health disparities as a specific goal. Second, they fail to acknowledge that partners face escalating profit-seeking mandates that may limit their capacity to provide charity services. Finally, they underemphasize the importance of seeking trusted partners, especially in working with communities that have been historically marginalized.
Contribution
There has been little discussion about equitably leveraging genetic advances that improve healthcare quality and efficacy. The role of State Health Agencies in mitigating disparities in access to genetic services has been largely ignored in the sociological literature.
Keywords: state health agencies, public health, public-private partnerships, rural health services, genetic services, health inequalities
INTRODUCTION
Unveiled to great acclaim in 2003, the Human Genome Project entailed a massive public investment. Although expectations are high that genetic advances will improve healthcare quality and efficacy (Collins, 1999; Guttmacher & Collins, 2005; Manolio, 2013), there has been much less discussion about how to leverage this significant public investment equitably, to mitigate health disparities (Burke et al., 2010; Khoury et al., 2009). The barriers in accessing genetic services are numerous: genetics providers tend to be concentrated in urban areas or academic medical centers, the workforce is small and unevenly distributed, and insurance providers often limit coverage for genetic testing and genetic counseling (Cooksey, Forte, Benkendorf, & Blitzer, 2005; Graf, Needham, Teed, & Brown, 2013; Secretary’s Advisory Committee on Genetics Health Society, 2006; Wang, Beattie, Ponce, & Phillips, 2011). State Health Agencies (SHAs) have historically sought to mitigate some of these barriers by providing clinical services (such as genetic testing or genetic counseling) directly, contracting with healthcare providers to provide care, or sharing these responsibilities with private payers and charities (Epstein, Erickson, Hall, & Golbus, 1975; Kelly, 2002; Lessick & Townes, 1979; MacDonald, Blazer, & Weitzel, 2010; Reid et al., 1976). SHAs have myriad other responsibilities, however, and the provision of direct clinical services makes up a very small proportion of their activities.
Policymakers in state, county, and local health departments rely on different conceptual frameworks or planning models to map out their responsibilities and to integrate their activities so that they can fulfill their mission efficiently and effectively (Ford, Duncan, & Ginter, 2005; Frieden, 2010; Institute of Medicine, 1988; Mays, Scutchfield, Bhandari, & Smith, 2010). Because they are so severely underfunded, however, public health policymakers must make strategic choices about how to allocate resources to fulfill their mission as best they can (Levi, Juliano, & Richardson, 2007; Levi, St Laurent, Segal, & Vinter, 2010). As a result, there are often gaps between the aspirations laid out in a planning model and the programs that are actually implemented. Understanding the connections and discontinuities between planning models and implementation is vitally important to improving system performance and service delivery (Leischow et al., 2008). SHAs may therefore play a vital role in mitigating disparities in access to specialty healthcare services (including genetic services), but to date this potential has been largely ignored in the sociological literature, and as a result, we understand relatively little about how policymakers apply planning models to solve public health problems.
This mixed-methods study examines one state’s experience sponsoring outreach clinics that provide clinical genetic services to improve access to specialty healthcare in underserved communities. We present quantitative data on utilization trends in genetic outreach clinics in Wisconsin over a twenty-year period, and show how the Wisconsin Department of Health Services (DHS) has supported these clinics with a combination of federal grants and partnerships with public- and private-sector institutions. We show how the program’s activities evolved as providers and policymakers struggled to simultaneously meet the needs of medically underserved populations in both rural and urban communities. Drawing on in-depth interviews with program administrators, we show how the public-private partnerships designed to deliver these services changed over time in response to changing financial and institutional constraints.
Our findings show that state health officials scaled back the portion of federal funding they invested in outreach clinic activities at the same time that healthcare providers in the partnering organizations experienced increasing pressure from administrators to reduce their investment in charity care. These external and internal constraints on outreach clinic activities led to a dramatic reduction in the overall number of patients served by the program statewide. We also find that redistribution of clinics to the Milwaukee area increased utilization among Hispanics but not among African-Americans. Interviewees attributed these somewhat disappointing results to historic tensions between Milwaukee’s black community and the city’s municipal and not-for-profit institutions. Our interviews also suggest, however, that these utilization patterns may be a function of the shortcomings embedded in the planning models that policymakers draw on to develop and coordinate programs.
The paper unfolds in three sections. We first explain the demand for clinical genetic services and describe geographic and socioeconomic barriers in access to care. In this section, we also describe some of the planning models that policymakers use in developing integrated public health programs, including the models that motivated development of genetic outreach clinics. In the next section, we describe Wisconsin’s genetic outreach clinics program and the quantitative and qualitative data we collected to assess its historical function. In the results section, we present data on financing and utilization trends in the clinics over a twenty-year period. We draw on in-depth interviews with the program’s original architects and current administrators, as well as clinicians who work in the clinics to show how external and internal constraints limited the program’s overall capacity. Drawing on interview data, we explore the degree of correspondence between the ideals embedded in the planning models and the program’s actual implementation. We close with some reflections on the relevance of our findings to public health theory and practice.
BACKGROUND
Recent advances in genetics have altered clinical genetic services in ways that will increase demand for them and may exacerbate disparities in access (Khoury et al., 2008). The expansion of genetic knowledge has made genetic services (herein defined as genetic testing and genetic counseling) relevant to a broader segment of the population and clinically relevant in diverse medical specialties. Once restricted to newborn screening or the diagnosis of rare diseases, genetic services have become increasingly important in diagnosing common, complex diseases such as cancer, cardiovascular disease, and neurological disorders (Goldman et al., 2011; Palma, Ristori, Ricevuto, Giannini, & Gulino, 2006; Petersen, Brensinger, Johnson, & Giardiello, 1999). Consequently, genetic services have become more relevant to diverse medical specialties and even in primary care, in part because these common diseases are a substantial burden on population health, and because primary care providers serve as gatekeepers to specialty care (Forrest, 2003; McCandless, Brunger, & Cassidy, 2004; National Coalition for Health Professional Education in Genetics, 2007).
Disparities in access to specialist healthcare services are pervasive and impact many populations and communities. Much of the literature on unmet healthcare needs has focused on core medical services (e.g., primary care, dental care, emergency medical services), and has assessed geographic barriers or insurance coverage as a predictor of access to basic and preventive healthcare (Casey, Thiede Call, & Klingner, 2001; Crump, Gaston, & Fergerson, 1999; Institute of Medicine, 2005; Zhang, Tao, & Irwin, 2000). Scholars have paid far less attention to how these barriers influence disparities in access to specialty healthcare services, although there are reasons to be concerned about inequitable access to them as well (Canin & Wunsch, 2009; Cook et al., 2007; Weissman et al., 2003). These disparities may be explained partly by geographic distribution of healthcare facilities, limited health insurance coverage, or provider bias (Armstrong, Micco, Carney, Stopfer, & Putt, 2005; Suther & Kiros, 2009). Rural populations face substantial access barriers, including spatial maldistribution of healthcare facilities; difficulties in recruiting and retaining providers; and demographic trends that have made rural populations older, culturally and ethnically diverse, less likely to have health insurance, more likely to have high rates of chronic illness and disabilities, and have lower levels of educational attainment (Casey et al., 2001; Zhang et al., 2000).
Barriers in accessing genetic services, in particular, are well documented and may be traced to many sources (Association of State and Territorial Health Officials, 2012; Hawkins & Hayden, 2011). The medical genetic workforce (i.e., board-certified physician medical geneticists and genetic counselors) remains small and unevenly distributed in the United States, and institutions that provide clinical genetic services remain concentrated in urban centers or near academic medical centers (Bernhardt & Pyeritz, 1992; Cooksey et al., 2005). Financing genetic services is a major challenge in both the public and private sectors. Reimbursement in private health systems is rarely sufficient to cover the cost of providing genetic services (McPherson et al., 2008; Secretary’s Advisory Committee on Genetics Health Society, 2006).
Health disparities among racial and ethnic minorities are especially problematic, for health services generally but for genetic services in particular. Racial and ethnic minorities may be less trusting of healthcare providers and institutions in general because of past discrimination (Forman & Hall, 2009; Gamble, 1993). Surveys and focus groups studying perceptions of genetic services, specifically, have shown that African-Americans are less likely than whites to identify benefits of genetic testing (e.g., will contribute to medical knowledge, will help my doctor provide better care) and were more likely to express concerns that genetic testing will contribute to racial discrimination (Furr, 2002; Peters, Rose, & Armstrong, 2004). Surveys of African-American healthcare professionals have documented similar findings, suggesting that the mistrust of genetics cannot be explained by differences in educational attainment (Laskey et al., 2003; Powell-Young & Spruill, 2013; Spruill, Coleman, & Collins-McNeil, 2009). Focus groups studying these ethical concerns have shown that African-Americans are also more likely than whites to link their concerns to specific episodes of discrimination or abuse at the hands of the health care system, such as the Tuskeegee syphilis study or practices of eugenics (Bates, Lynch, Bevan, & Condit, 2005). If healthcare administrators and policymakers do not assess and enhance their capacities and cooperate to mitigate all of these barriers in access, the potential benefits of genetic innovations may not be realized and inequities in access may worsen (Khoury et al., 2008).
State Health Agencies: Planning and Integration of Comprehensive Health Services
SHAs began operating outreach clinics in the 1970s to ensure that populations that are medically underserved—because of geographic, financial, or other barriers—would have access to genetic services (H. Chen & Wertelecki, 1994; Epstein et al., 1975; Lessick & Townes, 1979; Lowry & Bowen, 1990; Reid et al., 1976; Riccardi, 1976). These satellite clinics were located in local health departments, tribal health clinics, or private healthcare facilities, depending on the partnerships that fostered them (Buchanan et al., 2009; MacDonald et al., 2010). Outreach efforts relied heavily on the clinical expertise of a small number of clinicians based in academic medical centers. They were funded through multiple sources: grants from foundations concerned with genetic disease (e.g., March of Dimes), subsidies by public institutions or academic medical centers where medical geneticists had home appointments, or state and federal funds (Bernhardt & Pyeritz, 1992; H. Chen & Wertelecki, 1994). However, as federal and state public health investments have declined, competing priorities have driven states to direct funding toward mandated activities and have eroded support for any type of discretionary activity, such as clinical services for specialty care (Levi et al., 2007; Turnock & Atchison, 2002). Genetic services are offered in outreach clinics in many states to this day, although it is very unusual to see them funded exclusively by a state health agency. They are now more typically supported with modest state support, and with the lion’s share of funding being supplied by partners in the private sector, either philanthropic foundations or underwritten by academic medical centers (Genetic Services Policy Project Final Report, 2008).
Numerous planning models guide public health policymakers in their planning and implementation efforts. Planning models serve a number of functions for an organization. Their most explicit and visible function is that they specify goals for organizational action and give guidance on the allocation of resources toward meeting those goals (Fraser, 2012; Frieden, 2010). They also have important implicit functions. In specifying goals for organizational action, models reflect choices about what actions are (and are not) appropriate for an organization. They also reflect embedded institutional logics, or the “norms, values, and beliefs that structure the cognition of actors in organizations and provide a collective understanding of how strategic interests and decisions are formulated (Thornton, 2002).” The two planning models that are most relevant to understanding the barriers SHAs may face in mitigating disparities in access to genetic services are the Maternal and Child Health Services Pyramid (U.S. Maternal and Child Health Bureau, 2003) and the Core Public Health Functions (Harrell & Baker, 1994; Institute of Medicine, 1988).
The Health Resources and Services Administration (HRSA) developed the Maternal and Child Health Services pyramid (hereafter, the MCH pyramid) to identify the needs of women, children, and families, and to help the agency and its partners orchestrate a suite of comprehensive health services to meet those needs (U.S. Maternal and Child Health Bureau, 2003). The MCH pyramid identifies four levels of services that must be addressed. Infrastructure building services such as needs assessment, quality assurance, and program evaluation, form the foundation of the pyramid. The next tier is population-based services, such as newborn screening, lead poisoning prevention programs, and childhood immunizations. This is followed by enabling services such as transportation, translation, and case management services that make it possible for patients to utilize healthcare services. At the top of the pyramid are direct healthcare services (U.S. Maternal and Child Health Bureau, 2003). HRSA awards grants to states to support many types of maternal and child health services, most visibly through the Title V program. For more than 75 years, Title V programs have provided healthcare and supportive services to children with special healthcare needs, including birth defects or genetic disorders (Fraser, 2012). The genetic outreach clinic program we describe in this paper was motivated primarily by the MCH pyramid and funded through Title V.
There are two grounding assumptions implicit in the MCH pyramid. First, comprehensive and effective MCH healthcare delivery must comprise activities at all four levels. Second, state and local agencies should seek partnerships to meet these needs (Fraser, 2012). Despite this gesture toward the importance of comprehensive services, however, the pyramid prioritizes infrastructure and population-based services as foundational services, on the assumption that these are likely to reach the greatest number of patients with the fewest resources. Although the MCH pyramid does allow for the provision of some direct clinical care services, it is explicit that these services should be “gap filling,” meaning that private health systems should provide the majority of such services, with the state providing only minimal support as needed (Fraser, 2012). Notwithstanding the rhetoric about the importance of partnerships that often appears in supporting documentation from HRSA, the MCH pyramid does not include partnership development as a prioritized activity.
The Core Public Health Functions model grew out of a 1998 report by the Institute of Medicine (Institute of Medicine, 1988). The model has three goals: first, to define what public health is; second, to clarify the essential role of public health services in an overall comprehensive health system; and third, to make it possible to evaluate public health system performance in relation to specific health outcomes. The model posits that the three core functions of public health are: (1) assessment, or the identification of health problems, assessment of resources available to address them, evaluation of the efficacy of interventions, and presentation of results to decision makers; (2) assurance, or the provision of services to meet these policy goals; and (3) policy development, or planning, prioritization, and allocation of resources (Harrell & Baker, 1994). Linking communities to care and developing partnerships to promote population-based public health services are key activities in the assurance function. Consequently, SHAs have a vital role to play in coordinating healthcare delivery, even if they do not provide it directly (Derose, Gresenz, & Ringel, 2011). Efforts to link communities to genetic services are one example of how SHAs have tried to integrate a specific kind of specialty healthcare service into their Core Public Health Functions (Beskow, Khoury, Baker, & Thrasher, 2001; Kaye et al., 2001; Wang & Watts, 2007a).
These models share some common features, especially in their focus on broad-based and comprehensive activities to promote population health. However, they have some limitations that could hobble efforts to mitigate disparities in access to specialty healthcare services. First, they differ in the degree to which they prioritize partnerships as a means of achieving these goals. The Core Public Health Functions model identifies partnership development as an essential activity in the assurance realm. In contrast, the MCH pyramid does not include partnership development as a prioritized component. This is an important distinction, because there is rising enthusiasm for public-private partnerships that could mitigate many types of barriers in accessing health services (Keane & Weerasinghe, 2009; Reich, 2002), and yet the role that SHAs could play in fostering these partnerships remains underexamined. Second, both models are silent on the necessity of addressing health disparities. This is a curious omission, given the protracted nature of health disparities in the United States, and a decade of attention by policymakers in the public and private sectors (Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, 2009; Centers for Disease Control and Prevention [CDC], 2011).
Both planning models therefore argue for an integrated approach to comprehensive health services, but each has some limitations in guiding actual practice for policymakers. Providing comprehensive, population-based healthcare ultimately requires attention to both primary and specialty healthcare services, as well as innovative models that encourage public and private providers to cooperate, while recognizing the different economic, political, and institutional constraints that these partners face. In this paper, we explore how policymakers used the MCH pyramid to design a genetic services outreach program, and identify how the model contributed to difficulties in rallying support and coordinating effort by public and private partners in meeting the program’s goals.
DATA & METHODS
The Setting
We focused on Wisconsin for four reasons. First, approximately 30% of Wisconsin’s population is classified as rural and its major metropolitan center, Milwaukee, is a highly segregated city with high concentrations of poverty (U.S. Census Bureau, 2012). Milwaukee consistently ranks last or next-to-last among cities in Wisconsin on many key metrics of population health: all-cause mortality, infant mortality, self-reported health, and several important determinants of health (e.g., access to care, substance abuse, childhood lead poisoning). Reports by the city health department have shown that ZIP codes with high proportions of poor residents are (a) all clustered in central Milwaukee, and (b) that these communities have shown much worse health outcomes than wealthier ZIP codes on the city’s fringes (H.-Y. Chen et al., 2013; 2011). Geographic barriers to access and concerns about racial and ethnic health disparities are therefore prominent in both the city of Milwaukee and in the state’s historical and contemporary healthcare infrastructure.
These contemporary patterns of health inequalities are an especially cruel irony, given that between 1930 and 1940, the city won numerous awards as the “healthiest city” in the nation from professional and civic societies (Leavitt, 1996). In her history of public health activism in Milwaukee, Judith Walzer Leavitt (1996) charts how the city’s health department forged strategic alliances with private philanthropies and faith-based organizations to deliver a wide range of public health services. While many of these projects focused on sanitation, food safety, and halting the spread of infectious diseases such as smallpox, the city’s health department also supported a child health and welfare commission that provided comprehensive preventive care to children in the 14th ward on the city’s far southwest side. These early successes were eroded over succeeding decades, as the city’s industrial base declined, white majority populations fled for the surrounding suburbs, and new racial and ethnic minorities poured into the city during the Great Migration.
In his history of the civil rights movement in Milwaukee, David Jones (2009) argues that although northern civil rights activists shared a consciousness of racial politics with activists in the South, that northern cities had distinct legacies that made organizing there particularly challenging. Milwaukee exemplifies these barriers. On their face, some of the city’s traditions should have bolstered civil rights activism, such as the city’s legacy of socialist politics, the strong presence of the Catholic Church, and the relatively secure right to vote for African-Americans. However, Jones demonstrates how other aspects of the city’s history fostered racial discrimination and made civil rights organizing in Milwaukee especially difficult, such as the presence of white ethnic minorities and spatial patterns of racial segregation in the city’s inner core. By the 1960s, Milwaukee would become one of the most volatile and violent Northern centers of the civil rights movement (Jones, 2009), and concerns about race relations and social justice persist there to this day. The combination of this troubled history of racial politics in Milwaukee and the geographic maldistribution of healthcare services across rural and urban areas of the state make Wisconsin a good setting for studying how SHAs try to develop solutions to persistent health inequalities and the barriers they encounter.
A second reason to study outreach clinic services in Wisconsin is that the vast majority of Wisconsin residents had health insurance during the period of study, although having insurance does not necessarily assure coverage for specialty healthcare services (Office of the Commissioner of Insurance, 2009). Third, despite this high level of formal access, the state has perennially ranked very low in per capita public health spending (49th among all U.S. states in 2007–2008; Levi et al., 2010). Fourth, since 1979, the Wisconsin Department of Health Services (DHS) has provided clinical genetic services through a network of tertiary care medical centers and genetic outreach clinics. In 2001, the state obtained funding from HRSA to write a comprehensive genetic services plan (Henry, Pauli, & Katcher, 2005). One of the objectives in that plan concerned community-based access to genetic services:
Outreach activities have been and remain a central part of genetic care in Wisconsin. Such outreach not only provides services near the communities in which families live, but also strives to assure equality of access to services and to function as a ‘safety net’ for families who otherwise would be without this help (Henry et al., 2005).
Wisconsin thus features a mixture of the opportunities and challenges around access to specialty services that face the rest of the country, and the SHA has historically taken an active role in building partnerships to mitigate barriers in access to genetic services.
Since 1979, Wisconsin DHS has subsidized genetic services by using a portion of its Title V funding to underwrite a network of statewide outreach clinics. From 1979–2005, DHS awarded the outreach clinics contract to an academic medical center, which dispatched providers to run outreach clinics and subcontracted to other health systems that could offer outreach clinics in specific markets (e.g., Milwaukee). In 2005, DHS awarded the contract to a private, not-for-profit hospital system based in Milwaukee, which then subcontracted some outreach clinic activities to the academic medical center. This represented a major shift in the geographic distribution of outreach clinic services and the populations served. Our data encompassed both the era when the contract was administered by the academic medical center, and the period when it was steered by the private health system.
Data Collection and Analysis
This mixed-methods research draws upon quantitative data abstracted from archival records at the Wisconsin DHS and in-depth interviews with clinicians and policymakers. The genetic outreach clinics are funded from Wisconsin’s federal Maternal and Child Health Block Grant (the HRSA Title V grant). The genetic outreach clinics are administered in multi-year contracts, which require contractors and subcontractors to submit annual reports and budgets. The archive includes these reports and correspondence dating to 1990, and correspondence and other documents dating to 1977.
Archival documents contain aggregate information about services provided and the populations served through the outreach clinics, including the number screened, counseled, and referred for additional care. Reporting methods used in the annual reports changed during the period of follow up. For instance, the academic medical center consistently reported the number of families served annually in each clinic and reported individuals served in some years, while the private hospital system consistently reported individuals served annually by clinic and reported families served in some years. These variations in reporting may have been in response to instructions from state officials. We were therefore able to chart trends in utilization but were unable to directly compare characteristics of patients served over time. In this paper, we report the number of individuals served, either as documented by the contractors or imputed based on the number of families served.
Archival documents include aggregate demographic information on patients served by the genetic outreach clinics, although in a format that varied over time. The private health system reported aggregate race and ethnicity data for individual patients served in their outreach clinics beginning in late 1997, and for all patients served after they assumed responsibility for the contract in 2005. Demographic attributes reported by the academic medical center varied, but corresponded with the private hospital system’s reports from 2003–2004 and 2009–2010. Thus, there were two two-year periods of consistent demographic data reporting, one before and one after the contracting change that redistributed clinic locations. We abstracted these data from the annual reports filed to DHS and used them to chart utilization trends by clinic, services provided in the clinics, and other demographic characteristics (age, gender, income, and family size). We also examined healthcare status of the patients seen in outreach clinics (health insurance and dental insurance status, regular source of primary care; data not shown).
To determine whether the outreach clinics mitigated maldistribution of genetic services, we mapped the location of outreach clinics in 2004 and 2006, i.e., just before and just after the contract management changed and clinic locations were redistributed. We compared clinic location to county-level data on population density and minority population drawn from the 2000 U.S. Census. We plotted location of outreach clinics relative to the number of persons per square mile by county and to the number of minority persons per county. Minority status was defined as all non-white or Hispanic persons. We measured minority population at the county level in absolute numbers rather than percentages because some northern Wisconsin counties have high proportions of minority residents but small total populations. Using absolute number of minority population produced a more reliable estimate of their statewide distribution.
We conducted 35 confidential semi-structured interviews with healthcare providers and policymakers who were instrumental in founding, operating, or administering Wisconsin’s genetic outreach clinics. We sampled purposively, to ensure that we elicited the perspectives of a range of stakeholders involved with this program: authors of the state’s 2001 Genetic Services Plan, current members of the state’s Genetic Advisory Council, clinicians who provide care at outreach clinics, and clinicians who refer patients to them. Interviewees included genetic counselors, physician medical geneticists, other physicians, consumer advocates, personnel from state and local health agencies, and medical directors of healthcare organizations and clinical laboratories.
Interview questions probed about the origins of the outreach clinics, operational barriers, the goals of the 2001 Genetic Services Plan, the role of the state’s Genetic Advisory Council, and how the outreach clinics related to other objectives in the Genetic Services Plan. Interviews ranged from 60–90 minutes and were recorded and transcribed for analysis with participant consent. The University of Wisconsin-Madison Education and Social/Behavioral Science Institutional Review Board approved the study protocol.
Data were analyzed with QSR NVivo, a software package that facilitates analysis of qualitative data. Coding began with a pre-established set of codes drawn from themes in the interview guide. This set of codes was augmented with important themes introduced by the interviewees. The authors wrote analytical memos on emerging analytical categories and compared codes within and between interviews using the constant comparative method (Glaser & Strauss, 1965; Lincoln & Guba, 1985). Unreferenced quotations are drawn verbatim from interview transcripts.
RESULTS
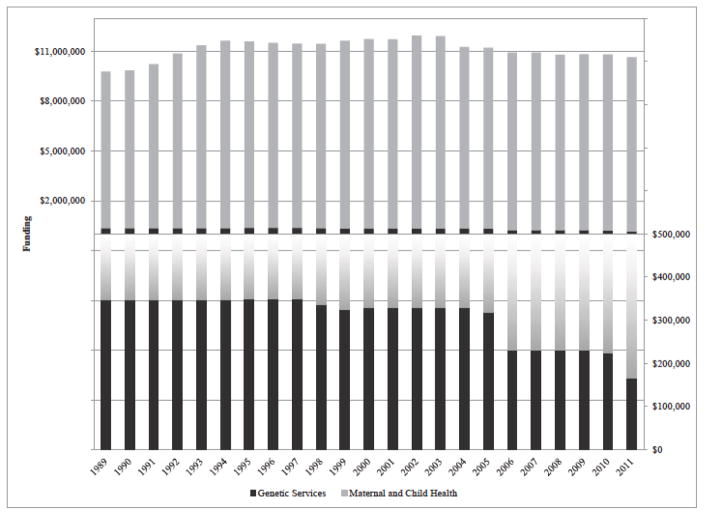
Figure 1 shows Wisconsin’s annual budget for maternal and child health services, including the portion earmarked for statewide genetic services, from 1989–2011. Other programs that received maternal and child health funding included a Family Planning and Reproductive Health Council, an infant death center, prenatal care coordination, and regional centers for children and youth with special healthcare needs. The genetic services portion funded the outreach clinics and some educational activities, although the bulk of those funds supported the outreach clinics. Between 2003 and 2011, the total Title V block grant declined by about 10% (from $11.6M to $10.5M). The genetic services portion declined disproportionately, however, from $330,000 to $165,000 (i.e., a cut of almost 50%). This underscores the intense competition for block grant funding, and illustrates that discretionary programs like the outreach clinics often must bear heavier cuts than programs that are federally or state mandated.
Figure 1.
Wisconsin Genetic Services Funding as a Portion of Maternal and Child Health Funding, 1989–2011
Note: The upper panel shows Wisconsin’s Maternal and Child Health budget by calendar year, omitting unspent funds. The lower panel shows the genetic services portion by calendar year.
Outreach Clinics: Services Provided and Utilization Trends
Outreach clinics provided screening, counseling and referral services to patients. More than 90% of patients received both screening and counseling, and approximately 35% of patients received referrals for follow-up specialty care. These proportions were consistent over time and did not vary depending on which organization oversaw the contract or the geographic distribution of outreach clinics (data not shown).
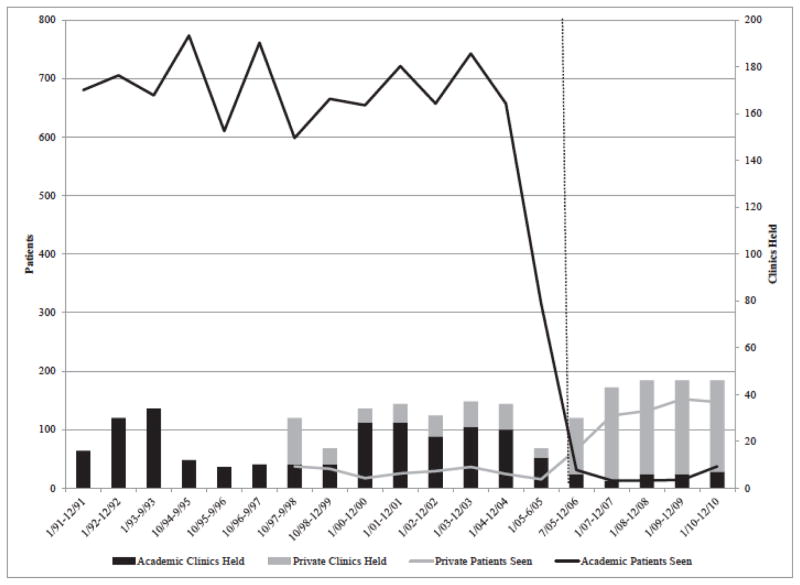
Figure 2 shows the number of clinics held and patients seen from 1991–2010 for each type of contractor—the academic medical center and the private, not-for-profit hospital system. The private hospital system joined the network as a subcontractor in 1997 and became the lead contractor in 2005. Prior to 2005, clinicians ran approximately 20–40 clinics per year, serving approximately 600–750 patients per year. After 2005, the number of annual clinics and the number of patients seen by the private medical center increased. However, the overall number of patients seen system-wide declined substantially, which resulted in much lower productivity per clinic. Significant operational changes (especially at the academic medical center), the closure or relocation of clinics, and reductions in state funding all contributed to this dramatic decline.
Figure 2.
Wisconsin Genetic Services Network Clinics Held and Patients Seen by Health System, 1991–2010
Note: Patients seen are plotted on the left vertical axis; clinics held are plotted on the right vertical axis. The vertical dotted line shows the change in lead contractor in July 2005. Data were reported by contract period, and contract periods are inconsistent. Academic Medical Center patients seen from 1/00–6/05 converts the reported number of families seen to individuals based on the rate of 2.67 individuals per family, which was extrapolated from the previous nine years, in which both individuals and families were reported.
Geographic Distribution of Clinics
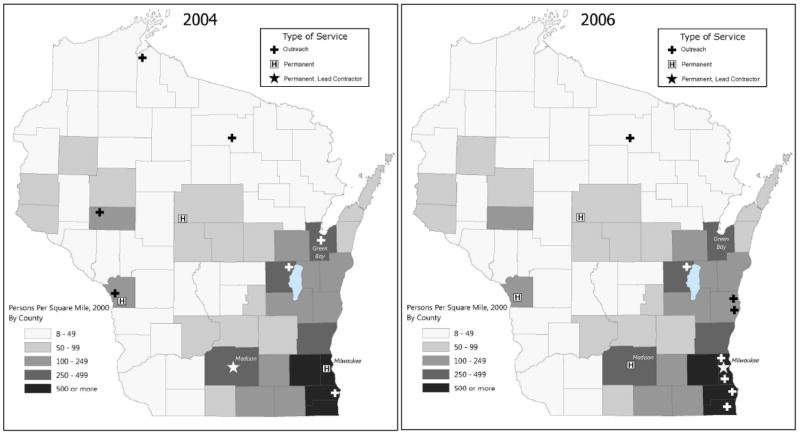
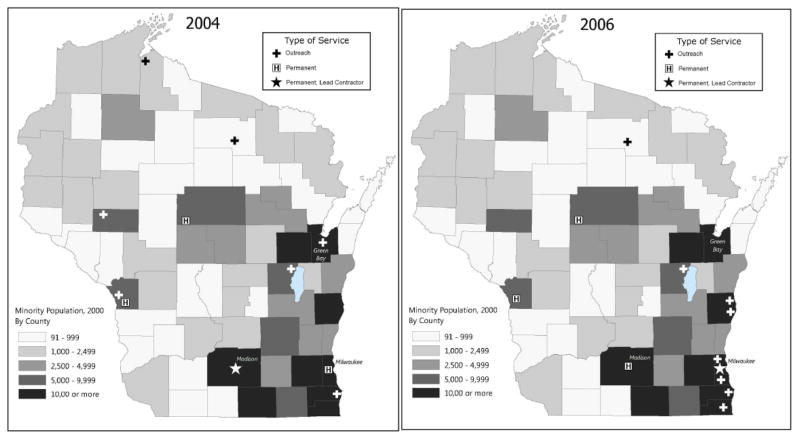
Figures 3 and 4 show the locations of genetic outreach clinics in 2004, when the outreach clinics were managed by the academic medical center, and 2006, when they were managed by the private health system, relative to population density (panels 3a and 3b) and minority population density (panels 4a and 4b). They also show locations of hospitals that offer genetic services on site. After the contract shift in 2005, the outreach clinics were relocated to regions of the state with higher concentrations of minority populations.
Figure 3.
Figure 3a and 3b: Wisconsin Genetic Outreach Clinics and Permanent Genetic Service Providers, With Population Density by County, 2004 and 2006
Figure 4.
Figure 4a and 4b: Wisconsin Genetic Outreach Clinics and Permanent Genetic Service Providers, With Minority Population Density by County, 2004 and 2006
Motivations for Locating Outreach Clinics
Our interviews revealed that original clinic locations were not based on a population-level analysis of statewide need, but primarily on anecdotally assessed local demand or the assumptions of state health officials about regional variations in need. This is one of the most significant ways that the program’s implementation diverged from the principles of the MCH pyramid. The foundation of the pyramid is infrastructure-building services, including needs assessment activities. In the original design of the program, administrators never surveyed the state’s population to assess demand for genetic services, nor did they systematically evaluate utilization trends as the program matured. We found no evidence that they estimated the incidence of genetic disorders in the population and attempted to predict their geographic distribution statewide. Nor did we find any evidence that they assessed unmet need by surveying wait times in scheduling visits with a geneticist or genetic counselor, or delays in obtaining follow-up care. In the program’s early days, personal connections, local champions, and logistics led clinics to be placed in communities that advocated most vigorously for them. After the 2005 contract shift, state officials hoped to regionalize outreach clinic services to better meet the needs of vulnerable populations, especially racial and ethnic minorities. This change was undertaken in response to widespread concern among public health practitioners about health disparities, but was not motivated by systematic, empirical evidence of unmet demand in the state’s urban centers. Although program planners viewed the outreach clinics as an activity that was motivated by one of the elements of their planning model (i.e., a type of direct clinical care given space at the top of the MCH pyramid), they did not link that program element to the needs assessment element at the base of the pyramid. Therefore, the application of a conceptual model that specifies and prioritizes certain activities does not necessarily require program planners to coordinate constituent elements of the program within the model.
In the earliest days of the program, the main factor motivating clinic placement was the presence of a local champion: a vocal primary care provider, nurse, or genetic counselor who recognized unmet need in their community. As one of the of the program’s early architects told us:
A lot of the early clinics were because there was one person [there] who was gung ho. … In ‘82 we began in [city] because [name], who was our pediatric neurologist there, said ‘We really have to have genetics. This is awful. I keep seeing kids who have genetic problems.’ … So it wasn’t that there was a map on the wall. It was who wanted it. We had to have [a] local contact.
Operational logistics were a second key concern in situating outreach clinics. As one clinician noted, “you have to pick a place where somebody is willing to let you have five clinic rooms for a day and office space and a computer.” In practice, this meant that certain kinds of local champions were more effective in seeking outreach services. One of the program’s early designers noted that the most persuasive local champion was “a local physician, because that just smooths the way a whole lot better.” Local physicians, as opposed to social workers, nurses, or genetic counselors, were better able to back up a request for specialty consults with resources.
Without a systematic, population-based analysis of the need for genetic services, however, it became difficult to judge whether the clinics were truly meeting demand. As Figure 1 demonstrates, the allocation of Title V funds dedicated to genetic services declined dramatically after 2003. A state administrator noted that, “We have so much to do with so little money. Basically over time, what we’ve learned is we need to put our money [where] we can get the most done with the least amount of funds.” This led to a tension between clinicians who served these clinics (or others who benefitted from having them in their communities) and program planners. Several clinicians reported to us that they wanted to see an increase in outreach clinic capacity. State planners, on the other hand, were skeptical about whether this would serve more patients:
It’s a question of whether we’ve really maximized what we can do in outreach. And that’s a hard thing to judge…If we doubled the number of clinics at [certain locations], you know whether we’d be able to fill them with local referrals…it’s hard to judge whether if we doubled everything—we’d probably eventually fill them.
This quote demonstrates uncertainty about the nature and extent of the demand for genetic services, and emphasizes the difficulty of planning outreach clinic activities without a systematic assessment of unmet need.
Clinic Locations: Serving Vulnerable Communities
When the academic medical center held the contract for the provision of outreach clinics, state planners were chiefly concerned with rural health disparities, and wanted to facilitate access to genetic services in rural communities in northern Wisconsin. One asserted, “[the grant] had primarily been used to provide services in a variety of communities in the state that had no genetic services,” because, as another described, “[people who live in] the upper tier of the state [have] to travel five plus hours to get to a center where they can access services.” Distance is a major barrier limiting utilization, especially for people who are disabled or who have a child with a genetic condition that limits their mobility (Hawkins & Hayden, 2011). One provider said, “There are some folks who lack the organization, the money, or the car to drive [five hours]. It’s just beyond them. They’re not going to be able to do that.” Over time, however, decreased advocacy from local champions in those rural communities (due to retirement or lack of support for participation in unpaid advisory activities) reduced pressure to keep outreach clinics at rural sites.
Moreover, by the early 2000s, the national dialogue about health disparities had turned to access barriers among racial and ethnic minorities (Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, 2009; Kaye et al., 2007). In Wisconsin, this focused attention on the city of Milwaukee. As the state’s largest population center, Milwaukee attracts significant attention for its well-documented health disparities. Program planners decided to close some genetic outreach clinics in northern Wisconsin and expand services in southeastern Wisconsin, but this decision was based on general discussion of health disparities, not based on any systematic assessment of demand for genetic services in Milwaukee. As one program planner said, “we were really trying to get services to the African-American community that show great disparities in health compared to the other racial and ethnic populations in Wisconsin.”
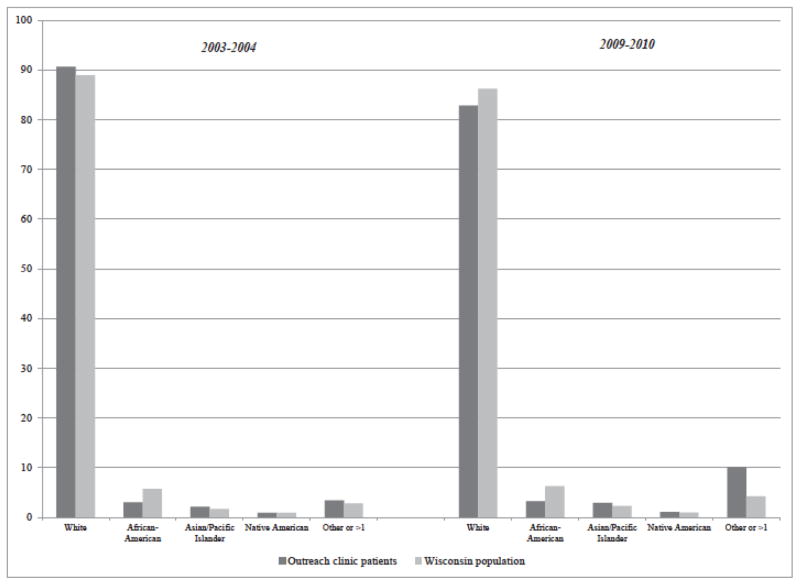
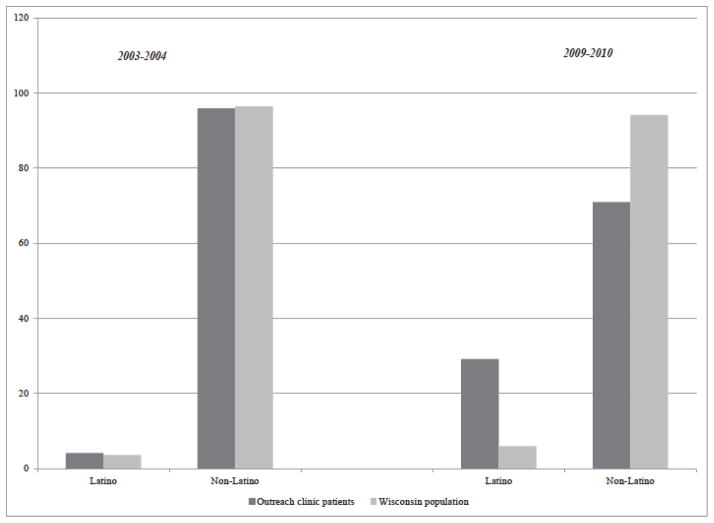
To determine whether the outreach clinics were meeting the stated goal of improving access to care among underserved populations, we compared the racial distribution of outreach clinic patients to the racial and ethnic distribution of the Wisconsin population, under the different contracting arrangements (Figures 5 and 6). While the Wisconsin population as a whole is predominantly white, Figure 5 shows that black patients were under-represented in outreach clinics. This remained true even after 2005, when the clinic locations were shifted to a region of the state where more black patients lived. In contrast, as Figure 6 shows, the relocated outreach clinics seemed to improve their capacity to serve Latino communities.
Figure 5.
Racial composition of outreach clinic patients vs. Wisconsin population.
Figure 6.
Ethnic composition of outreach clinic patients vs. Wisconsin population.
Outreach clinic staff and program administrators acknowledged these difficulties in reaching out to African-American neighborhoods:
When they tried to do it in the northern part of Milwaukee, they hit a lot of difficulty. And they didn’t have a lot of support from the site that they used. So as a result, they didn’t serve too many people in that area. And that’s been a long history in Milwaukee of people wanting to do good for the underserved population, but because for one reason or another, it was easier to work with the community health center on the south side [a predominantly Latino community]. A lot of the services were provided there, rather than on the north side where the African American population was.
This example demonstrates that local champions can be critically important to the success of outreach clinics, even if they are not initiating the demand for services. Because local champions articulated the demand for these services, these partnerships had little strategic direction from the program’s architects, and only a tenuous connection to statewide demand for genetic services. As noted, the rhetoric surrounding the MCH pyramid lauds the formation of partnerships as an unquestionable necessity, but the model itself gives planners little support or direction in thinking about how to cultivate partnerships strategically, to address gaps in services that have been systematically and empirically documented.
Economic Drivers in Outreach Clinic Activities
Healthcare, healthcare markets, and public health systems have changed dramatically in the two decades since the outreach clinic program was designed. Public health agencies have faced increasing pressure to accomplish more with less and less resources (Levi et al., 2007; Turnock & Atchison, 2002). Wisconsin is an especially acute example of this trend, and the state has ranked at or near the bottom of per-capita public health spending over the past decade (Levi et al., 2010). At the same time, however, public and private not-for-profit health systems have placed greater and greater emphasis on efficiency and quality, and clinicians in both settings have faced escalating expectations for productivity. Our interviews show that all partners involved in the outreach clinic program—state planners, and clinicians in both the academic medical center and the private not-for-profit health system—were caught up in these economic pressures.
As a labor-intensive, cognitive specialty, medical genetics generates relatively little income. A thorough work-up for a patient with a suspected genetic dysmorphology can take several hours, and reimbursements from public or private health insurance plans rarely cover the full cost (Secretary’s Advisory Committee on Genetics Health Society, 2006). The clinicians we interviewed recognized that they were able to participate in outreach clinic activity in part because of the university’s public service commitment, often described as “the Wisconsin Idea.” This ‘idea,’ which dates back to the early 20th century and Wisconsin’s progressive tradition, makes public service to the state a core mission of the university (Stark, 1996). As one early participant said:
We did this under our salaries here from the [university] … And I would always take a genetic counselor, meaning a staff person, students, and it was teaching medical students if they were interested. So it was always teaching, research, service, all in one. … We were so, in that respect, naïve also. We had salaries. So we did what we did. … So they would say okay, if we bring a family to you, what does all this cost? And I would say, nothing. Because we had our salaries. I mean it was ideal and stupid, but there was money in those days.
Clinicians who worked in the private healthcare system that now oversees the outreach clinics reported an entirely different philosophy. One physician said that his hospital administrator accepts only two rationales for non-remunerative services like genetics, “[he] tells me, ‘you either gotta make me rich or make me famous.’ ” Consequently, the outreach clinic activities are now directed more firmly toward capturing downstream revenue. Previous research has shown that, although medical genetic services often lose money for health systems, if they identify patients who require further specialist services (e.g., cardiology, neurology), they could generate significant downstream revenue (Ho, 2004). However, one genetic counselor argued that credit for generating downstream profit was rarely, if ever, credited back to medical genetics:
Once we can make a diagnosis, we can personalize that patient’s medical care by knowing what their diagnosis is and get them the right care, and they end up getting a lot of expensive stuff throughout our system in specialty care that maybe brings in money for the institution as a whole. But our medical genetics department isn’t the one doing those procedures. And they don’t track who referred for it. They track who is doing it, what services are actually being offered. So radiology is a department that brings in money because procedures cost money. It’s expensive equipment. And it’s a valuable ‘service’. So when it comes to budget time, it looks like they’re in the green. So when they want new staff and new support, it’s easy for them to argue to their administration, they need this.
The invisibility of downstream revenue makes it difficult for some institutions to justify expanding genetic services. While many clinicians have resigned themselves to economic realities of healthcare financing, some are uncomfortable with this shift.
Moreover, providers in the academic medical center reported that they believe the university’s commitment to the Wisconsin Idea has weakened over time, by aligning its goals (especially the university’s medical center) more closely with those of the private sector. Administrators in the university hospital have increased pressure for productivity on providers, subjecting providers to the same market pressures that have led private sector hospitals to chase downstream revenue. An academic clinician who provides care in genetic outreach clinics said:
We were [originally] very intent on having these things be community based. Who’s here who can provide these services that your child needs? Or what’s the easiest, least inconvenient place we can send you to where you can get those? Whereas now when … I go to [an outreach clinic], the understanding is, when kids need to see a cardiologist, they’re going to see one of ours.
Later in the interview, this clinician mused, “Our hospital’s outreach focus is becoming more and more concentrated on strategic partnerships. The emphasis is becoming more and more on what are we going to get out of this relationship instead of how can we best serve the people in the state of Wisconsin.”
State administrators believed the potential for downstream revenue should motivate hospital administrators to participate in outreach clinic activities. As one of the state planners said:
Although the geneticists themselves may not make money for the center, certainly if the people need referral for diagnostic studies, like MRIs or bone scans, or treatment, including surgery or medical treatment, then the center has an opportunity to make that into a profitable situation. So we’re thinking that it’s really up to the geneticists locally to sell this to their institutions, to sell their services.
In an environment where state and federal resources are shrinking, state planners are pressured to maximize every dollar they spend, eroding state support for discretionary activities like specialty outreach clinics. Accordingly, they have increasingly high expectations that academic and private healthcare systems will take on responsibilities for subsidizing genetic services, freeing up state program dollars for system building activities and program evaluation. As one state planner said:
What we’d like to see is that our money would provide the sort of grease or lubrication to make these services work but that the reimbursement really should come from insurance, or Medicaid, or national healthcare system of some sort, or from the institutions themselves. And I’m not sure exactly how that could be desirable for them if they’re not making money from it. But it doesn’t give us a chance to use our money to really get massive change. We’re affecting, I think, very few people the way we’re using the money.
These assumptions, however, may conflict with the focus that healthcare institutions of all kinds are taking on the bottom line, and accounting practices that render invisible the benefits generated by smaller specialties, such as downstream revenue. Because the MCH pyramid ignores partnership formation, it is not especially well suited to considering how broader environmental change may affect the ability and willingness of partners to cooperate.
Discussion
Recent expansions in genetic knowledge have increased pressure on healthcare professionals and healthcare institutions to provide genetic services, but as with other healthcare services, there are a host of barriers in expanding access. This close examination of one state’s attempts to improve access through state-supported outreach clinics has identified a number of common barriers. First, SHAs are subject to a variety of state and federal mandates to support certain public health programs and activities (e.g., family planning services, disease surveillance programs), which limit their ability to support discretionary programs. Second, expertise in healthcare specialties like genetics may be concentrated in academic medical centers and private health systems, where administrators and providers are increasingly subject to market-driven dynamics to maximize profit. Although genetic providers may identify patients who will require subsequent follow up and specialty care, cost accounting systems often make those contributions to the overall bottom line invisible.
Most importantly, our findings show that the way state agencies and healthcare institutions respond to these budgetary pressures and market dynamics can have a substantial impact on the number of people and the populations served by an outreach clinic program. In an era when expectations about the clinical relevance of genetics to general population health were expanding, the resources that officials could devote to outreach clinic activities were contracting. Our quantitative data and our interviews show that the program did not grow or evolve to accommodate the partners’ changing institutional capacity to support nonremunerative services. This illustrates how shifting organizational contexts, changing market dynamics, and motivations influence the formation of partnerships, shape their trajectories, and threaten their longevity.
The interview data shed light on the shortcomings of the planning models used by program planners. As we noted previously, this program was motivated primarily by the MCH pyramid. We have little evidence, however, to show that the program’s architects actually relied on the insights or principles of the MCH pyramid in developing the program. They did not survey the population to identify regions in the state with high demand or unmet need, and they did not systematically survey utilization trends over time to assess program performance. Our findings show that they made some assumptions about the desirability of public-private partnerships, but we also found that they were not able to think strategically about how to cultivate partnerships to best meet the unmet needs of the population, or how to form partnerships that would protect the interests of the participating stakeholders.
This shortcoming was most visible in the Milwaukee outreach clinics. By reassigning clinic locations, the program improved outreach to Latino communities, but they did not enjoy the same success in reaching African-American communities. This phenomenon could possibly reflect the mistrust that African-Americans hold toward healthcare institutions general, or toward genetic services specifically (Bates et al., 2005; Furr, 2002; Peters et al., 2004), but it probably also reflects the city’s specific and highly problematic history of race relations. Despite intense activism for civil rights and social justice throughout the 1960s, the city remains beleaguered by racial strife. Black political participation and voter turnout remain low, the city is one of the most heavily segregated in the US, and even throughout the economic boom of the 1990s, the city’s African-American neighborhoods were rife with crime, poverty, and unemployment (Jones, 2009). Whatever the specific reasons for the low turnout of African-Americans at Milwaukee’s clinics, the geographic redistribution of clinics came at a cost of greatly curtailed access to genetic services in rural communities. Taken together, these changes dramatically reduced the overall number of patients served. This suggests that in order to successfully mitigate access barriers, outreach clinic programs need to be designed not only in consideration of population-based data on unmet need, and in cooperation with trusted partners who are able to make programs locally successful.
Although some thought leaders in public health genomics have argued that the Core Public Health Functions model is useful for guiding the integration of genetics into state public health programs (Beskow et al., 2001; Wang & Watts, 2007a), it is not clear that the Core Functions model would have performed any better in averting the difficulties the Wisconsin program administrators encountered. In the course of doing this research, we asked the program administrators about their exclusive reliance on the MCH pyramid, and whether they thought the Core Public Health Functions model would give them more flexibility. They believed quite firmly that because the genomics activities were funded through the Title V block grant, they needed to hew to the MCH pyramid, and furthermore, that the source of their funding restricted their ability to spend funds on clinical services that would benefit adults. One of them said,
[Respondent]: Yeah but the focus is maternal and child. Because we have MCH priorities in the contract language as well. So it is maternal and child health focused. But yeah, it is kind of this [pause] challenge or I don’t know what exactly it would be. It’s kind of how things were set up compared to where things are now. It’s slightly different.
[Interviewer]: So that scares you off a little bit from doing too much adult stuff?
[Respondent]: Not necessarily. We can’t do certain objectives within the MCH contract. We’ve considered some in the past but have decided not to pursue them in that way. But I’m often trying to do them under my other duties versus putting them in the contract. Because we have to report our activities back to the feds. So. We always need to point out how it’s impacting mothers and children.
In any case, neither model specifically offers any mandate to address disparities in access—geographic or sociodemographic. And while the Core Functions model does include partnership development as an activity (in the Policy Development realm), it does not counsel policymakers on how to develop partnerships that are sensitive to the changing financial and institutional demands that their partners face. Over the past five years, some policymakers within HRSA have proposed revisiting the MCH pyramid to incorporate a life-course perspective or to address social and environmental determinants of health (Fine & Kotelchuck, 2010; Fine, Kotelchuck, Adess, & Pies, 2009; Fraser, 2012). In some respects, these proposals may be an improvement. Life course theory stresses how adverse events in childhood and early adolescence (such as poverty, economic inequality, and health disparities) have continued negative impacts well into adulthood (Fine et al., 2009; Fine & Kotelchuck, 2010; Fraser, 2012; Lu et al., 2010). But this ongoing dialogue is in its infancy, and it is not clear how it will change the models that guide program planning at HRSA or the way Title V grant dollars are spent at the state level.
These shortcomings of planning models can be more widely appreciated when we examine genetic outreach clinic activities in other states, and how variably states apply them. As noted in the introduction, many SHAs developed outreach clinic programs in the 1970s, and, like Wisconsin, some states continue those activities to this day. These clinics are typically supported by a mix of federal grants, state funds, service fees, or private sources (Genetic Services Policy Project Final Report, 2008). Washington State, for example, has a network of 15 genetic outreach clinics, 7 of which are state supported. A study of utilization trends at these clinics found that demand escalated significantly between 1995–2004, with growth being highest among patients 35 and older (Wang & Watts, 2007b). In 2009, Iowa passed legislation that established a Regional Genetic Consultation Service (RGCS; Iowa Admin. Code r. 641–4.5/136A 2009). The RGCS subcontracts with the University of Iowa to provide genetic counseling and comprehensive genetic services to individuals and families in the state of Iowa. Where possible, services are billed to a patient’s insurance, but if the patient is uninsured or if the insurance is inadequate, dedicated state funding fills the gap. In 2012, the RCGS provided services to some 585 patients, approximately 15% of who were over aged 20 (“Regional Genetic Consultation Service Annual Report, 2012,” 2013). But Iowa is fairly unusual in this respect—like Wisconsin, most SHAs are increasingly relying on outreach activities provided by academic medical centers, while still providing some gap-filling services in locations that remain underserved. The state genetics plan for Tennessee serves as a good example of this. Clinical genetic services are available at the three academic medical centers in the state (at Vanderbilt University, the University of Tennessee at Knoxville, and the University of Tennessee at Memphis) all of which provide on-site services, but which also offer outreach clinic services at satellite locations around the state. In addition to this network of clinics, the state funds a Sickle Cell clinic at Meharry Medical College, in Nashville (Tennesee Genetics Plan, 2003).
When we look at other types of outreach clinic activities funded through Title V funds, however, we see important contradictions in how the MCH pyramid is applied. For example, Kentucky supports a system of outreach clinics for its Children and Youth with Special Health Care Needs program (CYSHCN), but identifies these clinics as an “infrastructure building” activity, not as direct clinical care (Title V MCH Block Grant Five-Year Needs Assessment, 2010). This suggests that program planners have at least some discretion in determining which tier of the pyramid to classify program activities. While this may give the states some flexibility to tailor Title V activities according to the needs of their populations, it raises some question about the universality or applicability of the MCH pyramid as a planning model. Moreover, the documented increases in demand for genetic services among adults at these state-supported clinics lends further evidence to the assertion that genetics will become increasingly relevant in regular medical care, and yet the MCH pyramid does not accommodate programming oriented to adult populations.
The mixed methods design is a key strength of this study. Our quantitative data shows how changes in financing and clinic locations influenced the number of people served and the populations served. We were, however, limited in our ability to report more precisely the characteristics of the patients and communities benefiting from these outreach clinic activities. Future research should draw upon individual-level patient data to simultaneously control for factors that are hypothesized to limit access to services (e.g., race, income, health insurance status).
State health officials are potentially in a position to mitigate barriers in access to specialty healthcare services such as genetic services. They might do so through developing partnerships to meet some of these needs or they might provide gap-filling clinical services where needed. However, the institutional landscapes and rewards surrounding potential partners motivate or limit their ability to participate in programs to subsidize care for vulnerable populations. Public health policymakers hoping to build public-private partnerships need to pay close attention the changes in these landscapes. These findings also emphasize the importance of choosing partners who are highly trusted in the communities they serve, especially if those communities have been marginalized historically. Not all partnerships are created equal. Enhancing access to specialty healthcare services is both a legitimate function of state health agencies and crucially important in ensuring that the genetic innovations do not exacerbate current health inequalities.
Acknowledgments
This material is based upon work supported by the National Institute of Food and Agriculture, United States Department of Agriculture, under ID number MSN136524 and by the National Human Genome Research Institute of the National Institutes of Health, under award number 1K01HG006441-01A1. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the United States Department of Agriculture or the National Institutes of Health.
The authors thank Rozalynn Klaas for creating the maps that appear in Figures 3 and 4 and Jessica von Reyn, Phil Brown, Michelle Kempf-Weibel, Rachael Lee, Michael Shields, Berit Lindell, Lauren Nicoll, John Auerbach, and two anonymous reviewers for comments on a draft version of this paper. The authors also thank Pilar Ossorio and Daniel Kleinman at the University of Wisconsin-Madison and Deb Franko at Northeastern University for their support and mentorship in the execution of this research.
References
- Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293:1729–1736. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- Association of State and Territorial Health Officials. Newborn screening position statement. Arlington, VA: Association of State and Territorial Health Officials; 2012. Retrieved from http://www.astho.org/Policy-and-Position-Statements/Newborn-Screening-Position-Statement/ [Google Scholar]
- Bates BR, Lynch JA, Bevan JL, Condit CM. Warranted concerns, warranted outlooks: A focus group study of public understandings of genetic research. Social Science & Medicine. 2005;60:331–344. doi: 10.1016/j.socscimed.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Bernhardt BA, Pyeritz RE. The organization and delivery of clinical genetics services. Pediatric Clinics of North America. 1992;39:1–12. doi: 10.1016/s0031-3955(16)38259-1. [DOI] [PubMed] [Google Scholar]
- Beskow LM, Khoury MJ, Baker TG, Thrasher JF. The integration of genomics into public health research, policy and practice in the United States. Community Genetics. 2001;4:2–11. doi: 10.1159/000051150. [DOI] [PubMed] [Google Scholar]
- Buchanan AH, Skinner CS, Calingaert B, Schildkraut JM, King RH, Marcom PK. Cancer genetic counseling in rural North Carolina oncology clinics: Program establishment and patient characteristics. Community Oncology. 2009;6:70–77. [Google Scholar]
- Burke W, Burton H, Hall AE, Karmali M, Khoury MJ, Knoppers B, … Zimmern RL. Extending the reach of public health genomics: What should be the agenda for public health in an era of genome-based and “personalized” medicine? Genetics in Medicine. 2010;12:785–791. doi: 10.1097/GIM.0b013e3182011222. [DOI] [PubMed] [Google Scholar]
- Canin L, Wunsch B. Specialty care in the safety net: Efforts to expand timely access. Oakland, CA: California HealthCare Foundation; 2009. [Google Scholar]
- Casey MM, Thiede Call K, Klingner JM. Are rural residents less likely to obtain recommended preventive healthcare services? American Journal of Preventive Medicine. 2001;21:182–188. doi: 10.1016/s0749-3797(01)00349-x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. CDC Health Disparities and Inequalities Report—United States, 2011. MMWR. 2011;60(Suppl):1–2. [PubMed] [Google Scholar]
- Chen H, Wertelecki W. Genetic services in the United States. Japanese Journal of Human Genetics. 1994;39:275–288. doi: 10.1007/BF01876849. [DOI] [PubMed] [Google Scholar]
- Chen H-Y, Baumgardner DJ, Frazer DA, Kessler CL, Swain GR, Cisler RA. Milwaukee health report 2012: Health disparities in Milwaukee by Socioeconomic Status. Milwaukee, WI: Center for Urban Population Health; 2013. [Google Scholar]
- Chen H-Y, Baumgardner DJ, Galvao J, Rice J, Swain GR, Cisler RA. Milwaukee health report 2011: Health disparities in Milwaukee by socioeconomic status. Milwaukee, WI: Center for Urban Population Health; 2011. [Google Scholar]
- Collins FS. Shattuck lecture—Medical and societal consequences of the Human Genome Project. New England Journal of Medicine. 1999;341:28–37. doi: 10.1056/NEJM199907013410106. [DOI] [PubMed] [Google Scholar]
- Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC: Institute of Medicine; 2009. [Google Scholar]
- Cook NL, Hicks LS, O’Malley AJ, Keegan T, Guadagnoli E, Landon BE. Access to specialty care and medical services in community health centers. Health Affairs. 2007;26:1459–1468. doi: 10.1377/hlthaff.26.5.1459. [DOI] [PubMed] [Google Scholar]
- Cooksey JA, Forte G, Benkendorf J, Blitzer MG. The state of the medical geneticist workforce: Findings of the 2003 survey of American Board of Medical Genetics certified geneticists. Genetics in Medicine. 2005;7(6):439–443. doi: 10.1097/01.gim.0000172416.35285.9f. [DOI] [PubMed] [Google Scholar]
- Crump RL, Gaston MH, Fergerson G. HRSA’s Models That Work Program: Implications for improving access to primary health care. Public Health Reports. 1999;114:218–224. doi: 10.1093/phr/114.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derose KP, Gresenz CR, Ringel JS. Understanding disparities in health care access—and reducing them—Through a focus on public health. Health Affairs. 2011;30:1844–1851. doi: 10.1377/hlthaff.2011.0644. [DOI] [PubMed] [Google Scholar]
- Epstein CJ, Erickson RP, Hall BD, Golbus MS. The Center-satellite system for the wide-scale distribution of genetic counseling services. American Journal of Human Genetics. 1975;27:322–332. [PMC free article] [PubMed] [Google Scholar]
- Fine A, Kotelchuck M. Rethinking MCH: The Life Course Model as an Organizing Framework. Washington, DC: U.S. Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau; 2010. [Google Scholar]
- Fine A, Kotelchuck M, Adess N, Pies C. A new agenda for MCH policy and programs. Martinez, CA: Contra Costa Health Services; 2009. [Google Scholar]
- Ford EW, Duncan WJ, Ginter PM. Health departments’ implementation of public health’s core functions: An assessment of health impacts. Public Health. 2005;119:11–21. doi: 10.1016/j.puhe.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Forman AD, Hall MJ. Influence of race/ethnicity on genetic counseling and testing for hereditary breast and ovarian cancer. The Breast Journal. 2009;15(Suppl 1):S56–62. doi: 10.1111/j.1524-4741.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- Forrest CB. Primary care in the United States: Primary care gatekeeping and referrals: Effective filter or failed experiment? BMJ. 2003;326:692–695. doi: 10.1136/bmj.326.7391.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser MR. Bringing it all together: Effective maternal and child health practice as a means to improve public health. Maternal & Child Health Journal. 2012;17:767–775. doi: 10.1007/s10995-012-1064-1. [DOI] [PubMed] [Google Scholar]
- Frieden TR. A framework for public health action: The health impact pyramid. American Journal of Public Health. 2010;100:590–595. doi: 10.2105/AJPH.2009.185652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr LA. Perceptions of genetics research as harmful to society: Differences among samples of African-Americans and European-Americans. Genetic Testing. 2002;6:25–30. doi: 10.1089/109065702760093889. [DOI] [PubMed] [Google Scholar]
- Gamble VN. A legacy of distrust: African Americans and medical research. American Journal of Preventive Medicine. 1993;9(Suppl 6):35–38. [PubMed] [Google Scholar]
- Glaser B, Strauss A. The Discovery of Grounded Theory. Hawthorne, NY: Aldine Publishing; 1967. [Google Scholar]
- Goldman JS, Hahn SE, Catania JW, Larusse-Eckert S, Butson MB, Rumbaugh M, … Bird T. Genetic counseling and testing for Alzheimer disease: Joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genetics in Medicine. 2011;13:597–605. doi: 10.1097/GIM.0b013e31821d69b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf MD, Needham DF, Teed N, Brown T. Genetic testing insurance coverage trends: A review of publicly available policies from the largest US payers. Personalized Medicine. 2013;10:235–243. doi: 10.2217/pme.13.9. [DOI] [PubMed] [Google Scholar]
- Guttmacher AE, Collins FS. Realizing the promise of genomics in biomedical research. JAMA. 2005;294:1399–1402. doi: 10.1001/jama.294.11.1399. [DOI] [PubMed] [Google Scholar]
- Harrell JA, Baker EL. The essential services of public health. Leadership in Public Health. 1994;3:27–31. [Google Scholar]
- Hawkins AK, Hayden MR. A grand challenge: Providing benefits of clinical genetics to those in need. Genetics in Medicine. 2011;13:197–200. doi: 10.1097/GIM.0b013e31820c056e. [DOI] [PubMed] [Google Scholar]
- Henry S, Pauli RM, Katcher ML. Genetic services plan for Wisconsin. Wisconsin Medical Journal. 2005;104:13–15. [PubMed] [Google Scholar]
- Ho C. How to develop and implement a cancer genetics risk assessment program. Oncology Issues. 2004;19(Nov/Dec):22–26. [Google Scholar]
- Institute of Medicine. The Future of Public Health. Washington, DC: National Academies Press; 1988. [Google Scholar]
- Institute of Medicine. Quality through Collaboration: The Future of Rural Health. Washington, DC: National Academies Press; 2005. [Google Scholar]
- Jones PD. The Selma of the North: Civil Rights Insurgency in Milwaukee. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- Kaye CI, Laxova R, Livingston JE, Lloyd-Puryear MA, Mann M, McCabe ER, Therrell BL. Integrating genetic services into public health—Guidance for state and territorial programs from the National Newborn Screening and Genetics Resource Center (NNSGRC) Community Genetics. 2001;4:175–196. doi: 10.1159/000051179. [DOI] [PubMed] [Google Scholar]
- Kaye CI, Livingston J, Canfield MA, Mann MY, Lloyd-Puryear MA, Therrell BL., Jr Assuring clinical genetic services for newborns identified through US newborn screening programs. Genetics in Medicine. 2007;9:518–527. doi: 10.1097/gim.0b013e31812e6adb. [DOI] [PubMed] [Google Scholar]
- Keane CR, Weerasinghe MC. Public/private mix in health systems. In: Carrin G, editor. Health Systems Policy, Finance, and Organization. San Diego, CA: Elsevier; 2009. pp. 314–321. [Google Scholar]
- Kelly SE. “New” genetics meets the old underclass: Findings from a study of genetic outreach services in rural Kentucky. Critical Public Health. 2002;12:169–186. [Google Scholar]
- Kentucky Department for Public Health. Title V MCH Block Grant Five-Year Needs Assessment. Frankfort, KY: Kentucky Department for Public Health; 2010. Title V MCH Block Grant Five-Year Needs Assessment. [Google Scholar]
- Khoury MJ, Berg A, Coates R, Evans J, Teutsch SM, Bradley LA. The evidence dilemma in genomic medicine. Health Affairs. 2008;27:1600–1611. doi: 10.1377/hlthaff.27.6.1600. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Feero WG, Reyes M, Citrin T, Freedman A, Leonard D, et al. The genomic applications in practice and prevention network. Genetics in Medicine. 2009;11:488–494. doi: 10.1097/GIM.0b013e3181a551cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey SL, Williams J, Pierre-Louis J, O’Riordan M, Matthews A, Robin NH. Attitudes of African American premedical students toward genetic testing and screening. Genetics in Medicine. 2003;5:49–54. doi: 10.1097/00125817-200301000-00008. [DOI] [PubMed] [Google Scholar]
- Leavitt JW. The healthiest city. Madison, WI: University of Wisconsin Press; 1996. [Google Scholar]
- Leischow SJ, Best A, Trochim WM, Clark PI, Gallagher RS, Marcus SE, Matthews E. Systems thinking to improve the public’s health. American Journal of Preventive Medicine. 2008;35(Suppl 2):S196–S203. doi: 10.1016/j.amepre.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessick ML, Townes PL. Genetic health care: Community approach to delivery by demonstration satellite clinic project. New York State Journal of Medicine. 1979;79:199–203. [PubMed] [Google Scholar]
- Levi J, Juliano C, Richardson M. Financing public health: Diminished funding for core needs and state-by-state variation in support. Journal of Public Health Management and Practice. 2007;13(2):97–102. doi: 10.1097/00124784-200703000-00004. [DOI] [PubMed] [Google Scholar]
- Levi J, St Laurent R, Segal LM, Vinter S. Shortchanging America’s health: A state-by-state look at how public health dollars are spent and key state health facts. Washington, DC: Trust for America’s Health; 2010. [Google Scholar]
- Lincoln YS, Guba EG. Naturalistic Inquiry. Newbury Park, CA: Sage; 1985. [Google Scholar]
- Lowry RB, Bowen P. The Alberta Hereditary Diseases Program: A regional model for delivery of genetic services. CMAJ: Canadian Medical Association Journal. 1990;142:228–232. [PMC free article] [PubMed] [Google Scholar]
- Lu MC, Kotelchuck M, Hogan V, Jones L, Wright K, Halfon N. Closing the black-white gap in birth outcomes: A life-course approach. Ethnicity & Disease. 2010;20(1 Suppl 2):62–76. [PMC free article] [PubMed] [Google Scholar]
- MacDonald DJ, Blazer KR, Weitzel JN. Extending comprehensive cancer center expertise in clinical cancer genetics and genomics to diverse communities: The power of partnership. Journal of the National Comprehensive Cancer Network : JNCCN. 2010;8:615–624. doi: 10.6004/jnccn.2010.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA. Bringing genome-wide association findings into clinical use. Nature Reviews Genetics. 2013;14:549–558. doi: 10.1038/nrg3523. [DOI] [PubMed] [Google Scholar]
- Mays GP, Scutchfield FD, Bhandari MW, Smith SA. Understanding the organization of public health delivery systems: An empirical typology. The Milbank Quarterly. 2010;88:81–111. doi: 10.1111/j.1468-0009.2010.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless SE, Brunger JW, Cassidy SB. The burden of genetic disease on inpatient care in a children’s hospital. American Journal of Human Genetics. 2004;74:121–127. doi: 10.1086/381053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson E, Zaleski C, Benishek K, McCarty CA, Giampietro PF, Reynolds K, Rasmussen K. Clinical genetics provider real-time workflow study. Genetics in Medicine. 2008;10:699–706. doi: 10.1097/gim.0b013e318182206f. [DOI] [PubMed] [Google Scholar]
- National Coalition for Health Professional Education in Genetics. Core competencies in genetics for health professionals, third edition. Lutherville, MD: National Coalition for Health Professional Education in Genetics; 2007. [Google Scholar]
- Office of the Commissioner of Insurance. Health insurance coverage in Wisconsin. Madison, WI: State of Wisconsin; 2009. No. PI-094. [Google Scholar]
- Palma M, Ristori E, Ricevuto E, Giannini G, Gulino A. BRCA1 and BRCA2: The genetic testing and the current management options for mutation carriers. Critical Reviews in Oncology/Hematology. 2006;57:1–23. doi: 10.1016/j.critrevonc.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Peters N, Rose A, Armstrong K. The association between race and attitudes about predictive genetic testing. Cancer Epidemiology, Biomarkers & Prevention. 2004;13:361–365. [PubMed] [Google Scholar]
- Petersen GM, Brensinger JD, Johnson KA, Giardiello FM. Genetic testing and counseling for hereditary forms of colorectal cancer. Cancer. 1999;86(Suppl 11):2540–2550. doi: 10.1002/(sici)1097-0142(19991201)86:11+<2540::aid-cncr11>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Powell-Young YM, Spruill IJ. Views of Black nurses toward genetic research and testing. Journal of Nursing Scholarship. 2013;45:151–159. doi: 10.1111/jnu.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regional Genetic Consultation Service Annual Report, 2012. Des Moines, IA: Iowa Department of Public Health; 2013. [Google Scholar]
- Reich MR, editor. Public-Private Partnerships for Public Health. Cambridge, MA: Harvard Center for Population and Development Studies; 2002. [Google Scholar]
- Reid KJ, Sakati N, Prichard LL, Schneiderman LJ, Jones OW, Dixson BK. Genetic counseling. An evaluation of public health genetic clinics. The Western Journal of Medicine. 1976;124:6–12. [PMC free article] [PubMed] [Google Scholar]
- Riccardi VM. Health care and disease prevention through genetic counseling: A regional approach. American Journal of Public Health. 1976;66:268–272. doi: 10.2105/ajph.66.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secretary’s Advisory Committee on Genetics, Health, and Society. Coverage and Reimbursement of Genetic Tests and Services. Bethesda, MD: U.S. Department of Health and Human Services; 2006. [Google Scholar]
- Spruill I, Coleman B, Collins-McNeil J. Knowledge, beliefs and practices of African-American nurses regarding genetics/genomics. Journal of National Black Nurses’ Association: JNBNA. 2009;20:20–24. [PubMed] [Google Scholar]
- Stark J. The Wisconsin Idea: The University’s Service to the State. Madison, WI: Legislative Reference Bureau; 1996. [Google Scholar]
- Suther S, Kiros GE. Barriers to the use of genetic testing: A study of racial and ethnic disparities. Genetics in Medicine. 2009;11:655–662. doi: 10.1097/GIM.0b013e3181ab22aa. [DOI] [PubMed] [Google Scholar]
- Tennessee Department of Health. Tennessee genetics plan. Columbia, TN: Tennessee Department of Health; 2003. [Google Scholar]
- Thornton PH. The rise of the corporation in a craft industry: Conflict and conformity in institutional logics. Academy of Management Journal. 2002;45:81–101. [Google Scholar]
- Turnock BJ, Atchison C. Governmental public health in the United States: The implications of federalism. Health Affairs. 2002;21:68–78. doi: 10.1377/hlthaff.21.6.68. [DOI] [PubMed] [Google Scholar]
- University of Washington. Genetic services policy project final report. Seattle, WA: University of Washington; 2008. [Google Scholar]
- U.S. Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. 2012 Retrieved from http://www.census.gov/geo/reference/ua/urban-rural-2010.html.
- U.S. Maternal and Child Health Bureau. Maternal and Child Health Bureau Strategic Plan: FY 2003–2007. Rockville, MD: Author; 2003. [Google Scholar]
- Wang G, Watts C. The role of genetics in the provision of Essential Public Health Services. American Journal of Public Health. 2007a;97:620–625. doi: 10.2105/AJPH.2006.087791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Watts C. Utilization trends of genetic clinics excluding prenatal services in Washington State, 1995–2004. Genetics in Medicine. 2007b;9:713–718. doi: 10.1097/gim.0b013e318156abec. [DOI] [PubMed] [Google Scholar]
- Wang G, Beattie MS, Ponce NA, Phillips KA. Eligibility criteria in private and public coverage policies for BRCA genetic testing and genetic counseling. Genetics in Medicine. 2011;13:1045–1050. doi: 10.1097/GIM.0b013e31822a8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman JS, Moy E, Campbell EG, Gokhale M, Yucel R, Causino N, Blumenthal D. Limits to the safety net: Teaching hospital faculty report on their patients’ access to care. Health Affairs. 2003;22:156–166. doi: 10.1377/hlthaff.22.6.156. [DOI] [PubMed] [Google Scholar]
- Zhang P, Tao G, Irwin KL. Utilization of preventive medical services in the United States: A comparison between rural and urban populations. Journal of Rural Health. 2000;16:349–356. doi: 10.1111/j.1748-0361.2000.tb00485.x. [DOI] [PubMed] [Google Scholar]