Abstract
Objectives
The positive predictive value (PPV) of a single assessment of estimated glomerular filtration rate (eGFR) in the diagnosis of chronic kidney disease (CKD) is not known. Our objective was to determine the PPV of a single assessment of eGFR among adults with at least one eGFR <60 mL/min in their lifetime, using the Distributed Area Research and Therapeutics Network CKD natural history dataset.
Methods
In all, 47,104 adults who were cared for by 113 practices in the United States were included. Proportions of patients in eGFR categories at baseline were calculated using the following categories: <15 mL/min, 15 to 29.99 mL/min, 30 to 44.99 mL/min, and 45 to 59.99 mL/min. Comparisons were then made between the baseline and the endpoint to identify patients who had a follow-up eGFR that remained at <60 mL/min. The proportions of patients in each eGFR category were compared baseline to endpoint using cross-tabulations. To test the proposed cutpoint, the proportions of patients who had an eGFR that remained at <60 mL/min were measured, using the cutpoints that included the highest cumulative proportion of patients. The sensitivity and specificity of that cutpoint were calculated.
Results
A cutpoint of <45 mL/min was identified, yielding a PPV of 93% with a sensitivity of 28% and a specificity of 94%.
Conclusions
A valid cutpoint to screen for CKD was identified. This cutpoint may prove important to early screening for CKD while reducing the burden on the healthcare system and patients suspected of having CKD.
Keywords: chronic kidney disease, glomerular filtration rate, screening, positive predictive value
The US Preventive Services Task Force recommendation statement for screening of chronic kidney disease (CKD) states that adequate evidence does not exist to support the screening of asymptomatic adults for CKD.1 The American College of Physicians guideline on CKD stages 1 to 3 agrees that the validity of a single assessment of estimated glomerular filtration rate (eGFR) to screen for CKD is not known.1,2 The question remains whether a single eGFR <60 mL/min, independent of data on other risk factors, can accurately predict CKD in adults. In other words, the positive predictive value (PPV) of a single low eGFR for CKD at present is not known.2 Targeted screening is recommended for patients at higher risk for CKD, such as those with diabetes mellitus (DM), hypertension (HTN), older age, and obesity.3–5 Given the aging of the population and increasing prevalence of obesity-related disease, the future of screening for CKD will expand to populations not presently recommended to undergo screening. The serum creatinine test is widely preformed in primary care, making the predictive value of a single low eGFR clinically relevant and manageable. Using a large, national primary care dataset, the present study addresses the impact of opportunistic testing in primary care for detecting CKD.
We anticipated that among older patients (older than 60 years), a viable cutpoint could be identified and assessed in future studies. This is important to how we identify and classify patients, while minimizing the burden when diagnosing CKD. It also is integral in reducing the cost of care by more precisely identifying the most appropriate higher-risk patients and minimizing the use of unnecessary testing. In addition, understanding the utility of this assessment may influence the adoption of the 2012 Kidney Disease Improving Global Outcomes (KDIGO) guidelines.5
Methods
The source for this analysis is the Distributed Area Research and Therapeutics Network (DARTNet) CKD natural history dataset. This was a data extract of electronic health record (EHR) data from 113 practices from more than 20 states from New York to California. It included a number of federally qualified health centers and some predominantly African American and Hispanic practices. No Native American practices were part of this study. This study was deemed exempt by the institutional review board of the State University of New York at Buffalo. Data use agreements were obtained from the DARTNet Institute, which maintains business associate agreements with all of the sites included in this dataset. The extracted data from each clinical organization then were entered into a clinical data repository (CDR) maintained at each site for research and clinical support activities. These data were moved to a central location and merged into a single dataset. At this time, final local personal health identifiers were removed and replaced. For instance, the local patient identifier (in this case, the local CDR patient identification number) was converted to a random global universal identifier. The data underwent a series of data checks to ensure that no personal health identifiers were retained in the data fields, and the final limited dataset was securely transferred to the research team.
All periods of time were included in the local organizations’ EHRs, which varied from several years to >10. The study data were captured on November 1, 2011, and contained clinical data dating back to 2003, with an average of 3.5 years of data. The medical organizations included 19 different EHR products, with all data from each system standardized across sites at the time of local extraction from the EHR to the local CDR. Patients were included in the dataset if they had one eGFR under 60 mL/min in their lifetime. Data elements included in the data extraction are detailed in Table 1. For this analysis, creatinine, age, and sex (race was not available in this dataset) were used to calculate the eGFR. Because most of the creatinine values were not standardized in this data extract, the Modification of Diet in Renal Disease study equation for nonstandardized creatinine measures was used.6
Table 1.
Data elements included in the chronic kidney disease natural history dataset
| Alanine aminotransferase | Hemoglobin A1c |
| Aspartate aminotransferase | 25 OH vitamin D |
| Sex | Serum phosphorus |
| Age | Parathyroid hormone intact |
| Smoking status | All medications |
| Height | Diagnoses active |
| Weight | Diagnoses inactive |
| Body mass index | Blood pressure |
| Total no. physician visits | Estimated glomerular filtration rate |
| Hemoglobin | High-density lipoprotein |
| Urine:albumin creatinine ratio | Low-density lipoprotein |
Patient Inclusion Criteria
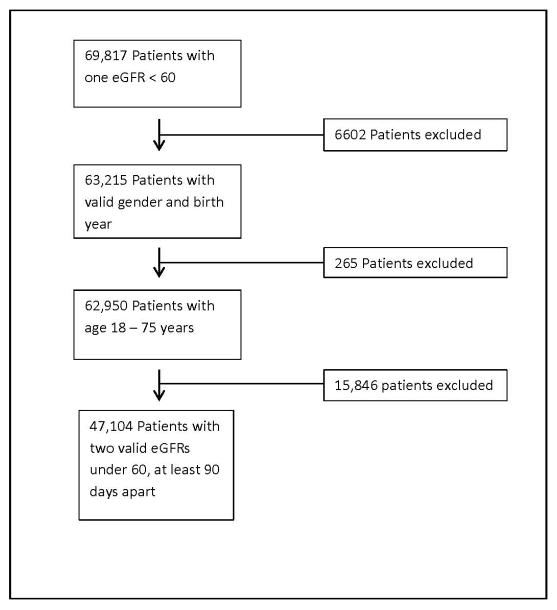
Patients were included if they had one eGFR in their lifetime <60 mL/min alone, which yielded 69,817 patients. Patients without a valid sex and birth year were excluded, yielding 63,215 patients. Furthermore, only patients 18 to 75 years were included, resulting in a total of 62,950 patients. eGFR was then calculated from creatinine. Only those patients having a baseline eGFR <60 mL/min were included. Finally, a follow-up eGFR was calculated at least 90 days later, yielding a total of 47,104 patients (Fig.).
Fig.
Flow of patient inclusion criteria. eGFR, estimated glomerular filtration rate.
To calculate eGFR from creatinine tests, the following procedure was used (Modification of Diet in Renal Disease study equation, nonstandardized):
Statistical Analysis
Descriptive data were assessed by mean ± standard deviation for continuous variables and cross-tabulation for categorical variables. Patients were categorized by eGFR cutpoints: <15 mL/min, 15 to 29.99 mL/min, 30 to 44.99 mL/min, and 45 to 59.99 mL/min. The proportion of patients in each category was compared with the proportion of patients having an eGFR that remained <60 mL/min at endpoint. Subgroup analyses by sex and age and DM HTN status were then performed using the same eGFR categories.
To test the cutpoint, the proportions of patients who had an eGFR that remained <60 mL/min at endpoint were studied using the cutpoints that cumulatively involved the highest proportion of patients. To assess the validity of this cutpoint, the sensitivity, specificity, and PPV were calculated. All of the analyses were conducted using SPSS version 23 (IBM SPSS Statistics, Armonk, NY).
Results
In all, 61% of patients were women (mean age 72 years). In addition, 31% had DM, 69% had HTN, and 28% had DM and HTN. Among patients with a baseline eGFR <15, 84% (confidence interval [CI] 0.82–0.86) had an eGFR that remained at <60 mL/min. In the 15 to 29.99 mL/min category, 97% (CI 0.96–0.99) had an endpoint eGFR <60 mL/min. Among patients with an eGFR of 30 to 44.99 mL/min, 95% (CI 0.90–0.99) had an eGFR <60 mL/min, and among patients with a baseline eGFR 45 to 59.99 mL/min, 77% (CI 0.69–0.78) had an endpoint eGFR <60 mL/min (Table 2). Similar results were discovered by sex (Table 3).
Table 2.
Proportion of patients with a baseline eGFR in each category who remained <60 mL/min/1.73 m2 at endpoint
| GFR range | n | % | |
|---|---|---|---|
| Males | <15 mL/min/1.73 m2 | 220 | 80 |
| 15–29.99 mL/min/1.73 m2 | 421 | 87 | |
| 30–44.99 mL/min/1.73 m2 | 1528 | 93 | |
| 45–59.99 mL/min/1.73 m2 | 4605 | 70 | |
| Females | <15 mL/min/1.73 m2 | 222 | 80 |
| 15–29.99 mL/min/1.73 m2 | 621 | 97 | |
| 30–44.99 mL/min/1.73 m2 | 2915 | 94 | |
| 45–59.99 mL/min/1.73 m2 | 8002 | 71 |
eGFR, estimated glomerular filtration rate.
Table 3.
Proportion of patients by sex with a baseline eGFR in each category who remained <60 mL/min/1.73 m2 at endpoint
| eGFR range | Total remaining <60 mL (n) | % | Total in eGFR group |
|---|---|---|---|
| <15 mL/min/1.73 m2 | 442 | 80 | 551 |
| 15–29.99 mL/min/1.73 m2 | 1011 | 97 | 1042 |
| 30–44.99 mL/min/1.73 m2 | 4443 | 94 | 4728 |
| 45–59.99 mL/min/1.73 m2 | 12,607 | 70 | 17,927 |
| 60–89 mL/min/1.73 m2 | 12,214 | 58 | 21,066 |
| >90 mL/min/1.73 m2 | 863 | 48 | 1788 |
eGFR, estimated glomerular filtration rate.
The results of the subgroup analyses by age (younger than 45, 45–60, and older than 60 years) reveal that among patients who were younger than 45 years old and in the <15 eGFR category, there was a PPV equal to 83% (CI 0.79–0.86). In the 15 to 29.99 mL/min and 30 to 44.99 mL/min categories, we observed PPVs of 86% (CI 0.76–0.93) and 85% (CI 0.78–0.90), respectively. In the same age group (younger than 45 years), patients in the baseline eGFR categories of 45 to 59.99 mL/min demonstrated a PPV of 72% (CI 0.67–0.76). Among patients in the 45 to 60 years old age group we found PPVs ranging from a high of 90% in the 30 to 44.99 mL/min group to a low of 62% in the 45 to 59.99 mL/min group. Finally, for patients in the older than 60 years age group, the PPVs ranged from a high of 98% (CI 0.93–0.99) to a low of 77% (CI 0.72–0.81), from the <15 mL/min category to the 45 to 59.99 mL/min category.
Analysis by DM and HTN Status
Subgroup analysis by DM status (n = 14,511, 31%) revealed similar results, with PPVs ranging from a high of 98% (CI 0.96–0.99) among patients with a beginning eGFR of 15 to 29.99 mL/min to a low of 74% (CI 0.72–0.75) for the eGFR range of 45 to 59.99 mL/min. Subgroup analysis by HTN status (n = 36,436, 77%) revealed similar results, with PPVs ranging from a high of 97% (CI 0.96–0.98) among patients with a beginning eGFR of 15 to 29.99 mL/min to a low of 73% (CI 0.72–0.74) for the eGFR range of 45 to 59.99 mL/min. Among patients with DM and HTN (n = 13,108, 28%), results again were similar, with PPVs ranging from a high of 98% to a low of 75%.
Identification of an eGFR Cutpoint
Upon visual determination of the proportion of patients with an eGFR remaining <60 mL/min, within each beginning eGFR category, a cutpoint of <45 mL/min was identified. This cutpoint was then tested by calculating the PPV, specificity, and sensitivity. The PPV of a single assessment of eGFR <45 mL/min was equal to 93%, with a specificity of 97% and sensitivity equal to 20%. Further analyses by age identified the same cutpoint of <45 mL/min, with a PPV equal to 93%, a specificity of 94%, and a sensitivity of 28%. In addition, we tested the PPV of 50 mL/min and found that it equaled 90%, with a specificity of 94% and sensitivity of 28%.
Discussion
eGFR is the most widely used assessment of kidney function, globally adopted to define and stratify CKD into stages by severity. The eGFR also is used to monitor disease progression.7 Adoption of the 2002 National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines sparked major changes to routine laboratory reporting to include eGFR with decision supports (a set of related computer programs and the data required to assist with analysis and decision making within an organization) regarding CKD classification.8 This new definition of generic “disease” resulted in increases in nephrology referrals for the evaluation of presumed CKD whenever an eGFR <60 mL/min was reported.7,9 The validity and utility of this stand-alone measure, independent of data on other risk factors, have not been extensively studied, however. Critics of the 2002 KDOQI guidelines also raise concerns about misdiagnosis and overdiagnosis and misclassification of stage based on eGFR.9–14 For this reason we sought to examine the utility of a single assessment of eGFR to predict CKD.
Using a sufficiently large dataset, we identified a cutpoint of <45 mL/min for eGFR that could be proposed in the prediction of patients with CKD. Patients with an eGFR <45 mL/min should be prioritized and monitored more closely for progression. At a time when financial resources are limited and there is an emphasis on reducing the cost of care while maintaining quality, this finding will inform clinical decision making with regard to diagnosis and monitoring of CKD.
Adoption of the 2002 KDOQI guidelines has had a profound impact on awareness, population health management, patient care, and public health policy surrounding CKD.10 Clinical research on kidney disease has grown exponentially, and the adoption of a unified nomenclature for chronic kidney disease allows for comparison of outcomes across studies.10,15 One of the major aims of the 2002 guidelines was to facilitate the diagnosis of patients with early signs of CKD.9,13,16–18 The ability to detect CKD early can have important implications for disease prognosis and therefore early detection remains of critical importance.2,9–11,19–21
Our findings suggest a valid eGFR cutpoint to be used for predicting CKD. It is unclear whether the PPV of a single assessment of eGFR can be effective in diagnosing CKD.10 Among the more than 40,000 patients, it was found that at an eGFR of <45 mL/min was associated with a PPV of 93%. Similar results were found for sex, indicating that the effect of sex is minimal and likely allowing for a uniform cutpoint for both male and female patients. It is important to note that this cutpoint has clinical significance that was demonstrated in a meta-analysis by Sharma et al,22 indicating that patients with an eGFR <45 mL/min demonstrated higher overall mortality rates and cardiovascular mortality rates than patients with an eGFR between 45 and 60 mL/min.22
Analysis by age group (younger than 45, 46–60, and older than 60 years) reveals similar findings among the oldest patient subgroup with a beginning eGFR of 15 to 45 mL/min, with reduced PPVs in the 46 to 60 and younger than 45 years old age groups. Overall, the finding that older patients with stages 3A, 3B, and 4 CKD demonstrated higher PPVs is consistent with previous findings of significant differences in CKD progression and rates of end-stage renal disease (ESRD) by age. O’Hare and colleagues23 found that among patients with comparable levels of eGFR, older patients had higher rates of death and lower rates of ESRD than did younger patients. Furthermore, the level of eGFR below which the risk of ESRD exceeded the risk of death varied by age, ranging from 45 mL/min for 18- to 44-year-old patients to 15 mL/min for 65- to 84-year-old patients. For patients 85 years or older, the risk of death always exceeded the risk of ESRD. The authors hypothesized that this may demonstrate that older patients are living with slowly progressive CKD, perhaps indicative of a more stage-stable disease.23
In the present study, a higher proportion of the oldest patients had a follow-up eGFR <60 mL/min. These findings confirm that age has a significant impact on disease progression; however, our results also demonstrate that this does not significantly affect the ability to screen for CKD using a single assessment of eGFR. Although the highest PPV was demonstrated in the older than 60 years age group, patients across all of the age groups had similar PPVs when patients with stages 3A, 3B, and 4 CKD were examined.
When examined in totality, our findings reveal a cutpoint of <45 mL/min for baseline estimation of eGFR to predict CKD. Fewer patients with baseline eGFRs >45 but <60 mL/min progress into CKD. This cutpoint is consistent with stages 3A, 3B, and 4 CKD.
As stated by Eckardt et al,10 several critics of the 2002 KDOQI guidelines question whether a single eGFR <60 mL/min alone, in the absence of other markers of kidney disease, is sufficient to define CKD.9,11–13,17,24 This criticism has resulted in efforts to revise the classification system. Based upon the examination of the PPV of a single assessment of GFR alone, our results suggest that patients with a beginning eGFR <45 mL/min and who are 60 years of age or older can be successfully screened for CKD with a single assessment of eGFR, necessitating closer follow-up to monitor progression. Our current analysis continues the discussion to better understand the utility of screening for CKD using a single assessment of eGFR. The similar results revealed by subgroup analysis by DM and HTN status indicate that the presence of DM and/or HTN does not exert a differential effect in predicting CKD.
Although the DARTNet dataset allowed for a robust sample size within a realistic context of the practice of primary care, some limitations must be recognized. This analysis sought to determine the PPV of a single assessment of eGFR for use in screening for CKD; however, the DARTNet dataset contains only patients having an eGFR <60 mL/min at some point in their lifetime as the baseline assessment. As such, a true control group is not present in the dataset. This analysis proposed a cutpoint among adult patients for eGFR that may be tested; however, patient demographic data that may affect CKD progression were limited to patient age and sex, and comorbidity data were limited to DM and HTN. Other more complex patient contextual factors were not included in this national dataset and therefore could not be investigated.
Effective screening for eGFR should not rely upon more complex patient contextual factors for which collection may prove prohibitively costly. As such, our simple methodology maximizes generalizability and ease of use in a healthcare environment that encourages strategic prioritization of resources. The data collected in the DARTNet national dataset are sufficient to identify this cutpoint for screening purposes. Our results indicate that a single cutpoint was identified that may inform future efforts to screen for CKD.
Another issue that must be recognized is that of missing data. The nature of the DARTNet national dataset, merging EMR data from many primary care practices throughout the United States, highlights the relatively high proportion of missing values for variables such as race and ethnicity; therefore, such factors could not be included in our analysis. We recognize that race has a differential impact on interpreting eGFR results and that this may limit the utility of this screening tool. Because this was a comparison of one test to a subsequent one in the same person, it should not have significantly affected our findings, however. Furthermore, when we increased the cutpoint of 50 mL/min we demonstrated a PPV of 90%, which can still be used effectively for screening and may be more appropriate for both African American and white patients for defining CKD. Future efforts to use the DARTNet national dataset should include collection and validation of race data.
Another limitation that we must acknowledge is the missing values of sex and age; however, in our study there is no evidence of missing values.
The DARTNet dataset provides a large population of >40,000 patients cared for in a geographically diverse national primary care practice environment. This setting is probably more generalizable to routine practice than large university academic clinical research settings. In addition, the clinical implications of our findings are significant and important to highlight given the ubiquitous application of the 2002 KDOQI guidelines. One of the major objectives of the 2002 guidelines was to facilitate easy, nonintrusive early screening for CKD, before the need for renal replacement therapy.
Conclusions
Our preliminary findings identified a valid cutpoint to screen for CKD with a PPV of 93%, with a specificity of 94% and sensitivity equal to 28% for both male and female patients having an initial eGFR <60 mL/min. This cutpoint may prove important to the efforts to screen early for CKD while reducing the burden on the healthcare system and patients suspected of having CKD.
Brief Description.
The objective of this study was to determine the positive predictive value of a single assessment of glomerular filtration rate in the diagnosis of chronic kidney disease. Using a large, national primary care dataset, the authors set out to determine the utility of this assessment in the context of diagnosis and classification of chronic kidney disease.
Key Points.
The utility of the positive predictive value of a single assessment of glomerular filtration rate in diagnosing chronic kidney disease (CKD) has not been fully explored.
Using the Distributed Area Research and Therapeutics Network CKD natural history dataset, our team identified a cutpoint of 45 mL/min, which could be used as a stand-alone screen for CKD.
Targeted screening for CKD has the potential to reduce the cost of care by more precisely identifying the most appropriate patients who would benefit while minimizing the use of unnecessary laboratory tests.
Acknowledgments
N.S. has received grants from the National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK090407-01A1 [funding agent of present study]); the University at Buffalo Center for Home Health and Wellbeing Through Adaptive Smart Environments; and the Agency for Healthcare Research and Quality (1R03HS023656-01A1). W.P. has received grants from the National Institutes of Health through the University at Buffalo, Mallinckrodt, Merck, Pfizer, and Sanofi; is a consultant to Novo Nordisk and Genova Diagnostics; and holds stock in Sanofi and Pfizer. G.M.A., M.Y., J.V., and P.G. were compensation through an institutional grant from the National Institutes of Health. The remaining authors have no financial relationships to disclose and no conflicts of interest to report.
Footnotes
To purchase a single copy of this article, visit http://sma.org/smj-home. To purchase larger reprint quantities, please contact reprints@wolterskluwer.com.
The findings from this study were presented as a poster for Translational Science 2015, Washington, DC, April 16–18.
References
- 1.Moyer VA. Screening for chronic kidney disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:567–570. doi: 10.7326/0003-4819-157-8-201210160-00533. [DOI] [PubMed] [Google Scholar]
- 2.Qaseem A, Hopkins RH, Jr, Sweet DE, et al. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:835–847. doi: 10.7326/0003-4819-159-12-201312170-00726. [DOI] [PubMed] [Google Scholar]
- 3.Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 4.Carville S, Wonderling D, Stevens P. Early identification and management of chronic kidney disease in adults: summary of updated NICE guidance. BMJ. 2014;349:g4507. doi: 10.1136/bmj.g4507. [DOI] [PubMed] [Google Scholar]
- 5.Agency for Healthcare Research and Quality. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 6.Poggio ED, Wang X, Greene T, et al. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16:459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 7.Glassock RJ. Referrals for chronic kidney disease: real problem or nuisance? JAMA. 2010;303:1201–1233. doi: 10.1001/jama.2010.315. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glassock RJ, Winearls C. Screening for CKD with eGFR: doubts and dangers. Clin J Am Soc Nephrol. 2008;3:1563–1568. doi: 10.2215/CJN.00960208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckardt KU, Berns JS, Rocco MV, et al. Definition and classification of CKD: the debate should be about patient prognosis—a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53:915–920. doi: 10.1053/j.ajkd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Eknoyan G. Kidney disease: wherefore, whence, and whereto? Kidney Int. 2007;71:473–475. doi: 10.1038/sj.ki.5002171. [DOI] [PubMed] [Google Scholar]
- 12.Eknoyan G, Lameire N, Barsoum R, et al. The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66:1310–1314. doi: 10.1111/j.1523-1755.2004.00894.x. [DOI] [PubMed] [Google Scholar]
- 13.Glassock RJ, Winearls C. An epidemic of chronic kidney disease: fact or fiction? Nephrol Dial Transplant. 2008;23:1117–1121. doi: 10.1093/ndt/gfn086. [DOI] [PubMed] [Google Scholar]
- 14.Rule AD, Rodeheffer RJ, Larson TS, et al. Limitations of estimating glomerular filtration rate from serum creatinine in the general population. Mayo Clin Proc. 2006;81:1427–1434. doi: 10.4065/81.11.1427. [DOI] [PubMed] [Google Scholar]
- 15.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 16.Bauer C, Melamed ML, Hostetter TH. Staging of chronic kidney disease: time for a course correction. J Am Soc Nephrol. 2008;19:844–846. doi: 10.1681/ASN.2008010110. [DOI] [PubMed] [Google Scholar]
- 17.Glassock RJ, Winearls C. The global burden of chronic kidney disease: how valid are the estimates? Nephron Clin Pract. 2008;110:c39–c47. doi: 10.1159/000151244. [DOI] [PubMed] [Google Scholar]
- 18.Moynihan R, Glassock R, Doust J. Chronic kidney disease controversy: how expanding definitions are unnecessarily labelling many people as diseased. BMJ. 2013;347:f4298. doi: 10.1136/bmj.f4298. [DOI] [PubMed] [Google Scholar]
- 19.Brown WW, Peters RM, Ohmit SE, et al. Early detection of kidney disease in community settings: the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2003;42:22–35. doi: 10.1016/s0272-6386(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 20.de Lusignan S, Chan T, Stevens P, et al. Identifying patients with chronic kidney disease from general practice computer records. Fam Pract. 2005;22:234–241. doi: 10.1093/fampra/cmi026. [DOI] [PubMed] [Google Scholar]
- 21.Locatelli F, Vecchio LD, Pozzoni P. The importance of early detection of chronic kidney disease. Nephrol Dial Transplant. 2002;17(Suppl 11):2–7. doi: 10.1093/ndt/17.suppl_11.2. [DOI] [PubMed] [Google Scholar]
- 22.Sharma P, McCullough K, Scotland G, et al. Does stage-3 chronic kidney disease matter?: a systematic literature review. Br J Gen Pract. 2010;60:e266–e276. [Google Scholar]
- 23.O’Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 24.Poggio ED, Rule AD. Can we do better than a single estimated GFR threshold when screening for chronic kidney disease? Kidney Int. 2007;72:534–536. doi: 10.1038/sj.ki.5002452. [DOI] [PubMed] [Google Scholar]