Abstract
Recent bioapplications of one-dimensional (1D) zinc oxide (ZnO) nanomaterials, despite the short development period, have shown promising signs as new sensors and assay platforms offering exquisite biomolecular sensitivity and selectivity. The incorporation of 1D ZnO nanomaterials has proven beneficial to various modes of biodetection owing to their inherent properties. The more widely explored electrochemical and electrical approaches tend to capitalize on the reduced physical dimensionality, yielding a high surface-to-volume ratio, as well as on the electrical properties of ZnO. The newer development of the use of 1D ZnO nanomaterials in fluorescence-based biodetection exploits the innate optical property of their high anisotropy. This review considers stimulating research advances made to identify and understand fundamental properties of 1D ZnO nanomaterials, and examines various biosensing modes utilizing them, while focusing on the unique optical properties of individual and ensembles of 1D ZnO nanomaterials specifically pertaining to their bio-optical applications in simple and complex fluorescence assays.
Keywords: zinc oxide nanomaterial, nanorod optical property, biomedical detection, biosensing, optical signal enhancement, enhanced fluorescence detection
1. INTRODUCTION
Nanoscale zinc oxide (ZnO) materials may offer many advantages to the burgeoning area of biomedical detection owing to the ease of synthesis and a wealth of micro/nanofabrication information available to engineer them into compact sensors. Various established growth procedures are available to create nanometer-scale ZnO materials of controlled dimensions and shapes (1, 2). Gas-phase growth approaches for synthesizing nanoscale ZnO structures include physical vapor deposition, pulsed laser deposition, chemical vapor deposition, metal-organic chemical vapor deposition, and vapor-liquid-solid epitaxy (3–10). Solution-based growth methods include homogeneous precipitation and hydrothermal decomposition processes (11–15). The physical, chemical, and optical properties of ZnO nanomaterials can be specifically tuned during growth to optimize their performance according to the needs of the ultimate bioapplications. With these considerations, the development of zero-dimensional (0D) ZnO nanomaterials, often referred to as ZnO quantum dots or nanoparticles, has largely been driven by their in vivo applications (16–19) so far. Alternatively, 1D ZnO nanomaterials, such as ZnO nanorods (NRs; often referring to rods with diameters greater than 100 nm) and nanowires (NWs; NRs less than 100 nm in diameter), have been utilized primarily for in vitro biomedical detection and sensing. Yet the development of ZnO nanomaterials for biosensing and biodetection is still considered to be largely in its infancy, especially pertaining to the use of 1D ZnO NRs compared to their particulate, thin film, and bulk counterparts. Despite the short lapse of time from initial development, significant and exciting research advances have been made on the fundamental properties of 1D ZnO nanomaterials specific to their biosensing applications. This review discusses these spearheading endeavors in depth while focusing on the contributions of 1D ZnO nanomaterials in substantially enhancing biodetection carried out in optical modes.
The extraordinary optical properties of ZnO NRs and NWs have led to extensive development and incorporation of the nanomaterials in optoelectronics and photonics (20, 21). However, potential impacts of the optical properties of 1D ZnO nanomaterials in biomedical sensing have not been realized till relatively recently. For example, it is well known that various 1D NRs and NWs are effective in absorbing (22), scattering (23), guiding (24, 25), emitting (26), collecting (25, 27), concentrating (28), and amplifying (29–31) light, and these properties have been exploited in numerous optoelectronic and optical devices, such as solar cells, light-emitting diodes, lasers, photodetectors, piezoelectrics, and waveguides. In basic biological research, as well as clinical testing environments, common optical modes of detection are based on the absorption, transmission, and emission of electromagnetic radiation. In particular, fluorescence-based methods are broadly utilized in biodetection settings, with examples including confocal fluorescence microscopy, fluorescence resonance energy transfer, fluorescence recovery after photobleaching, fluorescence correlation microscopy, and fluorescence lifetime imaging microscopy (32). With such wide applicability of fluorescence, there is much potential for the outstanding optical properties of ZnO NRs, similar to those already observed in optoelectronics and photonics, to contribute to biomedical detection and sensing applications. In this regard, much needed attempts have been made to optimize well-known optical properties of ZnO NRs during their syntheses. At the same time, researchers are steadily using combined experimental and computer simulation approaches to identify novel optical properties of ensembles of and individual ZnO NRs specific to bio-optical measurements and, furthermore, to elucidate the exact optical mechanisms leading to unique phenomena. Significant findings are discussed in the following section.
2. BIOMEDICALLY RELEVANT OPTICAL PROPERTIES OF ZnO NANORODS
2.1. Biomolecular Fluorescence Enhancement
Fluorescence is widely used in biomedical research and diagnostics as it offers reasonably high sensitivity to target components in complex biomolecular assemblies, versatility in accommodating a range of sample types, and modest instrumentation requirements for signal detection (33–36). The suitability of ZnO NRs in fluorescence measurements has been previously demonstrated (37, 38). Their ability to enhance fluorescence signals from nearby fluorophore-coupled biomolecules was first observed in a study comparing the fluorescence signal from model proteins and DNA measured while varying the underlying platform materials in a solid-state detection setting (39). In this study, ZnO NRs were employed as an alternative biodetection platform to conventional glass-, silicon-, and polymer-based supports. The fluorescence signal from the same test biomolecules was found to be a few to several orders of magnitude higher in intensity when using ZnO NRs relative to the cases of polystyrene (PS), polymethylmethacrylate (PMMA), silicon/silica, quartz, and glass substrates.
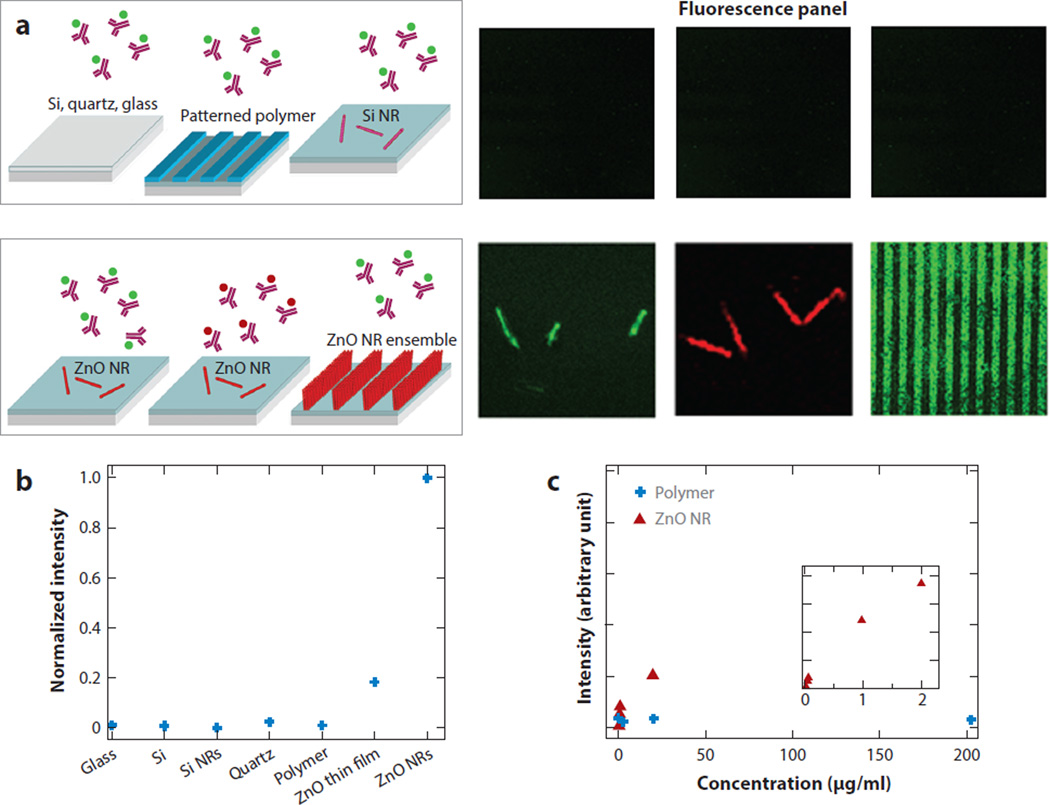
Figure 1 displays the enhanced fluorescence detection capability of ZnO NRs compared to the various control platforms by assessing the fluorescence intensity of model biosystems linked to fluorescein isothiocyanate (FITC) and tetramethyl rhodamine isothiocyanate (TRITC). Both the material and dimensional effects on signal enhancement are shown in Figure 1b. 2D ZnO thin films permitted improved fluorescence detection capability compared to other control substrates, but the measured signal was only 20% of that monitored on 1D ZnO NR platforms. However, reduced dimensions alone could not explain the fluorescence enhancement seen from ZnO NRs as no significant fluorescence signal was resolved on 1D silicon nanorods of similar physical dimensions. Therefore, the inherently higher surface-to-volume ratio of 1D materials compared to their 2D analogs, providing an increased surface footprint for biomolecule adherence, was not a dominant driving force for signal enhancement. In fact, the choice of material (i.e., the use of highly crystalline ZnO NRs) also plays a critical role for biomolecular fluorescence enhancement. Signal intensity variations observed on different platforms can be viewed as originating from different amounts of proteins on the material surfaces, resulting from varying degrees of protein-surface interactions, which in turn yield more or less protein adsorption to a given surface. The amounts of surface-bound proteins on ZnO NRs and polymeric surfaces were later evaluated directly and indirectly after an identical biodeposition process in atomic force microscopy (40) and spectroscopic (41) studies. The results revealed that proteins favored the control substrates of PS and PMMA to ZnO, resulting in a higher density of surface-bound proteins on the polymeric platforms. However, the fluorescence signal obtained from the ZnO NR platforms containing a smaller amount of biomolecules was still higher than that collected from the polymeric supports with a larger amount of biomolecules.
Figure 1.
Comparison of fluorescence emission monitored from 200-µg/ml fluorescein isothiocyanate (FITC)-conjugated or tetramethyl rhodamine isothiocyanate (TRITC)-conjugated antibovine immunoglobulin G on ZnO nanorods (NRs) versus control substrates after identical biodeposition. (a) No discernable fluorescence signal is detected from the biomolecules on the control substrates, including glass, quartz, native SiO2/Si, Si NRs, and polymeric surfaces of polystyrene (PS) and polymethylmethacrylate (PMMA). Conversely, a strong fluorescence signal is observed from individual and striped ZnO NR platforms. (b) Normalized fluorescence intensities observed from the biomolecules on various substrates are compared. (c) The fluorescence intensity was measured as a function of protein concentration on PMMA (blue) and ZnO NRs (red). The background fluorescence from PS and PMMA platforms inherent to these materials was subtracted from the fluorescence intensity measured after biodeposition. Figure adapted from Reference 39 with permission. Copyright 2006 American Chemical Society.
In another study comparing the time-dependent photostability of biomolecules on different material platforms under constant illumination, biomolecular photostability was determined to be much higher on ZnO NRs than on PS or PMMA (40). Table 1 presents the fluorescence decay profiles of 200-µg/ml 5-(4,6-dichlorotriazinyl) aminofluorescein (DTAF)-conjugated, antibovine IgG (anti-IgG) antibody with respect to time. Compared to PS and PMMA, the decay of biomolecular fluorescence is much slower on ZnO NRs, as evidenced by the extended T1/2, T1/5, and T1/10. Most organic fluorophores, exhibiting molar extinction coefficients of 5–10 × 104/(M·cm), typically have T1/2 values less than 15 s (42–45). At the same time, photochemical destruction of the fluorophores resulting in signal reduction can impose a major problem in many fluorescence-based biodetection techniques. Therefore, the prolonged photostability of biomolecules facilitated by ZnO NRs is significant and can be advantageous in temporally stable fluorescence measurements. These encouraging findings suggested that ZnO NR platforms may be able to provide the much-needed ultrahigh sensitivity in fluorescence assays to carry out low-abundance biomolecular screening, facilitating early biomarker detection.
Table 1.
Time-dependent decay of fluorescence intensity under constant excitation measured on polymeric and ZnO nanorod (NR) substrates with DTAF-anti-IgG
| Bioassay platforms | T1/2 (s) | T1/5 (s) | T1/10 (s) |
|---|---|---|---|
| PMMAa | 17 ± 2.1 | 72 ± 14.8 | 161 ± 10.2 |
| PSa | 32 ± 5.0 | 123 ± 15.4 | 205 ± 9.3 |
| ZnO NRb | 60 ± 5.3 | 163 ± 10.1 | 250 ± 15.9 |
Polystyrene (PS) and polymethylmethacrylate (PMMA) display autofluorescence with no coupled 5-(4,6-dichlorotriazinyl) aminofluorescein (DTAF)–antibovine IgG (anti-IgG); hence, this background fluorescence was subtracted from the raw fluorescence intensity measured in the presence of DTAF-anti-IgG. Biomolecular fluorescence measured on ZnO NRs persists much longer than the signal measured on the polymeric substrates.
The reported values are from the basal plane (NR end facet) of ZnO NRs.
T1/2, T1/5, and T1/10 denote the time taken for fluorescence intensity to lose 50%, 80%, and 90% of the initial intensity at T=0, respectively. Table adapted with permission from Reference 40. Copyright 2014 Royal Society of Chemistry.
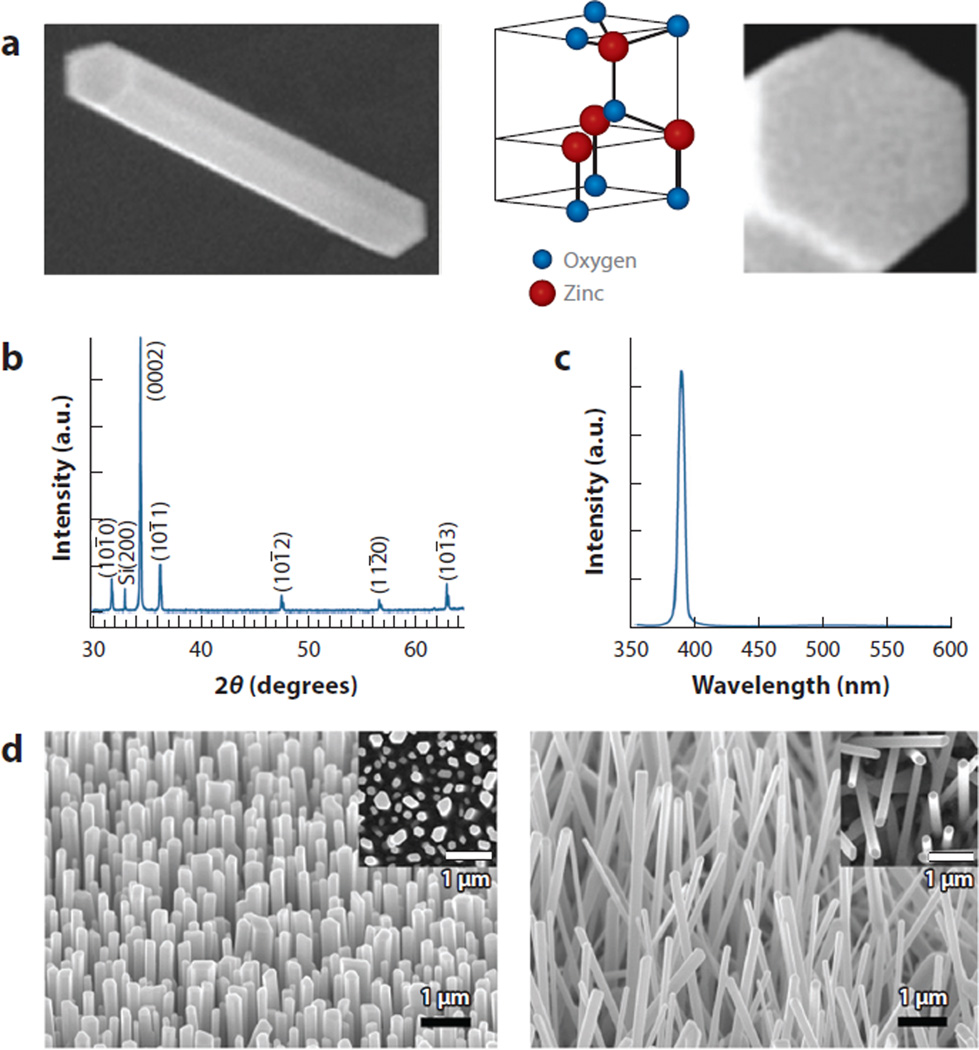
It is worthwhile to note that the highly crystalline, atomic defect-free nature of ZnO NRs plays an essential role in ultrasensitive fluorescence detection. To meet this requirement, ZnO NRs in the above-mentioned studies were synthesized via chemical vapor deposition to exhibit optical-quality 1D structures with well-defined physical dimensions. Diffraction and photoluminescence data confirmed the highly single-crystalline and atomic defect-free nature of these synthesized NRs. Figure 2 presents examples of such optical-quality ZnO NRs in scanning electron microscopy images as well as in X-ray diffraction and photoluminescence spectra. Typical ZnO NRs employed in biomedical fluorescence detection range from 100 to 500 nm in diameter with an aspect ratio (NR length/width) greater than 10 and exhibit a hexagonal wurtzite crystal structure. Taken directly from the synthesis chamber with no postmodification of the materials, these ZnO NRs yielded the desired optical and chemical properties suitable for biomedically pertinent fluorescence detection as the materials show no autofluorescence or spectroscopic overlap with common fluorophores used in biological assays (4, 39, 41, 46). However, depending on the growth and postsynthesis processing conditions, ZnO NRs may show autofluorescence because of the presence of atomic or chemical defects and produce intrinsic emission peaks in the visible (Vis) range (15, 47). Therefore, highly crystalline, optical-quality ZnO NRs serve as a critical factor not only in observing enhanced, background-free biomolecular fluorescence, but also in accurately correlating the collected fluorescence signal to biomolecular parameters, avoiding misleading signal interpretations.
Figure 2.
Gas-phase synthesized ZnO nanorods (NRs). (a) 763 nm × 1.2 µm and 200 nm × 200 nm scanning electron microscopy (SEM) images showing the side (prismic) and end (basal) facets of a wurtzite ZnO NR. The width and length of the ZnO NR are 180 nm and 1.2 µm, respectively. Panel a adapted from Reference 41 with permission. Copyright 2008 American Chemical Society. (b) X-ray diffraction data displaying the high crystalline quality of as-synthesized ZnO NRs showing the pronounced peak at 2θ = 34.5° corresponding to the preferential growth direction along 〈0001〉. (c) Room-temperature photoluminescence spectrum showing the extremely strong and narrow band-edge emission of ZnO NRs at 390 nm with no significant defect emission in the visible range. Panels b and c adapted from Reference 4 with permission. Copyright 2005 American Scientific Publishers. (d) SEM images of gas-phase-synthesized ZnO NRs revealing densely grown, vertically oriented ZnO NRs on the Si (100) growth substrate. (Insets) Plan-view images. Panel d adapted from Reference 48 with permission. Copyright 2005 American Chemical Society.
Although fluorescence-based detection is the technique of choice in biology and medicine, biomedical communities have long sought seamless, integration-ready methods while enabling rapid, ultrasensitive, and high-throughput detection that can lead to a breakthrough in the key development areas of population-level genetic screening, system-wide studies of proteins, and early disease diagnoses. Considering such needs, the newly identified ZnO NR platforms are particularly attractive as next-generation biodetection supports given that enhanced fluorescence detection using ZnO NRs could easily be realized with minimal changes to the existing fluorescence detection systems. Periodically patterned ZnO NR arrays can be easily fabricated during material growth via well-ordered catalyst delivery to assume the same dimensional specifications adopted in conventional fluorescence array and microplate readers (4, 39). The ability to achieve considerably high-detection capability with ZnO NR platforms, especially without additional improvements or modifications of fluorophores, filter sets, signal processing software, or instrumentation, could be also advantageous for their biodetection applications. Various advantageous properties of ZnO NRs with regard to fluorescence-based biodetection are summarized in Section 2.2.
Further studies have demonstrated that ZnO NR platforms allow the detection of the fluorescence signal solely from target biomolecules with no background emission from NRs in the Vis and near-infrared (NIR) range and that ZnO NRs enable signal enhancement from a variety of common fluorophores designed to show different absorption/emission profiles for biological measurements (39, 41, 46). In addition, the capability of increased signal detection persists regardless of the distance between a fluorophore and the NR surface, resulting in signal enhancement whether the fluorophore is placed directly on top of the NR surface or several tens of nanometers away from the NR (40, 41, 49–51). These unique properties of ZnO NRs, showing the fluorophore/NR distance-independent behavior and no spectroscopic overlap between the nanomaterials and fluorophores, separate them from metallic (gold, silver) nanoparticles utilized in biodetection for the metal-enhanced fluorescence effect (52). These additional findings signify that ZnO NR platforms have great potential for multiplexed detection in which multiple biological target molecules can be analyzed at the same time and that the origin of fluorescence enhancement produced in ZnO NRs lies beyond the well-known signal-enhancing mechanisms in fluorescence, such as energy transfer and radiative pathway engineering (52–55). Research efforts elucidating the underlying optical properties, which largely govern the ZnO NR-enabled, bio-optical fluorescence enhancement, are summarized in Sections 2.3 and 2.4.
Parallel research endeavors have been successfully made to utilize ZnO NRs in basic biological and applied biomedical detection. ZnO NRs have been employed in the optical, electrochemical, and electrical detection of various biomolecules, such as DNA, proteins, and cells. The development of new fluorescence assays involving analytes supported on ZnO NRs in simple and complex bioenvironments is discussed in detail in Sections 3.1–3.3. In addition, nonoptical biosensing applications of ZnO NRs primarily based on electrochemical and electrical modes of detection are summarized in Section 3.4. Subsequently, future efforts for this emerging research area are presented in Section 4.
2.2. Properties of ZnO Nanorod Platforms Suitable for Bio-Optical Detection
The above-discussed studies have demonstrated that nanoscale ZnO materials can serve as ideal optical signal-enhancing platforms for identifying and screening DNA and protein analytes in simple bioassays. With regard to biodetection and biosensing, the main advantage of ZnO NR platforms is the exquisite detection sensitivity enabled by the presence of high-quality ZnO NRs, which is several orders of magnitude higher in biomolecular fluorescence intensity relative to the signal observed on conventional substrates. Other key advantages of these platforms include the ease of array synthesis/fabrication, mechanical and chemical robustness, lack of autofluorescence, and direct correlation of the observed signal to protein concentration. The ZnO NR platform-enabled enhancement is present for common fluorophores used in bioassays; hence, the enhancement is not dependent on the spectroscopic characteristics of the fluorophores (40, 41,46, 50, 51). This feature was confirmed by coupling various fluorophores, such as FITC, DTAF, TRITC, indocarbocyanine, rhodamine 6G (R6G), phycoerythrin, and Alexa dyes, to ZnO NR platforms and then examining the presence/absence of increased fluorescence signal on the platforms.
Given the versatile and highly sensitive optical detection of many commonly used fluorophores, spanning different wavelengths, ZnO NR platforms can promote simultaneous detection of multiple analytes by coupling a different fluorophore to each protein for multiplexing. These biologically useful fluorophores exhibit absorption and emission bands in the Vis and NIR range. As high-quality ZnO NRs do not absorb or emit in this electromagnetic spectrum range, they also offer a background-free detection environment. Compared to other commonly used biosupport materials displaying varying degrees of autofluorescence, depending on the excitation wavelength, ZnO NRs can hence provide a higher signal-to-noise (SNR) ratio for detection. Because ZnO NRs exhibit no spectral overlap with fluorophores, the collected fluorescence signal can be directly correlated to the abundance of target biomolecules in fluorescence data analysis. This direct correlation of the optical signal to the biomolecules under investigation cannot be easily obtained by conventional detection schemes. For example, in enzyme-linked immunosorbent assays (ELISA), the abundance of analyte biomolecules is indirectly measured by monitoring the chromogenic signal from an enzyme-substrate reaction, rather than directly through a biomolecular reaction under study.
ZnO shows chemical stability in typical bioassay environments at normal biological pH (56), and at the same time, ZnO NRs offer chemical functionality via their surface hydroxyl group (50, 57) to covalently attach specific biomolecules of interest, increasing the specificity of detection. Besides the chemical modification of the ZnO NR surface via covalent derivatization, biologically recognizable moieties, such as biotin-streptavidin and polyvalent directed peptide, have also been incorporated to increase the NR selectivity toward specific target bioanalytes (58–60). In other cases, polymers and metal nanoparticles in conjunction with ZnO NRs have been used to increase biomolecular adsorption with the help of polymer brush chains (61) and to detect the signal more efficiently via the incorporation of a highly reflecting metal layer (62). In addition, ZnO NRs exhibiting an isoelectric point (pI) of ~9–10 allow for easy physical immobilization of low-pI biomolecules via electrostatic interactions at physiological pH (21).
In addition, the physical dimensions of the ZnO NR platforms can be tailored during synthesis so that an array can be seamlessly integrated into existing fluorescence array scanners and plate readers (37, 38, 63). High-density ZnO NR arrays can be straightforwardly fabricated by controlling the dimensions of surface-printed catalyst patterns prior to the NR synthesis (41, 46, 50), which is especially beneficial for their potential use in high-throughput detection, a more demanding application involving the simultaneous evaluation of a large number of biosamples. Extended target and signal amplification steps, such as polymerase chain reaction and enzyme coupling, are prerequisites in traditional biodetection methods involving analytes of low concentrations. But with ZnO NR platforms, such amplification steps are not necessary to achieve high-detection sensitivity of biomolecules. ZnO NRs enable the detection of hard-to-resolve optical signal through fluorescence enhancement without any costly and time-consuming procedures.
Furthermore, ZnO NRs offer a high surface-to-volume ratio and high aspect ratio through their physical dimensions, biocompatibility, chemical stability, electrochemical activity, and controllable electron flow through their semiconducting electrical characteristics (21, 58). The combined properties discussed in this section underpin the effectiveness of ZnO NRs as ideal platforms for easy, rapid, and extremely sensitive biodetection in an array format, especially for the identification of low-abundance, ultratrace-level biomolecules and the screening of a large library of biosamples.
2.3. Mechanism of Zinc Oxide Nanorod-Enabled Bio-Optical Signal Enhancement
The experimental observations discussed above and the impressively large degree of biomolecular fluorescence signal enhancement in ZnO NR systems indicate that the inherent optical properties of ZnO NRs are the main contributing factors of the signal increase, rather than reduced self-quenching of fluorophores or allowed energy transfer between the NR and fluorophore (52–55). The optical properties of 1D and 2D ZnO materials, proven useful in the area of optics and optoelectronics, may provide insight into the bio-optical signal-enhancing behavior of 1D ZnO NRs observed in biomolecular fluorescence detection.
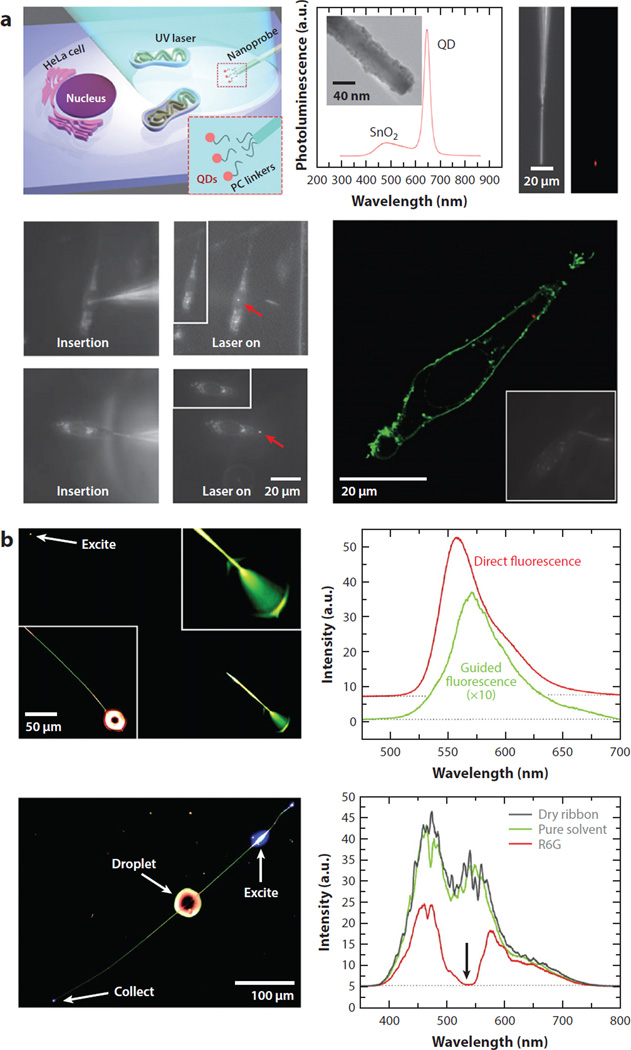
In optical and optoelectronic fields, 2D ZnO thin films have been fabricated into waveguiding planes (64–68) and antireflection coating layers (69, 70). In addition to these experimental works, numerical analyses performed on the thin film waveguides of TiO2 (71) and ZnO (72) have shown that the use of metal oxides in waveguide construction can enhance waveguide evanescent waves by several orders of magnitude. More recently, the light-amplifying and -guiding properties of 1D ZnO nanomaterials have been exploited as NR lasers (29–31, 73, 74) and subwavelength waveguides (24, 25,65, 73–75). Another study involving 1D SnO2 and ZnO nanomaterials exploited efficient light-coupling and -guiding properties of the nanomaterials as optical routers and sensors (25). A1D SnO2 NR has been further used as an illumination/collection/delivery source in a single-cell endoscopy study (27). Figure 3a shows that a payload of quantum dots can be delivered via a 1D SnO2 NR probe mounted at the end of a conventional optical fiber tip to a precise location of a cell with controllable release of the payload through a photoactivated chemical link. Figure 3b displays such applications of 1D SnO2 NRs in subwavelength wave guiding in order to produce light absorption and emission of a fluorophore, R6G.
Figure 3.
Exemplar applications of 1D NRs in guiding and collecting light. (a) Spatiotemporal delivery of QDs into a living cell is attempted using a photoactivatable SnO2 NR endoscope. Fluorescence confocal image of a HeLa cell shows the QDs (red dots in the cytoplasm) within the cell membrane (green) successfully delivered by the nanoprobe. Panel a adapted from Reference 27 with permission. Copyright 2012 Nature Publishing Group. (b) Fluorescence and absorbance spectra of R6G are recorded using an SnO2 NR waveguide (240 nm × 260 nm × 540 µm) excited at one end (with top panels showing fluorescence geometry) and near the middle of the NR (with bottom panels showing absorption geometry). Panel b adapted from Reference 25 with permission. Copyright 2005 National Academy of Sciences, USA. Abbreviations: NR, nanorod; PC, photocleavable; QD, quantum dot; R6G, rhodamine 6G; UV, ultraviolet.
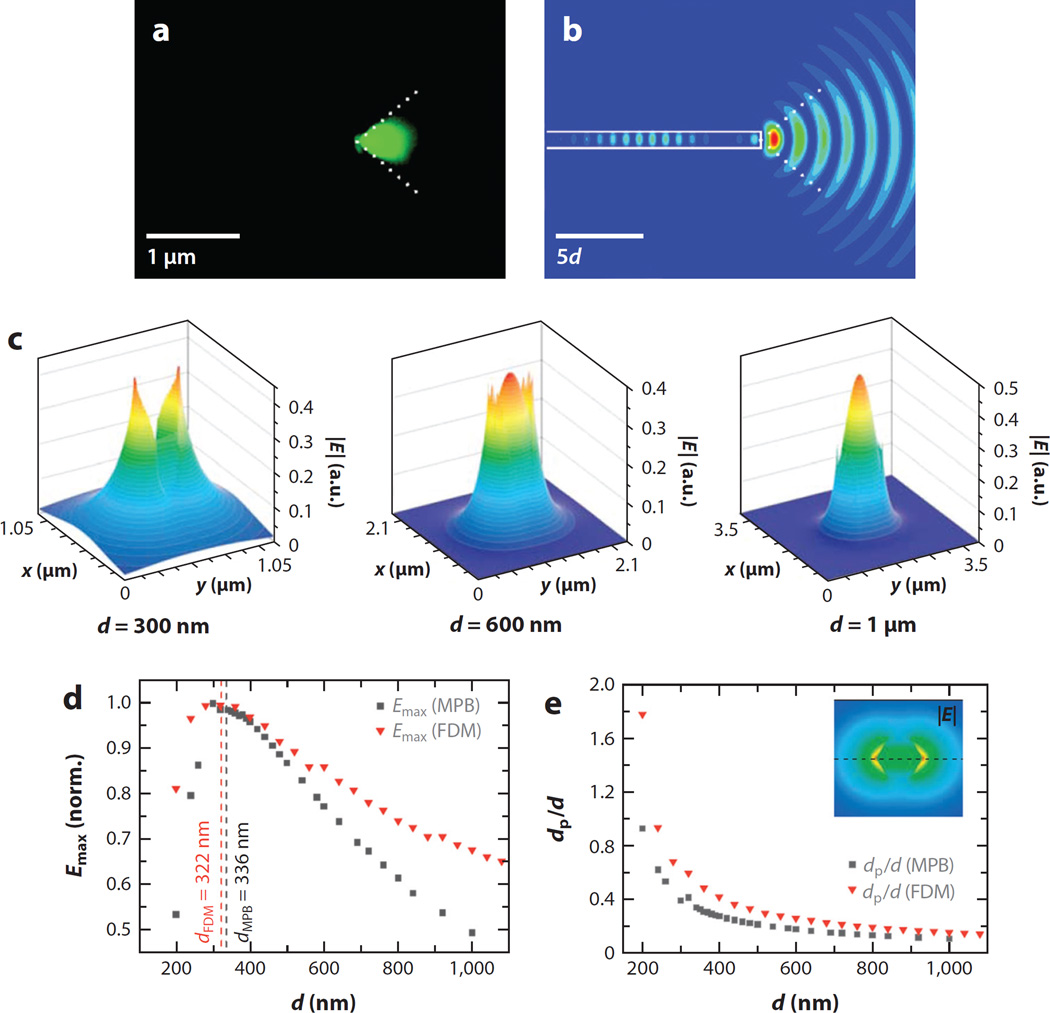
From these previous investigations on 1D subwavelength waveguides, it is well known that a larger fraction of light is guided outside the core than through the inside of the guiding medium as the physical dimension of the optical cavity is smaller than the wavelength of light (24, 25, 76). The waveguiding property of ZnO NRs in the diameter range of 200–250 nm was examined experimentally and through numerical simulations when light was coupled into the NR from a tapered silica fiber (75). Figure 4a,b illustrates the low-order mode waveguiding behavior of a ZnO NR for 532-nm light. The same study also reported that higher-order waveguide modes were frequently excited for the case of fiber-coupled light in the Vis range, with a significant amount of the intensity carried at the NR surface. For a 1D SnO2 NR of 250 nm in diameter, approximately 15% of the electric field intensity for 442-nm light is found to exist outside of the nanomaterial. In addition, the radial field intensity decays to 10% of its maximum value at approximately 135 nm into the surrounding medium, a significantly long distance away from the NR surface (25). 1D subwavelength waveguides not only carry a larger fraction of their modal power outside the core compared to conventional optical fibers, but also enhance the evanescent wave field and its penetration depth. The penetration depth of an evanescent field around a hexagonal ZnO NR, predicted by theoretical simulations based on the MIT Photonic-Bands program and finite-difference method, is approximately 125 nm for a NR of 500 nm in diameter for 761-nm light (77). Figure 4c displays 2D finite-difference time-domain (FDTD) calculation results of the electric field (E) of a light pulse emitted from a NR by varying the NR diameter (d) from 300 nm to 1 µm while considering E within the NR cavity as well as outside as a surface evanescent wave. This increased fraction of light outside the NR enhances the intensity of an evanescent field and its penetration depth into the surroundings, which can lead to more powerful excitation of nearby molecules (78, 79).
Figure 4.
Evidence of guided mode emission through a ZnO nanorod (NR) and computer-simulated results of an increased evanescent wave field and its penetration depth for ZnO NRs of various diameters. (a) Conical 90° emission of a low-order guided mode observed from one end of a ZnO NR. (b) 2D finite-difference time-domain calculation of the square of the electric field of a light pulse emitted from a NR of diameter d. Panels a and b adapted from Reference 75 with permission. Copyright 2007 American Chemical Society. (c) The electric field |E| of a ZnO NR graphed for NR diameters of 300 nm, 600 nm, and 1 µm at λ = 761 nm. (d) Normalized maximum magnitude of the evanescent field (Emax) plotted as a function of the NR diameter. (e) Penetration depth (dp) obtained by the MIT Photonic-Bands (MPB) program and the finite-difference method (FDM) as a function of diameter for λ = 761 nm. Panels c, d, and e adapted from Reference 77 with permission. Copyright 2007 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
An equation developed in fiber optics (80, 81) may be used to estimate the diameter range of ZnO NRs below which Vis and NIR light can be effectively guided:
When considering only single-mode guides by using the V(1,1) value of 2.405 (81), the cutoff diameter (dcutoff), which varies depending on the wavelength of light (λ) and the refractive index of the waveguide medium (nNR), can be calculated as shown in Table 2.
Table 2.
Cutoff diameter of ZnO nanorods (NRs) to guide single-mode wave of λ
| λ (nm) | ZnO NR refractive index (82) | ZnO NR dcutoff (nm) |
|---|---|---|
| 450 | 2.1058 | 185.9 |
| 500 | 2.0511 | 213.8 |
| 600 | 1.9985 | 265.5 |
| 700 | 1.9735 | 315.0 |
| 800 | 1.9597 | 363.4 |
Such insight from 1D and 2D waveguides underscores the role of the ZnO NR diameter in light guiding. To maximize light coupling and guiding axially along a ZnO NR, one can use the equation below for a cylindrical waveguide (74, 80) to estimate the fractional mode power (η), the ratio between the integrated electromagnetic field intensity axially guided inside the ZnO NR and the transversely diffracted light:
where V is the same as above,
The assessment indicates that thin NWs are not suitable to maximize light guiding because light cannot be effectively confined within the active medium because of diffraction. Greater than 80% of the electromagnetic field intensity of guided light of λ = 500 nm is expected to be retained within the NR for the lowest-order guided mode traveling through ZnO NRs with diameters larger than 200 nm, whereas less than 30% of the intensity is present for NRs less than 100 nm in diameter. In fact, the typical diameter of ZnO NRs employed in biomedical fluorescence detection ranges from 100 to 500 nm, which is well within the window for single-mode (or higher mode, depending on λ) guiding of Vis and NIR light with minimized diffraction lost to the surrounding. The predicted increase in the penetration depth of the evanescent wave in the ZnO NR waveguide, stretching well beyond 100 nm from the NR surface, may explain the distance-independent behavior between the fluorophore and ZnO NR observed in the biomolecular fluorescence enhancement discussed in Sections 2.1 and 2.2. The outcomes of these prior studies in optics and optoelectronics strongly suggest that the inherent optical properties of ZnO NRs with the specified dimensions that effectively couple, guide, and enhance light within and along the surface of the NRs may best explain the remarkable fluorescence sensitivity observed in ZnO NR-enabled biodetection.
2.4. Unique Optical Properties of Individual ZnO Nanorods
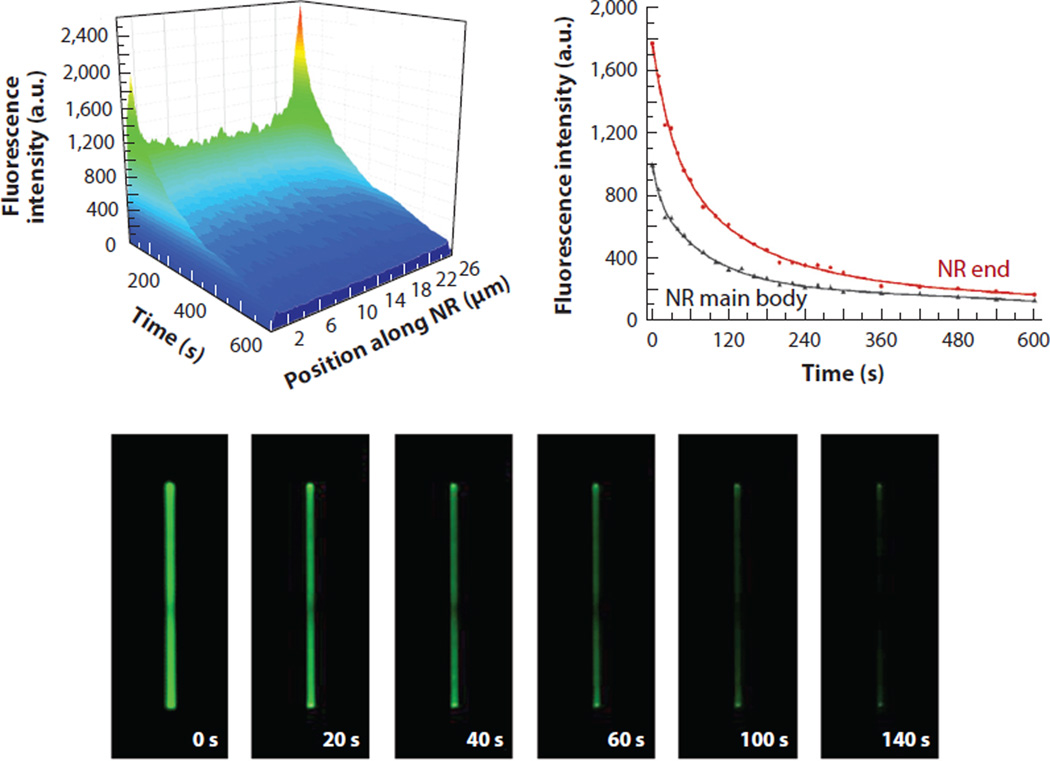
With valuable insight from optics and optoelectronics, experimental and computer simulation efforts have been recently made to determine the optical properties of ZnO NRs specifically pertaining to the biomolecular fluorescence enhancement effect (40, 51). When individual ZnO NRs were examined with fluorophore-coupled proteins for their temporal and spatial profiles of coupling and guiding fluorescence, a unique optical phenomenon of a highly localized, extremely intensified signal was observed on the two ends of the ZnO NRs, which was termed fluorescence intensification on nanorod ends (FINE). Additionally, the fluorescence signal on the NR ends not only is higher in intensity, but also lasts longer upon constant illumination relative to the signal on the NR main body. Therefore, the FINE phenomenon refers to the highly spatially localized and temporally extended biomolecular fluorescence emission on ZnO NRs. Figure 5 displays such spatial and temporal distributions of the biomolecular fluorescence signal measured from individual ZnO NRs. Plotted as a function of time and position along the NR length, biomolecular fluorescence emission patterns yield two intense peaks at each NR end, for which the intensity decays exponentially with a slower decay rate than for the NR main body. The NR facet-dependent photostability profile of biomolecular fluorescence is catalogued in Table 3.
Figure 5.
Highly spatially localized, temporally extended biomolecular fluorescence signal observed on single ZnO nanorods (NRs). The contour map and the time-lapse images display variations in the biomolecular fluorescence intensity along the long axis of a 25-µm-long ZnO NR. Differences in the time dependence of the fluorescence intensity decay under constant irradiation were clearly observed depending on the ZnO NR planes. Red and black data show biomolecular fluorescence measured from the NR end and main body, respectively. Figure adapted from Reference 40 with permission. Copyright 2014 Royal Society of Chemistry.
Table 3.
Averaged photostability characteristics of biomolecular fluorescence profiled by listing T1/2, T1/5, and T1/10 values for various single ZnO nanorod (NR) platforms
| Biomolecules | Test platforms | T1/2 (s) | T1/5 (s) | T1/10 (s) |
|---|---|---|---|---|
| 10-µg/ml DTAF-anti-IgG | V-ZnO NRef,g | Detector saturated | ||
| V-ZnO NRsf,g | 30 ± 2.9 | 120 ± 11.6 | 240 ± 20.5 | |
| L-ZnO NRef,g | 63 ± 7.7 | 174 ± 10.8 | 250 ± 21.8 | |
| L-ZnO NRsf,g | 43 ± 4.5 | 170 ± 10.0 | 279 ± 20.4 | |
| 1-µg/ml TRITC-anti-IgG | V-ZnO NRef,r | 40 ± 5.0 | 149 ± 12.1 | 220 ± 14.5 |
| V-ZnO NRsf,r | 35 ± 3.0 | 130 ± 13.5 | 205 ± 10.4 | |
| L-ZnO NRef,r | 60 ± 7.6 | 178 ± 18.3 | 282 ± 21.2 | |
| L-ZnO NRsf,r | 49 ± 7.7 | 180 ± 16.5 | 290 ± 12.6 | |
The photostability data were catalogued based on the two growth orientations of vertical (V) and lateral (L) with respect to the growth substrate, the position of the NR end facet (NRef) and NR side facet (NRsf), and the two types of fluorophores of 5-(4,6-dichlorotriazinyl) aminofluorescein (DTAF; g) and tetramethyl rhodamine isothiocyanate (TRITC; r) at different protein concentrations. Table adapted with permission from Reference 51. Copyright 2015 Royal Society of Chemistry.
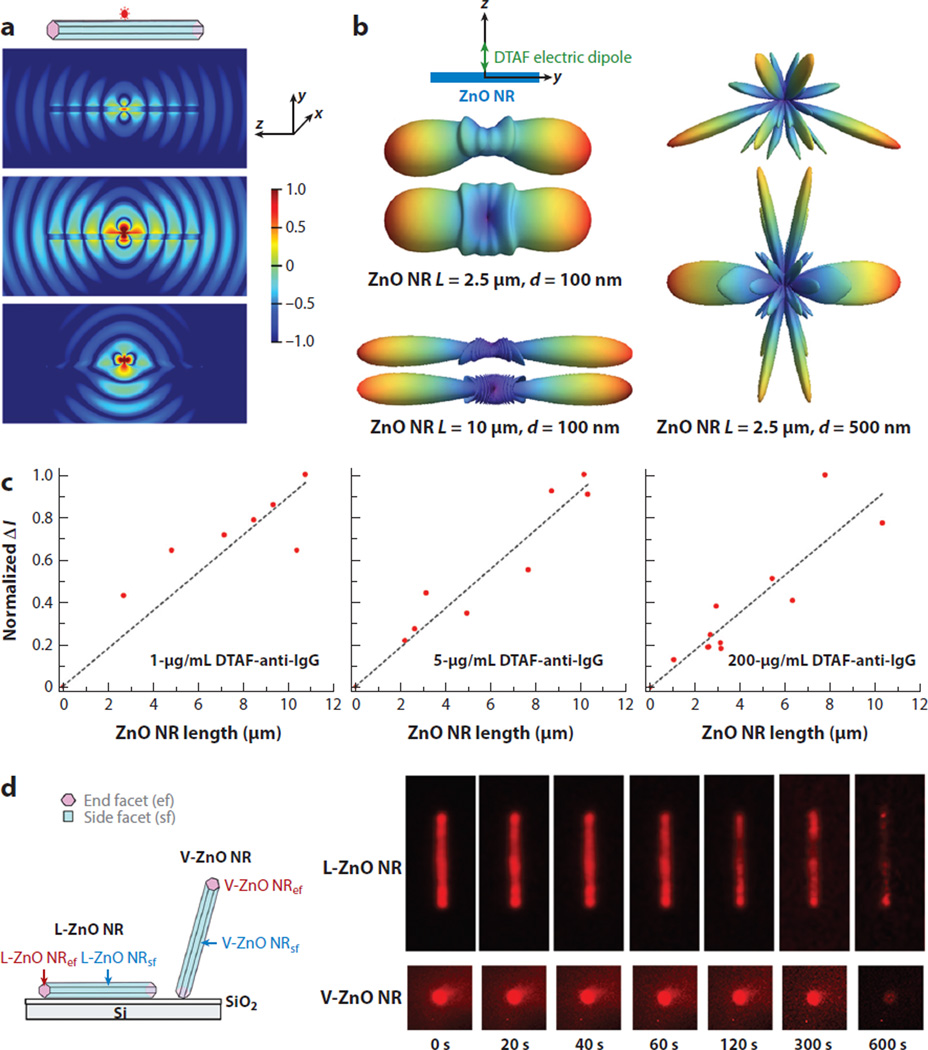
Considering that fluorophore-conjugated proteins were dispersed throughout the NR surface, this spatially nonuniform optical phenomenon was highly unusual. The origin of such unexpected behavior of FINE has since been scrutinized by systematically varying key experimental factors, such as the orientation of ZnO NRs with respect to the growth substrate plane, the physical dimensions of ZnO NRs, the spectroscopic profile of the fluorophores linked to biomolecules, and the concentration of biomolecules. When the signal distribution across the long axis of individual ZnO NRs was profiled, considerably higher signal was consistently observed on the NR ends relative to the NR main body, irrespective of the ZnO NR growth orientation and physical dimensions. In addition, FINE was present for all ZnO NRs regardless of the spectroscopic profiles of the fluorophores employed. The degree of FINE, ΔI (Iavg,NRef − Iavg,NRsf), defined as the average fluorescence intensity difference monitored on the NR ends versus the NR main body, became larger with increasing NR length. Conversely, the NR width did not play a significant role when assessing NRs in the diameter range of 50–600 nm. The magnitude of FINE increased with higher protein concentration. ZnO NRs vertically oriented with respect to the growth substrate showed a much greater degree of FINE compared to ZnO NRs laterally oriented, regardless of the fluorophore’s concentration or spectroscopic property. Moreover, the photostability of the biomolecular fluorescence signal observed from NR end facets was always higher than that from NR side facets.
With regard to numerical simulations, FDTD modeling efforts of a single fluorophore placed on a ZnO NR provided insight into the possible cause of the FINE behavior. In FDTD simulations, an electric dipole emitting at either 517 nm or 576 nm was placed at the end or in the middle of a ZnO NR with a side length of 100 nm and length of 2.5 µm; the simulations demonstrated the NR propensity of the coupling and guiding of fluorophore radiation through the cavity and surface of the NR before finally emanating from the two ends to the far field (40, 51). Figure 6 summarizes these experimental and FDTD simulation findings on the dependence of FINE with respect to the length, width, and growth orientation of the NRs, as well as the concentration and spectroscopic profile of biomolecules.
Figure 6.
Factors governing the occurrence, degree, and magnitude of fluorescence intensification on nanorod ends (FINE). (a) Finite-difference time-domain simulations were carried out to obtain the radiation patterns from a single emitter radiating at 576 nm polarized along the x (top), y (middle), and z (bottom) directions. (b) The dimensional effect of ZnO nanorods (NRs) on FINE was evaluated by simulating far-field radiation patterns of a 517-nm electric dipole. A pair of far-field patterns is shown for each NR of the specified length (L) and width (d), where the top/bottom simulation corresponds to the spatial patterns observed from the z/x axis. (c) ZnO NRs of various lengths and widths were analyzed after treatment with three different concentrations of 5-(4,6-dichlorotriazinyl) aminofluorescein (DTAF)–antibovine IgG (anti-IgG). In all cases, the normalized ΔI value of (Iavg,NRef − Iavg,NRsf) indicated that the degree of FINE increased as the NR length increased. (d) When the spatial and temporal emission behavior of 1-µg/ml TRITC-anti-IgG on a lateral (L)-ZnO NR (top) and a vertical (V)-ZnO NR (bottom) was monitored in time-lapse fluorescence panels, the presence of the FINE phenomenon was qualitatively confirmed in all cases. The magnitude and degree of FINE were higher for vertically oriented NRs relative to laterally oriented ones. Figure adapted from Reference 51 with permission. Copyright 2015 Royal Society of Chemistry.
With increasing miniaturization trends from the micro-to the nanoscale regime in biodetection technology (83–85), future applications of ZnO NRs may accordingly favor the use of single, rather than ensembles of, ZnO NRs as distinct detection elements, especially for in vivo imaging and in vitro assays requiring extremely low-volume and high-throughput analyses. Therefore, the previous studies discretely probing individual ZnO NRs may provide a basis for both the design of an optimized detection platform with large signal enhancement and accurate signal interpretation of fluorescence-based bioassays. The distinctive biomolecular emission profiles characterized for the different ZnO NR crystalline planes and growth orientations may further promote innovative biomedical applications based on the use of single ZnO NRs by harnessing their unique shape anisotropy for highly directional and locally enhanced optical detection.
3. ZnO NANOROD-BASED BIOMEDICAL ASSAYS
3.1. Single-Component Bioassay
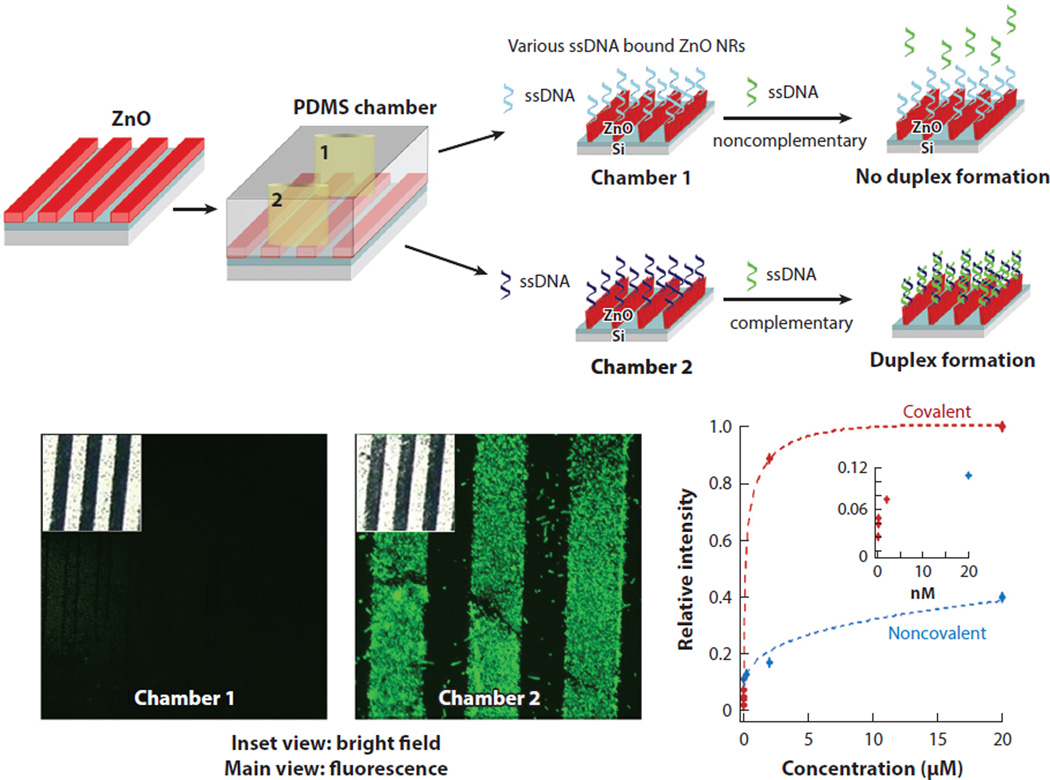
Based on experimental and computational findings that the use of ZnO NR platforms can facilitate highly sensitive detection of biomolecular emission, these platforms have been evaluated for enhanced fluorescence assays involving single-component biological systems of either DNA or proteins. The applicability of ZnO NR platforms in DNA sequence detection has been demonstrated by carrying out DNA duplex formation reactions in an elastomeric microfluidic chamber using fully complementary or noncomplementary probe oligonucleotides (5′-AGTGCGCGAGGAGCCT-3′ and 5′-GTTACGGAAAGAACCA-3′) linked to the NR surface covalently and noncovalently (50). Analyte DNA (3′-TCACGCGCTCCTCGGA-5′) preconjugated with 6-carboxyfluorescein was subsequently hybridized to the oligonucleotide probes on the ZnO NR platforms. The detection limit of the ZnO NR-assisted DNA fluorescence assay was down to a few femtomolars when using a covalent linking scheme via glycidoxylpropyltrimethoxysilane, whereas the lowest concentration of DNA assayed using a noncovalent, physisorption scheme was in the tens of nanomolars range. Figure 7 displays these results from DNA hybridization reactions carried out on a ZnO NR platform configured in a polydimethylsiloxane elastomeric chamber. The detection capability of ZnO NRs was also assessed in multilayer protein systems (46). In these tests, different pairs of interacting or noninteracting proteins were sequentially introduced to ZnO NR platforms and subsequently examined for fluorescence. The outcomes of the multilayer protein assays corroborated with the earlier results showing the effectiveness of ZnO NRs in monitoring the fluorescence signal from simple biological systems, even at ultratrace-level analyte concentrations.
Figure 7.
Enhanced fluorescence detection sensitivity demonstrated in a single-component bioassay. DNA hybridization reactions were performed on a stripe-patterned ZnO NR platform. Strong fluorescence emission was observed from a sample containing a fully complementary pair of single-stranded DNA fragments in chamber 2, whereas no signal was detected from noncomplementary strands in chamber 1. DNA duplex formation assays were also performed as a function of target DNA concentration to determine detection sensitivity. Data shown in red and blue were obtained from the hybridization assays employing a covalent and noncovalent linking scheme of probe DNA strands on ZnO NRs, respectively. Abbreviations: NR, nanorod; PDMS, polydimethylsiloxane. Figure adapted from Reference 50 with permission. Copyright 2006 Institute of Physics Publishing.
3.2. Multicomponent Bioassay
In model biological systems of single components, the biointeraction was limited to the same type of purified bioconstituents. ZnO NR platforms were further evaluated in the fluorescence detection of more biologically complex and clinically relevant systems to prove their full potential in biomedical screening and clinical diagnosis. Ensuing applications of ZnO NR platforms have enabled biomarker detection with regard to cancer and acute kidney disease. A fluorescence-based telomeric repeat elongation (TRE) assay was developed based on ZnO NRs and successfully employed for a fast and straightforward method to qualitatively determine telomerase activity in cancer cells (49). The TRE fluorescence assays showed that ZnO NR surfaces permit the monitoring of telomerase activity by preserving the physical integrity and biological functionality of various biocomponents involved in a complex assay mixture consisting of cell lysates, proteins, oligonucleotides, and deoxyribonucleotide triphosphates.
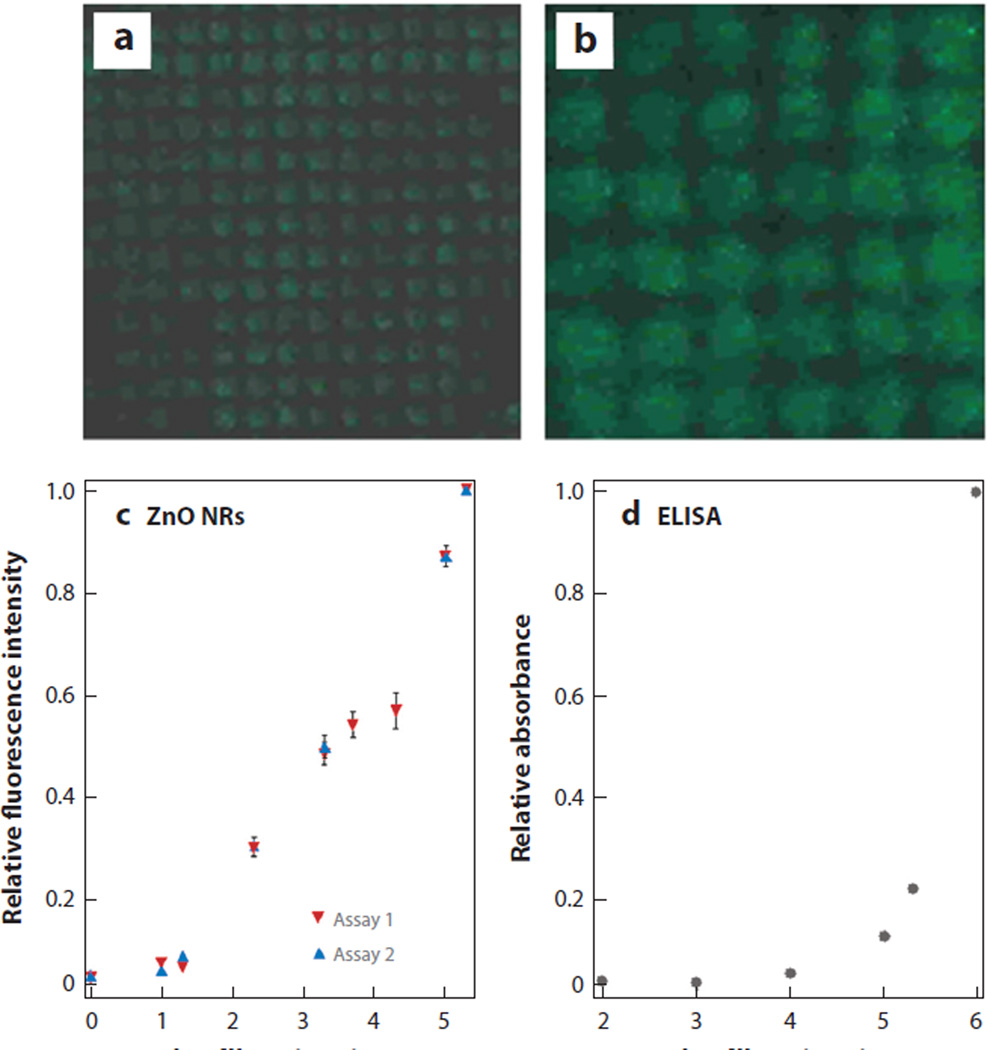
These promising outcomes led to a further demonstration of ZnO NR platforms in highly sensitive and quantitative cytokine detection. Although elevated levels of cytokines may serve as important markers of severity and diagnosis for acute kidney injury, these proteins are very difficult to detect in early stages of disease when their concentrations are well below the detection level of conventional detection kits based on ELISA. Encouraged by the unparalleled fluorescence detection sensitivity of ZnO NRs, the feasibility of ZnO NR platforms as an alternative cytokine screening platform has been evaluated (41). Cytokines evaluated in these initial tests were interleukin-18 and tumor necrosis factor-α, and the analytes were either carried in a phosphate buffered saline or spiked in urine from a healthy subject. The detection sensitivity of ZnO NRs was determined as low as several femtograms per milliliter, whereas a commercial detection kit showed sensitivity in the range of tens of picograms per milliliter. Figure 8 displays the results of these comparison assays carried out on a ZnO NR platform as well as on a commercial ELISA kit.
Figure 8.
ZnO nanorod (NR)-based fluorescence-based cytokine detection platform showing several orders of higher detection sensitivity beyond the capability of a commercial enzyme-linked immunosorbent assay (ELISA)-based kit. Cytokine assays were carried out on a ZnO NR platform in a physiologically relevant environment. Fluorescence panels of (a) 140 × 140 µm and (b) 60 × 60 µm were acquired from a sandwich assay involving 20 fg/ml of interleukin-18 (IL-18) spiked in urine. (c) Normalized fluorescence intensities from two independent runs were plotted against the logarithmic value of the IL-18 concentration. (d) As a comparison, normalized ELISA signals were plotted for various IL-18 concentrations. Figure adapted from Reference 41 with permission. Copyright 2008 American Chemical Society.
3.3. Physiological and Clinical Diagnostic Assays
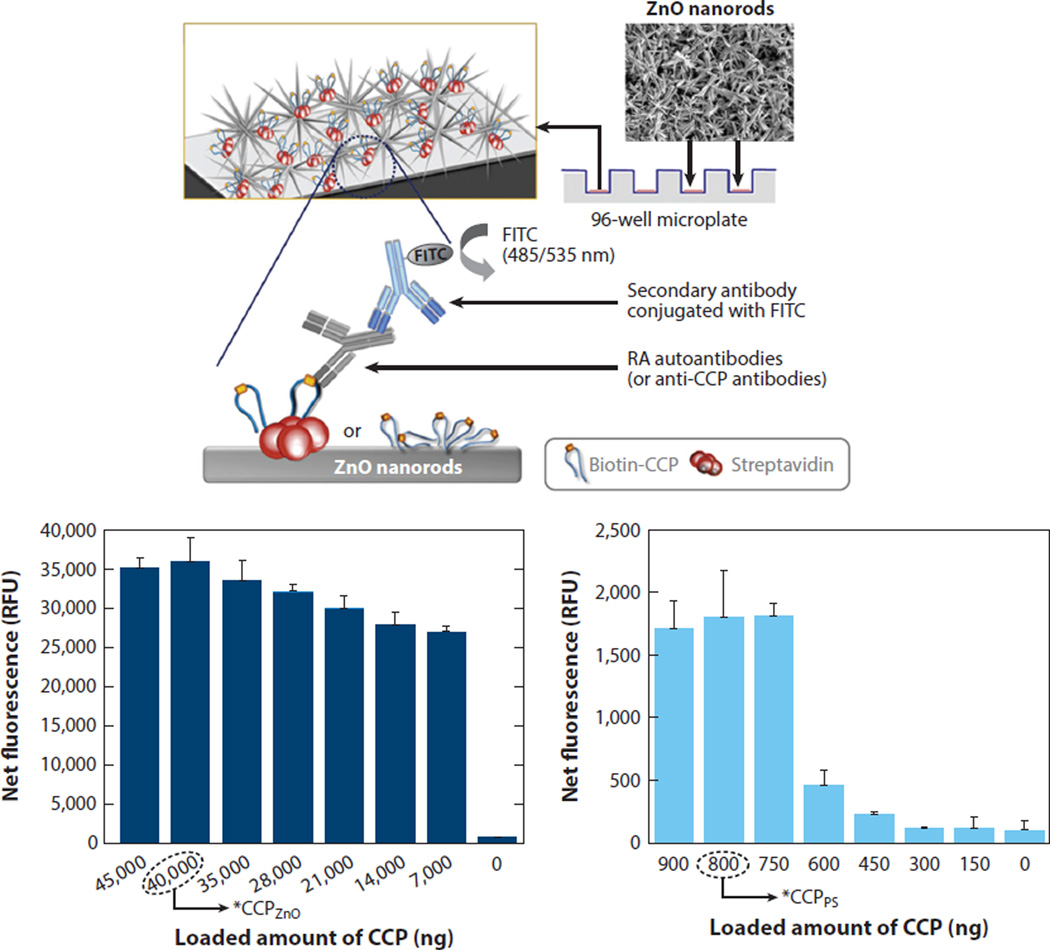
Although it is at a very early stage, research has been put forward to develop clinically relevant assays based on ZnO NRs. Diagnostic assays were performed on rheumatoid arthritis (RA) patient sera to detect the level of anticyclic citrullinated peptide (anti-CCP) RA autoantibodies in patients as well as healthy subjects. The RA assay results obtained on ZnO NR supports were compared to those of PS supports (see Figure 9) (86). ZnO NRs were capable of resolving a very low biomolecular fluorescence signal that could not be resolved on the polymeric support, even in the assays employing human sera.
Figure 9.
Clinically relevant assay performed on ZnO NR platforms. Net fluorescence from the anti-CCP RA autoantibody assay was measured on ZnO NRs and PS as a function of the loaded amount of CCP probe. Data from the ZnO NRs are displayed on the left, whereas those from a PS platform are shown on the right as a comparison. Abbreviations: CCP, cyclic citrullinated peptide; FITC, fluorescein isothiocyanate; NR, nanorod; PS, polystyrene; RA, rheumatoid arthritis. Figure adapted from Reference 86 with permission. Copyright 2011 Elsevier.
3.4. ZnO Nanorods in Nonfluorescence Biodetection
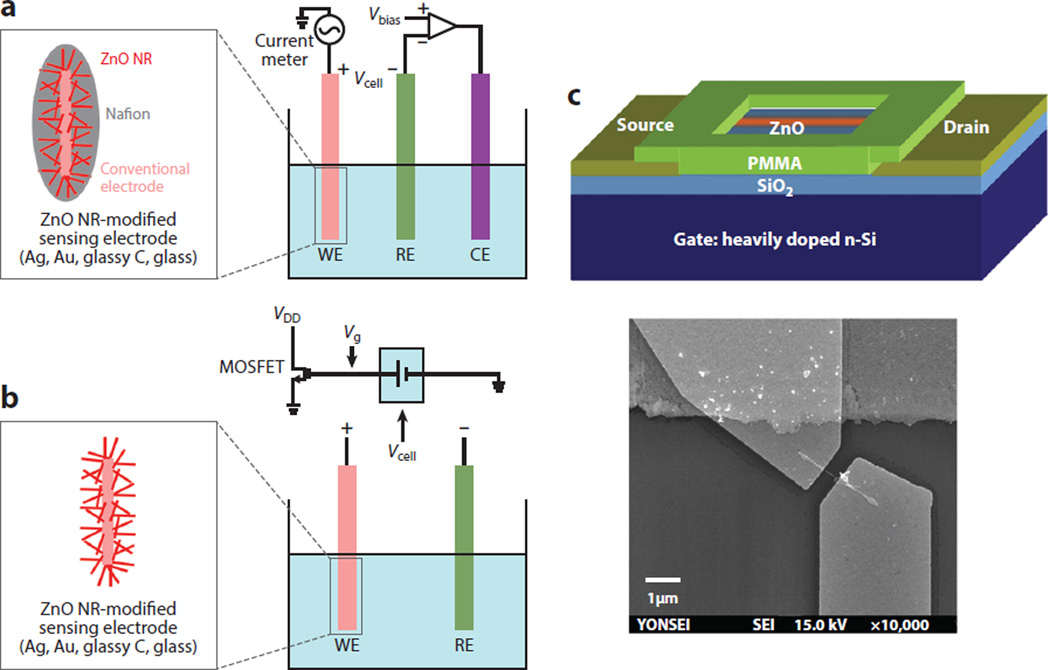
Although the scope of this review is primarily centered on the bioapplicability of ZnO NRs in fluorescence-based detection, the 1D nanomaterials have been widely utilized in nonoptical biosensing purposes as well. The mode of detection in the majority of nonoptical applications employs amperometric electrochemical biosensors with relatively few studies reporting on potentiometric approaches. Figure 10 displays typical electrochemical measurement configurations of amperometric and potentiometric sensor devices, as well as an electrical testing setup involving a field-effect transistor (FET). An amperometric biosensor comprises two or three electrodes. The former, consisting of a reference electrode (RE) and a working electrode (WE), has a limited application in biosensing given the difficulty in potential control associated with a high current flow as a result of a sizable ohmic drop (also known as an IR drop). The latter configuration is more commonly found in biosensors because the introduction of a counter electrode (CE) with a large surface area allows for most of the current to flow between the CE and WE for a voltage applied between the RE and WE. Potentiometric sensors include the metal-oxide-sensitive field-effect transistor, light-addressable potentiometric sensor, ion-sensitive field-effect transistor, and ion-selective electrode. In the potentiometric approach, the potential difference between the RE and WE is measured at zero current flow, by which measurements are carried out by recording the potentials between the two electrodes at zero current as a function of the concentrations of target analytes in a logarithmic manner.
Figure 10.
ZnO NR applications in nonoptical modes of biodetection. (a) A typical three-electrode amperometric electrochemical measurement setup. (b) MOSFET-based potentiometric electrochemical sensor utilizing extended-gate functionalized ZnO NRs as WEs. In both sensing approaches, conventional electrode surfaces of silver, gold, glassy carbon, or glass capillary are modified with a dense layer of ZnO NRs and exploited as a sensing electrode. (c) A single ZnO NR-based FET biosensor is shown. Figure adapted from Reference 60 with permission. Copyright 2010 Elsevier. Abbreviations: CE, counter electrode; FET, field-effect transistor; MOSFET, metal-oxide-semiconductor field-effect transistor; NR, nanorod; PMMA, polymethylmethacrylate; RE, reference electrode; WE, working electrode.
In FET-based electrical biosensors, the sensing channel served by either individual or groups of ZnO NRs is connected to two electrodes of the source and drain and the conductance between the source and drain is altered by the use of a gate electrode. Upon biomolecular binding, measurements are carried out by monitoring the current change between the source and drain, ISD, over time as a function of the analyte concentration. The fundamental sensing mechanism of FET-based electrical biosensors relies on a change in conductivity (or resistance) through the channel sensing layer caused by biomolecular interaction processes. 1D nanoscale FET biosensors fabricated from carbon nanotubes and silicon NWs offer high electrical sensitivity and real-time detection (87–93). The physical dimensions of 1D nanoscale materials are comparable to the sizes of biological analytes for sensing, serving as excellent transducers for signal generation to the detection level of single biomolecules. However, for use in these applications, the channel material surfaces should be stable without any formation of an insulating native oxide layer in air and with no chemical degradation in bioassay solution that may jeopardize the device reliability and sensitivity. ZnO NRs can be advantageous in this regard, leading to high electrical sensitivity with low power consumption (60).
ZnO NRs fabricated into pH sensors were used to monitor in vivo biological processes within single cells (94). ZnO NRs were also incorporated into a tyrosinase biosensor to detect phenolic compounds (95). The use of ZnO NRs was demonstrated for similar biosensing purposes, such as glucose (96–99), uric acid (100), H2O2 (101), pH (102, 103), cholesterol (104), penicillin (105), Ca2+ ion (103, 106), and l-lactic acid (107) detection. In many of these studies, a higher detection limit and extended linear range of analyte concentration were reported from ZnO NR-modified WEs compared to those measured in an electrochemical cell with a conventional WE, normally composed of materials such as metal wires of gold, silver, platinum, glassy carbon, and glass capillary. However, the majority of the studies concentrate on biosensor performance assessments, and the exact origin through which ZnO NR-derivatized electrodes can yield high-detection sensitivity is not yet clearly known. Mechanisms involved in the enhanced sensitivity demonstrated from n-type ZnO NR-FET biosensors operating in an electrical mode are better understood, largely owing to the insight from other 1D nanomaterial FETs such as carbon nanotube and silicon NW devices (60). Table 4 summarizes the benchmarking performance elements of these electrochemical and electrical biosensors successfully utilizing ZnO NRs for nonoptical detection schemes.
Table 4.
Various ZnO NR-based biosensors in nonoptical detection modes
| Physical dimensions of ZnO NRs |
Analyte | Detection mode | Detection sensitivity |
Linear range | Selectivity | Reference |
|---|---|---|---|---|---|---|
|
d = 30–100 nm L = 10–30 µm |
Glucose | Electrochemical | 0.7 µM (SNR > 3) | ~0.001–1 mM | Via GOx-modified ZnO NRs on Au electrode |
97 |
|
d = 300 nm L = 4 µm |
Glucose | Electrochemical | 10 µM (SNR > 3) | ~0.01–3.5 mM | Via GOx-modified ZnO NRs on Au electrode |
98 |
|
d = 100–120 nm L = 0.9–1 µm |
Intercellular glucose | Electrochemical | NA | 0.5 µM–1 mM | Via GOx-modified ZnO NRs on Ag-covered glass capillary |
99 |
|
d = 50 nm L > 1 µm |
Uric acid | Electrochemical | 2 × 10−6 M (SNR>3) |
5 × 10−6– 1 × 10−3 M |
Via uricase-modified ZnO NRs on glassy carbon electrode |
100 |
| NA | H2O2 | Electrochemical | 2 × 10−6 M (SNR>3) |
1 × 10−5– 1.8 × 10−3 M |
Via horseradish peroxidase/chitosan-modified ZnO NRs on glassy carbon electrode |
101 |
|
d = 125–250 nm L = ~1 µm |
Cholesterol | Electrochemical | 35.2 mV/decade | 1 × 10−6– 1 × 10−2 M |
Via cholesterol oxidase-modified ZnO NRs on Ag wire |
104 |
|
d = 100–150 nm L = 0.9–1 µm |
Ca2+ | EGFET | NA | 1 × 10−6– 1 × 10−3 M |
Via ionophore-containing membrane coupled ZnO NRs on Ag wire |
103 |
|
d = 500 nm at base L = 5 µm |
1-lactic acid | Electrochemical | 1.2 × 10−6 M | 3.6 × 10−6– 0.6 × 10−3 M |
Via lactate oxidase-modified ZnO NRs on Au electrode |
107 |
| NA | H+/OH− | EGFET | 38 mV/pH | pH 2–12 | Interfaced with MOSFET | 102 |
|
d = 80 nm L = 700 nm |
Intracellular H+/OH− |
Electrochemical | 52 mV/pH | pH 4–11 | Via ZnO NRs on Ag-covered glass capillary |
94 |
| NA | Penicillin | Electrochemical | ~121 mV/decade | 1 × 10−4– 1 × 10−1 M |
Via penicillinase-modified ZnO NRs grown on Au-coated glass |
105 |
|
d = 70 nm L = 8 µm |
Streptavidin | FET | ~2.5 nM | Up to 20 nM | Via biotinylated ZnO NRs | 60 |
Here d is the NR diameter, and L is the NR length. Abbreviations: EGFET, extended-gate field-effect transistor; FET, field-effect transistor; GOx, glucose oxidase; MOSFET, metal-oxide-semiconductor field-effect transistor; NA, not available; NR, nanorod; SNR, signal-to-noise ratio.
4. OUTLOOK
Fundamentally, further elucidation of light-matter interaction properties pertaining to both individual and ensembles of NRs is warranted to better capitalize on the ZnO NR-enabled high optical sensitivity in fluorescence-based biological detection. Although 1D NR systems have been previously examined for their light interaction behavior, the focus of these studies was largely on the band gap emission of the material in the ultraviolet range (73, 74, 108). Current understanding of ZnO NR optical properties in a biologically relevant framework is still in its infancy. The optical properties of both single and ensembles of ZnO NRs should be further evaluated carefully. Individual nanomaterials may possess substantially different optical behaviors than their ensemble-averaged response, as has been reported for single and multiple quantum dot and metallic nanoparticle systems (109–114). Additionally, optical signals collectively measured from an ensemble of NRs may not be accurately deduced from a simple sum of the intensity values obtained on single NRs making up the ensemble, as evidenced by metallic NR arrays and antennas (115, 116). Hence, in-depth studies probing interactions between ZnO NR/incident light as well as ZnO NR/fluorophore-coupled biomolecules should be carried out on both single and collective NR systems with respect to polarization direction, fluorescence lifetime, photostability, and NR specifications to deepen our understanding of the optical enhancement effect of ZnO NRs. Understanding the optical profiles of the ZnO NRs collectively contributing to the enhanced spot signal can further provide a basis for accurate signal processing and interpretation. Research endeavors to examine single ZnO NR optical characteristics can lead to discoveries of new properties that could not be resolved from ensemble studies.
The application of ZnO NRs in biosensing is at its very early stage, particularly pertaining to their use beyond laboratory-scale demonstrations. Despite this, it is expected that bio-optical applications of ZnO NRs will continue to grow and yield new development of clinically useful assays because of the many demonstrated advantages of ZnO NRs, such as the exquisite fluorescence detection capability, the lack of spectroscopic interference with the absorption and emission profiles of common fluorophores, easy growth and array configuration into patterned platforms, and the use of conventional fluorescence array scanners and plate readers as detectors,
In clinical research and diagnostics, the size of in vivo and in vitro detection platforms and devices is rapidly shrinking for low-cost, high-speed, and small-volume biomolecular analyses (38, 63, 117–119). Hence, the development of clinical assays based on single ZnO NRs may promote further miniaturization of the bio-optical detection platform, while maintaining a similar level of exquisite sensitivity and measurement simplicity in screening low-abundance bioanalytes in vitro. Additionally, the superior optical detection capability of ZnO NRs may be applicable to in vivo assays to measure locally hard-to-detect, low-signal-emitting bioconstituents. The feasibility of exploiting 1D nanomaterials in single-cell studies has been already demonstrated with SnO2 nanoribbon, carbon nanotube, and photonic nanocavity probes (27, 120, 121). The inherently small size of these 1D nanoprobes is known to offer less invasive, biocompatible, and spatially localized illumination to a specific cell location, without causing considerable cell death or stress (122). However, the fluorescence signal-enhancing capability still needs to be thoroughly examined not only from multiple NRs as a group, but also from individual NRs given that the optical behavior of individual and ensembles of ZnO NRs may differ significantly. The former system can serve as an attractive scaffold for in vitro optical detection, whereas the latter will be particularly useful as an optical signal carrier for in vivo biodetection.
5. CONCLUDING REMARKS
Although the bioapplications of 1D ZnO nanomaterials have only recently begun, forefront research endeavors have started to identify both well-known and new nanomaterial properties beneficial to the physical, optical, electrochemical, and electrical detection of bioconstituents. In particular, ZnO NRs in fluorescence-based detection have shown great promise for rapid, multiplexed, high-throughput, and highly sensitive platforms. Further elucidation of the optical properties of individual and ensembles of ZnO NRs can enlighten us on the nanomaterial’s unique optical phenomena and subsequently promote new development of highly miniaturized optical biosensors useful for new basic biology research and clinical diagnostics. Landmark efforts in the field of ZnO NR-based biosensors and biodetection platforms are highlighted in this review. The exceptional properties of ZnO NRs discussed above, making them suitable in biosensing and biodetection, still challenge us with plenty of room to grow in investigating novel properties, engineering the nanomaterials to tune for the desired properties, and devising new bioapplication capacities exploiting the distinctive properties.
Acknowledgments
The author acknowledges financial support of this work by the National Institutes of Health, with a National Research Service Award (1R01DK088016) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Kołodziejczak-Radzimska A, Jesionowski T. Zinc oxide—from synthesis to application: a review. Materials. 2014;7:2833–2881. doi: 10.3390/ma7042833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang ZL. Splendid one-dimensional nanostructures of zinc oxide: a new nanomaterial family for nanotechnology. ACS Nano. 2008;2:1987–1992. doi: 10.1021/nn800631r. [DOI] [PubMed] [Google Scholar]
- 3.Gao PX, Wang ZL. Substrate atomic-termination-induced anisotropic growth of ZnO nanowires/nanorods by the VLS process. J. Phys. Chem. B. 2004;108:7534–7537. [Google Scholar]
- 4.Kumar N, Dorfman A, Hahm JI. Fabrication of optically enhanced ZnO nanorods and microrods using novel biocatalysts. J. Nanosci. Nanotechnol. 2005;5:1915–1918. doi: 10.1166/jnn.2005.422. [DOI] [PubMed] [Google Scholar]
- 5.Park WI, Kim DH, Jung SW, Yi GC. Metalorganic vapor-phase epitaxial growth of vertically well-aligned ZnO nanorods. Appl. Phys. Lett. 2002;80:4232–4234. [Google Scholar]
- 6.Wang ZL. Zinc oxide nanostructures: growth, properties and applications. J. Phys. Condens. Matter. 2004;16:R829–R858. [Google Scholar]
- 7.Wu JJ, Liu SC. Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Adv. Mater. 2002;14:215–218. [Google Scholar]
- 8.Yang P, Yan H, Mao S, Russo R, Johnson J, et al. Controlled growth of ZnO nanowires and their optical properties. Adv. Funct. Mater. 2002;12:323–331. [Google Scholar]
- 9.Özgür Ü, Alivov YI, Liu C, Teke A, Reshchikov MA, et al. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005;98:041301. [Google Scholar]
- 10.Liu X, Wu X, Cao H, Chang RPH. Growth mechanism and properties of ZnO nanorods synthesized by plasma-enhanced chemical vapor deposition. J. Appl. Phys. 2004;95:3141–3147. [Google Scholar]
- 11.Greene LE, Law M, Goldberger J, Kim F, Johnson JC, et al. Low-temperature wafer-scale production of ZnO nanowire arrays. Angew. Chem. Int. Ed. 2003;42:3031–3034. doi: 10.1002/anie.200351461. [DOI] [PubMed] [Google Scholar]
- 12.Vayssieres L. Growth of arrayed nanorods and nanowires of ZnO from aqueous solutions. Adv. Mater. 2003;15:464–466. [Google Scholar]
- 13.Vayssieres L, Keis K, Hagfeldt A, Lindquist SE. Three-dimensional array of highly oriented crystalline ZnO microtubes. Chem. Mater. 2001;13:4395–4398. [Google Scholar]
- 14.Vayssieres L, Keis K, Lindquist SE, Hagfeldt A. Purpose-built anisotropic metal oxide material: 3D highly oriented microrod array of ZnO. J. Phys. Chem. B. 2001;105:3550–3552. [Google Scholar]
- 15.Tam KH, Cheung CK, Leung YH, Djurišić AB, Ling CC, et al. Defects in ZnO nanorods prepared by a hydrothermal method. J. Phys. Chem. B. 2006;110:20865–20871. doi: 10.1021/jp063239w. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Ai K, Yuan Q, Lu L. Fluorescence-enhanced gadolinium-doped zinc oxide quantum dots for magnetic resonance and fluorescence imaging. Biomaterials. 2011;32:1185–1192. doi: 10.1016/j.biomaterials.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Yuan Q, Hein S, Misra RDK. New generation of chitosan-encapsulated ZnO quantum dots loaded with drug: synthesis, characterization and in vitro drug delivery response. Acta Biomater. 2010;6:2732–2739. doi: 10.1016/j.actbio.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Kachynski AV, Kuzmin AN, Nyk M, Roy I, Prasad PN. Zinc oxide nanocrystals for nonresonant nonlinear optical microscopy in biology and medicine. J. Phys. Chem. C. 2008;112:10721–10724. doi: 10.1021/jp801684j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George S, Pokhrel S, Xia T, Gilbert B, Ji Z, et al. Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping. ACS Nano. 2010;4:15–29. doi: 10.1021/nn901503q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willander M, Nur O, Zhao QX, Yang LL, Lorenz M, et al. Zinc oxide nanorod based photonic devices: recent progress in growth, light emitting diodes and lasers. Nanotechnology. 2009;20:332001. doi: 10.1088/0957-4484/20/33/332001. [DOI] [PubMed] [Google Scholar]
- 21.Klingshirn CF, Waag A, Hoffmann A, Geurts J. Zinc Oxide: From Fundamental Properties Towards Novel Applications. Berlin: Springer; 2010. [Google Scholar]
- 22.Kupec J, Stoop RL, Witzigmann B. Light absorption and emission in nanowire array solar cells. Opt. Express. 2010;18:27589–27605. doi: 10.1364/OE.18.027589. [DOI] [PubMed] [Google Scholar]
- 23.Grange R, Brönstrup G, Kiometzis M, Sergeyev A, Richter J, et al. Far-field imaging for direct visualization of light interferences in GaAs nanowires. Nano Lett. 2012;12:5412–5417. doi: 10.1021/nl302896n. [DOI] [PubMed] [Google Scholar]
- 24.Law M, Sirbuly DJ, Johnson JC, Goldberger J, Saykally RJ, Yang P. Nanoribbon waveguides for subwavelength photonics integration. Science. 2004;305:1269–1273. doi: 10.1126/science.1100999. [DOI] [PubMed] [Google Scholar]
- 25.Sirbuly DJ, Law M, Pauzauskie P, Yan H, Maslov AV, et al. Optical routing and sensing with nanowire assemblies. PNAS. 2005;102:7800–7805. doi: 10.1073/pnas.0408641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grzela G, Paniagua-Domínguez R, Barten T, Fontana Y, Sánchez-Gil JA, Rivas JG. Nanowire antenna emission. Nano Lett. 2012;12:5481–5486. doi: 10.1021/nl301907f. [DOI] [PubMed] [Google Scholar]
- 27.Yan R, Park J-H, Choi Y, Heo C-J, Yang S-M, et al. Nanowire-based single-cell endoscopy. Nat. Nanotechnol. 2012;7:191–196. doi: 10.1038/nnano.2011.226. [DOI] [PubMed] [Google Scholar]
- 28.Carlo C, Peter K, Jesper N, Mark LB, Fontcuberta i Morral A. Engineering light absorption in single-nanowire solar cells with metal nanoparticles. New J. Phys. 2011;13:123026. [Google Scholar]
- 29.Johnson JC, Yan H, Schaller RD, Haber LH, Saykally RJ, Yang P. Single nanowire lasers. J. Phys. Chem. B. 2001;105:11387–11390. [Google Scholar]
- 30.Huang MH, Mao S, Feick H, Yan HQ, Wu YY, et al. Room-temperature ultraviolet nanowire nanolasers. Science. 2001;292:1897–1899. doi: 10.1126/science.1060367. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Zhang F, Xia T, Kumar N, Hahm J-I, et al. Low-threshold two-photon pumped ZnO nanowire lasers. Opt. Express. 2009;17:7893–7900. doi: 10.1364/oe.17.007893. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa-Ankerhold HC, Ankerhold R, Drummen GPC. Advanced fluorescence microscopy techniques—FRAP, FLIP, FLAP, FRET and FLIM. Molecules. 2012;17:4047–4132. doi: 10.3390/molecules17044047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pepperkok R, Ellenberg J. High-throughput fluorescence microscopy for systems biology. Nat. Rev. Mol. Cell Biol. 2006;7:690–696. doi: 10.1038/nrm1979. [DOI] [PubMed] [Google Scholar]
- 34.Lichtman JW, Conchello J-A. Fluorescence microscopy. Nat. Meth. 2005;2:910–919. doi: 10.1038/nmeth817. [DOI] [PubMed] [Google Scholar]
- 35.Waters JC. Accuracy and precision in quantitative fluorescencemicroscopy. J. Cell Biol. 2009;185:1135–1148. doi: 10.1083/jcb.200903097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lakowicz JR. Principles of Fluorescence Spectroscopy. New York: Springer; 2006. [Google Scholar]
- 37.Hahm J. Enhanced fluorescence detection enabled by zinc oxide nanomaterials. In: Geddes CD, editor. Metal-Enhanced Fluorescence. Hoboken, NJ: Wiley; 2010. pp. 363–391. [Google Scholar]
- 38.Hahm J. Zinc oxide nanomaterials for biomedical fluorescence detection. J. Nanosci. Nanotechnol. 2014;14:475–486. doi: 10.1166/jnn.2014.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorfman A, Kumar N, Hahm J. Highly sensitive biomolecular fluorescence detection using nanoscale ZnO platforms. Langmuir. 2006;22:4890–4895. doi: 10.1021/la053270+. [DOI] [PubMed] [Google Scholar]
- 40.Singh M, Song S, Hahm J-I. Unique temporal and spatial biomolecular emission profile on individual zinc oxide nanorods. Nanoscale. 2014;6:308–315. doi: 10.1039/c3nr05031a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adalsteinsson V, Parajuli O, Kepics S, Gupta A, Reeves WB, Hahm J-I. Ultrasensitive detection of cytokines enabled by nanoscale ZnO arrays. Anal. Chem. 2008;80:6594–6601. doi: 10.1021/ac800747q. [DOI] [PubMed] [Google Scholar]
- 42.Gao X, Yang L, Petros JA, Marshall FF, Simons JW, Nie S. In vivo molecular and cellular imaging with quantum dots. Curr. Opin. Biotechnol. 2005;16:63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Han XX, Kitahama Y, Tanaka Y, Guo J, Xu WQ, et al. Simplified protocol for detection of protein–ligand interactions via surface-enhanced resonance Raman scattering and surface-enhanced fluorescence. Anal. Chem. 2008;80:6567–6572. doi: 10.1021/ac800642g. [DOI] [PubMed] [Google Scholar]
- 44.Margineanu A, Hofkens J, Cotlet M, Habuchi S, Stefan A, et al. Photophysics of a water-soluble rylene dye: comparison with other fluorescent molecules for biological applications. J. Phys. Chem. B. 2004;108:12242–12251. [Google Scholar]
- 45.Song L, Hennink EJ, Young T, Tanke HJ. Photobleaching kinetics of fluorescein in quantitative fluorescence microscopy. Biophys. J. 1995;68:2588–2600. doi: 10.1016/S0006-3495(95)80442-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorfman A, Kumar N, Hahm J. Nanoscale ZnO-enhanced fluorescence detection of protein interactions. Adv. Mater. 2006;18:2685–2690. [Google Scholar]
- 47.Cui J. Defect control and its influence on the exciton emission of electrodeposited ZnO nanorods. J. Phys. Chem. C. 2008;112:10385–10388. [Google Scholar]
- 48.Greene LE, Law M, Tan DH, Montano M, Goldberger J, et al. General route to vertical ZnO nanowire arrays using textured ZnO seeds. Nano Lett. 2005;5:1231–1236. doi: 10.1021/nl050788p. [DOI] [PubMed] [Google Scholar]
- 49.Dorfman A, Parajuli O, Kumar N, Hahm J. Novel telomeric repeat elongation assay performed on ZnO nanorod array supports. J. Nanosci. Nanotechnol. 2008;8:410–415. doi: 10.1166/jnn.2008.146. [DOI] [PubMed] [Google Scholar]
- 50.Kumar N, Dorfman A, Hahm J. Ultrasensitive DNA sequence detection of Bacillus anthracis using nanoscale ZnO sensor arrays. Nanotechnology. 2006;17:2875–2881. [Google Scholar]
- 51.Singh M, Jiang R, Coia H, Choi DS, Alabanza A, et al. Insight into factors affecting the presence, degree, and temporal stability of fluorescence intensification on ZnO nanorod ends. Nanoscale. 2015;7:1424–1436. doi: 10.1039/c4nr06066k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aslan K, Lakowicz JR, Szmacinski H, Geddes CD. Metal-enhanced fluorescence solution-based sensing platform. J. Fluoresc. 2004;14:677–679. doi: 10.1023/b:jofl.0000047217.74943.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lakowicz JR. Radiative decay engineering: biophysical and biomedical applications. Anal. Biochem. 2001;298:1–24. doi: 10.1006/abio.2001.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lakowicz JR, Malicka J, D’Auria S, Gryczynski I. Release of the self-quenching of fluorescence near silver metallic surfaces. Anal. Biochem. 2003;320:13–20. doi: 10.1016/S0003-2697(03)00351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lakowicz JR, Shen Y, D’Auria S, Malicka J, Fang J, et al. Radiative decay engineering: 2. Effects of silver island films on fluorescence intensity, lifetimes, and resonance energy transfer. Anal. Biochem. 2002;301:261–277. doi: 10.1006/abio.2001.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z, Yang R, Yu M, Bai F, Li C, Wang ZL. Cellular level biocompatibility and biosafety of ZnO nanowires. J. Phys. Chem. C. 2008;112:20114–20117. [Google Scholar]
- 57.Grasset F, Saito N, Li D, Park D, Sakaguchi I, et al. Surface modification of zinc oxide nanoparticles by aminopropyltriethoxysilane. J. Alloys Compd. 2003;360:298–311. [Google Scholar]
- 58.Heo YW, Pearton SJ, Norton DP, Ren F. ZnO thin-film and nanowire-based sensing applications. In: Ren F, Pearton SJ, editors. Semiconductor Device-Based Sensors for Gas, Chemical, and Biomedical Applications. Philadelphia: Taylor & Francis; 2011. pp. 149–214. [Google Scholar]
- 59.Park H-Y, Gedi V, Kim J, Park H-C, Han S-H, Yoon M-Y. Ultrasensitive diagnosis for an anthrax-protective antigen based on a polyvalent directed peptide polymer coupled to zinc oxide nanorods. Adv. Mater. 2011;23:5425–5429. doi: 10.1002/adma.201103284. [DOI] [PubMed] [Google Scholar]
- 60.Choi A, Kim K, Jung H-I, Lee SY. ZnO nanowire biosensors for detection of biomolecular interactions in enhancement mode. Sens. Actuators B. 2010;148:577–582. [Google Scholar]
- 61.Hu W, Liu Y, Chen T, Liu Y, Li CM. Hybrid ZnO nanorod-polymer brush hierarchically nanostructured substrate for sensitive antibody microarrays. Adv. Mater. 2015;27:181–185. doi: 10.1002/adma.201403712. [DOI] [PubMed] [Google Scholar]
- 62.Yin Y, Sun Y, Yu M, Liu X, Jiang T, et al. ZnO nanorod array grown on Ag layer: a highly efficient fluorescence enhancement platform. Sci. Rep. 2015;5:8152. doi: 10.1038/srep08152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hahm J. Biomedical detection via macro- and micro-sensors fabricated with metallic and semiconducting oxides. J. Biomed. Nanotechnol. 2013;9:1–25. doi: 10.1166/jbn.2013.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao H, Xu JY, Zhang DZ, Chang SH, Ho ST, et al. Spatial confinement of laser light in active random media. Phys. Rev. Lett. 2000;84:5584–5587. doi: 10.1103/PhysRevLett.84.5584. [DOI] [PubMed] [Google Scholar]
- 65.Chu S, Wang G, Zhou W, Lin Y, Chernyak L, et al. Electrically pumped waveguide lasing from ZnO nanowires. Nat. Nanotechnol. 2011;6:506–510. doi: 10.1038/nnano.2011.97. [DOI] [PubMed] [Google Scholar]
- 66.Koch MH, Timbrell PY, Lamb RN. The influence of film crystallinity on the coupling efficiency of ZnO optical modulator waveguides. Semicond. Sci. Technol. 1995;10:1523–1527. [Google Scholar]
- 67.Yu SF, Yuen C, Lau SP, Lee HW. Zinc oxide thin-film random lasers on silicon substrate. Appl. Phys. Lett. 2004;84:3244–3246. [Google Scholar]
- 68.Yuen C, Yu SF, Leong ESP, Yang HY, Lau SP, et al. Low-loss and directional output ZnO thin-film ridge waveguide random lasers with MgO capped layer. Appl. Phys. Lett. 2005;86:031112. [Google Scholar]
- 69.Lee Y-J, Ruby DS, Peters DW, McKenzie BB, Hsu JWP. ZnO nanostructures as efficient antireflection layers in solar cells. Nano Lett. 2008;8:1501–1505. doi: 10.1021/nl080659j. [DOI] [PubMed] [Google Scholar]
- 70.Salman KA, Omar K, Hassan Z. Effective conversion efficiency enhancement of solar cell using ZnO/PS antireflection coating layers. Sol. Energy. 2012;86:541–547. [Google Scholar]
- 71.Kaiser R, Levy Y, Vansteenkiste N, Aspect A, Seifert W, et al. Resonant enhancement of evanescent waves with a thin dielectric waveguide. Opt. Commun. 1994;104:234–240. [Google Scholar]
- 72.Wang G, Spalding GC, Huang R, Luan L, Ketterson JB. Numerical analysis of waveguide-enhanced optical bistability. Opt. Quantum Electron. 2003;35:1357–1366. [Google Scholar]
- 73.Johnson JC, Yan H, Choi H-J, Knutsen KP, Petersen PB, et al. Single nanowire waveguides and lasers. Proc. SPIE. 2010;5223:187–196. [Google Scholar]
- 74.Johnson JC, Yan H, Yang P, Saykally RJ. Optical cavity effects in ZnO nanowire lasers and waveguides. J. Phys. Chem. B. 2003;107:8816–8828. [Google Scholar]
- 75.Voss T, Svacha GT, Mazur E, Müller S, Ronning C, et al. High-order waveguide modes in ZnO nanowires. Nano Lett. 2007;7:3675–3680. doi: 10.1021/nl071958w. [DOI] [PubMed] [Google Scholar]
- 76.Sirbuly DJ, Tao A, Law M, Fan R, Yang P. Multifunctional nanowire evanescent wave optical sensors. Adv. Mater. 2007;19:61–66. [Google Scholar]
- 77.Börner S, Rüter CE, Voss T, Kip D, Schade W. Modeling of ZnO nanorods for evanescent field optical sensors. Phys. Status Solidi A. 2007;204:3487–3495. [Google Scholar]
- 78.Pauzauskie PJ, Yang P. Nanowire photonics. Mater. Today. 2006;9:36–45. [Google Scholar]
- 79.Yan R, Gargas D, Yang P. Nanowire photonics. Nat. Photonics. 2009;3:569–576. [Google Scholar]
- 80.Snyder AW, Love JD. Optical Waveguide Theory. London: Chapman & Hall; 1983. [Google Scholar]
- 81.Buck JA. Fundamentals of Optical Fibers. Hoboken, NJ: Wiley; 2004. [Google Scholar]
- 82.Bond WL. Measurement of the refractive indices of several crystals. J. Appl. Phys. 1965;36:1674–1677. [Google Scholar]
- 83.Dahlin AB. Size matters: problems and advantages associated with highly miniaturized sensors. Sensors. 2012;12:3018–3036. doi: 10.3390/s120303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luong JH, Male KB, Glennon JD. Biosensor technology: technology push versus market pull. Biotechnol. Adv. 2008;26:492–500. doi: 10.1016/j.biotechadv.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 85.Melo MR, Clark S, Barrio D. Miniaturization and globalization of clinical laboratory activities. Clin. Chem. Lab. Med. 2011;49:581–586. doi: 10.1515/CCLM.2011.092. [DOI] [PubMed] [Google Scholar]
- 86.Ahn K-Y, Kwon K, Huh J, Kim GT, Lee EB, et al. A sensitive diagnostic assay of rheumatoid arthritis using three-dimensional ZnO nanorod structure. Biosens. Bioelectron. 2011;28:378–385. doi: 10.1016/j.bios.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 87.Chen RJ, Choi HC, Bangsaruntip S, Yenilmez E, Tang X, et al. An investigation of the mechanisms of electronic sensing of protein adsorption on carbon nanotube devices. J. Am. Chem. Soc. 2004;126:1563–1568. doi: 10.1021/ja038702m. [DOI] [PubMed] [Google Scholar]
- 88.Allen BL, Kichambare PD, Star A. Carbon nanotube field-effect-transistor-based biosensors. Adv. Mater. 2007;19:1439–1451. [Google Scholar]
- 89.Maehashi K, Katsura T, Kerman K, Takamura Y, Matsumoto K, Tamiya E. Label-free protein biosensor based on aptamer-modified carbon nanotube field-effect transistors. Anal. Chem. 2007;79:782–787. doi: 10.1021/ac060830g. [DOI] [PubMed] [Google Scholar]
- 90.Zheng G, Patolsky F, Cui Y, Wang WU, Lieber CM. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005;23:1294–1301. doi: 10.1038/nbt1138. [DOI] [PubMed] [Google Scholar]
- 91.Zheng G, Lieber CM. Nanowire biosensors for label-free, real-time, ultrasensitive protein detection. Methods Mol. Biol. 2011;790:223–237. doi: 10.1007/978-1-61779-319-6_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hahm J-I, Lieber CM. Direct ultrasensitive electrical detection of DNA and DNA sequence variations using nanowire nanosensors. Nano Lett. 2004;4:51–54. [Google Scholar]
- 93.Cui Y, Wei Q, Park H, Lieber CM. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science. 2001;293:1289–1292. doi: 10.1126/science.1062711. [DOI] [PubMed] [Google Scholar]
- 94.Al-Hilli SM, Willander M, Öst A, Stra°lfors P. ZnO nanorods as an intracellular sensor for pH measurements. J. Appl. Phys. 2007;102:084304. doi: 10.1007/978-1-59745-483-4_13. [DOI] [PubMed] [Google Scholar]
- 95.Zhao J, Wu D, Zhi J. A novel tyrosinase biosensor based on biofunctional ZnO nanorod microarrays on the nanocrystalline diamond electrode for detection of phenolic compounds. Bioelectrochemistry. 2009;75:44–49. doi: 10.1016/j.bioelechem.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 96.Zhao ZW, Chen XJ, Tay BK, Chen JS, Han ZJ, Khor KA. A novel amperometric biosensor based on ZnO: Co nanoclusters for biosensing glucose. Biosens. Bioelectron. 2007;23:135–139. doi: 10.1016/j.bios.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 97.Zang J, Li CM, Cui X, Wang J, Sun X, et al. Tailoring zinc oxide nanowires for high performance amperometric glucose sensor. Electroanalysis. 2007;19:1008–1014. [Google Scholar]
- 98.Wei A, Sun XW, Wang JX, Lei Y, Cai XP, et al. Enzymatic glucose biosensor based on ZnO nanorod array grown by hydrothermal decomposition. Appl. Phys. Lett. 2006;89:123902. [Google Scholar]
- 99.Asif MH, Ali SMU, Nur O, Willander M, Brännmark C, et al. Functionalised ZnO-nanorod-based selective electrochemical sensor for intracellular glucose. Biosens. Bioelectron. 2010;25:2205–2211. doi: 10.1016/j.bios.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 100.Zhang F, Wang X, Ai S, Sun Z, Wan Q, et al. Immobilization of uricase on ZnO nanorods for a reagentless uric acid biosensor. Anal. Chim. Acta. 2004;519:155–160. [Google Scholar]
- 101.Liu Y-L, Yang Y-H, Yang H-F, Liu Z-M, Shen G-L, Yu R-Q. Nanosized flower-like ZnO synthesized by a simple hydrothermal method and applied as matrix for horseradish peroxidase immobilization for electro-biosensing. J. Inorg. Biochem. 2005;99:2046–2053. doi: 10.1016/j.jinorgbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 102.Batista PD, Mulato M. ZnO extended-gate field-effect transistors as pH sensors. Appl. Phys. Lett. 2005;87:143508. [Google Scholar]
- 103.Asif MH, Nur O, Willander M, Danielsson B. Selective calcium ion detection with functionalized ZnO nanorods-extended gate MOSFET. Biosens. Bioelectron. 2009;24:3379–3382. doi: 10.1016/j.bios.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 104.Israr MQ, Sadaf JR, Asif MH, Nur O, Willander M, Danielsson B. Potentiometric cholesterol biosensor based on ZnO nanorods chemically grown on Ag wire. Thin Solid Films. 2010;519:1106–1109. [Google Scholar]
- 105.Ibupoto ZH, Ali SMU, Khun K, Chey CO, Nur O, Willander M. ZnO nanorods based enzymatic biosensor for selective determination of penicillin. Biosensors. 2011;1:153–163. doi: 10.3390/bios1040153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Asif MH, Fulati A, Nur O, Willander M, Brännmark C, et al. Functionalized zinc oxide nanorod with ionophore-membrane coating as an intracellular Ca2+ selective sensor. Appl. Phys. Lett. 2009;95:023703. [Google Scholar]
- 107.Lei Y, Luo N, Yan X, Zhao Y, Zhang G, Zhang Y. A highly sensitive electrochemical biosensor based on zinc oxide nanotetrapods for l-lactic acid detection. Nanoscale. 2012;4:3438–3443. doi: 10.1039/c2nr30334e. [DOI] [PubMed] [Google Scholar]
- 108.Wang J, Gudiksen MS, Duan X, Cui Y, Lieber CM. Highly-polarized photoluminescence and photodetection from single indium phosphide nanowires. Science. 2001;293:1455–1457. doi: 10.1126/science.1062340. [DOI] [PubMed] [Google Scholar]
- 109.Tang J, Marcus RA. Single particle versus ensemble average: from power-law intermittency of a single quantum dot to quasistretched exponential fluorescence decay of an ensemble. J. Chem. Phys. 2005;123:204511. doi: 10.1063/1.2128409. [DOI] [PubMed] [Google Scholar]
- 110.Ratchford D, Dziatkowski K, Hartsfield T, Li X, Gao Y, Tang Z. Photoluminescence dynamics of ensemble and individual CdSe/ZnS quantum dots with an alloyed core/shell interface. Appl. Phys. Lett. 2011;109:103509. [Google Scholar]
- 111.Dunn K, Derr J, Johnston T, Chaker M, Rosei F. Multiexponential photoluminescence decay of blinking nanocrystal ensembles. Phys. Rev. B. 2009;80:035330. [Google Scholar]
- 112.Pelton M, Liu M, Park S, Scherer NF, Guyot-Sionnest P. Ultrafast resonant optical scattering from single gold nanorods: large nonlinearities and plasmon saturation. Phys. Rev. B. 2006;73:155419. [Google Scholar]
- 113.Sau TK, Rogach AL, Jäckel F, Klar TA, Feldmann J. Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv. Mater. 2010;22:1805–1825. doi: 10.1002/adma.200902557. [DOI] [PubMed] [Google Scholar]
- 114.Zijlstra P, Orrit M. Single metal nanoparticles: optical detection, spectroscopy and applications. Rep. Prog. Phys. 2011;74:106401. [Google Scholar]
- 115.Lu L, Wang L-L, Zou C-L, Ren X-F, Dong C-H, et al. Doubly and triply coupled nanowire antennas. J. Phys. Chem. C. 2012;116:23779–23784. [Google Scholar]
- 116.Manjavacas A, García de Abajo FJ. Robust plasmon waveguides in strongly interacting nanowire arrays. Nano Lett. 2009;9:1285–1289. doi: 10.1021/nl802044t. [DOI] [PubMed] [Google Scholar]
- 117.Lindström S, Andersson-Svahn H. Miniaturization of biological assays: overview on microwell devices for single-cell analyses. Biochim. Biophys. Acta. 2011;1810:308–316. doi: 10.1016/j.bbagen.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 118.Vaddiraju S, Tomazos I, Burgess DJ, Jain FC, Papadimitrakopoulos F. Emerging synergy between nanotechnology and implantable biosensors: a review. Biosens. Bioelectron. 2010;25:1553–1565. doi: 10.1016/j.bios.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spindel S, Sapsford K. Evaluation of optical detection platforms for multiplexed detection of proteins and the need for point-of-care biosensors for clinical use. Sensors. 2014;14:22313–22341. doi: 10.3390/s141222313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singhal R, Orynbayeva Z, Kalyana Sundaram RV, Niu JJ, Bhattacharyya S, et al. Multifunctional carbon-nanotube cellular endoscopes. Nat. Nanotechnol. 2011;6:57–64. doi: 10.1038/nnano.2010.241. [DOI] [PubMed] [Google Scholar]
- 121.Shambat G, Kothapalli S-R, Provine J, Sarmiento T, Harris J, et al. Single-cell photonic nanocavity probes. Nano Lett. 2013;13:4999–5005. doi: 10.1021/nl304602d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee J, Kang BS, Hicks B, Chancellor J, Thomas F, et al. The control of cell adhesion and viability by zinc oxide nanorods. Biomaterials. 2008;29:3743–3749. doi: 10.1016/j.biomaterials.2008.05.029. [DOI] [PubMed] [Google Scholar]