KATP and cardiovascular disease: The theoretical case
Cardiovascular KATP and cardioprotection
Since their discovery in cardiac myocytes over 30 years ago, it has been recognized that KATP channels provide a very large potential ionic conductance in the surface membranes of all muscle cells. Under normal metabolic conditions, cardiac KATP channels are predominantly closed, and they do not significantly contribute to cell excitability. However, these channels can open when exposed to a severe metabolic stress such as anoxia, metabolic inhibition or ischemia. By shortening the action potential, KATP activation will reduce Ca2+ entry and inhibit contractility1, thereby reducing energy consumption, potentially protecting the cell. Such a preservation ‘strategy’ is naturally self-limiting - if too many myocytes stop contracting, the heart will stop pumping and the animal will die, but it has always been a reasonable notion that temporary protection of a small number of cells, or region of the heart, against the damage of Ca2+-overload during ischemia, is a likely beneficial consequence of KATP channel activation.
In the vasculature, activation of KATP channels will hyperpolarize the membrane potential, leading to inhibition of voltage-sensitive Ca2+-channels and lowering of intracellular Ca2+, resulting in vasodilation2.
Cardiac KATP channels and arrhythmia
Opening of cardiac KATP channels both shortens the action potential and reduces the refractory period, such that channel activation could establish an arrhythmogenic substrate supporting reentry. Hence inhibition of KATP could be a way to stop or even prevent arrhythmias. Because KATP channels tend to open only when cell metabolism is inhibited, any agents that inhibits KATP activity should specifically target channels only during ischemia, leaving non-ischemic myocardium unaffected. On the other hand activation of cardiac KATP channels has consistently been shown to protect the heart from damage during ischemia, by limiting Ca2+ entry.
The molecular basis of KATP channels
KATP channels are heterooctameric complexes of 4 pore-forming Kir6 channel-forming subunits, each associated with one regulatory SUR subunit. Two Kir6-encoding genes, KCNJ8 (Kir6.1) and KCNJ11 (Kir6.2)3,4, and two SUR genes, ABCC8 (SUR1) and ABCC9 (SUR2)4–6 encode mammalian KATP subunits, but alternative RNA splicing can give rise to multiple SUR protein variants (e.g. SUR2A and SUR2B) that confer distinct physiological and pharmacological properties on the channel complex7,8. Interestingly, the genes for Kir6.2 and SUR1 are located next to each other on human chromosome 11p15.14 suggesting an as yet unrecognized co-regulation at the gene level. In addition, the genes for Kir6.1 and SUR2 are also adjacent to one another on chromosome 12p12.16,9, implicating an evolutionary duplication. In heterologous expression, both Kir6.2 and SUR1 subunits co-assemble in a 4:4 stoichiometry4 to generate the functional KATP channel10–12. Similarly, biochemical studies confirm that the SUR2 protein variants, SUR2A and SUR2B, also coassemble with Kir6 subunits3,13–15, presumably in a similar octameric arrangement.
Crystallographic studies of bacterial and eukaryotic Kir channels16,17[new] demonstrate a conserved architecture of Kir channels with two transmembrane helices (M1, M2) bridged by an extracellular loop that generates the narrow portion of the pore and controls ion selectivity. As with other ABCC proteins, SURs contain two six-helix transmembrane domains, TMD1 and TMD2 and two cytoplasmic nucleotide binding folds (NBFs), but also contain an additional N-terminal TMD0 domain that is critical for trafficking and gating of the channel complex18. The details of the physical connection between Kir6 and SUR subunits remains unknown, but electron micrography and intersubunit FRET studies of complete KATP complexes suggest an intimate packing of 4 SUR and 4 Kir6.x subunits19,20.
The key regulatory features of KATP channels are rapid and reversible closure by cytoplasmic ATP, and activation by nucleotide tri- and diphosphates21. In the absence of other nucleotides, the free [ATP] that causes half-maximal channel inhibition is in the micromolar range. Since intracellular ATP concentrations are in the low millimolar range and change little under physiologic conditions, [ATP] is probably always sufficient to almost fully inhibit channel activity. Channel activation then arises from the activating effects of Mg-nucleotides, particularly MgADP, on the SUR subunit22. Nucleotide regulation is probably the key molecular regulator of KATP channel activities, although other second messenger systems and regulators23 may be involved in control of channel activity and channel-dependent pathologies.
Cardiovascular tissue distribution of KATP channel subunits
From studies in heterologous expression systems where SUR and Kir6 subunit expression can be controlled, it is apparent that all possible subunit combinations can and do occur. Post-translational quality control mechanisms have been described that ensure the appropriate octameric composition of the channel24,25, yet there is no evidence that these mechanisms discriminate between subunits. There have been relatively few studies to examine the transcriptional regulation of KATP subunits and still little is known about what specific factors might control KATP structure, although members of the forkhead transcription factor family and HIF-1α have been shown to regulate the expression of some subunits (as well as metabolic enzymes) 26,27.
Kir6.1 and Kir6.2, as well as SUR2 and SUR1, are all expressed in the heart3,28–30. There is now good evidence that in mouse hearts, SUR1 and Kir6.2 are major constituents of the atrial myocyte sarcolemmal KATP, whereas SUR2A and Kir6.2 generate ventricular KATP31,32. However, in hearts of larger animals, including humans, both SUR1 and SUR2A subunits probably contribute to sarcolemmal channels in both atrial and ventricular myocytes33 (Fig. 1). The situation may be more complex in critical subregions of the heart, including nodal and conduction cells. KATP channel currents have been detected throughout the pacemaking and conduction systems34–36. Low KATP single channel conductances in rabbit SA node cells and mouse conduction cells34 suggests a role for Kir6.1 in generating the channel pore in these tissues, yet sarcolemmal KATP is abolished in Kir6.2−/− SA node cells37 indicating a necessary requirement for Kir6.2. The identity of the SUR component of KATP in conducting and pacemaker tissues is unknown, although KATP channels in nodal cells do respond to the relatively SUR2-specific openers cromakalim and pinacidil, suggesting a major role for SUR234–36.
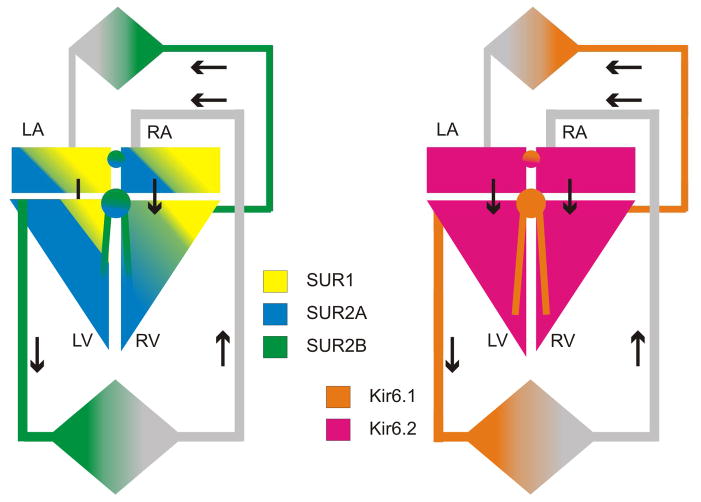
Figure 1. Cardiovascular KATP channel distribution.
Schematic representation of KATP channel subunit distribution in the cardiovascular system. SUR2A and to a lesser extent SUR1 are prominent in ventricular chambers (LV, RV), whereas SUR1 is more prominent in atrial chambers (LA, RA), and SUR2B is prominent throughout the vasculature. Kir6.2 is found throughout the myocardium, with Kir6.1 more prominent in conducting tissue and in the vasculature.
KATP channel density is relatively low in vascular smooth muscle (VSM) compared to cardiac myocytes38,39 and the biophysical and pharmacological properties are quite variable, reflecting variable expression of KATP subtypes between vascular beds40–47. There is considerable variation in reported single channel conductances43,44,48–52, although low-conductance channels (unitary conductances from 20–50 pS) may represent the predominant KATP channel subtype, with a more limited distribution of medium- and high conductance KATP channels (50–70 pS and >200 pS, respectively)53. Importantly, and unlike classic KATP channels of the heart3,54 or pancreas4,55, the predominant VSM KATP conductances are inactive in isolated membrane patches, and require nucleotide diphosphates (ADP, UDP, GDP) in the presence of Mg 2+ to open, leading to their functional designation as ‘nucleotide-dependent’ K+-channels, or KNDP channels46,56,57. Heterologously expressed Kir6.1/SUR2B channels recapitulate many of these biophysical properties of native VSM KATP/KNDP9,13,58–61. Thus the Kir6.1/SUR2B channel may represent the predominant VSM KATP, but other subtypes are also likely to be expressed in specific vascular beds, separately or in combination with Kir6.1/SUR2B subunits56 (Fig. 1).
Finally, it is important to note that KATP channels are also prominent in lymphatic muscle. While the classical understanding was that fluid flow in the lymphatic system was passive, it is now clear that lymphatic vessels are lined by smooth muscle. Contractility of these vessels is clearly sensitive to KATP activation62, with a pharmacological profile that is consistent with the major subunits expressed in lymphatic muscle being Kir6.1 and SUR263.
Cardiovascular disease and KATP mutations
Predictions from genetically modified animals
Murine knockout models of each of the four KATP channel genes have been generated and extensively analyzed. Knockout of Kir6.2 or SUR1 results in a loss of glucose-dependent insulin secretion, modeling features of hyperinsulinism in humans64,65. Conversely, knockout of Kir6.1 or SUR2 leads to a vascular hypercontractility phenotype30,66. The key features are baseline hypertension, coronary artery vasospasm and sudden cardiac death. SUR2−/− mice treated with the Ca2+ channel blocker nifedipine exhibit a reduction in coronary artery vasospasm, implicating abnormally elevated [Ca2+]i due to loss of hyperpolarizing KATP current as causal in the hypercontractility66. Collectively, these KATP-null mice recapitulate clinical features of the human disorder of Prinzmetal (or variant) angina, but several studies have failed to demonstrate any association of human coronary vasospasm or hypertension with LOF mutations in Kir6.1 or SUR267,68, even though linkage analysis indicates that there are associated genes within the same locus as Kir6.1 and SUR269.
We have extensively explored the potential for KATP gain-of-function (GOF) action in the heart and vasculature by transgenic introduction of mutant Kir6.1 and Kir6.2 channels that are very insensitive to closure by ATP70–72. Under aMHC control, GOF subunits expressed in the heart generate channels that still remain closed under all but extreme circumstances, and cause little overt malfunction, with no decrease in cardiac action potential duration, nor decrease in contractility70,72. Curiously, we find that in ventricular myocytes from these animals there is actually dramatically enhanced Ca2+ current,73 which may be a compensatory response to an initial or local action potential shortening. These studies also reveal that overexpressing the SUR1 isoform the myocardium has an effect to prolong the PR interval74, and that when Kir6.2 GOF is expressed together with SUR1, second and third degree AV block, progressing to ventricular and supra-ventricular arrhythmias and death74,75.
While the phenotype of animals expressing KATP GOF in the heart is complex, expression of Kir6.1 GOF mutants in smooth muscle (under smooth muscle HC promoter control) leads to enhanced KATP activity in vascular smooth muscle, and a clear reduction of systolic and diastolic blood pressures71, paralleling the effects of KCOs in human hypertensive patients.
KATP-associated human disease
Thus animal studies have provided a clear prediction of hypertensive or hypotensive consequences for KATP LOF or GOF, respectively, in smooth muscle, but rather complex and contradictory predictions regarding KATP mutations in the heart. This may help explain why, until recently, there has been little evidence for human cardiovascular disease resulting from KATP gene mutations (Table 1). Gain- and loss-of function mutations in KCNJ11 (Kir6.2) and ABCC8 (SUR1), which encode the predominant KATP channel subunits in pancreatic β-cells and in neurons76, are now well understood to underlie neonatal diabetes and congenital hyperinsulinism, respectively77. However, and despite evidence for expression of these subunits in cardiac myocytes, there is no published evidence for any cardiovascular problems in these patients.
Table 1.
REPORTED ASSOCIATION OF DISEASE WITH KATP CHANNEL MUTATIONS
| Gene | Clinical condition | Features | # of reported affected individuals | Refs |
|---|---|---|---|---|
| KCNJ8 (Kir6.1) | J-wave syndrome | S422L mutation. Reportedly gain-of- function (GOF). Abnormalities in the J- point of the ECG, and including Brugada syndrome (BrS) and early repolarization syndrome (ERS), including VF and AF | 9 | 81–83 |
| SIDS | In-frame deletion (E332del) and loss- of-function mutation (V346I), through as yet unexplained mechanisms. | 2 | 78 | |
| Cantu Syndrome | GOF mutations associated with complex multi-organ disease (See Table 2) | 2 | 89,90 | |
|
| ||||
| KCNJ11 (Kir6.2) | Neonatal diabetes | Multiple GOF mutations cause inhibition of insulin secretion. No cardiovascular phenotype | >100 | 128 |
| Congenital hyperinsulinism | LOF mutations cause hypersecretion of insulin. No cardiovascular phenotype | >10 | 77,128 | |
|
| ||||
| ABCC8 (SUR1) | Neonatal diabetes | Multiple GOF mutations cause inhibition of insulin secretion. No cardiovascular phenotype | >100 | 128 |
| Congenital hyperinsulinism | Multiple LOF mutations cause hypersecretion of insulin. No cardiovascular phenotype | >100 | 77,128 | |
|
| ||||
| ABCC9 (SUR2) | AF | Isolated case of LOF mutation assicated with AF originating in the vein of Marshal | 1 | 80 |
| Idiopathic dilated cardiomyopathy | Two cases with distinct LOF mutations associated with heart failure due to idiopathic dilated cardiomyopathy | 2 | 79 | |
| Cantu syndrome | GOF mutations associated with complex multi-organ disease (See Table 2) | >25 | 87,88 | |
Sequence analysis of DNA from necropsy tissue on sudden infant death syndrome (SIDS) cases identified coding mutations in KCNJ8 (Kir6.1), an in-frame deletion (E332del) and a missense mutation (V346I), both in the distal C-terminus of Kir6.1. Reduced channel activity was reported from expressed mutant channels, leading the authors to conclude that loss-of-function mutations in Kir6.1may be one cause of SIDS78, through as yet unexplained mechanisms. There have also been two reports of SUR2 loss of function mutations leading to cardiac disease79,80. In each case, the mutations were identified in the C-terminal exons and would therefore lead to a disruption of the second nucleotide binding fold of SUR2A, and hence reduction of nucleotide stimulation of channel activity, without affecting SUR2B. In the first report, the single patient with the mutation presented with long-standing atrial fibrillation originating in the vein of Marshall, with normal cardiac morphology and contractile features80. In the second report, two individuals with two distinct mutations presented with heart failure due to idiopathic dilated cardiomyopathy79. There have been no subsequent reports of similar genetic defects, and further evidence for causal association of Kir6.1 or SUR2 LOF mutations with disease is lacking.
Several studies reported a single KCNJ8 mutation (encoding S422L in Kir6.1) protein to be associated with the ‘J-wave’ phenomenon, characterized by abnormalities in the J-point of the ECG and early repolarization syndrome (ERS). First reported by Haissaguerre et al81, subsequent studies have reported association of this variant with atrial fibrillation (AF)82, as well as additional Brugada syndrome and early repolarization syndrome patients83,84. However, a recent study has reported that this variant is relatively common in individuals of Ashkenazi Jewish origin and it remains unclear whether the reported associations are causal85.
More recently, it has become clear that mutations in both ABCC9 (encoding SUR1) and KCNJ8 (Kir6.1) are associated with Cantu syndrome (CS)86. (MIM 239850), or hypertrichosis-osteochondrodysplasia-cardiomegaly syndrome, a distinctive multi-organ disease87–90. In many cases, the mutations are de novo, but autosomal dominant inheritance also occurs91. The conclusion that these mutations all lead to gain-of KATP channel function has been confirmed in several studies87,89,92, which demonstrate reduced sensitivity to ATP inhibition or enhanced activation by MgADP in each case.
Cantu Syndrome: Multiple tissue symptoms
Perhaps most striking about this recent discovery is that so many of the CS features are not trivially predictable, and in the heart, the resultant phenotypes are even counter to any naïve predictions. Since first being recognized as a unique syndrome in 198286, a constellation of features has been described in CS patients91,93–100 (Table 2). Multiple cardiovascular features include cardiac enlargement, concentric hypertrophy of the ventricles and pericardial effusion. Some patients have required pericardiocentesis and even pericardial stripping to prevent reaccumulation of the pericardial effusion. Multiple vascular consequences include pulmonary hypertension secondary to partial pulmonary venous obstruction has been reported, associated with severe mitral valve regurgitation that spontaneously resolved95. A significant number of patients have had patent ductus arteriosus (PDA) requiring surgical closure, as well as bicuspid aortic valves with and without stenosis. Lymphedema involving the lower extremities may develop over time, and in one patient, lymphangiogram demonstrated dilated lymphatic vessels in the legs with delayed lymphatic drainage101. Interestingly, diazoxide, minoxidil and other related KATP channel openers that are used to treat severe refractory hypertension can also result in similar features as unexplained side effects, including hypertrichosis, pericardial effusion, edema, and even coarsening of the facial featuresl102,103. Teratogenic effects of minoxidil, including marked hypertrichosis, dysmorphic facial features, low blood pressure, and transposition of the great vessels and pulmonary bicuspid valvular stenosis, have been reported in the offspring of minoxidil-treated mothers104,105. These observations first led to the suggestion that CS might result from gain-of-function (GOF) in K+ channel activity91.
Table 2.
MAJOR CLINICAL FEATURES OF CANTU SYNDROME
| Neonatal Features |
|
|
| Neonatal macrosomia |
| Maternal polyhydramnios |
| Macrocephaly |
|
|
| Craniofacial dysmorphology |
| Coarse facial appearance (can be confused with a storage disoder) |
| Epicanthal folds |
| Broad nasal bridge |
| Anteverted nostrils |
| Long philtrum |
| Wide mouth with full lips |
| Macroglossia |
| High or narrow palate |
| Gingival hyperplasia |
|
|
| Hair |
| Congenital generalized hirsutism |
| Thick scalp hair |
| Thick and/or curly eyelashes |
| Excessive hair growth on forehead, face, back and limbs |
|
|
| Cardiovascular |
| Cardiomegaly |
| Concentric hypertrophy of the ventricles |
| Normal ventricular contractility |
| Pericardial effusion |
| Pulmonary hypertension |
| Partial pulmonary venous obstruction |
| Mitral valve regurgitation |
| Congenital anomalies |
| Patent ductus arteriosus |
| Bicuspid and/or stenotic aortic valve |
|
|
| Skeletal abnormalities |
| Thickened calvarium |
| Narrow shoulders and thorax |
| Pectus carinatum |
| Broad ribs |
| Platyspondyly and ovoid vertebral bodies |
| Hypoplastic ischium and pubic bones |
| Erlenmeyer-flask-like long bones with metaphyseal flaring |
| Delayed bone age |
|
|
| Skin and joints |
| Loose and/or wrinkled skin, especially in neonates |
| Deep palmar and plantar creases |
| Persistent fingertip pads |
| Hyperextensibility of joints |
|
|
| Lymphatic system |
| Lymphedema, onset usually in adolescence or adulthood |
|
|
| Gastrointestinal |
| Pyloric stenosis |
| Increased risk for upper gastrointestinal bleeding |
Normally, abrupt increase in oxygen tension and falling PGE2 and PGI2 levels lead to inhibition of voltage-gated K channels and contraction of smooth muscle fibers in the ductus arteriosus, resulting in wall thickening and lumen obliteration after birth. Persistence of the PDA in Cantu syndrome patients may thus be readily explained as a consequence of maintained vessel dilation due to KATP overactivity. More generally, mechanisms of persistent PDA are not clear106, but the enhancement of a K current in smooth muscle presents an obvious potential explanation in Cantu syndrome patients. Altered vascular tone may also underlie pericardial effusion, but the reason for cardiomegaly is not obvious. Cardiomegaly reported in most cases of Cantu Syndrome is due to increased myocardial mass (hypertrophy) with larger cardiac chambers but with normal systolic function, and this does not fit the diagnostic criteria of dilated or hypertrophic cardiomyopathy107, and may be a secondary response to reduced vascular tone108. Similarly, the reason for osteochondrodysplasia and facial dysmorphology is not obvious, and the mechanism by which minoxidil causes hair growth has remained controversial109. While CS patients show no evidence of orthostatic blood pressure problems, systematic analysis of patient blood pressures does show that these are physiologically below the norm for age (G.K. Singh, M.D. Levin, D.K. Grange, C,G. Nichols, unpublished). Through opening vascular K channels and dilation of blood vessels, the supply of oxygen, blood and nutrients to the hair follicle may be increased, causing follicles in the telogen phase to shed and be replaced by new thicker hairs in a new anagen phase. However, there is also evidence that SUR2 isoforms are present in follicular dermal papillae 110 and while the new realization definitively ties the hair growth to an action on KATP channels, it does not immediately prove where the action is.
KATP manipulation in heart disease
Perhaps no other channels in the heart carries more potential and promise than KATP channels for breaking the link between myocardial ischemia and cardiac arrhythmia. Since the first report detailing the presence of KATP in cardiac myocytes was published111, the possibility that this channel 1) determines the electrical behavior of the heart during ischemia and 2) might protect the heart has been well recognized. Nevertheless, efforts to exploit the “cardiac KATP” channel to ameliorate arrhythmia and moderate damage of the myocardium during ischemia have yet to mature.
As genetic variation in humans, and manipulation in animals, has made clear, cardiac sarcolemmal KATP channels are normally predominantly closed in physiological conditions, and application of channel-blocking sulfonylureas generally has little or no effect on the ventricular action potential112. Because KATP channels in different regions of the heart have different composition, it is likely that they will be operative under different conditions in vivo. For example, shortening of the Purkinje action potential may be greater than that of the ventricular action potential at the same ATP/ADP ratio, given that SUR2B and Kir6.1 may be prominent in these cells113. KATP channels composed of SUR1 and Kir6.2, as in the mouse atrium32, will have still different activating conditions.
When metabolism is inhibited, the action potential can shorten markedly and contraction can be inhibited as a result of KATP activation1,114,115. KATP activation during ischemia is likely to be cardioprotective, since reduction of APD and contraction may preserve ATP stores that would otherwise be consumed during the contractile cycle. In support of this idea, treatment with the KATP opener pinacidil during ischemia increases cellular ATP and energy stored as creatine phosphate116. AP shortening is absent in Kir6.2−/− hearts, and the time to contractile failure is prolonged but the time to onset of rigor contracture is reduced117. Diastolic Ca2+ overload, myocardial damage, and increased mortality are also observed in isoproterenol-challenged Kir6.2−/− myocytes118. In addition to highlighting the acute protective effect of KATP activation, Kir6.2−/− animals show increased mortality and exaggerated hypertrophy in response to pressure overload 119,120, and to mineralocorticoid/salt challenge121. Together, these studies suggest that decreased KATP, by stopping the protective ‘unloading’ that KATP activation leads to, should tend to cause Ca overload and perhaps hasten the transition to heart failure under stressed conditions. However, other studies seem to contradict a cardioprotective role. Both SUR2- (SUR2−/−) and SUR1-knockout (SUR1−/−) mice were found to be more tolerant of global ischemia-reperfusion than control mice, with reduced infarct sizes122,123. Since the SUR2−/− mice have a marked reduction of ventricular sarcolemmal KATP channels, the enhanced cardioprotection is opposite the expected phenotype (i.e. impaired protection). Cardioprotection in SUR2−/− mice might conceivably be due to concomitant loss of the SUR2B component of vascular KATP channels, but similar cardioprotection in SUR1−/− mice123 could not be explained by such a mechanism.
Potential for therapeutic modulation of cardiovascular KATP activity
There is tremendous potential for modulation of KATP channel activity in general and more importantly perhaps, in a tissue-specific manner, since there is already a rich pharmacology, not only of channel inhibitors but also channel openers (KCOs). KCOs have been used in two major clinical settings: (1) to block insulin secretion in conditions of hyperinsulinema, and (2) as antihypertensives.
Sulfonylureas have seen widespread use as glucose lowering agents in type 2 diabetes. KATP channel inhibitory drugs have not reached clinical acceptance in the cardiovascular arena, the expectation being that blockade of cardiac KATP channels may be detrimental in conditions of myocardial ischemia, during which these channels can open and are presumed to be protective, as discussed above. This debate is still not resolved124,125. The association of Cantu Syndrome with KATP GOF holds the promise that sulfonylureas or other blockers should be an effective therapy. It is generally accepted that most sulfonylureas are physiologically more potent inhibitors of SUR1-dependent KATP than SUR2A-dependent channels, although there has been little careful comparison of effect on SUR1- versus SUR2B-dependent channels. There has been a long-standing dogma that the drug HMR1098 is a cardiac specific KATP blocker, although direct head-to-head comparison confirms that it is also a more effective blocker of SUR1-dependent than SUR2A-dependent KATP channels31,32,126. Relative efficacies of HMR1098 versus other sulfonylureas in specific physiological conditions may be important to understand, since it is conceivable that specific KATP inhibitors could successfully counteract the symptoms of Cantu syndrome, without significantly affecting blood glucose control, a key issue if KATP channel inhibition is to be a viable treatment for the disease.
Further implications and future prospects
It is now recognized that the subunit make-up of the family of KATP channels is more complex and labile than originally thought15,127. The growing association of Kir6.1 and SUR2 variants with specific cardiovascular electrical and contractile derangements and the clear association with Cantu syndrome firmly establish the importance of appropriate activity in normal function of the heart and vasculature. Further studies of patients with some or all symptoms of Cantu syndrome will reveal new mutations in KATP subunits and perhaps in proteins that regulate KATP synthesis, trafficking, or location, all of which may ultimately benefit therapeutically from the unique pharmacology of KATP channels.
Acknowledgments
Citation of financial support for the authors
Our own experimental work has been supported by NIH grants HL45742 and HL95010, and a grant from the Children’s Discovery Institute at Washington University(to CGN).
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lederer WJ, Nichols CG, Smith GL. The mechanism of early contractile failure of isolated rat ventricular myocytes subjected to complete metabolic inhibition. Journal of Physiology. 1989;413:329–349. doi: 10.1113/jphysiol.1989.sp017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. American Journal of Physiology. 1995;268:C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 3.Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 4.Inagaki N, Gonoi T, Clement JPt, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor [see comments] Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 5.Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JPt, Boyd AEr, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 6.Chutkow WA, Simon MC, Le Beau MM, Burant CF. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes. 1996;45:1439–1445. doi: 10.2337/diab.45.10.1439. [DOI] [PubMed] [Google Scholar]
- 7.Shi NQ, Ye B, Makielski JC. Function and distribution of the SUR isoforms and splice variants. Journal of Molecular & Cellular Cardiology. 2005;39:51–60. doi: 10.1016/j.yjmcc.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Chutkow WA, Makielski JC, Nelson DJ, Burant CF, Fan Z. Alternative splicing of sur2 Exon 17 regulates nucleotide sensitivity of the ATP-sensitive potassium channel. Journal of Biological Chemistry. 1999;274:13656–13665. doi: 10.1074/jbc.274.19.13656. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science (New York, NY) 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 10.Shyng S, Nichols CG. Octameric stoichiometry of the KATP channel complex. J Gen Physiol. 1997;110:655–664. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clement JPt, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J. Association and stoichiometry of K(ATP) channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 12.Inagaki N, Gonoi T, Seino S. Subunit stoichiometry of the pancreatic beta-cell ATP-sensitive K+ channel. FEBS Lett. 1997;409:232–236. doi: 10.1016/s0014-5793(97)00488-2. [DOI] [PubMed] [Google Scholar]
- 13.Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. Journal of Physiology. 1997;499:715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okuyama Y, Yamada M, Kondo C, Satoh E, Isomoto S, Shindo T, Horio Y, Kitakaze M, Hori M, Kurachi Y. The effects of nucleotides and potassium channel openers on the SUR2A/Kir6.2 complex K+ channel expressed in a mammalian cell line, HEK293T cells. Pflugers Archiv European Journal of Physiology. 1998;435:595–603. doi: 10.1007/s004240050559. [DOI] [PubMed] [Google Scholar]
- 15.Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Reconstituted human cardiac KATP channels: functional identity with the native channels from the sarcolemma of human ventricular cells. Circ Res. 1998;83:1132–1143. doi: 10.1161/01.res.83.11.1132. [DOI] [PubMed] [Google Scholar]
- 16.Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- 17.Tao X, Avalos JL, Chen J, MacKinnon R. Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 A resolution. Science. 2009;326:1668–1674. doi: 10.1126/science.1180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryan J, Vila-Carriles WH, Zhao G, Babenko AP, Aguilar-Bryan L. Toward linking structure with function in ATP-sensitive K+ channels. Diabetes. 2004;53(Suppl 3):S104–112. doi: 10.2337/diabetes.53.suppl_3.s104. [DOI] [PubMed] [Google Scholar]
- 19.Mikhailov MV, Campbell JD, de Wet H, Shimomura K, Zadek B, Collins RF, Sansom MS, Ford RC, Ashcroft FM. 3-D structural and functional characterization of the purified KATP channel complex Kir6.2-SUR1. Embo J. 2005;24:4166–4175. doi: 10.1038/sj.emboj.7600877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Makhina EN, Masia R, Hyrc KL, Formanack ML, Nichols CG. Domain organization of the ATP-sensitive potassium channel complex examined by FRET. J Biol Chem. doi: 10.1074/jbc.M112.388629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 22.Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JP, Gonzalez G, Aguilarbryan L, Permutt MA, Bryan J. Adenosine Diphosphate As an Intracellular Regulator Of Insulin Secretion. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- 23.Beguin P, Nagashima K, Nishimura M, Gonoi T, Seino S. PKA-mediated phosphorylation of the human K(ATP) channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO Journal. 1999;18:4722–4732. doi: 10.1093/emboj/18.17.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 25.Heusser K, Yuan H, Neagoe I, Tarasov AI, Ashcroft FM, Schwappach B. Scavenging of 14–3–3 proteins reveals their involvement in the cell-surface transport of ATP-sensitive K+ channels. J Cell Sci. 2006;119:4353–4363. doi: 10.1242/jcs.03196. [DOI] [PubMed] [Google Scholar]
- 26.Isidoro Tavares N, Philip-Couderc P, Papageorgiou I, Baertschi AJ, Lerch R, Montessuit C. Expression and function of ATP-dependent potassium channels in late post-infarction remodeling. J Mol Cell Cardiol. 2007;42:1016–1025. doi: 10.1016/j.yjmcc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Raeis-Dauve V, Philip-Couderc P, Faggian G, Tessari M, Roatti A, Milano AD, Bochaton-Piallat ML, Baertschi AJ. Increased expression of adenosine triphosphate-sensitive K+ channels in mitral dysfunction: mechanically stimulated transcription and hypoxia-induced protein stability? J Am Coll Cardiol. 2011;59:390–396. doi: 10.1016/j.jacc.2011.08.077. [DOI] [PubMed] [Google Scholar]
- 28.Morrissey A, Parachuru L, Leung M, Lopez G, Nakamura TY, Tong X, Yoshida H, Srivastava S, Chowdhury PD, Artman M, Coetzee WA. Expression of ATP-sensitive K+ channel subunits during perinatal maturation in the mouse heart. Pediatric Research. 2005;58:185–192. doi: 10.1203/01.PDR.0000169967.83576.CB. [DOI] [PubMed] [Google Scholar]
- 29.Morrissey A, Rosner E, Lanning J, Parachuru L, Chowdhury PD, Han S, Lopez G, Tong X, Yoshida H, Nakamura TY, Artman M, Giblin JP, Tinker A, Coetzee WA. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiology. 2005;5:1. doi: 10.1186/1472-6793-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 31.Glukhov AV, Flagg TP, Fedorov VV, Efimov IR, Nichols CG. Differential K(ATP) channel pharmacology in intact mouse heart. J Mol Cell Cardiol. 2009;48:152–160. doi: 10.1016/j.yjmcc.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flagg TP, Kurata HT, Masia R, Caputa G, Magnuson MA, Lefer DJ, Coetzee WA, Nichols CG. Differential structure of atrial and ventricular KATP: atrial KATP channels require SUR1. Circ Res. 2008;103:1458–1465. doi: 10.1161/CIRCRESAHA.108.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fedorov VV, Glukhov AV, Ambrosi CM, Kostecki G, Chang R, Janks D, Schuessler RB, Moazami N, Nichols CG, Efimov IR. Effects of KATP channel openers diazoxide and pinacidil in coronary-perfused atria and ventricles from failing and non-failing human hearts. J Mol Cell Cardiol. 2011;51:215–225. doi: 10.1016/j.yjmcc.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han X, Light PE, Giles WR, French RJ. Identification and Properties Of an Atp-Sensitive K+ Current In Rabbit Sino-Atrial Node Pacemaker Cells. Journal of Physiology. 1996;490:337–350. doi: 10.1113/jphysiol.1996.sp021148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakei M, Noma A. Adenosine-5′-triphosphate-sensitive single potassium channel in the atrioventricular node cell of the rabbit heart. Journal of Physiology London. 1984;352:265–284. doi: 10.1113/jphysiol.1984.sp015290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Light PE, Cordeiro JM, French RJ. Identification and properties of ATP-sensitive potassium channels in myocytes from rabbit Purkinje fibres. Cardiovasc Res. 1999;44:356–369. doi: 10.1016/s0008-6363(99)00218-7. [DOI] [PubMed] [Google Scholar]
- 37.Fukuzaki K, Sato T, Miki T, Seino S, Nakaya H. Role of sarcolemmal ATP-sensitive K+ channels in the regulation of sinoatrial node automaticity: an evaluation using Kir6.2-deficient mice. J Physiol. 2008;586:2767–2778. doi: 10.1113/jphysiol.2007.148932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dart C, Standen NB. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. J Physiol. 1993;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols CG, Lederer WJ. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. Journal of Physiology London. 1990;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanco-Rivero J, Gamallo C, Aras-Lopez R, Cobeno L, Cogolludo A, Perez-Vizcaino F, Ferrer M, Balfagon G. Decreased expression of aortic KIR6.1 and SUR2B in hypertension does not correlate with changes in the functional role of K(ATP) channels. Eur J Pharmacol. 2008;587:204–208. doi: 10.1016/j.ejphar.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 41.Cui Y, Tran S, Tinker A, Clapp LH. The molecular composition of K(ATP) channels in human pulmonary artery smooth muscle cells and their modulation by growth. Am J Respir Cell Mol Biol. 2002;26:135–143. doi: 10.1165/ajrcmb.26.1.4622. [DOI] [PubMed] [Google Scholar]
- 42.Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 43.Miyoshi Y, Nakaya Y, Wakatsuki T, Nakaya S, Fujino K, Saito K, Inoue I. Endothelin blocks ATP-sensitive K+ channels and depolarizes smooth muscle cells of porcine coronary artery. Circ Res. 1992;70:612–616. doi: 10.1161/01.res.70.3.612. [DOI] [PubMed] [Google Scholar]
- 44.Ottolia M, Toro L. Reconstitution in lipid bilayers of an ATP-sensitive K+ channel from pig coronary smooth muscle. J Membr Biol. 1996;153:203–209. doi: 10.1007/s002329900123. [DOI] [PubMed] [Google Scholar]
- 45.Beech DJ, Zhang H, Nakao K, Bolton TB. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol. 1993;110:573–582. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kajioka S, Kitamura K, Kuriyama H. Guanosine diphosphate activates an adenosine 5′-triphosphate-sensitive K+ channel in the rabbit portal vein. Journal of Physiology. 1991;444:397–418. doi: 10.1113/jphysiol.1991.sp018885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamouchi M, Kitamura K. Regulation of ATP-sensitive K+ channels by ATP and nucleotide diphosphate in rabbit portal vein. Am J Physiol. 1994;266:H1687–1698. doi: 10.1152/ajpheart.1994.266.5.H1687. [DOI] [PubMed] [Google Scholar]
- 48.Miyoshi Y, Nakaya Y. Angiotensin II blocks ATP-sensitive K+ channels in porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun. 1991;181:700–706. doi: 10.1016/0006-291x(91)91247-a. [DOI] [PubMed] [Google Scholar]
- 49.Wakatsuki T, Nakaya Y, Inoue I. Vasopressin modulates K(+)-channel activities of cultured smooth muscle cells from porcine coronary artery. Am J Physiol. 1992;263:H491–496. doi: 10.1152/ajpheart.1992.263.2.H491. [DOI] [PubMed] [Google Scholar]
- 50.Furspan PB, Webb RC. Decreased ATP sensitivity of a K+ channel and enhanced vascular smooth muscle relaxation in genetically hypertensive rats. J Hypertens. 1993;11:1067–1072. doi: 10.1097/00004872-199310000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Zhang HL, Bolton TB. Two types of ATP-sensitive potassium channels in rat portal vein smooth muscle cells. Br J Pharmacol. 1996;118:105–114. doi: 10.1111/j.1476-5381.1996.tb15372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cole WC, Malcolm T, Walsh MP, Light PE. Inhibition by protein kinase C of the K(NDP) subtype of vascular smooth muscle ATP-sensitive potassium channel. Circ Res. 2000;87:112–117. doi: 10.1161/01.res.87.2.112. [DOI] [PubMed] [Google Scholar]
- 53.Cole WC, Clement-Chomienne O. ATP-sensitive K+ channels of vascular smooth muscle cells. J Cardiovasc Electrophysiol. 2003;14:94–103. doi: 10.1046/j.1540-8167.2003.02376.x. [DOI] [PubMed] [Google Scholar]
- 54.Aguilar-Bryan L, Nichols CG, Rajan AS, Parker C, Bryan J. Co-expression of sulfonylurea receptors and KATP channels in hamster insulinoma tumor (HIT) cells. Evidence for direct association of the receptor with the channel. J Biol Chem. 1992;267:14934–14940. [PubMed] [Google Scholar]
- 55.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 56.Zhang HL, Bolton TB. Two types of ATP-sensitive potassium channels in rat portal vein smooth muscle cells. British Journal of Pharmacology. 1996;118:105–114. doi: 10.1111/j.1476-5381.1996.tb15372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beech DJ, Zhang H, Nakao K, Bolton TB. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. British Journal of Pharmacology. 1993;110:573–582. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farzaneh T, Tinker A. Differences in the mechanism of metabolic regulation of ATP-sensitive K+ channels containing Kir6.1 and Kir6.2 subunits. Cardiovasc Res. 2008;79:621–631. doi: 10.1093/cvr/cvn138. [DOI] [PubMed] [Google Scholar]
- 59.Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. Journal of Biological Chemistry. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- 60.Satoh E, Yamada M, Kondo C, Repunte VP, Horio Y, Iijima T, Kurachi Y. Intracellular nucleotide-mediated gating of SUR/Kir6.0 complex potassium channels expressed in a mammalian cell line and its modification by pinacidil. Journal of Physiology. 1998;511:663–674. doi: 10.1111/j.1469-7793.1998.663bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Babenko AP, Bryan J. A conserved inhibitory and differential stimulatory action of nucleotides on K(IR)6.0/SUR complexes is essential for excitation-metabolism coupling by K(ATP) channels. Journal of Biological Chemistry. 2001;276:49083–49092. doi: 10.1074/jbc.M108763200. [DOI] [PubMed] [Google Scholar]
- 62.von der Weid PY, Lee S, Imtiaz MS, Zawieja DC, Davis MJ. Electrophysiological properties of rat mesenteric lymphatic vessels and their regulation by stretch. Lymphatic research and biology. 2014;12:66–75. doi: 10.1089/lrb.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Telinius N, Kim S, Pilegaard H, Pahle E, Nielsen J, Hjortdal V, Aalkjaer C, Boedtkjer DB. The contribution of K(+) channels to human thoracic duct contractility. American journal of physiology. Heart and circulatory physiology. 2014;307:H33–43. doi: 10.1152/ajpheart.00921.2013. [DOI] [PubMed] [Google Scholar]
- 64.Seino S, Iwanaga T, Nagashima K, Miki T. Diverse roles of K(ATP) channels learned from Kir6.2 genetically engineered mice. Diabetes. 2000;49:311–318. doi: 10.2337/diabetes.49.3.311. [DOI] [PubMed] [Google Scholar]
- 65.Remedi MS, Nichols CG. Hyperinsulinism and diabetes: genetic dissection of beta cell metabolism-excitation coupling in mice. Cell Metab. 2009;10:442–453. doi: 10.1016/j.cmet.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels.[see comment] Journal of Clinical Investigation. 2002;110:203–208. doi: 10.1172/JCI15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ellis JA, Lamantia A, Chavez R, Scurrah KJ, Nichols CG, Harrap SB. Genes controlling postural changes in blood pressure: comprehensive association analysis of ATP-sensitive potassium channel genes KCNJ8 and ABCC9. Physiol Genomics. 2009;40:184–188. doi: 10.1152/physiolgenomics.00173.2009. [DOI] [PubMed] [Google Scholar]
- 68.Duan R, Cui W, Wang H. Mutational analysis of the Kir6.1 gene in Chinese hypertensive patients treated with the novel ATP-sensitive potassium channel opener iptakalim. Exp Ther Med. 2:757–760. doi: 10.3892/etm.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harrap SB, Cui JS, Wong ZY, Hopper JL. Familial and genomic analyses of postural changes in systolic and diastolic blood pressure. Hypertension. 2004;43:586–591. doi: 10.1161/01.HYP.0000118044.84189.44. [DOI] [PubMed] [Google Scholar]
- 70.Koster JC, Knopp A, Flagg TP, Markova KP, Sha Q, Enkvetchakul D, Betsuyaku T, Yamada KA, Nichols CG. Tolerance for ATP-insensitive K(ATP) channels in transgenic mice. Circ Res. 2001;89:1022–1029. doi: 10.1161/hh2301.100342. [DOI] [PubMed] [Google Scholar]
- 71.Li A, Knutsen RH, Zhang H, Osei-Owusu P, Moreno-Dominguez A, Harter TM, Uchida K, Remedi MS, Dietrich HH, Bernal-Mizrachi C, Blumer KJ, Mecham RP, Koster JC, Nichols CG. Hypotension Due to Kir6.1 Gain-of-Function in Vascular Smooth Muscle. J Am Heart Assoc. 2013;2:e000365. doi: 10.1161/JAHA.113.000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levin MD, Zhang H, Uchida K, Grange DK, Singh GK, Nichols CG. Electrophysiologic consequences of KATP gain of function in the heart: Conduction abnormalities in Cantu syndrome. Heart rhythm : the official journal of the Heart Rhythm Society. 2015;12:2316–2324. doi: 10.1016/j.hrthm.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flagg TP, Charpentier F, Manning-Fox J, Remedi MS, Enkvetchakul D, Lopatin A, Koster J, Nichols C. Remodeling of excitation-contraction coupling in transgenic mice expressing ATP-insensitive sarcolemmal KATP channels. American Journal of Physiology - Heart & Circulatory Physiology. 2004;286:H1361–1369. doi: 10.1152/ajpheart.00676.2003. [DOI] [PubMed] [Google Scholar]
- 74.Flagg TP, Patton B, Masia R, Mansfield C, Lopatin AN, Yamada KA, Nichols CG. Arrhythmia susceptibility and premature death in transgenic mice overexpressing both SUR1 and Kir6.2[DeltaN30,K185Q] in the heart. Am J Physiol Heart Circ Physiol. 2007;293:H836–845. doi: 10.1152/ajpheart.00011.2007. [DOI] [PubMed] [Google Scholar]
- 75.Toib A, Zhang HX, Broekelmann TJ, Hyrc KL, Guo Q, Chen F, Remedi MS, Nichols CG. Cardiac specific ATP-sensitive K(+) channel (K(ATP)) overexpression results in embryonic lethality. J Mol Cell Cardiol. 53:437–445. doi: 10.1016/j.yjmcc.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miki T, Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. Journal of Molecular & Cellular Cardiology. 2005;38:917–925. doi: 10.1016/j.yjmcc.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 77.Nichols CG, Koster JC, Remedi MS. beta-cell hyperexcitability: from hyperinsulinism to diabetes. Diabetes Obes Metab. 2007;9(Suppl 2):81–88. doi: 10.1111/j.1463-1326.2007.00778.x. [DOI] [PubMed] [Google Scholar]
- 78.Tester DJ, Tan BH, Medeiros-Domingo A, Song C, Makielski JC, Ackerman MJ. Loss-of-function mutations in the KCNJ8-encoded Kir6.1 K(ATP) channel and sudden infant death syndrome. Circ Cardiovasc Genet. 4:510–515. doi: 10.1161/CIRCGENETICS.111.960195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O’Cochlain F, Gao F, Karger AB, Ballew JD, Hodgson DM, Zingman LV, Pang YP, Alekseev AE, Terzic A. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nature Genetics. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olson TM, Alekseev AE, Moreau C, Liu XK, Zingman LV, Miki T, Seino S, Asirvatham SJ, Jahangir A, Terzic A. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nature Clinical Practice Cardiovascular Medicine. 2007;4:110–116. doi: 10.1038/ncpcardio0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haissaguerre M, Chatel S, Sacher F, Weerasooriya R, Probst V, Loussouarn G, Horlitz M, Liersch R, Schulze-Bahr E, Wilde A, Kaab S, Koster J, Rudy Y, Le Marec H, Schott JJ. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–98. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 82.Delaney JT, Muhammad R, Blair MA, Kor K, Fish FA, Roden DM, Darbar D. A KCNJ8 mutation associated with early repolarization and atrial fibrillation. Europace. 2012;14:1428–1432. doi: 10.1093/europace/eus150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Medeiros-Domingo A, Tan BH, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, Kroboth SL, Song C, Zhou Q, Kopp D, Schwartz PJ, Makielski JC, Ackerman MJ. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010;7:1466–1471. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barajas-Martinez H, Hu D, Ferrer T, Onetti CG, Wu Y, Burashnikov E, Boyle M, Surman T, Urrutia J, Veltmann C, Schimpf R, Borggrefe M, Wolpert C, Ibrahim BB, Sanchez-Chapula JA, Winters S, Haissaguerre M, Antzelevitch C. Molecular genetic and functional association of Brugada and early repolarization syndromes with S422L missense mutation in KCNJ8. Heart Rhythm. 2011;9:548–555. doi: 10.1016/j.hrthm.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Veeramah KR, Karafet TM, Wolf D, Samson RA, Hammer MF. The KCNJ8-S422L variant previously associated with J-wave syndromes is found at an increased frequency in Ashkenazi Jews. Eur J Hum Genet. 2013 doi: 10.1038/ejhg.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cantu JM, Garcia-Cruz D, Sanchez-Corona J, Hernandez A, Nazara Z. A distinct osteochondrodysplasia with hypertrichosis- Individualization of a probable autosomal recessive entity. Hum Genet. 1982;60:36–41. doi: 10.1007/BF00281261. [DOI] [PubMed] [Google Scholar]
- 87.Harakalova M, van Harssel JJ, Terhal PA, van Lieshout S, Duran K, Renkens I, Amor DJ, Wilson LC, Kirk EP, Turner CL, Shears D, Garcia-Minaur S, Lees MM, Ross A, Venselaar H, Vriend G, Takanari H, Rook MB, van der Heyden MA, Asselbergs FW, Breur HM, Swinkels ME, Scurr IJ, Smithson SF, Knoers NV, van der Smagt JJ, Nijman IJ, Kloosterman WP, van Haelst MM, van Haaften G, Cuppen E. Dominant missense mutations in ABCC9 cause Cantu syndrome. Nat Genet. 2012;44:793–796. doi: 10.1038/ng.2324. [DOI] [PubMed] [Google Scholar]
- 88.van Bon BW, Gilissen C, Grange DK, Hennekam RC, Kayserili H, Engels H, Reutter H, Ostergaard JR, Morava E, Tsiakas K, Isidor B, Le Merrer M, Eser M, Wieskamp N, de Vries P, Steehouwer M, Veltman JA, Robertson SP, Brunner HG, de Vries BB, Hoischen A. Cantu syndrome is caused by mutations in ABCC9. Am J Hum Genet. 2012;90:1094–1101. doi: 10.1016/j.ajhg.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cooper PE, Reutter H, Woelfle J, Engels H, Grange DK, van Haaften G, van Bon BW, Hoischen A, Nichols CG. Cantu syndrome resulting from activating mutation in the KCNJ8 gene. Human mutation. 2014;35:809–813. doi: 10.1002/humu.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brownstein CA, Towne MC, Luquette LJ, Harris DJ, Marinakis NS, Meinecke P, Kutsche K, Campeau PM, Yu TW, Margulies DM, Agrawal PB, Beggs AH. Mutation of KCNJ8 in a patient with Cantu syndrome with unique vascular abnormalities - Support for the role of K(ATP) channels in this condition. Eur J Med Genet. 2013;56:678–682. doi: 10.1016/j.ejmg.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grange DK, Lorch SM, Cole PL, Singh GK. Cantu syndrome in a woman and her two daughters: Further confirmation of autosomal dominant inheritance and review of the cardiac manifestations. Am J Med Genet A. 2006;140:1673–1680. doi: 10.1002/ajmg.a.31348. [DOI] [PubMed] [Google Scholar]
- 92.Cooper PE, Sala-Rabanal M, Lee SJ, Nichols CG. Differential mechanisms of Cantu syndrome-associated gain of function mutations in the ABCC9 (SUR2) subunit of the KATP channel. The Journal of general physiology. 2015;146:527–540. doi: 10.1085/jgp.201511495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scurr I, Wilson L, Lees M, Robertson S, Kirk E, Turner A, Morton J, Kidd A, Shashi V, Stanley C, Berry M, Irvine AD, Goudie D, Turner C, Brewer C, Smithson S. Cantu syndrome: report of nine new cases and expansion of the clinical phenotype. Am J Med Genet A. 2011;155A:508–518. doi: 10.1002/ajmg.a.33885. [DOI] [PubMed] [Google Scholar]
- 94.Garcia-Cruz D, Mampel A, Echeverria MI, Vargas AL, Castaneda-Cisneros G, Davalos-Rodriguez N, Patino-Garcia B, Garcia-Cruz MO, Castaneda V, Cardona EG, Marin-Solis B, Cantu JM, Nunez-Reveles N, Moran-Moguel C, Thavanati PK, Ramirez-Garcia S, Sanchez-Corona J. Cantu syndrome and lymphoedema. Clin Dysmorphol. 20:32–37. doi: 10.1097/MCD.0b013e32833d015c. [DOI] [PubMed] [Google Scholar]
- 95.Kobayashi D, Cook AL, Williams DA. Pulmonary hypertension secondary to partial pulmonary venous obstruction in a child with Cantu syndrome. Pediatr Pulmonol. 2010;45:727–729. doi: 10.1002/ppul.21215. [DOI] [PubMed] [Google Scholar]
- 96.Engels H, Bosse K, Ehrbrecht A, Zahn S, Hoischen A, Propping P, Bindl L, Reutter H. Further case of Cantu syndrome: exclusion of cryptic subtelomeric chromosome aberrations. Am J Med Genet. 2002;111:205–209. doi: 10.1002/ajmg.10560. [DOI] [PubMed] [Google Scholar]
- 97.Lazalde B, Sanchez-Urbina R, Nuno-Arana I, Bitar WE, de Lourdes Ramirez-Duenas M. Autosomal dominant inheritance in Cantu syndrome (congenital hypertrichosis, osteochondrodysplasia, and cardiomegaly) Am J Med Genet. 2000;94:421–427. doi: 10.1002/1096-8628(20001023)94:5<421::aid-ajmg15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 98.Concolino D, Formicola S, Camera G, Strisciuglio P. Congenital hypertrichosis, cardiomegaly, and osteochondrodysplasia (Cantu syndrome): a new case with unusual radiological findings. Am J Med Genet. 2000;92:191–194. [PubMed] [Google Scholar]
- 99.Robertson SP, Kirk E, Bernier F, Brereton J, Turner A, Bankier A. Congenital hypertrichosis, osteochondrodysplasia, and cardiomegaly: Cantu syndrome. Am J Med Genet. 1999;85:395–402. [PubMed] [Google Scholar]
- 100.Rosser EM, Kaariainen H, Hurst JA, Baraitser M, Hall CM, Clayton P, Leonard JV. Three patients with the osteochondrodysplasia and hypertrichosis syndrome--Cantu syndrome. Clin Dysmorphol. 1998;7:79–85. doi: 10.1097/00019605-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 101.Garcia-Cruz D, Mampel A, Echeverria MI, Vargas AL, Castaneda-Cisneros G, Davalos-Rodriguez N, Patino-Garcia B, Garcia-Cruz MO, Castaneda V, Cardona EG, Marin-Solis B, Cantu JM, Nunez-Reveles N, Moran-Moguel C, Thavanati PK, Ramirez-Garcia S, Sanchez-Corona J. Cantu syndrome and lymphoedema. Clin Dysmorphol. 2011;20:32–37. doi: 10.1097/MCD.0b013e32833d015c. [DOI] [PubMed] [Google Scholar]
- 102.Pennisi AJ, Takahashi M, Bernstein BH, Singsen BH, Uittenbogaart C, Ettenger RB, Malekzadeh MH, Hanson V, Fine RN. Minoxidil therapy in children with severe hypertension. The Journal of pediatrics. 1977;90:813–819. doi: 10.1016/s0022-3476(77)81260-2. [DOI] [PubMed] [Google Scholar]
- 103.Mehta PK, Mamdani B, Shansky RM, Mahurkar SD, Dunea G. Severe hypertension. Treatment with minoxidil. JAMA. 1975;233:249–252. [PubMed] [Google Scholar]
- 104.Kaler SG, Patrinos ME, Lambert GH, Myers TF, Karlman R, Anderson CL. Hypertrichosis and congenital anomalies associated with maternal use of minoxidil. Pediatrics. 1987;79:434–436. [PubMed] [Google Scholar]
- 105.Rosa FW, Idanpaan-Heikkila J, Asanti R. Fetal minoxidil exposure. Pediatrics. 1987;80:120. [PubMed] [Google Scholar]
- 106.Schneider DJ, Moore JW. Patent ductus arteriosus. Circulation. 2006;114:1873–1882. doi: 10.1161/CIRCULATIONAHA.105.592063. [DOI] [PubMed] [Google Scholar]
- 107.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 108.Mehta PA, Dubrey SW. High output heart failure. QJM. 2009;102:235–241. doi: 10.1093/qjmed/hcn147. [DOI] [PubMed] [Google Scholar]
- 109.Rossi A, Cantisani C, Melis L, Iorio A, Scali E, Calvieri S. Minoxidil use in dermatology, side effects and recent patents. Recent patents on inflammation & allergy drug discovery. 2012;6:130–136. doi: 10.2174/187221312800166859. [DOI] [PubMed] [Google Scholar]
- 110.Shorter K, Farjo NP, Picksley SM, Randall VA. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:1725–1736. doi: 10.1096/fj.07-099424. [DOI] [PubMed] [Google Scholar]
- 111.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 112.Faivre JF, Findlay I. Effects of tolbutamide, glibenclamide and diazoxide upon action potentials recorded from rat ventricular muscle. Biochim Biophys Acta. 1989;984:1–5. doi: 10.1016/0005-2736(89)90334-9. [DOI] [PubMed] [Google Scholar]
- 113.Bao L, Kefaloyianni E, Lader J, Hong M, Morley G, Fishman GI, Sobie EA, Coetzee WA. Unique properties of the ATP-sensitive K channel in the mouse ventricular cardiac conduction system. Circ Arrhythm Electrophysiol. 2011;4:926–935. doi: 10.1161/CIRCEP.111.964643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cole WC, McPherson CD, Sontag D. ATP-regulated K+ channels protect the myocardium against ischemia/reperfusion damage. Circ Res. 1991;69:571–581. doi: 10.1161/01.res.69.3.571. [DOI] [PubMed] [Google Scholar]
- 115.Venkatesh N, Lamp S-T, Weiss J-N IN Department of Medicine USoM. Sulfonylureas, ATP-sensitive K+ channels, and cellular K+ loss during hypoxia, ischemia, and metabolic inhibition in mammalian ventricle. Circ-Res. 1991 Sep;69(3):623–37. doi: 10.1161/01.res.69.3.623. IS 0009–7330. [DOI] [PubMed] [Google Scholar]
- 116.McPherson CD, Pierce GN, Cole WC. Ischemic cardioprotection by ATP-sensitive K+ channels involves high-energy phosphate preservation. Am J Physiol. 1993;265:H1809–1818. doi: 10.1152/ajpheart.1993.265.5.H1809. [DOI] [PubMed] [Google Scholar]
- 117.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yamada S, Kane GC, Behfar A, Liu XK, Dyer RB, Faustino RS, Miki T, Seino S, Terzic A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. The Journal of physiology. 2006;577:1053–1065. doi: 10.1113/jphysiol.2006.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu X, Xu X, Huang Y, Fassett J, Flagg TP, Zhang Y, Nichols CG, Bache RJ, Chen Y. Disruption of sarcolemmal ATP-sensitive potassium channel activity impairs the cardiac response to systolic overload. Circ Res. 2008;103:1009–1017. doi: 10.1161/CIRCRESAHA.107.170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kane GC, Behfar A, Dyer RB, O’Cochlain DF, Liu XK, Hodgson DM, Reyes S, Miki T, Seino S, Terzic A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Human Molecular Genetics. 2006;15:2285–2297. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- 122.Stoller D, Kakkar R, Smelley M, Chalupsky K, Earley JU, Shi NQ, Makielski JC, McNally EM. Mice lacking sulfonylurea receptor 2 (SUR2) ATP-sensitive potassium channels are resistant to acute cardiovascular stress. Journal of Molecular & Cellular Cardiology. 2007;43:445–454. doi: 10.1016/j.yjmcc.2007.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elrod JW, Harrell M, Flagg TP, Gundewar S, Magnuson MA, Nichols CG, Coetzee WA, Lefer DJ. Role of sulfonylurea receptor type 1 subunits of ATP-sensitive potassium channels in myocardial ischemia/reperfusion injury. Circulation. 2008;117:1405–1413. doi: 10.1161/CIRCULATIONAHA.107.745539. [DOI] [PubMed] [Google Scholar]
- 124.Schramm TK, Gislason GH, Vaag A, Rasmussen JN, Folke F, Hansen ML, Fosbol EL, Kober L, Norgaard ML, Madsen M, Hansen PR, Torp-Pedersen C. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 32:1900–1908. doi: 10.1093/eurheartj/ehr077. [DOI] [PubMed] [Google Scholar]
- 125.Gore MO, McGuire DK. Resolving drug effects from class effects among drugs for type 2 diabetes mellitus: more support for cardiovascular outcome assessments. Eur Heart J. 32:1832–1834. doi: 10.1093/eurheartj/ehr019. [DOI] [PubMed] [Google Scholar]
- 126.Zhang HX, Akrouh A, Kurata HT, Remedi MS, Lawton JS, Nichols CG. HMR 1098 is not an SUR isotype specific inhibitor of heterologous or sarcolemmal K ATP channels. J Mol Cell Cardiol. 2010;50:552–560. doi: 10.1016/j.yjmcc.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Flagg TP, Nichols CG. “Cardiac KATP”: a family of ion channels. Circ Arrhythm Electrophysiol. 2011;4:796–798. doi: 10.1161/CIRCEP.111.968081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Flanagan SE, Clauin S, Bellanne-Chantelot C, de Lonlay P, Harries LW, Gloyn AL, Ellard S. Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2009;30:170–180. doi: 10.1002/humu.20838. [DOI] [PubMed] [Google Scholar]