Abstract
Understanding the regulating mechanism of tumor invasion is of crucial importance for both fundamental cancer research and clinical applications. Previous in vivo experiments have shown that invasive cancer cells dissociate from the primary tumor and invade into the stroma, forming an irregular invasive morphology. Although cell movements involved in tumor invasion are ultimately driven by mechanical forces of cell-cell interactions and tumor-host interactions, how these mechanical properties affect tumor invasion is still poorly understood. In this study, we use a recently developed two-dimensional cellular model to study the effects of mechanical properties on tumor invasion. We study the effects of cell-cell adhesions as well as the degree of degradation and stiffness of extracellular matrix (ECM). Our simulation results show that cell-cell adhesion relationship must be satisfied for tumor invasion. Increased adhesion to ECM and decreased adhesion among tumor cells result in invasive tumor behaviors. When this invasive behavior occurs, ECM plays an important role for both tumor morphology and the shape of invasive cancer cells. Increased stiffness and stronger degree of degradation of ECM promote tumor invasion, generating more aggressive tumor invasive morphologies. It can also generate irregular shape of invasive cancer cells, protruding towards ECM. The capability of our model suggests it a useful tool to study tumor invasion and might be used to propose optimal treatment in clinical applications.
I. INTRODUCTION
Tumor invasion is of crucial importance for both fundamental cancer research and clinical applications. During tumor progression, cells invade into the surrounding host tissues, adapt to the environment, and develop resistance to therapies. Invasive cells are also left behind after resection and are responsible for tumor recurrence, ultimately resulting in human deaths [1]. Therefore, significant efforts have been made to understand the mechanism of regulating tumor invasion.
During tumor invasion, it is obvious that cell movements are ultimately driven by mechanical forces. Although significant efforts have been made to study the genetic and biochemical aspects of tumor invasion [2], how mechanical properties affect tumor invasion is still poorly understood.
The goal of this study is to use a mechanical cellular model to study the effects of mechanical properties on tumor invasion. We study the effects of cell-cell adhesions as well as the degree of degradation and stiffness of extracellular matrix (ECM). Our simulation results show that increased adhesion to ECM and decreased adhesion among tumor cells must be satisfied for tumor invasion. When tumor invasion occurs, ECM plays an important role for both promoting tumor morphology and generating the irregular shape of invasive cancer cells. This study also aims to suggest novel pharmaceutical targets for anticancer therapy by blocking these essential mechanical changes.
II. METHODS
A. Cellular Model
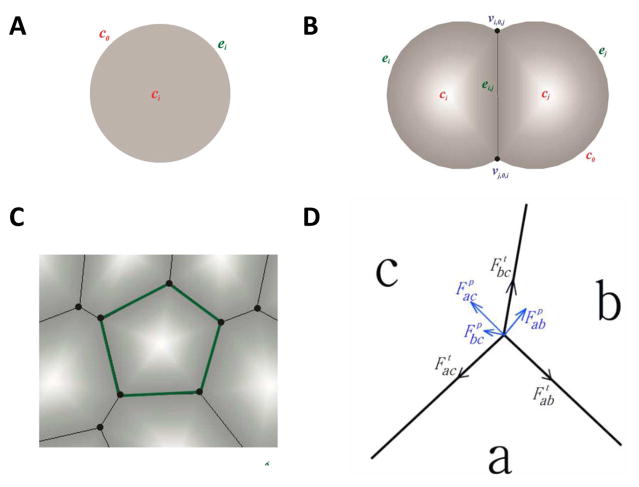
We use a previously developed cellular model to describe interacting cells [3]. This model represents accurately the geometric properties of a single cell as well as the topological properties of cells in a tissue in two dimensions (Fig. 1A–C). It can approximately model epithelial tumors that are largely two-dimensional in nature.
Fig. 1.
Two-dimensional cellular model. A) An isolated cell is modeled as a disk. B) A cell is modeled as a disk segment when contacting other cell(s). C) A cell completely surrounded by other cells is represented as a polygon. D) Forces at the junction vertex of three cells a, b, and c. Tension is tangential to the edge (black). Pressure is normal to the edge (blue).
In our model, cell movement depends on the mechanical forces a cell experiences. There are two types of forces in our model: tension and pressure. Tension models the compressional forces acting within a cell. These forces arise from cytoskeletal microfilaments, intermediate filaments, and cell membrane. For an edge between cell a and b, the direction of tension is tangential to edge ab (Fig. 1D):
where ηab is the tension coefficient, which may depend on the cell types of both cells, and eab is the edge vector. Pressure represents the forces resisting compression. These forces arise mainly from microtubules and extracellular matrix. The direction of pressure is normal to edge ab (Fig. 1D). The net force at a vertex is obtained by summing all the forces due to tension and pressure acting on the vertex (Fig. 1D) (more details can be found in ref. [4–8]).
B. Cell Types
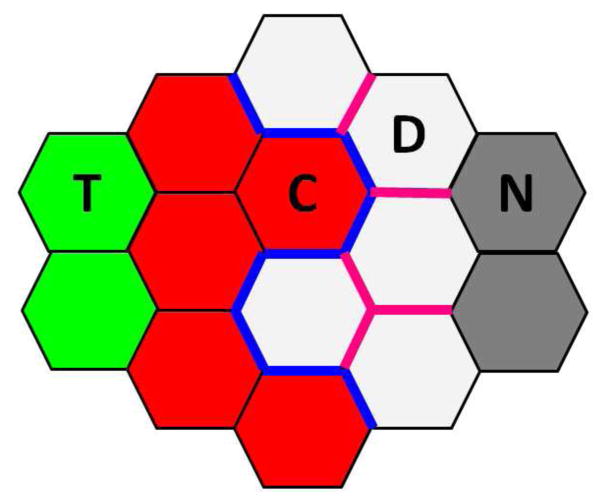
Cells in our model correspond to real tumor cells, cells in stroma or extracellular matrix (ECM). To study the effect of mechanical properties on tumor invasion, we model four types of cells involved in tumor and its microenvironment: non-invasive tumor cells, invasive cancer cells, degraded ECM treated as cells, and normal ECM treated as cells.
Non-invasive tumor cells (T) are inside the primary tumor, surrounded by invasive cancer cells. These cells cannot invade into the tumor stroma, thus cannot interact with the ECM.
Invasive cancer cells (C) are at the tumor-host interface. These cells can directly degrade the ECM, thus can invade into the tumor stroma.
Degraded ECM (D) are at the tumor-host interface. They can be degraded by invasive cancer cells directly.
Normal ECM (N) are at the outer region of tumor stroma, which cannot interact with tumor cells.
C. Mechanical Properties
Tumor exists in a constantly evolving microenvironment of diverse cell types and ECM structures [9]. ECM is a complex mixture of molecules that provide mechanical support for the tumor, and it also plays an important role for cell adhesion and motility [10, 11]. Our model represents ECM structures virtually as cells. Thus our model can explicitly take into account the mechanical interactions between tumor cells and ECM structures. In our current work, we focus on two types of mechanical properties:
Cell-cell adhesions: During tumor invasion, invasive cancer cells lose the adhesion to neighboring tumor cells and tend to adhere to ECM cells [12, 13]. In our model, tension coefficient ηCD reflects the difference in adhesion ability between cells. We set tension co-efficient η = 1 as default value for all edges. Larger ηCD (> 1) indicates invasive cancer cells have stronger adhesion to tumor cells and less adhesion to ECM cells. Smaller ηCD (< 1) indicates invasive cancer cells have less adhesion to tumor cells and stronger adhesion to ECM cells.
Degree of degradation and stiffness of ECM: During tumor invasion, invasive cancer cells can degrade ECM cells using matrix metalloproteinases (MMPs) in order to migrate [14, 15]. Previous studies have also shown that the stiffness of ECM promotes tumor invasion [9]. In this study, we model degree of degradation and stiffness of ECM by tension coefficient ηDD. Larger ηDD (> 1) indicates more tension on the edge between degraded ECM cells, thus ECM cells experience more compressional forces to degrade and become more stiff. Smaller ηDD (< 1) indicates less tension on the edge between degraded ECM cells, thus ECM cells experience less compressional forces to degrade and become less stiff.
D. Quantitative Measurement of Invasive Pattern
To quantitatively characterize the invasive pattern of simulations, we define Invasive Index IT to describe the degree of tumor invasiveness:
where NC and NT are the number of invasive cancer cells and non-invasive tumor cells, respectively. A larger invasive index IT value indicates more aggressively invasive pattern.
E. Simulation Methodology
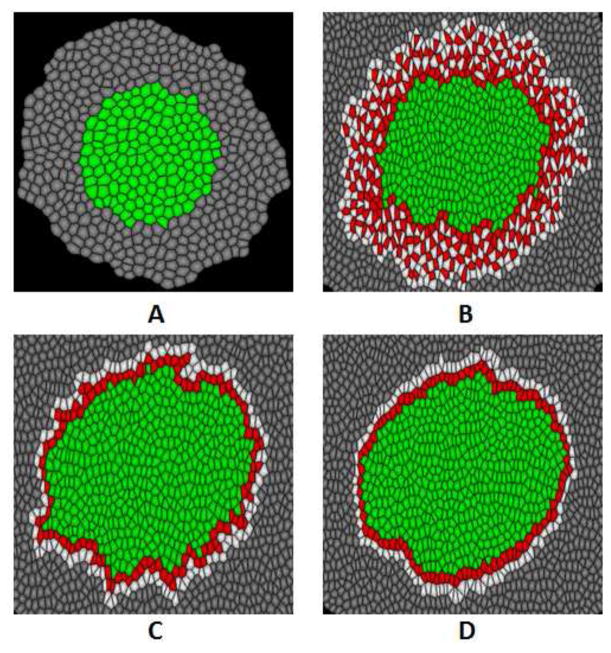
For a typical simulation, an initial tissue (Fig. 3A) consisting around 500 cells is constructed. This tissue is composed of two types of cells: non-invasive tumor cells in the center and normal ECM cells at the outside. Tension coefficients η are set to 1 on all edges at this point. When primary tumor intends to invade into ECM, non-invasive tumor cells at the tumor-host interface change to invasive cancer cells, and normal ECM cells connecting the invasive cancer cells change to degraded ECM cells. At the same time, cells begin to grow and divide. During cell growth, we increase cell volumes with random amounts. We explore the effects of cell-cell adhesions and degree of degradation and stiffness of ECM on tumor invasion with different value of tension coefficients ηCD and ηDD. We measure the invasive index IT, and check the tissue morphology and cell shape for visualization. For each set of parameters, we run simulations for 5 times, and take the average as our results.
Fig. 3.
Simulated tumor morphology with different tension coefficients ηCD. Tension coefficient ηDD = 1.0 for all simulations. (A) Initial tissue is composed of non-invasive tumor cells (green) and normal ECM cells (gray) before tumor invasion starts. (B) Invasive tumor morphology occurs with ηCD = 0.5. Invasive cancer cells invade into the extracellular matrix, forming an irregular and loose tumor-host interface. (C) Tumor morphology with ηCD = 1.0. Invasive cancer cells do not invade into the extracellular matrix, but the shape of tumor-host interface is slightly irregular and loose. (D) Tumor morphology with ηCD = 1.5. Invasive cancer cells do not invade into the extracellular matrix, restricted to the primary tumor and forming a stiff tumor-host interface.
III. RESULTS AND DISCUSSIONS
A. Effect of cell-cell adhesions
We first studied the effect of cell-cell adhesions on tumor invasion. Tension coefficient ηCD is simply set to three values: 0.5, 1.0, and 1.5, explicitly indicating invasive cancer cells with stronger adhesion to ECM (decreased adhesion to tumor cells), equal adhesion to ECM cells and tumor cells, and decreased adhesion to ECM (stronger adhesion to tumor cells), respectively.
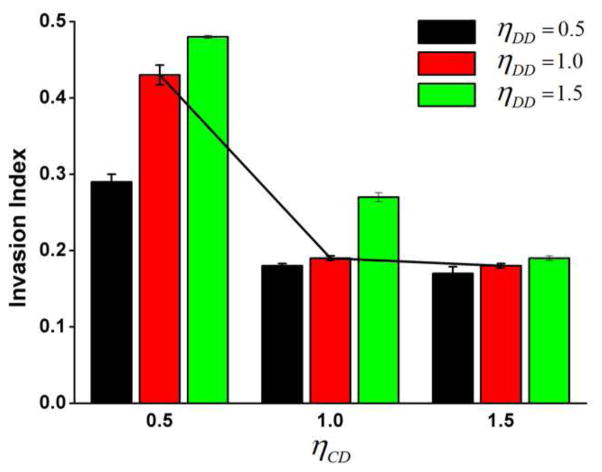
We found that invasive index IT decreases when ηCD increases despite of the value of ηDD (Fig. 4). Invasive index IT are 0.43, 0.20, and 0.18 when ηCD are set to 0.5, 1.0, and 1.5, respectively (ηDD = 1.0). When ηCD = 0.5, tumor experiences most invasive behaviors. Invasive cancer cells invade into the ECM, forming an irregular and loose tumor-host interface (Fig. 3B). When ηCD = 1.0, tumor experiences slightly invasive behaviors. Invasive cancer cells do not invade into the ECM, but the shape of tumor-host interface is slightly irregular and loose (Fig. 3C). When ηCD = 1.5, tumor does not show any invasive behaviors. Invasive cancer cells do not invade into the ECM, restricted in the primary tumor and forming a stiff tumor-host interface (Fig. 3D). These simulation results demonstrate that stronger adhesion to ECM cells and decreased adhesion to tumor cells can result in invasive tumor behaviors.
Fig. 4.
Invasive index with different tension coefficients ηCD and ηDD. Invasive index decreases when ηCD increases despite of the value of ηDD (black line for visual representation). When ηCD = 0.5, tumor is most invasive, indicating that increased adhesion to ECM cells and decreased adhesion to tumor cells result in invasive tumor behavior. Invasive index responds to ηDD differently dependent on the value of ηCD. When ηCD = 0.5, invasive index increases with ηDD significantly. When ηCD = 1.0, invasive index increases with ηDD slightly. When ηCD = 1.5, invasive index is not influenced by with ηDD.
B. Effect of extracellular matrix
We then studied the effect of extracellular matrix on tumor invasion. Tension coefficient ηDD is simply set to three values: 0.5, 1.0, and 1.5, explicitly indicating decreased stiffness with less degree of degradation of ECM, control group, and increased stiffness with stronger degree of degradation of ECM, respectively.
1) Tumor invasive morphology
We found that invasive index IT responds to ηDD differently dependent on tumor invasive behaviors (the value of ηCD) (Fig. 4). When aggressively invasive behaviors occur (ηCD = 0.5), invasive index IT increases with ηDD significantly. Invasive index IT are 0.29, 0.43, and 0.48 when ηDD are 0.5, 1.0, and 1.5, respectively (Fig. 4). Larger value of ηDD generate more aggressively invasive pattern (Fig. 5A). When slightly invasive behaviors occur (ηCD = 1.0), invasive index IT increases with ηDD slightly. Invasive index IT are 0.18, 0.19, and 0.27 when ηDD are 0.5, 1.0, and 1.5, respectively (Fig. 4). When invasive behaviors do not occur (ηCD = 1.5), invasive index IT is not influenced by ηDD (Fig. 4). These simulation results show that ECM affects the tumor invasive morphology only if the invasive behaviors occur. That is, cell-cell adhesion relationship needs to be satisfied to enable the invasive cancer cells to invade into ECM. Thus cancer invasive cells can be influenced by ECM in return, consequently affecting the tumor invasive pattern.
Fig. 5.
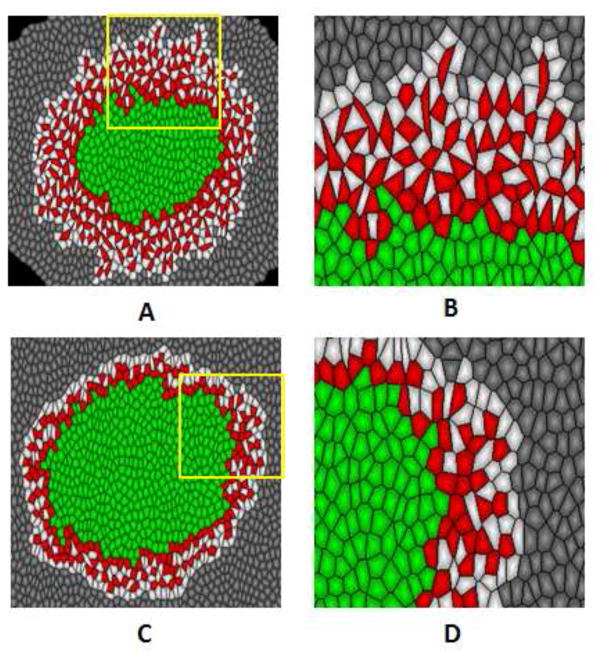
Simulated tumor morphology and shape of invasive cancer cells with tension coefficients ηDD = 1.5 and ηDD = 0.5. Tension coefficient ηCD = 0.5 for all simulations. (A) When ηDD = 1.5, tumor represents aggressively invasive behaviors with large number of invasive cancer cells invade into extracellular matrix. (B) Magnified picture of the yellow square region in (A). Invasive cancer cells forms elongated irregular cell shapes, protruding towards the extracellular matrix. (C) When ηDD = 0.5, tumor represents less invasive behaviors. (D) Magnified picture of the yellow square region in (C). Invasive cancer cells forms regular cell shapes as the non-invasive tumor cells.
2) Shape of cancer invasive cells
When aggressively invasive behavior occurs (ηCD = 0.5), we found that the shape of cancer invasive cells change with different values of tension coefficient ηDD. When ηDD = 1.5, invasive cancer cells form stretched, irregular cell shapes, protruding towards ECM (Fig. 5B). When ηDD = 0.5, invasive cancer cells does not deform, forming regular cell shapes (Fig. 5D). These simulation result is consistent with experimental observations that the deformability of cancer invasive cell (MCF-7) was significantly higher than that of normal tumor cells (MCF-10) [16]. We note that, to our knowledge, our model is the first to generate this stretched, irregular cell shapes during tumor invasion. This suggests our model a valid tool to study both the tissue morphology and cell shapes in tumor invasion.
IV. CONCLUSIONS
We present a cellular model to study the effects of mechanical properties on tumor invasion. Our simulation results show that decreased adhesion between tumor cells and increased adhesion to ECM is the prerequisite for tumor invasion. Following the change of cell-cell adhesion, the change of ECM may further promote tumor invasion, generating more aggressive tumor invasive morphologies and deformable cell shapes. These results may suggest novel pharmaceutical targets for cancer therapy by blocking these essential mechanical changes. Furthermore, the capability of our model suggests it a valid tool to study tumor invasion and might be used to propose optimal treatment in clinical applications.
Fig. 2.
Illustration of cell types and mechanical properties within tumor and its microenvironment. Non-invasive tumor cells (T, green) are surrounded by invasive cancer cells (C, red). Invasive cancer cells are at the tumor-host interface, directly degrading the ECM. Degraded ECM (D) are white, and normal ECM (N) far away from the invasive cancer cells are gray. During tumor invasion, tension on the edge between invasive cancer cells and degraded ECM (blue) changes due to the lost adhesion between tumor cells and intension to adhere to the ECM. Tension on the edge among degraded ECM (pink) changes due to degradation by invasive cancer cells and increased stiffness of the ECM.
Acknowledgments
This work was supported by NIH GM079804 and G-M086145, NSF DBI-1062328 and DMS-0800257, the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust, National Basic Research Program of China 2010CB834300, Research Fund of Science and Technology Commission of Shanghai Municipality 11DZ2211001, and 985 Project from Shanghai Jiao Tong University.
Contributor Information
Yingzi Li, Email: yingzili@sjtu.edu.cn, School of Biomedical Engineering and Med-X Research Institute, Shanghai Jiao Tong University, Shanghai, 200030, China and Department of Bioengineering, University of Illinois at Chicago, Chicago, IL 60607, USA.
Hammad Naveed, Email: hammadnaveed@yahoo.com, Department of Bioengineering, University of Illinois at Chicago, Chicago, IL 60607, USA and Computer, Electrical and Mathematical Sciences and Engineering Division, King Abdullah University of Science and Technology, Thuwal, 23955-6900, Saudi Arabia.
Jie Liang, Email: jliang@uic.edu, Department of Bioengineering, University of Illinois at Chicago, Chicago, IL 60607, USA and Shanghai Center for Systems Biomedicine, Shanghai Jiao Tong Univerisity, Shanghai, 200240, China.
Lisa X. Xu, Email: lisaxu@sjtu.edu.cn, School of Biomedical Engineering and Med-X Research Institute, Shanghai Jiao Tong University, Shanghai, 200030, China and Shanghai Center for Systems Biomedicine, Shanghai Jiao Tong Univerisity, Shanghai, 200240, China
References
- 1.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of Cancer: the Next Generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Naveed H, Li Y, Kachalo S, Liang J. Geometric Order in Proliferating Epithelia: Impact of Rearrangements and Cleavage Plane Orientation. Conf Proc IEEE Eng. Med. Biol. Soc; 2010. pp. 3808–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Naveed H, Kachalo S, Liang J. Mechanical forces mediate localized topological change in epithelia. Conf Proc IEEE Eng. Med. Biol. Soc; 2011. p. 178C181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Naveed H, Kachalo S, Xu LX, Liang J. Mechanisms of Regulating Cell Topology in Proliferating Epithelia: Impact of Division Plane, Mechanical Forces, and Cell Memory. PLoS ONE. 2012;7(8):e43108. doi: 10.1371/journal.pone.0043108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y, Liang C, Naveed H, Li Y, Chen M, Nie Q. Modeling spatial population dynamics of stem cell lineage in tissue growth. Conf Proc IEEE Eng. Med. Biol. Soc; 2012. pp. 5502–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y, Naveed H, Liang C, Liang J. Modeling Spatial Population Dynamics of Stem Cell Lineage in Wound Healing and Cancerogenesis. Conf Proc IEEE Eng. Med. Biol. Soc; 2013. pp. 5550–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Naveed H, Kachalo S, Xu LX, Liang J. Mechanisms of Regulating Tissue Elongation in Drosophila Wing: Impact of Oriented Cell Divisions, Oriented Mechanical Forces, and Reduced Cell Size. PLoS ONE. 2014;9(2):e86725. doi: 10.1371/journal.pone.0086725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H, Mouw JK, Weaver VM. Forcing Form and Function: Biomechanical Regulation of Tumor Evolution. Trends Cell Biol. 2011;21(1):47–56. doi: 10.1016/j.tcb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson AR. A hybrid mathematical model of solid tumour invasion: the importance of cell adhesion. Math Med Biol. 2005;22(2):163–186. doi: 10.1093/imammb/dqi005. [DOI] [PubMed] [Google Scholar]
- 11.Gevertz JL, Torquato S. A novel three-phase model of brain tissue microstructure. PLoS Comput Biol. 2008;4(8):e1000152. doi: 10.1371/journal.pcbi.1000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behrens J, Mareel MM, Van Roy FM, Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989;108(6):2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198(1):11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 14.Crawford HC, Matrisian LM. Tumor and stromal expression of matrix metalloproteinases and their role in tumor progression. Invasion Metastasis. 1994–1995;14(1–6):234–245. [PubMed] [Google Scholar]
- 15.Stetler-Stevenson WG, Liotta LA, Kleiner DE., Jr Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993;7(15):1434–1441. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- 16.Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, Romeyke M, Lenz D, Erickson HM, Ananthakrishnan R, Mitchell D, Kas J, Ulvick S, Bilby C. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J. 2005;88(5):3689–3698. doi: 10.1529/biophysj.104.045476. [DOI] [PMC free article] [PubMed] [Google Scholar]