Abstract
The development of new drugs for the treatment of depression is strategic to achieving clinical needs of patients. This study evaluates antidepressant-like effect and neural mechanisms of four oleanolic acid derivatives i.e. acrylate (D1), methacrylate (D2), methyl fumarate (D3) and ethyl fumarate (D4). All derivatives were obtained by simple one-step esterification of oleanolic acid prior to pharmacological screening in the forced swimming (FS) and open field (OF) tests. Pharmacological tools like α-methyl-p-tyrosine (AMPT, catecholamine depletor), p-chlorophenylalanine (serotonin depletor), prazosin (PRAZ, selective α1-receptor antagonist), WAY-100635 (selective serotonin 5-HT1A receptor antagonist) as well as monoamine oxidase (MAO) and functional binding assays were conducted to investigate possible neural mechanisms. In the FS test, D1 showed the most promising antidepressant-like effect without eliciting locomotor incoordination. Unlike group of mice pretreated with AMPT 100 mg/kg, PCPA 100 mg/kg or PRAZ 1 mg/kg, the effect of D1 was attenuated by WAY-100635 0.3 mg/kg pretreatment. D1 demonstrated moderate inhibition of MAO-A (IC50 = 48.848 ± 1.935 μM), potency (pEC50 = 6.1 ± 0.1) and intrinsic activity (Emax = 26 ± 2.0%) on 5-HT1A receptor. In conclusion, our findings showed antidepressant-like effect of D1 and possible involvement of 5-HT1A receptor.
Depression is a chronic psychiatric disorder that contributes substantially to mental impairment, physical disability and socioeconomic burden1. This disorder is capable of reducing quality of life, productivity, and increase suicidality. World Health Organization predicts, depression to become the second leading cause of disease by 20202,3. The symptoms of depression such as anhedonia, irritability, difficulties in concentrating and sleep are clear evidences of the complexity of its neurobiology4. The search for new drugs remains a desirable approach as the current agents including tricyclic antidepressants, selective serotonin reuptake and monoamine oxidase (MAO) inhibitors are still far from producing optimal effects without causing side effects5,6,7.
Preclinical and clinical studies of some medicinal plants such as Cimicifuga foetida, Hypericum perforatum, Pimenta pseudocaryophyllus among others have been reported to possess antidepressant property8,9,10,11,12. Oleanolic acid (OA), major active secondary metabolite isolated from Pimenta pseudocaryophyllus, has demonstrated numerous pharmacological activities such as hepatoprotection13,14, cytoprotection15, anti-oxidation16,17,18,19, anti-inflammation20, anti-cancer21,22, anti-ulcer23,24 and antidepressant7,25.
The diverse effects of OA are associated with its plurality of mechanism of action. The suppression of the inflammatory response by OA involves inhibition of C3-convertase26,27, reduction of prostaglandin PGE2 biosynthesis28 and exudates29. The hepatoprotective effect of OA involves inhibition of CYPB5, CYP1A and CYP2A, an increase in antioxidant substances such as glutathione, metallothionein, zinc, glutathione-S-transferase and glucuronosyltransferase13,30,31. Antidepressant-like effect of OA was found to be attenuated by depletion of indolamine and catecholamine7. However, unsuspecting health risks that are associated with the multiple interaction of OA constitute limitations to its therapeutic application. Relatively high availability of OA from plants offers immense opportunity for the synthesis and evaluation of its derivatives as potential antidepressant drugs. Previous biological studies of OA derivatives have shown their pharmacological potential32,33,34.
Herein we report the antidepressant-like effect of four new OA derivatives obtained by simple esterification reaction at C-3 with corresponding acyl chlorides.
Results
1H, 13C NMR and MS data for compounds D1-D4
NMR and MS data were obtained for all products purified by column chromatography.
Oleanolic acid acrylate (D1): White amorphous powder, yield 90%. 1H NMR (400 MHz, CDCl3): δ 6.44 (d, J = 16.4 Hz, 1H), 6.16 (dd, J = 16.4, 7.6 Hz, 1H), 5.85 (d, J = 7.6 Hz, 1H), 5.29 (dd, J = 5.8, 2.6 Hz, 1H), 4.53 (dd, J = 9.4, 6.2 Hz, 1H), 2.81 (dd, J = 14.2, 4.0 Hz, 1H), 1.86 (m, 1H), 1.74 (m, 1H), 1.65–1.54 (m, 6H), 1.44 (m, 1H), 1.38–1.27 (m, 3H), 1.22 (m, 1H), 1.15 (m, 1H), 1.14 (s, 3H), 1.07–0.97 (m, 7H), 0.96 (s, 3H), 0.93 (m, 1H), 0.92 (s, 3H), 0.90 (s, 3H), 0.88 (s, 3H), 0.87 (s, 3H), 0.85 (s, 3H), 0.76 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 185.1, 167.3, 144.7, 131.5, 129.3, 122.7, 80.3, 56.1, 47.9, 46.8, 46.1, 42.2, 41.1, 40.2, 38.3, 37.2, 33.9, 33.1, 32.7, 32.4, 30.6, 30.1, 29.2, 27.9, 26.2, 23.8, 23.1, 22.9, 18.4, 18.1, 17.4, 16.9, 15.7; HRESIMS (m/z): [M + H]+ calculated for C33H50O4, 511.3709; found, 511.3712.
Oleanolic acid methacrylate (D2): White amorphous powder, 83% yield. 1H NMR (400 MHz, CDCl3): δ 6.51 (s, 1H), 6.43 (s, 1H), 5.31 (dd, J = 5.8, 2.6 Hz, 1H), 4.55 (dd, J = 9.4, 6.2 Hz, 1H), 2.83 (dd, J = 14.2, 4.0 Hz, 1H), 1.99 (s, 3H), 1.85 (m, 1H), 1.73 (m, 1H), 1.66–1.53 (m, 6H), 1.45 (m, 1H), 1.37–1.26 (m, 3H), 1.21 (m, 1H), 1.16 (m, 1H), 1.13 (s, 3H), 1.06–0.98 (m, 7H), 0.96 (s, 3H), 0.94 (m, 1H), 0.92 (s, 3H), 0.89 (s, 3H), 0.88 (s, 3H), 0.87 (s, 3H), 0.86 (s, 3H), 0.77 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 185.7, 167.5, 144.3, 136.6, 131.8, 129.6, 125.7, 122.9, 80.4, 56.2, 47.8, 46.9, 46.3, 42.4, 41.2, 40.4, 38.5, 37.3, 33.9, 33.2, 32.8, 32.5, 30.8, 30.2, 29.3, 27.8, 26.3, 23.9, 23.2, 23.0, 18.5, 18.2, 17.8, 17.5, 16.8, 15.9; HRESIMS (m/z): [M + H]+ calculated for C34H52O4, 525.3866; found, 525.3871.
Oleanolic acid methyl fumarate (D3): White amorphous powder, 75% yield. 1H NMR (400 MHz, CDCl3): δ 6.29 (s, 2H), 5.28 (dd, J = 5.8, 2.6 Hz, 1H), 4.56 (dd, J = 9.4, 6.2 Hz, 1H), 3.81 (s, 3H), 2.84 (dd, J = 14.2, 4.0 Hz, 1H), 1.87 (m, 1H), 1.75 (m, 1H), 1.64-1.52 (m, 6H), 1.45 (m, 1H), 1.38–1.26 (m, 3H), 1.23 (m, 1H), 1.16 (m, 1H), 1.15 (s, 3H), 1.08–0.97 (m, 7H), 0.96 (s, 3H), 0.93 (m, 1H), 0.92 (s, 3H), 0.90 (s, 3H), 0.88 (s, 3H), 0.86 (s, 3H), 0.85 (s, 3H), 0.75 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 185.1, 168.3, 167.1, 144.7, 133.8, 130.1, 122.9, 80.5, 56.3, 52.3, 47.6, 46.9, 46.3, 42.8, 41.3, 40.6, 38.8, 37.5, 33.8, 33.1, 32.5, 32.4, 30.6, 30.1, 29.2, 27.9, 26.6, 23.8, 23.2, 22.8, 18.5, 18.2, 17.6, 16.3, 15.5; HRESIMS (m/z): [M + H]+ calculated for C35H52O6, 569.3764; found, 569.3769.
Oleanolic acid ethyl fumarate (D4): White amorphous powder, 80% yield. 1H NMR (400 MHz, CDCl3): δ 6.31 (s, 2H), 5.28 (dd, J = 5.8, 2.6 Hz, 1H), 4.58 (dd, J = 9.4, 6.2 Hz, 1H), 3.89 (q, 2H), 2.83 (dd, J = 14.2, 4.0 Hz, 1H), 1.89 (m, 1H), 1.76 (m, 1H), 1.65–1.53 (m, 6H), 1.45 (m, 1H), 1.38–1.26 (m, 3H), 1.23 (m, 1H), 1.21 (t, 3H), 1.16 (m, 1H), 1.15 (s, 3H), 1.08–0.97 (m, 7H), 0.96 (s, 3H), 0.93 (m, 1H), 0.92 (s, 3H), 0.90 (s, 3H), 0.88 (s, 3H), 0.86 (s, 3H), 0.85 (s, 3H), 0.75 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 185.4, 168.7, 167.3, 144.8, 132.9, 130.1, 122.9, 80.5, 61.5, 56.3, 47.6, 46.9, 46.3, 42.8, 41.3, 40.7, 38.9, 37.4, 33.8, 33.3, 32.5, 32.5, 30.8, 30.1, 29.2, 27.9, 26.6, 23.8, 23.2, 22.8, 18.5, 18.1, 17.8, 16.5, 15.3, 14.4; HRESIMS (m/z): [M + H]+ calculated for C36H54O6, 583.3921; found, 583.3927.
Effects of D1 treatment in the forced swimming (FS) and open field (OF) tests
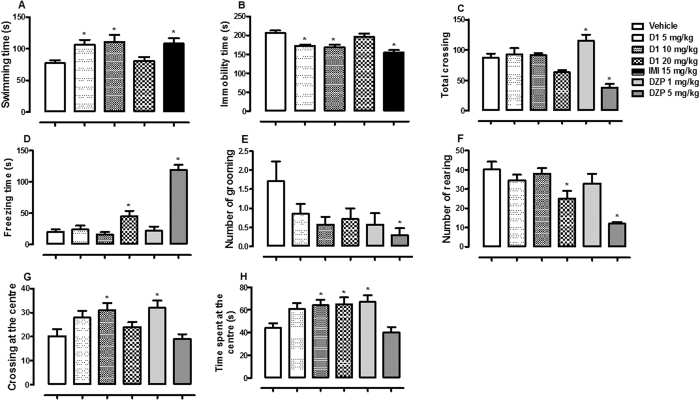
Oral administration of D1 elicited significant effect on swimming [F (4, 30) = 4.28, p < 0.01, Fig. 1A] and immobility time [F (4, 30) = 10.34, p < 0.001, Fig. 1B]. Dunnett´s test showed significant increase in swimming time by D1 5 or 10 mg/kg (p < 0.05) and reduction in immobility time at the same doses (p < 0.05). In the OF test, D1 and/or DZP elicited significant effects on total crossing [F (5, 36) = 13.60, p < 0.001, Fig. 1C], freezing time [F (5, 36) = 36.51, p < 0.001, Fig. 1D], number of grooming [F (5, 36) = 2.49, p < 0.05, Fig. 1E], number of rearing [F (5, 36) = 8.55, p < 0.001, Fig. 1F], crossing at the centre [F (5, 36) = 4.24, p < 0.01, Fig. 1G] and time spent at the centre [F (5, 36) = 5.10, p < 0.01, Fig. 1H] of OF. D1 elicited an increase in freezing time at dose 20 mg/kg (p < 0.05), crossing at the centre at dose 10 mg/kg (p < 0.05) and time spent at the centre at doses 10 and 20 mg/kg (p < 0.05), and a reduction in the number of grooming at dose 20 mg/kg (p < 0.05). However, other parameters were not altered by D1 (p > 0.05).
Figure 1.
The effect of oral administration of vehicle 10 mL/kg, acrylate (D1) 5, 10, 20 mg/kg or imipramine (IMI) 15 mg/kg on the swimming (A) and immobility time in the FS test (B). Figure 1 C, D, E, F, G and H showed the effect of oral administrations of vehicle 10 mL/kg, D1 5, 10, 20 mg/kg, diazepam (DZP) 1 or 5 mg/kg on the total crossing, freezing time, number of grooming, number of rearing, crossing at the centre, and time spent at the centre of the OF by mice, respectively. Each column represents the mean ± SEM of 10 animals. *p < 0.05 vs vehicle treated group.
Effects of D2 treatment in the FS and OF tests
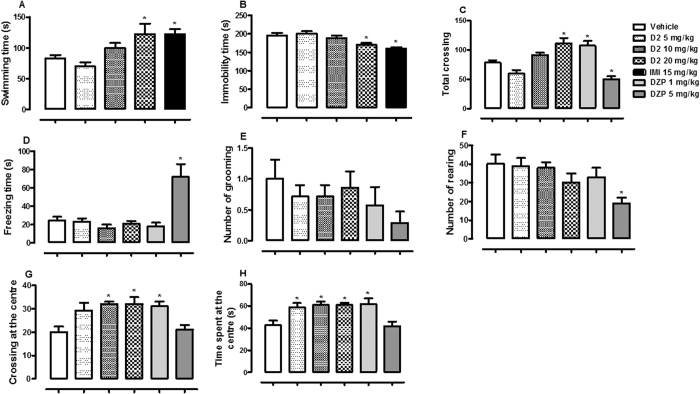
D2 elicited significant effect on swimming [F (4, 30) = 5.59, p < 0.01, Fig. 2A] and immobility time [F (4, 30) = 9.27, p < 0.001, Fig. 2B]. Dunnett´s test showed significant increase in swimming time and reduction in immobility time by D2 20 mg/kg (p < 0.05). Except for number of grooming [F (5, 36) = 1.03, p > 0.05, Fig. 2E] in the OF test, D2 and/or DZP showed significant effects on total crossing [F (5, 36) = 15.34, p < 0.001, Fig. 2C], freezing time [F (5, 36) = 10.24, p < 0.001, Fig. 2D], number of rearing [F (5, 36) = 3.39, p < 0.05, Fig. 2F], crossing at the centre [F (5, 36) = 5.35, p < 0.001, Fig. 2G] and time spent at the centre [F (5, 36) = 6.26, p < 0.001, Fig. 2H] of OF. D2 induced an increase in total crossing (at dose 20 mg/kg), crossing at the centre (at doses 10 and 20 mg/kg) and time spent at the centre (at doses 5, 10 and 20 mg/kg). However, other parameters were not altered by D2 (p > 0.05).
Figure 2.
The effect of oral administration of vehicle 10 mL/kg, methacrylate (D2) 5, 10, 20 mg/kg or imipramine (IMI) 15 mg/kg on the swimming (A) and immobility time in the FS test (B). Figure 2 C, D, E, F, G and H showed the effect of oral administrations of vehicle 10 mL/kg, D2 5, 10, 20 mg/kg, diazepam (DZP) 1 or 5 mg/kg on the total crossing, freezing time, number of grooming, number of rearing, crossing at the centrre, and time spent at the centre of the OF by mice, respectively. Each column represents the mean ± SEM of 10 animals. *p < 0.05 vs vehicle treated group.
Effects of D3 treatment in the FS and OF tests
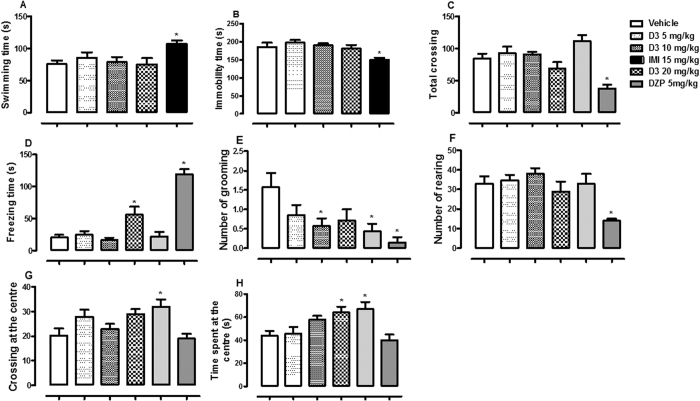
Oral administration of D3 did not elicit significant effect on swimming [F (4, 30) = 3.04, p > 0.05, Fig. 3A] and immobility time [F (4, 30) = 4.72, p > 0.05, Fig. 3B]. In the OF test, D3 and/or DZP showed significant effects on total crossing [F (5, 36) = 9.78, p < 0.001, Fig. 3C], freezing time [F (5, 36) = 28.99, p < 0.001, Fig. 3D], number of grooming [F (5, 36) = 3.67, p < 0.01, Fig. 3E], number of rearing [F (5, 36) = 5.23, p < 0.01, Fig. 3F], crossing at the centre [F (5, 36) = 4.35, p < 0.01, Fig. 3G] and time spent at the centre [F (5, 36) = 5.28, p < 0.01, Fig. 3H] of OF. D3 induced an increase in freezing time (at dose 20 mg/kg), time spent at the centre (at dose 20 mg/kg) and a reduction in the number of grooming (at dose 10 mg/kg). However, other parameters were not altered by D3 (p > 0.05).
Figure 3.
The effect of oral administration of vehicle 10 mL/kg, methyl fumarate (D3) 5, 10, 20 mg/kg or imipramine (IMI) 15 mg/kg on the swimming (A) and immobility time in the FS test (B). Figure 3 C, D, E, F, G and H showed the effect of oral administrations of vehicle 10 mL/kg, D3 5, 10, 20 mg/kg, diazepam (DZP) 1 or 5 mg/kg on the total crossing, freezing time, number of grooming, number of rearing, crossing at the centre and time spent at the centre of the OF by mice, respectively. Each column represents the mean ± SEM of 10 animals. *p < 0.05 vs vehicle treated group.
Effects of D4 treatment in the FS and OF tests
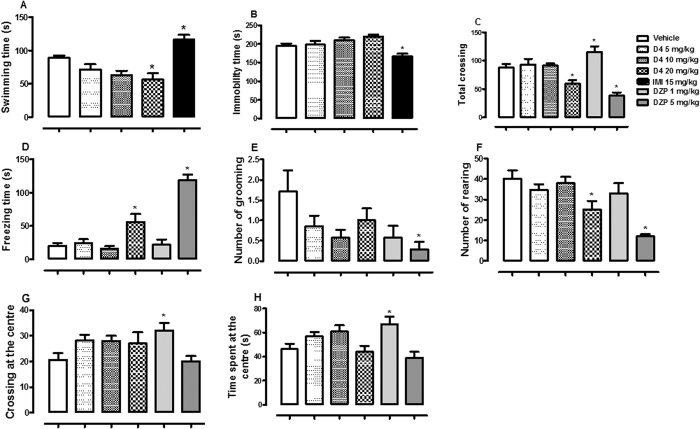
D4 elicited significant effect on swimming [F (4, 30) = 11.11, p < 0.001, Fig. 4A] without changes in immobility time [F (4, 30) = 7.35, p > 0.05, Fig. 4B]. Dunnett´s test showed significant reduction in swimming time by D4 20 mg/kg (p < 0.05). In the OF, statistical analysis showed the significant effects of D4 and/or DZP on total crossing [F (5, 36) = 13.36, p < 0.001, Fig. 4C], freezing time [F (5, 36) = 28.67, p < 0.001, Fig. 4D], number of grooming [F (5, 36) = 2.51, p < 0.05, Fig. 4E], number of rearing [F (5, 36) = 8.31, p < 0.001, Fig. 4F], crossing at the centre [F (5, 36) = 2.78, p < 0.05, Fig. 4G] and time spent at the centre [F (5, 36) = 4.98, p < 0.001, Fig. 4H] of OF. D4 induced a reduction in number of rearing, total crossing and an increase in freezing time at dose 20 mg/kg (p < 0.05).
Figure 4.
The effect of oral administration of vehicle 10 mL/kg, ethyl fumarate (D4) 5, 10, 20 mg/kg or imipramine (IMI) 15 mg/kg on the swimming (A) and immobility time in the FS test (B). Figure 3 C, D, E, F, G and H showed the effect of oral administrations of vehicle 10 mL/kg, D4 5, 10, 20 mg/kg, diazepam (DZP) 1 or 5 mg/kg on the total crossing, freezing time, number of grooming, number of rearing, crossing at the centre, and time spent at the centre of the OF by mice, respectively. Each column represents the mean ± SEM of 10 animals. *p < 0.05 vs vehicle treated group.
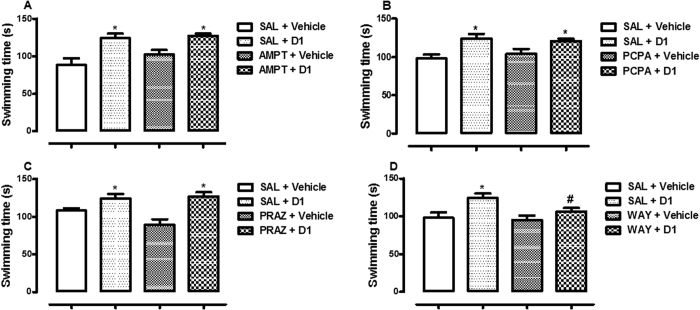
Effects of AMPT, PCPA PRAZ or WAY pretreatments on antidepressant-like property of D1
Figure 5 showed the effect of pretreatment (AMPT, PCPA, PRAZ or WAY; independent variable) and treatment (vehicle or D1; independent variable) on the swimming time (dependent variable and main effect) in the FS test. The data obtained on the main effect did not revealed interaction between the independent variables (p > 0.05). Pairwise comparisons with Bonferroni post hoc test showed that D1 administration increased the swimming time in the Fig. 5A–D (i.e. SAL + Vehicle versus SAL + D1, p < 0.05). Pretreatment with AMPT did not attenuate antidepressant-like effect of D1 (i.e. SAL + D1 versus AMPT + D1, p > 0.05, Fig. 5A). In addition, pretreatment with PCPA and PRAZ did not block antidepressant-like effect of D1 (i.e. SAL + D1 versus PCPA + D1, p > 0.05, Fig. 5B) and (SAL + D1 versus PRAZ + D1, p > 0.05, Fig. 5C), respectively. In contrary, WAY pretreatment induced a significant blockade of the antidepressant-like effect of D1 (i.e. SAL + D1 versus WAY + D1; p < 0.05, Fig. 5D). Pretreatments with AMPT, PCPA, PRAZ or WAY, prior to vehicle administration did not alter animal behaviour at the doses tested (i.e AMPT + Vehicle, PCPA + Vehicle, PRAZ + Vehicle, WAY + Vehicle versus SAL + Vehicle; p > 0.05).
Figure 5.
Alteration in swimming time following pretreatment with saline solution (SAL) 10 mL/kg or α-methyl-p-tyrosine (AMPT) 100 mg/kg, Fig. 5A; SAL 10 mL/kg or p-chlorophenylalanine methyl ester (PCPA) 100 mg/kg, Fig. 5B; SAL 10 mL/kg or prazosin (PRAZ) 1 mg/kg, Fig. 5C; SAL 10 mL/kg or WAY-100635 (WAY) 0.3 mg/kg, Fig. 5D prior to oral treatment with vehicle 10 mL/kg or acrylate (D1) 5 mg/kg. Data are expressed as mean ± SEM, n = 5; *p < 0.05 vehicle treated group versus other experimental groups; # p < 0.05 SAL + D1 versus WAY + D1.
Effects of D1 on enzymatic activity of MAO-A and -B
D1 showed an IC50 of 48.848 ± 1.935 μM for MAO-A (moderate inhibition) and IC50 value >100 μM for MAO-B. The reference compounds clorgyline and deprenyl showed potent inhibition of MAO-A and -B with IC50 of 0.003 ± 0.0001 and 0.047 ± 0.0041 μM, respectively.
Functional activity of D1 at 5-HT1A receptor
Unlike WAY, D1 produced an increase in [35S]GTPγS binding to mice hippocampal membranes. Potency and intrinsic activity of D1 on 5-HT1A receptor were close to that of buspirone (Table 1).
Table 1. The estimates of functional parameters (agonist potency and intrinsic activity) for D1 and standard compounds in a [35S]GTPγS binding assay.
| Compounds | Agonist potency (pEC50 ± SEM) | Intrinsic activity (Emax ± SEM in %) |
|---|---|---|
| (+) 8-OH-DPAT | 6.8 ± 0.2 | 94.0 ± 2.0 |
| Buspirone | 6.6 ± 0.3 | 33.0 ± 1.0 |
| D1 | 6.1 ± 0.1 | 26.0 ± 2.0 |
| WAY | 0 | 0 |
The values of pEC50 (negative logarithm of the concentration of agonist required to elicit a response halfway between the baseline and maximum responses) and Emax, (maximal drug effect on [35S]GTPγS binding baseline expressed as a percentage of that produced by 10 μM (+)8-OH-DPAT) were estimated using nonlinear regression analysis.
General pharmacological test of D1 effect on mice
In the general pharmacological test, D1 elicited a reduction in exploratory activity at the dose of 250 mg/kg after 30 minutes of subcutaneous (s.c) administration (Table 2). A reduction in exploratory activity and sedative effect were observed following intraperitoneal (i.p) and s.c administration of D1 at doses 10, 50 and 250 mg/kg between 30 minute and 1hour. Oral (p.o) administration of D1 elicited increase in exploratory activity starting from 30 minute to 1 hour at the doses of 10, 50 and 250 mg/kg (Table 2). All the behavioural manifestations disappeared within 4 hours of D1 administration without any record of toxicity or death.
Table 2. Report on behavioural alteration elicited by subcutaneous (s.c), intraperitoneal (i.p) or oral (p.o) administration of D1.
| Observation time after administration | Dose (mg/kg) |
Administration routes and observations |
||
|---|---|---|---|---|
| s.c | i.p | p.o | ||
| 15 min | 2, 10 or 50 | N | N | N |
| 250 | Reduced exploration | N | N | |
| 30 min | 2 or 10 | N | N | N |
| 50 | Reduced exploration | Reduced exploration | Increase exploratory | |
| 250 | Sedation | Sedation | Increase exploratory | |
| 1 hr | 2 or 10 | Effects after 30 min of administrations persisted | ||
| 50 | ||||
| 250 | ||||
| 4 hr – 7 days | Total recovery from the effects of D1 without any sign of toxicity, weight gain or death occurence | |||
N - No observable behavioural alteration as compared to vehicle treated group.
Discussion
Chemical modification remains an important strategy for new drug synthesis and development from natural products35. The rationale for modification of oleanolic acid structure by esterification with acrylic and fumaric acid chlorides was to introduce electrophilic centre through the formation of Michael acceptor-type OAD that could interact with nucleophilic residues of binding site of the receptor.
In order to investigate antidepressant-like effects, mice were treated orally with OAD and subjected to the FS test. The FS test is one of the most used behavioural tests for screening antidepressant-like activity of new compounds36,37. Oral administration of D1 or D2 increased the swimming time. In contrary, D4 reduced this parameter while D3 did not show any significant effect on this parameter. An increase in swimming time by D1 or D2 administration that was accompanied by a significant reduction in immobility time is a valid measure of antidepressant-like effect in this model.
The sensitivity of FS test to acute antidepressant treatment, stimulants, sedatives, myorelaxants or motor-impairing compounds can influence animal performance in this model and lead to false-negative or false-positive results38. Hence, it is imperative to assess locomotor activity of the mice in the OF test in order to ascertain that the antidepressant-like effect of OAD was not masked by other general pharmacological activity. An open field test provides simultaneous measurement of locomotion, exploratory and anxiolytic activities39. The effects of D4 in the FS (reduction of swimming time) and OF (increase in freezing time, decrease in total crossing and number of grooming activities) could be associated to a sedative or myorelaxant effect of this derivative. In contrary, a dose dependent increase in swimming time and reduction in immobility time in the FS test by D1 without any alteration in total crossing, grooming activity and freezing time in the OF test reinforced specificity of antidepressant-like effect of D1. The increase in total crossing (an effect that could suggests stimulation) by D2 20 mg/kg (a dose that induced increase in swimming time and reduction in immobility time) makes further investigation of D1 more promising. In addition to antidepressant-like effect, an increase in crossing and time spent at the centre of OF suggests anxiolytic-like effect of D1 at the dose of 10 mg/kg. Anti-anxiety drugs like diazepam (benzodiazepine receptor agonist) or buspirone (partial agonist of 5-HT1A receptor) are known to alter these parameters40. The choice of reference drugs and their respective doses in the FS and OF tests was based on previous study7. The IMI 15 mg/kg elicited antidepressant-like effect in FS test without altering locomotor activity of mice in the OF test while the DZP 1 or 5 mg/kg induced anxiolytic and sedative like effect, respectively.
Having demonstrated antidepressant-like effect of D1 at the dose of 5 mg/kg, pharmacological tools like AMPT and PCPA (tyrosine and tryptophan hydroxylase enzyme inhibitors, respectively), PRAZ and WAY-100635 (selective antagonist of α1- adrenoceptor and 5HT1A receptors, respectively) were used to investigate possible mechanism of antidepressant-like effect of D1. The modulation of monoamine neurotransmission, transport and/or inhibition of their oxidative deamination by MAO have been associated with the mechanism of action of many clinically available antidepressant drugs41. In the present study, depletion of serotonin with PCPA or catecholamine with AMPT did not attenuate the antidepressant-like effect of D1. Unlike PRAZ, WAY-100635 pretreatment blocked the effect of D1, thereby suggesting involvement of 5HT1A receptor. Unlike previous study that showed the blockade of antidepressant-like effect of OA by PCPA, AMPT, NAN, PRAZ and WAY1006357, the effects of D1 seems to be devoid of plural mechanisms.
The inhibition and/or activation of metabolic enzymes that are involved in the synthesis or breakdown of monoamine are critical targets of some antidepressant drugs. Although selective inhibitors of MAO-B are clinically use for the treatment of neurological disorders such as Parkinson’s disease and Alzheimer’s disease, the isoenzyme MAO-A inhibitors are currently in use for the treatment of depression42. MAO is an outer mitochondrial membrane bound flavin adenine dinucleotide-linked enzyme involved in the oxidative deamination of biogenic amines and xenobiotic arylalkylamines to aldehydes43. Serotonin (5-HT) and norepinephrine are preferentially metabolized by MAO-A isoform whereas MAO-B isoform deaminates benzylamine as a substrate. Previous study showed that antidepressant-like effect of OA was independent of MAO activity and that the levels of 4-hydroxy-3-methoxyphenylglycol and 3,4-dihydroxyphenylacetic acid in the brain hippocampus and cortex remained unaltered after administration of OA7,25. Consistent with these results, D1 showed only moderate inhibitory effect on MAO-A and no effect on MAO B. Unlike D1, clorgyline and deprenyl showed significant inhibition of MAO isoenzymes. Clorgyline and (R)-deprenyl have been reported to be potent and selective inhibitors of MAO-A and -B, repectively42,44.
Although the antidepressant-like effect of D1 could be associated to the inhibition of MAO A, the preservation of antidepressant-like effect of D1 despite AMPT and PCPA pretreatments suggests that the enzymes that are involved in the synthesis of monoamines (tyrosine and tryptophan hydroxylase, respectively) are possibly not critical to the effect of D1. Hence, we hypothesized that the antidepressant-like effect of the acute oral administration of this new oleanolic acid derivative is independent of monoamine synthesis. The inhibition of MAO A by D1 could increase synaptic concentration of 5-HT, bioavailability, prolongation of its interaction with 5-HT1A receptor and subsequent potentiation of serotonergic transmission. The blockade of D1 effect by WAY-100635, which seems to be consistent with serotonergic mechanism, is not sufficient to discriminate between direct activity of D1 on the 5-HT1A receptor or indirect effect through inhibition of MAO A to facilitate high level of 5-HT.
The interaction of 5-HT with diverse serotonergic receptors has been hypothesized in the regulation of neuronal activity45. As 5-HT1A receptor seems to be involved in the effect of D1, a functional characterization of D1 at this serotonergic receptor subtype in vitro became necessary. Our findings showed similar potency and intrinsic activity of D1 and buspirone. The variations in the intrinsic activity could differentiate among full agonists, partial agonists, and antagonists, based on their high, intermediate, and zero intrinsic activity, respectively46. Unlike (+)8-OH-DPAT which is full agonist of 5-HT1A receptors, D1 could be considered as a partial agonist of this receptor on the basis of its the intrinsic activity. The 5-HT1A receptors are relevant to the clinical response to antidepressant drugs. They are located presynaptically in the raphe nuclei (where they act as cell body autoreceptors to inhibit serotonergic transmision) and postsynaptically in limbic and cortical regions to attenuate firing activity47. The azapirones are full agonists at 5-HT1A autoreceptors and are generally partial agonists at postsynaptic 5-HT1A receptors (e.g buspirone).
In addition to the promising therapeutic value of D1, the data on general pharmacological test showed that this OAD did not elicit any sign of toxicity or behavioural alterations that could constitute harm to the mice even at the highest dose of 250 mg/kg. Except for the mild sedation that was observed within 4 hours of the administration at the highest dose, there was no record of weight gain or animal’s death during the 7 days of observation. These observations demonstrate that the chemical modification of OA did not make the derivative unsafe for administration.
In conclusion, four new Michael acceptor-type oleanolic acid derivatives esterified on C-3 were synthesized and their antidepressant-like activity investigated. It is important to reiterate that the therapeutic application of OA or its derivatives is still very limited due to dearth of pharmacological data. The overall findings showed promising antidepressant-like property of D1. The mechanism of antidepressant-like effect of this compound suggested the involvement of 5HT1A receptor.
Methods
Experimental animals
Male Swiss mice (weighing between 25–30 g; 6–8 weeks old), provided by Central Animal House of the Federal University of Goiás, were used in all behavioural models. The experimental animals were kept in an intra-laboratory facility cage (32 × 18 × 16 cm) under controlled environmental conditions (23 ± 1 °C, 12 hr light-dark cycle) with access to food and water ad libitum. The experimental protocols (number 104/08) were approved by Ethical Committee of Federal University of Goiás in accordance to the international laws on the care and use of laboratory animals.
Drugs and Treatment
Oleanolic Acid (OA), kynuramine, clorgyline, deprenyl, buspirone, Tween 80 (2% polyoxyethylenesorbitan monooleate), p-chlorophenylalanine (PCPA), N-{2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl}-N-2-pyridinylcyclohexane carboxamide (WAY-100635 or WAY), Tris-HCl, ethylenediaminetetraacetic acid (EDTA), bovine serum albumin (BSA), polyethylenimine, 8-OH-DPAT, [35S]-GTPγS and dimethyl Sulfoxide (DMSO) were purchased from Sigma-Aldrich, St Louis, MO, USA. Diazepam (DZP), imipramine (IMI) and prazosin (PRAZ) were purchased from Cristália, Itapira, SP, Brazil. Recombinant Human Monoamine Oxidase-A and -B (MAO-A and -B) were purchased from BD Biosciences Bedford, MA, USA. Oleanolic acid derivatives (OAD); oleanolic acid acrylate (D1), oleanolic acid methacrylate (D2), oleanolic acid methyl fumarate (D3) and oleanolic acid ethyl fumarate (D4) were synthesized by esterification of OA with corresponding acyl chlorides. For in vivo assay, drugs were dissolved in a vehicle [a mixture of 0.9% NaCl and 5% Tween-80 (v/v)] and administered orally (p.o.) or intraperitonealy (i.p) in a volume of 0.1 mL per 10 g of mice body weight. All control animals received vehicle on the same regimen as the treated groups. For in vitro assay, drugs were dissolved in DMSO to yield a final DMSO concentration of 1.0% in the reaction mixture.
General Experimental Procedures:
All commercially available reagents were used without further purification. All these reactions were performed under Argon atmosphere and anhydrous dichloromethane was used as solvent, which was purchased from Sigma-Aldrich. The 1H NMR spectra were recorded on a Bruker Avance–400 spectrometer using CDCl3 as solvent, δ values in ppm and coupling constant J (Hz) assignments of 1H resonance coupling. Thin-layer chromatography (TLC) was performed on 250 μm layer plates Whatmann PE SIL G/UV silica gel (backing polyester) plates using n-hexane/EtOAc, 1:1 solvent system. Spots on TLC visualized with anisaldehyde / H2SO4 in methanol. Column chromatography was performed with silica gel (230 × 400 mesh) from GA, USA. Analytical HPLC was carried out on Waters 2487 with duel λ Absorbance detector system on Phenomenex Luna-C18 column (4.6 × 250 mm, 5 μm) with elution at a flow rate 1.0 mL/min. All biologically evaluated compounds were found to possess more than 95% purity by HPLC.
Synthesis of OAD
General procedure for the synthesis of OAD (D1, D2, D3 and D4): Oleanolic acid (100 mg, 0.05 mmol) and a catalytic amount of trimethylamine were dissolved in dichloromethane (15 mL). An appropriate acid chloride (0.125 mmol) was added, and the reaction mixture was stirred for 4 h at room temperature. After TLC indicated completion of the reaction, the reaction mixture was quenched with water and the organic layer separated. The organic phase was washed with dilute aqueous HCl (0.01 mol/L, 2 mL) followed by saturated NaHCO3 (2 mL). The organic layer was dried over anhydrous MgSO4, evaporated, and the residue was purified by column chromatography (SiO2; eluent: CHCl3/MeOH) to obtain the target products with 75–90% yield. The synthetic scheme for the designed compounds D1-D4 is shown in Fig. 6 with (a) reagents and conditions - appropriate acid chloride, Et3N, dry.
Figure 6.
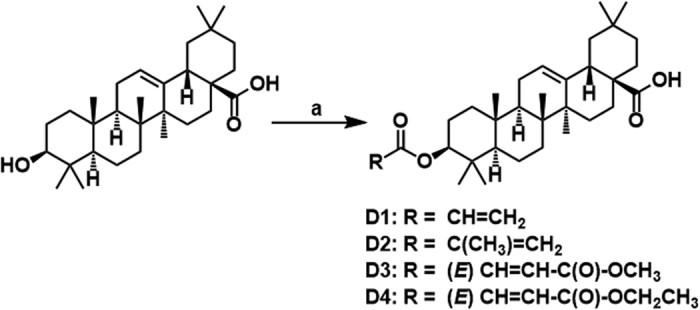
The synthetic scheme for the designed compounds D1-D4 with (a) reagents and conditions - appropriate acid chloride, Et3N, dry, DCM, N2, rt, 4 h.
FS test
In the FS model, mice were randomly divided into 5 groups [control (vehicle), 3 different doses of OAD and IMI]. Mice were exposed to a modified version of FS test48 after oral administration of vehicle, OAD (5, 10 or 20 mg/kg) or IMI 15 mg/kg. FS apparatus is a cylindrical water container (42 cm in height, 18 cm in diameter) filled with water up to 30 cm (total volume (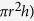 of 7635.06 cm3 at 24 ± 2 °C). In this model, the initial escape-directed behaviour like swimming and climbing by mouse are generally followed with a relatively passive and immobile posture. The container was cleaned with 10% ethanol solution prior to the exposure of naïve mouse. This test session (6-min duration) was videotaped and the swimming and immobility time was later scored and analyzed.
of 7635.06 cm3 at 24 ± 2 °C). In this model, the initial escape-directed behaviour like swimming and climbing by mouse are generally followed with a relatively passive and immobile posture. The container was cleaned with 10% ethanol solution prior to the exposure of naïve mouse. This test session (6-min duration) was videotaped and the swimming and immobility time was later scored and analyzed.
OF test
In the OF, animals were randomly divided into six groups [control (vehicle), 3 different doses of OAD, DZP 1 or 5 mg/kg]. Animals were treated with vehicle, OAD (5, 10 or 20 mg/kg, p.o), DZP (1 or 5 mg/kg, p.o) as described in our previous work7,49, and placed in a circular open field [base area (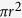 ) = 62.80 cm2 which is divided into 8 equal sectors enclosed in a 50 cm high wooden wall]. The test session (5-min duration) was videotaped in a sound-proof experimental room. Parameters like total crossing, freezing time, number of grooming and rearing activity, crossing at the centre and time spent at the centre of the open field were observed and scored.
) = 62.80 cm2 which is divided into 8 equal sectors enclosed in a 50 cm high wooden wall]. The test session (5-min duration) was videotaped in a sound-proof experimental room. Parameters like total crossing, freezing time, number of grooming and rearing activity, crossing at the centre and time spent at the centre of the open field were observed and scored.
Mechanism of antidepressant-like effect of D1
Mice were pretreated (i.p) with saline solution or AMPT 100 mg/kg (catecholamine depletor) 4 h prior to the oral administration of vehicle 10 mL/kg or D1 5 mg/kg. It was demonstrated that AMPT (tyrosine hydroxylase inhibitor) reduced 57% of dopamine and 53% of noradrenaline levels in mice without affecting the levels of serotonin50. In a separate experiment, pretreatment (i.p) of mice with saline solution or PCPA 100 mg/kg (serotonin depletor) for four consecutive days prior to oral administration of vehicle or D1 5 mg/kg was used to investigate effect of serotonin depletion on antidepressant-like activity of this OAD. The regimen of PCPA in this study has been reported to deplete about 60% of endogenous serotonin content without altering the noradrenaline or dopamine levels51,52,53. Two separate experiment were also conducted where mice were pretreated with PRAZ (α1- adrenoceptor antagonist) 1 mg/kg or WAY-100635 - WAY (a selective antagonist of 5-HT1A) 0.3 mg/kg prior to the oral administration of vehicle 10 mL/kg or D1 5 mg/kg (30 minutes interval between i.p. pretreatment and p.o. treatment). In all the four experiments, oral administration of vehicle or D1 5 mg/kg was followed by FS test (60 min interval between p.o. treatment and behavioural testing).
Inhibition assay using recombinant human MAO-A and -B
In order to investigate the effect of OAD on the activities of recombinant human MAO-A and -B, the kynuramine deamination assay was performed in 96-well plates as described earlier54. The assays were performed at a fixed concentration of the substrate (kynuramine) and varying inhibitor concentrations to determine the IC50 values. The substrate concentration of 80 and 50 μM kynuramine was chosen for MAO-A and -B, respectively, based on the previously reported apparent Km values (the substrate concentration at half Vmax) for substrate binding55. The assay was performed with the addition of inhibitor. Inhibition was calculated as percent of product formation compared to the corresponding control (enzyme-substrate reaction) without the inhibitors. The reactions were carried out in 0.1 M potassium phosphate buffer at pH 7.4. Incubation mixtures contained 5 μg/mL of MAO-A (50 μL in buffer) or 12.5 μg/mL of MAO-B (50 μL in buffer). The compounds were dissolved in DMSO. The total reaction volume was 200 μL yielding a final DMSO concentration of 1.0% in the reaction mixture. The reaction mixtures were pre-incubated for 10 min at 37 °C followed by the addition of MAO-A or MAO-B to initiate the enzymatic reactions. The assay plates were incubated for 20 min at 37 °C and enzymatic reactions were stopped by the addition of 75 μL of 2N NaOH. The formation of 4-hydroxyquinoline was determined fluorometrically by SpectraMax M5 fluorescence plate reader (Molecular Devices, Sunnyvale, CA) with an excitation and emission wavelength of 320 nm and 380 nm, respectively, using the Soft Max Pro program.
Binding of [35S]-GTPγS in mice hippocampus
The hippocampi of mice were dissected and homogenized in 5 volumes of ice-cold buffer (25 mM Tris-HCl, 0.5 mM dithiothreitol, and 0.5 mM EGTA, pH 7.4). The homogenate was centrifuged (1,000 × g for 5 min at 4 °C) and the resultant supernatant was removed. The remaining pellet was resuspended in buffer, homogenized, and centrifuged. The two supernatants obtained were combined and centrifuged at 1,000 × g for 20 min at 4 °C and the pellets were resuspended in membrane preparation buffer. The volumes were adjusted prior to the determination of protein concentration by Bradford method56 and stored at −80 °C until required. The (+)8-OH-DPAT, buspirone, WAY-100635 or D1 was incubated for 30 min at 30 °C with hippocampal membranes (approximately 10 μg protein) and 225 μl buffer (50 mM NaCl, 0.5 mM EGTA, 50 mM Tris–HCl, 0.5 mM MgCl2 and 50 μM GDP, 0.5 mM dithiothreitol, pH 7.5). The reactions were initiated by the addition of 25 μl [35S]-GTPγS, followed by a 20 min incubation at 20 °C. The reactions were terminated by rapid filtration. The filters were placed in liquid scintillation cocktail in counting vials. The liquid scintillation spectroscopy was used to measure the radioactivity bound to the filter paper after 10 hour.
General pharmacological test
A modified method that was adopted by Malone57 was used in the present study to evaluate general pharmacological alterations in mice following administration of D1. This test permits the observation of behavioural change, report of possible toxic effect or death occurrence in addition to variations in pharmacological responses to different route of drug administrations. Group of animals were subjected to s.c, i.p, or p.o route of D1 administrations at the doses of 2, 10, 50 or 250 mg/kg or vehicle 10 mL/kg and observed for 7 days.
Statistical analyses
Data were expressed as group mean ± S.E.M. A one-way analysis of variance (ANOVA) was used to detect behavioural changes elicited by drug treatment. Pairwise comparisons of individual treatment group to vehicle treated group were subsequently carried out with Dunnett´s test (post hoc test). A two-way ANOVA was used to detect the effect of treatment or pretreatment on the swimming time. This was followed up by pairwise comparisons of individual treatment group with Bonferroni test (post hoc test). The estimation of potency and efficacy were determined by nonlinear regression analysis using GraphPad Prism version 5.00 (GraphPad Software, San Diego, CA, USA). The p value of less than 0.05 was considered significant58.
Additional Information
How to cite this article: Fajemiroye, J. O. et al. Oleanolic acid acrylate elicits antidepressant-like effect mediated by 5-HT1A receptor. Sci. Rep. 5, 11582; doi: 10.1038/srep11582 (2015).
Acknowledgments
The authors thank Coordination for the Improvement of Higher Education Personnel (CAPES) for study fellowship. The MAO inhibition assays were partly supported by NIH COBRE grant CORE-NPN Core C supported by the National Institute of General Medical Sciences (NIGMS), a component of the National Institutes of Health (NIH) (Grant # P20GM104931), and USDA-ARS specific cooperative research agreement No. 58-6408-2-0009.
Footnotes
Author Contributions J.O.F. performed the in vivo assays of the experiments and wrote the manuscript; P.R.P. synthesized OAD. N.D.C. and B.L.T. complemented the in vivo assays with data from in vitro assays. J.K.Z. and E.A.C. designed the research contributed materials and revised the manuscript.
References
- Kern N., Sheldrick A. J., Schmidt F. M. & Minkwitz J. Neurobiology of depression and novel antidepressant drug targets. Curr. Pharm. Design 18, 5791–5801 (2012). [DOI] [PubMed] [Google Scholar]
- Murray C. J. & Lopez A. D. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 349, 1498–1504 (1997). [DOI] [PubMed] [Google Scholar]
- World Health Organization. Strengthening Mental Health Promotion. Geneva, World Health Organization (Fact sheet no. 220), 2001. [Google Scholar]
- Nestler E. J. et al. Neurobiology of depression. Neuron 34, 13–25 (2002). [DOI] [PubMed] [Google Scholar]
- Marks D. M., Pae C.-U. & Patkar A. A. Triple reuptake inhibitors: the next generation of antidepressants. Curr. Neuropharmacol. 6, 338–343 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaosu G. et al. Antidepressant-like effects of auraptenol in mice. Scientific Reports 4, 4433, 10.1038/srep04433 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajemiroye J. O. et al. Plurality of anxiety and depression alteration mechanism by oleanolic acid. J. Psychopharmacol. 28, 928–934 (2014). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Landa J. F. & Contreras C. M. A review of clinical and experimental observations about antidepressant actions and side effects produced by Hypericum perforatum extracts. Phytomedicine 10, 688–699 (2003). [DOI] [PubMed] [Google Scholar]
- Nowak G. Herbal medicines with anti-anxiety and antidepressant activity. Herba Polonica 55, 84–97 (2009). [Google Scholar]
- El-Alfy A. T., Abourashed E. A. & Matsumoto R. R. Nature against depression. Curr. Med. Chem. 19, 2229–2241 (2012). [DOI] [PubMed] [Google Scholar]
- Ye L. et al. Antidepressant-like effects of the extract from Cimicifuga foetida L. J. Ethnopharmacol. 144, 683–691 (2012). [DOI] [PubMed] [Google Scholar]
- Fajemiroye J. O. et al. Antidepressive like property of dichloromethane fraction of Pimenta pseudocaryophyllus and relevance of monoamine metabolic enzymes. Evid Based Complement Alternat Med 2013, 10.1155/2013/659391 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 49, 57–68 (1995). [DOI] [PubMed] [Google Scholar]
- Wang X. et al. Antioxidant activities of oleanolic acid in vitro: possible role of Nrf2 and MAP kinases. Chem. Biol. Interact. 184, 328–337 (2010). [DOI] [PubMed] [Google Scholar]
- Reisman S. A., Aleksunes L. M. & Klaassen C. D. Oleanolic acid activates Nrf2 and protects from acetaminophen hepatotoxicity via Nrf2-dependent and Nrf2-independent processes. Biochem. Pharmacol. 77, 1273–1282 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somova L. I., Shode F. O., Ramnanan P. & Nadar A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies Africana leaves. J. Ethnopharmacol. 84, 299–305 (2003). [DOI] [PubMed] [Google Scholar]
- Somova L. O., Nadar A., Rammanan P. & Shode F. O. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 10, 115–121 (2003). [DOI] [PubMed] [Google Scholar]
- Ovesná Z., Kozics K. & Slamenová D. Protective effects of ursolic acid and oleanolic acid in leukemic cells. Mutat. Res. 600, 131–137 (2006). [DOI] [PubMed] [Google Scholar]
- Sultana N. & Ata A. Oleanolic acid and related derivatives as medicinally important compounds. J. Enzyme Inhib. Med. Chem. 23, 739–756 (2008). [DOI] [PubMed] [Google Scholar]
- Dzubak P. et al. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 23, 394–411 (2006). [DOI] [PubMed] [Google Scholar]
- Laszczyk M. N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 75, 1549–1560 (2009). [DOI] [PubMed] [Google Scholar]
- Petronelli A., Pannitteri G. & Testa U. Triterpenoids as new promising anticancer drugs. Anti-Cancer Drugs 20, 880–892 (2009). [DOI] [PubMed] [Google Scholar]
- Farina C., Pinza M. & Pifferi G. Synthesis and anti-ulcer activity of new derivatives of glycyrrhetic, oleanolic and ursolic acids. Farmaco 53, 22 (1998). [DOI] [PubMed] [Google Scholar]
- Rodríguez J. A., Astudillo L. & Schmeda-Hirschmann G. Oleanolic acid promotes healing of acetic acid-induced chronic gastric lesions in rats. Pharmacol. Res. 48, 291–4 (2003). [DOI] [PubMed] [Google Scholar]
- Yi L. T. et al. Antidepressant-like effect of oleanolic acid in mice exposed to the repeated forced swimming test. J. Psychopharmacol. 27, 459–468 (2013). [DOI] [PubMed] [Google Scholar]
- Kapil A. & Sharma S. Anti-complement activity of oleanolic acid. An inhibitor of C3 convertase of the classical chemical pathway. J. Pharm. Pharmacol. 46, 922–923 (1994). [DOI] [PubMed] [Google Scholar]
- Kapil A. & Sharma S. Effect of oleanolic acid on complement in adjuvant and carrageenan induced inflammation in rats. J. Pharm. Pharmacol. 47, 585–587 (1995). [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K., Michaluart P., Sporn M. B. & Dannenberg A. J. Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res. 60, 2399 (2000). [PubMed] [Google Scholar]
- Singh G. B., Singh S., Bani S., Gupta B. D. & Banerjee S. K. Antiinflammatory activity of oleanolic acid in rats and mice. J. Pharm. Pharmacol. 44, 456–458 (1992). [DOI] [PubMed] [Google Scholar]
- Liu J. Oleanolic acid and ursolic acid: research perspectives. J. Ethnopharmacol. 100, 92–94 (2005). [DOI] [PubMed] [Google Scholar]
- Xinhui T. et al. Inhibition of ursolic acid on calcium-induced mitochondrial permeability transition and release of two proapoptotic proteins. Biochem. Biophys. Res. Commun. 337, 320 (2005). [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Honda T., Finlay H. J., Gribble G. W. & Suh N. New enone derivatives of oleanolic acid and ursolic acid as inhibitors of nitric oxide production in mouse macrophages. Bioorg. Med. Chem. Lett. 7, 1623–1628 (1997). [Google Scholar]
- Sporn M. B. et al. New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J. Nat. Prod. 74, 537–545 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Espinosa J. J. et al. Synthesis of oleanolic acid derivatives; in vitro, in vivo and in silico studies for PTP-1B inhibition. Eur. J. Med. Chem. 87, 316–327 (2014). [DOI] [PubMed] [Google Scholar]
- Newman D. J. & Cragg G. M. Natural Products as Sources of New Drugs Over the 30 Years from 1981 to 2010”. J. Nat. Prod. 75, 311–335 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Demouliere B., Chenu F. & Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berlin) 177, 245–55 (2005). [DOI] [PubMed] [Google Scholar]
- Can A. et al. The Mouse Forced Swim Test. J Vis Exp 59, 3638 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery D. A. & Cryan J. F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nature Protocols 7, 1009–1014 (2012). [DOI] [PubMed] [Google Scholar]
- Walsh R. N. & Cummins R. A. The open-field test: a critical review. Psychological Bulletin 83, 482–504 (1976). [PubMed] [Google Scholar]
- Prut L. & Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463, 3–33 (2003). [DOI] [PubMed] [Google Scholar]
- Blier P. & de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci 15, 220–226 (1994). [DOI] [PubMed] [Google Scholar]
- Wouters J. Structural aspects of monoamine oxidase and its reversible inhibition. Curr Med Chem 5, 137–62 (1998). [PubMed] [Google Scholar]
- Bolasco A., Carradori S. & Fioravanti R. Focusing on new monoamine oxidase inhibitors. Expert Opin Ther Pat 20, 909–939 (2010). [DOI] [PubMed] [Google Scholar]
- Singer T. P., Von Korff R. W. & Murphy D. L. Monoamino oxidase: structure, function and altered functions. Academic, New York, 1979. [Google Scholar]
- Andrade R. Regulation of membrane excitability in the central nervous system by serotonin receptor subtypes. Ann N Y Acad Sci 861, 190–203 (1998). [DOI] [PubMed] [Google Scholar]
- Ariëns E. J. Intrinsic activity: partial agonists and partial antagonists. J Cardiovasc Pharmacol 5, 8–15 (1983). [PubMed] [Google Scholar]
- Pierre B. & Nick M. W. Is there a role for 5-HT1A agonists in the treatment of depression? Biological Psychiatry 53, 193–203 (2003). [DOI] [PubMed] [Google Scholar]
- Porsolt R. D., Bertin A. & Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Archives Internationales de Pharmacodynamie et de Therapie 229, 327–336 (1977). [PubMed] [Google Scholar]
- Fajemiroye J. O. et al. Involvement of 5-HT1A in the anxiolytic-like effect of dichloromethane fraction of Pimenta pseudocaryophyllus. J Ethnopharmacol 3, 872–877 (2012). [DOI] [PubMed] [Google Scholar]
- Mayorga J. A. et al. Antidepressant-Like Behavioral Effects in 5-Hydroxytryptamine1A and 5-Hydroxytryptamine 1B Receptor Mutant Mice. JPET 298, 1101–1107 (2001). [PubMed] [Google Scholar]
- Redrobe J. P., Bourin M., Colombel M. C. & Baker G. B. Dose-dependent noradrenergic and serotonergic properties of venlafaxine in animal models indicative of antidepressant activity. Psychopharmacology 138, 1–8 (1998). [DOI] [PubMed] [Google Scholar]
- Redrobe J. P., Bourin M., Colombel M. C. & Baker G. B. Psychopharmacological profile of the selective serotonin reuptake inhibitor, paroxetine: implication of noradrenergic and serotonergic mechanisms. J. Psychopharmacol. 12, 348–55 (1998). [DOI] [PubMed] [Google Scholar]
- Kwon S. et al. Antidepressant-like effect of the methanolic extract from Bupleurum falcatum in the tail suspension test. Progress in Neuro-Psychopharmacology & Biological Psychiatry 34, 265–270 (2010). [DOI] [PubMed] [Google Scholar]
- Chaurasiya N. D., Ibrahim M. A., Muhammad I., Walker L. A., Tekwani B. L. Monoamine oxidase inhibitory constituents of propolis: kinetics and mechanism of inhibition of recombinant human MAO-A and MAO-B. Molecules 19, 18936–52. 10.3390/molecules191118936 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh S., Hanscom S., Gagne P., Crespi C. & Patten C. A Fluorescent-Based, High-Throughput Assay for Detecting Inhibitors of Human Monoamine Oxidase A and B; S02T081R2; BD Biosciences Discovery Labware: Woburn, MA, USA (2002). [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- Malone M. H. Pharmacological approaches to natural products screening and evaluation, in Wagner H., Wolf P. (eds), Natural products and plant drugs with pharmacological, biological or therapeutical activity. Springer-Verlag, Berlin, 23–53 (1977). [Google Scholar]
- Drummond G. B. &Tom B. D. M. Statistics, probability, significance, likelihood: words mean what we define them to mean. Br. J. Pharmacol. 164, 1573–1576 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]