Abstract
Background
To examine the relationship between reported high serum or red blood cell (RBC) folate status and adverse health outcomes.
Methods
We systematically searched PubMed/Medline and EMBASE (to May 2013), with no limits by study type, country or population, to identify studies reporting high folate concentrations in association with adverse health outcomes. Two reviewers screened studies and extracted data. Study quality was assessed.
Results
We included 51 articles, representing 46 studies and 71 847 participants. Quantiles were used by 96% of studies to identify high folate concentrations. Eighty-three percent of serum folate and 50% of RBC folate studies reported a high folate cutoff that corresponded with a clinically normal concentration. Increasing values of reported high folate concentration did not demonstrate a consistent association with risk of adverse health outcomes. Overall, reported high folate concentrations appeared to be associated with a decreased risk of adverse health outcomes, though substantial methodological heterogeneity precluded complex analyses.
Conclusions
Our interpretation was complicated by methodological variability. High folate cutoffs varied and often corresponded with normal or desirable blood concentrations. In general, a negative association appeared to exist between reported high folate status and adverse health outcomes. Consistent methods and definitions are needed to examine high folate status and ultimately inform public health interventions.
Keywords: health, high folate status, red blood cell folate, serum folate, systematic review
Background
Folate is the generic term for both naturally occurring folates in food and folic acid. Folic acid is the most oxidized, monoglutamate form that is used in dietary supplements and fortified foods.1 Consuming synthetic folic acid—from supplements and fortified food—is more effective in quickly elevating blood concentrations and tissue folate stores than intake of folate from natural sources.2,3 There is a well-established link between folate status and the occurrence of neural tube defects (NTDs).4,5 The Institute of Medicine guidelines recommend that women of childbearing age consume 0.4 mg/day of folic acid from fortified foods, supplements or both, in addition to natural food sources of folate.3
The neural tube is formed in the first 4 weeks post-conception, and since >50% of pregnancies are unplanned, the opportunity to reduce NTD risk may be limited for women who do not consume folic acid preconceptually.6–8 Thus, >50 countries have reported policies for folic acid fortification of the food supply; however, regulations may not be implemented in all countries.9–11 These policies have had a positive impact on NTD births. In Canada, for example, folic acid fortification was implemented in 1998, and there has since been a documented 46% reduction in the prevalence of NTDs.12
Increased blood folate concentrations have been documented in countries where the population is exposed to folic acid through fortification of the food supply and common usage of folic acid supplements.13,14 For folate, the Tolerable Upper Intake Level (UL)—the highest intake of a nutrient thought to pose no adverse health effects in health individuals—is set at 1000 µg/day of synthetic folic acid.3 This is based on the lowest observed adverse effect level (5000 µg/day), a dose that could potentially mask neurological symptoms of vitamin B12 deficiency and an uncertainty factor of 5, which accounts for the limited evidence and uncertainty in the process of defining a UL.3 Though many positive associations have been made between safe doses of folic acid and health outcomes,10 reports of a potential link between high synthetic folic acid intake and adverse health effects, such as an increased risk of cancer in those with pre-existing neoplasms, have resulted in significant media attention and controversy.15–17 Further, the relationship between high folate concentrations in the blood, or high folate status, with adverse outcomes is poorly understood. Folate status research is challenged by a lack of consensus on a high folate cutoff14 and a growing lack of confidence in the validity of folate measurement methods, in particular red blood cell (RBC) folate concentrations, which can vary depending on the assay method used, for example by 30–40% when measured by Bio-Rad radioassay versus microbiologic assay.18–20 The aim of this research was to conduct a systematic review to examine the relationship between reported high serum or RBC folate status and adverse health outcomes.
Methods
This systematic review followed a protocol registered with PROSPERO CRD42013004622.
Study inclusion and exclusion criteria
We sought to identify studies examining the association between reported high folate concentrations in the blood and adverse health outcomes in humans. A broad range of adverse health outcomes—various cancers, cognitive issues, cardiovascular and offspring health outcomes—were chosen for inclusion a priori by the study authors (see Supplementary data, Table S1 for details). These adverse health outcomes represent those that, according to the literature, have a potential association with blood folate concentrations or folic acid intake.17,21–27 For example, outcomes in the general population included neoplasms17 (e.g. gastrointestinal, brain, breast), cardiovascular diseases21 and cognition disorders.22 To examine adverse outcomes in offspring, we included congenital abnormalities,23–25 neoplasms26 (e.g. central nervous system, trophoblastic) and diabetes mellitus.26 We excluded studies that examined folic acid intake (as opposed to blood concentrations); did not report a specific high folate cutoff or did not refer to the concentration of folate being studied as high or elevated; were published in languages other than English; or were not primary studies.
Search strategy
We conducted a comprehensive search of the literature to identify relevant studies of high folate concentrations and adverse health outcomes. A search was performed in May 2013 using PubMed/Medline and EMBASE. The search strategy was conceptualized by C.K.C. with the assistance of an external consultant. No filters were applied to limit the retrieval by study type, publication years, country or population. The search was limited to English language and human research studies. In addition to the search of electronic databases, reference lists of relevant articles were reviewed and four key content experts were contacted and asked to identify the most influential papers examining the association between blood folate concentrations and adverse health outcomes. Previous reviews and meta-analyses were also used to identify articles. Upon completion of screening, a simplification of the MEDLINE search was validated as able to identify the included studies with a sensitivity of 0.92. The search was updated 18 August 2014 using the simplified Boolean search, limited to records created since April 2013. As well, PubMed related article searches were performed based on the three newest and three largest included studies for each of cancer, cognitive, offspring and child health and cardiovascular outcome. Search strategies for the original searches and update are presented in Supplementary data, Table S2.
Data extraction and analyses
Two reviewers screened all eligible titles and abstracts (C.K.C. and B.W.). Articles identified by either author were retrieved for full-text review. Articles were excluded when the exposure or outcome of interest was not included; folate status was measured inconsistently within the study (e.g. the participants were grouped either by radiobinding or microbiologic assay); high folate status was not stated or included in the analyses or the study did not include human subjects. Where required, folate concentration was converted to nmol/l from ng/ml using a conversion factor of 2.265. For each study, we extracted the following information: study year, country, study design, sample size, folate concentration characteristics (measurement method, fasting status, definition of high folate concentration) and effect size. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach was used to guide the review.28–34 Quality of evidence for each study was assessed for risk of bias, indirectness of evidence, and inconsistency and imprecision of results. One reviewer (C.K.C.) assessed the quality of evidence for all studies. Meta-analyses were planned for data that were sufficiently homogeneous in terms of statistical, clinical and methodological characteristics using Review Manager Software 5.0 (The Cochrane Collaboration, Copenhagen, Denmark). Otherwise, narrative syntheses were conducted for remaining studies. A priori comparisons for subgroup analyses were planned as follows: by serum or RBC folate; by population subgroup (e.g. age, sex); by adverse health outcome and by study risk of bias assessment. Following the review, it was determined that serum and RBC folate concentrations should be examined separately by reported value for high folate status within assay method.
Results
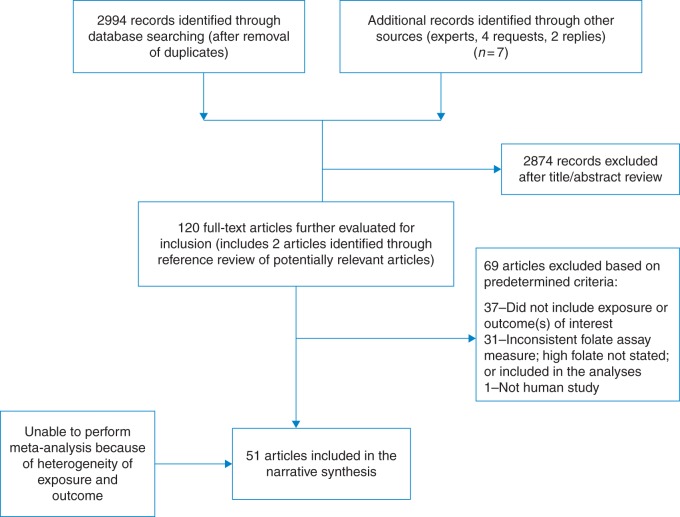
After de-duplication, the preliminary search of electronic databases, reference lists and expert contributions identified 2994 potentially relevant records (Fig. 1). After a preliminary review of titles and abstracts, 120 were selected for a full-text review. Of these, 51 articles, representing 46 studies, met the inclusion criteria (50 observational studies [22 case–control; 28 cohort] and 1 RCT). Of the 51 studies included, a total of 13 studies utilized the same cohort: 8 cancer (4 from the Alpha-Tocopherol, Beta-Carotene Cancer Prevention [ATBC] Study35–38; 2 from the Nurses Health Study39,40; and 2 from the European Prospective Investigation into Cancer and Nutrition [EPIC])41,42; 3 cardiovascular disease (Kuopio Ischaemic Heart Disease Risk Factor [KIHD])43–45; and the NHANES I Epidemiologic Follow-up Study [NEFUS] was used to examine cancer in one study and cardiovascular disease in another study.46,47 All participants were included in the determination of the final sample size. Though cases were identified as distinct health outcomes in these studies, there may be some duplication in controls that could lead to the population being overestimated.
Fig. 1.
Flowchart of study selection process.
The final studies included 71 847 participants in four broad adverse outcome categories (cancer: 24 observational studies [n = 23 002]; cognition: 1 RCT [n = 369] and 7 observational studies [n = 12 254]; offspring and child health: 9 observational studies [n = 20 063]; and cardiovascular and kidney disease: 10 observational studies [n = 16 159]). Key characteristics of these studies can be found in Supplementary data, Tables S3–S6.
The included studies reported results from 27 different countries. Seven of these studies involved population data from the USA following fortification of the food supply with folic acid. The largest study was a birth cohort involving 8742 pregnant women (the Netherlands),48 while the smallest was a cohort, also from the Netherlands (N = 77).49 The oldest study involved data collected in 1970–72 (Canada)50 and the most recent study involved data from 2010–2012 (China).51
In the included articles, 9 studies used immunoassay,48,51–58 21 used radioimmunoassay,22,35–40,43–45,59–69 11 used microbiologic assay41,42,47,50,70,71–76 and 3 used another method77–79 to study serum folate. For RBC folate, one study used immunoassay,49 four used radioimmunoassay27,64,69,80 and four used microbiologic assay.72,74,75,81 Ninety-one percent of studies—that examined serum folate—reported a high serum folate cutoff35–48,50,52–68,71–74,76–79,82,83 that was within the range of values considered clinically normal, or possibly deficient, using macrocytic anemia as a metabolic indicator (7–45 nmol/l).3 There is no established normal range for RBC folate, but 33% of the studies examining RBC folate reporting a high cutoff64,74,80 concentrations were close to 305 nmol/l, the deficiency cutoff established by the Institute of Medicine.3
Considering the bias that may be introduced due to variation in folate measurement methods, including unknown fasting status and variability in the reported cutoff for high folate concentration, we examined significant findings by creating categories for serum and RBC folate within each adverse health outcome (Table 1). We then established four broad folate assay method groups: immunoassay, radioimmunoassay, microbiologic assay and other assay methods. Regardless of folate assay method, no consistent association was observed between increasing values of reported high folate concentration cutoffs and adverse health outcomes. For reported high serum folate, one study measured by immunoassay demonstrated an increased risk of prostate cancer52 and another found an increased risk of atopic dermatitis in offspring.48 Four studies, measuring high folate cutoff by immunoassay, demonstrated a decreased risk of cancer (colorectal,53,54 breast,56 esophageal squamous cell carcinoma51). Those measured by radioimmunoassay showed a decreased risk of cancer (pancreatic,38 colorectal39); high-risk human papillomavirus,60 cardiovascular disease43–45; allergy, atopy and wheeze62; respiratory tract infections at 6 months and atopic dermatitis at 24 months in the offspring82; NTD63 in the offspring; and cognitive issues.65 Increased risk of colorectal cancer59 was observed in one study. At the highest cutoff reported (>59 nmol/l) in this group, an increased risk of cognitive impairment was observed in those with concurrently low vitamin B12 status.22 Reported high serum folate, measured by microbiologic assay, showed a persistent decrease in adverse health outcome risk at all reported high folate cutoffs for lung cancer,41 cardiovascular disease,47,50 cognitive impairment73 and cleft lip/palate in offspring,75 though one study showed an increase in cardiovascular disease risk for those older than 55 years of age.47 Other measurement methods for reported high serum folate cutoff showed a decreased risk of cognitive impairment,78 prostate cancer77 and anencephaly79 in offspring of women with the methylenetetrahydrofolate reductase (MTHFR) TT genotype, though an increased risk in stroke risk was found in one of these studies.78 There were five studies with significant results in the RBC folate category, three measured by radioimmunoassay,27,64,80 one by microbiologic assay81 and one by immunoassay.49 The highest reported RBC folate cutoff (≥1853 nmol/l), which demonstrated a significant association, was measured by microbiologic assay and was positively associated with the risk of advanced hepatocellular carcinoma.81 Another, measured by immunoassay, reported an association between high RBC folate (≥1813 nmol/l) and decreased embryonic size.49 For those measured by radioimmunoassay, one demonstrated an increase in prevalence of insulin resistance and central adiposity at a cutoff of ≥1269 nmol/l,27 while the other two reported high folate cutoffs (≥320 and ≥544 nmol/l) with a decrease in colorectal cancer80 and NTD birth in those with high homocysteine,64 respectively.
Table 1.
Risk of adverse health outcome by folate assay method and reported high folate concentration cutoff
| Citation | Assay method |
High folate definition |
Change in risk with highest folate concentration versus lowest folate concentration |
||||
|---|---|---|---|---|---|---|---|
| Health outcome | Fasted (Y/N) | Value (nmol/l)a | Rationale | Adverse health outcome | Direction | Risk (95% CI) | |
| Cancer serum folate | |||||||
| 52 | Immunoassay (Immulite analyser) | N | ≥11.0 | Lower bound of highest tertile | Prostate | ↑ | OR 5.82 (3.12 to 10.88) P = 0.0001 |
| 53 | Immunoassay (ADVIA Centaur) | Y | ≥18.1 | Stratified after grouping by gender and serum folate concentration | Colorectal | ↓ | RR 0.49 (0.32 to 0.66) in males RR 0.77 (0.50 to 1.05) in females |
| 56 | Competitive immunoassay | Not stated | >20.4 | Lower bound of highest quartile | Breast | ↓ | OR 0.23 (0.09 to 0.54) P < 0.01 |
| 54 | Immunoassay | N | ≥31.0 | Lower bound of highest quartile | Colorectal | ↓ | OR 0.52 (0.27 to 0.97) P = 0.04 |
| 51 | Enzyme-linked immunoassay (ELISA) kit | Y | >61.0 | Lower bound of highest quartile | Esophageal squamous cell carcinoma | ↓ | OR 0.11 (0.04 to 0.33) P < 0.05 |
| 38 | Bio-Rad radioassay | Y | >10.1 | Lower bound of highest tertile | Pancreas | ↓ | OR 0.45 (0.24 to 0.82) P = 0.009 |
| 59 | Radioimmunoassay | Not stated | ≥12.5 | Above the median | Colorectal | ↑ | OR 4.9 (1.4 to 17.7) P = 0.01 |
| 60 | Competitive radiobinding assay | Not stated | >13.6 | Lower bound of highest tertile | High-risk human papillomavirus | ↓ | OR 0.26 (0.08 to 0.89) P = 0.03 |
| 39 | Bio-Rad radioassay | Not stated | ≥34.2 | Median of highest quintile | Colorectal cancer-specific mortality | ↓ | 0.42 (0.20 to 0.88) P = 0.01 |
| Overall mortality | ↓ | 0.46 (0.24 to 0.88) P = 0.02 |
|||||
| 41 | Microbiologic assay | Not stated | ≥22.5 | Lower bound of highest quartile | Lung | ↓ | Lung OR 0.68 (0.51 to 0.90), P (for trend) = 0.001 |
| 77 | pABG equivalents following oxidation and mild acid | N | ≥17.5 | Lower bound of the highest quintile | Prostate | ↑ | ≥50 years of age OR 1.40 (1.07 to 1.84), P-trend = 0.02 |
| Cognition serum folate | |||||||
| 65 | Bio-Rad radioassay | Not stated | ≥23.8 | Lower bound of highest quartile | Reading | ↓ | Mean difference ± SE 3.05 ± 1.24, P < 0.05 |
| Block design | ↓ | 0.64 ± 0.28, P < 0.05 | |||||
| 67 | Bio-Rad radioassay | Not stated | >44.8 | Lower bound of highest tertile | Depression | ↓ | OR 0.52 (0.35 to 0.76), P < 0.05 |
| 22 | Bio-Rad radioassay | Not stated | >59.0 | 80th percentile of the distribution of seniors | Cognition | ↑ | Anemia High folate/low B12 OR 4.8 (2.3 to 10.4) |
| ↓ | Cognitive impairment High folate/normal B12 OR 0.5 (0.2 to 0.96) |
||||||
| ↑ | High folate/low B12 OR 4.9 (2.6 to 9.2) |
||||||
| 73 | Microbiologic assay | N | ≥30.0 | No rationale given | Cognition | Q3 as reference anemia | |
| ≥60.0 | ↓ | OR 2.02 (1.46 to 2.80) | |||||
| ≥42.1 | Lowest bound of the highest tertile | ↓ | Cognitive impairment OR 1.55 (1.28 to 1.87) |
||||
| 78 | Lab method varied since no central lab | Not stated | >14.0 | Lower bound of highest quartile | Mild cognitive impairment | ↓ | Q3 as reference OR 3.1 (1.3 to 8.1) P = 0.007 |
| Dementia | ↓ | OR 3.8 (1.3 to 11.2) P = 0.018 |
|||||
| Stroke | ↑ |
b2 = 0.22 P = 0.007 |
|||||
| Dementia | ↓ | Q1 64.6%, Q4 45.7% P < 0.0001 |
|||||
| Alzheimer's disease | ↓ | Q1 41.5%, Q4 31.3% P = 0.01 |
|||||
| Other types of dementia | ↑ | Q1 4.3%, Q4 1.5% P = 0.05 |
|||||
| Health in offspring serum folate | |||||||
| 48 | Immunoassay | N | ≥23.2 | Lower bound of highest quartile | Atopic dermatitis | ↑ | OR 1.18 (1.05 to 1.33) P < 0.05 |
| 62 | Bio-Rad radioassay | Not stated | ≥40.8 | Lower bound of highest quintile | Total IgE | ↓ | OR 0.70 (0.53 to 0.92) |
| Atopy | ↓ | OR 0.69 (0.57 to 0.85) | |||||
| Wheeze past 12 months | ↓ | OR 0.60 (0.44 to 0.82) | |||||
| 63 | Bio-Rad radioassay | Not stated | >44.4 | Lower bound of highest quartile | NTD | ↓ | OR 0.2 (0.08 to 0.62) P = 0.002 |
| 82 | SimulTRAC-SNB radioassay kit | Y | ≥21.5 | Lower bound of median value in mid-pregnancy | Respiratory tract infections at 6 months | ↓ | aOR 0.50 (0.28 to 0.91) |
| Atopic dermatitis at 24 months | ↓ | aOR 0.52 (0.31 to 0.88) | |||||
| 79 | Ionic capture using an IMx analyser | Y | >31.9 | Lower bound of highest tertile | Anencephaly | ↓ | MTHFR TT genotype OR 0.05 (0.01 to 0.37) |
| 75 | Microbiologic assay | Not stated | ≥74.1 | Lower bound of highest quartile (controls) | Isolated CL/P | ↓ | OR 0.34 (0.21 to 0.56) P < 0.001 |
| Isolated CP | ↓ | OR 0.35 (0.18 to 0.68) P = 0.002 |
|||||
| Combined CL/P + CP | ↓ | OR 0.35 (0.23 to 0.53) P < 0.001 |
|||||
| Cardiovascular serum folate | |||||||
| 43 | Bio-Rad radioassay | Not stated | ≥11.2 | Lower bound of highest tertile | All strokes | ↓ | Hazard rate ratios 0.35 (0.14 to 0.87) P = 0.046 |
| 44 | Bio-Rad radioassay | Y | >11.3 | Lower bound of highest tertile | Acute coronary events | ↓ | 0.39 (0.18 to 0.83) P = 0.016 |
| 45 | Bio-Rad radioassay | Y | >11.3 | Lower bound of highest tertile | Acute coronary events | ↓ | Q3 as reference (versus Q1/Q2) RR 0.03 (0.10 to 0.84) P = 0.023 |
| 68 | Radioimmunoassay | Y | >16.8 | Lower bound of highest tertile | Retinal vein occlusion | ↓ | Q3 as reference (versus Q1) OR 5.41 (3.08 to 9.51) |
| 50 | Microbiologic assay | Not stated | >13.6 | Lower bound of highest quartile | Fatal coronary heart disease | ↓ | Q4 as reference Rate ratio 1.69 (1.10 to 2.61) |
| 47 | Microbiologic assay | Not stated | ≥21.8 | Lower bound of highest quartile | Acute coronary events | Q4 as reference Age adjusted |
|
| ↓ | 35–55 years of age RR 2.4 (1.1 to 5.2) |
||||||
| ↑ | ≥55 years of age RR 0.5 (0.3 to 0.8) |
||||||
| Cancer RBC folate | |||||||
| 80 | Bio-Rad radioassay | Not stated | ≥320 | Lower bound of highest tertile | Colorectal | ↓ | Q3 as reference OR 3.05 (1.34 to 6.96) P-trend = 0.006 |
| 81 | Microbiologic assay (Lactobacillus casei) | Y | ≥1853 | Lower bound of upper tertile | Advanced HCC stages (III and IV) | ↑ | Q3 HR 2.05 (1.11 to 3.78) |
| Health in offspring RBC folate | |||||||
| 49 | Electrochemiluminescence immunoassay | N | ≥1813 | Lower bound of upper quartile | Crown-rump length length | ↑ | Q3 as reference −0.29 (−0.49 to −0.09) |
| Embryonic size | ↑ | Q4 versus Q3, 1.1 mm (23.5%) and 4.5 mm (7.4%) smaller at 6 and 12 weeks gestations, respectively | |||||
| 64 | Bio-Rad radioassay | Not stated | ≥544 | Lowest bound of second quintile | NTD | ↓ | Low Hcy/high folate as reference High Hcy/High folate OR 2.9 (1.1 to 7.4) |
| 27 | Radioassay | Y | ≥1269 | Lowest bound of highest quartile | HOMA-IR | ↑ | Median (intraquartile range) Q1: 0.52 (0.28 to 0.81) Q2: 0.65 (0.36 to 0.92) Q3: 0.71 (0.39 to 1.12) Q4: 0.85 (0.51 to 1.27), P-trend < 0.001 |
| Fat mass | ↑ | Mean (SD) Q1: 3.0 (1.0) Q2: 3.1 (0.9) Q3: 3.2 (1.2) Q4: 3.4 (1.1) |
|||||
pABG, p-aminobenzoylglutamate; OR, odds ratio; RR, relative risk; CI, confidence interval; SD, standard deviation; HOMA-IR, homeostasis model assessment insulin resistance; MTHFR, methylenetetrahydrofolate reductase; IgE, immunoglobulin E; Hcy, homocysteine; NTD, neural tube defect; CP, cleft palate; CL/CP, cleft lip/cleft palate; HCC, hepatocellular carcinoma.
aFolate concentration converted to nmol/l from ng/ml (2.265×) when needed.
Meta-analyses and subgroup analyses were not possible for any outcomes due to substantial heterogeneity in the measurement methods for serum or RBC folate; population under study; definition of high folate status; adverse health outcome examined; and study quality (Table 2). For example, when reporting on the relationship between high folate concentration and colorectal cancer, one study may report the relationship with a folate concentration defined as the upper quartile, whereas another may examine folate concentrations above the mean. Further, some would report serum folate measured by Bio-Rad radioassay while another would report serum folate measured by microbiologic assay. In addition, some studies reported on data for males or females only, while others reported only overall estimates. As a result, we were unable to determine a summary measure for a high folate concentration cutoff to determine common point estimates and associated measures of error for the studies included in this review.
Table 2.
Association between reported high folate concentrations and adverse health outcomes: quality assessment
| Outcome |
Quality assessment |
No. of participantsa | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | |||
| Cancer | 24 | Observational studyb | Somewhat serious risk of biasc | Some inconsistency evidentd,e | No serious indirectness | No serious imprecisionf | None | 23 002 (7601 cases/9769 controls and 5632 cohort) |
Low |
| Adverse cognitive outcome | 1 | RCT | Somewhat serious risk of biasc–g | Not applicable | No serious indirectness | No serious imprecisionh | None | 369 | Moderate |
| Adverse cognitive outcome | 7 | Observational studyi | Somewhat serious risk of biasc | Some inconsistency evidentc | No serious indirectness | No serious imprecision | None | 12 254 | Low |
| Adverse offspring outcome | 9 | Observational studyj | Somewhat serious risk of biasc | Some inconsistency evidentc | No serious indirectness | No serious imprecisionh,k | None | 20 063 (655 cases/974 controls and 18 472 cohort) |
Low |
| Adverse cardiovascular outcome | 10 | Observational studyl | Somewhat serious risk of biasc | Some inconsistency evidentc | No serious indirectness | No serious imprecision | None | 16 159 (393 cases/451 controls and 15 315 cohort) |
Very low |
Quality of evidence assessed using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach.
aNine studies demonstrated duplication in use of cohorts (four cancer (Alpha-Tocopherol, Beta-Carotene Cancer Prevention [ATBC] Study),35–38 three cardiovascular disease (Kuopio Ischaemic Heart Disease Risk Factor [KIHD]),43–45 and the NHANES I Epidemiologic Follow-up Study [NEFUS] was used to examine cancer in one study and cardiovascular disease in another study.46,47 All participants were included in the final sample size calculation; therefore, the population may be overestimated.
bIncludes 16 case–control35–38,40–42,51,52,54–56,59,61,71,77 and 8 cohort39,46,53,60,70,72,80,81 studies.
cMeasurement (assay method) of RBC folate variable,27,64,69,72,74,75,80,81 no indication of fasting status for serum folate measurement22,39–41,43,46,49,50,55,56,59,60,62–65,67,72,76,78,83 and variable definition of reported high folate concentration.
eOne study showed positive association between study definition of high folate concentrations and colorectal tumor59 or hepatocellular carcinoma.81
gBlinding not clearly described.74
Discussion
Main findings of this study
This review indicates that, overall, reported high folate concentrations appear to be related to a decreased risk of adverse health outcomes without consistent association observed with increasing concentrations. However, high-quality evidence describing the association between reported high folate concentrations and adverse health outcomes is scarce. The majority of reported high folate cutoffs could be considered indicative of a clinically normal folate status and not necessarily representative of elevated values. Further, variability was observed in several factors, including participant fasting status; country fortification policies; folate assay methodology; and the cutoff selected to define high folate status. As a result, the evidence was insufficient to infer any solid conclusions. Consistent methods are needed to compare the results of health research related to high folate status.
What is already known on this topic
The health outcomes observed in this review of folate status were similar to those that have been identified in the folic acid intake literature. For example, folic acid intake has been associated with many positive health outcomes, including a decreased risk of NTD and other congenital anomalies, including cardiovascular defects, oral cleft, urinary tract abnormalities, congenital hydrocephalus and limb defects.23–26 Safe doses of folic acid, as defined by the dietary reference intakes, have also been implicated in lower risk of colorectal cancer—and other cancers such as breast, lung and prostate—in the general population.3,10 A meta-analyses involving 26 RCTs (n = 58 804) did not support an association between folic acid supplementation and decreased risk of cardiovascular disease, but a potential link was made to a decreasing trend in stroke risk.21 Though the many benefits of folic acid intake have been studied rigorously, the safety of higher doses of folic acid has been questioned and there is on-going debate about the association with negative health outcomes.10,84–89 For example, folic acid may mask vitamin B12 deficiency when consumed at unsafe levels (≥5 mg/day).85 This is of particular concern in the elderly population; however, it has been suggested that current recommended intakes of folic acid pose a minimal threat of masking or exacerbating neuropathies.10 The influence of folic acid consumed during pregnancy on DNA methylation has been implicated in epigenetic changes that could alter the way genes are expressed—without changing the DNA sequence—potentially leading to adverse outcomes in the offspring, including childhood wheeze or asthma.87,88 However, a 2012 cohort study did not support an association between folic acid supplementation in early pregnancy and the development of asthma in offspring at 6 years of age.89 Conflicting evidence has also emerged following evidence of a possible association between folic acid intake and increased risk of cancer in individuals with pre-existing neoplasms,86 though several subsequent systematic reviews and meta-analyses did not demonstrate a relationship between folic acid supplement intake and most cancers.15–17 For example, a 2013 meta-analysis included individual participant datasets from 13 trials (n = 49 969) that compared folic acid supplementation (in amounts ranging from 0.5 to 5 mg/day, with one trial using 40 mg/day) with placebo, had a duration of at least 1 year, included at least 500 participants and recorded data on cancer incidence.17 In the folic acid-supplemented participants, no short-term effects on overall or site-specific cancer incidence were demonstrated.
What this study adds
Though several of the adverse health outcomes that have been associated with folic acid intake were also identified in this systematic review of folate concentrations, there were few that supported an association with increased risk at high folate concentration cutoffs. However, since the majority of reported high folate concentration cutoffs are considered to be normal values, it is difficult to determine the clinical relevance of these findings. Overall, the studies captured in this review demonstrated mixed results. The majority of the included studies used quantiles of the study population—referring to the upper quantile(s) as high—to examine folate concentrations categorically in the absence of a consensus definition of high folate status. For example, based on the captured studies, it appears that reported high folate status is negatively associated with colorectal cancer prevalence.35,39,53,54,59,80 However, there were marked inconsistencies among the colorectal cancer studies captured in our review, including variable or unreported fasting status; different folate measurement method (an immunoassay or a radioimmunoassay); and cutoffs for the reported high serum folate status that ranged from 11.8 to 34.2 nmol/l based on different quantiles (e.g. above the mean, upper tertile, upper quartile or upper quintile) and notably in a normal serum folate range. Thus, any overarching conclusion is limited by these inconsistencies. This methodological heterogeneity complicated any comparisons and precluded conclusions from being drawn regarding the relationship between folate status and adverse health outcomes.
A consistent cutoff for high folate status—defined in association with a clinical outcome—was not observed in this review. Several papers have proposed folate concentration cutoffs that do not represent upper quantiles, but these do not report adverse health outcomes thus were not captured by our search strategy.14,90,91 For example, Fazili et al.90 assigned a cutoff of 45 nmol/l to define high serum folate status as this value represented the upper end of the calibration curve, as well as an observed increase in folic acid concentrations at greater than ∼50 nmol/l total folate. Another example is our examination of the Canadian Health Measures Survey data, in which the cutoff was determined based on the 97th percentile of Bio-Rad 1999–2004 NHANES, as recent national Canadian data were unavailable.14
Further methodological variability was observed in folate measurement. The majority of studies in our review used serum folate status, which is considered a sensitive indicator of folic acid intake. This was difficult to interpret since fasting status was often not reported. RBCs build folate stores during erythropoeisis that are retained through the 120-day life span of the RBC; thus, RBC folate concentrations are a common measure of long-term folate status.92 Few studies in our review reported RBC folate status cutoffs. Further, significant interassay differences exist in folate measurement, which can impair the ability to define high folate concentrations and compare serum and RBC folate measurement methods.18–20 These differences exist due to multiple complications; for example, reduced folates are unstable at extreme pH values and when exposed to elevated temperatures, oxygen or light. Fazili et al.18 conducted a methods comparison study analyzing whole blood hemolysates, from an American and a European blood bank, to examine differences in total folate concentrations measured by liquid chromatography–tandem mass spectrometry (LC/MS/MS), microbiologic assay (using Lactobacillus Rhamnosus) and Bio-Rad radioassay. The microbiologic assay was comparable to the LC/MS/MS within ±10%, but the values were 45% lower for Bio-Rad assay in whole blood samples. Similarly, considerably lower results (29%) were observed with Bio-Rad assay, in comparison to LC/MS/MS, for serum folate, potentially due to under recovery of certain folate forms. It is notable that polymorphisms in the enzyme MTHFR C677T can impair folate metabolism and decrease circulating folate by 10–25% in those with the homozygous TT variant. This could impact laboratory measures of RBC folate.18,93 However, MTHFR genotype was not accounted for consistently in the studies included in our review, therefore was not considered in the present examination. Standardization of folate measurement methodology would allow for high folate status to be more reliably defined and, subsequently, for studies to be more readily compared.
Limitations of this study
Strengths of this review included a comprehensive search strategy; and a priori inclusion and exclusion criteria and analyses. We included both serum and RBC folate concentrations, and a range of health outcomes that have previously been associated with high folate supplement intake. The review was large in scope and included numerous health outcomes and measurement methods. The quality of the captured studies was a limitation of this review. The majority of studies included in this review were observational studies; thus, causation cannot be inferred and results should be interpreted with caution. A detailed meta-analysis would have allowed us to estimate the overall effect sizes for each outcome. However, due to the heterogeneity of the data, it was impossible to complete such an analysis. Many studies grouped their variables into quantiles, and although it was still possible to ascertain information regarding the association between folate concentrations and health outcomes, it made it impossible to compare the information across studies. We did not attempt to determine a grouping by ‘high folate concentration’ since cutoffs were arbitrary and had the potential to lead to false conclusions.
Conclusions
The scientific value of this review is the demonstrated variance in how researchers are studying and defining high folate status, which affects the ability to interpret associations with adverse health outcomes. Further, these variable definitions can be misleading as high folate is often indicative of a clinically normal concentration; thus, if higher values had been selected as being ‘high’, different conclusions could be drawn. Our results demonstrate that there is no consistent relationship between increasing folate concentrations and the adverse health outcomes we examined. This review provides evidence that standard methods are needed to examine the intersection of folate status and adverse health outcomes. Further, using a consistent, evidence-based cutoff to define high folate status may enhance the ability to examine associations with adverse health outcomes and compare study results. This would provide clinicians with direction for assessing patient folate status and ultimately inform public health interventions and clinical guidelines for folic acid.
Authors' contributions
C.K.C., M.S.T. and D.L.O. conceptualized and designed the search strategy with input from M.S. M.S. reviewed and oversaw the implementation of the search strategy. C.K.C. and B.W. reviewed the data and conducted all aspects of data capture. All authors assisted in the interpretation of the results. C.K.C. drafted the manuscript, and all authors provided critical review and feedback. All authors read and approved the final manuscript.
Supplementary data
Funding
This study was funded by a Canadian Institutes of Health Research Operating Grant (funding reference #218776).
Supplementary Material
Acknowledgements
The authors thank Jessie Cunningham for her assistance in designing and implementing the search strategy.
References
- 1.McNulty H, Pentieva K. Folate bioavailability. In: Bailey L. (ed). Folate in Health and Disease, 2nd edn Boca Raton, FL: Taylor & Francis Group, L.L.C., 2010. [Google Scholar]
- 2.Goh YI, Koren G. Folic acid in pregnancy and fetal outcomes. J Obstet Gynaecol 2008;28(1):3–13. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine. DRI Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press, 1998. [PubMed] [Google Scholar]
- 4.MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 1991;338(8760):131–7. [PubMed] [Google Scholar]
- 5.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327(26):1832–5. [DOI] [PubMed] [Google Scholar]
- 6.Koren G, Goh I. Increasing folate supplementation for selected groups of Canadian women. J Obstet Gynaecol Can 2007;29(12):992–6. [DOI] [PubMed] [Google Scholar]
- 7.Kallen B. Congenital malformations in infants whose mothers reported the use of folic acid in early pregnancy in Sweden. A prospective population study. Congenit Anom (Kyoto) 2007;47(4):119–24. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Oz B, Koren G, Nguyen P et al. Folate fortification and supplementation–are we there yet? Reprod Toxicol 2008;25(4):408–12. [DOI] [PubMed] [Google Scholar]
- 9.Global progress mandatory wheat flour fortification legislation - September 2014. http://www.ffinetwork.org/global_progress/index.php (13 October 2014, date last accessed).
- 10.Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011;3(3):370–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly F, Gibney ER, Boilson A et al. Folic acid levels in some food staples in Ireland are on the decline: implications for passive folic acid intakes? J Public Health (Oxf) 2016;382:265–9. [DOI] [PubMed] [Google Scholar]
- 12.De Wals P, Tairou F, Van Allen MI et al. Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med 2007;357(2):135–42. [DOI] [PubMed] [Google Scholar]
- 13.Pfeiffer CM, Johnson CL, Jain RB et al. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988 2004. Am J Clin Nutr 2007;86(3):718–27. [DOI] [PubMed] [Google Scholar]
- 14.Colapinto CK, O'Connor DL, Tremblay MS. Folate status of the population in the Canadian Health Measures Survey. CMAJ 2011;183(2):E100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fife J, Raniga S, Hider PN et al. Folic acid supplementation and colorectal cancer risk: a meta-analysis. Colorectal Dis 2011;13(2):132–7. [DOI] [PubMed] [Google Scholar]
- 16.Baggott JE, Oster RA, Tamura T. Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol 2012;36(1):78–81. [DOI] [PubMed] [Google Scholar]
- 17.Vollset SE, Clarke R, Lewington S et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet 2013;381(9871):1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fazili Z, Pfeiffer CM, Zhang M et al. Influence of 5,10-methylenetetrahydrofolate reductase polymorphism on whole-blood folate concentrations measured by LC-MS/MS, microbiologic assay, and bio-rad radioassay. Clin Chem 2008;54(1):197–201. [DOI] [PubMed] [Google Scholar]
- 19.Gunter EW, Bowman BA, Caudill SP et al. Results of an international round robin for serum and whole-blood folate. Clin Chem 1996;42(10):1689–94. [PubMed] [Google Scholar]
- 20.Owen WE, Roberts WL. Comparison of five automated serum and whole blood folate assays. Am J Clin Pathol 2003;120(1):121–6. [DOI] [PubMed] [Google Scholar]
- 21.Yang HT, Lee M, Hong KS et al. Efficacy of folic acid supplementation in cardiovascular disease prevention: an updated meta-analysis of randomized controlled trials. Eur J Intern Med 2012;23(8):745–54. [DOI] [PubMed] [Google Scholar]
- 22.Morris MS, Jacques PF, Rosenberg IH et al. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr 2007;85(1):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardiner HM, Fouron JC. Folic acid fortification and congenital heart disease. BMJ 2009;338:b1144. [DOI] [PubMed] [Google Scholar]
- 24.Czeizel AE. Periconceptional folic acid and multivitamin supplementation for the prevention of neural tube defects and other congenital abnormalities. Birth Defects Res A Clin Mol Teratol 2009;85(4):260–8. [DOI] [PubMed] [Google Scholar]
- 25.Godwin KA, Sibbald B, Bedard T et al. Changes in frequencies of select congenital anomalies since the onset of folic acid fortification in a Canadian birth defect registry. Can J Public Health 2008;99(4):271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goh YI, Bollano E, Einarson TR et al. Prenatal multivitamin supplementation and rates of congenital anomalies: a meta-analysis. J Obstet Gynaecol Can 2006;28(8):680–9. [DOI] [PubMed] [Google Scholar]
- 27.Yajnik CS, Deshpande SS, Jackson AA et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the pune maternal nutrition study. Diabetologia 2008;51(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyatt GH, Oxman AD, Vist G et al. GRADE guidelines: 4. rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 2011;64(4):407–15. [DOI] [PubMed] [Google Scholar]
- 29.Guyatt GH, Oxman AD, Montori V et al. GRADE guidelines: 5. rating the quality of evidence—publication bias. J Clin Epidemiol 2011;64(12):1277–82. [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Oxman AD, Kunz R et al. GRADE guidelines 6. rating the quality of evidence—imprecision. J Clin Epidemiol 2011;64(12):1283–93. [DOI] [PubMed] [Google Scholar]
- 31.Guyatt GH, Oxman AD, Kunz R et al. GRADE guidelines: 7. rating the quality of evidence—inconsistency. J Clin Epidemiol 2011;64(12):1294–302. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt GH, Oxman AD, Kunz R et al. GRADE guidelines: 8. rating the quality of evidence—indirectness. J Clin Epidemiol 2011;64(12):1303–10. [DOI] [PubMed] [Google Scholar]
- 33.Guyatt GH, Oxman AD, Sultan S et al. GRADE guidelines: 9. rating up the quality of evidence. J Clin Epidemiol 2011;64(12):1311–6. [DOI] [PubMed] [Google Scholar]
- 34.Balshem H, Helfand M, Schunemann HJ et al. GRADE guidelines: 3. rating the quality of evidence. J Clin Epidemiol 2011;64(4):401–6. [DOI] [PubMed] [Google Scholar]
- 35.Glynn SA, Albanes D, Pietinen P et al. Colorectal cancer and folate status: a nested case-control study among male smokers. Cancer Epidemiol Biomarkers Prev 1996;5(7):487–94. [PubMed] [Google Scholar]
- 36.Hartman TJ, Woodson K, Stolzenberg-Solomon R et al. Association of the B-vitamins pyridoxal 5′-phosphate (B(6)), B(12), and folate with lung cancer risk in older men. Am J Epidemiol 2001;153(7):688–94. [DOI] [PubMed] [Google Scholar]
- 37.Gibson TM, Weinstein SJ, Mayne ST et al. A prospective study of one-carbon metabolism biomarkers and risk of renal cell carcinoma. Cancer Causes Control 2010;21(7):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stolzenberg-Solomon RZ, Albanes D, Nieto FJ et al. Pancreatic cancer risk and nutrition-related methyl-group availability indicators in male smokers. J Natl Cancer Inst 1999;91(6):535–41. [DOI] [PubMed] [Google Scholar]
- 39.Wolpin BM, Wei EK, Ng K et al. Prediagnostic plasma folate and the risk of death in patients with colorectal cancer. J Clin Oncol 2008;26(19):3222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang SM, Willett WC, Selhub J et al. Plasma folate, vitamin B6, vitamin B12, homocysteine, and risk of breast cancer. J Natl Cancer Inst 2003;95(5):373–80. [DOI] [PubMed] [Google Scholar]
- 41.Johansson M, Relton C, Ueland PM et al. Serum B vitamin levels and risk of lung cancer. JAMA 2010;303(23):2377–85. [DOI] [PubMed] [Google Scholar]
- 42.Fanidi A, Relton C, Ueland PM et al. A prospective study of one-carbon metabolism biomarkers and cancer of the head and neck and esophagus. Int J Cancer 2014;1364:915–27. [DOI] [PubMed] [Google Scholar]
- 43.Virtanen JK, Voutilainen S, Happonen P et al. Serum homocysteine, folate and risk of stroke: Kuopio ischaemic heart disease risk factor (KIHD) study. Eur J Cardiovasc Prev Rehabil 2005;12(4):369–75. [DOI] [PubMed] [Google Scholar]
- 44.Voutilainen S, Virtanen JK, Rissanen TH et al. Serum folate and homocysteine and the incidence of acute coronary events: the Kuopio ischaemic heart disease risk factor study. Am J Clin Nutr 2004;80(2):317–23. [DOI] [PubMed] [Google Scholar]
- 45.Voutilainen S, Lakka TA, Porkkala-Sarataho E et al. Low serum folate concentrations are associated with an excess incidence of acute coronary events: the Kuopio ischaemic heart disease risk factor study. Eur J Clin Nutr 2000;54(5):424–8. [DOI] [PubMed] [Google Scholar]
- 46.Ford ES, Byers TE, Giles WH. Serum folate and chronic disease risk: findings from a cohort of United States adults. Int J Epidemiol 1998;27(4):592–8. [DOI] [PubMed] [Google Scholar]
- 47.Giles WH, Kittner SJ, Croft JB et al. Serum folate and risk for coronary heart disease: results from a cohort of US adults. Ann Epidemiol 1998;8(8):490–6. [DOI] [PubMed] [Google Scholar]
- 48.Kiefte-de Jong JC, Timmermans S, Jaddoe VW et al. High circulating folate and vitamin B-12 concentrations in women during pregnancy are associated with increased prevalence of atopic dermatitis in their offspring. J Nutr 2012;142(4):731–8. [DOI] [PubMed] [Google Scholar]
- 49.van Uitert EM, van Ginkel S, Willemsen SP et al. An optimal periconception maternal folate status for embryonic size: the rotterdam predict study. BJOG 2014;121(7):821–9. [DOI] [PubMed] [Google Scholar]
- 50.Morrison HI, Schaubel D, Desmeules M et al. Serum folate and risk of fatal coronary heart disease. JAMA 1996;275(24):1893–6. [DOI] [PubMed] [Google Scholar]
- 51.Huang GL, Wang SK, Su M et al. Serum folate, MTHFR C677T polymorphism and esophageal squamous cell carcinoma risk. Biomed Environ Sci 2013;26(12):1008–12. [DOI] [PubMed] [Google Scholar]
- 52.Jackson MD, Tulloch-Reid MK, McFarlane-Anderson N et al. Complex interaction between serum folate levels and genetic polymorphisms in folate pathway genes: biomarkers of prostate cancer aggressiveness. Genes Nutr 2013;8(2):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujimori S, Gudis K, Takahashi Y et al. Determination of the minimal essential serum folate concentration for reduced risk of colorectal adenoma. Clin Nutr 2011;30(5):653–8. [DOI] [PubMed] [Google Scholar]
- 54.Kato I, Dnistrian AM, Schwartz M et al. Serum folate, homocysteine and colorectal cancer risk in women: a nested case-control study. Br J Cancer 1999;79(11–12):1917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ito Y, Wakai K, Suzuki K et al. Serum carotenoids and mortality from lung cancer: a case-control study nested in the Japan collaborative cohort (JACC) study. Cancer Sci 2003;94(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beilby J, Ingram D, Hahnel R et al. Reduced breast cancer risk with increasing serum folate in a case-control study of the C677T genotype of the methylenetetrahydrofolate reductase gene. Eur J Cancer 2004;40(8):1250–4. [DOI] [PubMed] [Google Scholar]
- 57.Weng LC, Yeh WT, Bai CH et al. Is ischemic stroke risk related to folate status or other nutrients correlated with folate intake? Stroke 2008;39(12):3152–8. [DOI] [PubMed] [Google Scholar]
- 58.Hishida A, Okada R, Guang Y et al. MTHFR, MTR and MTRR polymorphisms and risk of chronic kidney disease in Japanese: cross-sectional data from the J-MICC study. Int Urol Nephrol 2013;45(6):1613–20. [DOI] [PubMed] [Google Scholar]
- 59.Mokarram P, Naghibalhossaini F, Saberi Firoozi M et al. Methylenetetrahydrofolate reductase C677T genotype affects promoter methylation of tumor-specific genes in sporadic colorectal cancer through an interaction with folate/vitamin B12 status. World J Gastroenterol 2008;14(23):3662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piyathilake CJ, Badiga S, Paul P et al. Indian women with higher serum concentrations of folate and vitamin B12 are significantly less likely to be infected with carcinogenic or high-risk (HR) types of human papillomaviruses (HPVs). Int J Womens Health 2010;2:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Potischman N, Brinton LA, Laiming VA et al. A case-control study of serum folate levels and invasive cervical cancer. Cancer Res 1991;51(18):4785–9. [PubMed] [Google Scholar]
- 62.Matsui EC, Matsui W. Higher serum folate levels are associated with a lower risk of atopy and wheeze. J Allergy Clin Immunol 2009;123(6):1253–9. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cech I, Burau KD. Serological differences in folate/vitamin B12 in pregnancies affected by neural tube defects. South Med J 2010;103(5):419–24. [DOI] [PubMed] [Google Scholar]
- 64.Felkner M, Suarez L, Canfield MA et al. Maternal serum homocysteine and risk for neural tube defects in a Texas-Mexico border population. Birth Defects Res A Clin Mol Teratol 2009;85(6):574–81. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen CT, Gracely EJ, Lee BK. Serum folate but not vitamin B-12 concentrations are positively associated with cognitive test scores in children aged 6–16 years. J Nutr 2013;143(4):500–4. [DOI] [PubMed] [Google Scholar]
- 66.Quadri P, Fragiacomo C, Pezzati R et al. Homocysteine and B vitamins in mild cognitive impairment and dementia. Clin Chem Lab Med 2005;43(10):1096–100. [DOI] [PubMed] [Google Scholar]
- 67.Beydoun MA, Shroff MR, Beydoun HA et al. Serum folate, vitamin B-12, and homocysteine and their association with depressive symptoms among U.S. adults. Psychosom Med 2010;72(9):862–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sofi F, Marcucci R, Bolli P et al. Low vitamin B6 and folic acid levels are associated with retinal vein occlusion independently of homocysteine levels. Atherosclerosis 2008;198(1):223–7. [DOI] [PubMed] [Google Scholar]
- 69.Siri PW, Verhoef P, Kok FJ. Vitamins B6, B12, and folate: association with plasma total homocysteine and risk of coronary atherosclerosis. J Am Coll Nutr 1998;17(5):435–41. [DOI] [PubMed] [Google Scholar]
- 70.Tomaszewski JJ, Cummings JL, Parwani AV et al. Increased cancer cell proliferation in prostate cancer patients with high levels of serum folate. Prostate 2011;71(12):1287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gylling B, Van Guelpen B, Schneede J et al. Low folate levels are associated with reduced risk of colorectal cancer in a population with low folate status. Cancer Epidemiol Biomarkers Prev 2014;23(10):2136–44. [DOI] [PubMed] [Google Scholar]
- 72.Welzel TM, Katki HA, Sakoda LC et al. Blood folate levels and risk of liver damage and hepatocellular carcinoma in a prospective high-risk cohort. Cancer Epidemiol Biomarkers Prev 2007;16(6):1279–82. [DOI] [PubMed] [Google Scholar]
- 73.Clarke R, Sherliker P, Hin H et al. Folate and vitamin B12 status in relation to cognitive impairment and anaemia in the setting of voluntary fortification in the UK. Br J Nutr 2008;100(5):1054–9. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen PH, Grajeda R, Melgar P et al. Micronutrient supplementation may reduce symptoms of depression in guatemalan women. Arch Latinoam Nutr 2009;59(3):278–86. [PubMed] [Google Scholar]
- 75.Munger RG, Tamura T, Johnston KE et al. Oral clefts and maternal biomarkers of folate-dependent one-carbon metabolism in Utah. Birth Defects Res A Clin Mol Teratol 2011;91(3):153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loria CM, Ingram DD, Feldman JJ et al. Serum folate and cardiovascular disease mortality among US men and women. Arch Intern Med 2000;160(21):3258–62. [DOI] [PubMed] [Google Scholar]
- 77.de Vogel S, Meyer K, Fredriksen A et al. Serum folate and vitamin B12 concentrations in relation to prostate cancer risk—a Norwegian population-based nested case-control study of 3000 cases and 3000 controls within the JANUS cohort. Int J Epidemiol 2013;42(1):201–10. [DOI] [PubMed] [Google Scholar]
- 78.Ebly EM, Schaefer JP, Campbell NR et al. Folate status, vascular disease and cognition in elderly Canadians. Age Ageing 1998;27(4):485–91. [DOI] [PubMed] [Google Scholar]
- 79.Lacasana M, Blanco-Munoz J, Borja-Aburto VH et al. Effect on risk of anencephaly of gene-nutrient interactions between methylenetetrahydrofolate reductase C677T polymorphism and maternal folate, vitamin B12 and homocysteine profile. Public Health Nutr 2012;15(8):1419–28. [DOI] [PubMed] [Google Scholar]
- 80.Ulvik A, Evensen ET, Lien EA et al. Smoking, folate and methylenetetrahydrofolate reductase status as interactive determinants of adenomatous and hyperplastic polyps of colorectum. Am J Med Genet 2001;101(3):246–54. [DOI] [PubMed] [Google Scholar]
- 81.Kuo CS, Huang CY, Kuo HT et al. Interrelationships among genetic C677T polymorphism of 5,10-methylenetetrahydrofolate reductase, biochemical folate status, and lymphocytic p53 oxidative damage in association with tumor malignancy and survivals of patients with hepatocellular carcinoma. Mol Nutr Food Res 2014;58(2):329–42. [DOI] [PubMed] [Google Scholar]
- 82.Kim JH, Jeong KS, Ha EH et al. Relationship between prenatal and postnatal exposures to folate and risks of allergic and respiratory diseases in early childhood. Pediatr Pulmonol 2014;502:155–63. [DOI] [PubMed] [Google Scholar]
- 83.Hooshmand B, Solomon A, Kareholt I et al. Associations between serum homocysteine, holotranscobalamin, folate and cognition in the elderly: a longitudinal study. J Intern Med 2012;271(2):204–12. [DOI] [PubMed] [Google Scholar]
- 84.Refsum H. Folate, vitamin B12 and homocysteine in relation to birth defects and pregnancy outcome. Br J Nutr 2001;85(Suppl. 2):S109–13. [PubMed] [Google Scholar]
- 85.Cuskelly GJ, Mooney KM, Young IS. Folate and vitamin B12: friendly or enemy nutrients for the elderly. Proc Nutr Soc 2007;66(4):548–58. [DOI] [PubMed] [Google Scholar]
- 86.Cole BF, Baron JA, Sandler RS et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 2007;297(21):2351–9. [DOI] [PubMed] [Google Scholar]
- 87.Haberg SE, London SJ, Stigum H et al. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child 2009;94(3):180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whitrow MJ, Moore VM, Rumbold AR et al. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol 2009;170(12):1486–93. [DOI] [PubMed] [Google Scholar]
- 89.Martinussen MP, Risnes KR, Jacobsen GW et al. Folic acid supplementation in early pregnancy and asthma in children aged 6 years. Am J Obstet Gynecol 2012;206(1):72.e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fazili Z, Pfeiffer CM, Zhang M. Comparison of serum folate species analyzed by LC-MS/MS with total folate measured by microbiologic assay and bio-rad radioassay. Clin Chem 2007;53(4):781–4. [DOI] [PubMed] [Google Scholar]
- 91.MacFarlane AJ, Greene-Finestone LS, Shi Y. Vitamin B-12 and homocysteine status in a folate-replete population: results from the Canadian Health Measures Survey. Am J Clin Nutr 2011;94(4):1079–87. [DOI] [PubMed] [Google Scholar]
- 92.Shane B. Folate status assessment history: implications for measurement of biomarkers in NHANES. Am J Clin Nutr 2011;94(1):337S–42S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Christensen KE, Rozen R. Genetic variation: effect on folate metabolism and health. In: Bailey L. (ed). Folate in Health and Disease, 2nd edn Boca Raton, FL: Taylor & Francis Group, L.L.C., 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.