Abstract
The aim of the present study was to investigate alterations in gene expression of opioid system components induced by extended access (18 h) cocaine self-administration and to determine the impact of genetic background in the vulnerability to escalate cocaine intake. Comparing two inbred rat strains, we previously reported that Lewis rats progressively escalated cocaine consumption compared to Fischer rats, in a new translational model of intravenous cocaine self-administration, which included 14 sessions of 18-h operant sessions in which rats were allowed to select the cocaine unit dose to self-administer. We compare here Fischer and Lewis rats in the gene expression of endogenous opioid peptides (Pomc, Penk, Pdyn) and cognate receptors (Oprm, Oprk and Oprd) in reward-related brain regions, after exposure to either cocaine self-administration or yoked-saline, in the aforementioned translational paradigm. We performed a correlation analysis between the mRNA level, found in the Dorsal Striatum (DS), Nucleus accumbens (NAcc) shell and core respectively, and individual cocaine intake.
Our findings show that the gene expression of all the aforementioned opioid genes exhibit strain-dependent differences in the DS, in absence of cocaine exposure. Also, different strain-specific cocaine-induced mRNA expression of Oprm and Oprk was found in DS. Only few differences were found in the ventral parts of the striatum. Moreover, gene expression level of Pdyn, Penk, Oprk, and Oprm in the DS was significantly correlated with cocaine intake only in Fischer rats. Overall, these data shed light on potential genetic differences which may predispose of subjects to initiate and escalate cocaine consumption.
1. Introduction
Addiction to drugs of abuse is a chronic brain disease with behavioral manifestation such as drug seeking and taking, with intermittent phases of withdrawal and relapse (Kreek and Koob, 1998). Addiction to cocaine and other psychostimulants is a major public health issue because it causes medical, psychological and social problems including crime and violence, and at present there are no approved pharmacotherapeutic approaches for its management (World Health Organization Report 2012). Cocaine addiction is often associated with comorbidity with anxiety, depression and/or dependence to other drugs of abuse (Fernandez-Calderon et al., 2015). Drug dependence is a multifactorial brain disease with genetic, epigenetic, environmental and drug-induced components (Butelman et al., 2012; Nestler, 2014). Therefore, one current and critical goal of scientific research is to determine which factors make individuals more vulnerable to develop addiction. One approach is to identify genetic traits that predict increased sensitivity to the effects of the drug of abuse. In this regard, human studies present obvious ethical and practical limitations; conversely, preclinical animal models allow for experimental manipulations and analysis of neurobiological and genetic factors which may predict the onset of specific addictive diseases. Inbred strains of rodents are particularly helpful, since their homogenous genotype allow the identification of genetic factors involved in behavioral phenotypes (Crabbe and Belknap, 1992; Valenza et al., 2015). Among these, Fischer and Lewis rats have often been studied in comparison for their different behavioral profile in response to drugs of abuse and other phenotypes (Kosten and Ambrosio, 2002). Fischer rats are generally considered “addiction-resistant”, while Lewis rats are thought to have “addiction-prone” phenotype (Meyer and Bardo, 2015). Thus, Lewis rats acquire cocaine, morphine, methamphetamine, nicotine and heroin self-administration or conditioned place preference more rapidly and/or at higher rates/doses compared with Fischer rats (Ambrosio et al., 1995; Cadoni et al., 2015; Kosten et al., 1994; Kosten et al., 1997; Kruzich and Xi, 2006a, b; Nylander et al., 1995; Picetti et al., 2012; Picetti et al., 2010). Fischer and Lewis rats differ also in their stress response (Dhabhar et al., 1993; Ergang et al., 2015) and impulsivity score (Hamilton et al., 2014; Madden et al., 2008), two features related to vulnerability to addiction. Overall, comparisons between Fischer and Lewis rats in cocaine self-administration have been carried out primarily in relatively short access self-administration paradigm (≤ 6h), rather than extended access (Freeman et al., 2009; Miguens et al., 2015; Rivera et al., 2013). Our laboratory has recently reported that Lewis rats escalate both cocaine and heroin intake to a greater extent than Fischer rats, in a new model of extended access (18h/day for 14 sessions) intravenous self-administration, in which rats are allowed to select the unit dose of drug to self-administer (Picetti et al., 2012; Picetti et al., 2010). This model was developed to approach more closely the human natural history of cocaine exposure, with respect to daily availability and self-selection of unit doses, thus exploring endogenous mechanisms that may lead to escalation of cocaine exposure.
Endogenous opioid systems have major roles in mediating the direct and downstream properties of drugs of abuse, including cocaine. For example, polymorphisms in genes encoding opioid receptors and ligands have been associated with drug addiction in humans (Bond et al., 1998; LaForge et al., 2000; Levran et al., 2014). Cocaine acts primarily by increasing dopamine overflow, by inhibition of its reuptake. Endogenous opioid systems modulate dopaminergic circuitry; the activation of mu opioid receptor (MOP-r) triggers the release of dopamine, whereas the kappa opioid receptor (KOP-r) activation by the endogenous dynorphins has the opposite effect, inhibiting dopamine release (Di Chiara and Imperato, 1988b; Spanagel et al., 1990, 1992; Zhang et al., 2004). Moreover, chronic cocaine and withdrawal from its administration profoundly affects the expression endogenous opioid system components (Bailey et al., 2005; Kreek, 1996; Spangler et al., 1996; Zhang et al., 2013).
The present study investigated the hypothesis that differential gene expression of opioid peptides and receptors contribute to the vulnerability to escalate cocaine intake in Lewis rats, compared to the non-escalating Fischer rats. Therefore, we compared Fischer and Lewis rats in the gene expression of endogenous opioid peptides [proopiomelanocortin (Pomc), prodynorphin (Pdyn), proenkephalin (Penk)], and cognate receptors [mu opioid receptor (Oprm), kappa opioid receptor (Oprk), and delta opioid receptor (Oprd)] 24 h in withdrawal from exposure to either extended access cocaine self-administration or yoked-saline, in the aforementioned translational paradigm. We studied brain reward-related regions such as the nucleus accumbens core and shell and the dorsal striatum, terminal areas of the major dopaminergic pathways, postulated to be prominently involved in addictive-like properties of cocaine, either in initial exposure or during escalation.
Here we studied gene expression 24 h after the last self-administration session, in order to explore mRNA expression in early withdrawal to examine the role of opioid peptides and receptors in the escalation of drug consumption over time. Cocaine levels and those of its metabolites have half-lives <2 h in rats, therefore cocaine levels were likely to have considerably diminished by the 24 h time point (Allain et al., 2015; Booze et al., 1997; Mets et al., 1999).
In order to study the relationship between gene expression and drug intake we then performed a correlation analysis between the mRNA level, found in the Dorsal Striatum (DS), Nucleus accumbens (NAcc) shell and core respectively, and individual rat cocaine intake.
2. Materials and Methods
2.1. Subjects
Adult male Fischer and Lewis rats purchased from Charles River (Wilmington, MA) were single housed upon arrival in a 12-h reverse light/dark cycle in an AAALAC-approved humidity-(60%) and temperature-(22°C) controlled vivarium. Rats had ad libitum access to food and water at all times.
The animal data reported herein are a subset of those included in Picetti et al., 2010 (n=11 of 19 Fischer rats, and n=10 of 23 Lewis rats). The subjects constitute a cohort of rats intended a priori for the present dual behavioral and gene expression analysis, and were not selected based on any specific behavioral characteristics.
All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Principles of Laboratory Animal Care and were approved by the Institutional Animal Care and Use Committee of The Rockefeller University.
2.2. Surgery and intravenous self-administration procedure
Surgery and cocaine self-administration procedures were described in detail in Picetti et al. (2010). Briefly, rats were implanted with an indwelling jugular catheter and, upon recovery, were trained in the self-administration procedure (FR1, 2h/day). Once responses were stable, rats were run in the extended access procedure (18 h/day) with subject-controlled unit dose selection. Briefly, rats were allowed to press three levers (two active and one inactive). Pressing the two active levers (FR1) resulted in delivery of a low and high dose of cocaine, respectively (0.2 mg/kg and 0.5 mg/kg). Once rat lever presses were ≥ 70 % on the lever associated with the high dose for two consecutive sessions, the low dose (0.2 mg/kg) was discontinued, and rats had access to the 0.5 mg/kg (new low dose) and 1.25 mg/kg (new high dose) of cocaine. Following the same criterion, when rats pressed ≥ 70 % on the lever associated with the high dose for two consecutive sessions, the low dose 0.5 mg/kg was discontinued, and rats had access to 1.25 mg/kg (new low dose) and 2.5 mg/kg (new high dose) of cocaine. Responding for cocaine was associated with a light cue and a dose-specific tone cue. Rats were typically run 5 days/week and had a 2-day interval, except for one time in which the interval was 5 days.
As context-control group, saline-yoked rats (n=10/strain) underwent the same procedure as cocaine rats, but received isotonic saline (0.9%) in place of cocaine (see Picetti et al, 2010 for full description). Both cocaine and saline-yoked rats were placed in the self-administration chambers with ad libitum food and water, starting 3 h after the onset of the dark cycle. The light/dark cycle in the self-administration chambers was synchronized with the one in the housing room; therefore rats were exposed to 12-h reverse light/dark cycle. At conclusion of the 18-h operant session, rats were returned to the home cage for 6 h.
2.3. Analysis of opioid system gene expression
2.3.1. Dissection procedure, RNA extraction and cDNA synthesis
Procedures were carried out as previously published with minor modifications (Valenza et al., 2015). Twenty-four hours after the end of the last self-administration session, rats were decapitated and the brain was rapidly extracted, sliced on an ice-cold brain matrix, and under an optical microscope, the regions of interest were dissected following a rat brain atlas (Paxinos and Watson 1986). Tissue collected was immediately frozen in dry ice and stored at −70 °C.
Brain region samples were homogenized in Qiazol solution (Qiagen, Valencia, CA, USA) and the total mRNA extraction was performed using the miRNeasy Kit (Qiagen) according to the manufacturer’s protocol. Following RNA isolation, all samples were treated with DNase (Turbo DNA-free™, Ambion [ABI], Austin, TX) to purify samples from possible DNA contamination. The quantity and quality of RNA in each extract was determined with the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Since qRT-PCR performance is affected by the RNA integrity (Bustin, 2002; Tricarico et al., 2002), an accepted careful practices and good quality control for RNA quality assessment is the RNA Integrity Number (RIN, developed by Agilent Technologies) for the lab-on-chip capillary gel-electrophoresis used in the Bioanalyzer 2100. Fleige and Pfaffl (2006) recommend a RNA Integrity Number (RIN)>5 as “good quality” total RNA, and RIN> 8 as “perfect” total RNA for qPCR application. Our samples had RIN ≥8 in all three regions under investigation. Single-stranded cDNA was synthesized using the same amount of RNA for each sample of a given brain region (approximately 1µg), using the QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA), which includes a genomic DNA removal step as previously published (Dore et al., 2014). All samples from a given brain region were reverse transcribed at the same time. Samples were then stored at −70 °C until further processing.
2.3.2. Quantitative Real-time Polymerase Chain Reaction
Quantification of the mRNA level of six opioid genes encoding respectively for proopiomelanocortin (Pomc), prodynorphin (Pdyn), proenkephalin (Penk), mu opioid receptor (Oprm), kappa opioid receptor (Oprk), and delta opioid receptor (Oprd) was performed by quantitative real-time polymerase chain reaction (qRT-PCR).
cDNA amplification and analysis were performed as previously published (Valenza et al., 2015; Yuferov et al., 2013). cDNA (1 µl) was amplified in a 10-µl solution that contained the Fast SYBRGreen Master Mix (Qiagen) and 1 uM of primers. The list of primers used to amplify target and housekeeping genes can be found in the Supplemental Table 1, in the online Supplemental File. Gene sequences were amplified using a two-temperature protocol, which included an initial step at 5 min at 95 °C to activate the polymerase, followed by 40 denaturation cycles at 94 °C for 10 sec, annealing and extension at 60 °C for 1 min. Experimental samples were amplified simultaneously with a standard curve constructed using serial 10-fold dilutions of purified fragments (QIAquick PCR purification kit, Qiagen) of the target gene. The concentration of the purified cDNA was estimated by OD260 using a Nanodrop spectrophotometer ND-1000, and the number of copies/mL of standard were calculated according to the following formula (Rose’Meyer et al., 2003):
where C = 5 × 10−5 g/ml cDNA; the Molecular Weight = base pairs × 6.58 × 102 g (Yin et al., 2001). A master concentration (1 × 109 copies/mL) of standard cDNA for each gene to analyze was aliquoted and stored at - 20 °C. Serial dilutions were made fresh in sterile water right before the experiment.
Standards and samples from each brain region were run in duplicate concurrently in the same plate, in the ABI Prism 7900HT Sequence Detection System (Applied Biosystem, Carlsbad, CA, USA). The copy number of cDNA of target and housekeeping genes was determined by interpolating the threshold cycles (Ct) of an experimental sample to those in standard curves for the corresponding gene using the SDS 2.3 software (Applied Biosystem).
The gene-specific amplification was determined by melting curve analysis as one peak at the expected melting temperature and by agarose gel electrophoresis of the expected size of the PCR products.
Results were analyzed as ratio between copy number of the target gene and the mean copy number of two housekeeping genes, beta-2-microglobulin (B2m) and hypoxanthine phosphoribosyltransferase (Hprt). B2m and Hprt were selected among a set of housekeeping genes assayed for tissue normalization (B2m, Gapdh, Hprt, Trfr, 18S) since they were both unaffected by either Strain or Treatment (Bustin et al., 2010). This is considered a careful and conservative approach (Bustin et al., 2009). Therefore it is less likely that a different amount of tissue between the three regions is a factor affecting the results. Normalized gene expression levels, measured as the ratio between the target gene copy number and the housekeeping genes’ mean copy number, in the Dorsal Striatum and the Nucleus Accumbens core and shell of both yoked-saline Fischer and yoked-saline Lewis rats are shown in Supplemental Table 2 in the online Supplemental File.
2.4. Statistical analysis
To verify that the behavioral profile of the cohort of Fischer and Lewis rats (n=10–11/strain) designated a priori for the gene expression analysis was representative of the whole population used in Picetti et al. (2010) (n=19–23/strain), their behavioral data were separately reanalyzed using a two-way mixed ANOVA, with Strain as a between-subjects factor, and Time (session 1–14) as the within-subject factor. Separate repeated measure one-way ANOVA on data from each Strain were performed and, upon detection of a main effect, post-hoc analyses were run using the Dunnet’s test. The Student t test was used to compare two groups, and the Student Newman-Keuls (SNK) test was used for all the other post-hoc comparisons, where appropriate.
The results from the gene expression analysis were examined with a factorial two-way ANOVA with Strain and Treatment as between-subjects factors. When a statistically significant overall effect and/or interaction were observed, data were further analyzed for post-hoc comparisons with the SNK test.
Separate Pearson’s correlations were performed on each data set, using the normalized gene expression level (ratio between copy number of the target gene and the mean copy number of two housekeeping genes) versus the amount of cocaine self-administered during the last operant session (mg/kg). Additional Pearson’s correlations performed on each data set using the total cocaine intake during the 14 sessions of self-administration, as well as the escalation index are shown in the Supplemental Tables 3, 4 and 5 in the online Supplemental File. A p value <0.05 was considered statistically significant.
The Statistical software used were Statistica 7.0 (StatSoft. Inc., Tulsa, OK, USA) and GraphPad Prism 6 (GraphPad, San Diego, CA, USA). The graphical software used was SigmaPlot 12.5 (Systat Software, Inc.).
3. Results
3.1. Different pattern of cocaine self-administration between Fischer and Lewis rats
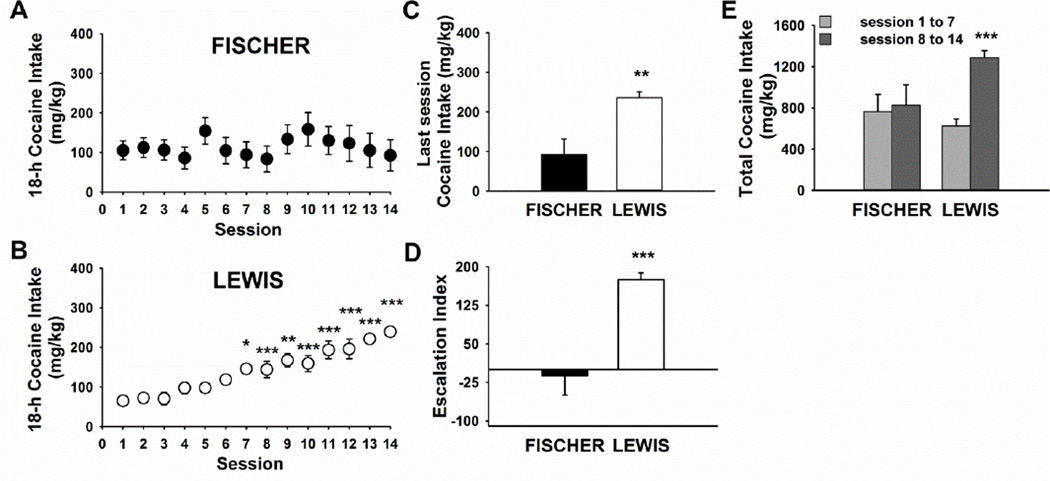
As shown in Figure 1, Fischer and Lewis rats differed in their pattern of cocaine intake during extended access intravenous cocaine self-administration. Two-way mixed ANOVA on daily intake during 14 sessions of 18-h operant sessions showed a statistically significant main effect of Session [F(13,260)=5.2, p<0.0001] and a significant interaction Strain*Session [F(13,260)=4.8, p<0.0001]. Separate repeated measure one-way ANOVA on data from each Strain revealed the effect of Session was present only in Lewis [F(14,56)=24.7, p<0.0001, Figure 1B], but not in Fischer rats [F(13,130)=0.9, NS, Figure 1A], demonstrating that only Lewis rats escalated their cocaine intake progressively over the course of the study. Indeed, the analysis of the intake on the last self-administration session showed a statistically significant difference between the two strains (t(19)=3.35, p≤0.003, Figure 1C).
Figure 1.
Cocaine intake in Fischer (A) and Lewis (B) rats during 14 (18-h) self-administration sessions. Graphs shows the Mean±SEM of cocaine intake (n= 10–11/group) * p< 0.05, ** p< 0.01, *** p< 0.001 versus session 1 (Dunnet’s post-hoc test). Panel C shows the Mean±SEM of cocaine intake during the last self-administration session before sacrifice. ** p< 0.01 versus Fischer rats (Student’s t test). Panel D shows the Mean±SEM of the escalation index, calculated as the difference in cocaine intake between the last and first self-administration session. *** p< 0.001 versus Fischer rats (Student’s t test). Panel E shows the Mean±SEM of cumulative cocaine intake. *** p< 0.001 versus Lewis rats sessions 1 to 7 (SNK post-hoc test).
The cumulative 14-session intake did not differ between strains (t(19)=1.05, NS, not shown), likely due to the greater intake seen in Fischer rats over the initial days (see also below). Overall, the behavioral profile of this subset of Fischer and Lewis rats used for gene expression (n=10/strain) was highly consistent with the whole population (n=19–23/strain) published in Picetti et al., 2010.
A more detailed analysis of the cocaine intake was then performed for the first time herein. The “escalation index” was calculated as the “Δintake” (e.g. the difference in cocaine intake between the last and first session). The escalation index was statistically different between the two strains (t(19)=4.56, p≤0.0002, Figure 1D). Two-way ANOVA on cumulative intake during the two weeks (i.e., sessions 1–7 vs. 8–14) of self-administration showed a significant interaction of Strain*Week (F[1,20]=20.0, p<0.0001). A post-hoc comparison revealed Lewis rats had a significantly higher intake in the second week of access compared to the first week, while Fischer rats were equal over the two weeks (Figure 1E).
Moreover, observations of rats’ selection of unit dose during the overall self-administration period showed that Fischer rats selected preferentially the 0.5 mg/kg unit dose, by contrast Lewis rats preferred to self-administer the 1.25 mg/kg unit doses of cocaine (data not shown).
3.2. Expression of opioid peptide and receptor genes in the dorsal striatum
Integrity of mRNA samples
The Mean±SD RNA Integrity Number (RIN) values of RNA samples in the DS were 8.3± 0.3, confirming the purity of the samples analyzed.
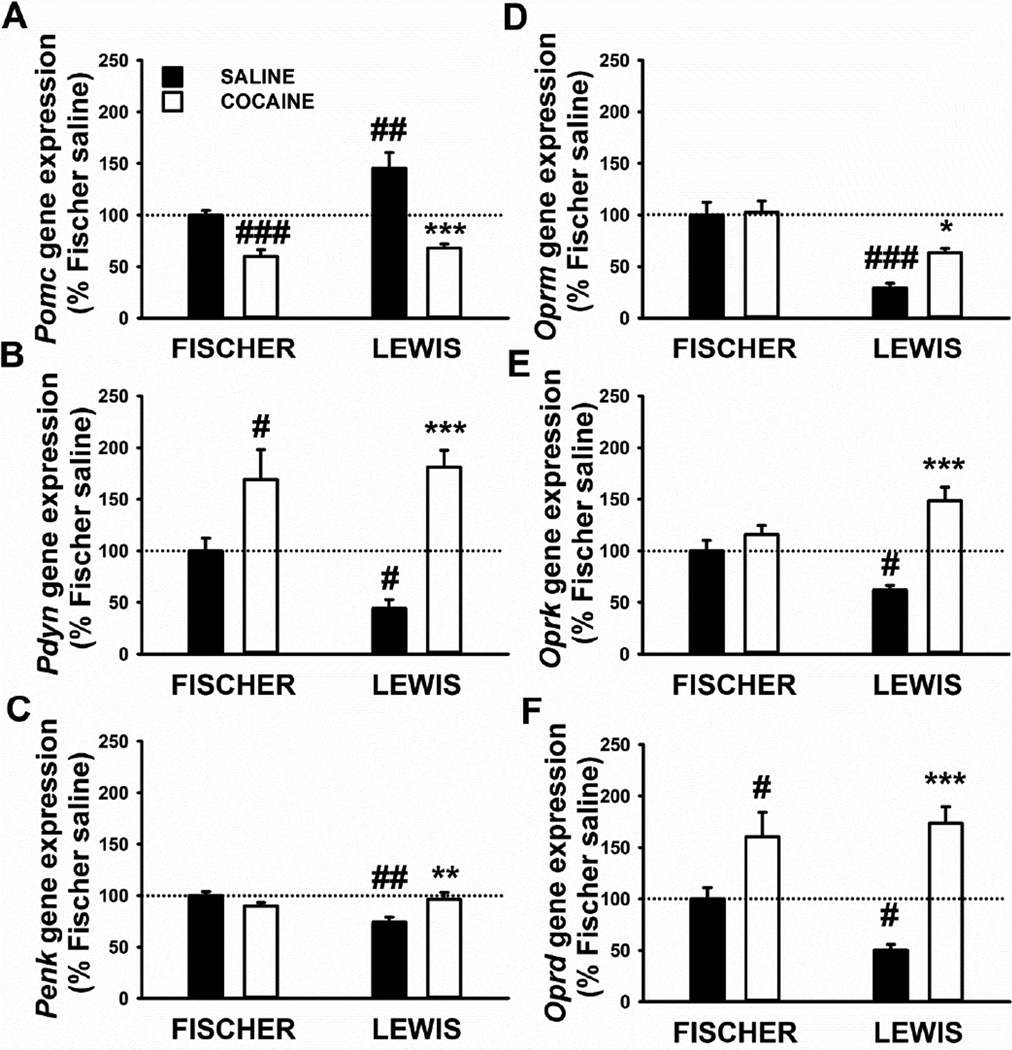
As shown in Figure 2, the gene expression analysis in the dorsal striatum of Fischer and Lewis rats revealed clear differences between the two strains, in the absence of cocaine exposure (i.e., in yoked-saline subjects) in all the gene targets studied. Moreover gene expression alterations due to cocaine exposure were detected too.
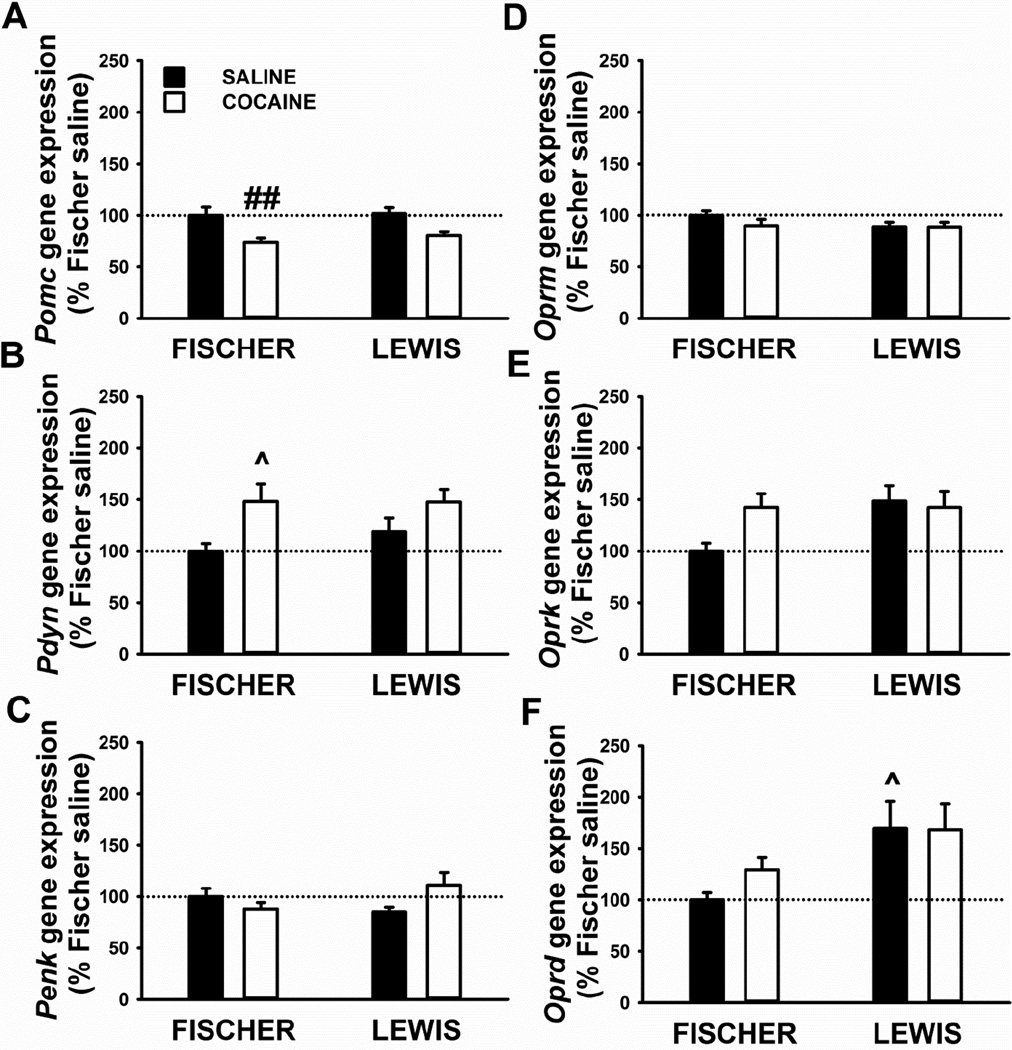
Figure 2.
Gene expression of three opioids peptides, proopiomelanocortin (Pomc, A), prodynorphin (Pdyn, B), proenkephalin (Penk, C), and three opioid receptors, mu (Oprm, D), kappa (Oprk, E), and delta (Oprd, F), in the dorsal striatum of Fischer and Lewis rats sacrificed 24 h after the last 18-h operant session (cocaine). Control rats underwent same self-administration procedure, but with saline in place of cocaine (yoked-saline). Panels represent Mean±SEM expressed as the percent of Fischer yoked-saline. # p<0.05, ## p<0.01, ### p<0.001 versus Fischer yoked-saline. * p<0.05, ** p<0.01, *** p<0.001 versus Lewis yoked-saline (SNK post-hoc test).
Analysis of Pomc data: factorial two-way ANOVA showed a main effect of Strain [F(1,33)=8.3, p=0.007], Treatment [F(1,33)=40.0, p<0.0001] and a strong trend in the interaction [F(1,33)=4.1, p=0.052]. Post-hoc analysis showed Pomc mRNA to be lower in rats exposed to extended cocaine self-administration in both strains, and the percent reduction compared to respective yoked-saline control was −40 % in Fischer, and −46 % in Lewis rats. As shown in Figure 2A, the basal Pomc level was significantly greater in Lewis yoked-saline than in Fischer yoked-saline rats.
Analysis of Pdyn data: factorial two-way ANOVA showed a main effect of Treatment [F(1,35)=30.3, p<0.0001] and a trend in the interaction Strain*Treatment [F(1,35)=3.2, p=0.08]. Pdyn expression was higher in rats that had cocaine self-administration exposure than yoked-saline rats, irrespective of Strain [F(1,35)=1.4, NS, Figure 2B]. However, the percent increase was +69 % in Fischer and +309 % in Lewis rats, compared to the respective yoked-saline groups. Lewis yoked-saline rats showed lower (<50 %) Pdyn mRNA expression than Fischer yoked-saline rats.
Analysis of Penk data: factorial two-way ANOVA showed a trend of Strain [F(1,34)=4.0, p=0.055] and a main effect in the interaction Strain*Treatment [F(1,34)=11.0, p=0.002]. As shown in Figure 2C, Penk mRNA expression was higher (+30 %) selectively in Lewis rats exposed to cocaine compared to yoked-saline. At basal level, Lewis had significantly lower Penk mRNA levels, versus Fischer rats.
Analysis of Oprm data: factorial two-way ANOVA showed a main effect of Strain [F(1,35)=34.4, p<0.0001] and a trend in the Treatment [F(1,35)=3.8, p=0.058], but without an interaction between the two [F(1,35)=2.8, p=0.1]. As shown in Figure 2D, Oprm gene expression was not different in Fischer saline versus cocaine-exposed rats. However, the basal Oprm level was greater (<70 %) in Fischer yoked-saline than in Lewis yoked-saline rats, and this difference was statistically significant. In Lewis we saw higher Oprm in cocaine-exposed compared to saline-yoked rats.
Analysis of Oprk data: factorial two-way ANOVA showed a main effect Treatment [F(1,35)=25.9, p<0.0001] and in the interaction Strain*Treatment [F(1,35)=12.4, p=0.001]. As shown in Figure 2E, Post-hoc analysis showed Oprk mRNA expression was significantly higher in Lewis rats that had cocaine self-administration exposure, compared to yoked-saline rats, difference not seen in Fischer rats. Lewis yoked-saline rats showed significantly lower Oprk mRNA expression (<50 %) than Fischer yoked-saline rats.
Analysis of Oprd data: factorial two-way ANOVA showed a main effect of Treatment [F(1,33)=32.9, p<0.0001] and a strong trend in the interaction Strain*Treatment [F(1,33)=3.81, p=0.06]. As shown in Figure 2F, Oprd gene expression was affected by cocaine self-administration exposure in both strains. However, the percent of increase relative to yoked-saline subjects was much higher in Lewis (+245 %) than in Fischer rats (+52 %), considering also that at yoked-saline condition level, Lewis had lower Oprd mRNA level than Fischer rats.
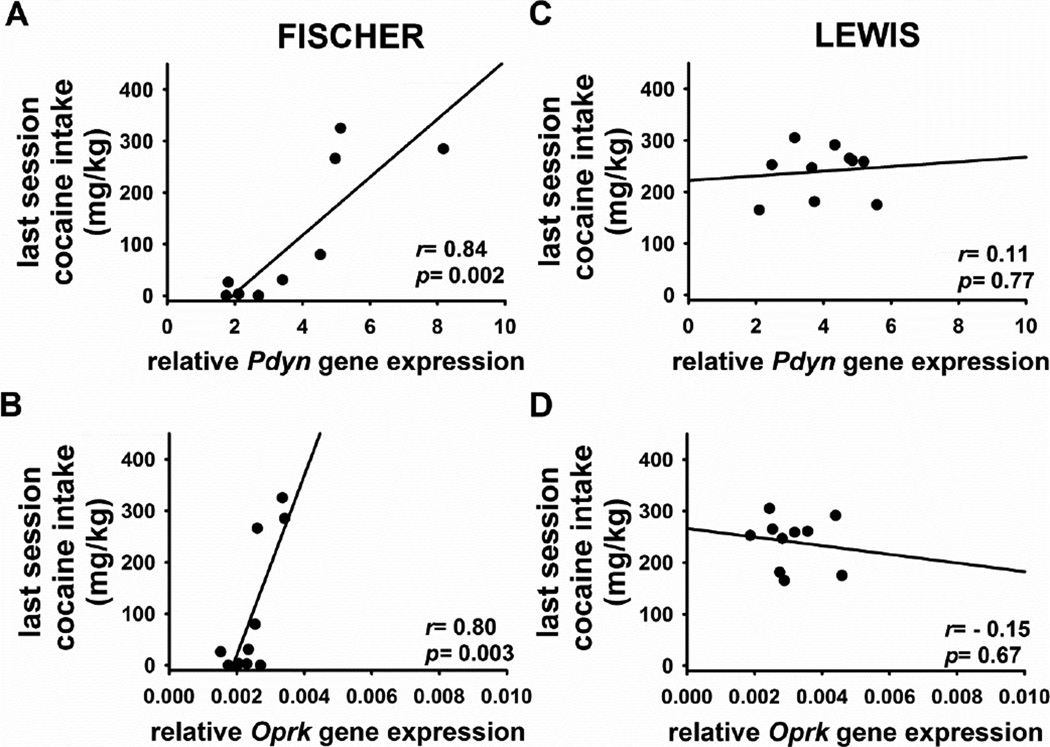
3.3. Correlation between expression of opioid peptide and receptor genes in the DS and individual rat cocaine intake
Separate Pearson correlation analyses were performed between the mRNA level of a target gene, and the intake (mg/kg) of cocaine self-administered during the last 18-h intravenous self-administration session. The amount of cocaine consumed during the last self-administration session was significantly and positively correlated with the level of gene expression of Pdyn (r=0.84, p=0.002; Figure 3A), Oprk (r=0.80, p=0.003; Figure 3B), Penk (r=0.79, p=0.007), and Oprm (r=0.68, p=0.02) in Fischer rats; Lewis rats did not show any significant correlations in Pdyn (r=0.11, NS; Figure 3C), Oprk (r=−0.15, NS; Figure 3D), Penk (r=−0.60 NS), and Oprm (r=0.20, NS) data. In either strains, no significant correlation with cocaine intake was found in Pomc (Lewis r=0.04, NS; Fischer r=−0.36, NS) and Oprd data (Lewis r=0.01, NS; Fischer r=−0.38, NS).
Figure 3.
Graph shows the correlation between the cocaine self-administered (mg/kg) during their last session before sacrifice and either Pdyn (A, C) or Oprk (B, D) mRNA expression, measured as ratio between the target gene copy numbers of and the mean copy numbers of two housekeeping genes, B2m and Hprt, in the dorsal striatum in Fischer (left) and Lewis (right) rats (n=10/11), respectively.
3.4. Expression of opioid peptides and opioid receptors genes in the Nucleus accumbens shell
Integrity of mRNA samples
In nucleus accumbens shell samples the Mean±SD RNA Integrity Number (RIN) values of the samples analyzed were 7.9± 0.5.
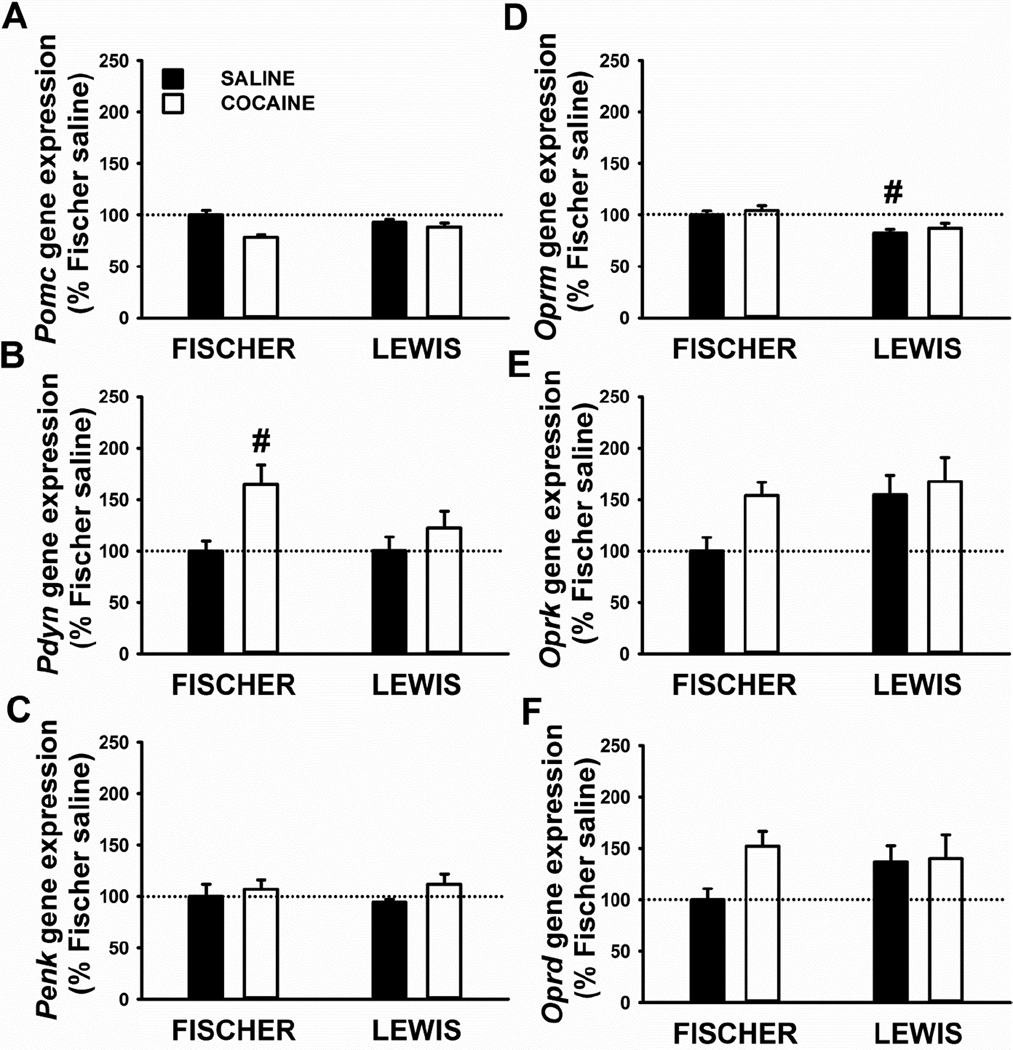
As shown in Figure 4, the gene expression analysis in the nucleus accumbens shell revealed limited differences between the two strains.
Figure 4.
Gene expression of three opioids peptides, proopiomelanocortin (Pomc, A), prodynorphin (Pdyn, B), proenkephalin (Penk, C), and three opioid receptors, mu (Oprm, D), kappa (Oprk, E), and delta (Oprd, F), in the nucleus accumbens shell of Fischer and Lewis rats sacrificed 24 h after the last 18-h operant session (cocaine). Control rats underwent same self-administration procedure, but with saline in place of cocaine (yoked-saline). Panels represent Mean±SEM expressed as the percent of Fischer yoked-saline. # p< 0.05 versus Fischer yoked-saline (SNK post-hoc test).
Analysis of Pomc data: factorial two-way ANOVA revealed a main effect of Treatment [F(1,32)=12.3, p=0.001] the interaction Strain*Treatment [F(1,32)=5.3, p=0.03]. Nonetheless, Post-hoc analysis did not detect any statistical difference in Pomc mRNA expression between groups (Figure 4A).
Analysis of Pdyn data: factorial two-way ANOVA revealed a main effect of Treatment [F(1,34)=7.5, p=0.01] but no interaction Strain*Treatment [F(1,34)=1.8, p=0.19]. As shown in Figure 4B, Pdyn expression was significantly higher selectively in Fischer rats exposed to cocaine, compared to their yoked-saline control (+65 %), with no difference seen in Lewis rats.
Analysis of Penk data: no main effects or interaction were detected.
Analysis of Oprm data: a main effect of the Strain [F(1,34)=16.1, p<0.001] was revealed by the two-way ANOVA. Indeed, Lewis yoked-saline rats showed lower Oprm mRNA level than Fischer yoked-saline rats (Figure 4D).
Analysis of Oprk data: no overall effects or interaction were detected by factorial two-way ANOVA although strong trend towards a significance was found in Strain [F(1,32)=3.7, p=0.06] and Treatment [F(1,32)=3.5, p=0.06]. As shown in Figure 4E, Oprk expression was higher in Fischer rats exposed to cocaine, compared to their yoked-saline control (+54 %), with no difference seen in Lewis rats.
Analysis of Oprd data: no main effects or interaction were detected. As shown in Figure 4F, Oprd expression was higher in Fischer rats exposed to cocaine, compared to their yoked-saline control (+52 %), with no difference seen in Lewis rats.
3.5. Gene expression of opioid peptides and receptors in the Nucleus accumbens core
Integrity of mRNA samples
In nucleus accumbens core samples the Mean±SD RNA Integrity Number (RIN) values of the samples analyzed were 8.3± 0.5.
As shown in Figure 5, the gene expression analysis in the nucleus accumbens core of Fischer and Lewis rats revealed limited differences between the two strains.
Figure 5.
Gene expression of three opioids peptides, proopiomelanocortin (Pomc, A), prodynorphin (Pdyn, B), proenkephalin (Penk, C), and three opioid receptors, mu (Oprm, D), kappa (Oprk, E), and delta (Oprd, F), in the nucleus accumbens core of Fischer and Lewis rats sacrificed 24 h after the last 18-h operant session (cocaine). Control rats underwent same self-administration procedure, but with saline in place of cocaine (yoked-saline). Panels represent Mean±SEM expressed as the percent of Fischer yoked-saline. ## p< 0.01 versus Fischer yoked-saline. * p< 0.05 versus Lewis yoked-saline. ^ statistical trend toward significance, 0.05≤p≤0.08 versus Fischer yoked-saline (SNK post-hoc test).
Analysis of Pomc data: factorial two-way ANOVA revealed a main effect of Treatment [F(1,35)=17.7, p<0.001]. Indeed, Post-hoc analysis confirmed Pomc mRNA expression to be affected by cocaine self-administration exposure in both strains, as shown in Figure 5A. The percent difference between cocaine and saline rat were −21 % and −26 %, in Fischer and Lewis rats, respectively.
Analysis of Pdyn data: factorial two-way ANOVA revealed a main effect of Treatment [F(1,36)=7.5, p=0.008], however no difference between groups was found in Pdyn mRNA expression except for a trend towards the significance between yoked-saline and cocaine (p=0.08, Figure 5B) selectively in Fischer rats.
Analysis of Penk data: a significant interaction Strain*Treatment was revealed by the two-way ANOVA [F(1,35)=5.0, p=0.03], however no difference between groups was found with further analysis.
Analysis of Oprm data: no overall effects or interaction were detected.
Analysis of Oprk data: no overall effects or interaction were detected by factorial two-way ANOVA although a trend towards a significance was found in Strain [F(1,36)=3.1, p=0.08] and interaction Strain*Treatment [F(1,36)=3.1, p=0.08].
Analysis of Oprd data: factorial two-way ANOVA revealed a main effect of Strain [F(1,33)=6.7, p=0.014], however no difference between groups was found except for a trend towards the significance between Fischer and Lewis rats in yoked-saline condition (p=0.07, Figure 5F).
4. Discussion
Results presented here demonstrate region-specific and strain-dependent differences in gene expression of components of the opioid system, induced by exposure to a novel protocol of chronic extended access cocaine self-administration, including subject-controlled selection of unit doses. We focused on gene expression of Oprm, Oprk and Oprd, encoding respectively for mu (MOP-r), kappa (KOP-r) and delta (DOP-r) opioid receptors, and on gene expression of Pomc, Pdyn and Penk, which encode neuropeptidic precursors of endogenous ligands, with relative selectivity for the three opioid receptors. Cocaine-induced gene expression changes were observed primarily in dorsal rather than in ventral parts of the striatum, since in the dorsal striatum (DS) all the gene targets analyzed were statistically different compared to yoked-saline control, especially in the “escalation-prone” Lewis rats. Interestingly, results demonstrate differences in DS gene expression level of all opioid peptides and receptors analyzed between Fischer and Lewis in the yoked-saline condition (i.e., in the absence of cocaine exposure).
Behavioral analysis of the cohort of subjects used for this genetic analysis is highly consistent with a previously reported larger data set (Picetti et al., 2010). Thus, while Fischer rats displayed stable intake over 2 weeks of 18-h cocaine self-administration sessions, Lewis rats escalated progressively their intake, especially during the second week of access. We also found that Lewis rats were more homogenous in their cocaine intake distribution (last session cocaine intake CV= 17 %) compared to Fischer rats (last session cocaine intake CV= 141%), indeed three of the eleven Fischer rats analyzed showed high cocaine intake during the last self-administration day, similar to the mean of Lewis rats’ intake.
Several reports have compared Fischer and Lewis rats for differential response to several drug of abuse and stress challenges, suggesting Lewis rats to be “addiction-prone” compared to “addiction-resistant” Fischer rats (Ambrosio et al., 1995; Kosten et al., 1994; Meyer and Bardo, 2015; Picetti et al., 2012; Picetti et al., 2010). Extended-access cocaine self-administration results in escalation of cocaine intake, behavior shown here selectively by Lewis rats, which is thought to model the transition from initial use to the more advanced stages in addiction trajectory seen in human patients, characterized by compulsivity and dysregulated drug taking behavior (Ahmed and Koob, 1998; Kreek and Koob, 1998; Wise and Koob, 2014).
Cocaine activates the mesolimbic/mesocortical and nigrostriatal dopaminergic pathways primarily by inhibiting dopamine re-uptake. Endogenous opioid peptides and receptors are expressed in areas mediating reward, motivation, learning and stress-responsiveness, therefore the opioid system may play key roles in development and maintenance of cocaine addiction (Kreek, 1996). Gene expression results presented here are in line with this postulation, since DS gene expression of all opioid targets was different in cocaine-exposed versus yoked-saline Lewis rats. While only one gene (Pdyn) was found altered by cocaine exposure in the NAcc shell and only one gene (Pomc) in the NAcc core, the expression of all opioid gene targets was different in cocaine and yoked-saline rats in the DS, which suggests that neuroadaptations of the endogenous opioid system may have occurred in this brain area following this chronic extended access escalating self-administration. Taken together, these findings support the hypothesis that a shift from the ventral to the dorsal striatum may occur in more advanced stages in addiction-like trajectory, after prolonged exposure to cocaine (Everitt and Robbins, 2005; Fagergren et al., 2003; Vanderschuren et al., 2005). Whereas drugs of abuse, including cocaine, initially lead to dopamine release into the ventral striatum, as well as dorsal striatum (Di Chiara and Imperato, 1988a; Goeders and Smith, 1983; Pontieri et al., 1995), it has been postulated that repeated exposure in the context of extended access self-administration lead to compulsive-like habit formations, mediated at least in part by the DS (Ito et al., 2002; Veeneman et al., 2012; Volkow et al., 2006; Willuhn et al., 2014).
The possibility that gene expression alterations in cocaine-exposed rats were induced by acute withdrawal (24 h) cannot be ruled out. Such changes in mRNA expression following extended access cocaine self-administration are present 24 h later than the last self-administration session, when plasma and brain cocaine levels are likely to be very low, and may indicate mechanisms that are involved in further cocaine intake escalation. Indeed, Lewis rats did not reach a plateau of intake during our behavioral paradigm. Future studies could assess if and when they reached a plateau using an even more prolonged (> 14 sessions) experiment.
Dynorphin/KOP-r results
Higher Pdyn mRNA was found in the DS of cocaine-exposed rats, irrespective of strain. This has been consistently reported from our and other laboratories in different rodent models of cocaine exposure or other drug of abuse (Gieryk et al., 2010; Sivam, 1989; Spangler et al., 1996), as well as in Fischer and Lewis rats exposed to experimenter-injected cocaine (20 mg/kg daily for 7 days) (Werme et al., 2000). Furthermore, an increase in striatal Pdyn gene expression after chronic cocaine self-administration has been documented in primates as well as in humans (Fagergren et al., 2003; Hurd and Herkenham, 1993). Previous studies have shown that level of dynorphin and prodynorphin peptides and mRNA are correlated suggesting that an upregulation in mRNA increases the content of the corresponding peptide (Sivam, 1989, 1996; Trifilieff and Martinez, 2013).
Prodynorphin (pDyn) is the precursor of the Dynorphins, endogenous agonists of the Kappa opioid receptor (KOP-r). KOP-r activation decreases dopamine release and therefore the reinforcing properties of drugs of abuse, therefore KOP-r/dynorphin tone thought to be part of a counter-regulatory mechanism. Also, increased KOP-r/dynorphin tone results in anhedonia, dysphoria-like and depression-like effects, which may also contribute to continued drug-taking and relapse (Bruchas et al., 2010; Knoll and Carlezon, 2010; Kreek, 1996; Lalanne et al., 2014; Shippenberg et al., 2007). Mice with a constitutive deletion of Pdyn gene show greater reinstatement to cocaine self-administration compared to their wild-type littermates (Gutierrez-Cuesta et al., 2014). In our study, the DS mRNA levels of both Pdyn and Oprk were positively correlated with cocaine intake during the last day of self-administration in Fischer, but not in Lewis rats. This result is also strengthened by the fact that striatal Pdyn mRNA level was positively correlated also with the escalation index selectively in Fischer rats (Suppl. Table 3). Furthermore, Pdyn mRNA expression was upregulated in the NAcc shell of rats exposed to cocaine compared to saline selectively in Fischer rats, which is in accordance to a previous finding using a not-operant “binge” protocol, with 3 daily injections of cocaine 15 mg/kg for 14 days (Unterwald et al., 1994). Based on these results, it can be hypothesized that the “anti-reward” system in Fischer rats is still active and responsive to cocaine despite the prolonged exposure to extended access self-administration, thus it dampens dopamine release and therefore drug rewarding effects and, ultimately, intake escalation. Indeed, it has been recently reported that the suppression of dopaminergic neurotransmission in the DS reduces the rewarding properties of cocaine (Trifilieff and Martinez, 2013; Veeneman et al., 2012). Lewis rats showed a lower striatal mRNA expression of both Pdyn and Oprk mRNA compared to Fischer, in their saline-yoked condition. Previous finding in drug naïve rats reported Fischer rats to have higher endogenous dynorphin peptide levels in striatum, ventral tegmental area and substantia nigra compared to Lewis rats (Nylander et al., 1995). The two strains do not differ in their basal levels of dopamine in the NAcc (Cadoni and Di Chiara, 2007), or in the increase in extracellular dopamine measured by microdialysis in the NAcc after acute cocaine challenge, although Lewis rats had a smaller dopamine elevation peak and a slower return to basal levels (Strecker et al., 1995). Thus, Lewis rats lower “anti-reward” signaling may predispose them to greater escalation of cocaine intake, as observed herein.
A strain selective increase of Oprk mRNA expression was found in the DS of cocaine versus yoked-saline in Lewis rats, but this was not correlated with the amount of cocaine self-administered during the last operant session, or with cumulative 14-session intake. This result support the KOP-r receptor as a potentially therapeutic target for addiction (Butelman et al., 2012), since the upregulation was seen only in rats that escalated cocaine consumption, thus model the late stage of human addiction. This is also corroborated by the demonstration that the long-acting KOPr antagonist nor-BNI reduces motivation for cocaine selectively in rats who escalated cocaine intake by an extended access (6-h) cocaine self-administration protocol (Wee et al., 2009).
Proopiomelanocortin/MOP-r results
We also found that gene expression of all endogenous opioid peptides and receptors studied here, with the exception of Pomc, are lower in the striatum of Lewis compared to Fischer rats, in the yoked-saline condition. Therefore, differences in other opioid peptide and receptor targets may also be involved in escalation of cocaine intake.
The lower level of Oprm mRNA expression, gene encoding for the mu opioid receptor (MOP-r), detected in the DS and NAcc Shell of Lewis rats in saline condition, compared to Fischer yoked-saline rats is consistent with Sanchez-Cardoso et al. (2007), who reported the same difference by autoradiography in experimentally naïve Fischer and Lewis rats. A low level of MOP-r indirectly reduces dopamine signaling, which in turn reduces the rewarding properties of the drug of abuse; therefore Lewis rats may need high doses of cocaine to reach the same level of reward perceived by Fischer rats at lower doses. Indeed, we observed Lewis rats to prefer higher unit doses than Fischer rats.
In the DS of cocaine-exposed Lewis rats, we found higher Oprm mRNA compared to yoked-saline control rats. This upregulation was not seen in Fischer rats and was not detected either in the shell or in the core of the NAcc. However, previous animal studies have shown that in Fischer rats chronic, intermittent cocaine administration increases NAcc MOP-r binding (Branch et al., 1992; Unterwald et al., 1994) in studies where cocaine was administered intraperitoneally using a “binge” protocol. mRNA expression of Oprm in the NAcc, but not DS, was increased even after only 1 or 3 days of “binge” protocol (Azaryan et al., 1996; Yuferov et al., 1999). To note, no operant behavior was involved in these studies, while in our study rats underwent cocaine self-administration for 18 h/day for 14 sessions. Therefore differences in this finding may be due to the impact of extended access intravenous cocaine self-administration and its escalation, leading to upregulation of Oprm transcription. Consistent with this, the novel MOP-r antagonist decreases cocaine seeking in operant self-administration (Giuliano et al., 2013). Moreover, Berger and Whistler (2011) suggested a causal relationship between MOP-r trafficking and compulsive behaviors, occurring after prolonged cocaine and morphine intravenous self-administration in rats. Another possible explanation is that Oprm mRNA expression was upregulated to counterbalance for lower beta-endorphin levels (encoded by the Pomc gene). Indeed, we found a Pomc mRNA downregulation both in the dorsal and ventral striatum in cocaine-exposed compared to yoked-saline rats, irrespective of Strain. Similar evidence was reported by Sweep et al. (1988) showing that rats self-administering heroin or cocaine have decreased amounts of beta-endorphin in DS and NAcc, measured by high-pressure liquid chromatography, compared to saline rats. However, another report showed higher release of the peptide in the NAcc of rats exposed to cocaine self-administration compared to saline-control rats using microdialysis and ELISA assays (Roth-Deri et al., 2003). Differences in strains of rats studied, cocaine self-administration protocols (short versus our extended access), and peptide detection techniques used may account for these discrepancies. Of translational relevance, PET neuroimaging studies show that MOP-r receptor populations are increased in the DS of cocaine addicted patients in early withdrawal (Zubieta et al., 1996) and this would be congruent with findings observed herein (i.e., due to decreased occupancy by the endogenous ligand b-endorphin).
Proenkephalin/DOP-r results
We found lower Penk mRNA expression in DS of Lewis rats compared to Fischer rats, in the yoked-saline condition. This is similar to prior reports by Martin et al. (1999), as well as Sanchez-Cardoso et al. (2007). Furthermore, gene expression of Penk was higher in the DS of cocaine exposed Lewis rats compared to their yoked-saline control, which is in accordance with a previous paper by Crespo et al. (2001).
The role of delta opioid receptor (DOP-r) in cocaine self-administration and the impact of DOP-r agonism and antagonism on cocaine self-administration in rodents are not fully resolved (de Vries et al., 1995; Negus et al., 1995; Reid et al., 1995; Shippenberg and Heidbreder, 1995; Simmons and Self, 2009). Results here reported showed higher Oprd mRNA in DS of both Fischer and Lewis rats exposed to cocaine, versus their yoked-saline controls. A previous report on Fischer rats with experimenter-administered “binge” cocaine found decreased Oprd mRNA in the DS (Unterwald et al., 1994), and also exhibited anxiety-like and depressive-like behavioral phenotypes, and these were blocked by exogenous DOP-r agonist administration (Perrine et al., 2008). However, difference between behavioral protocols used (experiment-administered versus extended access cocaine self-administration) may explain the discrepancy. It has been suggested a role for DOP-r in anxiety-like and depressive-like behavior, since DOP-r KO mice show higher anxiety-like and depressive like behavior compared to their wild-type littermates (Filliol et al., 2000). In the present study, strain difference in the mRNA level of Oprd were found in the dorsal part of the striatum, with Lewis rats having lower striatal Oprd mRNA expression compared to Fisher rats at yoked-saline condition. This might help explain results collected by Cohen et al. (2006) who showed Lewis rats have higher basal anxiety-like behavior compared to Fischer rats. Indeed, naïve Lewis rats show higher anxiety-like behavior in the elevated plus maze and compulsive-like behavior, measured by marble burying test, compared to naïve Fischer rats (Valenza, M., unpublished results).
5. Conclusions
The study here presented characterizes for the first time differential gene expression response in two extensively used inbred rat strains with different genetic background, Fischer and Lewis, in a translational model of extended access cocaine self-administration. A current goal of scientific research is to determine which factors make individuals more vulnerable than others to develop addiction, and selected inbred strains such as Fischer and Lewis rats are a powerful setting to examine this experimentally. Data support the hypothesis that the interaction between a predisposing genetic background (Lewis) and environmental history (exposure to extended access cocaine self-administration) may lead to the development of an addictive-like state, here modeled as escalation of cocaine intake. The differences shown in opioid system peptides and receptors gene expression are consistent with a key role of opioid system signaling in our model of late stage cocaine addiction. Differences in other neurotransmitter systems may also contribute to the different behavioral profiles observed between these two rat strains. For example, Lewis and Fischer rats show differences in tyroxine hydroxylase levels, both in the ventral tegmental area and the nucleus accumbens shell (Beitner-Johnson et al., 1991). Flores et al. (1998) demonstrated that in both dorsal and ventral parts of the striatum there are no strain difference in the dopamine receptor 1, whereas Lewis rats showed lower levels of Dopamine receptor 2 (D2R) and dopamine transporter in the Shell compared to Fischer rats. Similarly, Sanchez-Cardoso et al. (2009) reported Lewis rats to have higher D2R binding in the dorsal striatum, but not in the ventral compared to Fischer rats. Of note, the aforementioned studies did not characterize gene expression changes following cocaine self-administration, one major focus of this study.
Here we highlight that basal and drug-induced differential expression of opioid genes in the dorsal part of the striatum, in comparisons to the ventral parts, may represent predisposing factors to develop addiction. Although our investigation was focused on gene expression, which does not necessarily correspond to peptide expression, the present results raise specific hypotheses that may be studied in appropriate follow-up experiments, on neurobiological differences between rats displaying escalated cocaine intake (in our case Lewis) and those that do not (in our case Fischer), within and across strains. Based on the present results, it may be hypothesized that the higher striatal Pdyn found in Fischer rats at basal level may protect them to escalate cocaine intake, and vice versa the lower level found in Lewis rats may predispose them to escalate cocaine intake, in view of the known regulatory function of KOPr/dynorphin signaling on cocaine reward.
Supplementary Material
Highlights.
Lewis rats, but not Fischer rats, escalate cocaine intake during 14 extended access (18 h) self-administration sessions.
Cocaine-induced gene expression changes were observed primarily in dorsal and not ventral parts of the striatum.
Strain differences were observed in basal striatal level of Pomc, Pdyn, Penk, Oprm, Oprk, and Oprd mRNA.
Pdyn, Penk, Oprk, and Oprm mRNA level in the Dorsal Striatum correlates with individual cocaine intake during the last self-administration session selectively in Fischer rats.
Acknowledgments
The authors gratefully acknowledge the financial support from The Dorothea Dix Fellowship Fund (MV), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (MJK), the NIDA P60-DA05130 (MJK), and The Arcadia Charitable Trust (MJK). The authors gratefully thank Ms. Hilary Briggs who proof-read the final manuscript and all the members of The Laboratory of Addictive Diseases at The Rockefeller University for constructive discussion of data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare not to have any conflict of interest to disclose.
REFERENCES
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Allain F, Minogianis EA, Roberts DC, Samaha AN. How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev. 2015;56:166–179. doi: 10.1016/j.neubiorev.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Ambrosio E, Goldberg SR, Elmer GI. Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav Pharmacol. 1995;6:229–237. [PubMed] [Google Scholar]
- Azaryan AV, Coughlin LJ, Buzas B, Clock BJ, Cox BM. Effect of chronic cocaine treatment on mu- and delta-opioid receptor mRNA levels in dopaminergically innervated brain regions. J Neurochem. 1996;66:443–448. doi: 10.1046/j.1471-4159.1996.66020443.x. [DOI] [PubMed] [Google Scholar]
- Bailey A, Yuferov V, Bendor J, Schlussman SD, Zhou Y, Ho A, Kreek MJ. Immediate withdrawal from chronic “binge” cocaine administration increases mu-opioid receptor mRNA levels in rat frontal cortex. Brain Res Mol Brain Res. 2005;137:258–262. doi: 10.1016/j.molbrainres.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Guitart X, Nestler EJ. Dopaminergic brain reward regions of Lewis and Fischer rats display different levels of tyrosine hydroxylase and other morphine- and cocaine-regulated phosphoproteins. Brain Res. 1991;561:147–150. doi: 10.1016/0006-8993(91)90759-o. [DOI] [PubMed] [Google Scholar]
- Berger AC, Whistler JL. Morphine-induced mu opioid receptor trafficking enhances reward yet prevents compulsive drug use. EMBO Mol Med. 2011;3:385–397. doi: 10.1002/emmm.201100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booze RM, Lehner AF, Wallace DR, Welch MA, Mactutus CF. Dose-response cocaine pharmacokinetics and metabolite profile following intravenous administration and arterial sampling in unanesthetized, freely moving male rats. Neurotoxicol Teratol. 1997;19:7–15. doi: 10.1016/s0892-0362(96)00180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch AD, Unterwald EM, Lee SE, Kreek MJ. Quantitation of preproenkephalin mRNA levels in brain regions from male Fischer rats following chronic cocaine treatment using a recently developed solution hybridization assay. Brain Res Mol Brain Res. 1992;14:231–238. doi: 10.1016/0169-328x(92)90178-e. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Beaulieu JF, Huggett J, Jaggi R, Kibenge FS, Olsvik PA, Penning LC, Toegel S. MIQE precis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol. 2010;11:74. doi: 10.1186/1471-2199-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Yuferov V, Kreek MJ. kappa-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci. 2012;35:587–596. doi: 10.1016/j.tins.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Differences in dopamine responsiveness to drugs of abuse in the nucleus accumbens shell and core of Lewis and Fischer 344 rats. J Neurochem. 2007;103:487–499. doi: 10.1111/j.1471-4159.2007.04795.x. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Simola N, Espa E, Fenu S, Di Chiara G. Strain dependence of adolescent Cannabis influence on heroin reward and mesolimbic dopamine transmission in adult Lewis and Fischer 344 rats. Addict Biol. 2015;20:132–142. doi: 10.1111/adb.12085. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, Kozlovsky N, Kaplan Z. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry. 2006;59:1208–1218. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Belknap JK. Genetic approaches to drug dependence. Trends Pharmacol Sci. 1992;13:212–219. doi: 10.1016/0165-6147(92)90066-f. [DOI] [PubMed] [Google Scholar]
- Crespo JA, Manzanares J, Oliva JM, Corchero J, Palomo T, Ambrosio E. Extinction of cocaine self-administration produces a differential time-related regulation of proenkephalin gene expression in rat brain. Neuropsychopharmacology. 2001;25:185–194. doi: 10.1016/S0893-133X(01)00221-4. [DOI] [PubMed] [Google Scholar]
- de Vries TJ, Babovic-Vuksanovic D, Elmer G, Shippenberg TS. Lack of involvement of delta-opioid receptors in mediating the rewarding effects of cocaine. Psychopharmacology (Berl) 1995;120:442–448. doi: 10.1007/BF02245816. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Stress response, adrenal steroid receptor levels and corticosteroid-binding globulin levels--a comparison between Sprague-Dawley, Fischer 344 and Lewis rats. Brain Res. 1993;616:89–98. doi: 10.1016/0006-8993(93)90196-t. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988a;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988b;244:1067–1080. [PubMed] [Google Scholar]
- Dore R, Valenza M, Wang X, Rice KC, Sabino V, Cottone P. The inverse agonist of CB1 receptor SR141716 blocks compulsive eating of palatable food. Addict Biol. 2014;19:849–861. doi: 10.1111/adb.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergang P, Vodicka M, Sotak M, Klusonova P, Behuliak M, Rehakova L, Zach P, Pacha J. Differential impact of stress on hypothalamic-pituitary-adrenal axis: gene expression changes in Lewis and Fisher rats. Psychoneuroendocrinology. 2015;53:49–59. doi: 10.1016/j.psyneuen.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur J Neurosci. 2003;17:2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Calderon D, Fernandez F, Ruiz-Curado S, Verdejo-Garcia A, Lozano OM. Profiles of substance use disorders in patients of therapeutic communities: Link to social, medical and psychiatric characteristics. Drug Alcohol Depend. 2015 doi: 10.1016/j.drugalcdep.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta-and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006;27:126–139. doi: 10.1016/j.mam.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Flores G, Wood GK, Barbeau D, Quirion R, Srivastava LK. Lewis and Fischer rats: a comparison of dopamine transporter and receptors levels. Brain Res. 1998;814:34–40. doi: 10.1016/s0006-8993(98)01011-7. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Kearns DN, Kohut SJ, Riley AL. Strain differences in patterns of drug-intake during prolonged access to cocaine self-administration. Behav Neurosci. 2009;123:156–164. doi: 10.1037/a0013727. [DOI] [PubMed] [Google Scholar]
- Gieryk A, Ziolkowska B, Solecki W, Kubik J, Przewlocki R. Forebrain PENK and PDYN gene expression levels in three inbred strains of mice and their relationship to genotype-dependent morphine reward sensitivity. Psychopharmacology (Berl) 2010;208:291–300. doi: 10.1007/s00213-009-1730-1. [DOI] [PubMed] [Google Scholar]
- Giuliano C, Robbins TW, Wille DR, Bullmore ET, Everitt BJ. Attenuation of cocaine and heroin seeking by mu-opioid receptor antagonism. Psychopharmacology (Berl) 2013;227:137–147. doi: 10.1007/s00213-012-2949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Cuesta J, Burokas A, Mancino S, Kummer S, Martin-Garcia E, Maldonado R. Effects of genetic deletion of endogenous opioid system components on the reinstatement of cocaine-seeking behavior in mice. Neuropsychopharmacology. 2014;39:2974–2988. doi: 10.1038/npp.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Potenza MN, Grunberg NE. Lewis rats have greater response impulsivity than Fischer rats. Addict Behav. 2014;39:1565–1572. doi: 10.1016/j.addbeh.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Chi S, Nestler EJ. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J Pharmacol Exp Ther. 1994;269:137–144. [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Haile CN, DeCaprio JL, Jatlow PI, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778:418–429. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Cocaine, dopamine and the endogenous opioid system. J Addict Dis. 1996;15:73–96. doi: 10.1300/J069v15n04_05. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Xi J. Differences in extinction responding and reinstatement of methamphetamine-seeking behavior between Fischer 344 and Lewis rats. Pharmacol Biochem Behav. 2006a;83:391–395. doi: 10.1016/j.pbb.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Xi J. Different patterns of pharmacological reinstatement of cocaine-seeking behavior between Fischer 344 and Lewis rats. Psychopharmacology (Berl) 2006b;187:22–29. doi: 10.1007/s00213-005-0264-4. [DOI] [PubMed] [Google Scholar]
- LaForge KS, Yuferov V, Kreek MJ. Opioid receptor and peptide gene polymorphisms: potential implications for addictions. Eur J Pharmacol. 2000;410:249–268. doi: 10.1016/s0014-2999(00)00819-0. [DOI] [PubMed] [Google Scholar]
- Lalanne L, Ayranci G, Kieffer BL, Lutz PE. The kappa opioid receptor: from addiction to depression, and back. Front Psychiatry. 2014;5:170. doi: 10.3389/fpsyt.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Randesi M, Li Y, Rotrosen J, Ott J, Adelson M, Kreek MJ. Drug addiction and stress-response genetic variability: association study in African Americans. Ann Hum Genet. 2014;78:290–298. doi: 10.1111/ahg.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Smith NG, Brewer AT, Pinkston JW, Johnson PS. Steady-state assessment of impulsive choice in Lewis and Fischer 344 rats: between-condition delay manipulations. J Exp Anal Behav. 2008;90:333–344. doi: 10.1901/jeab.2008.90-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Manzanares J, Corchero J, Garcia-Lecumberri C, Crespo JA, Fuentes JA, Ambrosio E. Differential basal proenkephalin gene expression in dorsal striatum and nucleus accumbens, and vulnerability to morphine self-administration in Fischer 344 and Lewis rats. Brain Res. 1999;821:350–355. doi: 10.1016/s0006-8993(99)01122-1. [DOI] [PubMed] [Google Scholar]
- Mets B, Diaz J, Soo E, Jamdar S. Cocaine, norcocaine, ecgonine methylester and benzoylecgonine pharmacokinetics in the rat. Life Sci. 1999;65:1317–1328. doi: 10.1016/s0024-3205(99)00367-7. [DOI] [PubMed] [Google Scholar]
- Meyer AC, Bardo MT. Amphetamine self-administration and dopamine function: assessment of gene x environment interactions in Lewis and Fischer 344 rats. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-014-3854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguens M, Kastanauskaite A, Coria SM, Selvas A, Ballesteros-Yanez I, DeFelipe J, Ambrosio E. The effects of cocaine self-administration on dendritic spine density in the rat hippocampus are dependent on genetic background. Cereb Cortex. 2015;25:56–65. doi: 10.1093/cercor/bht200. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lukas SE, Mendelson JH. Role of delta opioid receptors in the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1995;273:1245–1256. [PubMed] [Google Scholar]
- Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76(Pt B):259–268. doi: 10.1016/j.neuropharm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander I, Vlaskovska M, Terenius L. Brain dynorphin and enkephalin systems in Fischer and Lewis rats: effects of morphine tolerance and withdrawal. Brain Res. 1995;683:25–35. doi: 10.1016/0006-8993(95)00279-y. [DOI] [PubMed] [Google Scholar]
- Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety-and depression-like behaviors in the rat. Neuropharmacology. 2008;54:355–364. doi: 10.1016/j.neuropharm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picetti R, Caccavo JA, Ho A, Kreek MJ. Dose escalation and dose preference in extended-access heroin self-administration in Lewis and Fischer rats. Psychopharmacology (Berl) 2012;220:163–172. doi: 10.1007/s00213-011-2464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picetti R, Ho A, Butelman ER, Kreek MJ. Dose preference and dose escalation in extended-access cocaine self-administration in Fischer and Lewis rats. Psychopharmacology (Berl) 2010;211:313–323. doi: 10.1007/s00213-010-1899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid LD, Glick SD, Menkens KA, French ED, Bilsky EJ, Porreca F. Cocaine self-administration and naltrindole, a delta-selective opioid antagonist. Neuroreport. 1995;6:1409–1412. doi: 10.1097/00001756-199507100-00012. [DOI] [PubMed] [Google Scholar]
- Rivera P, Miguens M, Coria SM, Rubio L, Higuera-Matas A, Bermudez-Silva FJ, de Fonseca FR, Suarez J, Ambrosio E. Cocaine self-administration differentially modulates the expression of endogenous cannabinoid system-related proteins in the hippocampus of Lewis vs. Fischer 344 rats. Int J Neuropsychopharmacol. 2013;16:1277–1293. doi: 10.1017/S1461145712001186. [DOI] [PubMed] [Google Scholar]
- Rose’Meyer RB, Mellick AS, Garnham BG, Harrison GJ, Massa HM, Griffiths LR. The measurement of adenosine and estrogen receptor expression in rat brains following ovariectomy using quantitative PCR analysis. Brain Res Brain Res Protoc. 2003;11:9–18. doi: 10.1016/s1385-299x(02)00219-2. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Zangen A, Aleli M, Goelman RG, Pelled G, Nakash R, Gispan-Herman I, Green T, Shaham Y, Yadid G. Effect of experimenter-delivered and self-administered cocaine on extracellular beta-endorphin levels in the nucleus accumbens. J Neurochem. 2003;84:930–938. doi: 10.1046/j.1471-4159.2003.01584.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cardoso P, Higuera-Matas A, Martin S, del Olmo N, Miguens M, Garcia-Lecumberri C, Ambrosio E. Modulation of the endogenous opioid system after morphine self-administration and during its extinction: a study in Lewis and Fischer 344 rats. Neuropharmacology. 2007;52:931–948. doi: 10.1016/j.neuropharm.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cardoso P, Higuera-Matas A, Martin S, Miguens M, Del Olmo N, Garcia-Lecumberri C, Ambrosio E. Strain differences between Lewis and Fischer 344 rats in the modulation of dopaminergic receptors after morphine self-administration and during extinction. Neuropharmacology. 2009;57:8–17. doi: 10.1016/j.neuropharm.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C. The delta-opioid receptor antagonist naltrindole prevents sensitization to the conditioned rewarding effects of cocaine. Eur J Pharmacol. 1995;280:55–61. doi: 10.1016/0014-2999(95)00185-n. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D, Self DW. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology. 2009;34:1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivam SP. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J Pharmacol Exp Ther. 1989;250:818–824. [PubMed] [Google Scholar]
- Sivam SP. Dopaminergic regulation of postnatal development of dynorphin neurons in rat striatum. Neuropeptides. 1996;30:103–107. doi: 10.1016/s0143-4179(96)90062-1. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R, Ho A, Zhou Y, Maggos CE, Yuferov V, Kreek MJ. Regulation of kappa opioid receptor mRNA in the rat brain by "binge' pattern cocaine administration and correlation with preprodynorphin mRNA. Brain Res Mol Brain Res. 1996;38:71–76. doi: 10.1016/0169-328x(95)00319-n. [DOI] [PubMed] [Google Scholar]
- Strecker RE, Eberle WF, Ashby CR., Jr Extracellular dopamine and its metabolites in the nucleus accumbens of Fischer and Lewis rats: basal levels and cocaine-induced changes. Life Sci. 1995;56:PL135–PL141. doi: 10.1016/0024-3205(94)00913-9. [DOI] [PubMed] [Google Scholar]
- Sweep CG, Van Ree JM, Wiegant VM. Characterization of beta-endorphin-immunoreactivity in limbic brain structures of rats self-administering heroin or cocaine. Neuropeptides. 1988;12:229–236. doi: 10.1016/0143-4179(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Tricarico C, Pinzani P, Bianchi S, Paglierani M, Distante V, Pazzagli M, Bustin SA, Orlando C. Quantitative real-time reverse transcription polymerase chain reaction: normalization to rRNA or single housekeeping genes is inappropriate for human tissue biopsies. Anal Biochem. 2002;309:293–300. doi: 10.1016/s0003-2697(02)00311-1. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Martinez D. Kappa-opioid receptor signaling in the striatum as a potential modulator of dopamine transmission in cocaine dependence. Front Psychiatry. 2013;4:44. doi: 10.3389/fpsyt.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterwald EM, Rubenfeld JM, Kreek MJ. Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. Neuroreport. 1994;5:1613–1616. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- Valenza M, Steardo L, Cottone P, Sabino V. Diet-induced obesity and diet-resistant rats: differences in the rewarding and anorectic effects of D-amphetamine. Psychopharmacology (Berl) 2015;232:3215–3226. doi: 10.1007/s00213-015-3981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeneman MM, Broekhoven MH, Damsteegt R, Vanderschuren LJ. Distinct contributions of dopamine in the dorsolateral striatum and nucleus accumbens shell to the reinforcing properties of cocaine. Neuropsychopharmacology. 2012;37:487–498. doi: 10.1038/npp.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology (Berl) 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Thoren P, Olson L, Brene S. Running and cocaine both upregulate dynorphin mRNA in medial caudate putamen. Eur J Neurosci. 2000;12:2967–2974. doi: 10.1046/j.1460-9568.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Groblewski PA, Phillips PE. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat Neurosci. 2014;17:704–709. doi: 10.1038/nn.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JL, Shackel NA, Zekry A, McGuinness PH, Richards C, Putten KV, McCaughan GW, Eris JM, Bishop GA. Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for measurement of cytokine and growth factor mRNA expression with fluorogenic probes or SYBR Green I. Immunol Cell Biol. 2001;79:213–221. doi: 10.1046/j.1440-1711.2001.01002.x. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Ho A, Morgello S, Yang Y, Ott J, Kreek MJ. Expression of ephrin receptors and ligands in postmortem brains of HIV-infected subjects with and without cognitive impairment. J Neuroimmune Pharmacol. 2013;8:333–344. doi: 10.1007/s11481-012-9429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Zhou Y, Spangler R, Maggos CE, Ho A, Kreek MJ. Acute “binge” cocaine increases mu-opioid receptor mRNA levels in areas of the rat mesolimbic mesocortical dopamine system. Brain Res Bull. 1999;48:109–112. doi: 10.1016/s0361-9230(98)00155-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the endogenous kappa opioid agonist dynorphin A(1–17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 2004;172:422–429. doi: 10.1007/s00213-003-1688-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schlussman SD, Rabkin J, Butelman ER, Ho A, Kreek MJ. Chronic escalating cocaine exposure, abstinence/withdrawal, and chronic re-exposure: effects on striatal dopamine and opioid systems in C57BL/6J mice. Neuropharmacology. 2013;67:259–266. doi: 10.1016/j.neuropharm.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ. Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med. 1996;2:1225–1229. doi: 10.1038/nm1196-1225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.