Abstract
Background
Illicit drug use in pregnancy is a complex social and public health problem. The consequences of drug use in pregnancy are high for both the woman and her child. Therefore, it is important to develop and evaluate effective treatments. There is evidence for the effectiveness of psychosocial interventions in drug treatment but it is unclear whether they are effective in pregnant women. This is an update of a Cochrane review originally published in 2007.
Objectives
To evaluate the effectiveness of psychosocial interventions in pregnant women enrolled in illicit drug treatment programmes on birth and neonatal outcomes, on attendance and retention in treatment, as well as on maternal and neonatal drug abstinence. In short, do psychosocial interventions translate into less illicit drug use, greater abstinence, better birth outcomes, or greater clinic attendance?
Search methods
We conducted the original literature search in May 2006 and performed the search update up to January 2015. For both review stages (original and update), we searched the Cochrane Drugs and Alcohol Group Trial's register (May 2006 and January 2015); the Cochrane Central Register of Trials (CENTRAL; the Cochrane Library 2015, Issue 1); PubMed (1996 to January 2015); EMBASE (1996 to January 2015); and CINAHL (1982 to January 2015).
Selection criteria
We included randomized controlled trials comparing any psychosocial intervention vs. a control intervention that could include pharmacological treatment, such as methadone maintenance, a different psychosocial intervention, counselling, prenatal care, STD counselling and testing, transportation, or childcare.
Data collection and analysis
We used standard methodological procedures expected by the Cochrane Collaboration. We performed analyses based on three comparisons: any psychosocial intervention vs. control, contingency management (CM) interventions vs. control, and motivational interviewing based (MIB) interventions vs. control.
Main results
In total, we included 14 studies with 1298 participants: nine studies (704 participants) compared CM vs. control, and five studies (594 participants) compared MIB interventions vs. control. We did not find any studies that assessed other types of psychosocial interventions. For the most part, it was unclear if included studies adequately controlled for biases within their studies as such information was not often reported. We assessed risk of bias in the included studies relating to participant selection, allocation concealment, personnel and outcome assessor blinding, and attrition.
The included trials rarely captured maternal and neonatal outcomes. For studies that did measure such outcomes, no difference was observed in pre‐term birth rates (RR 0.71, 95% confidence interval (CI) 0.34 to 1.51; three trials, 264 participants, moderate quality evidence), maternal toxicity at delivery (RR 1.18, 95% CI 0.52 to 2.65; two trials, 217 participants, moderate quality evidence), or low birth weight (RR 0.72, 95% CI 0.36 to 1.43; one trial, 160 participants, moderate quality evidence). However, the results did show that neonates remained in hospital for fewer days after delivery in CM intervention groups (RR ‐1.27, 95% CI ‐2.52 to ‐0.03; two trials, 103 participants, moderate quality evidence). There were no differences observed at the end of studies in retention or abstinence (as assessed by positive drug test at the end of treatment) in any psychosocial intervention group compared to control (Retention: RR 0.99, 95% CI 0.93 to 1.06, nine trials, 743 participants, low quality evidence; and Abstinence: RR 1.14, 95% CI 0.75 to 1.73, three trials, 367 participants, low quality evidence). These results held for both CM and MIB combined. Overall, the quality of the evidence was low to moderate.
Authors' conclusions
The present evidence suggests that there is no difference in treatment outcomes to address drug use in pregnant women with use of psychosocial interventions, when taken in the presence of other comprehensive care options. However, few studies evaluated obstetrical or neonatal outcomes and rarely did so in a systematic way, making it difficult to assess the effect of psychosocial interventions on these clinically important outcomes. It is important to develop a better evidence base to evaluate psychosocial modalities of treatment in this important population.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Psychotherapy; Length of Stay; Patient Dropouts; Patient Dropouts/statistics & numerical data; Pregnancy Complications; Pregnancy Complications/psychology; Pregnancy Complications/therapy; Pregnancy Outcome; Pregnant Women; Pregnant Women/psychology; Premature Birth; Premature Birth/epidemiology; Randomized Controlled Trials as Topic; Reinforcement, Psychology; Substance‐Related Disorders; Substance‐Related Disorders/psychology; Substance‐Related Disorders/therapy
Plain language summary
Psychosocial interventions for pregnant women in outpatient illicit drug treatment programmes compared to other interventions
Review question
We reviewed the evidence about the effect of psychosocial interventions, such as contingency management (CM) and motivational interviewing based (MIB) techniques vs. usual care for pregnant women in outpatient illicit drug treatment programmes.
Background
Women who use illicit drugs while pregnant are more likely to give birth early and have low birthweight infants. A pregnant woman can reduce the risk of these complications by undergoing drug treatment during pregnancy.
Psychosocial interventions, such as CM and MIB techniques, may help them to overcome the many barriers to staying in a drug treatment programme and reduce illicit drug use. CM uses positive supportive reinforcement with incentives, such as monetary vouchers, awarded based on pre‐determined endpoints such as treatment attendance or drug abstinence. MIB is a form of patient‐centred counselling used to resolve uncertainty in their drug use, treatment, or cessation.
Study characteristics
Researchers from the Cochrane Collaboration examined the evidence published up to January 2015 and included 14 studies with 1298 pregnant women in this Cochrane review. The 1298 pregnant women received either CM or MIB techniques in adjunct to other comprehensive care options; women in the control group received usual care that included pharmacological treatment such as methadone maintenance, counselling, prenatal care, STD counselling and testing, transportation, and/or childcare. Nine studies used CM techniques vs. usual care, while five studies involved MIB techniques vs. usual care.
All of the studies were completed in the United States of America and most participants were African American. Most studies used the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐R) criteria to determine drug dependence.
Key results
There were no differences in retention or abstinence between CM or MIB techniques and usual care. There were also no differences in birth outcomes between the groups.
Overall, there is low to moderate quality of evidence from the included studies. Allocation methods were often described in very limited manner. Furthermore, many studies lacked attrition information which could have impacted results. While further information related to these methods could be helpful, future randomized trials using psychosocial interventions are unlikely to show a benefit. In addition, there was significant heterogeneity in terms of methods for measuring outcomes.
Summary of findings
Summary of findings for the main comparison. Summary of findings table 1.
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) |
|
Patients: Pregnant women enrolled in illicit drug treatment programs for any treatment of substance abuse or dependence of any drug Settings: Outpatient treatment facilities Intervention: Psychosocial interventions of any kind (including Contingency Management methods and Motivational Interviewing based techniques) alone or given in addition to usual care Comparison: Comprehensive usual care such as methadone maintenance, counselling, prenatal care (PNC), STD counselling and testing, transportation, and/or childcare | |||
|
Preterm birth (< 37 weeks gestation) (Any psychosocial intervention vs. control) |
RR 0.71 (95% CI 0.34 to 1.51) | 264 (3 studies) | ⊕⊕⊕⊝ moderate 1 |
|
Low birth weight (< 2500 g) (Any psychosocial intervention vs. control) |
RR 0.72 (95% CI 0.36 to 1.43) | 160 (1 study) | ⊕⊕⊕⊕ high |
|
Days hospitalized after delivery (Any psychosocial intervention vs. control) |
MD ‐1.27 (95% CI ‐2.52 to ‐0.03) | 103 (2 studies) | ⊕⊕⊕⊝ moderate 2 |
|
Retention at treatment completion (Any psychosocial intervention vs. control) |
RR 0.99 (95% CI 0.93 to 1.06) | 743 (9 studies) | ⊕⊕⊝⊝ low 3 |
|
Short term treatment retention (Any psychosocial intervention vs. control) |
RR 1.00 (95% CI 0.90 to 1.10) | 514 (6 studies) | ⊕⊕⊝⊝ low 4 |
|
Positive urine at delivery (Any psychosocial intervention vs. control) |
RR 1.18 (95% CI 0.52 to 2.65) | 217 (2 studies) | ⊕⊕⊕⊕ high |
|
Positive urine drug test (end of treatment) (Any psychosocial intervention vs. control) |
RR 1.14 (95% CI 0.75 to 1.73) | 367 (3 studies) | ⊕⊕⊕⊝ moderate 5 |
|
Retention at treatment completion (CM vs. control) |
RR 1.03 (95% CI 0.92 to 1.16) | 388 (6 studies) | ⊕⊕⊝⊝ low 6 |
|
Retention at treatment completion (MIB interventions vs. control) |
RR 0.97 (95% CI 0.89 to 1.06) | 355 (3 studies) | ⊕⊕⊝⊝ low 7 |
| CI: Confidence interval; RR: Risk ratio; MD: Mean difference; CM: contingency management; MIB: motivational interviewing based. | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1 Downgraded by one due to possible selection bias in one of the three included studies.
2 Downgraded by one due to possible attrition bias associated with one of the two studies.
3 Downgraded by two due to possible selection bias, attrition bias, and detection bias in majority of the included studies (all but two).
4 Downgraded by two due to possible selection bias, attrition bias, and detection bias in majority of the included studies (all but two).
5 Downgraded by one due to possible selection bias associated with one of the three studies.
6 Downgraded by two due to possible selection bias associated with four of the included studies.
7 Downgraded by two due to possible selection bias associated with two of the included studies.
Background
Description of the condition
Illicit drug use among pregnant women is an important and complex public health concern. Since the early 1990s, there has been a gradual increase in the incidence of illicit drug use in the United States of America (USA), especially among women of reproductive age (http://oas.samhsa.gov/women.htm). The most current data in the USA is from the 2012 National Survey on Drug Use and Health (http://oas.samhsa.gov/nhsda), in which 5.9% of pregnant women reported current illicit drug use. Furthermore, the data showed that 18.3% of pregnant women aged 15 to 17, 9.0% of women aged 18 to 25, and 3.4% of women aged 26 to 44 were current users of illicit drugs. A 2010 Australian survey also found that 4.2% of women who were pregnant, breastfeeding, or both in the past 12 months had used an illicit drug while pregnant (AIHW 2011).
Women who use illicit drugs are more likely to experience adverse obstetrical and perinatal outcomes than women in the general population (Ludlow 2004). Illicit drug use in pregnancy has been associated with preterm delivery, low birth weight infants, placental abruption, neonatal abstinence syndrome (NAS), and Neonatal Intensive Care Unit (NICU) admission (Sherwood 1999; Ludlow 2004). Cocaine, amphetamines, opioids, and marijuana use has been linked to premature labour, placental abruption, uterine rupture, fetal distress, neonatal addiction syndrome, and delay in cognitive development (Kuczkowski 2007). There is also conflicting evidence on long term adverse outcomes, especially among cocaine exposed infants. Although some studies have shown cognitive deficits at two years of life (Singer 2004), the bulk of the research points to minimal or absent effects in early childhood, as summarized well in a systematic review (Frank 2001).
Description of the intervention
Drug treatment interventions can be grouped into either pharmacological or psychosocial methods. This Cochrane review focuses only on psychosocial interventions, which involve the use of contingency management (CM) methods, including vouchers and other incentives, as well as manual‐based techniques such as motivational interviewing based (MIB) techniques. There are additional psychosocial interventions including cognitive behavioural therapy (CBT) and individual psychotherapy. We focused on CM and MIB techniques because they are the most common.
Contingency management
CM treatments are based on the principle of positive reinforcement as a means of operant conditioning that influences behaviour change. It is grounded in the work of Thorndike, especially in his "Law of Effect", which states that behavioural responses which produce a "satisfying" effect are "stamped in" by the experience and likely to occur more frequently than responses which produce an "annoying" effect (Thorndike 1898). This theory was elaborated by B.F. Skinner who examined the relationship between positive vs. negative reinforcement and positive vs. negative punishment in relation to behavioural outcome. Like Thorndike, his research (initially) involved the use of animals (a pigeon in a box rather than a cat in a maze). He demonstrated that punishment, regardless of whether positive or negative, decreases behaviour whereas reinforcement increases behaviour, and he attempted to describe the psycho‐dynamic mechanism by which this behaviour change was affected (Skinner 1947). The premise behind CM is to systematically use reinforcement techniques to modify behaviour in a positive and supportive manner. It has been used in the treatment of substance abuse since the 1970s (for a good review see Sitzer 2006). The most common form of CM has been the use of monetary vouchers, although prize reinforcers have also been used. CM was first demonstrated to be efficacious in both treatment retention and substance abstinence in cocaine‐dependent individuals (Higgins 1991), but has subsequently been studied in opioids, marijuana, cigarettes, alcohol, benzodiazepines, and multiple drugs. Recently it has been used in populations of pregnant illicit drug‐dependent women.
Motivational interviewing based
MIB techniques are cognitive‐behavioural interventions that are standardized and reproducible. They are based on motivational interviewing, a concept initially developed for the treatment of problem drinkers (Miller 2003). It is a directive, client‐centred counselling style for eliciting behaviour change by helping clients explore and resolve the ambivalence surrounding their substance use (Rollnick 1995). It draws from the trans‐theoretical model of change (DiClemente 1998) in order to improve treatment readiness and retention. The four included trials each employed a relatively brief MIB intervention. In Mullins 2004, participants in the intervention group received three one‐hour MIB sessions. In O'Neill 1996, the participants received a total of six sessions lasting 60 to 90 minutes each. The first session was MIB, whereas the subsequent sessions addressed strategies for avoiding high risk behaviours, including relaxation techniques and problem‐solving techniques. In Haug 2004, the most standardized form of MIB was employed: motivational enhancement therapy (MET). This involved four sessions each tailored to the person's stage of change. Winhusen 2008 employed three sessions, the first of which emphasized rapport building, reflective listening, and affirmation. The second session focused on providing feedback to the patient and a discussion of the benefits and consequences of substance use and pregnancy. The final session centred on either planning for behaviour change or strengthening commitment to behaviour change.
How the intervention might work
Drug treatment in pregnancy has been shown to reduce the maternal and fetal complications associated with illicit drug use (Kukko 1999; Armstrong 2003). Pregnancy can be considered as a "window of opportunity" for drug treatment intervention (Daley 1998). Maternal concern for the pregnancy has been thought of as a motivator to seek drug treatment. Although qualitative studies have documented maternal motivation (Murphy 1999; Dakof 2003), they have also described the many structural and social barriers to both receiving and remaining in treatment (Boyd 1999; Murphy 1999). To date, empirical research has failed to demonstrate better adherence rates to treatment among pregnant women compared with other people in drug treatment (Hser 1998). In fact, pregnant women may have higher dropout rates (Howell 2000). Since length of time in treatment is related to positive outcomes (Grella 2000; Howell 2000), it is important to identify modalities of treatment retention that are successful in this specific population. Psychosocial interventions can increase individual motivation to remain in treatment and decrease drug use through the use of incentives, or therapeutic techniques, or both (Amato 2011a; Amato 2011b)
Why it is important to do this review
The consequences of drug use in pregnancy are great for both the woman and her child (Ludlow 2004). Psychosocial interventions are widely used in drug treatment but it is unclear whether or not they work in pregnant women. As no other systematic reviews have been done on this subject since the first version of this Cochrane review (Terplan 2007), this update provides a valuable re‐evaluation of the effect of such interventions in this important population.
Objectives
To assess the effectiveness of psychosocial interventions for women enrolled in outpatient illicit drug treatment and to evaluate the effect of such interventions on increasing maternal and neonatal abstinence, and/or improving attendance and retention.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Pregnant women enrolled in illicit drug treatment programmes for any treatment of substance abuse or dependence of any drug. Illicit drugs include illegal substances such as cannabis, heroin, cocaine, amphetamines, etc. We also included women on methadone treatment.
Types of interventions
Experimental
Psychosocial interventions of any kind alone or given in addition to usual care.
Control
Comprehensive usual care that included pharmacological treatment such as methadone maintenance, counselling, prenatal care (PNC), STD counselling and testing, transportation, and/or childcare;
A different psychosocial intervention;
No intervention.
Types of outcome measures
Primary outcomes
1. Neonatal outcomes:
Preterm birth (gestational age at birth < 37 weeks);
‐Neonatal toxicology at delivery;
Low birth weight (birth weight < 2500 g);
Length of time spent in hospital post delivery.
2. Maternal drug use measured by:
Maternal toxicology;
Maternal self‐reported drug use.
3. Adverse events for the mother of the child.
Secondary outcomes
4. Retention in treatment measured as number of subjects retained at the end of the study; or 5. Short term retention in treatment measured as number of subjects retained at the end of one month after enrolment in the study or greater but before study completion.
Search methods for identification of studies
Electronic searches
We have listed the search methods we used in the original version of this Cochrane review (Terplan 2007) in Appendix 1.
For the update performed up to 28 January 2015, we searched the following databases:
Cochrane Drugs and Alcohol Group Specialized Register (searched January 2015);
Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library 2015, Issue 1) (Appendix 2);
PubMed (August 2006 to January 2015) (Appendix 3);
EMBASE (August 2006 to January 2015) (Appendix 4);
CINAHL (August 2006 to January 2015) (Appendix 5).
We combined the PubMed search with the Cochrane highly sensitive search strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version; PubMed format (2008 revision) (Lefebvre 2011).
In addition, we searched for ongoing clinical trials and unpublished studies via internet searches on the following sites:
ClinicalTrials.gov: www.clinicaltrials.gov (search date: 28 January 2015);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) www.who.int/ictrp/en/ (search date: 10 January 2015).
Searching other resources
We reviewed the reference lists of all articles obtained as full reports to identify any further studies not retrieved by our electronic searches. We sought personal communication, conference abstracts, and unpublished trials from books chapters on treatment of opioid dependence. In addition, we checked the database of Selective Dissemination (SDI) and the National Institute on Health (NIH), Bethesda, USA to identify additional studies. We scanned internet websites, including the National Institute on Drug Abuse (USA), National Institute on Alcohol Abuse and Alcoholism (USA) and National Treatment Agency for Substance Misuse (UK).
We identified trials by handsearching a wide range of healthcare/addiction journals, including those not indexed in the main electronic databases and those published in non‐English languages. We did not apply any language restrictions to this Cochrane review.
Data collection and analysis
Selection of studies
All review authors independently assessed studies for inclusion. We resolved any conflicts by consensus.
Data extraction and management
All review authors independently extracted data. We discussed any disagreements and resolved them by consensus. We sought information from studies including: participant demographics, what and when interventions were performed, how and when drug screening was performed, screening results, measures of retention and abstinence, and prenatal and maternal outcomes (gestation length, birth weight, maternal and neonatal toxicity, and neonatal hospital stay length).
Assessment of risk of bias in included studies
We performed the 'Risk of bias' assessment for included RCTs and CCTs using the criteria recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The 'Risk of bias' tool for assessing risk of bias in included studies is a two‐part tool, addressing seven specific domains, namely: sequence generation and allocation concealment (selection bias), blinding of participants and providers (performance bias), blinding of outcome assessor (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias. The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry, in terms of either low, high, or unclear risk. We used the criteria indicated by the Cochrane Handbook for Systematic Reviews of Interventions adapted to the addiction field.
We assessed the domains of sequence generation and allocation concealment (avoidance of selection bias) using the 'Risk of bias' tool by a single entry for each study.
We considered blinding of participants, personnel and outcome assessor (avoidance of performance bias and detection bias) separately for objective outcomes (e.g. drop out, use of substance of abuse measured by urine‐analysis, subjects relapsed at the end of follow‐up, subjects engaged in further treatments) and subjective outcomes (e.g. duration and severity of signs and symptoms of withdrawal, patient self‐reported use of substance, side effects, social functioning as integration at school or at work, family relationship). Blinding of participants and personnel was generally not feasible given the type of intervention, so we did not consider this item. In addition, we assessed detection bias based on blinding of outcome assessors.
Incomplete outcome data (avoidance of attrition bias) was considered for all outcomes except for drop out from the treatment, which is very often the primary outcome measure in trials on addiction. We also considered whether exclusion criteria could be a source of bias and if intention‐to‐treat analysis was used.
Measures of treatment effect
For most of the included studies, retention and abstinence outcomes were reported in terms of proportion (given as a percent) of participants in each intervention arm to have specific outcomes. When possible, we analysed the results as dichotomous outcomes (e.g. retention vs. non‐retention in treatment) and reported them as a risk ratio (RR) with a 95% confidence intervals (CIs).
Dealing with missing data
When questions regarding data arose, we contacted the primary study author. We attempted to contact six study authors and four responded. However, none had data that would have allowed additional analyses.
Assessment of heterogeneity
We analysed heterogeneity by means of the I2 statistic and Chi2 test for heterogeneity. The cut‐off points were: I2 statistic value > 50% and the Chi2 test P < 0.1. Also, we evaluated the occurrence of heterogeneity and reported it qualitatively for included studies because of variability between chosen control populations in different studies. For the outcomes measured, different studies used many types of control groups including pharmacological, placebo, and other psychosocial interventions.
Data synthesis
We combined the RR value from each trial through meta‐analysis using a random‐effects model. Otherwise, we reported the mean difference for outcomes that measured average measures (birth weight and days hospitalized after delivery).
Sensitivity analysis
We did not use methodological quality as a criterion for trial inclusion. However, in order to assess the effect of the low quality studies we planned to do a sensitivity analysis, either including or excluding the studies at high risk of bias from meta‐analysis.
Results
Description of studies
Results of the search
The original Cochrane review yielded 263 records of which 44 were considered eligible and were reviewed as full texts (Terplan 2007). We included a total of nine articles. Updated searches done in January 2015 identified a further 385 papers. We screened these articles by title/abstract and of these we retrieved and assessed 21 full text articles. We included five of these trials. In total we included 14 trials in this review update (Figure 1).
1.

Study flow diagram.
Included studies
Fourteen RCTs (1298 participants) met the inclusion criteria. All included studies were in the English language. For substantive descriptions of each of the included trials see the Characteristics of included studies section.
Trial duration
The duration of the included trials ranged from 14 days to 24 weeks.
Participants
We included a total of 1298 participants. Thirteen trials reported age and the mean age for those was 28.8 years. Two trials did not report the ethnicity of participants (O'Neill 1996; Yonkers 2012). All but three RCTs had a majority of African American participants. Carroll 1995 did not report ethnicity specifics, but most participants were non‐minority (11/14, 78.6%). Mullins 2004 reported 50% of participants were Caucasian and only 33% were African American. Winhusen 2008 reported 34.7% participants were African American women and 39.7% were Caucasian. Of the trials that reported ethnicity, on average 63.12% of participants were African American. All trials but O'Neill 1996 and Yonkers 2012 reported marital status. Overall, 79.8% of participants were single, never married, or divorced. All trials except O'Neill 1996 reported employment status for all participants. Overall, 88.6% of the trial participants were unemployed. Three studies did not report the educational level of participants (Carroll 1995; Jones 2001; Mullins 2004). Of the remaining studies, most participants had at least some high school education, either measured as a proportion (> 50%) or > 10 mean years of education. In Haug 2004, most participants had less than high school education (94%). Most trials did not mention gestational age at enrolment. Of the remaining trials, on average participants were enrolled during the second trimester, with the exception of Carroll 1995 in which the average gestational age was eight (± 6) weeks.
All but four studies (Carroll 1995; Winhusen 2008; Jones 2011; Tuten 2012a) used the Diagnostic and Statistical Manual of Mental Disorders (DSM‐III‐R or DSM‐IV‐R) criteria in the assessment of substance use among participants. With Carroll 1995, all participants can be assumed to be opioid dependent as they were receiving methadone maintenance. Many were also using cocaine (mean days of cocaine = 2.7 in 30 days prior to enrolment). Jones 2011 included participants if they had self‐reported heroin or cocaine use in the past 30 days. Participants reported an average of 14.9 days of opiate use over the past 30 days and an average of 13.4 days of cocaine use over the past 30 days. For Winhusen 2008, participants were identified by the participating treatment programmes as requiring substance abuse treatment, and no mention of DSM‐III‐R criteria was made. In Tuten 2012a, participants had an average of 13.4 days of use in the past 30 days for cocaine and 14.9 days of use in the past 30 days for opiates. In the remaining nine RCTs, 70% of participants were cocaine dependent, and 66.9% were opiate dependent. Only Mullins 2004 had no heroin‐using participants. Seven studies recorded both marijuana and alcohol use (O'Neill 1996; Svikis 1997; Jones 2001; Silverman 2001; Haug 2004;Mullins 2004; Svikis 2007). Within these studies, 23.7% of participants were marijuana dependent, and 17.9% were alcohol dependent. Only two trials recorded nicotine dependence (O'Neill 1996; Haug 2004), which was 99%. All included RCTs had high compliance with reporting.
Setting and country of origin
All included RCTs took place in the USA except for O'Neill 1996, which took place in Australia. All trials took place in drug treatment facilities that were either academic‐based, or hospital‐based, or both. All included trials were predominately in the outpatient setting. Five studies began in an inpatient setting (Jones 2000; Jones 2001; Svikis 2007; Jones 2011; Tuten 2012a) during which time randomization took place. All participants in these trials transitioned to outpatient management after seven days where they received the study intervention. Several trial sites provided free transportation and childcare to their participants. Jones 2000, Jones 2011, and Tuten 2012a also included on‐site PNC and psychiatric consultation. Mullins 2004 described their centre as providing "gender‐specific" treatment.
Study size
Study sizes ranged from 12 to 168 participants.
Types of interventions
Trials fell into two types of experimental interventions: nine with 704 participants used CM (Carroll 1995; Svikis 1997; Elk 1998; Jones 2000; Jones 2001; Silverman 2001; Svikis 2007; Jones 2011; Tuten 2012a), and five with 594 participants used MIB methods that involved motivational approaches (O'Neill 1996; Haug 2004; Mullins 2004; Winhusen 2008; Yonkers 2012).
With the exception of Silverman 2001 and Jones 2011, all RCTs applied CM in the form of monetary vouchers. In Silverman 2001, the concept of abstinent reinforcement contingencies were integrated into an employment setting referred to as the Therapeutic Workplace. The participants received work and salary only when they remained abstinent. For Jones 2011, CM methods were applied to housing. A key piece of the treatment plan in this study involved abstinence‐contingent housing.
Among the included trials, vouchers were tied to negative urine toxicology (Carroll 1995; Silverman 2001; Jones 2011; Tuten 2012a), treatment attendance (Svikis 1997; Svikis 2007), or both (Elk 1998; Jones 2000; Jones 2001). Although the original applications of CM to substance treatment involved a schedule of escalating reinforcement for sustained behaviours (e.g. Higgins 1991), only Jones 2000, Jones 2001, Svikis 2007, and Tuten 2012a used an escalating schedule.
Ten trials provided psychosocial interventions in addition to comprehensive usual care that included pharmacological treatment such as methadone maintenance, counselling, PNC, STD counselling and testing, transportation, and/or childcare (Carroll 1995; O'Neill 1996; Svikis 1997; Elk 1998; Jones 2000; Silverman 2001; Haug 2004; Mullins 2004; Jones 2011; Tuten 2012a). Eight RCTss (Carroll 1995; O'Neill 1996; Svikis 1997; Jones 2000; Silverman 2001; Haug 2004; Jones 2011; Tuten 2012a) also had patients in both the intervention and control groups receive methadone maintenance treatment (MMT).
For the control interventions, eight studies utilized usual care or comprehensive usual care (O'Neill 1996; Elk 1998; Jones 2000; Jones 2001; Silverman 2001; Haug 2004; Winhusen 2008; Jones 2011). Five of the fourteen studies used other psychosocial interventions for the control group (Carroll 1995; Svikis 1997; Mullins 2004; Tuten 2012a; Yonkers 2012). O'Neill 1996 used only MMT with the control group and no other interventions.
Excluded studies
We excluded 52 studies in total from this review. The reasons for exclusion were: study design did not meet inclusion criteria (i.e. it was not a RCT) (37 studies); study participants did not meet inclusion criteria, either not pregnant or not in treatment (seven studies); paper was a re‐analysis of already published data, in this case the primary paper that matched the inclusion criteria best was included (four studies); or intervention was not within the scope of the review (three studies). For further details, see the Characteristics of excluded studies section.
Risk of bias in included studies
Allocation
Only five studies reported an adequate method of randomization and were judged as having low risk of selection bias (Jones 2001; Silverman 2001; Winhusen 2008; Jones 2011; Yonkers 2012). In Jones 2001, randomization was performed via coloured chip selection from a hat (with replacement of the chip following each selection). Jones 2011 and Yonkers 2012 used a computer programme to generate randomized assignments. In Silverman 2001, a modified dynamic balanced randomization (Signorini 1993) was used to randomize patients sequentially to the treatment conditions. Winhusen 2008 used an urn randomization protocol, the details of which are limited, but which was likely sufficient to limit risk of bias. All other included trials simply reported that the groups were randomized, so were judged as having unclear risk of bias.
Only two trials adequately described an adequate method of allocation concealment (Jones 2011; Yonkers 2012). The remainder did not describe a method for allocation concealment and were judged as having an unclear risk of bias.
Blinding
Blinding of participants and providers was not considered because, given the type of interventions reviewed, blinding of the study participants and providers was impossible. There was only one mention in the included RCTs as to whether the outcomes were assessed in a blinded fashion. O'Neill 1996 stated that follow‐up assessments were conducted by an interviewer who was blind to the participants' assignments. Yonkers 2012 used an objective measure (urine toxicology) unlikely to be biased by lack of blinding. All other RCTs were judged as having an unclear risk of detection bias.
Incomplete outcome data
Seven studies were judged to have low risk of bias (Elk 1998; Jones 2001; Silverman 2001; Svikis 2007; Winhusen 2008; Jones 2011; Yonkers 2012). Carroll 1995 and Haug 2004 were judged as high risk of attrition bias. The other trials did not provide information about attrition from the study and were judged as having an unclear risk of bias
Other potential sources of bias
All included trials collected and reported baseline characteristics, such as demographic data. This allowed for the comparison of the study sample between intervention and control groups in all but two trials. Svikis 1997 reported information stratified by methadone maintenance status. Therefore, it was impossible to evaluate differences between intervention and control groups. Svikis 2007 only provided summary statistics for the entire study group, and we were unable to separate them by intervention vs. control group assignment. In the remaining studies, the groups were judged to be similar in terms of baseline drug use. With the exception of Haug 2004, demographic data between the groups were similar. In Haug 2004, the groups differed with respect to race; there was a higher proportion of Caucasians in the intervention group (23% vs. 6%).
We have provided summary figures related to the risk of bias of included studies (Figure 2; Figure 3).
2.
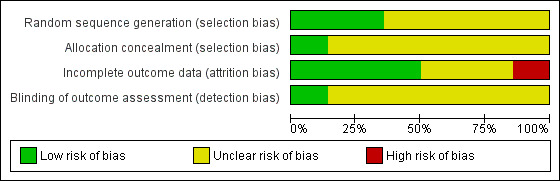
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
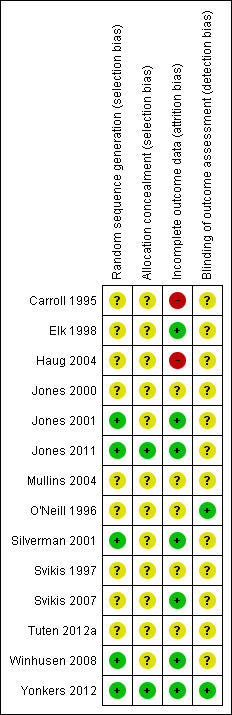
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
We considered the following types of comparisons:
Comparison 1: Any psychosocial vs. control, 14 RCTs, 1298 participants (Carroll 1995; O'Neill 1996; Svikis 1997; Elk 1998; Jones 2000; Jones 2001; Silverman 2001; Haug 2004; Mullins 2004; Svikis 2007: Winhusen 2008; Jones 2011; Tuten 2012a; Yonkers 2012);
Comparison 2: CM vs. control, nine RCTs, 704 participants (Carroll 1995; Svikis 1997; Elk 1998; Jones 2000; Jones 2001; Silverman 2001; Svikis 2007; Jones 2011; Tuten 2012a);
Comparison 3: MIB vs. control, five RCTs, 594 participants (O'Neill 1996; Haug 2004; Mullins 2004; Winhusen 2008; Yonkers 2012).
We summarized the results and compared them quantitatively when possible.
Comparison 1: Any psychosocial Intervention vs. control neonatal outcomes
See Table 1.
Neonatal outcomes
Neonatal outcomes were rarely captured. Four RCTs reported neonatal outcomes (Carroll 1995; Elk 1998; Jones 2011; Yonkers 2012).
Preterm birth
Three trials (Elk 1998; Jones 2011; Yonkers 2012) with 264 participants (RR 0.71, 95% CI 0.34 to 1.51; three trials, 264 participants; Analysis 1.1) showed no difference in preterm birth. Carroll 1995 measured median gestational age at delivery. Women in the intervention group (both contingency management and motivational interviewing methods) had slightly longer gestations (40 vs. 38 weeks) than the control groups. Null hypothesis testing was not provided and our attempts to obtain further data from trial authors were not fruitful.
1.1. Analysis.

Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 1 Preterm birth (< 37 weeks gestation).
Positive neonatal toxicology at delivery
One trial (Jones 2011) with 89 participants (RR 1.91, 95% CI 0.86 to 4.24; one trial, 89 participants; Analysis 1.2).
1.2. Analysis.
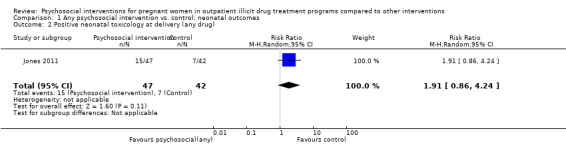
Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 2 Positive neonatal toxicology at delivery (any drug).
Low birth weight
One trial (Yonkers 2012) (RR = 0.72, 95% CI 0.36 to 1.43; one trial, 160 participants; Analysis 1.3) showed no difference in low birth weight. Carroll 1995 measured median birth weight. Women in the intervention group had heavier infants (3.348 gm vs. 2.951 gm). Null hypothesis testing was not provided and our attempts to obtain data from the trial authors were not fruitful.
1.3. Analysis.

Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 3 Low birth weight (< 2500 g).
Days hospitalized after delivery
Two studies (Carroll 1995, Jones 2011) (RR = ‐1.27, 95% CI ‐2.52 to ‐0.03; two trials, 103 participants; Analysis 1.4; Figure 4) showed a decrease in days hospitalized after surgery. Elk 1998 stated that there was no difference in length of hospital detoxification for newborns between the intervention and control groups, although mean days or other summary statistics were not reported.
1.4. Analysis.
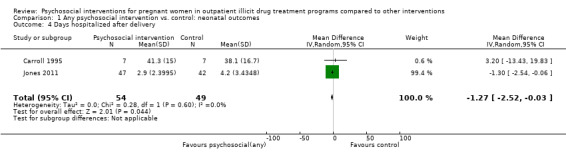
Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 4 Days hospitalized after delivery.
4.
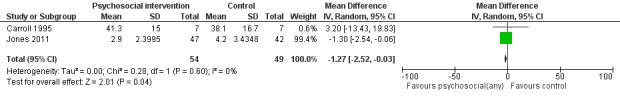
Forest plot of comparison: 1 Neonatal outcomes any psychosocial intervention vs. control, outcome: 1.5 Mean days hospitalized after delivery.
Adverse events
Elk 1998 reported adverse perinatal events between the groups (although "adverse perinatal events" were not clearly defined). Nobody in the intervention group had an adverse event, whereas 80% of the control group did: two had preterm labour and two delivered pre‐term (prior to 37 weeks). However, this difference was not statistically significant (P = 0.22).
Comparison 2: Any psychosocial Intervention vs. control neonatal outcomes maternal outcomes
Maternal drug use measured by maternal toxicology
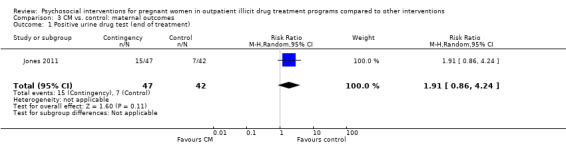
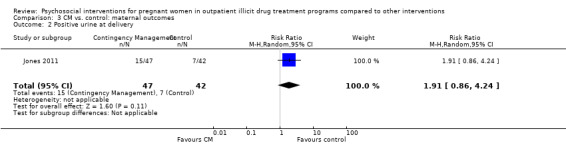
All but two included trials reported that urine toxicology was measured (Svikis 1997; Svikis 2007). However, only three RCTs included these data. We received raw data from the authors of Haug 2004 but were unable to incorporate these data into the meta‐analysis. The authors of Winhusen 2008 measured positive urine toxicology at one month of treatment (25.4% ‐ intervention group vs. 27.8% ‐ control group) and at treatment completion (16.7% intervention and 14.3% control). Jones 2011 and Yonkers 2012 also captured urine toxicology at the end of treatment. We performed a meta‐analysis from the available data for these three studies (Winhusen 2008; Jones 2011; Yonkers 2012): RR 1.14, 95% CI 0.75 to 1.73; three trials, 367 participants; Analysis 2.1). Pooling urine toxicology results at delivery only (Jones 2011; Yonkers 2012) (RR 1.18, 95% CI 0.52 to 2.65; two trials, 217 participants; Analysis 2.3).Overall for the four trials that employed MIB, there were no differences related to substance use. Among the trials employing CM, two trials showed a reduction in drug use measured by urine toxicology although the magnitude of this reduction was minimal and transient (Jones 2001) or difficult to assess (Silverman 2001).
2.1. Analysis.
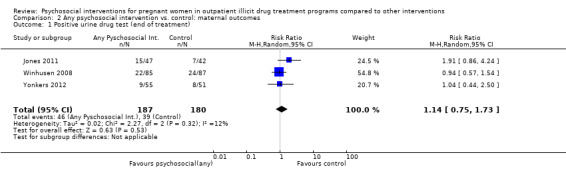
Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 1 Positive urine drug test (end of treatment).
2.3. Analysis.
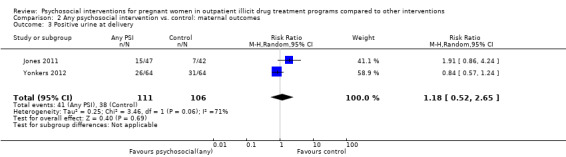
Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 3 Positive urine at delivery.
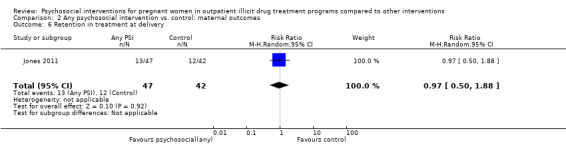
Retention at treatment completion
Ten trials measured retention at the end of study treatment (O'Neill 1996; Svikis 1997; Elk 1998; Jones 2000; Jones 2001; Haug 2004; Mullins 2004; Svikis 2007; Winhusen 2008; Jones 2011): RR 0.99 (95% CI 0.93 to 1.06; 10 trials, 819 participants; Analysis 2.4).
2.4. Analysis.
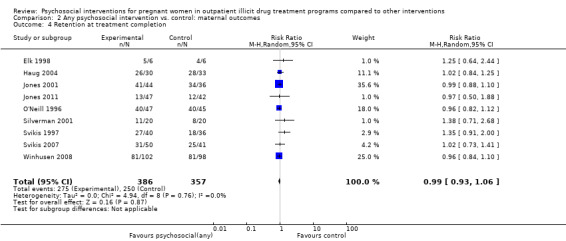
Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 4 Retention at treatment completion.
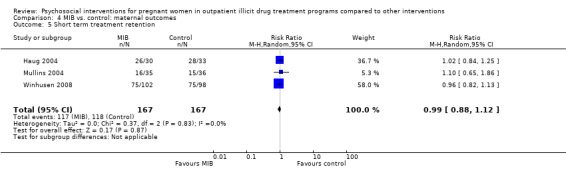
Short term treatment retention
Early treatment retention was assessed by looking at retention at longer than one month but less than the time of treatment completion. Six RCTs including 514 participants had data beyond one month but less than the end of treatment date (O'Neill 1996; Svikis 1997; Elk 1998, Haug 2004; Mullins 2004; Winhusen 2008). The pooled estimates across these studies yielded: RR 1.00, 95% CI 0.90 to 1.10; six trials, 514 participants; Analysis 2.5. We performed sensitivity analysis by excluding Mullins 2004, but this did not alter the results.
2.5. Analysis.
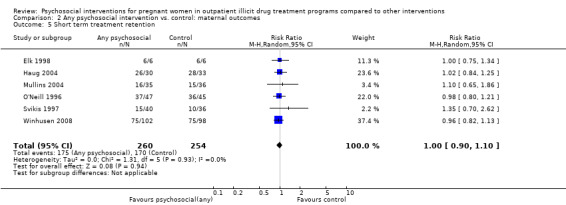
Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 5 Short term treatment retention.
Comparison 3 : CM interventions vs. control
For this comparison, only single studies addressed primary outcomes and therefore meta‐analysis was not possible.
Retention at treatment completion
As previously described in the aggregate retention at treatment completion (Analysis 2.4), six studies utilizing CM interventions reported information related to retention in treatment (Svikis 1997; Elk 1998; Jones 2001; Silverman 2001; Svikis 2007; Jones 2011). RR 1.03, 95% CI 0.92 to 1.16; six trials, 388 participants; Analysis 3.3; Figure 5. Short term retention was described in two different studies (Elk 1998 and Svikis 1997). RR 1.10 (95% CI 0.70, 1.73; 2 trials, 88 participants; Analysis 3.4).
3.3. Analysis.

Comparison 3 CM vs. control: maternal outcomes, Outcome 3 Retention at treatment completion.
5.

Forest plot of comparison: 2 CM vs. control, outcome: 3.1 Retention in treatment at the end of study.
3.4. Analysis.

Comparison 3 CM vs. control: maternal outcomes, Outcome 4 Short term treatment retention.
Comparison 4 : MIB interventions vs. control
Maternal drug use measured by maternal toxicology
Related to the primary outcome of positive urine drug tests at the end of treatment only two MIB method trials (Winhusen 2008 and Yonkers 2012) had data, demonstrating no substantial difference in urine toxicology between the MIB intervention groups and the control groups. RR 0.96 (95% CI 0.63, 1.48; 2 trials 278 participants; Analysis 4.1)
4.1. Analysis.
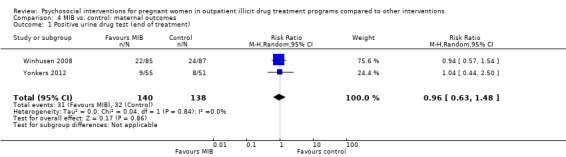
Comparison 4 MIB vs. control: maternal outcomes, Outcome 1 Positive urine drug test (end of treatment).
Retention at treatment completion
Three trials provided data on retention in treatment using MIB techniques (O'Neill 1996; Haug 2004; Winhusen 2008). Pooling these data via meta‐analysis resulted in: RR 0.97, 95% CI 0.89 to 1.06; three trials, 355 participants; Analysis 4.4).
4.4. Analysis.
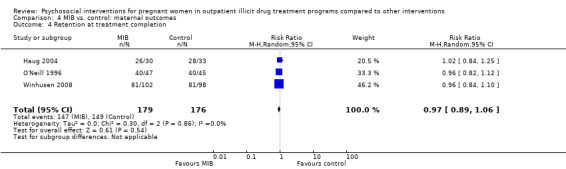
Comparison 4 MIB vs. control: maternal outcomes, Outcome 4 Retention at treatment completion.
Discussion
Summary of main results
We included 14 RCTs, nine that employed a form of CM and five that used a manual‐based intervention, namely MIB techniques. We did not identify any trials that assessed other types of psychosocial interventions. All interventions were compared with varied comprehensive usual care control.
Our main interest was in both obstetrical and neonatal outcomes of treatment. Illicit drug use is associated with a myriad of complications for both the pregnant woman and her newborn. These complications are costly and identifying a means of reducing their prevalence would be beneficial. Unfortunately, the included RCTs rarely reported on obstetrical or neonatal outcomes. Furthermore, four trials listed obstetrical events, such as early delivery and miscarriage, as exclusion criteria. Only four trials reported birth outcomes (Carroll 1995; Elk 1998; Jones 2011; Yonkers 2012). The three CM trials showed a benefit with intervention. There was also inconsistency between the studies with regards to the obstetrical outcomes measured. Carroll 1995 and Jones 2011 measured both mean gestational age and mean birth weight. However, Elk 1998 counted "adverse perinatal events", a category that included both preterm delivery, a serious obstetrical event, as well as preterm labour, a clinical event of far less significance.
Eleven included trials reported continued illicit drug use, as measured primarily by urine toxicology. All trials had abstinence as a goal of treatment except O'Neill 1996 which employed a harm reduction model. This was also the only study which relied exclusively on client self‐report to assess continued drug use. The frequency of urine sampling varied between studies, as did the methods of reporting the results. It was often unclear how the summary proportions had been generated and, with the exception of Elk 1998, none mentioned statistical adjustment for multiple testing. Overall, there appears to be little evidence that psychosocial interventions reduce continued illicit drug use in pregnant women enrolled in drug treatment. None of the trials that employed MIB techniques reported an effect of the intervention on drug abstinence. Of the two RCTs that reported an effect, both employed CM. However, the magnitude of the reduction was difficult to assess (Silverman 2001), and its effect was small and temporary (Jones 2001).
A primary goal of CM is to alter, if not to eliminate, drug use behaviours. Over the last decade, the clinical evidence demonstrating CM efficacy in reinforcing drug abstinence has been established (for a good summary see Sitzer 2006). This Cochrane review is the first to specifically address drug treatment in pregnancy. It appears that the benefits of CM on drug abstinence are not as profound in pregnant women as in the general population of people in drug treatment. MIB methods in contradistinction have not been shown to have consistent effects on subsequent abstinence (Miller 2003) in drug treatment. Similarly, our results show no benefit of MI in pregnancy, with a trend towards a negative effect.
Included trials reported retention in treatment most consistently of all outcomes. As with any surrogate outcome, its clinical utility is dependent upon the evidence of it as an intermediary to the important clinical outcome (Grimes 2005). In pregnancy, attendance in drug treatment has been shown to lead to increase birth weight, increase in one minute Apgars, and overall lower costs (Hubbard 1989; McCaul 1996; Svikis 1998). We found no difference in treatment retention either by CM or MIB techniques. Psychosocial interventions, either CM or MIB, did not improve treatment retention.
Overall, our Cochrane review identified few experimental studies of psychosocial interventions in pregnancy for illicit drug use. This is surprising given the wide use of such interventions in clinical treatment. Furthermore, it is unlikely that manual based interventions, such as MI, are used in isolation in clinical practice. Counsellors most likely use different parts of multiple modalities simultaneously. Even if a particular psychosocial intervention is found to be beneficial, its application may be limited by the realities of drug treatment.
It is important to note that sites where these psychosocial treatments were delivered also included supplementary services for both the experimental and control groups, including child care services, transportation, and housing. These additional services are likely to have contributed an overall effect to drug treatment outcomes, hence the differential contribution of the psychosocial intervention might have been difficult to observe. The drug treatment locations from where this review's data were obtained are likely more comprehensive than standard drug treatment centres that enrol pregnant women. We cannot assess whether psychosocial interventions in the absence of these additional services are beneficial to pregnant women.
Overall completeness and applicability of evidence
There were several limitations of the included RCTs. Overall trials were heterogeneous, differing in the details of both the intervention and the control, and employing different lengths of treatment. Some studies were quite small and likely underpowered, especially for obstetrical outcomes. The study settings were all similar (academic specialist drug dependence units primarily in the USA), as were the included participants. Although this decreases heterogeneity between trials, the results of this review may not be generalizable to other settings.
One of the limitations to CM is the cost. On average, CM participants in the included trials cost up to $1364 per client (Tuten 2012a). Clearly this limits the applicability of CM on a large scale. For this reason non‐cash incentives, such as vouchers, have been used. Silverman 2001 extended this concept to include job training.
It is important to acknowledge that many pregnant women are referred to drug treatment from the criminal justice system (Terplan 2010). Unfortunately, no included trials discussed the referral pattern of their respective patient populations. Manual based interventions, such as MI, are likely less effective among coerced people. In fact, they may be counterproductive. Actively engaging a pregnant woman before she is ready to change may be detrimental, especially when the intervention involves MIB techniques (Hettema 2005).
It is also important to note the characteristics of the included participants. The patients were overwhelmingly unemployed, poor, African American, and with low education levels. This limits the generalizability of the study results. The population of women who use illicit drugs in pregnancy is different from those enrolled in drug treatment, which differs from the population of women in treatment who participate in experimental trials. In general, the efficacy of psychosocial interventions may vary between these different patient subsets.
Quality of the evidence
The quality of reporting in the included studies was very poor so that for most items and studies a judgment of unclear risk of bias was given. A recurring issue was an observed heterogeneity between the included trials in terms of outcomes measurements. For example, trials measured urine toxicology differently (and at different time points). Similarly, retention in treatment definitions varied and were assessed at different time points between studies. See also Table 1.
Potential biases in the review process
The data extraction and input process were consensus driven and several review authors verified this independently. We believe that we identified all relevant studies as we searched the grey literature, and contacted trial authors and field experts. However, we were unable to obtain additional data from some trial authors which would have enriched analysis of neonatal and urine toxicology outcomes. Given the absence of any effect of the interventions on retention or urine toxicology, it is unlikely that these additional data would have changed our summary meta‐analysis.
Agreements and disagreements with other studies or reviews
Since our first version of this Cochrane review (Terplan 2007), no other systematic reviews have been performed on this topic.
Authors' conclusions
Implications for practice.
In conclusion, psychosocial interventions when taken in the presence of other comprehensive care options do not translate into better neonatal or obstetrical outcomes, nor are they associated with greater illicit drug abstinence or increased treatment retention among pregnant women. The included trials rarely captured maternal and neonatal outcomes and there was no data on cost benefit. Though psychosocial interventions were found to reduce days neonates were hospitalized after delivery, it is unclear if this observation is reflective of real effect or delivery practices. Therefore, there is insufficient evidence to evaluate the effect of psychosocial interventions on birth or neonatal outcomes among pregnant women in treatment. Overall, the current quality of evidence was low to moderate and future studies should measure these outcomes systematically.
Implications for research.
Large RCTs with obstetrically meaningful endpoints and longer follow‐up times are required to examine whether psychosocial interventions help pregnant women with illicit drug dependence. Poor obstetrical outcomes should not be exclusion criteria to study participation, as these events are essential to capture as they may be related to illicit drug use. Ideally these studies should have multiple sites in order to capture a greater diversity of study patients which would increase the generalizability of the findings. The reporting of criminal justice referral into treatment is especially important, as the efficacy of psychosocial interventions may be different between people who have been coerced into treatment and those who enter voluntarily.
Questions that should be considered include:
Is one psychosocial intervention more effective than another?
What covariate, such as drug use history, time in treatment, or gestational age upon enrolment, are associated with treatment effectiveness?
What is the optimal reimbursement for CM and its overall cost effectiveness?
Overall the trials should state clearly the method of randomization and allocation concealment. Although blinding of participants is impossible, those assessing outcomes and performing the analyses should be blinded (and this should be clearly stated). Intention to treat (ITT) analysis should be performed and power calculations used a priori.
What's new
| Date | Event | Description |
|---|---|---|
| 2 April 2015 | Amended | Contact person email updated |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 16 February 2015 | New citation required and conclusions have changed | Conclusions changed. |
| 28 January 2015 | New search has been performed | We updated the review to include reviewer recommendations and new studies identified from the updated literature search up to January 2015. |
| 3 August 2007 | New citation required and conclusions have changed | Substantive amendment |
Appendices
Appendix 1. Search strategy
For the original search executed in May 2006, we searched the Cochrane Drugs and Alcohol Group Register (May 2006); CENTEAL, (The Cochrane Library, Issue 3, 2005); MEDLINE (1996 to August 2006); EMBASE (January 1996 to August 2006); and CINAHL (January 1982 to August 2006). We followed the 'optimal' MEDLINE and EMBASE sensitive search strategies devised by the Cochrane Collaboration for RCTs as published in Section 6.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) in order to identify studies relevant to this Cochrane review.
Search strategy to locate drug abuse
1. SUBSTANCE ADJ RELATED ADJ DISORDERS 2. SUBSTANCE‐RELATED‐DISORDERS#.DE. 3. ADDICT$4.TI,AB. 4. (OVERDOS$2 OR OVER‐DOS$2).TI,AB. 5. INTOXICAT$3.TI,AB. 6. (ABSTINEN$2 OR ABSTAIN$2).TI,AB. 7. WITHDRAW$2.TI,AB. 8. (ABUSE$2 OR USE).TI,AB. 9. (EXCESSIVE$2 ADJ USE$1).TI,AB. 10. (USE$2 ADJ DISORDER$2).TI,AB. 11. PSYCHOSES‐SUBSTANCE‐INDUCED 12. PSYCHOSES‐SUBSTANCE‐INDUCED#.DE. 13. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12
Search strategy to identify drugs
14.HEROIN or HEROIN#.W..DE. or HEROIN.TI,AB. 15.NARCOTICS or NARCOTICS#.W..DE. 16.OPIOID.TI,AB. Or OPIATE.TI,AB. Or OPIATE.RN. 17.ANTI‐ANXIETY‐AGENTS#.DE. 18.BENZODIAZEPINE or BENZODIAZEPINES#.W..DE. 19.BARBITURATES or BARBITURATES#.W..DE. or BARBITURATES.TI,AB. 20.AMPHETAMINES or AMPHETAMINE#.W..DE. or AMPHETAMINE.TI,AB. 21.DESIGNER ADJ DRUGS or DESIGNER‐DRUGS#.DE. or (DESIGNER ADJ DRUGS).TI,AB. or (DESIGNER ADJ DRUGS).RN. 22.HALLUCINOGENS or HALLUCINOGENS#.W..DE. or HALLUCINOGENS.TI,AB. or HALLUCINOGENS.RN. 23.STREET ADJ DRUGS or STREET‐DRUGS#.DE. or(STREET ADJ DRUGS).TI,AB. or STREET‐DRUGS.TI,AB. 24.COCAINE or COCAINE#.W..DE. or COCAINE.TI,AB. or COCAINE.RN. 25.ALOCHOLS or ALCOHOLS#.W..DE. or ALCOHOL.TI,AB. 26.LYSERGIC ADJ ACID or LYSERGIC‐ACID#.DE. or (LYSERGIC ADJ ACID).TI,AB. or (LYSERGIC ADJ ACID).RN. or LSD.TI,AB. or LSD.RN. or LSD or LYSERGIC‐ACID‐DIETHYLAMIDE#.DE. 27.KETAMINE or KETAMINE#.W..DE. or KETAMINE.TI,AB. or KETAMINE.RN. 28.CANNABIS or CANNABIS#.W..DE. or CANNABIS.TI,AB. or CANNABIS.RN. 29.MARIHUANA.TI,AB. or MARIHUANA.RN. or MARIJUANA or MARIJUANA‐SMOKING#.DE. or MARIJUANA‐ABUSE#.DE. or MARIJUANA.TI,AB. 30.HASHISH or HASHISH.TI,AB. 31.OPIUM or OPIUM#.W..DE. or OPIUM.TI,AB. 32.INHALANT$2.TI,AB. or (INHALANT$2 ADJ ABUSE$2).TI,AB. 33.SOLVENT or SOLVENTS#.W..DE. or SOLVENT$2.TI,AB. or SOLVENT$2.RN. 34.(STEROID$2 ADJ ABUSE).TI,AB. or ANABOLIC ADJ STEROIDS or ANABOLIC‐AGENTS#.DE. 35.(ANABOLIC ADJ AGENT$2).TI,AB. AND PERFORM$6.TI,AB. 36.METHADONE or METHADONE#.W..DE. or METHADONE.TI,AB. METHADONE.RN. 37.14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36
Search strategy to locate interventions
38.PATIENT ADJ COMPLIANCE or PATIENT‐COMPLIANCE#.DE. 39.(ATTENDANCE ADJ INCENTIVE$2).TI,AB. 40.INCENTIVE$2.TI,AB. or VOUCHER$2.TI,AB. 41.PSYCHOTHERAPY or PSYCHOTHERAPY#.W..DE. 42.BEHAVIOR‐THERAPY#.DE. or BEHAVIOR ADJ THERAPY 43.REINFORCEMENT ADJ PSYCHOLOGY or REINFORCEMENT‐PSYCHOLOGY#.DE. or REINFORCEMENT.TI,AB. 44.MOTIVATION or MOTIVATION#.W..DE. or MOTIVATION ADJ INTERVIEWING 45.(CONTINGENCY ADJ MANAGEMENT).TI,AB. 46.38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45
Search strategy to identify pregnancy
47.PREGNANCY or PREGNANCY#.W..DE. or PREGNAN$4.TI,AB.
Search strategy to locate RCTs and different types of studies
48.PT=RANDOMIZED‐CONTROLLED‐TRIAL or RANDOMIZED ADJ CONTROLLED ADJ TRIAL or RANDOMIZED‐CONTROLLED‐TRIALS#.DE. 49.PT=CONTROLLED‐CLINICAL‐TRIAL or RANDOM ADJ ALLOCATION or RANDOM‐ALLOCATION.DE. 50.DOUBLE ADJ BLIND ADJ METHOD or DOUBLE‐BLIND‐METHOD.DE. 51.SINGLE‐BLIND‐METHOD.DE. 52.PT=CLINICAL‐TRIAL$ or CLINICAL ADJ TRIALS or CLINICAL‐TRIALS#.DE. or (CLINIC$2 ADJ TRIAL$2).TI,AB. 53.((SINGL$2 OR DOUBL$2 OR TREBL$2 OR TRIPL$2) ADJ (BLIND$2 OR MASK$2)).TI,AB. 54.PLACEBOS or PLACEBOS#.W..DE. or PLACEBO$2.TI,AB. 55.RANDOM$4.TI,AB. 56.RESEARCH ADJ DESIGN or RESEARCH‐DESIGN#.DE. 57.COMPARATIVE ADJ STUDY or COMPARATIVE‐STUDY.DE. 58.EVALUATION‐STUDIES#.DE. 59.PROSPECTIVE‐STUDIES#.DE. 60.48 OR 49 OR 51 OR 52 OR 53 OR 54 OR 55 OR 57 OR 59
Search strategy to locate studies for this review
61.13 and 37 and 46 and 47 and 60
Appendix 2. CENTRAL search strategy
MeSH descriptor Substance‐Related Disorders explode all trees
((stimulant* or polydrug* or drug* or substance) near (abstain* or abstinen* or abus* or addict* or dependen* or disorder* or intoxicat* or misuse* or over dos* or overdos* or withdraw*)):ab,ti
(#1 OR #2)
(abstain* or abstinen* or abus* or addict* or drug user* or dependen* or inject* drug* or intoxicat* or misus* or overdos* or illicit use* or withdraw*):ti,ab,kw
MeSH descriptor Heroin explode all trees
(heroin or morphine* or diamorphine or diacetylmorphine or morfin* or narcotic* or methadone):ti,ab,kw
MeSH descriptor Methadone explode all trees
(opioid* or opiate* or opium):ti,ab,kw
MeSH descriptor Narcotics explode all trees
MeSH descriptor Anti‐Anxiety Agents explode all trees
MeSH descriptor Benzodiazepines explode all trees
(benzodiazepine):ti,ab,kw
MeSH descriptor Barbiturates explode all trees
(barbiturates):ti,ab,kw
MeSH descriptor Amphetamine explode all trees
(amphetamine* or methamphetamine*):ti,ab,kw
(Designer near/2 Drug*):ti,ab,kw
MeSH descriptor Hallucinogens explode all trees
(ecstasy or MDMA or hallucinogen*):ti,ab,kw
MeSH descriptor Street Drugs explode all trees
MeSH descriptor Cocaine explode all trees
(crack or cocaine):ti,ab,kw
(alcohol*):ti,ab,kw
MeSH descriptor Lysergic Acid explode all trees
(Lysergic NEXT Acid):ti,ab,kw
(LSD):ti,ab,kw
(ketamine):ti,ab,kw
MeSH descriptor Ketamine explode all trees
MeSH descriptor Cannabis explode all trees
(cannabis or marijuana or marihuana or hashish):ti,ab,kw
(Inhalant NEXT abuse ):ti,ab,kw
(Solvent*):ti,ab,kw
MeSH descriptor Solvents explode all trees
(anabolic near steroid*):ti,ab,kw
MeSH descriptor Anabolic Agents explode all trees
(steroid* near/3 abuse):ti,ab
(anabolic near agent*):ti,ab
(#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37)
(#4 AND #38)
(#3 OR #39)
MeSH descriptor Patient Compliance explode all trees
(patient NEXT compliance):ti,ab,kw
MeSH descriptor Psychotherapy explode all trees
MeSH descriptor Reinforcement (Psychology) explode all trees
MeSH descriptor Motivation explode all trees
(behavi* near/3 therap*):ti,ab
(psychotherap* or psychosocial or voucher* or incentive* or reinforcement or motivation*):ti,ab,kw
(contingency near management):ti,ab,kw
(#41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48)
MeSH descriptor Pregnancy explode all trees
(pregnan*):ti,ab,kw
(#50 OR #51)
(#40 AND #49 AND #52)
Appendix 3. PubMed search strategy
Substance‐related disorders[MeSH]
Psychoses, Substance‐Induced[MeSH]
(abstain*[tiab] OR abstinen*[tiab] OR abus*[tiab] OR addict*[tiab] OR dependen*[tiab] OR disorder*[tiab] OR intoxicat*[tiab] OR misuse*[tiab] OR use*[tiab] OR over‐dos*[tiab] OR overdos*[tiab] OR withdraw*[tiab])
#1 OR #2 OR #3
Heroin [MH]
Anti‐anxiety agents [MeSH]
Benzodiazepines [MeSH]
Barbiturates [MeSH]
Amphetamines [MeSH]
Designer drugs [MeSH]
"designer drug"[tiab]
"illicit drug"[tiab]
Antidepressive agents [MeSH]
Hallucinogens [MeSH]
Street drugs [MeSH] OR street‐drug* [tiab]
Cocaine [MeSH]
Lysergic acid [MeSH] or lysergic‐acid* [tiab] or LSD[tiab]
Ketamine [MeSH]
Cannabis [MeSH]
Opium [MeSH]
"steroid abuse"[tiab]
Anabolic steroids [MeSH]
drug*[tiab] OR substance[tiab] OR polidrug*[tiab] OR alcohol*[tiab] OR amphetamine*[tiab] OR cannabis[tiab] OR marihuana[tiab] or marijuana[tiab] OR "hash oil*"[tiab] OR hashish[tiab] OR cocaine[tiab] OR hallucinogen* [tiab] OR heroin[tiab] OR mdma[tiab] OR ecstasy[tiab] OR methamphetamine*[tiab] OR narcotic* [tiab] OR ketamine[tiab] OR opioid*[tiab] OR opiate* [tiab] OR opium[tiab] OR tranquilizer*[tiab] OR tranquiliser*[tiab] OR inhalant*[tiab] OR barbiturate*[tiab] OR solvent*[tiab] OR stimulant*[tiab]
#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22
Patient Compliance[MH]
Psychotherapy[MH]
reinforcement psychology[MeSH Terms]
motivation[mh]
(behavi*[tiab] AND therap*[tiab])
(psychotherap*[tiab] OR psychosocial[tiab] OR voucher*[tiab] OR incentive*[tiab] OR reinforcement[tiab] OR motivation*[tiab])
(contingency[tiab] AND management[tiab])
#25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31
"pregnancy"[MeSH Terms]
pregnan*[tiab]
#33 OR #34
randomized controlled trial [pt]
controlled clinical trial [pt]
randomized [tiab]
placebo [tiab]
drug therapy [sh]
randomly [tiab]
trial [tiab]
groups [tiab]
#36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43
animals [mh] NOT humans [mh]
#44 NOT #45
#4 AND #24 AND #32 AND #35 AND #46
Appendix 4. EMBASE search strategy
'addiction'/exp
dependen*:ab,ti OR addict*:ab,ti OR overdos*:ab,ti OR intoxicat*:ab,ti OR abstin*:ab,ti OR abstain:ab,ti OR withdraw*:ab,ti OR abus*:ab,ti OR use*:ab,ti OR misus*:ab,ti OR disorder*:ab,ti
#1 OR #2
'diamorphine'/exp
diamorphine:ab,ti OR heroin:ab,ti OR narcotic*:ab,ti OR drug*:ab,ti OR polydrug:ab,ti OR substance:ab,ti OR opioid:ab,ti OR opiate:ab,ti OR opium:ab,ti OR hallucinogen*:ab,ti OR amphetamine*:ab,ti OR barbiturate:ab,ti OR inhalant*:ab,ti OR morphine:ab,ti OR ecstasy:ab,ti OR mdma:ab,ti
'street drug'/exp
'designer drug'/exp
'lysergic acid'/exp OR 'lysergic acid':ab,ti OR lsd:ab,ti
'cocaine'/exp OR cocaine:ab,ti
'alcohol'/exp OR alcohol:ab,ti
'ketamine'/exp OR ketamine:ab,ti
'cannabis'/exp OR cannabis:ab,ti OR hashish:ab,ti OR marihuana:ab,ti OR marijuana:ab,ti
'inhalant abuse'/exp OR inhalant:ab,ti
'solvent'/exp OR solvent:ab,ti
'methadone'/exp OR methadone:ab,ti
'anabolic agent'/exp
steroid*:ab,ti AND abuse:ab,ti
anabolic:ab,ti AND agent*:ab,ti
'benzodiazepine'/exp OR benzodiazepine:ab,ti
'amphetamine'/exp OR amphetamine:ab,ti
#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20
'patient compliance'/exp
'psychotherapy'/exp
'reinforcement'/exp
'motivation'/exp
incentive*or:ab,ti OR voucher*:ab,ti OR psychotherap*:ab,ti OR psychosocial*:ab,ti OR reinforcement:ab,ti OR motivation*:ab,ti
#22 OR #23 OR #24 OR #25 OR #26
'pregnancy'/exp
pregnan*:ab,ti
#28 OR #29
'crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR 'controlled clinical trial'/exp OR 'clinical trial'/exp OR placebo:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR (cross:ab,ti AND over:ab,ti) OR 'randomized controlled trial'/exp
#3 AND #21 AND #27 AND #30 AND #31
Appendix 5. CINAHL search strategy
(MH "Substance Use Disorders+")
(MH "Psychoses, Substance‐Induced+")
TX(drug N3 addict*) or TX(drug N3 dependen*) or TX(drug N3 abuse*) or TX(drug N3 misus*)
TX(substance N3 addict*) or TX(substance N3 dependen*) or TX(substance N3 abuse*) or TX(substance N3 misus*)
TX(addict* OR overdos* OR intoxicat* OR abstin* OR abstain OR withdraw* OR abus* OR misus* OR disorder* OR dependen*)
TX(use* N2 drug) or TX(use* N2 disorder) or TX(use* N2 illicit)
TX(use* N2 drug) or TX(use* N2 disorder) or TX(use* N2 illicit)
S1 or S2 or S3 or S4
S5 or S6 or S7
TX(polydrug or alcohol or opioid or opiate or opium or hallucinogen or cocaine or benzodiazepine* or amphetamine*or “anti‐anxiety‐agents” or barbiturate* or “lysergic acid” or ketamine or cannabis or marihuana or marijuana or hashish or inhalant* or solvent or steroid* or methadone or morphine)
MH "Narcotics"
MH "Designer Drugs"
(MH "Hallucinogens+")
(MH "Methadone")
(MH "Amphetamines+")
(MH "Ketamine")
S10 or S11 or S12 or S13 or S14 or S15 or S16
S9 and S17
S8 or S18
(MH "Patient Compliance+")
(MH "Motivational Interviewing") OR (MM "Counseling")
(MH "Psychotherapy+")
TI incentive* OR voucher OR psychotherap* OR psychosocial* OR reinforcement OR motivation* OR contingent* OR advice
AB incentive* OR voucher OR psychotherap* OR psychosocial* OR reinforcement OR motivation* OR contingent* OR advice
TI (contingency N1 management) OR AB (contingency N1 management)
TI (behaviour* N2 therapy) OR AB (behaviour* N2 therapy)
(MH "Reinforcement (Psychology)+")
S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27
(MH "Pregnancy")
TX pregnan*
S29 or S30
MH "Clinical Trials+"
PT Clinical trial
TI clinic* N1 trial* or AB clinic* N1 trial*
TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
TI randomi?ed control* trial* or AB randomi?ed control* trial*
MH "Random Assignment"
TI random* allocat* or AB random* allocat*
MH "Placebos"
TI placebo* or AB placebo*
MH "Quantitative Studies"
S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42
S19 and S28 and S31 and S43
Data and analyses
Comparison 1. Any psychosocial intervention vs. control: neonatal outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth (< 37 weeks gestation) | 3 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.34, 1.51] |
| 2 Positive neonatal toxicology at delivery (any drug) | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.86, 4.24] |
| 3 Low birth weight (< 2500 g) | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.36, 1.43] |
| 4 Days hospitalized after delivery | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐2.52, ‐0.03] |
Comparison 2. Any psychosocial intervention vs. control: maternal outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Positive urine drug test (end of treatment) | 3 | 367 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.75, 1.73] |
| 2 Positive urine at 1 month+ | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.55, 2.31] |
| 3 Positive urine at delivery | 2 | 217 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.52, 2.65] |
| 4 Retention at treatment completion | 9 | 743 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.06] |
| 5 Short term treatment retention | 6 | 514 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.90, 1.10] |
| 6 Retention in treatment at delivery | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.50, 1.88] |
2.2. Analysis.
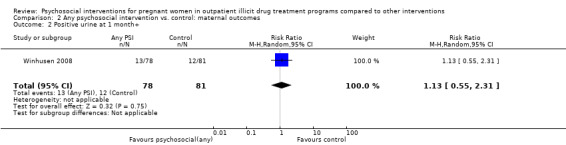
Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 2 Positive urine at 1 month+.
2.6. Analysis.
Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 6 Retention in treatment at delivery.
Comparison 3. CM vs. control: maternal outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Positive urine drug test (end of treatment) | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.86, 4.24] |
| 2 Positive urine at delivery | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.86, 4.24] |
| 3 Retention at treatment completion | 6 | 388 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.92, 1.16] |
| 4 Short term treatment retention | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.70, 1.73] |
| 5 Retention at delivery | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.50, 1.88] |
3.1. Analysis.
Comparison 3 CM vs. control: maternal outcomes, Outcome 1 Positive urine drug test (end of treatment).
3.2. Analysis.
Comparison 3 CM vs. control: maternal outcomes, Outcome 2 Positive urine at delivery.
3.5. Analysis.

Comparison 3 CM vs. control: maternal outcomes, Outcome 5 Retention at delivery.
Comparison 4. MIB vs. control: maternal outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Positive urine drug test (end of treatment) | 2 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.63, 1.48] |
| 2 Positive urine drug test at three months (follow‐up) | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.55, 2.31] |
| 3 Positive urine at delivery | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.57, 1.24] |
| 4 Retention at treatment completion | 3 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.89, 1.06] |
| 5 Short term treatment retention | 3 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.12] |
4.2. Analysis.

Comparison 4 MIB vs. control: maternal outcomes, Outcome 2 Positive urine drug test at three months (follow‐up).
4.3. Analysis.

Comparison 4 MIB vs. control: maternal outcomes, Outcome 3 Positive urine at delivery.
4.5. Analysis.
Comparison 4 MIB vs. control: maternal outcomes, Outcome 5 Short term treatment retention.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carroll 1995.
| Methods | RCT. No difference between groups on baseline drug use or demographic characteristics. | |
| Participants | 20 pregnant women enrolled in methadone maintenance. Mean age 27.6; 78.6% non‐minority (11/14); 78.6% single; 100% unemployed; 8(± 6) weeks gestational age upon entry into MMT; 2.7 mean days cocaine use in past 30 days. Exclusion: > 28 weeks pregnant. | |
| Interventions | Daily MMT, weekly group counselling, three times/week urine toxicology screening for all participants.
No difference between groups in terms of MMT dose (mean 50 mg). Duration: average 23 weeks (range 13 to 31 weeks). |
|
| Outcomes | Attendance was measured in terms of % number of groups attended. Infant outcomes measured as mean gestational age at delivery, mean weight, and mean number of days in the hospital. Urine toxicology was measure as % positive for cocaine, opiates, or other drugs. | |
| Notes | Unable to measure retention as not reported. We attempted to contact trial authors but data was unavailable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "a total of 20 women provided informed consent and were randomly enrolled…" No details were provided related to randomization methods. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "A total of 20 women provided informed consent and were randomly enrolled. Of these, one delivered within 1 week of providing consent, one was hospitalized for sedative detoxification, and four, all of whom had been on the methadone programme for several months or years when they became pregnant, did not participate in any groups or study assessments and were considered dropouts." 20 patients randomized and only 14 analysed. 6 dropouts (unclear from which randomized groups). Exclusions in analysis were lost to follow‐up because they did not attend meetings or because of early labour. These are all possible outcomes of the review and their exclusion biases results. Also analysis was per protocol not ITT. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No references to outcome assessor blinding made. |
Elk 1998.
| Methods | RCT. | |
| Participants | 12 cocaine‐dependent pregnant women: 58% African American, 8% Hispanic, 33% White; 92% never married or separated/widowed/divorced; 75% high school; 83% unemployed; 67% gravida 5 or more, 25% gravida 3 to 4; 75% in second trimester; DSM‐III‐R: 92% (10/11) cocaine primary drug of abuse, 8% (1/11) both heroin and cocaine dependent. Exclusion: Cessation of cocaine use greater than 30 days prior to enrolment. |
|
| Interventions | All received PNC, behaviourally‐based drug counselling, nutritional education, and HIV counselling.
|
|
| Outcomes | Retention in treatment as number remaining in treatment. Abstinence as average of individual %. Attendance in PNC as number of visits. Perinatal outcomes as number of preterm labour, or preterm delivery, or both. ASI composite scores presented as a bar graph. All outcomes reported as chi square with P value only when significant. | |
| Notes | Treatment facility provided free transportation and child care. We attempted to contact trial authors but received no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Following stratification on referral source (self vs. court or probation or parole), subjects were randomly assigned to one of two treatment groups…" No details provided related to randomization methods. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition details outlined for the 12 women, all 12 carried throughout the analysis. Results have detailed participant numbers for each outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No references to outcome assessor blinding made. |
Haug 2004.
| Methods | RCT. Groups significantly different for race. No difference in drug use and psychiatric comorbidity. |
|
| Participants | 77 pregnant opioid‐dependent women randomized with 14 disqualified post‐randomization, ≤ 26 weeks gestational age, receiving MMT and ≥5 cigarettes/day. Mean age 29.7; 84% African American; 79% single or never married; 97% unemployed; 94% less than high school education. DSM‐III‐R: all heroin dependent (100%), 41 (35%) cocaine dependent, 10 (16%) marijuana dependent, 17 (27%) alcohol dependent, all (100%) nicotine dependent. Exclusion criteria: not stated. |
|
| Interventions | For all MMT, no information on urine monitoring, or counselling/therapy. All received USD 10 voucher after initial battery and USD20 when 10‐week interview completed. Mean MMT dose 65.2 mg. All received PNC and substance abuse counselling ‐ no details described.
Duration 10 weeks. |
|
| Outcomes | Retention in treatment as % attrition. Stage of change and stage movement. Urine toxicology done at the 10‐week follow‐up. | |
| Notes | Randomization occurred during residential treatment. 14 disqualified post randomization for spontaneous abortion. 21 excluded for reasons not stated. We received raw data for urine toxicology after contacting the trial authors for information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Seven women refused study participation and 77 patients completed baseline assessment and were randomized". No specific methods for randomization outlined. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "At the 10‐week follow‐up, participant attrition was 14% (n = 9), with missing participants evenly distributed between the MET (n = 4) and SC (n = 5) groups. The only difference between completers and those lost at follow‐up was average methadone dose during treatment (M=50.8mg vs. 36.3 mg, respectively), t(61)=‐6.34,p<.0001." Some information was provided related to attrition (total number of individuals who dropped out). Outcome measures did not explicitly state the number of participants analyses were based on. Furthermore, details were not provided related to which groups the initially disqualified participants came from, making attrition data more unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors. |
Jones 2000.
| Methods | RCT. No difference between groups on baseline drug use. | |
| Participants | 93 pregnant women enrolled in substance use treatment, 18 years of age or older, meeting DSM‐III‐R criteria. Average age 28.9; 88% African American; 75% single; 50% less than HS education; 97% unemployed; DSM‐III‐R: 75% cocaine dependence, 74% opiate dependence, 50% both; 25 women (27%) received MMT. Exclusion criteria: not stated. |
|
| Interventions | For all participants, treatment services included a 7‐day residential stay followed by a 30‐day intensive treatment (7 days/week, 6.5 hours/day). For individuals receiving MMT, no information on doses.
For MMT subgroup: could receive USD5 for each negative urine and USD25 or USD50 bonus for 5 or 7 days drug‐free urine, and additional USD20 if completed all urine samplings and or attendance card monitoring. For non MMT: USD5 each day attended at least 4 hours treatment, USD25 or USD50 bonus for attending 5 to 6 or 7 days. (n = 40) Duration: 37 days. |
|
| Outcomes | Attendance was measured in days and hours. Urine toxicology was not reported. Retention or loss to follow‐up was not reported. | |
| Notes | Transportation and childcare provided. Although randomized to incentive vs. no incentive, the nature of the disbursement of the incentives varied between MMT and not MMT subgroups. We contacted the trial author who was unable to provide original data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "On admission, both MM and AT subjects were recruited and randomly assigned to one of two incentive conditions…" No explicit methods of randomization outlined. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details related to attrition provided. No detailed results included, unclear which participant numbers outcomes are based on. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No references to outcome assessor blinding made. |
Jones 2001.
| Methods | RCT. No difference between groups on baseline drug use or demographic characteristics. | |
| Participants | 80 pregnant women on MMT, greater than 18 years of age, meeting DSM‐II‐R criteria for opiate dependence with cocaine abuse, admitted for first time for substance abuse treatment. Mean age 28; mean gestational age 23.4; 96% unemployed; 85% single/never married; 76% African American; 20% chronic medical conditions (HTN, DM, HIV); DSM‐III‐R: 100% opiate dependent, 69% cocaine, 5% marijuana, 10% alcohol. | |
| Interventions | For all participants treatment consisted of a 7‐day residential followed by 7 days of intensive outpatient (7 days/week, 6.5 hours/day). Treatment consisted of group counselling and at least once a week individual psychotherapy. All received MMT, mean dose 42.
Duration 14 days. |
|
| Outcomes | Attendance was measured as mean full day attendance as well as "perfect treatment attendance" defined as attendance on at least 13 or 14 full days of treatment. Retention was measured as the % drop out. Urine samples were collected daily from days 8 to 14 and reported as % positive. | |
| Notes | Transportation, child care, on site PNC and psychiatric consultation provided. We contacted the trial authors who were unable to provide raw data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization procedure involved patients selecting one of two different color chips from a hat with replacement following each selection…" Specific process outlined that provides participants with equal chance of assignment. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition data specified and descriptions of different analytic methods to account for missing data outlined. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No references to outcome assessor blinding made. |
Jones 2011.
| Methods | RCT. | |
| Participants | 89 women at least 18 years old, with a single fetus, self‐reported heroin or cocaine use in the past 30 days, completion of a 7‐night inpatient stay on an assisted living unit and willingness to live in recovery housing or other drug‐free housing. | |
| Interventions | Both groups received usual care involving group and individual counseling and psychoeducation, medically‐assisted withdrawal for patients either refusing methadone maintenance or not meeting current opioid dependence criteria, methadone maintenance for qualifying opioid‐dependent patients, care management, obstetrical care, psychiatric evaluation and treatment, general medical management.
|
|
| Outcomes | One month post‐randomization treatment outcomes included days retained in CAP treatment before delivery. Measures at baseline and 1 month after: number of days in recovery housing; heroin and cocaine use; employment status; illegal activity. Maternal and neonatal outcomes at delivery: Maternal: enrolment at CAP at delivery; urine screening positive for any illegal drug at delivery. Neonatal outcomes: estimated gestational age at delivery; prematurity (< 37 weeks); birth weight, number of days of hospitalization after birth. | |
| Notes | On‐site child care and paediatric care also provided to all participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random assignment of participants to one of the two treatment conditions was performed by a staff member with no participant contact who generated a random condition assignment with a computer program…" Type of computer programme is unknown, but was likely sufficient for randomization. |
| Allocation concealment (selection bias) | Low risk | "random assignment of participants to one of the two treatment conditions was performed by a staff member with no participant contact who generated a random condition assignment with a computer program…" Staff member had no contact with participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From caption of Figure 1: "All analyses reported in this paper are based on the data from these 89 participants and their respective 89 neonates". Detailed attrition data with flowchart. 89 patients randomized and 89 patients data at follow‐up. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors. |
Mullins 2004.
| Methods | RCT. No difference between groups in baseline drug use. | |
| Participants | 71 pregnant women who used illicit drugs in pregnancy, aged 18 years or older. (1) 35 (2) 36. Average age 27.1; 73% single/never married; 88% unemployed; 82% receiving government income; 32% African American, 50% Caucasian; DSM‐III‐R: 49% cocaine dependent, 28% marijuana, 4% alcohol. Exclusion: no obvious impairment (acute psychosis or organic illness). |
|
| Interventions | For all participants, substance treatment counselling provided. Nature of counselling not elaborated.
Study duration 2 months. |
|
| Outcomes | Attendance reported as number and % attended. Urine was screened at intake and then randomly once per week and were reported as a mean proportion of negative screens. | |
| Notes | Gender‐specific treatment centre. Transportation and childcare included. Treatment integrity and MI proficiency were evaluated. Likert scores used to asses inter‐rater reliability. We attempted to contact the trial authors but received no answer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | We used a stratified random sample procedure based on ethnicity and drug of choice (cocaine/crack cocaine, marijuana, amphetamine/methamphetamine, alcohol, or PCP) to assign participants to conditions. Comment: No specific descriptions of randomization methods were made. |
| Allocation concealment (selection bias) | Unclear risk | No references to allocation concealment procedures made. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Specific attrition data was not included. Some information related to number of sessions attended and no‐show rates for specific sessions were detailed, but overall study attrition was not outlined clearly. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors. |
O'Neill 1996.
| Methods | RCT. | |
| Participants | 92 pregnant women enrolled in MMT who injected drugs. Mean age 26.2; mean years education 10.2; 53% ever sex worker; mean gestational age 22 weeks; DSM‐III‐R: 85% opiate dependent, 15% cocaine, 59% marijuana, 32% alcohol, 98% nicotine. | |
| Interventions | All participants received MMT (mean methadone dose 49 mg) and counselling about HIV risk.
Duration 9 months. |
|
| Outcomes | Retention was measured as a proportion. Attendance was measured as the average number of missed appointments. | |
| Notes | Researchers attempted to contact patients lost to follow‐up through the Department of Social Security and Department of Health. We attempted to contact the trial authors but received no answer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study states that subjects were randomly allocated to the intervention or control group, but does not outline specific randomization methods. |
| Allocation concealment (selection bias) | Unclear risk | No specific references to allocation concealment methods were made. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Five subjects dropped out of the intervention condition; three before any sessions and four after two or more sessions. There were no differences in any of the variables (demographic, drug use or HIV‐risk taking behaviour) between those who remained in the study and those who dropped out. There were 40 subjects in each group at the post‐intervention assessment. Of these 80 subjects, 74 (92.5%) were contactable 9 months later. One refused to participate further, giving a 91% follow‐up rate of those who complete pre‐ and post‐intervention assessments." Attrition information provided, but differences between the intervention and control group not detailed. In addition, specific participant numbers for different outcomes not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Assessments occurred at pre‐intervention, post‐intervention and at 9‐month follow‐up. Follow‐up assessments were conducted by an interviewer blind to the subject’s group membership." Specific references to the blinding of the interviewer conducting follow‐up assessments makes the risk of detection bias low. |
Silverman 2001.
| Methods | RCT. Groups similar in terms of demographic and baseline drug use. | |
| Participants | 40 pregnant, unemployed, women 18 to 50 years old on MMT, and with positive urine toxicology for opiates within 6 weeks prior to enrolment. Mean age 32; 83% African America; 65% HS or greater education; 7.5% married; 100% unemployed; 100% used cocaine; 75% used cocaine. Exclusion: at risk for suicide, psychiatric disorder. | |
| Interventions | For all participants, substance abuse counselling and MMT provided. Details of counselling not given. No mention of MMT doses or schedule.
Duration 24 weeks. |
|
| Outcomes | Retention in treatment defined as remaining in the study through 24 weeks and reported as N and %. Urine toxicology reported as % negative over total study period for each group, and reported as overall positive and drug‐specific positive. Attendance in Therapeutic Workplace was calculated and presented in a bar graph for each woman. | |
| Notes | Transportation and childcare provided at no cost. We attempted to contact trial authors but received no answer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A modified dynamic balanced randomization was used to randomize patients sequentially to the treatment conditions." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition details outlined, reasons for leaving also outlined. Different methods utilized to attempt to account for missing data. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No references to outcome assessor blinding made. |
Svikis 1997.
| Methods | RCT. Unable to assess baseline difference between groups as results reported in terms of MMT vs. not MMT. |
|
| Participants | 142 pregnant women in a comprehensive substance abuse treatment programme, > 18 years of age, and who met DSM‐III‐R criteria for opiate or cocaine dependence, or both. Mean age 28.4; mean years of education 11; estimated gestational age 22 weeks; 53(70%) unemployed; 81.4% single/never married; DSM‐III‐R: 79.6% used cocaine; 83.8% used cocaine. Exclusion: gestational age ≥ 34 weeks, psychiatric disorder, delivered, or had miscarriage during the study time. | |
| Interventions | For all participants, treatment consisted of 7 days residential care followed by 30 days of day treatment (7 days/week, 6.5 hours/day). Group counselling with once/week individual counselling. Obstetrics/Gynecology services on site. For individuals on MMT, no mention of doses.
Duration 30 days. |
|
| Outcomes | Retention in treatment defined as remaining in treatment for 30 days and reported as number of days. Mean number of days also calculated. | |
| Notes | Results were not reported in terms of intervention vs. control group, rather in terms of MMT vs. not MMT. All individuals were randomized to USD0, USD1, USD5 or USD10 group, however USD0 and USD1 were grouped together as "control". Only USD5 and USD10 groups were analyzed as "intervention". We attempted to contact the trial authors but received no answer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were told that within 24 h prior to transfer from residential to IDT, they would be randomly assigned to one of the four incentive groups…" No description of specific randomization process used. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only breakdown of methadone vs. non‐methadone maintained provided, limited information related to incentive condition assigned. Presumably data is for all randomized individuals, given fact that retention and attendance are primary outcomes, unclear if this impacts outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No references to outcome assessor blinding made. |
Svikis 2007.
| Methods | RCT. | |
| Participants | 91 pregnant drug‐dependent women enrolled in comprehensive drug use treatment programme ‐ 18 years of age or older who met the DSM‐III‐R criteria for opiate, or cocaine dependence, or both. Exclusion: Received methadone pharmacotherapy, delivered prematurely or aborted during study period, were administratively discharged from study, had extended or reduced residential stays, had acute psychiatric distress that prohibited study participation. Mostly in early 30s, 74% single/never married, 84% African American, and 95% unemployed, mean education of 11.2 years (SD = 1.9). 79.0% cocaine dependence, 37.1% opiate dependence, 17.7% alcohol dependence, and 17.7% cannabis dependence. |
|
| Interventions | For all participants, treatment included 7 days of residential care followed by 30 days intensive outpatient care.
|
|
| Outcomes | Rate of premature residential treatment dropout against medical advice (AMA). Length of stay in treatment. Mean days prior to AMA. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Those providing informed consent were randomly assigned to either standard care or an escalating voucher schedule.." No specific description of randomization process used. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | As attrition is a primary outcome, incomplete data does not seem to be an issue. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not mentioned. |
Tuten 2012a.
| Methods | RCT. | |
| Participants | 222 pregnant women provided written consent, but 143 were randomized. Pregnant women with an estimated gestational age of < 28 weeks who were opioid dependent and methadone stabilized. Average of 30.0 years old (SD = 5.2), 71.4% African American, 69.9% never married, 11.6 (SD = 1.5) mean years of education, 6.0% currently employed. Exclusion: not receiving methadone maintenance, non‐compliant with study or CAP procedures, had a miscarriage or terminated the pregnancy, transferred programmes, or had a negative pregnancy test. |
|
| Interventions |
Study duration was 13 weeks or until delivery with 1 week of inpatient treatment (when participants could earn two vouchers and 12 outpatient weeks during which participants could earn three vouchers weekly). |
|
| Outcomes | Drug abstinence (number of urine screening tests negative for both opiates and cocaine, number of negative urine tests prior to the first positive test and longest consecutive number of negative urine tests), opioid use (with similar parameters as drug abstinence), cocaine use (with similar parameters). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "participants were randomly assigned to one of the treatment conditions within 5 days of program admission…" No specific description of randomization used. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment processes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Ten cases were missing data on one or more of the concomitant variables so were dropped from the sample, reducing the final sample to 133 cases." Some information about attrition given, but breakdown of assignment groups and attrition was not provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of outcome assessor blinding. |
Winhusen 2008.
| Methods | RCT. | |
| Participants | 200 women participating in the pregnant women treatment programmes at the four participating community treatment programmes (CTPs). At least 18 years of age and pregnant. Identified as needing substance use treatment via the CTP's usual screening procedure. Participants mostly unmarried, unemployed, and had, on average, high school education. Sample was fairly diverse in terms of race and ethnicity. Significant baseline differences between the intervention and control participants. Intervention condition had significantly older participants, significantly more minority participants, with significantly more years of education, and with significantly more participants with cocaine as their primary drug of choice (marijuana was primary drug of choice in the control group). Exclusion: not pregnant, did not need to enter substance abuse treatment, under 18, not interested in participating, had unstable living arrangements, had plans to relocate within 4 months, pending legal charges, required inpatient treatment, suicidal/homicidal risks, more than 32 weeks pregnant. | |
| Interventions |
Active study phase was four weeks (three sessions of MET or TAU provided in the 28 day period). |
|
| Outcomes | Treatment utilization, defined as ratio of number of outpatient treatment hours attended to the number of hours scheduled. Number of weeks in which at least one treatment session was attended. Number of weeks until treatment dropout, dropout defined as failure to attend any treatment provided by the CTP for three consecutive weeks. Substance use measured by self‐report and qualitative toxicology results. | |
| Notes | Participants in both conditions were encouraged to participate in other treatment services offered by the CTP (group treatment, case management). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Urn randomization to balance three dichotomous variables: pressure to attend treatment, self report of drug and alcohol use and need for methadone maintenance". Unclear what type of urn randomization was used, but method likely adequate. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition information provided (e.g. 200 randomized, 162 completed the 1‐month active phase) and statistical analyses related to attrition showed no difference between intervention and control group (P > 0.5). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No references to outcome assessor blinding made. |
Yonkers 2012.
| Methods | RCT. | |
| Participants | 183 women attending two hospital‐based reproductive health clinics in Connecticut between June 2006 and July 2010. Women met inclusion criteria if they: were aged > 16 years of age, were fluent in English or Spanish, had not yet completed 28 weeks of pregnancy at screening, were planning to deliver at a collaborating hospital, and using alcohol or an illicit drug other than opiates during the 28 days prior to screening or scored at least a "3" on the modified TWEAK survey. Women were excluded if they: were already engaged in substance use treatment, endorsed nicotine or opiates as their only substance, had plans to relocate, were not willing to provide consent, were an imminent danger to themselves or their fetus, or if they required inpatient general medical or psychiatric treatment. |
|
| Interventions | Women were randomized to one of two groups:
|
|
| Outcomes | Outcomes were assessed as measured at intake, delivery, and 3 months post‐delivery. The primary outcome was the percentage of days of any alcohol or drug use in the prior 28 days. Secondary outcomes measured abstinence from substances (alcohol and drugs) according to self‐report, urine toxicology and combined self‐report and urine. Birth outcomes were also analyzed. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated block randomization used. |
| Allocation concealment (selection bias) | Low risk | A statistician or project member who had no direct contact with subjects maintained allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis not performed. Women were excluded from analysis if there were no follow‐up assessments and if there were early deliveries. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Urine toxicology used. |
CBT = Cognitive behavioural therapy; MET = Motivational enhancement therapy; MI = Motivational interviewing; MMT = Methadone maintenance therapy; OB/Gyn = Obstetrical/gynecological;
BA = brief advice therapy.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amato 2006 | Inappropriate study design: a systematic review. |
| Ashley 2003 | Inappropriate study design: review article. |
| Bolnick 2003 | Inappropriate study design: review article. |
| Brady 1993 | Inappropriate study design: matched case control, not randomized. |
| Brigham 2010 | Uses Winhusen 2008 data and looks at consecutive weeks attended (as proxy for retention). |
| Chang 1992 | Inappropriate study design: not randomized. |
| Chang 2005 | Excluded as the type of participants were not in the inclusion criteria: alcohol dependent, not illicit drug‐using. |
| Chazotte 1995 | Inappropriate study design: retrospective cohort. |
| Clark 2001 | Inappropriate study design: not randomized; and no intervention was analysed. |
| Daley 2005 | Inappropriate study design: not a RCT; and because there was no intervention studies. This article concerned the development of a Quality of Life index. |
| Day 2003 | Inappropriate study design: retrospective case review. |
| Drozdick 2002 | Excluded as the intervention was not within the scope of the review: comparison of methadone trough levels between symptomatic and asymptomatic women. |
| Egelko 1998 | Inappropriate study design: cohort study with non‐perinatal controls. |
| Elk 1995 | Inappropriate study design: cohort study. |
| Elk 1997 | Inappropriate study design: cohort study. |
| Erickson 2008 | Uses Winhusen 2008 data and looks at impact of therapist on abstinence. |
| Finnegan 2005 | Inappropriate study design: review article. |
| Fischer 1999 | Excluded as the intervention was not in scope of the review: pharmacological intervention. |
| Funai 2003 | Inappropriate study design: retrospective cohort. |
| Fundaro 2004 | Excluded as the study design and participants were not in the scope of the review: cohort study with comparison group and the participants were infants with perinatal illicit drug exposure. |
| Greenfield 2011 | Excluded as the study design and intervention were not in the scope of the review: review article. |
| Hodnett 2010 | Excluded as the study design and intervention were not in the scope of the review: Cochrane review. |
| Hulse 2002 | Excluded as the study design and intervention were not in the scope of the review: case series evaluating a pharmacological intervention. |
| Jansson 2003 | Inappropriate study design: retrospective case series. |
| Jones 2002 | Inappropriate study design: cohort study. |
| Jones 2004 | Inappropriate study design: cohort study. |
| Jones 2011a | Excluded as the study participants were not in the scope of the review: targets male partners of pregnant women. |
| Kastner 2002 | Inappropriate study design: cohort study. |
| Kropp 2010 | Uses Winhusen 2008 data with birth outcomes, but does not stratify. |
| Kukko 1999 | Inappropriate study design: case series. |
| Nishimoto 2001 | Excluded as the study participants and intervention were not in the scope of the review: postpartum patients and no psychosocial intervention. |
| Ondersma 2005 | Excluded as the study participants were not in the scope of the review: postpartum. |
| Ondersma 2007 | Excluded as the study participants were not in the scope of the review: targeted postpartum women. |
| Ondersma 2009 | Uses Winhusen 2008 and looks at impact of baseline motivation on abstinence. |
| Rayburn 2004 | Inappropriate study design: review article. |
| Roy 2011 | Inappropriate study design. |
| Schottenfeld 2011 | Excluded as no data related to pregnant women was specifically referenced and data could not be stratified. |
| Seracini 1996 | Inappropriate study design: cohort study. |
| Shieh 2002 | Inappropriate study design: cohort study. |
| Svikis 1998 | Inappropriate study design: cohort study. |
| Sweeney 2000 | Inappropriate study design: cohort study. |
| Tuten 2006 | Excluded as citation was only for oral session. |
| Tuten 2012b | Analyzes cigarette smoking and treatment for cigarette use (no an illicit substance). |
| Unger 2011 | Inappropriate study design: case series. |
| Weisdorf 1999 | Inappropriate study design: cohort study with historical control. |
| Wexler 1998 | Inappropriate study design: case series. |
| Whicher 2012 | Inappropriate study design: cohort study. |
| Winhusen 2003 | Inappropriate study design: review article. |
| Winklbaur‐Hausknost 2013 | Review article comparing a pilot study and a RCT. |
| Wong 2011 | Inappropriate study design: review article. |
| Yonkers 2009 | Inappropriate study design: cohort study. |
| Zlotnick 1996 | Inappropriate study design: cohort study. |
Differences between protocol and review
None
Contributions of authors
All review authors screened the abstracts of all identified articles. Review authors independently assessed all relevant studies for inclusion. We resolved any conflicts by consensus.
Declarations of interest
Mishka Terplan declares no known conflicts of interest. Shaalini Ramanadhan declares no known conflicts of interest. Abigail Locke declares no known conflicts of interest. Nyaradzo Longinaker declares no known conflicts of interest. Steve Lui declares no known conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Carroll 1995 {published data only (unpublished sought but not used)}
- Carroll KM, Chang G, Behr H, Clinton B, Kosten TR. Improving treatment outcome in pregnant, methadone‐maintained women: Results from a randomized controlled trial. American Journal on Addictions 1995;4(1):56‐9. [Google Scholar]
Elk 1998 {published data only}
- Elk R, Mangus L, Rhoades H, Andres R, Grabowski J. Cessation of cocaine use during pregnancy: effects of contingency management interventions on maintaining abstinence and complying with prenatal care. Addictive Behaviors 1998;23(1):57‐64. [DOI] [PubMed] [Google Scholar]
Haug 2004 {published data only}
- Haug NA, Svikis DS, DiClemente C. Motivational enhancement therapy for nicotine dependence in methadone‐maintained pregnant women. Psychology of Addictive Behaviors 2004;18(3):289‐92. [DOI] [PubMed] [Google Scholar]
Jones 2000 {published data only}
- Jones HE, Haug NA, Stitzer ML, Svikis DS. Improving treatment outcomes for pregnant drug‐dependent women using low‐magnitude voucher incentives. Addictive Behaviors 2000;25(2):263‐7. [DOI] [PubMed] [Google Scholar]
Jones 2001 {published data only}
- Jones HE, Haug N, Silverman K, Stitzer M, Svikis D. The effectiveness of incentives in enhancing treatment attendance and drug abstinence in methadone‐maintained pregnant women. Drug and Alcohol Dependence 2001;61(3):297‐306. [DOI] [PubMed] [Google Scholar]
Jones 2011 {published data only}
- Jones HE, O'Grady KE, Tuten M. Reinforcement‐based treatment improves the maternal treatment and neonatal outcomes of pregnant patients enrolled in comprehensive care treatment. American Journal on Addictions 2011;20(3):196‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mullins 2004 {published data only}
- Mullins SM, Suarez M, Ondersman SJ, Page MC. The impact of motivational interviewing on substance abuse treatment retention: a randomised control trial of women involved with child welfare. Journal of Substance Abuse Treatment 2004;27(1):51‐8. [DOI] [PubMed] [Google Scholar]
O'Neill 1996 {published data only}
- O'Neill K, Baker A, Cooke M, Collins E, Heather N, Wodak A. Evaluation of a cognitive‐behavioural intervention for pregnant injecting drug users at risk of HIV infection. Addiction 1996;91(8):1115‐25. [DOI] [PubMed] [Google Scholar]
Silverman 2001 {published data only}
- Silverman K, Svikis D, Robles E, Stitzer ML, Bigelow GE. A reinforcement‐based therapeutic workplace for the treatment of drug abuse: six‐month abstinence outcomes. Experimental and Clinical Psychopharmacology 2001;9(1):14‐23. [DOI] [PubMed] [Google Scholar]
Svikis 1997 {published data only}
- Svikis D, Lee JH, Haug NA, Stitzer ML. Attendance incentives for outpatient treatment: effects in methadone‐ and non methadone‐maintained pregnant drug dependent women. Drug and Alcohol Dependence 1997;48(1):33‐41. [DOI] [PubMed] [Google Scholar]
Svikis 2007 {published data only}
- Svikis D, Silverman K, Haug NA, Stitzer M, Keyser‐Marcus L. Behavioral strategies to improve treatment participation and retention by pregnant drug‐dependent women. Substance Use & Misuse 2007;42(10):1527‐35. [DOI] [PubMed] [Google Scholar]
Tuten 2012a {published data only}
- Tuten M, Svikis DS, Keyser‐Marcus L, O'Grady KE, Jones HE. Lessons learned from a randomized trial of fixed and escalating contingency management schedules in opioid‐dependent pregnant women. American Journal of Drug and Alcohol Abuse 2012;38(4):286‐92. [DOI] [PubMed] [Google Scholar]
Winhusen 2008 {published data only}
- Winhusen T, Kropp F, Babcock D, Hague D, Erickson SJ, Renz C, et al. Motivational enhancement therapy to improve treatment utilization and outcome in pregnant substance users. Journal of Substance Abuse Treatment 2008;35(2):161‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yonkers 2012 {published data only}
- Yonkers KA, Forray A, Howell HB, Gotman N, Kershaw T, Rounsaville BJ, et al. Motivational enhancement therapy coupled with cognitive behavioral therapy versus brief advice: a randomized trial for treatment of hazardous substance use in pregnancy and after delivery. General Hospital Psychiatry 2012;34(5):439‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Amato 2006 {published data only}
- Amato L, Minozzi S, Davoli M, Vecchi S, Ferri M, Mayet S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005031] [DOI] [PubMed] [Google Scholar]
Ashley 2003 {published data only}
- Ashley OS, Marsden ME, Brady TM. Effectiveness of substance abuse treatment programming for women: a review. American Journal of Drug and Alcohol Abuse 2003;29(1):19‐53. [DOI] [PubMed] [Google Scholar]
Bolnick 2003 {published data only}
- Bolnick JM, Rayburn WF. Substance use disorders in women: special considerations during pregnancy. Obstetrics and Gynecology Clinics of North America 2003;30(3):545‐58, vii. [DOI] [PubMed] [Google Scholar]
Brady 1993 {published data only}
- Brady KT, Grice DE, Dustan L, Malcom R, Killeen T. Characteristics of pregnant cocaine abusers. NIDA Research Monograph 1993;132:114. [Google Scholar]
Brigham 2010 {published data only}
- Brigham G, Winhusen T, Lewis D, Kropp F. Incentives for retention of pregnant substance users: a secondary analysis. Journal of Substance Abuse Treatment 2010;38(1):90‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 1992 {published data only}
- Chang G, Carroll KM, Behr HM, Kosten TR. Improving treatment outcome in pregnant opiate‐dependent women. Journal of Substance Abuse Treatment 1992;9(4):327‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2005 {published data only}
- Chang G, McNamara TK, Orav EJ, Koby D, Lavinge A, Ludman B, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstetrics and Gynecology 2005;105(5 Pt 1):991‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chazotte 1995 {published data only}
- Chazotte C, Youchah J, Freda MC. Cocaine use during pregnancy and low birth weight: the impact of prenatal care and drug treatment. Seminars in Perinatology 1995;19(4):293‐200. [DOI] [PubMed] [Google Scholar]
Clark 2001 {published data only}
- Clark KA, Dee DL, Bale PL, Martin SL. Treatment compliance among prenatal care patients with substance abuse problems. American Journal of Drug and Alcohol Abuse 2001;27(1):121‐36. [DOI] [PubMed] [Google Scholar]
Daley 2005 {published data only}
- Daley M, Shepard DS, Bury‐Maynard DB. Changes in quality of life for pregnant women in substance user treatment: developing a quality of life index for the addictions. Substance Use and Misuse 2005;40(3):375‐94. [DOI] [PubMed] [Google Scholar]
Day 2003 {published data only}
- Day E, Porter L, Clarke A, Allen D, Moselhy H, Copello A. Drug misuse in pregnancy: the impact of a specialist treatment service. Psychiatric Bulletin 2003;27:99‐101. [Google Scholar]
Drozdick 2002 {published data only}
- Drozdick J 3rd, Berghella V, Hill M, Kaltenbach K. Methadone trough levels in pregnancy. American Journal of Obstetrics and Gynecology 2002;187(5):1184‐8. [DOI] [PubMed] [Google Scholar]
Egelko 1998 {published data only}
- Egelko S, Galanter M, Dermatis H, DeMaio C. Evaluation of a multisystems model for treating perinantal cocaine addiction. Journal of Substance Abuse Treatment 1998;15(3):251‐9. [DOI] [PubMed] [Google Scholar]
Elk 1995 {published data only}
- Elk R, Schmitz J, Spiga R, Rhoades H, Andres R, Grabowski J. Behavioral treatment of cocaine‐dependent pregnant women and TB‐exposed patients. Addictive Behaviors 1995;20(4):533‐42. [DOI] [PubMed] [Google Scholar]
Elk 1997 {published data only}
- Elk R, Mangus LG, LaSoya RJ, Rhoades HM, Andres RL, Grabowski J. Behavioral interventions: effective and adaptable for the treatment of pregnant cocaine‐dependent women. Journal of Drug Issues 1997;27(3):625‐58. [Google Scholar]
Erickson 2008 {published data only}
- Erickson SJ, Tonigan JS, Bogenschulz MP. Therapist effects in the treatment of pregnant substance abusers: clinical trials network. Proceedings of the 70th Annual Scientific Meeting of the College on Problems of Drug Dependence; 2008 June 14‐19; San Juan, Puerto Rico. http://www.cpdd.vcu.edu/Pages/Meetings/CPDD08AbstractBook2.pdf. 2008.
Finnegan 2005 {published data only}
- Finnegan LP, Amass L, Jones HE, Kaltenbach K. Addiction and pregnancy. Heroin Addiction and Related Clinical Problems 2005;7(4):5‐22. [Google Scholar]
Fischer 1999 {published data only}
- Fischer G, Jagsch R, Eder H, Gombas W, Etzersdorfer P, Schmidl‐Mohl K, et al. Comparison of methadone and slow‐release morphine maintenance in pregnant addicts. Addiction 1999;94(2):231‐9. [DOI] [PubMed] [Google Scholar]
Funai 2003 {published data only}
- Funai EF, White J, Lee MJ, Allen M, Kuczynski E. Compliance with prenatal care visits in substance abusers. Journal of Maternal‐Fetal and Neonatal Medicine 2003;14(5):329‐32. [DOI] [PubMed] [Google Scholar]
Fundaro 2004 {published data only}
- Fundaro C, Genovese O, Baldieri F, Miccinesi N. Long‐term neurodevelopmental outcome of children exposed to illicit substances during pregnancy: the role of home environmental factors. Italian Journal of Pediatrics 2004;30(2):118‐24. [Google Scholar]
Greenfield 2011 {published data only}
- Greenfield SF, Rosa C, Putnins SI, Green CA, Brooks AJ, Calsyn DA, et al. Gender research in the National Institute on Drug Abuse National Treatment Clinical Trials Network: a summary of findings. American Journal of Drug and Alcohol Abuse 2011;37(5):301‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodnett 2010 {published data only}
- Hodnett ED, Fredericks S, Weston J. Support during pregnancy for women at increased risk of low birthweight babies (Review). Cochrane Collaboration 2010, (6). [DOI] [PubMed] [Google Scholar]
Hulse 2002 {published data only}
- Hulse G, O'Neil G. Using naltrexone implants in the management of the pregnant heroin user. Australian and New Zealand Journal of Obstetrics and Gynaecology 2002;42(5):569‐73. [DOI] [PubMed] [Google Scholar]
Jansson 2003 {published data only}
- Jansson LM, Svikis DS, Beilenson P. Effectiveness of child case management services for offspring of drug‐dependent women. Substance Use and Misuse 2003;38(14):1933‐52. [DOI] [PubMed] [Google Scholar]
Jones 2002 {published data only}
- Jones HE, Svikis DS, Tran G. Patient compliance and maternal/infant outcomes in pregnant drug‐using women. Substance Use and Misuse 2002;37(11):1411‐22. [DOI] [PubMed] [Google Scholar]
Jones 2004 {published data only}
- Jones HE, Svikis D, Rosado J, Tuten M, Kulstad JL. What if they do not want treatment?: lessons learned from intervention studies of non‐treatment‐seeking, drug‐using pregnant women. American Journal on Addictions 2004;13(4):342‐57. [DOI] [PubMed] [Google Scholar]
Jones 2011a {published data only}
- Jones HE, Tuten M, O'Grady KE. Treating the partners of opioid‐dependent pregnant patients: feasibility and efficacy. American Journal of Drug and Alcohol Abuse 2011;37(3):170‐8. [DOI] [PubMed] [Google Scholar]
Kastner 2002 {published data only}
- Kastner R, Hartl K, Lieber A, Hahlweg BC, Knobbe A, Grubert T, et al. Opiate addiction during pregnancy: Results of a psychosomatic treatment strategy with replacement therapy. Geburtshilfe und Frauenheilkunde 2002;32(1):32‐6. [Google Scholar]
Kropp 2010 {published data only}
- Kropp F, Winhusen T, Lewis D, Hague D, Somoza E. Increasing prenatal care and healthy behaviors in pregnant substance users. Journal of Psychoactive Drugs 2010;42(1):73‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kukko 1999 {published data only}
- Kukko H, Halmesmäki E. Prenatal care and counseling of female drug abusers: effects on drug abuse and perinatal outcome. Acta Obstetricia et Gynecologica Scandinavica 1999;78(1):22‐6. [PubMed] [Google Scholar]
Nishimoto 2001 {published data only}
- Nishimoto RH, Roberts AC. Coercion and drug treatment for postpartum women. American Journal of Drug and Alcohol Abuse 2001;27(1):161‐81. [DOI] [PubMed] [Google Scholar]
Ondersma 2005 {published data only}
- Ondersma SJ, Chase SK, Svikis D, Schuster CR. Computer‐based brief motivational intervention for perinatal drug use. Journal of Substance Abuse Treatment 2005;28(4):305‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ondersma 2007 {published data only}
- Ondersma SJ, Svikis DS, Schuster CR. Computer‐based brief intervention. A randomized trial with postpartum women. American Journal of Preventive Medicine 2007;32(3):231‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ondersma 2009 {published data only}
- Ondersma SJ, Winhusen T, Erickson SJ, Stine SM, Wang Y. Motivation Enhancement Therapy with pregnant substance‐abusing women: does baseline motivation moderate efficacy?. Drug and Alcohol Dependence 2009;101(1‐2):74‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rayburn 2004 {published data only}
- Rayburn WF, Bogenschutz MP. Pharmacotherapy for pregnant women with addictions. American Journal of Obstetrics and Gynecology 2004;191(6):1885‐97. [DOI] [PubMed] [Google Scholar]
Roy 2011 {published data only}
- Roy J, Toubin R, Mazurier E, Chanal C, Misraoui M, Brulet C, et al. Developmental outcome of 5‐year‐old children born to opiate‐dependent mothers: effects of a multidisciplinary intervention during pregnancy [Devenir a 5 ans des enfants de meres dependantes aux opiace's: effets d'un suivi multidisciplinaire pendant la grossesse]. Archives de Pediatrie 2011;18(11):1130‐8. [DOI] [PubMed] [Google Scholar]
Schottenfeld 2011 {published data only}
- Schottenfeld RS, Moore B, Pantalon MV. Contingency management with community reinforcement approach or twelve‐step facilitation drug counseling for cocaine dependent pregnant women or women with young children. Drug and Alcohol Dependence 2011;118(1):48‐55. [DOI] [PubMed] [Google Scholar]
Seracini 1996 {published data only}
- Seracini AM, Nunes E, Tross S, Spano C. Achieving cocaine abstinence in preinatal cocaine‐dependent women: A contingency management approach. Problems of Drug Dependence 1996: Proceedings of the 58th Annual Scientific Meeting. Washington DC: NIH 97‐4236, 1996:261.
Shieh 2002 {published data only}
- Shieh C, Kravitz M. Maternal‐fetal attachment in pregnant women who use illicit drugs. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2002;31(2):156‐64. [PubMed] [Google Scholar]
Svikis 1998 {published data only}
- Svikis D, McCaul M, Feng T, Stuart M, Fox M, Stokes E. Drug dependence during pregnancy: Effect of an on‐site support group. Journal of Reproductive Medicine 1998;43(9):799‐805. [PubMed] [Google Scholar]
Sweeney 2000 {published data only}
- Sweeney PJ, Schwartz RM, Mattis NG, Vohr B. The effect of integrating substance abuse treatment with prenatal care on birth outcome. Journal of Perinatology 2000;20(4):219‐24. [DOI] [PubMed] [Google Scholar]
Tuten 2006 {published data only}
- Tuten M, Jones HE. Reinforcement‐based treatment is an effective treatment for drug dependence during pregnancy. Proceedings of the 68th Annual Scientific Meeting of the College on Problems of Drug Dependence; 2006; Orlando, Florida. 2006.
Tuten 2012b {published data only}
- Tuten M, Fitzsimons H, Chisolm MS, Nuzzo PA, Jones HE. Contingent incentives reduce cigarette smoking among pregnant, methadone‐maintained women: results of an initial feasibility and efficacy randomized clinical trial. Addiction 2012;107(10):1868‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Unger 2011 {published data only}
- Unger A, Jagsch R, Jones H, Arria A, Leitich H, Rohrmeister K, et al. Randomized controlled trials in pregnancy: scientific and ethical aspects. Exposure to different opioid medications during pregnancy in an intra‐individual comparison. Addiction 2011;106(7):1355‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weisdorf 1999 {published data only}
- Weisdorf T, Parran TV Jr, Graham A, Snyder C. Comparison of pregnancy‐specific interventions to a traditional treatment program for cocaine‐addicted pregnant women. Journal of Substance Abuse Treatment 1999;16(1):39‐45. [DOI] [PubMed] [Google Scholar]
Wexler 1998 {published data only}
- Wexler HK, Cuadrado M, Stevens SJ. Residential treatment for women: behavioral and psychological outcomes. In: Stevens S, Wexler HK editor(s). Women and Substance Abuse. New York: Hawthorne Medical Press, 1998:213‐33. [Google Scholar]
Whicher 2012 {published data only}
- Whicher EV, Utku F, Schirmer G, Davis P, Abou‐Saleh MT. Pilot project to evaluate the effectiveness and acceptability of single‐session brief counseling for the prevention of substance misuse in pregnant adolescents. Addictive Disorders and their Treatment 2012;11(1):43‐9. [Google Scholar]
Winhusen 2003 {published data only}
- Winhusen TM, Kropp F. Psychosocial treatments for women with substance use disorders. Obstetrics and Gynecology Clinics of North America 2003;30(3):483‐99, vi. [DOI] [PubMed] [Google Scholar]
Winklbaur‐Hausknost 2013 {published data only}
- Winklbaur‐Hausknost B, Jagsch R, Graf‐Rohrmeister K, Unger A, Baewert A, Langer M, et al. Lessons learned from a comparison of evidence‐based research in pregnant opioid‐dependent women. Human Psychopharmacology: Clinical and Experimental 2013;28(1):15‐24. [DOI] [PubMed] [Google Scholar]
Wong 2011 {published data only}
- Wong S, Ordean A, Kahan M. Substance use in pregnancy. International Journal of Gynecology & Obstetrics 2011;114(2):190‐202. [DOI] [PubMed] [Google Scholar]
Yonkers 2009 {published data only}
- Yonkers KA, Howell HB, Allen AE, Ball SA, Pantalon MV, Rounsaville BJ. A treatment for substance abusing pregnant women. Archives of Women's Mental Health 2009;12(4):221‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zlotnick 1996 {published data only}
- Zlotnick C, Franchino K, Claire N, Cox K, John M. The impact of outpatient drug services on abstinence among pregnant and parenting women. Journal of Substance Abuse Treatment 1996;13(3):195‐202. [DOI] [PubMed] [Google Scholar]
Additional references
AIHW 2011
- Australian Institute of Health and Welfare. National Drug Strategy Household Survey Report. Drug Statistics Series No. 25. www.aihw.gov.au (accessed 12/09/15).
Amato 2011a
- Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD004147.pub4] [DOI] [PubMed] [Google Scholar]
Amato 2011b
- Amato L, Minozzi S, Pani PP, Solimini R, Vecchi S, Zuccaro P, et al. Dopamine agonists for the treatment of cocaine dependence. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD003352.pub3] [DOI] [PubMed] [Google Scholar]
Armstrong 2003
- Armstrong MA, Gonzales Osejo V, Lieberman L, Carpenter DM, Pantoja PM, Escobar GJ. Perinatal substance abuse intervention in obstetric clinics decreases adverse neonatal outcomes. Journal of Perinatology 2003;23(1):3‐9. [DOI] [PubMed] [Google Scholar]
Boyd 1999
- Boyd SC. Mothers and Illicit Drugs: Transcending the Myths. Toronto: University of Toronto Press, 1999. [Google Scholar]
Dakof 2003
- Dakof GA, Quille TJ, Tejeda MJ, Alberga LR, Bandstra E, Szapocznik J. Enrolling and retaining mothers of substance‐exposed infants in drug abuse treatment. Journal of Consulting and Clinical Psychology 2003;71(4):764‐72. [DOI] [PubMed] [Google Scholar]
Daley 1998
- Daley M, Argeriou M, McCarty D. Substance abuse treatment for pregnant women: a window of opportunity?. Addictive Behaviors 1998;23(2):239‐49. [DOI] [PubMed] [Google Scholar]
DiClemente 1998
- DiClemente CC, Prochaska JO. Towards a comprehensive, trans theoretical model of change: Stages of change and addictive behaviours. In: Miller WR, Heather N editor(s). Treating Addictive Behaviours. 2. New York: Plenum Press, 1998:3‐24. [Google Scholar]
Frank 2001
- Frank DA, Augustyn M, Knight WG, Pell T, Zuckerman B. Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review. JAMA 2001;285(12):1613‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEPro [Computer program]
- Cochrane Collaboration. GRADEPro. Cochrane Collaboration, 2014.
Grella 2000
- Grella CE, Joshi V, Hser YI. Program variation in treatment outcomes among women in residential drug treatment. Evaluation Reviews 2000;24(4):364‐83. [DOI] [PubMed] [Google Scholar]
Grimes 2005
- Grimes DA, Schulz KF. Surrogate end points in clinical research: hazardous to your health. Obstetrics and Gynecology 2005;105(5 Pt 1):1114‐8. [DOI] [PubMed] [Google Scholar]
Hettema 2005
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annual Review of Clinical Psychology 2005;1:91‐111. [DOI] [PubMed] [Google Scholar]
Higgins 1991
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, et al. A behavioral approach to achieving initial cocaine abstinence. American Journal of Psychiatry 1991;148(9):1218‐24. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at www.cochrane‐handbook.org.
Howell 2000
- Howell EM, Heiser N, Cherlow A, Mason M, Ewell D, Potwein S. Identifying pregnant substance abusers and studying their treatment using birth certificates, Medicaid claims, and state substance abuse treatment data. NIDA Research Monograph 2000;1:225‐41. [Google Scholar]
Hser 1998
- Hser YI, Maglione M, Polinsky ML, Anglin MD. Predicting drug treatment entry among treatment‐seeking individuals. Journal of Substance Abuse Treatment 1998;15(3):213‐20. [DOI] [PubMed] [Google Scholar]
Hubbard 1989
- Hubbard RL, Mardsen ME, Rachal JV, Harwood HJ, Cavanaugh ER, Ginzburg HM. Drug Abuse Treatment: A national study of effectiveness. Chapel Hill: University of North Carolina Press, 1989. [Google Scholar]
Kuczkowski 2007
- Kuczkowski KM. The effects of drug abuse on pregnancy. Obstetrics and Gynecology 2007;19(6):578‐85. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ludlow 2004
- Ludlow JP, Evans SF, Hulse G. Obstetric and perinatal outcomes in pregnancies associated with illicit substance abuse. Australian and New Zealand Journal of Obstetrics & Gynaecology 2004;44(4):302‐6. [DOI] [PubMed] [Google Scholar]
McCaul 1996
- McCaul ME, Svikis DS. Service utilization measures in drug treatment research. In: Rahdert E editor(s). Treatment for D Exposed Women and Their Children: Advances in Research Methodology. Washington DC: U.S. Government Printing Office, 1996:225‐41. [Google Scholar]
Miller 1995
- Miller WR. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. National Institute on Alcohol Abuse and Alcoholism 1995;2. [Google Scholar]
Miller 2003
- Miller WR, Yahne CE, Tonigan JS. Motivational interviewing in drug abuse services: a randomized trial. Journal of Consulting and Clinical Psychology 2003;71(4):754‐63. [DOI] [PubMed] [Google Scholar]
Murphy 1999
- Murphy S, Rosenbaum M. Pregnant Women on Drugs: Combating Stereotypes and Stigma. New Brunswick: Rutgers University Press, 1999. [Google Scholar]
RevMan 2014 [Computer program]
- Cochrane Collaboration. Review Manager 5.3. RevMan 2014. Cochrane Collaboration, 2014.
Rollnick 1995
- Rollnick S, Miller WR. What is Motivational Interviewing?. Behavioural and Cognitive Psychotherapy 1995;23:325‐34. [DOI] [PubMed] [Google Scholar]
Sherwood 1999
- Sherwood RA, Keating J, Kavvadia V, Greenough A, Peters TJ. Substance misuse in early pregnancy and relationship to fetal outcome. European Journal of Pediatrics 1999;158(6):488‐92. [DOI] [PubMed] [Google Scholar]
Signorini 1993
- Signorini DF, Leung O, Simes RJ, Beller E, Gebski VJ, Callaghan T. Dynamic balanced randomization for clinical trials. Statistics in Medicine 1993;12(24):2343‐50. [DOI] [PubMed] [Google Scholar]
Singer 2004
- Singer LT, Minnes S, Short E, Arendt R, Farkas K, Lewis B, et al. Cognitive outcomes of preschool children with prenatal cocaine exposure. JAMA 2004;291(20):2448‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sitzer 2006
- Sitzer M, Petry N. Contingency management for treatment of substance abuse. Annual Review of Clinical Psychology 2006;2:411‐34. [DOI] [PubMed] [Google Scholar]
Skinner 1947
- Skinner BF. Superstition in the Pidgeon. Journal of Experimental Psychology 1947;38:168‐72. [DOI] [PubMed] [Google Scholar]
Terplan 2010
- Terplan M, Smith EJ, Kozloski MJ, Pollack HA. "Compassionate coercion": factors associated with court‐mandated drug and alcohol treatment in pregnancy 1994‐2005. Journal of Addiction Medicine 2010;4(3):147‐52. [DOI] [PubMed] [Google Scholar]
Thorndike 1898
- Thorndike EL. Animal intelligence: An experimental study of the associative processes in animals. Psychological Review Monograph Supplement 1898;2(4):109. [Google Scholar]
References to other published versions of this review
Terplan 2007
- Terplan M, Lui S. Psychosocial interventions for pregnant women in outpatient illicit drug treatment programs compared to other interventions. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006037.pub2] [DOI] [PubMed] [Google Scholar]


