Abstract
In a previous cross sectional study, we found that non-demented elderly participants from the Rush Religious Orders Study (RROS) displayed a wide range of Braak neurofibrillary tangle and amyloid plaque pathology similar to that seen in prodromal and frank AD. Here, we examined longitudinal changes in cognitive domains in subjects from this RROS cohort grouped by Braak stage using linear mixed effects models. We found that the trajectory of episodic memory (EMC), executive function (EFC) and global cognitive composite scores (GCS: average of EMC and EFC scores) was significantly associated with age at visit over time, but not with Braak stage, APOE ε4 status or plaque pathology alone. By contrast, the combined effects of Braak stage, APOE status and age at visit were strongly correlated with the trajectory of EMF, EFC and GCS performance over time. These data suggest that age and APOE ε4 status, rather than AD-related pathology, play a more prominent role in the trajectory of cognitive decline over time in this elderly non-demented population. However, the findings reported require confirmation in a larger cohort of cases.
Keywords: Aging, Alzheimer’s, dementia, longitudinal, neurofibrillary tangles, cognition
Introduction
Older people without dementia display neurofibrillary tangles (NFTs) and beta amyloid (A β) plaques, the two neuropathology hallmarks of Alzheimer’s disease (AD) (Bennett et al., 2002; Guillozet et al., 2003; Markesbery, 2010; Morris and Price, 2001; Morris et al., 2001; Mufson et al., 2016, 1999; Price et al., 2009; Tomlinson et al., 1970; Wilson et al., 2006). However, there are relatively few prospective studies on the association between premortem cognitive function and AD pathology in people who died with a clinical diagnosis of no cognitive impairment but display extensive NFT deposition within the medial temporal lobe (MTL) memory circuit. The Braaks (Braak and Braak, 1991) proposed a neuropathological staging to differentiate initial, intermediate and advanced AD based upon the spread of NFTs within the MTL memory circuit: Braak Stage 0 corresponds to an absence of NFTs, Stages I–II displays NFTs within the entorhinal-perirhinal cortex, Stages III–IV shows NFTs additionally in hippocampus and, Stages V-VI, NFTs are widely distributed in neocortical areas. Although previous studies evaluated the association between the Consortium to Establish a Registry in Alzheimer’s Disease (CERAD) (Mirra et al., 1991) and NIA Reagan AD (The National Institute on Aging, 1997) pathological criteria and clinical findings in older people without cognitive impairment (Bennett et al., 2002; Guillozet et al., 2003; Markesbery, 2010; Morris and Price, 2001; Morris et al., 2001), there is limited information on the relationship between Braak staging and longitudinal neurocognitive measure changes in this type of population (Bennett et al., 2002; Erten-Jones et al., 2009; Guillozet et al., 2003; Markesbery, 2010; Morris et al., 2001; Mufson et al., 1999; Nelson et al., 2009).
Previously, we performed clinical molecular pathobiological investigations regarding the onset of dementia in the elderly using tissue obtained from the Rush Religious Orders Study (RROS), a longitudinal clinic-pathologic investigation of aging and AD (Mufson et al., 2012b, 2008, 1999). In these studies, subjects with a premortem clinical diagnosis of no cognitive impairment (NCI) were classified postmortem with a wide range of Braak scores (I–V) (Gilmor et al, 1999; Mufson et al, 2012b, 1999; Perez et al, 2015). Recently, we reported in a cross section study that elderly RROS participants without dementia and free of other neurological disorders or pathologies who at autopsy were classified as Braak NFT stages of I–V displayed preserved cognitive function (Mufson et al., 2016). Here, we extend this investigation to a longitudinal examination of changes in cognitive domains in this cohort of cases grouped by Braak stage. Linear mixed effects models were used to assess differences in the trajectory of change for cognitive composite scores between the Braak stage groups and to determine the association between amyloid load and neuritic and diffuse plaque counts with longitudinal changes in episodic memory and executive function.
Methods
Braak staging and cognitive status were examined in 106 older deceased and autopsied persons with no cognitive impairment and no coexisting clinical or neurological condition judged to contribute to cognitive impairment at the last clinical evaluation (Bennett et al., 2005; Mufson, et al., 1999). The participants agreed to annual clinical evaluations and signed an informed consent and an Anatomic Gift Act donating their brains at time of death (see Table 1). Data from these subjects have been used in numerous clinical pathological studies supported by our ongoing NIA program project grant entitled the “Neurobiology of Mild Cognitive Impairment in the Elderly” (PO1AG14449) over the last almost twenty years. Individuals were chosen from all RROS brains that came to autopsy during a rolling admission (Bennett et al., 2006). Neuropathological procedures used to determine these conditions have been reported (Bennett et al., 2006, 2005, 2004). The cases chosen for this study were derived from the RROS cohort based upon a strict exclusion criteria. Cases with small or large vascular infarcts, strokes, Parkinson’s disease, Lewy body pathology or hippocampal sclerosis were excluded. In addition, those taking anticholinesterases or medication for depression were also excluded. The average interval from last evaluation to brain autopsy was 0.73+0.79 years. The Human Investigation Committee of Rush University Medical Center approved the study.
Table 1.
Demographic characteristics by Braak Stage.
| Braak Stage 0 – II | Braak Stage III | Braak Stage IV – V | Total | p-value | |
|---|---|---|---|---|---|
| N | 39 | 30 | 37 | 106 | na |
| Gender (Male/Female) | 26/13 | 12/18 | 16/21 | 54/52 | 0.05 |
| APOE ε4 (2/3, 3/3, 3/4, 4/4) | 2, 30, 6, 0* | 5, 23, 1, 1 | 5, 22, 10, 0 | 12, 75, 17, 1 | 0.07 |
| Time Between Last Assessment and Death (Years) | 0.75±0.71 | 0.67±0.61 | 0.67±0.56 | 0.70±0.62 | 0.98 |
| Age at Death (Years) | 81.42±5.65 | 85.68±5.66 | 86.13±4.25 | 84.27±5.58 | <0.001† |
| Education (Years) | 18.21±3.89 | 17.10±4.16 | 18.46±2.53 | 18.02±3.57 | 0.28 |
| PMI (Hours) | 8.12±10.29 | 6.70±4.10 | 6.30±3.89 | 7.12±6.93 | 0.71 |
| MMSE | 28.41±1.33 | 28.20±1.47 | 28.24±1.16 | 28.29±1.31 | 0.77 |
APOE genotype was not available for one individual; mean±standard deviation;
Braak Stage 0–II < Braak Stage III, Braak Stage IV–V; Chi-square was used for gender and APOE ε4 frequency; Kruskall-Wallis test was used for Time Between Last Assessment and Death, Age at Death, Education, postmortem interval (PMI), and mini mental status examination (MMSE)
Clinical Evaluation
Participants underwent a uniform, structured, clinical evaluation and self-report medical history obtained by a team led by a neurologist, and cognitive function was determined by a trained neuropsychological test technician (Bennett, et al., 2006, 2005). Medications used by the subjects within the previous fourteen days of the examination were reviewed and classified. After review of all clinical data and examination of the participant, a clinical diagnosis was made by a board certified neurologist or geriatrician with expertise in the evaluation of elderly persons with dementia. Diagnostic classification of no cognitive impairment was performed as described previously (Bennett et al., 2006, 2005). A neurologist reviewed the medical history, medication use, neurologic examination, results of cognitive performance testing and the neuropsychologist’s opinion of cognitive impairment and dementia. Each participant was evaluated in their home, emphasizing findings deemed clinically relevant.
Cognitive Composite Scores
The Episodic Memory Composite (EMC) score was derived from the results of the following tests: WMS-R Logical Memory Story, Immediate and Delayed Recall, CERAD Word List Immediate Recall, CERAD Word List Delayed Recall, CERAD Word List Recognition. The Executive Function Composite (EFC) used the Symbol Digit Modalities Test, Category Fluency Test, and Ravens Progressive Matrices. Composite scores were derived by first converting raw test scores into z-scores using the sample mean and sample standard deviation for each test. The resulting z-scores from each test were then averaged to create the composite score (Episodic Memory and Executive Function). The Global Cognitive Score (GCS) was created by taking an average of the Episodic and Executive Composite scores.
Tissue Accruement and Neuropathological Diagnosis
Brain accruement and processing have been described previously (Mufson, et al., 1999). Briefly, one hemisphere of the brain was cut into 1 cm thick coronal slabs using a plastic brain slicer and immersion fixed in 4% paraformaldehyde for at least 72 hours. Tissue blocks including the midfrontal cortex, middle or superior temporal cortex, entorhinal cortex, hippocampus, inferior parietal cortex were paraffin embedded and cut at 6 μm. Examination for cerebral infarctions was conducted on these fixed slabs (Bennett et al., 2006) and the Bielschowsky silver stain was used to visualize NPs, DPs and NFTs (Schneider et al., 2009). Paired helical filament tau (AT8; 1:800, Covance) immunohistochemistry (Bennett et al., 2004) was also used to label NFTs and for quantitation (see below). Other blocks were used to collect data on amyloid load from the hippocampus and entorhinal cortex. Sections were stained for amyloid load using A β antibodies (6F/3D, 1:50; DAKO, CA and 4G8, 1:9000, Covance, WI) (Bennett et al., 2004). Neuropathological diagnoses were determined according to CERAD (Mirra et al., 1991) and Braak staging (Braak et al., 2006; Braak and Braak, 1991) as recommended by the NIA-Reagan criteria (1997).
Pathologic Quantitation
A board-certified neuropathologist or trained technician blinded to all clinical data counted total number of NPs, DPs, and NFTs in one square mm area (100x magnification) per cortical region examined as reported previously (Mufson et al., 1999). Quantitation of amyloid load was performed using a scheme to capture images of A β stained sections employing a custom algorithm as described previously (Mitchell, et al., 2000). Briefly, following camera and illumination calibration, 24-bit color images obtained at each sampling site were converted to 8-bit gray scale images. Calculation of percent area occupied by A β immunopositive pixels using the public domain Object-Image 1.62p15. This analysis algorithm segmented labeled images and background compartments using 1 of 2 histogram-dependent automatic thresholding methods (Iterative Self-Organizing Data Analysis) and triangulation. The percent areas for each section were averaged and the number used for analyses. Since the distribution of plaques and NFTs count values did not follow a normal distribution, standardized plaque and tangle count from each area were converted to standard scores by dividing the standard deviation (SD) of mean raw counts per marker and region from the entire deceased cohort. Scaled scores for NPs and DPs, and NFTs for each region were averaged across the four brain regions examined to develop a summary AD pathology score for each subject. Cronbach’s coefficient alpha, a measure of internal consistency, was 0.90 for the 12 postmortem indices, supporting the formation of the global measure of AD pathology.
Following pathological evaluation, the cases were divided into three groups based on each individual’s Braak score (0 to II, III, IV to V). None of the individuals in the sample had a Braak stage of VI.
Statistical Analysis
Chi-square analyses were used to determine significant differences in gender and APOE ε4 allele status among the different clinical groups. The non-parametric Kruskall-Wallis test was used to examine differences in age at death, education, and post-mortem interval between the Braak stage groups.
Linear mixed effects models that were fit by restricted maximum likelihood (REML) were used to assess differences in the trajectory of change for the cognitive composite scores between the Braak stage groups. Braak stage group, age at visit, education, gender, APOE ε4 carrier status, and time between last clinical assessment and death were used as fixed effects with the following interaction terms: Braak stage group x APOE ε4 carrier status and Braak stage group x APOE ε4 carrier status x age at visit. Three separate models were run which used the EMC, EFC, and the GCS each as outcome variables. Additional linear mixed effects models that used CERAD neuropathological diagnoses and NIA Reagan diagnoses in place of Braak stage were also carried out. For the NIA Reagan analyses, subjects were collapsed into two groups (Not AD and Low Likelihood, Intermediate and High Likelihood) due to the small number of individuals in the Not AD and High Likelihood groups. CERAD and NIA-Reagan analyses were performed separately using the same covariates and interaction terms in place of Braak stage. Linear mixed models using the z-scores for the individual cognitive tests were also used to determine their relative sensitivity to cognitive decline with age. The first model used the five EMC tests as predictors with age at visit as the outcome while a second model used the three EFC tests as predictors with age at visit as the outcome.
Linear mixed effects models were also used to assess the association between neuritic and diffuse plaque counts with longitudinal changes in episodic memory and executive function. For each model, age at visit, gender, education, APOE ε4 status, and time between last assessment and death were included to account for their effects. A mean composite of the hippocampal and entorhinal cortex plaque counts was used with the EMC score while mid-frontal plaque counts was used with the EFC score. Linear mixed models that were adjusted for the same covariates examined in the plaque count analysis were used to test the association between amyloid load and the cognitive domains. Statistical analyses were carried out using JMP 10.0 with the significance level set at α ≤ 0.05.
Results
Demographics
A total of 842 person-years were contributed by the 106 individuals in the sample. The average duration of follow-up was 7.94±4.47 years, average age at death was 84.42±6.97 years and average post-mortem interval was 5.64±6.87 hours. The sample cohort had an average of 18.02±3.57 years of education, average time between last clinical assessment and death was less than one year (0.70±0.62 years) and had a relatively even gender distribution (51% male) (see Table 1). Quantile-quantile (QQ) plots for episodic memory, executive function, and GCS composite scores did not deviate from the normal distribution.
There was a significant gender imbalance among the Braak groups (χ2 = 6.17, p = 0.05) as females were more likely to be in the Braak III group [OR = 3.00, 95% CI: (1.12, 8.06), p = 0.03] or the Braak IV–V group [OR = 2.62, 95% CI: (1.03, 6.66), p = 0.04]. There was no significant difference in the distribution of APOE ε4 carriers and non-carriers among the three Braak groups. However, when the Braak 0–II and III groups were combined the Braak IV–V group had a significantly higher frequency of ε4 carriers (p = 0.05). Post-mortem interval and education level were not significantly different between the groups. Age at death for the Braak 0–II (81.42±5.65) was significantly lower than the Braak III (85.68±5.66), (p<0.001) and IV–V groups (86.13±4.25), (p < 0.001; see Table 1). Frequencies for the different CERAD and NIA-Reagan neuropathologic diagnoses among the Braak groups are shown in Tables 2A and 2B, respectively. According to CERAD classifications, 39% were No AD, 37% were Probable AD, 13% Possible AD and 11% Definite AD. For NIA-Reagan diagnosis, 52% had the Intermediate Likelihood classification while 44% were classified as Low Likelihood of AD. The Not AD and High Likelihood classifications consisted of less than 2%.
Table 2A.
Frequency of CERAD Neuropathological Diagnoses by Braak Stage.
| Braak 0–II | Braak III | Braak IV–V | |
|---|---|---|---|
| No AD | 23 | 11 | 7 |
| Possible AD | 7 | 6 | 1 |
| Probable AD | 6 | 11 | 22 |
| Definite AD | 3 | 2 | 7 |
Table 2B.
Frequency of NIA-Reagan Neuropathological Diagnoses by Braak Stage.
| Braak 0–II | Braak III | Braak IV–V | |
|---|---|---|---|
| Not AD | 2 | 0 | 0 |
| Low Likelihood | 34 | 13 | 8 |
| Intermediate Likelihood | 3 | 17 | 27 |
| High Likelihood | 0 | 0 | 2 |
Cognitive test scores and Braak pathology
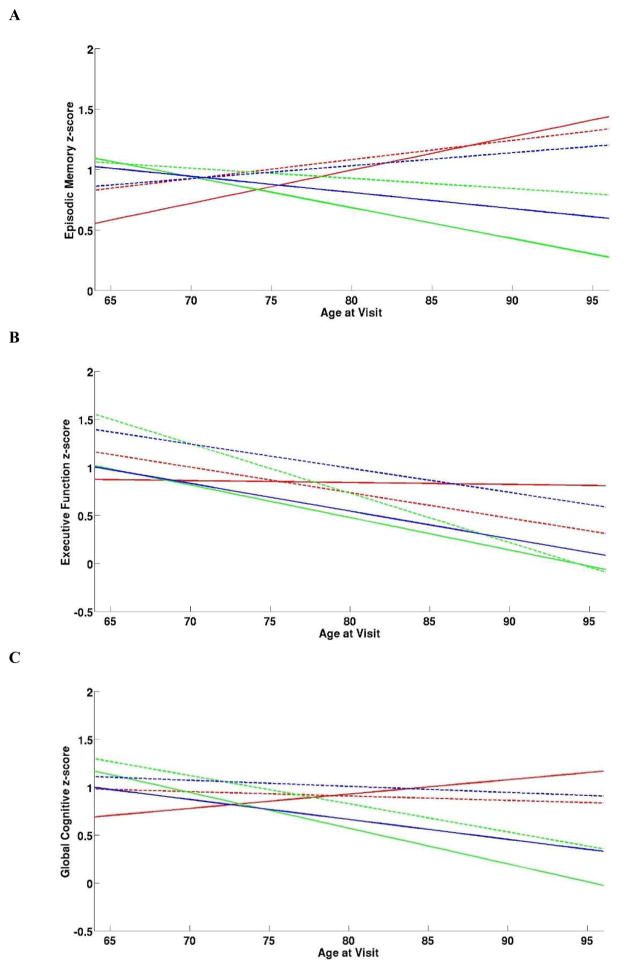
Linear mixed effect models for the different cognitive domains revealed a significant interaction between EMC score and age at visit (p = 0.03; Table 3), Braak stage, APOE ε4 carrier status and age at visit (p = 0.04; Table 3). Braak stage 0–II APOE ε4 carriers and non-carriers as well as Braak stage IV to V non-carriers showed an increase in EMC performance over time (Figure 1A), whereas EMC performance declined over time in the Braak stage IV–V APOE ε4 carriers (Figure 1A). Braak stage III APOE ε4 carriers and non-carriers declined over time with carriers showing a steeper decline (Figure 1A). Among the five EMC tests, the CERAD word list delayed recall was the most sensitive to cognitive decline over time (p = 0.003), while the other four tests had p-values above 0.05.
Table 3.
Linear Mixed Effect Models for Global Cognitive, Episodic Memory, and Executive Function
| Cognitive Domain | Variable | F-value | p-value |
|---|---|---|---|
| Episodic Memory | Braak Stage | 0.65 | 0.52 |
| APOE ε4 Status | 0.75 | 0.39 | |
| Age at Visit | 4.67 | 0.03 | |
| Gender | 0.54 | 0.46 | |
| Education | 1.41 | 0.24 | |
| Time Between Last Assessment and Death | 1.95 | 0.17 | |
| Braak Group x APOE ε4 Status | 0.14 | 0.87 | |
| Braak Stage x APOE ε4 Status x Age at Visit | 3.36 | 0.04 | |
| Executive Function | Braak Stage | 0.55 | 0.58 |
| APOE ε4 Status | 0.08 | 0.78 | |
| Age at Visit | 35.18 | <0.001 | |
| Gender | 6.88 | 0.01 | |
| Education | 5.78 | 0.02 | |
| Time Between Last Assessment and Death | 0.02 | 0.90 | |
| Braak Group x APOE ε4 Status | 1.02 | 0.36 | |
| Braak Stage x APOE ε4 Status x Age at Visit | 6.96 | 0.001 | |
| Global Cognitive Score | Braak Stage | 0.72 | 0.49 |
| APOE ε4 Status | 0.40 | 0.53 | |
| Age at Visit | 4.90 | 0.03 | |
| Gender | 3.82 | 0.05 | |
| Education | 4.36 | 0.04 | |
| Time Between Last Assessment and Death | 0.71 | 0.40 | |
| Braak Group x APOE ε4 Status | 0.60 | 0.55 | |
| Braak Stage x APOE ε4 Status x Age at Visit | 8.91 | <0.001 |
Figure 1.
Trajectory of Episodic Memory (A), Executive Function (B), and Global Cognitive (C) Scores for Braak Stage Groups by APOE ε4 Carrier Status.
—Braak 0–II + ε4 Carriers, ---Braak 0–II + ε4 Non-Carriers, —Braak III + ε4 Carriers, ---Braak III+ ε4 Non-Carriers, —Braak IV–V+ ε4 Carriers, ---Braak IV–V + ε4 Non-Carriers
EFC score, age at visit (p < 0.001), education (p = 0.02), gender (p = 0.01), and interaction between APOE ε4 status, Braak stage, and age at visit (p = 0.001) showed statistically significant effects (Table 3). All groups showed a descending trajectory of EFC score over time except that the Braak stage 0 to II ε4 carriers maintained stable EFC performance over time (Figure 1B). Category fluency and the symbol digit modalities test components of the EFC showed significant decline over time (p<0.001), while the Ravens progressive matrices did not (p = 0.11).
GCS showed significant effects for age at visit (p = 0.03), education (p = 0.04) and gender (p = 0.05) (Table 3) as well as an interaction between APOE ε4 status, Braak stage, and age at visit (p = 0.001) (Table 3). GCS scores declined over time for both Braak stage III ε4 carriers and non-carriers and the Braak stage IV–V ε4 carriers (Figure 1C). Braak stages 0–II and IV–V APOE ε4 non-carriers showed stable GCS performance over time while ε4 carriers in the Braak 0 to II group improved over time (Figure 1C). Slope estimates for the Braak stage/APOE subgroups in each of the cognitive domains are shown in Table 4.
Table 4.
Linear Mixed Model Slope for Braak, CERAD and NIA-Reagan Diagnosis
| Neuropathological Criteria | Episodic Memory | Executive Function | Global Cognitive | |||
|---|---|---|---|---|---|---|
| Slope | 95% CI | Slope | 95% CI | Slope | 95% CI | |
| Braak Stage/APOE | ||||||
| Braak 0–II ε 4 Carriers | 0.03 | (−0.01, 0.07) | 0.00 | (−0.05, 0.05) | 0.02 | (−0.02, 0.05) |
| Braak 0–II ε 4 Non-Carriers | 0.02 | (0.01, 0.03) | −0.03 | (−0.04, −0.01) | 0.00 | (−0.01, 0.00) |
| Braak III ε 4 Carriers | −0.03 | (−0.17, 0.11) | −0.03 | (−0.19, 0.12) | −0.03 | (−0.16, 0.09) |
| Braak III ε 4 Non-Carriers | −0.01 | (−0.02, 0.01) | −0.05 | (−0.06, −0.04) | −0.03 | (−0.04, −0.02) |
| Braak IV–V ε 4 Carriers | −0.01 | (−0.04, 0.01) | −0.03 | (−0.06, 0.00) | −0.02 | (−0.04, 0.00) |
| Braak IV–V ε 4 Non-Carriers | 0.01 | (0.00, 0.02) | −0.02 | (−0.01, −0.03) | 0.00 | (−0.01, 0.00) |
|
| ||||||
| CERAD | ||||||
|
| ||||||
| No AD | 0.01 | (0.00, 0.02) | −0.03 | (−0.04, −0.02) | −0.02 | (−0.03, 0.01) |
| Possible AD | −0.02 | (−0.04, 0.00) | −0.04 | (−0.06, −0.02) | −0.03 | (−0.05, −0.01) |
| Probable AD | 0.01 | (0.00, 0.02) | −0.03 | (−0.04, −0.02) | −0.01 | (−0.02, 0.00) |
| Definite AD | 0.01 | (0.00, 0.03) | −0.04 | (−0.06, −0.02) | −0.01 | (−0.03, 0.00) |
|
| ||||||
| NIA-Reagan | ||||||
|
| ||||||
| Not AD / Low Likelihood | 0.01 | (0.00, 0.02) | −0.03 | (−0.02, −0.04) | −0.01 | (−0.02, 0.00) |
| Intermediate/High Likelihood | 0.00 | (−0.01, 0.01) | −0.03 | (−0.02, 0.03) | −0.01 | (−0.02, −0.01) |
CI, confidence interval
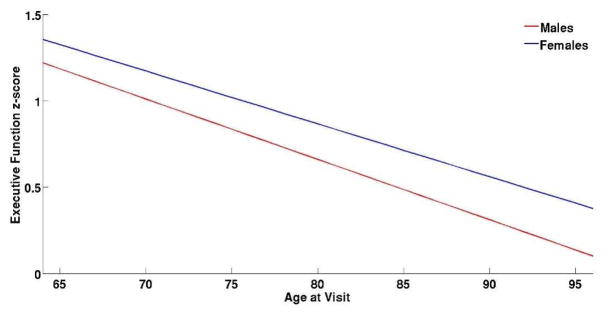
A linear mixed-effects model using EFC as the outcome and gender as the predictor variable revealed significant effects for age at visit (p<0.001), education (p = 0.01) and APOE ε4 carrier status (p = 0.01). None of the interaction terms showed significant associations suggesting that gender is independently associated with EFC performance with females performing significantly better than males (p=0.05) (Figure 2).
Figure 2.
Longitudinal Executive Function Composite Score for Males and Females.
Cognitive Test Scores, CERAD, NIA-Reagan and APOE Status
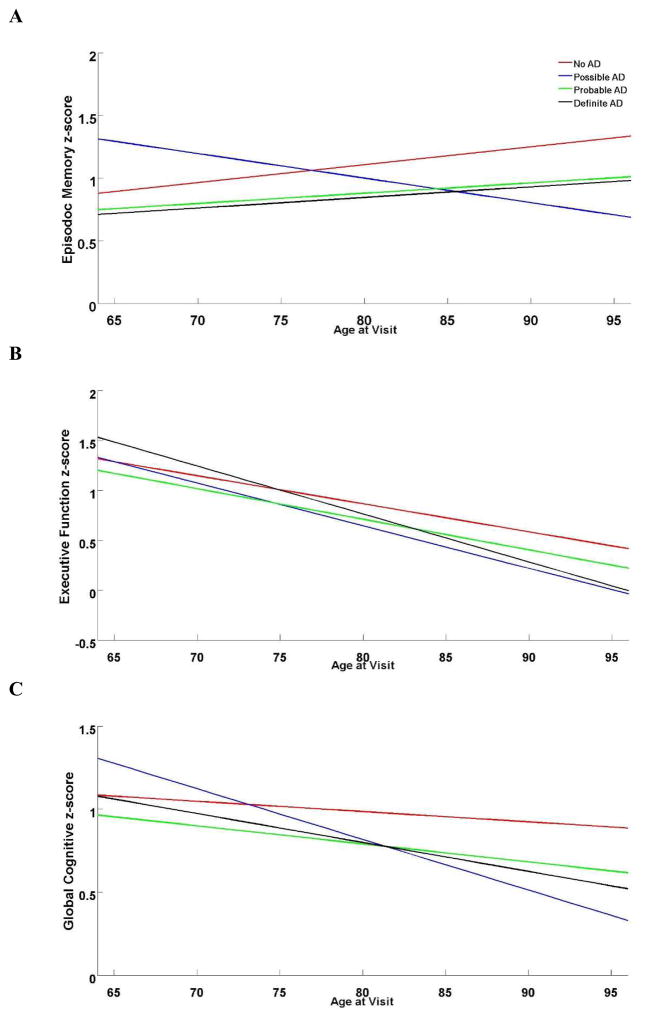
We found a significant interaction between CERAD diagnosis, APOE ε4 carrier status, and age at visit with EMC (p = 0.005) and GCS (p = 0.006), but not EFC (p = 0.16). Although the Possible AD group declined with age, the Not, Probable, and Definite AD groups showed improved EMC performance (Figure 3A), whereas EFC declined in all groups (Figure 3B). GCS declined for all CERAD criteria with age, but Possible AD declined faster than the other groups (Figure 3C). Slope estimates for each of the CERAD diagnoses in each of the cognitive domains are shown in Table 4.
Figure 3.
Trajectory of Episodic Memory (A), Executive Function (B), and Global Cognitive (C) Scores for Braak Stage Groups by CERAD Neuropathological Diagnosis.
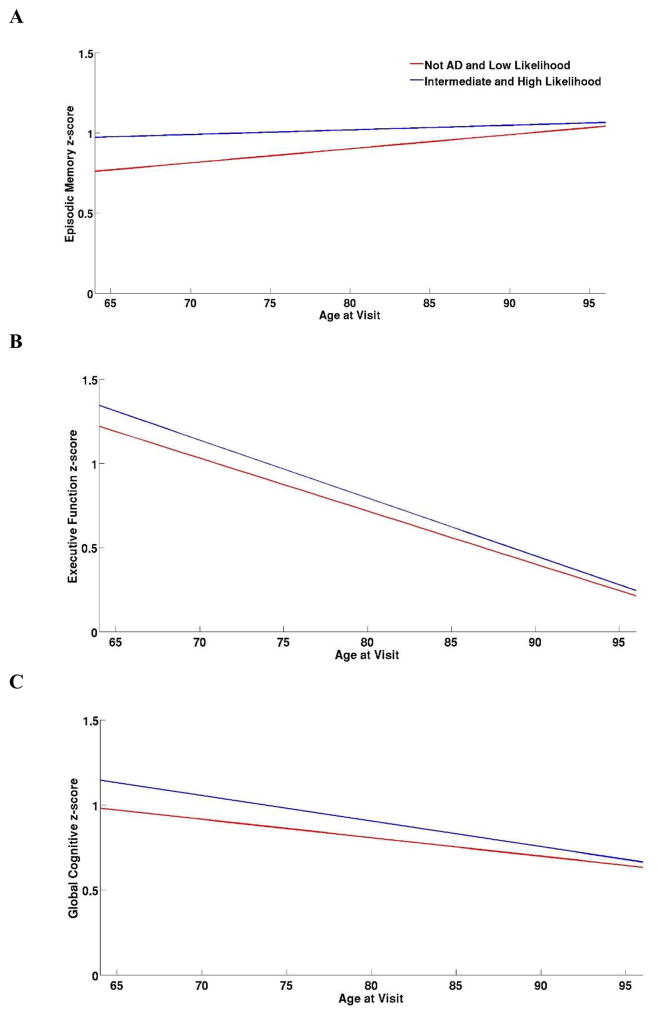
There was a significant three-way interaction between NIA-Reagan diagnosis, APOE ε4 carrier status, and age at visit for performance on EMC (p = 0.03), but not EFC (p = 0.74) or GCS (p = 0.09) (Figure 4). EMC performance in the Not AD/Low Likelihood group was more pronounced relative to the Intermediate/High Likelihood group (Figure 4A) but declined over time for the Not AD/Low Likelihood and Intermediate/High Likelihood groups (Figure 4B). GCS scores in AD/Low Likelihood and Intermediate/High Likelihood groups showed a slower declined compared to EFC (Figure 4C). Slope estimates for each of the NIA-Reagan diagnoses in each of the cognitive domains are shown in Table 4.
Figure 4.
Trajectory of Episodic Memory (A), Executive Function (B), and Global Cognitive (C) Scores for Braak Stage Groups by NIA-Reagan Neuropathological Diagnosis.
Plaque Count and Amyloid Load Associations with Cognition
Analyses of the NPs and DPs counts with the cognitive domains failed to reveal any significant associations (Table 5). In addition, no interactions were found between plaque counts, Braak stage, and APOE ε4 status with any of the cognitive domains. Amyloid load alone and its interactions with Braak stage or APOE status were not significantly associated with any of the cognitive domains examined (Table 6).
Table 5.
Linear Mixed Effect Models for Neuritic and Diffuse Plaque Count Interactions with Braak Group and APOE ε 4 Carrier Status on Episodic Memory and Executive Function.
| Cognitive Domain | Variable | F-value | p-value |
|---|---|---|---|
| Episodic Memory | EH Neuritic Plaque Count | 0.07 | 0.79 |
| EH Diffuse Plaque Count | 0.12 | 0.73 | |
| Braak Group x EH Neuritic Plaque Count | 0.30 | 0.74 | |
| Braak Group x EH Diffuse Plaque Count | 0.60 | 0.55 | |
| APOE ε 4 Status x EH Neuritic Plaque Count | 0.02 | 0.90 | |
| APOE ε 4 Status x EH Diffuse Plaque Count | 0.51 | 0.48 | |
| Executive Function | MF Neuritic Plaque Count | 1.74 | 0.19 |
| MF Diffuse Plaque Count | 0.13 | 0.72 | |
| Braak Group x MF Neuritic Plaque Count | 0.01 | 0.99 | |
| Braak Group x MF Diffuse Plaque Count | 0.62 | 0.54 | |
| APOE ε 4 Status x MF Neuritic Plaque Count | 0.01 | 0.94 | |
| APOE ε 4 Status x MF Diffuse Plaque Count | 0.33 | 0.57 |
Both models included age at visit, gender, education, and time between last assessment and death; EH – entorhinal-hippocampal; MF – mid-frontal
Table 6.
Linear Mixed Effect Models for Amyloid Load Interactions with Braak Group and APOE ε 4 Carrier Status on Episodic Memory, Executive Function, and Global Cognitive Score.
| Cognitive Domain | Variable | F-value | p-value |
|---|---|---|---|
| Episodic Memory | Amyloid Load | 1.12 | 0.29 |
| Braak Group x Amyloid Load | 0.76 | 0.47 | |
| APOE ε 4 Status x Amyloid Load | 1.03 | 0.31 | |
| Executive Function | Amyloid Load | 0.18 | 0.67 |
| Braak Group x Amyloid Load | 0.49 | 0.62 | |
| APOE ε 4 Status x Amyloid Load | 0.07 | 0.79 | |
| Global Cognitive Score | Amyloid Load | 0.14 | 0.71 |
| Braak Group x Amyloid Load | 0.44 | 0.65 | |
| APOE ε 4 Status x Amyloid Load | 0.17 | 0.68 |
All models included age at visit, gender, education, and time between last assessment and death
Discussion
The present study, found that changes in episodic memory, executive function, and global cognition are dependent upon the combined effects of age, NFT pathology and APOE ε4 status in elderly non-cognitively impaired individuals. Braak 0–II cases tended to display better longitudinal cognitive performance than Braak III and Braak IV–V subjects among the cognitive domains examined. APOE ε4 carrier status also effected the trajectories of cognitive performance within each Braak group. In this regard, the Braak 0–II ε4 carriers and non-carriers and Braak IV–V ε4 non-carriers showed an increase in episodic memory performance similar to previous reports (Balasubramanian et al., 2012). Interestingly, there was a more pronounced decline in EMC performance for Braak III compared to Braak IV–V in the APOE ε4 carriers, suggesting that APOE ε4 status in conjunction with age play a greater role in cognitive decline than AD-related pathology alone. On the other hand, longitudinal decreases in executive function performance were found in most Braak groups with the exception of Braak 0–II APOE ε4 carriers that remained stable. Interestingly, it has been suggested that lower Braak stage (I and II) cases without Aβ deposition represent a pathologic classification termed primary age-related tauopathy (PART) and are not representative of early AD (Giaccone, 2015). Since the present subjects displayed both Aβ and tau pathology, they would not fall into this new category of tauopathy.
Although others have shown that increased levels of AD pathology are associated with significantly lower domain-specific cognitive performance over time (Balasubramanian et al., 2012; Bennett et al., 2006; Boyle et al., 2006; Caselli et al., 2009; Monsell et al., 2014; Price et al., 2009), APOE ε4 status was not considered. However, reports indicate that cognitively normal ε4 carriers are more likely to have significant AD-related pathology relative to non-carriers, but rates of cognitive decline were not significantly different between carriers and non-carriers (Vos et al., 2013). By contrast, others found that both the onset and trajectory of cognitive decline is effected by the mediation of AD-related neuropathology via APOE status (Caselli et al., 2009; Yu et al., 2014). APOE ε4 carriers display a significantly greater amyloid load measured by PiB retention and significantly lower cognitive test scores relative to non-carriers in non-demented elderly subjects (Yu et al., 2014) suggesting that APOE ε4 modifies the harmful effect of Aβ on cognition (Kantarci et al., 2012). It has been suggested that the effect of APOE on late life cognitive decline depends on Aβ and NFTs within the cortex (Yu et al., 2014). This interaction is not supported by the current findings showing that neuritic and diffuse plaque counts were not associated with cognitive decline and that there were no interactions between plaque counts, amyloid load, Braak stage, and APOE ε4 status with any of the test of cognition examined.
Braak stage and APOE ε4 status were not independently associated with longitudinal change in any of the cognitive domains tested, however, age played a more prominent role in the underlying cognitive decline observed in our cohort of non-demented elderly individuals. Therefore, the interaction between cognition and AD-pathology alone may not be sufficient to affect the trajectory of cognitive change over time in cognitively intact older adults. Taken together, these results suggest that cognitive decline in the elderly is dependent upon an interaction between Braak stage, APOE status, and age. Perhaps some type of brain resilience/reserve allows individuals to withstand a significant degree of AD pathology without concomitant dementia (Dickson et al., 1992; Negash et al., 2013; O’Brien et al., 2009; Stern, 2009). Brain reserve postulates that these individuals have a greater quantity of either neurons or synapses at the initiation of the disease allowing the performance of cognitive tasks (Katzman et al., 1988; Stern, 2009). On the other hand, cognitive reserve may involve the recruitment of other brain regions not severely affected by the disease process to aid in task performance and even in the creation of new brain connections (Stern, 2009). Although the mechanism(s) initiating these constructs remain unknown, the MTL memory circuit is highly neuroplastic (DeKosky et al., 2002; Ikonomovic et al., 2003; Mufson, et al., 2015). For example, we reported similarities in entorhinal cortex amyloid load between aged non-cognitively impaired individuals, MCI and AD cases obtained from the RROS (Mufson et al., 1999). Other studies using hippocampal tissue revealed a preservation of synapse number (Scheff et al., 2007), synaptic protein levels (Counts et al., 2012), and neurotrophic factor receptor levels (Mufson et al., 2012a) in non-demented aged subjects with a wide range of Braak stages as well as an increase in dendritic spine size in prodromal AD (Mufson et al., 2015). Clinical pathological investigations also indicate preserved numbers and larger neuronal size in the hippocampus in “asymptomatic AD” compared with normal, MCI and clinical AD (Iacono et al., 2009; Riudavets et al., 2007) indicating early reorganizational responses to the AD process. The hippocampus displays a significant up regulation of choline acetyltransferase activity during the prodromal phase of AD (DeKosky et al., 2002). Together, these biochemical and structural compensatory responses in the face of extensive NFT pathology underscores the neural reorganizational capacity of the MTL. Early MTL plasticity may represent a time point for potential therapeutic strategies aimed at restoring or maintaining neuronal function in the elderly (Mufson et al., 2015). Moreover, whether the presence of the APOE ε2 allele, which is thought to be protective against the risk of AD (Strittmatter and Roses, 1995) has a mitigating effect on AD pathology, cognitive decline (Driscoll et al., 2011) and brain plasticity remains an open question. In addition to neural plasticity, educational level may play a role in masking the effects of the AD related neuropathology upon cognition. In this study, virtually all of the subjects had at least 18 years of education. However, it is possible that individuals with only a high school education or less, would have reduced cognitive reserve and thus reduced ability to mask the effect of AD pathology upon cognition (Satizabal et al., 2016).
We further examined the interaction of CERAD and NIA-Reagan neuropathological criteria and APOE ε4 status, age, AD pathology and cognition. Episodic memory performance was the only cognitive domain to show a significant interaction between CERAD and NIA-Reagan criteria as well as with APOE ε4 status and age. Interestingly, age also mediates the effect of macroscopic infarcts upon cognition, whereas APOE ε4 status influences the effect of Lewy body pathology and cognitive status (Yu et al., 2014). AD pathologies and macroscopic infarcts were also reported to be associated with declines in episodic and semantic memory (Bennett et al., 2012). Since the cases used here were screened for Lewy bodies, vascular pathologies and other neurodegenerative disorders that may affect cognition, our findings suggest that longitudinal changes in cognition observed in non-cognitively impaired older people are significantly affected by the interaction between AD-related tau pathology, age and APOE status. Interestingly, we found cases with probable/definite AD vs intermediate/high likelihood AD vs low (0–III) Braak scores. These discrepancy may be related to the way these pathological criteria are derived. Both the CERAD and NIA-Reagan criterion include measures of amyloid pathology whereas Braak stage is independent of these lesions and only depends upon the topographical distribution of NFTs beginning within the medial temporal lobe. Moreover, it is possible that classic AD pathology is not a direct correlate of clinical AD. This issue remains to be fully investigated.
Finally, this study has several limitations. The individuals were from a community based cohort of highly educated retired clergy who had excellent health care and nutrition. People who volunteer may introduce bias by decreasing pathology but this is partially mitigated by high RROS follow-up and autopsy rates (Schneider et al., 2009). None of the cases evaluated were Braak stage VI, which is highly associated with AD-dementia (Braak and Braak, 1991). Although subtle changes in neuropsychological testing may fail to detect cognitive impairment in non-demented people (Bennett et al., 2005), our cognitive tests are standard in the field (Bennett et al., 2006). Data interpretation was limited by the use a three-way interaction term (i.e., categorical and continuous variables), which allows for the analysis of the combined effects of age, Braak stage, and APOE status upon cognitive decline over time, but does not provide post-hoc comparisons involving the interaction term. When the sample was grouped by both APOE ε4 status and Braak stage, select subgroups contained small subject numbers, which may affect the reliability of the reported cognitive decline estimates, although others have reported similar findings (Riley et al., 2011; Vos et al., 2013). However, we are aware that the small sample size examined in this study may affect the reliability of the reported associations, particularly the evaluation of the influence of APOE status. The findings reported here require confirmation in a larger cohort of subjects. Despite these caveats, study strengths include uniform premortem clinical and postmortem pathological evaluation and that final pathologic classification was performed without knowledge of clinical evaluation. The subjects here have been used in multiple clinical pathological investigations (Mufson et al., 2012b). Overall, these characteristics lend strength to our previous observation that elderly people can withstand the onslaught of AD pathology without a significant degradation in cognition (Mufson et al., 2016).
The present findings indicate that intermediate to high levels of NFT pathology in the MTL alone may not be sufficient to affect cognition in non-demented elderly individuals. On the other hand, our data suggest that age and APOE ε4 status play a more prominent role in the trajectory of cognitive decline over time in elderly non-demented individuals. The cascade(s) of neurobiological events/factors that underlie the onset of frank dementia in the elderly remains to be determine.
Highlights.
Braak stage or APOE ε4 status was not associated with changes in the trajectory of cognitive decline over time in non-demented elders.
Age at visit was significantly associated with the trajectory of cognitive scores over time in non-demented elders.
Combining Braak stage, age and APOE ε4 status revealed a strong correlation with the trajectory of cognitive performance over time in non-demented elders.
Acknowledgments
Supported by National Institute on Aging grants PO1AG14449, P30AG10161, P30AG019610, RO1AG43775 and Barrow Neurological Institute Barrow and Beyond. The authors thank the participants in the Religious Orders Study, the faculty and staff who work on this project at the Rush Alzheimer’s Disease Center.
Footnotes
Disclosure statement
None of the authors have any disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balasubramanian AB, Kawas CH, Peltz CB, Brookmeyer R, Corrada MM. Alzheimer disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurology. 2012;79(9):915–21. doi: 10.1212/WNL.0b013e318266fc77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res. 2012;9(6):628–45. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64(5):834–41. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61(3):378–84. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198–05. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67(3):441–45. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, Locke DE, Snyder CH, Alexander GE, Rademakers R, Reiman EM. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361(3):255–63. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, He B, Nadeem M, Wuu J, Scheff SW, Mufson EJ. Hippocampal drebrin loss in mild cognitive impairment. Neuro-degenerative diseases. 2012;10(1–4):216–19. doi: 10.1159/000333122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, Cochran EJ, Kordower JH, Mufson EJ. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51(2):145–55. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13(1):179–89. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Zhou Y, An Y, Sojkova J, Davatzikos C, Kraut MA, Ye W, Ferrucci L, Mathis CA, Klunk WE, et al. Lack of association between 11C-PiB and longitudinal brain atrophy in non-demented older individuals. Neurobiol Aging. 2011;32(12):2123–30. doi: 10.1016/j.neurobiolaging.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erten-Jones D, Woltjer RL, Dodge H, Nixon R, Vorobik R, Calvert JF, et al. Factors associated with resistance to dementia despite high Alzheimer disease pathology. Neurology. 2009;72:354–60. doi: 10.1212/01.wnl.0000341273.18141.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccone G. The Existence of Primary Age-Related Tauopathy Suggests that not all the Cases with Early Braak Stages of Neurofibrillary Pathology are Alzheimer’s Disease. J Alzheimers Dis. 2015;48(4):919–21. doi: 10.3233/JAD-150435. [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Erickson JD, Varoqui H, Hersh LB, Bennett DA, Cochran EJ, Mufson EJ, Levey AI. Preservation of nucleus basalis neurons containing choline acetyltransferase and the vesicular acetylcholine transporter in the elderly with mild cognitive impairment and early Alzheimer’s disease. J Comp Neurol. 1999;411(4):693–704. [PubMed] [Google Scholar]
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol. 2003;60(5):729–36. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- Iacono D, Markesbery WR, Gross M, Pletnikova O, Rudow G, Zandi P, Troncoso JC. The Nun study: clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology. 2009;73(9):665–73. doi: 10.1212/WNL.0b013e3181b01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic MD, Mufson EJ, Wuu J, Cochran EJ, Bennett DA, DeKosky ST. Cholinergic plasticity in hippocampus of individuals with mild cognitive impairment: correlation with Alzheimer’s neuropathology. J Alzheimers Dis. 2003;5(1):39–48. doi: 10.3233/jad-2003-5106. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Yang C, Schneider JA, Senjem ML, Reyes DA, Lowe VJ, Barnes LL, Aggarwal NT, Bennett DA, Smith GE, et al. Antemortem amyloid imaging and beta-amyloid pathology in a case with dementia with Lewy bodies. Neurobiol Aging. 2012;33(5):878–85. doi: 10.1016/j.neurobiolaging.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Annals of neurology. 1988;23(2):138–44. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. Neuropathologic alterations in mild cognitive impairment: a review. J Alzheimers Dis. 2010;19(1):221–28. doi: 10.3233/JAD-2010-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiol Aging. 1997;18(4 Suppl):S91–94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- Mitchell TW, Nissanov J, Han LY, Mufson EJ, Schneider JA, Cochran EJ, Bennett DA, Lee VM, Trojanowski JQ, Arnold SE. Novel method to quantify neuropil threads in brains from elders with or without cognitive impairment. J Histochem Cytochem. 2000;48(12):1627–38. doi: 10.1177/002215540004801206. [DOI] [PubMed] [Google Scholar]
- Monsell SE, Mock C, Hassenstab J, Roe CM, Cairns NJ, Morris JC, Kukull W. Neuropsychological changes in asymptomatic persons with Alzheimer disease neuropathology. Neurology. 2014;83(5):434–40. doi: 10.1212/WNL.0000000000000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J Mol Neurosci. 2001;17(2):101–18. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, He B, Nadeem M, Perez SE, Counts SE, Leurgans S, Fritz J, Lah J, Ginsberg SD, Wuu J, et al. Hippocampal proNGF signaling pathways and beta-amyloid levels in mild cognitive impairment and Alzheimer disease. J Neuropathol Exp Neurol. 2012a;71(11):1018–29. doi: 10.1097/NEN.0b013e318272caab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Binder L, Counts SE, DeKosky ST, de Toledo-Morrell L, Ginsberg SD, Ikonomovic MD, Perez SE, Scheff SW. Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol. 2012b;123(1):13–30. doi: 10.1007/s00401-011-0884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Chen EY, Cochran EJ, Beckett LA, Bennett DA, Kordower JH. Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp Neurol. 1999;158(2):469–90. doi: 10.1006/exnr.1999.7086. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert review of neurotherapeutics. 2008;8(11):1703–18. doi: 10.1586/14737175.8.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Mahady L, Waters D, Counts SE, Perez SE, DeKosky ST, Ginsberg SD, Ikonomovic MD, Scheff SW, Binder LI. Hippocampal plasticity during the progression of Alzheimer’s disease. Neuroscience. 2015;309:51–67. doi: 10.1016/j.neuroscience.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Malek-Ahmadi M, Perez SE, Chen K. Braak staging, plaque pathology and APOE status in elderly persons without cognitive impairment. Neurobiol Aging. 2016;37:147–53. doi: 10.1016/j.neurobiolaging.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negash S, Xie S, Davatzikos C, Clark CM, Trojanowski JQ, Shaw LM, Wolk DA, Arnold SE. Cognitive and functional resilience despite molecular evidence of Alzheimer’s disease pathology. Alzheimers Dement. 2013;9(3):e89–95. doi: 10.1016/j.jalz.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68(1):1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L, Crain BJ, Pletnikova O, Rudow G, Iacono D, Riudavets MA, Driscoll I, et al. Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA) J Alzheimers Dis. 2009;18(3):665–75. doi: 10.3233/JAD-2009-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, He B, Nadeem M, Wuu J, Scheff SW, Abrahamson EE, Ikonomovic MD, Mufson EJ. Resilience of precuneus neurotrophic signaling pathways despite amyloid pathology in prodromal Alzheimer’s disease. Biol Psychiatry. 2015;77(8):693–703. doi: 10.1016/j.biopsych.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30(7):1026–36. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riudavets MA, Iacono D, Resnick SM, O’Brien R, Zonderman AB, Martin LJ, Rudow G, Pletnikova O, Troncoso JC. Resistance to Alzheimer’s pathology is associated with nuclear hypertrophy in neurons. Neurobiol Aging. 2007;28(10):1484–92. doi: 10.1016/j.neurobiolaging.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley KP, Jicha GA, Davis D, Abner EL, Cooper GE, Stiles N, Smith CD, Kryscio RJ, Nelson PT, Van Eldik LJ, Schmitt FA. Prediction of preclinical Alzheimer’s disease: Longitudinal rates of change in cognition. J Alzheimers Dis. 2011;25(4):707–17. doi: 10.3233/JAD-2011-102133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68(18):1501–08. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18(3):691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–28. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer disease. Proc Natl Acad Sci USA. 1995;92(11):4725–27. doi: 10.1073/pnas.92.11.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11(3):205–42. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, Cairns NJ, Morris JC, Holtzman DM, Fagan AM. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12(10):957–65. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Li Y, Bienias JL, Bennett DA. Cognitive decline in old age: separating retest effects from the effects of growing older. Psychol Aging. 2006;21(4):774–89. doi: 10.1037/0882-7974.21.4.774. [DOI] [PubMed] [Google Scholar]
- Yu L, Boyle PA, Leurgans S, Schneider JA, Bennett DA. Disentangling the effects of age and APOE on neuropathology and late life cognitive decline. Neurobiol Aging. 2014;35(4):819–26. doi: 10.1016/j.neurobiolaging.2013.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]