Abstract
Sphingolipids serve important structural and functional roles in cellular membranes and myelin sheaths. Plasma sphingolipids have been shown to predict cognitive decline and Alzheimer’s disease (AD). However, the association between plasma sphingolipid levels and brain white matter (WM) microstructure has not been examined. We investigated whether plasma sphingolipids (ceramides, sphingomyelins) were associated with MRI-based diffusion measures, fractional anisotropy (FA) and mean diffusivity (MD), 10.5 years later in 17 WM regions of 150 cognitively normal adults (mean age 67.2). Elevated ceramide species (C20:0, C22:0, C22:1, and C24:1) were associated with lower FA in multiple WM regions, including total cerebral WM, anterior corona radiata, and the cingulum of the cingulate gyrus. Higher sphingomyelins (C18:1, C20:1) were associated with lower FA in regions such as the anterior corona radiata and body of the corpus callosum. Furthermore, lower sphingomyelin to ceramide ratios (C 22:0, C24:0, C24:1) were associated with lower FA or higher MD in regions including the superior and posterior corona radiata. However, while these associations were significant at the a priori p<0.05, only associations with some regional diffusion measures for ceramide C22:0 and sphingomyelin C18:1 survived correction for multiple comparisons. These findings suggest plasma sphingolipids are associated with variation in WM microstructure in cognitively normal aging.
Keywords: DTI, ceramides, sphingomyelin, white matter, fractional anisotropy, sphingolipids
1.0 Introduction
Sphingolipids such as sphingomyelin and ceramide contribute to the structural integrity and fluidity of cell membranes and are highly enriched in the central nervous system. Indeed, the specific concentrations of, and interaction between, sphingomyelin with cholesterol affect membrane permeability (Needham and Nunn, 1990) and are critical for the formation and structure of lipid rafts (Brown and London, 2000; Cremesti et al., 2002). Sphingomyelins are a type of phospholipid also found in myelin. Ceramide is both a precursor and a metabolite of sphingomyelin. While ceramides do act as structural lipids, they are important bioactive lipids necessary for regulating signaling cascades involved in cellular senescence, apoptosis, and inflammation (Hannun and Obeid, 2008; Kolesnick, 1998). Both sphingomyelins and ceramides increase with age (Cutler et al., 2004; Mielke et al., 2015a, 2015b). Further, these lipids, individually and the ratio of sphingomyelin to ceramide, have been implicated in the development and progression of neurodegenerative diseases (Ben-David and Futerman, 2010; Cutler et al., 2004; Haughey, 2010).
Several postmortem studies have found higher concentrations of ceramides and sphingomyelins in frontal and temporal brain regions in Alzheimer’s disease (AD) patients compared with normal controls (Chan et al., 2012; Cutler et al., 2004; Han et al., 2002), and in persons with Lewy body pathology and fronto-temporal lobar degeneration (Filippov et al., 2012). We have previously reported that high blood ceramide and sphingomyelin levels predicted cognitive impairment and AD among cognitively normal (CN) individuals (Mielke et al., 2012, 2010a) and memory decline and hippocampal volume loss among amnestic MCI patients(Mielke et al., 2010b). Notably, in AD patients, the ratio of sphingomyelin to ceramide was most predictive of rate of cognitive decline compared to sphingomyelin or ceramide levels alone (Mielke et al., 2011). Given the high concentration of sphingolipids in WM, it is possible that changes in circulating sphingolipids could be related to changes in microstructural diffusion measures such as fractional anisotropy (FA) and mean diffusivity (MD). However, the relationship of circulating sphingolipid concentrations on WM microstructure, as measured with Diffusion Tensor Imaging (DTI), has not been reported.
A number of cross-sectional neuroimaging studies have shown lower FA or higher MD with increasing age in WM tracts (Abe et al., 2002; Bennett et al., 2010; Grieve et al., 2007; Head, 2004; Kennedy et al., 2009; Pfefferbaum et al., 2000; Salat et al., 2005), with the most significant associations often found in frontal regions. These decreases in FA with age remain significant even after correcting for WM volume loss (Hugenschmidt et al., 2008), suggesting these microstructural changes could precede WM atrophy. Identifying blood markers that predict deficits in WM integrity of cognitively normal individuals would improve our understanding of modifiers of healthy brain aging. The prospective design of the Baltimore Longitudinal Study of Aging (BLSA) allowed us to evaluate the association of peripheral sphingolipid levels with subsequent brain structure. Given the important structural and functional roles of sphingolipids in cellular membranes and within myelin sheaths, the goal of the present study was to examine whether plasma sphingolipids were associated with global and region-specific WM microstructure ten years later.
2.0 Methods and Materials
2.1 Participants
The present study included 150 cognitively normal BLSA participants who had (3T) DTI scans and available plasma ceramide and sphingomyelin levels an average of 10.5 years prior to the scan. We initially identified 168 BLSA participants with available plasma ceramide and sphingomyelin levels and a 3T DTI scan. The average time between the baseline plasma sphingolipid assays and the DTI scan was 17 years (SD =6 years) with a range of 8.5 to 32.0 years. To reduce the variability in time to DTI scan, we a priori decided to examine the sphingolipid measurement that was nearest to 10 years prior to each participant’s DTI scan (mean=10.2 years; SD=2.5). Thus, we excluded 8 participants with a follow-up interval of less than 5 years and 3 participants over 15 years. Scans were also excluded from an additional six participants for whom WM hyperintensity volumes concurrent with the DTI scan were not available and another participant because the image processing pipeline failed to generate diffusion metrics for several brain regions. Compared to participants with a sphingolipid measurement but without a 3T DTI scan (N=824), the 150 participants included in this study were slightly younger at their initial blood draw (one-way ANOVA, F(1,972)=15.74), were more likely to be female (Fishers exact test, odds ratio = 2.37 (95%: 1.64–3.42)) and Caucasian (Fishers exact test, odds ratio = 5.7 (95%: 3.68–8.82)), had slightly lower systolic blood pressure (one-way ANOVA, F(1,964)=3.92), had no prevalence of myocardial infarction compared with 3% in the larger group, and lower total cholesterol (one-way ANOVA, F(1,928)=19.47). However, the samples did not differ (p>0.05) in the frequency of diabetes, hypertension, cancer, use of statins, body mass index, diastolic blood pressure, triglycerides, apolipoprotein E ε4 (APOE ε4) allele carrier status, or total sphingomyelin and ceramide levels.
All participants were cognitively normal at the time of both the blood and MRI assessments. Diagnoses of dementia and Alzheimer’s disease were determined by Diagnostic and Statistical Manual (DSM)-III-R (1987) and the National Institute of Neurological and Communication Disorders—Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., 1984), respectively. Mild cognitive impairment (MCI) was based on the Petersen criteria (Petersen et al., 1999) and diagnosed when (1) cognitive impairment was evident for a single domain (typically memory) or (2) cognitive impairment in multiple domains occurred without significant functional loss in activities of daily living. Furthermore, all participants had no medical history of stroke at the time of blood sample or at the time of DTI. Blood samples were drawn at all visits from the antecubital vein between 7–8 AM after an overnight fast (Shock et al., 1984). Participants were not allowed to smoke, engage in physical activity, or take medications before the sample was collected. Plasma samples were immediately processed, catalogued and stored at −80°C.The proto col was approved by the local Institutional Review Board and all participants provided written informed consent.
2.2 Lipid extraction and LC/ESI/MS/MS analysis
The lipid extractions and methods for measuring plasma ceramide and sphingomyelin levels in the BLSA have previously been described in detail, including the inter- and intra-day coefficients of variation (Mielke et al., 2015a, 2015b). Briefly, a crude lipid extraction of plasma was conducted using a modified Bligh and Dyer procedure with ceramide or sphingomyelin C12:0 included as an internal standard (Avanti Polar Lipids, Alabaster, Alabama) (Bandaru et al., 2013; Haughey et al., 2004). Plasma extracts were dried in a nitrogen evaporator (Organomation Associates Inc., Berlin, MA, USA), and re-suspended in pure methanol just prior to analysis. An autosampler (LEAP technologies Inc., Carrboro, NC) injected extracts into an HPLC (PerkinElmer, MA, USA) equipped with a reverse phase C18 column (Phenomenex, Torrance, CA). The eluted sample was then injected into an electrospray ion source coupled to a triple quadrupole mass spectrometer (API3000, AB Sciex Inc. Thornhill, Ontario, Canada) (Bandaru et al., 2013, 2011, 2007). Analyses were conducted by multiple reaction monitoring (MRM). Eight point calibration curves (0.1 to 1,000 ng/ml) were constructed by plotting area under the curve (AUC), separately for ceramides and sphingomyelins, for each calibration standard d18:1/C16:0, d18:1/C18:0, d18:1/C20:0, d18:1/C22:0, d18:1/C24:0 (Avanti polar lipids, Alabaster, AL, USA) normalized to the internal standard. Correlation coefficients (R2) obtained were >0.999. Ceramide concentrations were determined by fitting the identified ceramide species to these standard curves based on acyl-chain length. Internal standards were run daily, and AUCs plotted weekly, to track instrument efficiency. Plasma extracts were re-analyzed if the internal standard deviated more than 25% of the median value. Instrument control and quantitation of spectral data was performed using Analyst 1.4.2 and MultiQuant software (AB Sciex Inc. Thornhill, Ontario, Canada). All sphingolipids are expressed in μg/ml for statistical analyses, and descriptive statistics for sphingomyelins and ceramides for the current sample are shown in Supp. Table 1.
2.3 MRI protocol and image processing
The neuroimaging component of the BLSA incorporated diffusion weighted imaging on 3T scanners in 2008. Data acquired at each BLSA visit included T1-weighted magnetization prepared rapid gradient recalled echo (MPRAGE) and DTI scans. This study pooled imaging data from three different Philips Achieva 3T scanners (Best, Netherlands) with similar protocols. Two of these scanners were located at the Kennedy Krieger Institute (KKI) and the third was located at the clinical research program of the National Institute on Aging (NIA). The MPRAGE protocol for the KKI scanners are: number of slices = 170, voxel size = 1 mm*1 mm*1.2 mm, acquisition matrix size = 256*240*170, reconstruction matrix = 256*256, flip angle = 8 degrees, and TR/TE = 6.8 msecs/3.1 msecs. The NIA scanner had an identical MPRAGE protocol with the exception of using a TR/TE of 6.5 msecs/3.1 msecs. The DTI protocol for all scanners included two acquisitions of the following: number of gradients = 32, echo time = 75 msecs, flip angle = 90 degrees, slice thickness = 2.2 mm, and b-factor = 700 s/mm2. The KKI scanner had TR = 6801 msecs, number of slices = 65, reconstructed matrix = 256*256, voxel size= 0.83 mm * 0.83 mm and NIA scanner had TR = 7454 msecs, number of slices = 70, reconstructed matrix = 320*320, voxel size= 0.81mm * 0.81mm. Each DTI acquisition had two b0 images, which were averaged in k-space into a single b0 image. Two separate DTI acquisitions each with NSA = 1 were obtained and then combined offline for an effective NSA = 2 to improve signal-to-noise ratio. Of the 150 participants included in the current analyses, 44 were assessed on KKI scanners and 106 on the NIA scanner. All analyses included a covariate to adjust for differences due to scanners and respective variation in scanning parameters.
DTI processing followed standard practice for tensor fitting and quality assessment and is explained in detail in our earlier publication (Lauzon et al., 2013). Briefly, the individual diffusion weighted volumes were affine co-registered to a minimally weighted (b0) target to compensate for eddy current effects and physiological motion. The gradient tables were corrected for the identified rotational component using finite strain (Alexander et al., 2001). To combine the two DTI scans acquired within each session, with different (unknown) intensity normalization constants, each diffusion weighted image was normalized by its own reference image (b0) prior to tensor fitting. To segment gray matter regions, we used multi-atlas registration using 35 manually labeled atlases from NeuroMorphometrics with the BrainCOLOR protocol (Klein et al., 2010). To segment the WM, the Eve White Matter atlas (Lim et al., 2013) was registered to each subject using multi-channel registration using the T1-weighted structural volume and the FA map. To minimize the impacts of warping and partial volume effects, region of interest analysis were computed in subject space. The process of warping the Eve WM labels to the subject space was carefully constructed to limit the negative impacts of label transformation and issues of partial volume effects with gray matter. Whole brain segmentation was performed on the T1-weighted structural volume, which resulted in white/gray matter separation as well as specific regions of interest for gray matter labels. The Eve atlas was registered/warped to each subject’s T1-weighted volume and the labels were propagated. However, there were two sources of inconsistency - (1) warped Eve labels showed WM tracts in subject’s gray matter areas, and (2) some subject’s WM areas had no Eve label. To address (1), Eve labels outside of the subject’s WM were ignored (defaulted to the gray matter ROI labels from the structural scans). To address (2), Eve labels were grown to fill all WM areas of the subject via minimum distance propagation. The white and gray matter ROI labels obtained from the T1 image for each visit were affine registered to the FA image and used to extract region-specific mean FA and MD measures from left and right regions separately. This analysis focused on 17 ROIs (Table 1), 16 averaged across left and right hemispheres defined by the Eve White Matter atlas and one ROI, the average of left and right total cerebral white matter (CWM), defined by the BrainColor atlas.
Table 1.
White Matter Regions
| Abbreviation | Region Name |
|---|---|
| CWM | Cerebral White Matter |
| SCR | Superior Corona Radiata |
| ACR | Anterior Corona Radiata |
| SLF | Superior Longitudinal Fasciculus |
| PTR | Posterior Thalamic Radiation (Include Optic Radiation) |
| Scc | Splenium of Corpus Callosum |
| Bcc | Body of Corpus Callosum |
| PLIC | Posterior Limb of Internal Capsule |
| Gcc | Genu of Corpus Callosum |
| SS | Sagittal stratum (Includes Inferior Longitudinal fasciculus and Inferior fronto occipital fasciculus) |
| CG | Cingulum of Cingulate Gyrus |
| ALIC | Anterior Limb of Internal Capsule |
| PCR | Posterior Corona Radiata |
| IFOF | Inferior Fronto Occipital Fasciculus |
| CH | Cingulum of Hippocampus |
| FX | Fornix (body and column) |
| UF | Uncinate Fasciculus |
The ROI for cerebral white matter was taken from the BrainColor atlas, whereas all other ROIs are from the EVE Johns Hopkins Atlas.
To obtain measures of intracranial volume and WM hyperintensity, we used a separate, validated automated approach (Davatzikos et al., 2001). The total ICV was estimated by calculating the volume of a subject's brain mask. A multi-atlas label fusion based automated segmentation method was applied for extracting a brain mask on the T1-weighted image of each subject (Doshi et al., 2013). The brain mask included gray matter (GM), WM, and ventricular and cortical cerebrospinal fluid (CSF). Each brain mask was visually inspected for quality, and the masks with errors were manually corrected by a trained rater. White matter lesion (WML) volume was segmented using MPRAGE, T2 and FLAIR images based on a support vector machine classifier approach previously published (Lao et al., 2008; Zacharaki et al., 2008). Because the mean FA and MD calculations within each ROI include WMLs, we used the ratio of WML volume to ICV as a covariate to account for differences in diffusion measures due to inter-individual variation in global WML volume.
2.4 Covariates
Demographic variables included age, sex, race, and years of education. Height (in meters) and weight (in kilograms) were measured to calculate body mass index (BMI). Medical history information included hypertension, myocardial infarction, cancer, and statin use. The diagnoses of diabetes at each visit were established by combining information on medications, fasting glucose and glucose levels at 2-hours of a standard glucose tolerance test. In particular, participants who were taking anti-diabetes medication or had a fasting glucose >126 mg/dL and/or a 2-hour glucose >200 mg/dL were defined as diabetic. Plasma total cholesterol and triglycerides were determined by an enzymatic method (Abbott Laboratories ABA-200 ATC Biochromatic Analyzer, Irving, Texas). APOE ε4 genotype was determined using polymerase chain reaction amplification of leukocyte deoxyribonucleic acid followed by HhaI digestion and product characterization (Hixson and Vernier, 1990) or TaqMan, relying on several single nucleotide polymorphisms around the APOE gene (Koch et al., 2002).
2.5 Statistical Analyses
We used simultaneous multiple linear regression to assess the relationship between the plasma sphingolipid measurements and future regional diffusion measures. Specifically, we implemented robust multiple linear regression using iterated re-weighted least squares to prevent outliers in the response variables from biasing results (via foreign and MASS packages built under R version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/). The dependent variables were future regional diffusion measures and independent variables of interest were plasma sphingolipid measurements. The covariates for multiple regression models were selected because they significantly correlated with FA or MD in large WM regions (age, sex, ratio of WML to ICV), have been shown to affect sphingolipid levels (i.e., diabetes, BMI), or to control for limitations in data collection (i.e., multiple scanners and variable follow-up interval between blood sample and DTI). As previous work has shown levels of peripheral ceramides and sphingomyelins increase with age (Mielke et al., 2015a, 2015b), we also examined models including age X sphingolipids. However, we only present models without the addition of this interaction as the simpler model yielded similar results. To determine the specificity of the sphingolipids relative to other lipids and genetic predispositions known to modify lipid metabolism, we conducted secondary analyses controlling for the effects of total cholesterol, triglycerides, and APOE ε4 allele carrier status. Furthermore, we also investigated the extent that sphingolipids were associated with WML volume. The same independent variables were included as before, with the exception of the ratio of WML to ICV. Because the distribution for WML volume was right skewed, the data were transformed using the natural log transformation (plus the constant 1, to account for a single subject that had no detected lesion volume). All dependent variables were standardized by creating z-scores to facilitate comparisons across measures, and all continuous independent variables were mean-centered. As this is the first study to examine plasma sphingolipids and WM microstructure we considered p<0.05 significant. To address the issue of multiple comparisons, we also determined which sphingolipid measures survived a False Discovery Rate (FDR) threshold of p<0.05 for a given diffusion measure.
3.0 Results
Sample characteristics at the visit in which the sphingolipids were assayed are shown in Table 2. Mean (standard deviation [SD]) age of the participants was 67.2 (6.7) years and 55% were women. The mean (SD) time between the sphingolipid measure and DTI scan was 10.5 (1.6) years.
Table 2.
Participant Characteristics
| Demographics and Health Information | N | Visit 1 | N | Visit 2 |
|---|---|---|---|---|
| Women N (%) | 150 | 82 (54.7) | - | - |
| Baseline Age (years) | 150 | 67.2 (6.7) | 150 | 77.7 (6.7) |
| White N (%) | 150 | 98 (65.3) | - | - |
| Time between blood and DTI (years) | 150 | 10.5 (1.6) | - | - |
| Body Mass Index (kg/m) | 150 | 27.3 (4.2) | 135 | 26.8 (4.2) |
| Diabetes N (%) | 150 | 9 (6.0) | 135 | 11(8.1) |
| Hypertension N (%) | 150 | 58 (38.7) | 135 | 53 (39.3) |
| Systolic BP (mmHg) | 134 | 134.4 (19.5) | 135 | 115.8 (14.5) |
| Diastolic BP (mmHg) | 134 | 77.9 (10.8) | 135 | 62.3 (9.1) |
| APOE E4 Carrier N (%) | 150 | 38 (25.3) | - | - |
| Total cholesterol (mg/dl) | 145 | 199.2 (34.8) | 134 | 183.7 (36.1) |
| Triglycerides (mg/dl) | 142 | 109.1 (66.0) | 133 | 95.7 (44.9) |
| Statin use N (%) | 150 | 30 (20.0) | 135 | 49 (36.3) |
| Cancer N (%) | 150 | 18 (12.0) | 135 | 25 (18.5) |
| Myocardial infarction N (%) | 150 | 3 (2.0) | 135 | 6 (4.4) |
| Education (years) | 150 | 16.6 (2.5) | - | - |
| MMSE | 124 | 29.0 (1.2) | 133 | 28.6 (1.5) |
Mean and standard deviation unless otherwise noted. Visit 1 information is concurrent with the visit at which the sphingolipids were assayed, and visit 2 is concurrent with DTI. APOE E4 = apolipoprotein E4. BP = blood pressure. MMSE = Mini-mental state examination.
3.1 Associations between ceramides and white matter diffusion measures
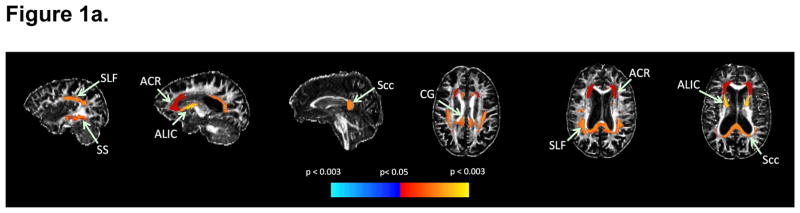
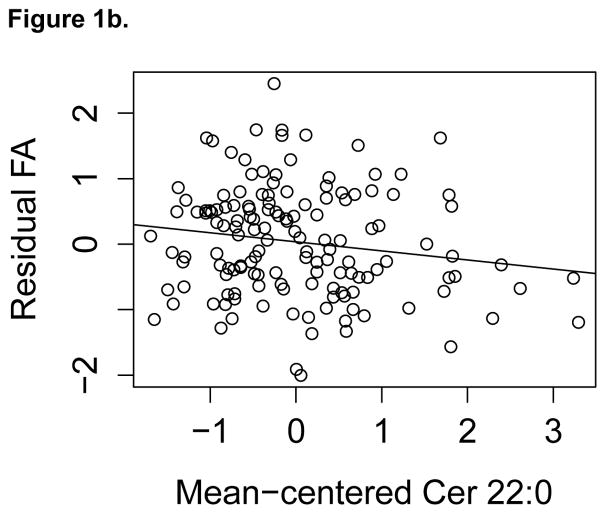
All relationships significant at the p<0.05 level between specific ceramide acyl chain lengths and subsequent WM integrity, assessed by FA and MD, are shown in Table 3. Higher ceramide concentrations were associated with lower FA or higher MD, with the majority of results involving FA rather than MD. Higher ceramides C20:0 and C22:0 were associated with lower FA in the greatest number of WM regions, including total CWM, superior longitudinal fasciculus, anterior corona radiata, and the cingulum of the cingulate gyrus. Results for C22:0 are displayed on a single subject’s FA image shown in Figure 1a and a scatterplot for anterior corona radiata is shown in Figure 1b. Higher C24:1 was associated both with lower FA and higher MD in the anterior corona radiata and with lower FA in the genu of the corpus callosum. The associations between higher plasma C22:0 and lower regional FA survived FDR p<0.05 for the total CWM, sagittal stratum, anterior limb of the internal capsule, superior longitudinal fasciculus, cingulum of the cingulate gyrus, and splenium of the corpus callosum.
Table 3.
Significant effects of ceramides on white matter regions
| Ceramide | Region | Diffusion Measure | N | β | p-Value |
|---|---|---|---|---|---|
| C16:0 | |||||
| - | - | - | - | - | |
| C18:0 | |||||
| ACR | FA | 150 | −3.440 | 0.030 | |
| C20:0 | |||||
| CWM | FA | 149 | −1.534 | 0.026 | |
| ACR | FA | 149 | −1.551 | 0.046 | |
| ACR | MD | 149 | 1.243 | 0.049 | |
| SLF | FA | 149 | −1.650 | 0.040 | |
| CG | FA | 149 | −1.790 | 0.044 | |
| C22:0 | |||||
| CWM | FA | 144 | −0.213 | 0.004* | |
| ACR | FA | 144 | −0.170 | 0.043 | |
| SLF | FA | 144 | −0.212 | 0.012* | |
| Scc | FA | 144 | −0.188 | 0.012* | |
| SS | FA | 144 | −0.201 | 0.014* | |
| CG | FA | 144 | −0.226 | 0.014* | |
| ALIC | FA | 144 | −0.247 | 0.005* | |
| C22:1 | |||||
| ACR | FA | 150 | −12.092 | 0.040 | |
| ALIC | FA | 150 | −15.708 | 0.017 | |
| CH | FA | 150 | −12.420 | 0.015 | |
| C24:0 | |||||
| - | - | - | - | - | |
| C24:1 | |||||
| ACR | FA | 148 | −0.660 | 0.007 | |
| ACR | MD | 148 | 0.568 | 0.005 | |
| Gcc | FA | 148 | −0.513 | 0.014 | |
| C26:0 | |||||
| - | - | - | - | - | |
For each ceramide, only the diffusion measure from each white matter region that was significant at p<0.05 is displayed. The model adjusted for baseline age, sex, interval between blood draw and DTI, diabetes, BMI, scanner, and ratio of white matter lesions to intracranial volume. Values represent mean differences in Z-scores of FA or MD per 1 ug/ml increase of sphingolipids concentration. Corresponding p-values are shown and asterisks indicate associations survive FDR p<0.05.
Figure 1.
Figure 1a. Significant associations with plasma C22:0 and subsequent FA
Figure 1a. Red-yellow indicates higher plasma C22:0 associated with lower FA in white matter regions, while cyan-blue indicates higher plasma C22:0 associated with higher FA. Regional statistics are overlaid onto an FA image from a single subject; results are not corrected for multiple comparisons.
Figure 1b. Anterior Corona Radiata FA by mean-centered Cer 22:0
Figure 1b. Association between FA in ACR and Cer 22:0. The residual FA of ACR was calculated by regressing standardized FA on baseline age, sex, interval between blood draw and DTI, diabetes, BMI, scanner, and ratio of white matter lesions to intracranial volume. This is shown plotted against mean-centered Cer 22:0 with a linear fit overlaid.
In secondary analyses examining the specificity of sphingolipids, most of the results in Table 3 remained even after adjusting for other blood lipids (cholesterol, triglycerides) and APOE genotype. The associations between ceramides and diffusion measures remained for C22:0, C22:1 and C24:1 after controlling for total cholesterol. While results for C18:0 and C20:0 were similar, associations with diffusion measures were no longer significant at the p<0.05 level. Associations for the majority of C22:0 and C24:1 remained unchanged after controlling for triglycerides. The results for C18:0, C20:0 and C22:1 were no longer significant but showed similar trends. Adjusting for the presence of an APOE ε4 allele did not affect any of the associations. Additionally, none of the ceramide species were associated with WML volume.
3.2 Associations between sphingomyelins and white matter diffusion measures
Significant associations between sphingomyelins of varying chain lengths and FA and MD for WM regions are shown in Table 4. Most associations indicated that higher levels of sphingomyelins were associated with lower FA. Sphingomyelin C18:1 was associated with FA in the greatest number of regions, including total CWM. The only chain length associated with MD was C24:0, with higher levels of C24:0 related to lower MD in the uncinate fasciculus. Sphingomyelins C16:0, C20:0, C22:0, and C24:1 were not associated with diffusion measures in any WM regions examined. The association between higher sphingomyelin C18:1 and lower FA in the body of the corpus callosum survived FDR p<0.05.
Table 4.
Significant effects of sphingomyelins on white matter regions
| SM | ROIs | Diffusion Measure | N | β | p-Value |
|---|---|---|---|---|---|
| C16:0 | |||||
| - | - | - | - | - | |
| C16:1 | |||||
| Bcc | FA | 150 | −7.98E-03 | 0.042 | |
| C18:0 | |||||
| ACR | FA | 150 | −6.40E-03 | 0.033 | |
| C18:1 | |||||
| CWM | FA | 146 | −2.32E-03 | 0.009 | |
| ACR | FA | 146 | −1.96E-03 | 0.042 | |
| Bcc | FA | 146 | −2.89E-03 | 0.003* | |
| PLIC | FA | 146 | −2.44E-03 | 0.011 | |
| FX | FA | 146 | 1.93E-03 | 0.042 | |
| C20:0 | |||||
| - | - | - | - | - | |
| C20:1 | |||||
| ACR | FA | 150 | −1.11E-02 | 0.017 | |
| Bcc | FA | 150 | −1.07E-02 | 0.027 | |
| C22:0 | |||||
| - | - | - | - | - | |
| C22:1 | |||||
| ACR | FA | 150 | −2.94E-03 | 0.042 | |
| C24:0 | |||||
| UF | MD | 148 | −1.93E-03 | 0.039 | |
| C24:1 | |||||
| - | - | - | - | - | |
For each sphingomyelin, only the diffusion measure from each white matter region that was significant at p<0.05 is displayed. The model adjusted for baseline age, sex, interval between blood draw and DTI, diabetes, BMI, scanner, and ratio of white matter lesions to intracranial volume. Values represent mean differences in Z-scores of FA or MD per 1 ug/ml increase of sphingolipids concentration. Corresponding p-values are shown and asterisks indicate associations survive FDR p<0.05.
Secondary analyses adjusting for triglycerides, cholesterol, and APOE ε4 allele showed similar associations. Results remained substantially unchanged after controlling for triglycerides. However, after adjusting for total cholesterol levels, while trends remained similar for chain lengths C16:1, C18:0, C22:1, and C24:0, most associations were not significant at the p<0.05 level. Results for the majority of C18:1 and C20:1 with FA remained after adjusting for total cholesterol. After controlling for APOE ε4, associations for C24:0 with the uncinate fasciculus and C18:1 with the fornix were no longer significant, however results for other chain lengths remained. Additionally, only species C22:0 and C24:0 were associated with WML volume, where higher levels were associated with greater WML volume (Supp. Table 2).
3.3 Effects of the ratio of sphingomyelin to ceramide on white matter diffusion measures
We also investigated whether the ratios of sphingomyelin to ceramide were associated with subsequent FA and MD (Table 5). Lower ratios of sphingomyelin to ceramide for C22:0, C22:1, and C24:0 were related to lower FA for superior and posterior corona radiata, cingulum of the hippocampus, and the posterior corona radiata and anterior limb of the internal capsule, respectively. A lower ratio of C24:1 was also associated with higher MD in the anterior corona radiata and inferior fronto-occipital fasciculus. These results did not survive correction for multiple comparisons with FDR p<0.05.
Table 5.
Significant effects of ratios of SM/Cer on white matter regions
| ROIs | Diffusion Measure | N | β | p-value | |
|---|---|---|---|---|---|
| SM/Cer C16:0 | |||||
| - | - | - | - | - | |
| SM/Cer C18:0 | |||||
| - | - | - | - | - | |
| SM/Cer C20:0 | |||||
| - | - | - | - | - | |
| SM/Cer C22:0 | |||||
| SCR | FA | 141 | 4.41E-03 | 0.044 | |
| PCR | FA | 141 | 4.80E-03 | 0.017 | |
| SM/Cer C22:1 | |||||
| CH | FA | 150 | 2.91E-05 | 0.016 | |
| SM/Cer C24:0 | |||||
| ALIC | FA | 147 | 2.24E-02 | 0.043 | |
| PCR | FA | 147 | 2.05E-02 | 0.032 | |
| SM/Cer C24:1 | |||||
| ACR | MD | 148 | −7.05E-05 | 0.033 | |
| IFOF | MD | 148 | −8.68E-05 | 0.023 | |
For each lipid outcome, only the diffusion measure from each white matter region that was significant at p<0.05 is displayed. The model adjusted for baseline age, sex, interval between blood draw and DTI, diabetes, BMI, scanner, and ratio of white matter lesions to intracranial volume. Corresponding p-values are shown and asterisks indicate associations survive FDR p<0.05.
Results after controlling for triglycerides remained unchanged for ratios C22:0 and C22:1, however associations were no longer significant for ratios C24:0 and C24:1 but exhibited similar trends. Results for ratio measures remained substantially unchanged after controlling for total cholesterol and APOE ε4. Greater ratios of C22:0 and C24:0 were associated with greater WML volume (Supp. Table 3).
4.0 Discussion
In this study, we found that higher concentrations of circulating ceramides were associated with lower FA or higher MD 10.5 years later in several large WM regions. These findings are consistent with worse microstructural WM integrity. Higher sphingomyelins were also associated with lower FA in several brain areas. Notably, a lower sphingomyelin to ceramide ratio, indicating higher levels of ceramides, was also associated with diffusion indices consistent with worse WM integrity. Results remained after adjustment for other circulating lipids and APOE genotype. However, after correction for multiple comparisons, associations with DTI metrics remained significant for only a few sphingolipid chain lengths.
This is the first study to identify associations between peripheral measures of sphingolipids and subsequent WM microstructure. A previous voxel-wise analysis (Williams et al., 2013) investigating concurrent serum lipids and DTI measures showed that among different serum lipid measures, elevated low density lipoprotein (LDL) was most robustly associated with lower FA across several WM tracts. This study found LDL was associated with disrupted WM microstructure in right frontal and temporal regions, such as anterior and superior corona radiata and superior longitudinal fasciculus. While some of our findings with sphingolipids show similar regional overlap, many of our results remained significant after controlling for total cholesterol and triglycerides, suggesting differences in microstructure are not solely driven by changes in global lipids and may be specific to perturbations of sphingolipid metabolism.
Our findings are consistent with existing literature implicating elevated ceramides in neurodegeneration and cognitive impairment. Previous pathological studies have identified elevated levels of ceramides in AD patients compared with normal controls in overall frontal and temporal brain regions (Chan et al., 2012; Cutler et al., 2004; Han et al., 2002). Associations of higher ceramides with disrupted WM integrity in cognitively normal individuals suggest a role of sphingolipids in brain aging. Additionally, the association of lower FA in the ACR with multiple ceramide chain lengths is consistent with studies that have identified the strongest associations between older age and lower FA for frontal and anterior regions (Head, 2004; Salat et al., 2005). Furthermore, previous studies have identified elevated peripheral measures of ceramides and sphingomyelins and risk of subsequent cognitive impairment (Mielke et al., 2012, 2010a). In particular, older cognitively normal women, followed for nine years, with higher levels of ceramides C16:0, C18:0, C22:0, C24:0, and C24:1 were at greater risk of memory impairment (Mielke et al., 2010a). As several of these ceramides, such as C20:0, C22:0, and C24:1 were associated with diffusion measures in our study of cognitively normal individuals, future follow-up studies will determine the extent to which our findings predict future cognitive impairment.
We also found that higher levels of sphingomyelin, especially C18:1, were associated with lower FA in total CWM and some large WM tracts. Most of these associations survived after adjusting for triglycerides and APOE ε4 carrier status. However, the relationships between several sphingomyelin chain lengths (C16:1, C18:0, C22:1, C24:0) and diffusion measures were attenuated and were no longer significant at p<0.05 after controlling for total cholesterol. The lipid composition of myelin is enriched in sphingomyelin to a greater extent than ceramide, and associations with increased circulating sphingomyelin could reflect a degenerative process of WM.
Sphingomyelinase (SMase) metabolizes sphingomyelin to ceramide and phosphocholine. Previous research has suggested that SMase activity may contribute to neurodegenerative diseases. Sphingomyelinases are over-expressed in AD patients compared with normal controls (He et al., 2010), and higher sphingomyelin to ceramide ratios were strongly associated with slower cognitive decline as measured by longitudinal Mini-Mental Status Exam and the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (Mielke et al., 2011). Indeed, our results are congruent with these findings. When we examined the sphingomyelin to ceramide ratio, lower levels of both saturated and unsaturated ratios for C22 and C24 were associated with either decreased FA or increased MD in projecting and association WM tracts. Associations with diffusion measures were more pronounced for ceramides than sphingomyelins for these chain lengths. These results suggest that the observed associations between sphingolipids and lower WM microstuctural integrity may be driven by the elevated ceramides, and that a lower ratio of plasma sphingomyelin to ceramide may be implicated in worsening WM microstructure. However, higher ratios of sphingomyelin to ceramide for chain lengths C22:0 and C24:0 were also associated with greater WML volume. While elevated sphingomyelin chain lengths C22:0 and C24:0 were associated with greater WML volume, ceramides showed no such association, suggesting the associations between these ratios and WML volume may be driven by elevated sphingomyelins, which again could reflect pronounced WM damage. Clearly, additional studies with serial blood and MRI measures across the AD spectrum are needed to clarify the relationship between sphingolipids and white matter integrity and ischemic lesion burden.
The majority of associations for ceramides, sphingomyelins, and their ratio appeared to be with FA rather than MD. FA is sensitive to the extent diffusion occurs along a particular direction, whereas MD is not. Our results could indicate that circulating sphingolipids are associated with variability in axon bundle coherence. However, the mechanism linking peripheral sphingolipids and brain WM microstructure is not well understood. While a previous study in rats showed smaller chain bioactive ceramides in the periphery could cross the blood-brain barrier to mediate cognitive impairment (Monte et al., 2010), it is unclear if this holds true for the longer-chain ceramides analyzed in the present study. Even if peripheral and brain ceramide measures are not correlated, peripheral ceramides contribute to inflammation, insulin resistance, and vascular disease, all of which have unique contributions to brain aging, loss of WM integrity, and neurodegenerative diseases (Mielke and Haughey, 2012). Furthermore, because of the correlative nature of this study design, we cannot exclude the possibility that worsening WM integrity alters the levels of circulating sphingolipids.
Limitations of the study warrant consideration. First, we took an exploratory approach that tested a large number of regions with several sphingolipid measures, and the majority of results for each sphingolipid measure did not survive correction for multiple comparisons. However, all results for ceramides and ratio of sphingomyelins to ceramides significant at the a priori p<0.05 were in directions consistent with worse WM integrity. Future studies will be required to replicate the robustness of these findings. There is a considerable time interval between the blood sample and DTI scans (10.5 years). Thus, we cannot eliminate the possibility that other physiological changes during this period may confound our observed effects. Our study includes scans from three different scanners, and while they are all 3T Philips Achieva with similar protocols, adding scanner as a covariate may not fully account for scanner differences. We have addressed the issue of different scanners extensively in prior work (Venkatraman et al., 2015) . Our regions of interest contain voxels with WM lesions, and while we included WML/ICV ratio as a covariate, it cannot completely account for possible effects on diffusion measures. With regards to model selection, we did not examine non-linear effects of age on diffusion measures, as we wanted to apply the same model to all diffusion measures and such non-linear effects may vary across sphingolipid species. Lastly, the BLSA consists of community-dwelling volunteers who may be healthier and more educated compared to the general population, limiting generalizability of results. However, our study also has a number of strengths, including the well-characterized cohort followed over many years, allowing assessment of previous sphingolipid levels in relation to subsequent cognitive and brain aging.
4.1 Conclusion
In conclusion, we have shown that elevated levels of peripheral ceramides and lower levels of sphingomyelin to ceramide ratio were associated with lower FA or higher MD in several large white matter regions ten years later. These findings are important for characterizing the role of peripheral sphingolipids in normative brain aging and white matter disease. Future studies should examine how sphingolipid measures relate to diffusion measures at the voxel-level, as averages over large regions may not reflect differences in local microstructure. Furthermore, longitudinal DTI studies are needed to determine whether peripheral sphingolipids predict or are serially associated with subsequent change in diffusion measures across white matter tracts. Such studies are important for characterizing the timeline between the dysregulation of sphingolipid metabolism, white matter changes, and cognitive decline and impairment.
Supplementary Material
Highlights.
Higher levels of plasma ceramides predict lower FA in white matter 10.5 years later
Higher ratios of plasma sphingomyelin to ceramide predicts higher FA and higher MD
Trends are similar but reduced after adjusting for cholesterol and triglycerides
Plasma sphingolipids may predict variation in white matter microstructure in aging
Acknowledgments
This research was supported by a grant from the National Institutes of Health/National Institute on Aging (U01 AG37526) and by the Intramural Research Program of the National Institute on Aging, NIH.
Footnotes
Financial Disclosures
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher E. Gonzalez, Email: Christopher.gonzalez@nih.gov.
Vijay K. Venkatraman, Email: vijay.venkatraman@nih.gov.
Yang An, Email: anya@grc.nia.nih.gov.
Bennett A. Landman, Email: bennett.landman@vanderbilt.edu.
Christos Davatzikos, Email: christos@rad.upenn.edu.
Veera Venkata Ratnam Bandaru, Email: vbandar2@jhmi.edu.
Norman J. Haughey, Email: nhaughe1@jhmi.edu.
Luigi Ferrucci, Email: ferruccilu@mail.nih.gov.
Susan M. Resnick, Email: resnicks@grc.nia.nih.gov.
References
- Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, Yoshikawa T, Okubo T, Ohtomo K. Normal aging in the central nervous system: Quantitative MR diffusion-tensor analysis. Neurobiol Aging. 2002;23:433–441. doi: 10.1016/S0197-4580(01)00318-9. [DOI] [PubMed] [Google Scholar]
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans Med Imaging. 2001;20:1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Bandaru VVR, DP, Mcarthur JC, Sacktor N, GR, Sc CM, Knapp EL, Mattson MP, Haughey NJ. Associative and Predictive Biomarkers of Dementia in HIV-1 Infected Patients. Neurology. 2007;68:1481–1488. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VVR, Mielke MM, Sacktor N, McArthur JC, Grant I, Letendre S, Chang L, Wojna V, Pardo C, Calabresi P, Munsaka S, Haughey NJ. A lipid storage-like disorder contributes to cognitive decline in HIV-infected subjects. Neurology. 2013;81:1492–1499. doi: 10.1212/WNL.0b013e3182a9565e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VVR, Patel N, Ewaleifoh O, Haughey NJ. A failure to normalize biochemical and metabolic insults during morphine withdrawal disrupts synaptic repair in mice transgenic for HIV-gp120. J Neuroimmune Pharmacol. 2011;6:640–649. doi: 10.1007/s11481-011-9289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David O, Futerman AH. The role of the ceramide acyl chain length in neurodegeneration: involvement of ceramide synthases. Neuromolecular Med. 2010;12:341–50. doi: 10.1007/s12017-010-8114-x. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010;31:378–90. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- Chan RB, Oliveira TG, Cortes EP, Honig LS, Duff KE, Small Sa, Wenk MR, Shui G, Di Paolo G. Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J Biol Chem. 2012;287:2678–2688. doi: 10.1074/jbc.M111.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremesti AE, Goni FM, Kolesnick R. Role of sphingomyelinase and ceramide in modulating rafts: Do biophysical properties determine biologic outcome? FEBS Lett. 2002;531:47–53. doi: 10.1016/S0014-5793(02)03489-0. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen Wa, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatzikos C, Genc a, Xu D, Resnick SM. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage. 2001;14:1361–1369. doi: 10.1006/nimg.2001.0937. [DOI] [PubMed] [Google Scholar]
- Doshi J, Erus G, Ou Y, Gaonkar B, Davatzikos C. Multi-Atlas Skull-Stripping. Acad Radiol. 2013;20:1566–1576. doi: 10.1016/j.acra.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov V, Song MA, Zhang K, Vinters HV, Tung S, Kirsch WM, Yang J, Duerksen-Hughes PJ. Increased ceramide in brains with alzheimer’s and other neurodegenerative diseases. J Alzheimer’s Dis. 2012;29:537–547. doi: 10.3233/JAD-2011-111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive Aging, Executive Function, and Fractional Anisotropy: A Diffusion Tensor MR Imaging Study. AJNR Am J Neuroradiol. 2007;28:226–235. 28/2/226 [pii] [PMC free article] [PubMed] [Google Scholar]
- Han X, Holtzman D, McKeel DW, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: Potential role in disease pathogenesis. J Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Haughey NJ. Sphingolipids in neurodegeneration. Neuromolecular Med. 2010;12:301–5. doi: 10.1007/s12017-010-8135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo Ca, Turchan J, Nath A, Mattson MP. Perturbation of Sphingolipid Metabolism and Ceramide Production in HIV-Dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiol Aging. 2010;31:398–408. doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D. Differential Vulnerability of Anterior White Matter in Nondemented Aging with Minimal Acceleration in Dementia of the Alzheimer Type: Evidence from Diffusion Tensor Imaging. Cereb Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, Maldjian Ja, Laurienti PJ. Relating imaging indices of white matter integrity and volume in healthy older adults. Cereb Cortex. 2008;18:433–442. doi: 10.1093/cercor/bhm080. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. Age-related differences in regional brain volumes: A comparison of optimized voxel-based morphometry to manual volumetry. Neurobiol Aging. 2009;30:1657–1676. doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Canton TD, Ghosh SS, Landman BA, Lee J, Worth A. Open labels: Online feedback for a public resource of manually labeled brain images. 16th Annu Meet Organ Hum Brain Mapp 2010 [Google Scholar]
- Koch W, Ehrenhaft A, Griesser K, Pfeufer A, Müller J, Schömig A, Kastrati A. TaqMan systems for genotyping of disease-related polymorphisms present in the gene encoding apolipoprotein E. Clin Chem Lab Med. 2002;40:1123–1131. doi: 10.1515/CCLM.2002.197. [DOI] [PubMed] [Google Scholar]
- Kolesnick RN. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998 doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- Lao Z, Shen D, Liu D, Jawad AF, Melhem ER, Launer LJ, Bryan RN, Davatzikos C. Computer-Assisted Segmentation of White Matter Lesions in 3D MR Images Using Support Vector Machine. Acad Radiol. 2008;15:300–313. doi: 10.1016/j.acra.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon CB, Asman AJ, Esparza ML, Burns SS, Fan Q, Gao Y, Anderson AW, Davis N, Cutting LE, Landman Ba. Simultaneous analysis and quality assurance for diffusion tensor imaging. PLoS One. 2013;8:e61737. doi: 10.1371/journal.pone.0061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim IA, Faria AV, Li X, Hsu JT, Airan RD, Mori S, van Zijl PC. Human brain atlas for automated region of interest selection in quantitative susceptibility mapping: application to determine iron content in deep gray matter structures. Neuroimage. 2013;82:449–469. doi: 10.1016/j.neuroimage.2013.05.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–939. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Bandaru VVR, Han D, An Y, Resnick SM, Ferrucci L, Haughey NJ. Demographic and clinical variables affecting mid- to late-life trajectories of plasma ceramide and dihydroceramide species. Aging Cell. 2015a doi: 10.1111/acel.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Bandaru VVR, Han D, An Y, Resnick SM, Ferrucci L, Haughey NJ. Factors affecting longitudinal trajectories of plasma sphingomyelins: the Baltimore Longitudinal Study of Aging. Aging Cell. 2015b;14:112–121. doi: 10.1111/acel.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Bandaru VVR, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging. 2010a;31:17–24. doi: 10.1016/j.neurobiolaging.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Bandaru VVR, Haughey NJ, Xia J, Fried LP, Yasar S, Albert M, Varma V, Harris G, Schneider EB, Rabins PV, Bandeen-Roche K, Lyketsos CG, Carlson MC. Serum ceramides increase the risk of Alzheimer disease: The Women’s Health and Aging Study II. Neurology. 2012;79:633–41. doi: 10.1212/WNL.0b013e318264e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Haughey NJ. Could plasma sphingolipids be diagnostic or prognostic biomarkers for Alzheimer’s disease? Clin. Lipidol. 2012;7:525–536. doi: 10.2217/clp.12.59.Could. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Haughey NJ, Bandaru VVR, Weinberg DD, Darby E, Zaidi N, Pavlik V, Doody RS, Lyketsos CG. Plasma sphingomyelins are associated with cognitive progression in Alzheimer’s disease. J Alzheimers Dis. 2011;27:259–69. doi: 10.3233/JAD-2011-110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Haughey NJ, Ratnam Bandaru VV, Schech S, Carrick R, Carlson MC, Mori S, Miller MI, Ceritoglu C, Brown T, Albert M, Lyketsos CG. Plasma ceramides are altered in mild cognitive impairment and predict cognitive decline and hippocampal volume loss. Alzheimers Dement. 2010b;6:378–85. doi: 10.1016/j.jalz.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte SM, de la Tong M, Nguyen V, Setshedi M, Longato L, Wands JR. Ceramide-Mediated Insulin Resistance and Impairment of Cognitive-Motor Functions. J Alzheimer’s Dis. 2010;21:967–984. doi: 10.3233/JAD-2010-091726.Ceramide-Mediated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham D, Nunn RS. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys J. 1990;58:997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–309. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::AID-MRM13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe aJW, Hevelone ND, Zaleta aK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale aM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–27. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Shock NW, Greulich RC, Andres R, Arenberg D, Costa PT, Lakatta EG, Tobin JD. Normal human aging: The Baltimore longitudinal study of aging 1984 [Google Scholar]
- Venkatraman VK, Gonzalez CE, Landman B, Goh J, Reiter DA, An Y, Resnick SM. Region of interest correction factors improve reliability of diffusion imaging measures within and across scanners and field strengths. Neuroimage. 2015;119:406–416. doi: 10.1016/j.neuroimage.2015.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams VJ, Leritz EC, Shepel J, Mcglinchey RE, Milberg WP, Rudolph JL, Lipsitz La, Salat DH. Interindividual variation in serum cholesterol is associated with regional white matter tissue integrity in older adults. Hum Brain Mapp. 2013;34:1826–1841. doi: 10.1002/hbm.22030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharaki EI, Kanterakis S, Bryan RN, Davatzikos C. Measuring brain lesion progression with a supervised tissue classification system. Proc Int Conf Med Image Comput Comput Assist Interv. 2008;11:620–627. doi: 10.1007/978-3-540-85988-8_74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.