SUMMARY
Crohn's disease is a chronic inflammatory bowel disease of unknown cause, affecting approximately 1.4 million North American people. Due to the similarities between Crohn's disease and Johne’s disease, a chronic enteritis in ruminant animals caused by Mycobacterium avium paratuberculosis (MAP) infection, MAP has long been considered to be a potential cause of Crohn's disease. MAP is an obligate intracellular pathogen that cannot replicate outside of animal hosts. MAP is widespread in dairy cattle and because of environmental contamination and resistance to pasteurization and chlorination, humans are frequently exposed through contamination of food and water. MAP can be cultured from the peripheral mononuclear cells from 50 to 100% of patients with Crohn's disease, and less frequently from healthy individuals. Association does not prove causation. We discuss the current data regarding MAP as a potential cause of Crohn's disease and outline what data will be required to firmly prove or disprove the hypothesis.
Keywords: Mycobacterium avium subspecies paratuberculosis (MAP), Inflammatory bowel disease, Crohn's disease, Johne’s disease, Helicobacter pylori, treatment trials, prevention
Introduction
Mycobacterium avium subspecies paratuberculosis (MAP) is a bacterium in the Mycobacteriaceae family. The Mycobacteriaceae family includes the pathogenic bacteria that cause tuberculosis (Mycobacterium tuberculosis) and leprosy (Mycobacterium leprae). MAP is included in the Mycobacterium avium complex (MAC), along with M. avium and M. intracelluare. MAP is not a distinct species, as it is sub-species of M. avium. [1–3]. MAP are rod-shaped and acid-fast. As with other mycobacteria, it contains a thick and hydrophobic cell wall that resists decolorization with acidic alcohol causing the cells to be “acid fast” [4].
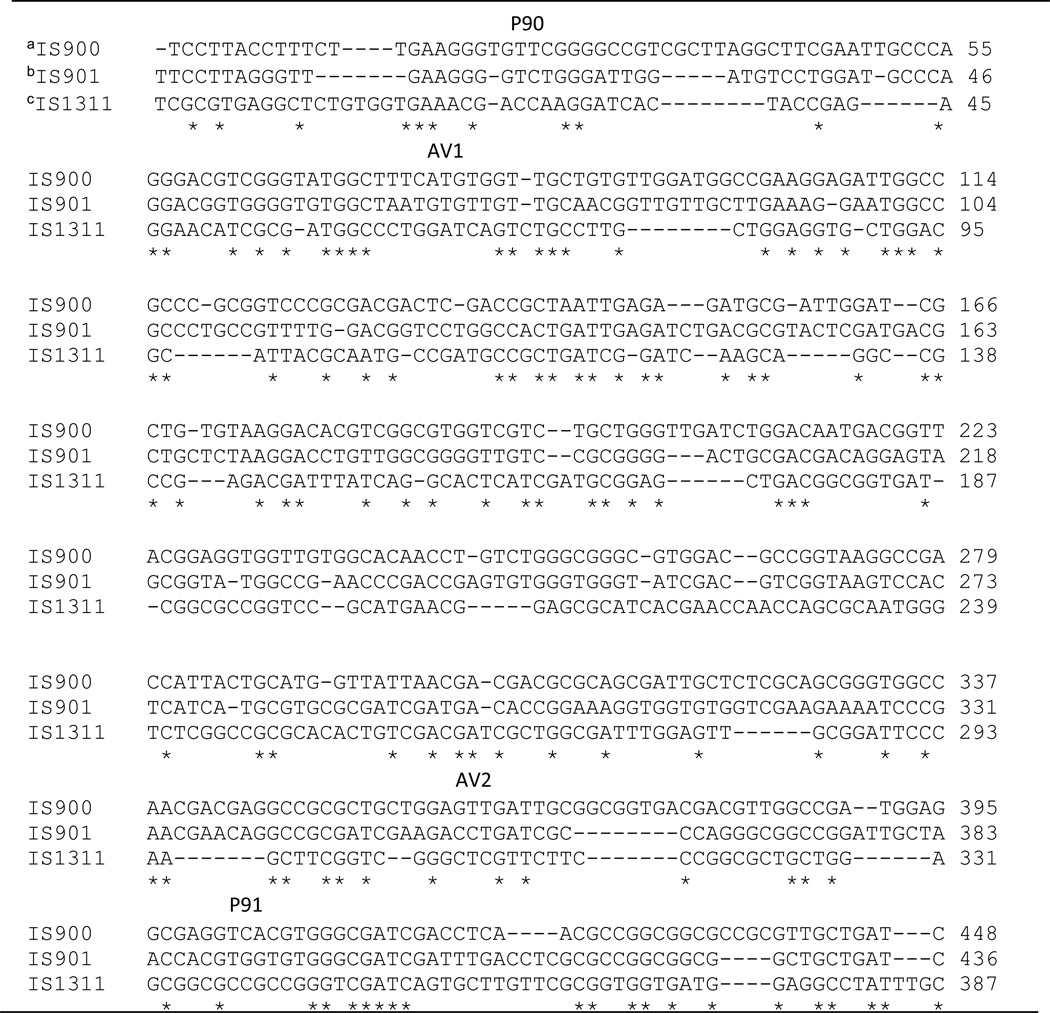
The MAP genome contains a species-specific insertion element (IS 900) that can be used to detect the presence of the organism and separate it from other Mycobacteria using polymerase chain reaction (PCR) assays or in situ hybridization [5]. This genetic sequence (Table 1) was identified in a MAP isolate from a Crohn’s disease patient and is used in most PCR assays designed to distinguish MAP from the other MAC species. IS900 contains 1451 bp with ~66% G+C content. Each MAP genome contains 14 to 20 copies of the insertion element [5].
Table 1.
Insertion sequences in Mycobacterium avium
Multiple sequences alignments are compared using CLUSTAL 2.1.
IS900 (GenBank accession no. X16293) is unique to M. avium subspecies paratuberculosis (MAP).
IS901 (GenBank accession no. X59272) is in all M. avium subspecies.
IS1311 (GenBank accession no. U16276) is in M. avium subspecies except M. intracelluare.
MAP was first isolated and described as the etiological agent of a chronic inflammatory enteric disease in cattle by Heinrich Albert Johne, a German veterinarian, in 1895 [6]. Johne’s disease was subsequently identified in both North America and Europe and is present worldwide. Johne’s disease is a chronic enteritis of ruminant animals (e.g., cattle and sheep) and clinically presents as weight loss and diarrhea. It is often fatal for infected animals [7]. A similar disease has also been occasionally observed in other animals including deer, goats, rabbits, monkeys, and chimpanzees [8,9]. MAP is unable to synthesize mycobactin, a siderophore required for the bacterium to obtain iron from environmental sources. It therefore cannot replicate outside of an animal host and as such is an obligate intracellular pathogen. In cattle MAP is trophic for the mucosa of the distal small intestine. Following entry into the host, MAP replicates in the cytoplasm of monocytes/macrophages. The presence of persisting mycobacterial antigens trigger inflammation and in some animals leads to clinical disease [10].
Once shed into the environment, MAP can exist as either a cell wall-containing form or [11] remain dormant [12] in a vegetative state or in a spore-like form [13]. The spore-like form can survive heat and may contribute to the cells being stable in both host organisms and the environment. In human tissues MAP is difficult or often impossible to identify microscopically. However, its presence can be identified by culture of the organism or by molecular techniques such as PCR or in situ hybridization [6]. The difficulty in identifying MAP with the usual stains for acid fast bacteria is ascribed to the belief that, in human tissues, it exists primarily in an intracellular and cell-wall deficient form as a spheroplast [11,14]. The lack of a cell wall makes spheroplasts unable to accept the typical staining methods such as the Ziehl Neelsen stain and is thus difficult or impossible to detect using standard light microscopy[15]. However, the acid-fast negative form is thought to be a robust phenotype and is associated with persistent infections, perhaps due in part to altered immune recognition. It has also been shown that the intracellular form can be taken up, at least in vitro, by environmental protists such as Acanthamoeba polyphaga [16]. Passage of MAP through bovine macrophages and epithelial cells and possibly in environmental protists has been suggested to enhance virulence and persistence [16–19]. However, the role of environmental protists, if any, in vivo is unknown.
MAP grows very slowly in culture and three to many months are required for colonies to appear [20]. Human-derived cultures are often very slow growing and may take a year or more before colonies are seen. The bacterial cells in vitro can develop typical mycobacterial cell walls and become acid-fast.
MAP infection of ruminants
Johne’s disease caused by MAP infection results in significant economic losses for the dairy and cattle industries with annual estimates of millions of dollars [21]. In ruminants, MAP infection is transmitted primarily by the fecal-oral route [22]. Most animals become infected within the first month of life; susceptibility to MAP infection decreases with age [10]. Infection is followed by a long latency period and symptoms rarely appear before 3 to 5 years of age. Johne’s disease is a progressive disease with 4 stages progressing from silent, to subclinical, clinical, and finally to advanced stage disease [22]. Infected animals shed MAP during the subclinical stages, despite absence of symptoms. The clinical stages are manifest by increasing diarrhea and weight loss. The advanced disease is associated with decreased milk production and the presence of granulomas in regional lymph nodes and small intestines, and wasting followed by death.
Most calves exposed to MAP develop persistent infection and only 10 to 15% will develop fatal clinical disease [10,23] This suggests the infection can often be limited by the bovine immune system. Early phase of infection involves invasion of gut epithelium and late phase of infection is persistence of MAP in macrophages facilitated by immune evasion mechanisms. Despite the portal of entry (e.g., nasal, intravenous or oral) MAP is trophic for intestinal tissue. Following ingestion MAP is internalized in the small intestine of ruminants by microfold cells (M cells) or by enterocytes. It can then be carried by dendritic cells or macrophages to mesenteric or regional lymph nodes [24]. Entry of MAP into bovine Peyer’s patch tissue is followed by suppression of genes that maintain intestinal barriers such as those controlling tight junctions, gap junctions, and adherens junctions [25]. It is thought that this damage to the tight junctions also facilitates the passage of MAP between enterocytes into the lamina propria. MAP infection also activates epithelial cells to recruit macrophages to the infection site. As with other Mycobacterial infections, MAP infection can persist in macrophages where phagosomal maturation processes are inhibited and antigen processing and presentation are altered [10] [23]. MAP infection is also associated with down regulation of inflammatory cytokine expression, including TNF, IL-6, and IL-12, effectively quieting the immune response to infection [25].
The bovine immune response to subclinical MAP infection begins with a Th1 type, or cell mediated response, against infected macrophages [26–28]. A low level humoral response may be detectable. If maintained infection is cleared; however, MAP has several strategies to counter these responses [23]. In later stages of infection when MAP shedding is measurable, the immune response switches from a Th1 to a Th2, or humoral response, which cannot eliminate intracellular pathogens.
The standard methods for detection of MAP infection in cattle are by fecal culture or the use of ELISA assays for antibodies [29]. The sensitivity of both methods is low in subclinically infected animals making eradication by culling difficult.
MAP in the environment
The major source of MAP in the environment is related to shedding of MAP in the feces of infected ruminants, especially from cows. MAP along with feces is deposited onto pastures where subsequent runoff can contaminate water [30]. Secondarily infected animals include rabbits and wild ruminants such as deer, which also shed MAP into the environment via their feces [8]. As noted above, MAP is an obligate intracellular pathogen and cannot replicate outside of host cells. However, once shed it can survive in the environment for between 12 weeks and at least a year in soil or water [14,32,33]. Susceptible animals typically become infected following exposure to MAP-containing feces that can also contaminate animal food or milk. Transmission of MAP from infected cattle to sheep grazing on the same pastures is also recognized [31]. Estimates of MAP-infected U.S. dairy herds range from 68% [32] to 91% [33]. It has also been reported 8% of beef herds in the US have evidence of MAP infection [34].
MAP is also present in the human food supply, especially in dairy and meat products [35]. The thick lipid cell wall allows the bacteria to survive pasteurization [36,37], and live MAP has been detected in retail milk and cheese products [37–41].
The environmental prevalence of MAP is widespread such that humans face chronic exposure. In some areas, river and municipal water supplies can become heavily contaminated. For example, cow manure containing MAP is widely used as compost or fertilizer for crops and landscaping, which increases the probability of contaminating ground water sources [42]. It is therefore not surprising that MAP can be detected in surface water sources [43–45]. Environmental aerosols represent another possible exposure source because viable MAP has been recovered from air samples collected over rivers that drain livestock pastures [46]. As noted previously, MAP can survive chlorine disinfection treatment used for treating municipal water sources [47] and has been detected in drinking water systems [48–50].
MAP in Crohn's disease
Inflammatory bowel disease refers to a group of chronic inflammatory diseases of the gastrointestinal tract, including ulcerative colitis and Crohn's disease. Once thought to be an autoimmune disease, Crohn's disease is currently thought to result from the interactions between environmental and genetic factors and persisting antigens [51]. It is unclear whether these persisting antigens reflect inappropriate responses to the normal gut microbiome, to pathogenic organisms, or both. Ulcerative colitis is primarily a mucosal disease involving the colon whereas Crohn's disease involves the full thickness of the intestine and can present with lesions from the mouth to the anus.
Crohn's disease is clinically characterized by symptoms such as abdominal pain, diarrhea, bleeding, bowel obstruction, as well as a variety of systemic symptoms. More than 1.4 million persons in North America are thought to suffer from Crohn's disease incurring an estimated annual healthcare costs >$1.7 billion dollars. Clinical features in common between Johne’s and Crohn’s disease include intermittent diarrhea, weight loss, primary disease site as the illeocecal area, mucosal ulcerations, and granulomas. Given the strong similarities between Johne’s and Crohn's disease, it has long been hypothesized that MAP could be the etiological agent of Crohn's disease [6]. The first report of a possible link between MAP and Crohn's disease was made even before the original descriptions by Crohn [52]. In 1913, the Scottish surgeon Thomas Kennedy Dalziel noted that the clinical and gross appearances of the disease in his patients were very similar to those reported in cattle with Johne’s disease [53]. The zoonotic capacities of MAP, including epidemiology, transmission routes to humans, similar pathobiologies, and associations with human diseases, have all been reviewed recently [54]. Genetic evidence for zoonotic transmission comes from whole genome sequence comparisons of MAP isolates from humans with inflammatory bowel disease and animals [55], and adaptations of strains between sheep and camels [56].
A number of early attempts to culture MAP from Crohn's tissues were unsuccessful. In the 1980s, evidence to support the hypothesis that MAP is a causative agent for Crohn's disease was reported when, a then unclassified Mycobacterium sp. was cultured from several patients with Crohn's disease [13,16,61]. The organism was initially reported to be different from MAP. Despite samples not being made available for analysis subsequent studies by Yoshimura et al. using DNA/DNA hybridization techniques proved that the organism was in fact a Mycobacterium paratuberculosis sp. [57]. There remained concern about whether the original isolation was due to laboratory contamination in that the original isolation was made in a laboratory where MAP was commonly grown and subsequent isolations from the original site or other sites initially proved very difficult or impossible. Approximately 20 years later, the link between MAP and Crohn’s disease was significantly strengthened when Naser et al. was able to culture MAP from peripheral blood macrophages and the breast milk of patients with Crohn's disease [58,59]. PCR testing for MAP in peripheral blood macrophages has subsequently become widely used in research [60] and MAP DNA has been detected with variable frequency (46%-100%) in blood and biopsies of Crohn's disease patients [6,61]. MAP is detected less often in patients with ulcerative colitis and normal individuals than in Crohn's patients. Although the detectable frequencies of MAP associated with CD vary among reports, two meta-analysis studies of 65 papers have confirmed that detection of MAP is significantly greater in CD patients compared to controls [62,63]. The most commonly quoted frequency for detection of MAP is approximately 7 times more from Crohn's disease patients than those with ulcerative colitis or normal controls [62]. The presence of MAP DNA in peripheral blood mononuclear cells from normal control patients also confirmed that humans are often exposed to MAP and that exposure is widespread [61,64]. Currently, MAP DNA can be identified by PCR or viable MAP can be cultured from the peripheral blood in 50%-100% of Crohn's disease patients [58,65]. The fact that MAP can be found in individuals without Crohn's disease does not exclude it as the cause of the disease. For example, most calves exposed to MAP become subclinically infected and only approximately 10% subsequently develop Johne’s disease. Host factors, such as genetics, and environmental stress likely contribute to loss of protective immunity that controls MAP infection during the latency phase. This is a typical outcome for chronic bacterial infectious diseases where only as subset of infected individuals develop clinical disease. For example, approximately one-third of the world population is infected with Mycobacterium tuberculosis yet clinical disease develops in 5–10% infected people [66].
Genetic Susceptibility to IBD overlaps with susceptibility to intracellular pathogens
There has been considerable interest in identifying biomarkers for susceptibility to inflammatory bowel disease, specifically to Crohn's disease. Many reports describe associations of genetic variants with Crohn’s disease [67]. More than 160 genes have been associated with Crohn's disease in genome wide association studies (GWAS) [73–75]. The majority of these genes encode components important to innate immune response functions, and in particular, mechanisms related to responding to intracellular parasites and mycobacterial infections.
A genetic association with Crohn's disease was identified in the Nucleotide-binding Oligomerization Domain-containing protein2, or NOD2 encoded by the CARD15 gene. This protein functions as an intracellular pattern recognition receptor for microbial pathogens, especially for Mycobacteriaceae [68]. NOD2 activates NFkB signaling following binding of microbial peptidoglycans. NOD2 mutations are thought to confer susceptibility to Crohn's disease by altering the receptors’ recognition of pathogens or the downstream activation of NFkB in monocytes [69].
The SLC11A1 (Solute carrier 11A1), formerly identified as NRAMP (natural resistance-associated macrophage protein 1), is an ion transporter across phagosomal membranes and induces microbicidal functions in macrophages [70]. It plays a role in the innate immune response to mycobacterial infections. Polymorphisms at locus 823 C/T are strongly associated with CD [71], and the −237C/T polymorphism is significantly associated with IBD [72]. Genetic variants of SLC11A1 at 469+14G/C, 1730G/A, and 1729+55del4 are also associated with increased susceptibility to tuberculosis [72].
GWAS in cattle also identify genetic associations to MAP infection [81,82], with polymorphisms, which vary among breeds, in NOD2/CARD15 and in SLC11A1 [83–85].
Autophagy is an important component in innate immunity and contributes to clearance of intracellular microbes [73]. The genes ATG16L1 and IRGM encode proteins involved in autophagy show a strong association with Crohn's disease susceptibility [74].
Genetic studies have identified loci as risk factors for susceptibility to Crohn’s disease, rather than genetic causes of disease. These gene polymorphisms are not associated with Crohn's disease in all populations and are not significantly associated with the detection of MAP in Crohn’s patients [72,88–90]. These associations have in common genes that are important in anti-bacterial responses of the innate immune system and genetic studies may be useful in predicting disease phenotype and treatment outcomes in inflammatory bowel diseases.
MAP and Susceptibility
Current thinking is that MAP is widespread in the environment and most individuals are exposed repeatedly. The interaction of MAP and the immune response is complex and modified by factors, such as early life experiences related to the hygiene hypothesis (proposed by Strachan [75], and later updated to describe microbial exposures and immunological diseases from evolutionary points of view [76,77]), exposure, and to alterations in the host's microbiome. The natural history of MAP exposure in humans remains unknown. In many instances, the association is likely transient. In animals, Johne's disease begins as a latent MAP infection that in some animals ultimately results in clinical disease [22]. Young animals are most susceptible. The triggers for transition from transient or latent infection to clinical illness are also unknown and are likely related to environmental and host genetic factors. A similar phenomena is seen in other chronic infectious diseases in humans such as tuberculosis [66], syphilis [78], and H. pylori gastritis [79].
Human immune response to MAP
MAP infection in humans probably begins via the fecal oral route following consumption of contaminated water or food. Little is known about the human immune response to MAP. Studies of MAP infection in cattle show similarities to the immune response during Mycobacterium tuberculosis latent infection, including the development of cellular mediated immune response [28]. However, MAP-specific cellular responses are detected at a higher frequency in intestinal T cells in Crohn’s disease patients compared to controls. Gut mucosal organ cultures from Crohn’s disease patients [80] and T cell lines established from biopsies have been shown to be reactive to MAP antigens [81]. The frequency of MAP-reactive tissues is also significantly higher in IS900 PCR-positive samples [80].
MAP-specific antibodies are also detected in Crohn's disease patients. The prevalence of seropositivity to MAP in Crohn's disease, ulcerative colitis and, in non-IBD normals varies geographically possibly reflecting different exposures [98–100]. A meta-analysis of serology studies in Crohn’s disease patients found a higher prevalence of MAP specific antibodies compared to controls [62].
MAP epidemiology and its relation to Crohn's disease
It has been suggested that the presence of MAP in Crohn's disease is not causal but reflects the fact that MAP colonizes and invades an already inflamed bowel [82]. This same argument was made for the association of H. pylori with duodenal ulcer disease. That conundrum was only solved with H. pylori when it was shown that cure of the H. pylori infection resulted in healing of the inflammation and subsequent cure of chronic recurrent peptic ulcers. As discussed below, proof of causation requires more than similarity and association, no matter how strong. However, there are multiple strong associations between MAP and Crohn's disease.
A correlation exists between rising worldwide incidence of IBD [83] and the increased incidence of Johne's disease in dairy cattle over the last 100 years [84]. Although numerous reports of higher than average incidences of Crohn's disease in agricultural areas have been published, those working with infected livestock do not experience an increased risk. Examples of Crohn's disease in agricultural areas include Winnipeg, Canada, where the Crohn's disease incidence is 3.5 times higher than nearby areas possibly due to high levels of waterborne MAP from agricultural runoff in local rivers [85]. Cardiff, South Wales [43], and the Canterbury region of New Zealand [86] are other places that have rivers carrying runoff from agricultural pastures and higher incidences of Crohn's disease. Two recent possibly waterborne outbreaks have been reported in the U.S, one from a dairy farming area of Virginia and the other related to wild sheep in the Rocky mountains [87,88]. A clustering of Crohn's disease cases was reported in unrelated students from the high school graduating class in 1980 in Mankato, Minnesota, [89] and are thought to have possibly been linked to the students swimming in waters contaminated with high levels of bacteria.
The importation of MAP-infected animals has also been documented to precede an increase in Crohn's disease incidence. In 1933, epidemics of Johne's disease in Iceland were reported in sheep followed by epidemics in cattle [90]. The Crohn's disease incident rates has risen 18-fold between 1960 and 1992. A similar scenario has been described in the Czech Republic with a 4.5 fold increase in Crohn's disease between 1995 and 2007 following an increase in Johne's disease after the importation of asymptomatic infected cattle.
Crohn's disease may occur as clusters or outbreaks. Such “outbreaks” of Crohn's disease in both related and unrelated individuals argues that a heritable disposition to the disease is not absolutely required and is consistent with the presence of an environmental etiology. Clusters of Crohn's disease have also been observed in groups of related individuals but the timing of disease onset suggests exposure to infectious organisms. For example, parents and children in two French families developed disease within years of each other with no history of Crohn's disease in previous generations [91]. Development of IBD in non-consanguineous married couples also argues for the importance of exposure to a common environmental factor [92–94].
However, as noted above, occupations that allow high exposure to MAP such as in dairy farmers and veterinarians do not show a high incidences of IBD despite potential exposure to MAP that is excreted by animals in the trillions [95–97]. Further studies are needed to explore the importance of the method of exposure (e.g., in food or water), host genetic predisposition, the frequency of exposure, the infectious dose in humans, and the importance of exposure to other environmental organisms that may confer some protective immunity via mechanisms proposed in the hygiene hypothesis. As noted earlier, with other chronic infectious diseases such as tuberculosis, syphilis, and H. pylori, clinical disease only occurs in a small subset of individuals.
Treatment and outcomes
Current conventional treatments of Crohn's disease focuses on reducing the inflammatory response and includes corticosteroids, anti-TNF-alpha antibodies such as infliximab, adalimumab, or certolizumab pegol, immune modulators including azathioprine, 6-mercaptopurine (6-MP), methotrexate, and cyclosporine A. IBD drugs reported to inhibit MAP growth in vitro in a dose-dependent manner include 5-aminosalicylic acid (5-ASA), mercaptopurine, methotrexate, cyclosporine A, rapamycin, and tacrolimus [98]. Azathioprine added to MAP culture also inhibits its growth [99].
Given the suspected role of commensal gut bacteria, or infectious bacteria, in causing inflammation or pathogenesis in inflammatory bowel disease, antibiotic regimens have been used to treat Crohn’s disease with various outcomes, although clinical improvements or remission are reported in numerous studies [100,101]. A meta-analysis of placebo-controlled trials of broad spectrum antibiotics shows improved clinical outcome in Crohn’s patients [102]. MAP is a member of the M. avium complex. These "atypical" mycobacteria are notoriously difficult to cure with the usual antimicrobial regimens that are successful in M. tuberculosis. For example, treatment of Crohn's disease patients with traditional anti-tuberculosis drugs (isoniazid, ethambutol, and ripampicin) evaluated during a five year study including two years of clinical trial treatment with and a three-year follow up period did not show consistent improvement [103]. There are however data reporting remission of Crohn's disease following MAP-specific treatments. Meta-analysis studies of randomized placebo controlled trials show benefits from treatment with nitroimidazoles and macrolides (clofazimine) [104] and antibiotic therapies using antimycobacterial combinations can induce remission in active Crohn’s disease [105]. Current therapies include combinations of antimicrobials typically used for treatment of these atypical Mycobacteria and to prevent multi-drug resistance. Several trials using combinations of intracellular active antibiotics including rifabutin, clarithromycin, and clofazimine have reported clinical and histological remissions [124–128] (Table 2). Two case reports describe Crohn’s disease patients that were either MAP-positive by PCR in blood before treatment with rifabutin, clarithromycin, and levoflaxin [106], or that had MAP-specific antibodies, and MAP-positive blood cultures and PCR before treatment with rifabutin and clarithromycin [107]. Both patients progressed to complete remission and MAP was no longer detected in their blood. Case reports do not establish effectiveness of antimycobacterial therapy for treating Crohn’s disease or that MAP causes Crohn’s disease. Clinical trials are needed to examine the presence of MAP and correlate it to disease before, during, of after treatments, or after relapse.
Table 2.
Summary of trials for treatment of Crohn’s disease with anti-MAP antibiotics
| Study [Source] | No. Patients |
Drugs | Months of Treatment |
Clinical Outcomes |
|---|---|---|---|---|
| Gui et al., 1997 [124] |
46 | Rifabutin Clarithromycin or Azithromycin |
6–35 | Clinical remission in 88.5% Steroid independence in 89.5% |
| Borody et al., 2002 [125] |
12 | Rifabutin Clarithromycin Clofazimine |
24 6–54 |
Clinical remission in 50% |
| Shafran et al., 2002 [127] |
36† | Rifabutin Clarithromycin Clofazimine |
4–17 | Clinical improvement in 58.3% |
| Selby et al., 2007 [126] |
213 | Rifabutin Clarithromycin Clofazimine |
12 | Relapse in 59% who completed |
| Borody et al., 2007 [128] |
39 | Rifabutin Clarithromycin Clofazimine |
6–108 | Mucosal healing 56.4% |
Patients were seropositive for MAP before treatment
Given that M. avium infections are extremely difficult to eradicate, it is unclear whether and how long the benefits of treatment will be sustained in Crohn's disease patients. Considering the widespread contamination of our food and water supplies with MAP, the risk of reinfection may also be high following continued exposure to the pathogen. As with H. pylori, confirmation of a definite etiologic role of MAP in Crohn's disease will require the ability to confirm healing of the mucosal lesions and cure of the disease associated with cure or long term suppression of the infection. The ideal study would confirm patients with Crohn’s disease are infected with MAP prior to starting treatment, show that healing of the disease during therapy associated with disappearance of the organism, and if the disease recurred, confirm that the recurrence was associated with recurrence of active infection. No such study is currently available. However, we are currently participating in multi-center studies treating Crohn’s disease patients with rifabutin, clarithromycin, and clofazimine, compared to placebo controls, in a double blind fashion [108]. Patient samples are being tested for MAP throughout the trial. Hopefully these studies will inform whether observed improvements in clinical disease are concurrent with curing MAP infection.
Future considerations
Preventing MAP from causing Crohn's disease will require development of methods to prevent or eliminate environmental MAP exposures to humans. For example, UV light can be used to inactivate Mycobacteria in drinking water systems [109]. Importantly, the feasibility of controlling mycobacterium infections in cattle that are pathogenic to humans has been demonstrated by the bovine tuberculosis program in the U.S. [110]. Herd infection prevalence has been lowered from 5% to <0.001%. The program produced significant benefits to public health and yielded economic returns greater than implementation costs. Similarly, MAP control programs such as culling of infected animals would reduce the current global economic impact of Johne’s disease in dairy and beef cattle.
Theoretically, cattle can be treated with vaccines to prevent MAP infection and vaccine preparations of heat-killed, live attenuated, or recombinant MAP subunits have been tested in several animal species [111]. A meta-analysis of 118 paratuberculosis vaccination experiments from 63 reports concludes that most studies showed reductions in the clinical symptoms of Johne’s disease including isolation of MAP from tissue or feces, histopathological lesions, and mortality rates [112]. Overall, the data suggest vaccination limits MAP infection symptoms, but it is not clear if the proportion of animals with subclinical infections is reduced. Fecal shedding still occurs so a vaccine approach has not yet been developed that will eliminate MAP infection from food animals. Concerns over the pathogenicity of MAP in humans have supported the progress of planned Phase 1 and Phase 2a clinical trials to determine the safety and efficacy in humans of a vaccine that encodes two secreted and two surface proteins of MAP [113,114].
If MAP cannot be eradicated due to constraints of economy, policy, or farming practices, then vaccines may be effective in controlling infection, while strategies are put into place that will reduce or remove MAP from food products. Development of biomarkers to identify subclinical MAP-infected cattle, separately or in combination with vaccine use, might prevent further progression of contaminated food products into human and animal food chains [115]. Lessons for effective strategies in controlling Johne’s disease might be learned from comparing the diverse approaches of private organizations and governments in 6 countries with endemically MAP-infected animals [116].
EXPERT COMMENTARY.
Increasing research evidence links human inflammatory bowel disease to MAP infection in food animals. Crohn's disease may well be a by-product of modern animal husbandry practices in use by the dairy industry. Proof that MAP is causing a significant human disease will require improved management of cattle and dairy industries and a revision of USDA regulations. The lack of proof has led the USDA to be reluctant to require culling as a major strategy to eliminate MAP from dairy herds. Proof that it is involved in even a subset of patients with Crohn's disease will require a complete reconsideration of their current thinking. Carefully designed clinical trials in Crohn’s patients that specifically confirm the presence of MAP and relate the responses to treatment and recurrence can establish a causal zoonotic link between the pathogen, a major food industry, and a devastating chronic human gastrointestinal illness. We learned from H. pylori that no matter how strong the associations were, proof only came when the diseases it caused could be healed, cured and prevented by H. pylori eradication. If this is accomplished for MAP, then the priority will be to devise and implement control measures to eliminate MAP from the environment and human food supplies.
FIVE-YEAR VIEW.
The focus on developing new drugs for multi-drug resistant tuberculosis should provide new and better therapies useful for infection with other mycobacterial infection. The ongoing treatment trial, if positive, will provide an impetus for additional studies and a change from anti-inflammatory therapy to treatment of specific causes. In the meantime, metagenomic analyses to identify and characterize MAP strains in infected animals and Crohn’s disease patients should provide further evidence for or against the hypothesis that MAP is an important etiological agent. Such studies may be especially informative during “outbreaks” of Crohn’s disease, or when environmental sources of MAP have been confirmed locally, or epidemiologically linked to newly diagnosed cases.
KEY ISSUES.
Mycobacterium avium subspecies paratuberculosis (MAP) is a ubiquitous pathogen that cannot replicate in the environment and is widely present in food and water sources of humans.
MAP causes Johne’s disease, a chronic enteritis, in cattle and other ruminant animals.
Accumulating evidence supports a causative association between MAP and Crohn's disease in humans.
Viable MAP and its DNA can be detected in high percentages of blood and tissue from Crohn's disease patients.
Crohn's disease may occur in clusters or epidemics in which MAP is detected in the environment.
Genes associated with susceptibility to Crohn's disease and to MAP infection overlap significantly.
MAP-specific treatments appear to promote resolution of symptoms and healing of mucosal damage. Placebo controlled trials are needed to confirm the specificity of these finding.
Proof that MAP causes Crohn's disease will result in a major change in the cattle and dairy industries similar to those that occurred during the program to eliminate bovine tuberculosis.
Acknowledgments
D.Y. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH. D.Y. Graham is a paid consultant for Red Hill Biopharma regarding novel H. pylori therapies and is the Principal Investigator for the ongoing Red Hill clinical trial entitled A Phase III Randomized, Double Blind, Placebo-controlled, Multicenter, Parallel Group Study to Assess the Efficacy and Safety of Fixed-dose Combination RHB-104 in Subjects with Moderately to Severely Active Crohn’s Disease.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Thorel MF, Krichevsky M, Levy-Frebault VV. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium and description of Mycobacterium avium subsp. avium subsp nov, Mycobacterium avium subsp. paratuberculosis subsp nov, Mycobacterium avium subsp. silvaticum subsp. nov. Int. J Syst. Bacteriol. 1990;40:254–260. doi: 10.1099/00207713-40-3-254. [DOI] [PubMed] [Google Scholar]
- 2.Turenne CY, Wallace R, Jr, Behr MA. Mycobacterium avium in the postgenomic era. Clin. Microbiol. Rev. 2007;20:205–229. doi: 10.1128/CMR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rindi L, Garzelli C. Genetic diversity and phylogeny of Mycobacterium avium. Infect. Genet. Evol. 2014;21:375–383. doi: 10.1016/j.meegid.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Niederweis M, Danilchanka O, Huff J, Hoffmann C, Engelhardt H. Mycobacterial outer membranes: in search of proteins. Trends Microbiol. 2010;18:109–116. doi: 10.1016/j.tim.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green EP, Tizard ML, Moss MT, et al. Sequence and characteristics of IS900, an insertion element identified in a human Crohn's disease isolate of Mycobacterium paratuberculosis. Nucleic. Acids Res. 1989;17:9063–9073. doi: 10.1093/nar/17.22.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naser SA, Sagramsingh SR, Naser AS, Thanigachalam S. Mycobacterium avium subspecies paratuberculosis causes Crohn's disease in some inflammatory bowel disease patients. World. J. Gastroenterol. 2014;20:7403–7415. doi: 10.3748/wjg.v20.i23.7403. **Recent summary of studies that support or do not support an association of MAP with Crohn's disease by evidence of viable MAP in culture or detection of MAP DNA.
- 7.Chacon O, Bermudez LE, Barletta RG. Johne's disease, inflammatory bowel disease, and Mycobacterium paratuberculosis. Annu. Rev. Microbiol. 2004;58:329–363. doi: 10.1146/annurev.micro.58.030603.123726. [DOI] [PubMed] [Google Scholar]
- 8.Greig A, Stevenson K, Henderson D, et al. Epidemiological study of paratuberculosis in wild rabbits in Scotland. J Clin. Microbiol. 1999;37:1746–1751. doi: 10.1128/jcm.37.6.1746-1751.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke CJ. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J Comp. Pathol. 1997;116:217–261. doi: 10.1016/s0021-9975(97)80001-1. [DOI] [PubMed] [Google Scholar]
- 10.Koets AP, Eda S, Sreevatsan S. The within host dynamics of Mycobacterium avium ssp. paratuberculosis infection in cattle: where time and place matter. Vet. Res. 2015;46:61. doi: 10.1186/s13567-015-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiodini RJ, Van Kruiningen HJ, Thayer WR, Coutu JA. Spheroplastic phase of mycobacteria isolated from patients with Crohn's disease. J. Clin. Microbiol. 1986;24:357–363. doi: 10.1128/jcm.24.3.357-363.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whittington RJ, Marshall DJ, Nicholls PJ, Marsh IB, Reddacliff LA. Survival dormancy of Mycobacterium avium subsp. paratuberculosis in the environment. Appl. Environ. Microbiol. 2004;70:2989–3004. doi: 10.1128/AEM.70.5.2989-3004.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamont EA, Bannantine JP, Armien A, Ariyakumar DS, Sreevatsan S. Identification characterization of a spore-like morphotype in chronically starved Mycobacterium avium subsp. paratuberculosis cultures. PLoS One. 2012;7:e30648. doi: 10.1371/journal.pone.0030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiodini RJ, Van Kruiningen HJ, Thayer WR, Merkal RS, Coutu JA. Possible role of mycobacteria in inflammatory bowel disease I. An unclassified Mycobacterium species isolated from patients with Crohn's disease. Dig. Dis. Sci. 1984;29:1073–1079. doi: 10.1007/BF01317078. **The first report of isolating MAP from two Crohn's disease pateints.
- 15.Seiler P, Ulrichs T, Bandermann S, et al. Cell-wall alterations as an attribute of Mycobacterium tuberculosis in latent infection. J Infect. Dis. 2003;188:1326–1331. doi: 10.1086/378563. [DOI] [PubMed] [Google Scholar]
- 16.Mura M, Bull TJ, Evans H, et al. Replication long-term persistence of bovine and human strains of Mycobacterium avium subsp. paratuberculosis within Acanthamoeba polyphaga. Appl. Environ. Microbiol. 2006;72:854–859. doi: 10.1128/AEM.72.1.854-859.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cirillo JD, Falkow S, Tompkins LS, Bermudez LE. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 1997;65:3759–3767. doi: 10.1128/iai.65.9.3759-3767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel D, Danelishvili L, Yamazaki Y, et al. The ability of Mycobacterium avium subsp. paratuberculosis to enter bovine epithelial cells is influenced by preexposure to a hyperosmolar environment and intracellular passage in bovine mammary epithelial cells. Infect. Immun. 2006;74:2849–2855. doi: 10.1128/IAI.74.5.2849-2855.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salgado M, Alfaro M, Salazar F, et al. Application of cattle slurry containing Mycobacterium avium subsp. paratuberculosis (MAP) to grassland soil and its effect on the relationship between MAP and free-living amoeba. Vet. Microbiol. 2015;175:26–34. doi: 10.1016/j.vetmic.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Collins MT. Paratuberculosis: review of present knowledge. Acta Vet. Scand. 2003;44:217–221. [PubMed] [Google Scholar]
- 21.Lombard JE. Epidemiology and economics of paratuberculosis. Vet. Clin. North Am. Food. Anim Pract. 2011;27:525–35. doi: 10.1016/j.cvfa.2011.07.012. v. [DOI] [PubMed] [Google Scholar]
- 22.Salem M, Heydel C, El-Sayed A, Ahmed SA, Zschock M, Baljer G. Mycobacterium avium subspecies paratuberculosis: an insidious problem for the ruminant industry. Trop. Anim Health Prod. 2013;45:351–366. doi: 10.1007/s11250-012-0274-2. [DOI] [PubMed] [Google Scholar]
- 23.Arsenault RJ, Maattanen P, Daigle J, Potter A, Griebel P, Napper S. From mouth to macrophage: mechanisms of innate immune subversion by Mycobacterium avium subsp. paratuberculosis. Vet. Res. 2014;45:54. doi: 10.1186/1297-9716-45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bannantine JP, Bermudez LE. No holes barred: invasion of the intestinal mucosa by Mycobacterium avium subsp. paratuberculosis. Infect. Immun. 2013;81:3960–3965. doi: 10.1128/IAI.00575-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khare S, Lawhon SD, Drake KL, et al. Systems biology analysis of gene expression during in vivo Mycobacterium avium paratuberculosis enteric colonization reveals role for immune tolerance. PLoS One. 2012;7:e42127. doi: 10.1371/journal.pone.0042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coussens PM. Mycobacterium paratuberculosis and the bovine immune system. Anim Health Res. Rev. 2001;2:141–161. [PubMed] [Google Scholar]
- 27.Sohal JS, Singh SV, Tyagi P, et al. Immunology of mycobacterial infections: with special reference to Mycobacterium avium subspecies paratuberculosis. Immunobiology. 2008;213:585–598. doi: 10.1016/j.imbio.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Davis WC, Madsen-Bouterse SA. Crohn's disease Mycobacterium avium subsp. paratuberculosis: the need for a study is long overdue. Vet. Immunol. Immunopathol. 2012;145:1–6. doi: 10.1016/j.vetimm.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitlock RH, Wells SJ, Sweeney RW, Van TJ. ELISA and fecal culture for paratuberculosis (Johne's disease): sensitivity and specificity of each method. Vet. Microbiol. 2000;77:387–398. doi: 10.1016/s0378-1135(00)00324-2. [DOI] [PubMed] [Google Scholar]
- 30.Salgado M, Alfaro M, Salazar F, et al. Effect of soil slope on the appearance of Mycobacterium avium subsp. paratuberculosis in water running off grassland soil after application of contaminated slurry. Appl. Environ. Microbiol. 2013;79:3544–3552. doi: 10.1128/AEM.00610-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muskens J, Bakker D, de BJ, van KL. Paratuberculosis in sheep: its possible role in the epidemiology of paratuberculosis in cattle. Vet. Microbiol. 2001;78:101–109. doi: 10.1016/s0378-1135(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 32.Veterinary Services U. Johne's Disease on U.S. Dairies 1991–2007. 2008. [Google Scholar]
- 33.Lombard JE, Gardner IA, Jafarzadeh SR, et al. Herd-level prevalence of Mycobacterium avium subsp. paratuberculosis infection in United States dairy herds in 2007. Prev. Vet. Med. 2013;108:234–238. doi: 10.1016/j.prevetmed.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Dargatz DA, Byrum BA, Hennager SG, et al. Prevalence of antibodies against Mycobacterium avium subsp paratuberculosis among beef cow-calf herds. J Am Vet. Med Assoc. 2001;219:497–501. doi: 10.2460/javma.2001.219.497. [DOI] [PubMed] [Google Scholar]
- 35. Eltholth MM, Marsh VR, Van WS, Guitian FJ. Contamination of food products with Mycobacterium avium paratuberculosis: a systematic review. J. Appl. Microbiol. 2009;107:1061–1071. doi: 10.1111/j.1365-2672.2009.04286.x. *Review of contamination of food products with MAP
- 36.Chiodini RJ, Hermon-Taylor J. The thermal resistance of Mycobacterium paratuberculosis in raw milk under conditions simulating pasteurization. J Vet. Diagn. Invest. 1993;5:629–631. doi: 10.1177/104063879300500424. [DOI] [PubMed] [Google Scholar]
- 37.Grant IR, Ball HJ, Rowe MT. Incidence of Mycobacterium paratuberculosis in bulk raw and commercially pasteurized cows' milk from approved dairy processing establishments in the United Kingdom. Appl. Environ. Microbiol. 2002;68:2428–2435. doi: 10.1128/AEM.68.5.2428-2435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corti S, Stephan R. Detection of Mycobacterium avium subspecies paratuberculosis specific IS900 insertion sequences in bulk-tank milk samples obtained from different regions throughout Switzerland. BMC Microbiol. 2002;2:15. doi: 10.1186/1471-2180-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donaghy JA, Totton NL, Rowe MT. Persistence of Mycobacterium paratuberculosis during manufacture and ripening of cheddar cheese. Appl. Environ. Microbiol. 2004;70:4899–4905. doi: 10.1128/AEM.70.8.4899-4905.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Millar D, Ford J, Sanderson J, et al. IS900 PCR to detect Mycobacterium paratuberculosis in retail supplies of whole pasteurized cows' milk in England and Wales. Appl. Environ. Microbiol. 1996;62:3446–3452. doi: 10.1128/aem.62.9.3446-3452.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ellingson JL, Anderson JL, Koziczkowski JJ, et al. Detection of viable Mycobacterium avium subsp. paratuberculosis in retail pasteurized whole milk by two culture methods and PCR. J. Food. Prot. 2005;68:966–972. doi: 10.4315/0362-028x-68.5.966. *Recent detection of MAP in commercial milk
- 42. Grewal SK, Rajeev S, Sreevatsan S, Michel FC., Jr Persistence of Mycobacterium avium subsp. paratuberculosis and other zoonotic pathogens during simulated composting, manure packing, and liquid storage of dairy manure. Appl. Environ. Microbiol. 2006;72:565–574. doi: 10.1128/AEM.72.1.565-574.2006. *Study of ability of MAP to survive composting and it dairy manure
- 43.Pickup RW, Rhodes G, Arnott , et al. Mycobacterium avium subsp. paratuberculosis in the catchment area and water of the River Taff in South Wales, United Kingdom, and its potential relationship to clustering of Crohn's disease cases in the city of Cardiff. Appl. Environ. Microbiol. 2005;71:2130–2139. doi: 10.1128/AEM.71.4.2130-2139.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pickup RW, Rhodes G, Bull TJ, et al. Mycobacterium avium subsp. paratuberculosis in lake catchments, in river water abstracted for domestic use, and in effluent from domestic sewage treatment works: diverse opportunities for environmental cycling and human exposure. Appl. Environ. Microbiol. 2006;72:4067–4077. doi: 10.1128/AEM.02490-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whan L, Ball HJ, Grant IR, Rowe MT. Occurrence of Mycobacterium avium subsp. paratuberculosis in untreated water in Northern Ireland. Appl. Environ. Microbiol. 2005;71:7107–7112. doi: 10.1128/AEM.71.11.7107-7112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhodes G, Richardson H, Hermon-Taylor J, Weightman A, Higham A, Pickup R. Mycobacterium avium Subspecies paratuberculosis: Human Exposure through Environmental and Domestic Aerosols. Pathogens. 2014;3:577–595. doi: 10.3390/pathogens3030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor RH, Falkinham JO, III, Norton CD, LeChevallier MW. Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl. Environ. Microbiol. 2000;66:1702–1705. doi: 10.1128/aem.66.4.1702-1705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beumer A, King D, Donohue M, Mistry J, Covert T, Pfaller S. Detection of Mycobacterium avium subsp. paratuberculosis in drinking water and biofilms by quantitative PCR. Appl. Environ. Microbiol. 2010;76:7367–7370. doi: 10.1128/AEM.00730-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klanicova B, Seda J, Slana I, Slany M, Pavlik I. The tracing of mycobacteria in drinking water supply systems by culture, conventional, and real time PCRs. Curr. Microbiol. 2013;67:725–731. doi: 10.1007/s00284-013-0427-1. [DOI] [PubMed] [Google Scholar]
- 50.Aboagye G, Rowe MT. Occurrence of Mycobacterium avium subsp. paratuberculosis in raw water and water treatment operations for the production of potable water. Water Res. 2011;45:3271–3278. doi: 10.1016/j.watres.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 51.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 52.Crohn BB, Ginzburg L, Oppenheimer GD. Regional ileitis: a pathologic and clinical entity. J Am Med Assoc. 1932;99:1323–1329. [Google Scholar]
- 53.Dalziel TK. Chronic interstitial enteritis. BMJ. 1913;2:1068–1070. [Google Scholar]
- 54.Atreya R, Bulte M, Gerlach GF, et al. Facts, myths and hypotheses on the zoonotic nature of Mycobacterium avium subspecies paratuberculosis. Int. J. Med. Microbiol. 2014;304:858–867. doi: 10.1016/j.ijmm.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Wynne JW, Bull TJ, Seemann T, et al. Exploring the zoonotic potential of Mycobacterium avium subspecies paratuberculosis through comparative genomics. PLoS One. 2011;6:e22171. doi: 10.1371/journal.pone.0022171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghosh P, Hsu C, Alyamani EJ, et al. Genome-wide analysis of the emerging infection with Mycobacterium avium subspecies paratuberculosis in the Arabian camels (Camelus dromedarius) PLoS One. 2012;7:e31947. doi: 10.1371/journal.pone.0031947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yoshimura HH, Graham DY. Nucleic acid hybridization studies of mycobactin-dependent mycobacteria. J. Clin. Microbiol. 1988;26:1309–1312. doi: 10.1128/jcm.26.7.1309-1312.1988. ** First proof that the first mycobacterial isolates recovered from two Crohn's disease patients were MAP.
- 58. Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet. 2004;364:1039–1044. doi: 10.1016/S0140-6736(04)17058-X. ** Confirmation that viable MAP were present in the blood of Crohn's disease patients.
- 59. Naser SA, Schwartz D, Shafran I. Isolation of Mycobacterium avium subsp paratuberculosis from breast milk of Crohn's disease patients. Am. J. Gastroenterol. 2000;95:1094–1095. doi: 10.1111/j.1572-0241.2000.01954.x. ** First isolation of MAP from breast milk of humans providing a potential route of transmission within families.
- 60.Timms VJ, Gehringer MM, Mitchell HM, Daskalopoulos G, Neilan BA. How accurately can we detect Mycobacterium avium subsp. paratuberculosis infection? J Microbiol. Methods. 2011;85:1–8. doi: 10.1016/j.mimet.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 61. Chiodini RJ, Chamberlin WM, Sarosiek J, McCallum RW. Crohn's disease and the mycobacterioses: a quarter century later. Causation or simple association? Crit. Rev. Microbiol. 2012;38:52–93. doi: 10.3109/1040841X.2011.638273. **Recent summary of the evidence for and against a causative association of MAP with Crohn's disease.
- 62.Feller M, Huwiler K, Stephan R, et al. Mycobacterium avium subspecies paratuberculosis and Crohn's disease: a systematic review and meta-analysis. Lancet Infect. Dis. 2007;7:607–613. doi: 10.1016/S1473-3099(07)70211-6. [DOI] [PubMed] [Google Scholar]
- 63.Abubakar I, Myhill D, Aliyu SH, Hunter PR. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn's disease using nucleic acid-based techniques: a systematic review and meta-analysis. Inflamm. Bowel. Dis. 2008;14:401–410. doi: 10.1002/ibd.20276. [DOI] [PubMed] [Google Scholar]
- 64.Carol Nacy, Merry Buckley. Mycobacterium avium paratuberculosis: Infrequent human pathogen or public health threat? A report from the American Academy of Microbiology. 2008;2008:1–37. [PubMed] [Google Scholar]
- 65.Mendoza JL, San-Pedro A, Culebras E, et al. High prevalence of viable Mycobacterium avium subspecies paratuberculosis in Crohn's disease. World. J. Gastroenterol. 2010;16:4558–4563. doi: 10.3748/wjg.v16.i36.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stewart GR, Robertson BD, Young DB. Tuberculosis: a problem with persistence. Nat. Rev. Microbiol. 2003;1:97–105. doi: 10.1038/nrmicro749. [DOI] [PubMed] [Google Scholar]
- 67.Naser SA, Arce M, Khaja A, et al. Role of ATG16L, NOD2 and IL23R in Crohn's disease pathogenesis. World. J Gastroenterol. 2012;18:412–424. doi: 10.3748/wjg.v18.i5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 69.Inohara N, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 70.Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes. Infect. 2007;9:1662–1670. doi: 10.1016/j.micinf.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 71.Sechi LA, Gazouli M, Sieswerda LE, et al. Relationship between Crohn's disease, infection with Mycobacterium avium subspecies paratuberculosis and SLC11A1 gene polymorphisms in Sardinian patients. World. J. Gastroenterol. 2006;12:7161–7164. doi: 10.3748/wjg.v12.i44.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Archer NS, Nassif NT, O'Brien BA. Genetic variants of SLC11A1 are associated with both autoimmune and infectious diseases: systematic review and meta-analysis. Genes. Immun. 2015;16:275–283. doi: 10.1038/gene.2015.8. [DOI] [PubMed] [Google Scholar]
- 73.Jo EK. Autophagy as an innate defense against mycobacteria. Pathog. Dis. 2013;67:108–118. doi: 10.1111/2049-632X.12023. [DOI] [PubMed] [Google Scholar]
- 74.Parkes M, Barrett JC, Prescott NJ, et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat. Genet. 2007;39:830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rook GA, Raison CL, Lowry CA. Microbial 'old friends', immunoregulation and socioeconomic status. Clin. Exp. Immunol. 2014;177:1–12. doi: 10.1111/cei.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okada H, Kuhn C, Feillet H, Bach JF. The 'hygiene hypothesis' for autoimmune and allergic diseases: an update. Clin. Exp. Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lafond RE, Lukehart SA. Biological basis for syphilis. Clin. Microbiol. Rev. 2006;19:29–49. doi: 10.1128/CMR.19.1.29-49.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lina TT, Alzahrani S, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Immune evasion strategies used by Helicobacter pylori. World. J Gastroenterol. 2014;20:12753–12766. doi: 10.3748/wjg.v20.i36.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clancy R, Ren Z, Turton J, Pang G, Wettstein A. Molecular evidence for Mycobacterium avium subspecies paratuberculosis (MAP) in Crohn's disease correlates with enhanced TNF-alpha secretion. Dig. Liver Dis. 2007;39:445–451. doi: 10.1016/j.dld.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 81.Olsen I, Tollefsen S, Aagaard C, et al. Isolation of Mycobacterium avium subspecies paratuberculosis reactive CD4 T cells from intestinal biopsies of Crohn's disease patients. PLoS One. 2009;4:e5641. doi: 10.1371/journal.pone.0005641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sartor RB. Does Mycobacterium avium subspecies paratuberculosis cause Crohn's disease? Gut. 2005;54:896–898. doi: 10.1136/gut.2004.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 84.Hermon-Taylor J. Mycobacterium avium subspecies paratuberculosis, Crohn's disease and the Doomsday scenario. Gut Pathog. 2009;1:15. doi: 10.1186/1757-4749-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Green C, Elliott L, Beaudoin C, Bernstein CN. A population-based ecologic study of inflammatory bowel disease: searching for etiologic clues. Am. J. Epidemiol. 2006;164:615–623. doi: 10.1093/aje/kwj260. [DOI] [PubMed] [Google Scholar]
- 86.Gearry RB, Richardson A, Frampton CM, et al. High incidence of Crohn's disease in Canterbury, New Zealand: results of an epidemiologic study. Inflamm. Bowel. Dis. 2006;12:936–943. doi: 10.1097/01.mib.0000231572.88806.b9. [DOI] [PubMed] [Google Scholar]
- 87. Pierce ES. Possible transmission of Mycobacterium avium subspecies paratuberculosis through potable water: lessons from an urban cluster of Crohn's disease. Gut Pathog. 2009;1:17. doi: 10.1186/1757-4749-1-17. *Recent cluster of IBD possibly related to waterborne infection from dairy farming in the US
- 88. Pierce ES. Free-ranging Rocky Mountain bighorn sheep and an outbreak of inflammatory bowel disease along the Clark Fork River in Plains, Montana. Virulence. 2012;3:546–550. doi: 10.4161/viru.22121. * Recent cluster of IBD possibly related to waterborne infection from wild animals in the US
- 89.Van Kruiningen HJ, Freda BJ. A clustering of Crohn's disease in Mankato, Minnesota. Inflamm. Bowel. Dis. 2001;7:27–33. doi: 10.1097/00054725-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 90.Fridriksdottir V, Gunnarsson E, Sigurdarson S, Gudmundsdottir KB. Paratuberculosis in Iceland: epidemiology and control measures, past and present. Vet. Microbiol. 2000;77:263–267. doi: 10.1016/s0378-1135(00)00311-4. [DOI] [PubMed] [Google Scholar]
- 91.Van Kruiningen HJ, Colombel JF, Cartun RW, et al. An in-depth study of Crohn's disease in two French families. Gastroenterology. 1993;104:351–360. doi: 10.1016/0016-5085(93)90401-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh K, Saunders JH, Foley RJ. Inflammatory bowel disease in married couples. Gut. 1995;37:158. doi: 10.1136/gut.37.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rhodes JM, Marshall T, Hamer JD, Allan RN. Crohn's disease in two married couples. Gut. 1985;26:1086–1087. doi: 10.1136/gut.26.10.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holmes GK, Painter NS. Crohn's disease in married couples. Gut. 1986;27:350. doi: 10.1136/gut.27.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Radon K, Windstetter D, Poluda AL, et al. Contact with farm animals in early life and juvenile inflammatory bowel disease: a case-control study. Pediatrics. 2007;120:354–361. doi: 10.1542/peds.2006-3624. [DOI] [PubMed] [Google Scholar]
- 96.Cucino C, Sonnenberg A. Occupational mortality from inflammatory bowel disease in the United States 1991–1996. Am. J. Gastroenterol. 2001;96:1101–1105. doi: 10.1111/j.1572-0241.2001.03747.x. [DOI] [PubMed] [Google Scholar]
- 97.Jones PH, Farver TB, Beaman B, Cetinkaya B, Morgan KL. Crohn's disease in people exposed to clinical cases of bovine paratuberculosis. Epidemiol. Infect. 2006;134:49–56. doi: 10.1017/S0950268805004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Greenstein RJ, Su L, Juste RA, Brown ST. On the action of cyclosporine A, rapamycin, tacrolimus on M. avium including subspecies paratuberculosis. PLoS One. 2008;3:e2496. doi: 10.1371/journal.pone.0002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shin SJ, Collins MT. Thiopurine drugs azathioprine and 6-mercaptopurine inhibit Mycobacterium paratuberculosis growth in vitro. Antimicrob. Agents. Chemother. 2008;52:418–426. doi: 10.1128/AAC.00678-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hulten K, Almashhrawi A, El-Zaatari FA, Graham DY. Antibacterial therapy for Crohn's disease: a review emphasizing therapy directed against mycobacteria. Dig. Dis. Sci. 2000;45:445–456. doi: 10.1023/a:1005453409445. [DOI] [PubMed] [Google Scholar]
- 101.Chamberlin W, Borody TJ, Campbell J. Primary treatment of Crohn's disease: combined antibiotics taking center stage. Expert. Rev. Clin. Immunol. 2011;7:751–760. doi: 10.1586/eci.11.43. [DOI] [PubMed] [Google Scholar]
- 102.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. A meta-analysis of broad-spectrum antibiotic therapy in patients with active Crohn's disease. Clin. Ther. 2006;28:1983–1988. doi: 10.1016/j.clinthera.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 103.Thomas GA, Swift GL, Green JT, et al. Controlled trial of antituberculous chemotherapy in Crohn's disease: a five year follow up study. Gut. 1998;42:497–500. doi: 10.1136/gut.42.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Feller M, Huwiler K, Schoepfer A, Shang A, Furrer H, Egger M. Long-term antibiotic treatment for Crohn's disease: systematic review and meta-analysis of placebo-controlled trials. Clin. Infect. Dis. 2010;50:473–480. doi: 10.1086/649923. *A meta-analysis of randomized controlled trials shows treating Crohn's disease patients with nitroimidazoles or clofazimine is effective.
- 105. Khan KJ, Ullman TA, Ford AC, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am. J. Gastroenterol. 2011;106:661–673. doi: 10.1038/ajg.2011.72. *A comprehensive meta-analysis of randomized controlled trials treating Crohn's disease patients with antibiotics. A statistically significant effect was seen for use of antimycobacterial drugs preventing replapse of Crohn's disease.
- 106. Chamberlin W, Ghobrial G, Chehtane M, Naser SA. Successful treatment of a Crohn's disease patient infected with bacteremic Mycobacterium paratuberculosis. Am. J. Gastroenterol. 2007;102:689–691. doi: 10.1111/j.1572-0241.2007.01040_7.x. *A case report of a Crohn's disease patient with evidence of MAP infection whose disease went into remission following anti-MAP antibiotic therapy.
- 107. Kuenstner JT, Chamberlin W, Naser SA, et al. Resolution of Crohn's disease and complex regional pain syndrome following treatment of paratuberculosis. World. J. Gastroenterol. 2015;21:4048–4062. doi: 10.3748/wjg.v21.i13.4048. *A case report of a Crohn's disease patient with evidence of MAP infection whose disease went into remission following anti-MAP antibiotic therapy.
- 108. Efficacy and Safety of Anti-MAP Therapy in Adult Crohn's Disease. Available from https://clinicaltrials.gov/ct2/show/NCT01951326. *Ongoing clinical trial to study MAP infection and response to treatment during anti-MAP therapy in Crohn's disease patients.
- 109.Hayes SL, Sivaganesan M, White KM, Pfaller SL. Assessing the effectiveness of low-pressure ultraviolet light for inactivating Mycobacterium avium complex (MAC) micro-organisms. Lett. Appl. Microbiol. 2008;47:386–392. doi: 10.1111/j.1472-765X.2008.02442.x. [DOI] [PubMed] [Google Scholar]
- 110.Palmer MV, Waters WR. Bovine tuberculosis and the establishment of an eradication program in the United States: role of veterinarians. Vet. Med Int. 2011;2011:816345. doi: 10.4061/2011/816345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosseels V, Huygen K. Vaccination against paratuberculosis. Expert. Rev. Vaccines. 2008;7:817–832. doi: 10.1586/14760584.7.6.817. [DOI] [PubMed] [Google Scholar]
- 112.Bastida F, Juste RA. Paratuberculosis control: a review with a focus on vaccination. J. Immune. Based. Ther. Vaccines. 2011;9:8. doi: 10.1186/1476-8518-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bull TJ, Gilbert SC, Sridhar S, et al. A novel multi-antigen virally vectored vaccine against Mycobacterium avium subspecies paratuberculosis. PLoS One. 2007;2:e1229. doi: 10.1371/journal.pone.0001229. *A MAP vaccine for use in humans is under development.
- 114.Antrobus RD, Coughlan L, Berthoud TK, et al. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved Influenza A antigens. Mol. Ther. 2014;22:668–674. doi: 10.1038/mt.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De BJ, Shaykhutdinov R, Barkema HW, Vogel HJ. Metabolomic profiling in cattle experimentally infected with Mycobacterium avium subsp. paratuberculosis. PLoS One. 2014;9:e111872. doi: 10.1371/journal.pone.0111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Geraghty T, Graham DA, Mullowney P, More SJ. A review of bovine Johne's disease control activities in 6 endemically infected countries. Prev. Vet. Med. 2014;116:1–11. doi: 10.1016/j.prevetmed.2014.06.003. *A recent summary of measures in use worldwide to control Johne's disease.