Abstract
Contributions of differential behavioral (executive functions) and electrophysiological (frontal-temporal electroencephalogram or EEG coherence) measures to episodic memory performance were examined during middle childhood. Cognitive flexibility and right frontotemporal functional connectivity during encoding (F4/T8), as well as left frontotemporal functional connectivity during retrieval (Fp1/T7), contributed to episodic memory performance in a sample of 9–12 year olds. These results suggest that executive functions differentially influence episodic memory, as does left and right frontotemporal functional connectivity during different portions of the memory task.
Keywords: episodic memory, executive functions, EEG coherence, middle childhood
Improvements in episodic memory (EM) during middle childhood have been attributed to neural development of the hippocampus and prefrontal cortex, and associated increases in the ability to organize and elaborate information (Chiu, Schmithorst, Brown, Holland, & Dunn, 2006; Ghetti & Bunge, 2012). Because EM has been associated with positive learning outcomes in math and reading (Broek, 2005; Mirandola, Del Prete, Ghetti, & Cornoldi, 2011; Stevenson & Newman, 1986), we examined cognitive and neural processes related to EM during middle childhood. In particular, we used electroencephalogram (EEG) coherence to examine frontotemporal functional connectivity during encoding and retrieval. We also examined the contributions of individual executive functions (EF; inhibitory control, working memory, and cognitive flexibility) to EM because EFs are involved in the management of cognitive processes.
EM is detail-rich memory for specific events or episodes, including what, when, where, and other relevant information (Tulving, 1972). Storing episodic events in a way that may be handled by memory systems is referred to as encoding, while reactivation of these events is retrieval (Tulving, 1972). EM involves the ability to encode and retrieve a specific event, as well as the contextual information associated with the episode. Development of EM continues throughout childhood and into adolescence (Ghetti, DeMaster, Yonelinas, & Bunge, 2010). During middle childhood improvements are observed in strategy use during cognitive processing (Bjorklund & Jacobs, 1985), resulting in benefits to EM performance. The improvements in use of strategies have been attributed to children’s ability to semantically organize information, and to their successful regulation of memory traces, thus improving memory accuracy (Ghetti & Alexander, 2004; Ornstein et al, 2006). Advances in the ability to successfully use strategies, along with the increased implementation of those strategies, make middle childhood a prime developmental period to examine EM. Development of hippocampal and frontal areas during middle childhood also make this period of development critical for the study of EM.
Adult neuroimaging studies have suggested that both the medial temporal lobe (MTL) and the prefrontal cortex (PFC) are activated during encoding of EMs (Buckner, Kelley, & Peterson, 1999; Schacter & Wagner, 1999; Takahashi, Ohki, & Kim, 2007). Additionally, similar activation patterns have been found within the PFC during working memory and during the retrieval phase of EM (Nyberg et al., 2003). Such findings suggest that EFs and EM may operate using similar neural mechanisms, implying that the PFC and the MTL interact during EM encoding and retrieval (for review on PFC and MTL interactions see Simons & Spiers, 2003).
PFC appears to develop into young adulthood (Diamond, 2002). Thus, developments within the PFC were originally believed to drive performance improvements in EM observed across childhood because the hippocampus was thought to reach full development by middle childhood (Ofen, Kao, Sokol-Hessner, Kim, Whitfield-Gabrieli, & Gabrieli, 2008). The most current research examining MTL activation in children and young adolescents, however, suggests that the functional organization of the MTL, specifically the hippocampus, continues to develop into adolescence. Work by Ghetti and colleagues (2010) has demonstrated that although the dimensions of the hippocampus remain stable, anterior volume decreases while posterior volume increases during childhood. The implications for this change in children are currently unknown. In adults, however, small anterior hippocampi and large posterior hippocampi have been associated with increased EM performance (Maguire et al., 2000). Such findings suggest that hippocampal developments continue to influence performance in EM throughout middle childhood. Because of the simultaneous development of PFC and MTL, and their contributions to encoding and retrieval, examining EM during middle childhood may provide insight on the development of the cross communication between frontal and temporal regions.
Much of the research examining the brain during EM encoding and retrieval has used the electroencephalogram (EEG) in the study of source memory during early (Riggins et al., 2009; Marshall, Drummey, Fox, & Newcombe, 2002) and middle childhood (Rajan & Bell, 2014). Such studies suggest that electrophysiological measures (brain activation measured via EEG power or event-related potentials) at separate frontal and temporal scalp locations may be used to distinguish correct from incorrect EM responses and to assess developmental changes in relation to EM performance. Considering that both PFC and MTL regions have been associated with EM, we used EEG coherence across frontal and temporal regions as our measure of brain function during task performance. Coherence is the frequency-dependent squared cross-correlation of electrical signals between two scalp electrode sites (Nunez, 1981; Thatcher, Krause, & Hrybyk, 1986). Theoretically, lower and higher levels of coherence reflect functional connectivity between two brain areas (Thacher, Krause, & Hrybyk, 1986; Thatcher, North, & Biver, 2008). Unlike EEG power values, EEG coherence is not affected by arousal, opening or closing of eyes, or changes in state (Thatcher, 1994), making it a conservative electrophysiological measure.
With respect to EEG activity, theta band oscillations (4–7 Hz) are correlated with EM in adults (Klimesch et al, 2001). Less is known about the implication of theta EEG activity for EM during childhood; however, a recent study reported event-related increases in theta activity at frontal and temporal, as well as parietal, scalp locations in 6- and 8-year-old children during the recall portion of an EM task (Rajan & Bell, 2014). Because of our focus on PFC and MTL interactions during EM, we used EEG coherence within the theta band to examine the functional connectivity between frontal and temporal scalp locations during both encoding and retrieval while children performed an EM task.
As implicated by neuroimaging work with adults, EFs are involved in monitoring and manipulating information during encoding and retrieval of EMs (Miyake & Shah, 1999; Marsh, Beaman, Hughes, & Jones, 2012). Developments in EF ability may explain some improvements observed in EM performance during childhood (for review see Raj & Bell, 2010). For instance, the ability to strategically organize information to be encoded is likely attributed to developing EF ability (Luciana, Conklin, Hooper, & Yarger, 2005). These developing cognitive organizational abilities translate into developmental improvements in EM (Bjorklund & Jacobs, 1985). We focused on three core EFs in our study (i.e., working memory, inhibitory control, and cognitive flexibility; Miyake, Friedman, Emerson, Witzki, Howerter, & Wager, 2000).
Working memory, or a set of cognitive processes that maintain information available for analysis and manipulation, aids in the process of encoding through short term contextual and item binding (i.e., source binding; Ruffman, Rustin, Garnham, & Parkin, 2001). EM requires that a connection exists between item and context and working memory provides that function. This involvement of one of the EFs in the formation of EM is further supported by Baddeley’s classic model of working memory incorporating the episodic buffer (for review see Baddeley, 2000). The episodic buffer is responsible for integration of information (i.e. short-term source binding). Information within the episodic buffer differs from EM in that it is short-term. Information must be transferred from the episodic buffer into long term EM in order for it to be consolidated.
Inhibitory control, or the ability to inhibit a dominant response in choice of another, aids in the retrieval of relevant over irrelevant information (Marsh et al., 2012). The ability to suppress irrelevant information is critical for successful encoding and retrieval. For example, during encoding it is necessary to focus attentional resources on the information that is pertinent, allowing for that information to be stored and later retrieved. Additionally, during retrieval the ability to suppress interfering information is essential. Otherwise, one would be prone to inaccurate recall. For example, Ruffman and colleagues (2001) reported that 8 and 10 year olds who demonstrated high inhibitory control abilities also generated less source-based episodic memory errors; children were able to suppress interfering sources in order to make correct responses.
Cognitive flexibility, also known as set shifting, is the ability to switch between tasks or mental sets. Although less examined than working memory and inhibitory control, cognitive flexibility does predict spatial and short-term binding of episodic free recall in children (Picard, Cousin, Cuillery-Girars, Eustavche, & Piolino, 2012). Cognitive flexibility may play a role in encoding through enhancement and flexibility of the to-be-encoded information. To our knowledge, no study has examined all three core EFs in a study of EM in childhood. It is more typical to create a composite measure of EF in the study of EM (e.g., Raj, Cuevas, & Bell, 2014; Rajan & Bell, 2014). We examined the unique contributions of all three EFs to EM during middle childhood.
Overview of Current Study
Middle childhood is characterized by vast improvements in the use of encoding strategies, such as rehearsal techniques (Hulme, Thompson, Muir, & Lawerence, 1984) and organization (Chiu et al., 2006). These EM strategies, in turn, contribute to academic achievement in reading and math (Broek, 2005; Mirandola, Del Prete, Ghetti, & Cornoldi, 2011; Stevenson & Newman, 1986), thus making middle childhood a prime period of development to study EM. Development of PFC and MTL, and interactions between frontal and hippocampal areas, are the likely foundations to improvements in EM during this time. Middle childhood is also a period of continuing development in the core EFs. Although most studies tend to examine EF as a composite score rather than examining the individual components, we took a more detailed approach and teased apart the individual contributions of three components of EF: working memory, inhibitory control, and cognitive flexibility. Thus, our two main research questions were: 1) How does frontotemporal functional connectivity (assessed via EEG coherence) during encoding and retrieval contribute to EM performance during middle childhood? 2) How do EFs differentially contribute to EM performance during middle childhood?
Method
Participants
Our sample consisted of 81 children (52% female; 9–12 years, M=10.38, SD = .73) who represented one cohort of an on-going longitudinal study focused on cognition and emotion development. Eleven of the 81 participants had parent reports of ADHD. These participants did not differ from non-ADHD participants on any of the measures used in this study (all p’s > .10). A majority of the children had been participating in the longitudinal study since infancy (n = 57), whereas others were newly recruited for this particular lab visit (n = 24). The children from the longitudinal study were originally recruited using a commercial mailing list, whereas the new participants were recruited from existing participant siblings, local working mother listserv, university announcements, and a local online family newsletter. Children were predominantly Caucasian (89%) with highly educated parents; 99% of mothers and 91% of fathers had at least some education beyond high school. Of the 81 children, three refused to wear the EEG cap and three had insufficient EEG due to artifacts. Seven children were missing EF data due to experimenter error (n=5) and computer malfunctions (n=2). There was overlap among children missing data such that no variable of interest had data contributed by fewer than 72 participants (see Table 1). Of the participants with missing data, 36% were male and 64% were female and had a mean age of 10.4. As compensation for participation, which also included a booklet of questionnaires not included in this report, parents received a $50 gift card and children received an inexpensive toy and a $10 gift card.
Table 1.
Descriptive Statistics and Bivariate Correlation
| Task | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1. Episodic Memory |
-- | ||||||||||||||||
|
2. EF Cognitive Flexibility |
40*** | -- | |||||||||||||||
|
3. EF Inhibitory Control |
38*** | .31** | -- | ||||||||||||||
|
4. EF Working Memory |
.33** | −.11 | −.35** | -- | |||||||||||||
| 5. F3/T7 Enc. | .01 | .12 | −.04 | .05 | -- | ||||||||||||
| 6. F4/T8 Enc. | .28* | −.07 | −.11 | .07 | .30** | -- | |||||||||||
| 7. F3/T7 Ret. | .13 | .20 | −.22 | .22 | .32** | .11 | -- | ||||||||||
| 8. F4/T8 Ret. | .12 | .10 | −.28* | .11 | .25* | .26* | 74*** | -- | |||||||||
| 9. Fp1/T7 Enc. | −.03 | .08 | −.20 | −.03 | .21 | .03 | .36** | .36** | -- | ||||||||
| 10. Fp2/T8 Enc. | .13 | .03 | −.08 | −.01 | .23* | .35** | .32** | .32** | .58*** | -- | |||||||
| 11. Fp1/T7 Ret. | .17 | .12 | −.20 | .03 | .05 | −.03 | 46*** | 46*** | .62*** | 54*** | -- | ||||||
| 12.Fp2/T8Ret. | −.01 | .21 | −.19 | −.04 | .17 | .08 | 57*** | 5 7*** | 6 7*** | 67*** | 75*** | -- | |||||
| 13. F7/T7 Enc. | .04 | −.01 | −.05 | −.05 | .08 | .13 | .27* | .27* | .52*** | 45*** | 39*** | 41*** | -- | ||||
| 14. F8/T8 Enc. | .02 | .00 | −.01 | −.14 | .04 | .28* | .15 | .15 | .34** | 43*** | .28* | .31** | .65*** | -- | |||
| 15. F7/T7 Ret. | .08 | −.04 | −.07 | −.07 | −.03 | .15 | .34** | .34** | 45*** | 48*** | 54*** | 47*** | 83*** | 64*** | -- | ||
| 16. F8/T8 Ret. | −.07 | .19 | .00 | −.20 | .04 | .09 | .35** | .35** | 39*** | 38*** | 44*** | .55*** | .63*** | 77*** | 71 *** | -- | |
|
17. PPVT (proxy for verbal IQ) |
.24* | −.08 | −.10 | .13 | −.07 | .25* | .18 | .19 | −.00 | .01 | .07 | .01 | −.02 | −.05 | −.03 | .19 | -- |
| n | 8 1 | 7 3 | 79 | 81 | 75 | 75 | 75 | 75 | 73 | 73 | 73 | 73 | 77 | 77 | 77 | 77 | 81 |
| M | 16.21 | 7.60 | 2163.76 | 4.39 | .12 | .11 | .17 | .17 | .13 | .15 | .16 | .18 | .45 | .46 | .45 | .48 | 72.78 |
| Min | 7.0 | 2.0 | 1292.04 | 2.0 | .02 | .02 | .08 | .08 | .03 | .04 | .06 | .07 | .05 | .05 | .04 | .05 | 16.0 |
| Max | 27.0 | 30.0 | 3935.56 | 6.0 | .23 | .28 | .41 | .39 | .63 | .48 | .56 | .69 | .76 | .68 | .73 | .71 | 99.70 |
Note: Enc = EEG coherence during memory task encoding. Ret = EEG coherence during memory task retrieval.
p<.05,
p<.01,
p<.001 (two-tailed)
Memory Task
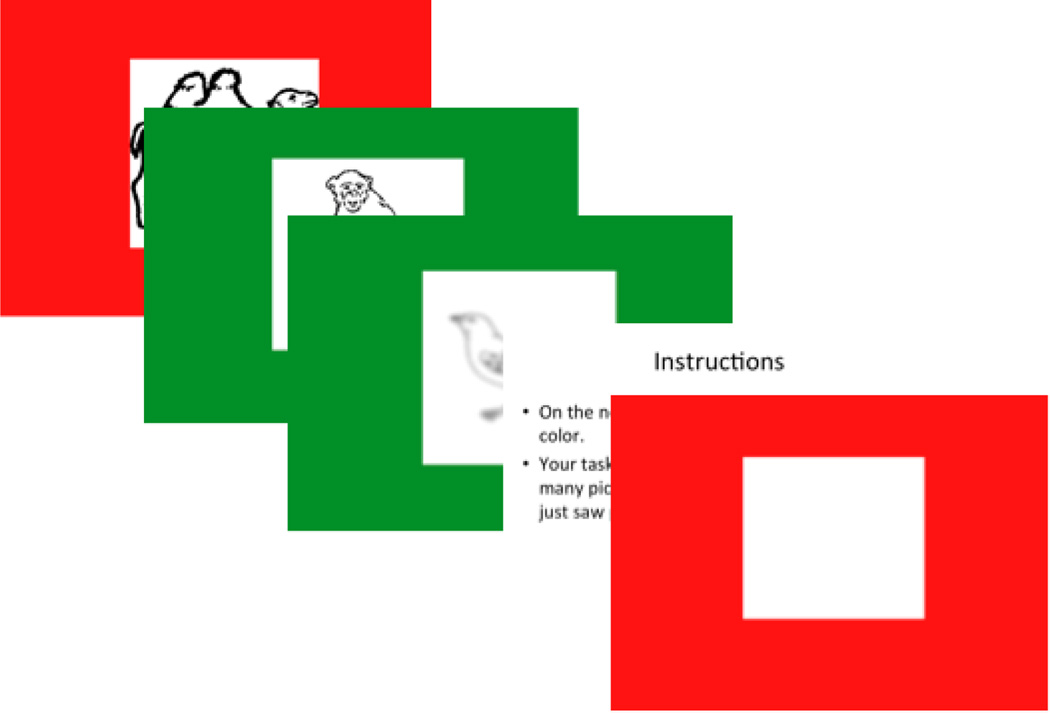
The EM task was adapted from work by DeMaster and Ghetti (2013). Children viewed black and white line drawings surrounded by a color border. The stimuli were taken from an existing set of 244 line drawings (Szekely, et al., 2003). Four blocks, each with eight line drawings, were used. The four blocks were comprised of the categories of foods, vehicles, animals, and outdoor activities. The groupings were used to allow for the potential use of a mnemonic technique (categorical clustering); however, this technique was not suggested to the children. The line drawings were surrounded by one of two colors with each block having a different pair: red/green, blue/yellow, purple/green, or brown/pink. The stimuli were displayed on a computer monitor one at a time, each for 4 seconds with an inter-trial interval of 1.5 seconds.
Prior to the encoding phase, children were instructed to attend to the drawings as well as the color of the surrounding borders. They were also told that they would later be asked to name the drawings associated with each color border. During the encoding phase the children viewed a total of eight line drawings. Immediately after each block, children were shown a color border from that block and prompted to verbally recall the drawings associated with that color (see Figure 1). They were then shown the other color from that block and asked to recall those line drawings. The variable of interest was the total number of recalled images across all blocks. Maximum possible score was 32. The internal consistency (split-half) for this task was acceptable (α = .62, average inter-item = .45). An average inter-item correlation between .15 and .50 is considered ideal and may be a more appropriate measure of the unidimensionality of a scale, particularly with few items (Clark & Watson, 1995).
Figure 1.
Example of the presentation of images and the question slides. There were white blank slides that are not presented in this example shown between images that lasted 1.5 seconds.
EEG Acquisition, Processing and Analysis
EEG data were collected during all of the tasks; the focus here is on the EEG collected during the EM task. Recordings were made from 26 left, right, and midline scalp sites [frontal pole (Fp1, Fp2), frontal (F3, F4, Fz, F7, F8), central (C3, C4), central frontal (FC1, FC2, FC5, FC6), temporal (T7, T8), parietal (P3, P4, Pz, P7, P8), central parietal (CP1, CP2, CP5, CP6), occipital (O1, O2)]. All electrodes were referenced to Cz during the recordings. We recorded EEG using a stretch cap (Electro-Cap, Inc.; Eaton, OH; E1-series cap) with electrodes in the 10/20 system pattern. We placed a small amount of abrasive gel into each recording site and gently rubbed the scalp. We then added conductive gel to the recording sites. Electrode impedances were measured and accepted if they were below 20 KΩ. The electrical activity from each lead was amplified using separate James Long Company Bioamps (James Long Company; Caroga Lake, NY). The EEG activity for each scalp electrode was displayed on the monitor of the acquisition computer. The signal was digitized on-line at 512 samples per second for each channel in order to eliminate the effects of aliasing. This calibration signal was digitized for 30 seconds and stored for subsequent analysis. The acquisition software used was Snapshot-Snapstream (HEM Data Corp., Southfield, MI) and the raw data were stored for later analyses.
EEG data were examined and analyzed using EEG Analysis software developed by the James Long Company. Average reference EEG data were then artifact scored for eye movements using a peak-to-peak criterion of 100µV or greater. Gross motor movements over 200µV peak to peak were also scored. These artifact scored epochs were eliminated from all analyses. The data were then analyzed with a discrete Fourier transform (DFT) using a Hanning window of 1 second width and 50% overlap. Coherence was computed for the theta 4–7 Hz band using an algorithm by Saltzberg, Burton, Burch, Fletcher, and Michaels (1986; equation 9). Based on research regarding episodic memory in childhood, we focused on frontal and temporal scalp locations in each hemisphere and in a number of electrode pairs (Fp1/T7, Fp2/T8, F3/T7, F4/T8, F7/T7, F8/T8) in an attempt to capture frontal-temporal functioning connectivity during our memory task. The electrode pairs represent the 6 possible intrahemispheric frontotemporal combinations for our particular EEG caps. EEG coherence was examined during encoding and retrieval conditions within each block of the memory task. Encoding EEG was collected during the presentation of each line drawing and then averaged across the four encoding blocks. Retrieval EEG was collected during the presentation of each individual question (e.g., Please tell me the drawings with a red border.) and then averaged across the four retrieval blocks. Encoding and retrieval EEG coherence composites were weighted by the amount of DFT windows associated with the stimuli within each block.
Executive Function Tasks
Working Memory
A backwards digit span (BDS) task was administered to assess working memory. This task was based on the version given in the Wechsler Intelligence Scale for Children-Revise (WISC-R; Wechsler, 1986). Children were initially presented with two digits and instructed to repeat the sequence backwards. Two practice trials were given to ensure understanding and then the task began. Attempt at recall of the same digit span with at least one correct trial for two trials was required before lengthening the span by one digit. The digit span was lengthened until errors were produced on two consecutive trials of the same span. The variable of interest was highest digit, which refers to the highest number span reached.
Inhibitory Control
A number-based computerized Stroop task was used to assess inhibitory control (Ruffman et al., 2001). The Stroop task had three conditions: letters, numbers, and mixed. Emphasis was placed on the mixed condition, which is considered to induce the most conflict because it includes trials from both the letters and numbers conditions. Children were told to count either letters (“AAA”= 3) or number digits (“555”=3) that appeared on the computer screen and to indicate their response on the keyboard. Practice trials were provided. The variable of interest was mean reaction time for mixed trials.
Cognitive Flexibility
A computerized version of the Wisconsin Card Sorting Task (WCST-64; Hearton & PAR staff, 2003) was used to assess cognitive flexibility. Children were instructed to sort cards by matching the stimulus card, at the bottom of the screen, with one of four key cards at the top of the screen. Children were further instructed to sort cards based on three dimensions (color, shape, and quantity). Once the children sorted the cards correctly for a period of time the dimension used to sort the cards changed, and they had to flexibly change their strategy in order to be successful on the task. The variable of interest was perseverative errors. Perseverative errors are made by continuously using the same matching rule even after receiving feedback that the rule was no longer correct. This variable is considered to tap into cognitive flexibility, providing a more process pure measure than other variables (Miyake, Friedman, Emerson, Witzki, & Howerter, 2000).
Verbal IQ Proxy
The Peabody Picture Vocabulary Test IV (PPVT; Dunn & Dunn, 2012) was administered to the children to determine their receptive vocabulary and verbal comprehension; the measure is often used as a proxy for verbal IQ. Because intelligence is typically correlated with EFs and memory performance, we controlled for this variable in our analyses. The PPVT is a nationally standardized instrument, and the measure of interest was participants’ percentile rank based on age. Because the age range in our sample was 9 to 12 years, we selected this age-based measure of the PPVT.
Results
Correlations
Refer to Table 1 for descriptive statistics and correlations. The EM task was correlated with all three executive function tasks and with F4/T8 during encoding. Hierarchical regression was used to examine the contributions of EEG coherence during encoding and retrieval, as well as the contributions of individual EFs to EM performance.
EEG Coherence Predicting Episodic Memory Performance
F7/T7 and F8/T8
PPVT percentile rank was entered in the first step of the regression equation along with right (F7/T7) and left (F8/T8) frontotemporal coherence during encoding. Right and left frontotemporal coherence values during retrieval were entered into the second step of the equation. The regression equation was not significant (see Table 2).
Table 2.
Hierarchical Regression Analyses of F7/T7 and F8/T8 EEG Coherence Predicting Episodic Memory Performance
| R | R2 | R2Δ | FΔ | F | β | t | sr2 | |
|---|---|---|---|---|---|---|---|---|
| Step 1. | .25 | .06 | 1.66 | |||||
| Verbal IQ Proxy | .25 | 2.21* | .06 | |||||
| F7/T7 Encoding | .03 | .22 | .00 | |||||
| F8/T8 Encoding | .02 | .08 | .00 | |||||
| Step 2: | .32 | .11 | .04 | 1.63 | 1.67 | |||
| Verbal IQ Proxy | .24 | 2.28* | .06 | |||||
| F7/T7 Encoding | −.13 | −.61 | .00 | |||||
| F8/T8 Encoding | .15 | .80 | .01 | |||||
| F7/T7 Retrieval | .32 | 1.42 | .03 | |||||
| F8/T8 Retrieval | −.31 | −1.58 | .03 |
Note:
p ≤.001
p ≤ .01;
p≤.05. (n= 77)
F3/T7 and F4/T8
The same predictors were entered into this regression equation using F3/T7 and F4/T8 coherence pairs. Step 1 accounted for 12% of the variance in EM performance, with PPVT (3%) and right frontotemporal coherence during encoding (5%) contributing unique variance. The retrieval coherence predictors added in Step 2 did not contribute additional variance to EM performance (see Table 3).
Table 3.
Hierarchical Regression Analyses of F3/T7 and F4/T8 EEG Coherence Predicting Episodic Memory Performance
| R | R2 | R2Δ | FΔ | F | β | t | sr2 | |
|---|---|---|---|---|---|---|---|---|
| Step 1. | .35 | .12 | 3.22* | |||||
| Verbal IQ Proxy | .20 | 1.67 | .03 | |||||
| F3/T7 Encoding | −.05 | −.46 | .00 | |||||
| F4/T8 Encoding | .25 | 2.03* | .05 | |||||
| Step 2: | .36 | .13 | .01 | .40 | 2.06 | |||
| Verbal IQ Proxy | .17 | 1.44 | .03 | |||||
| F3/T7 Encoding | −.09 | −.72 | .01 | |||||
| F4/T8 Encoding | .27 | 2.08* | .05 | |||||
| F3/T7 Retrieval | .15 | .82 | .01 | |||||
| F4/T8 Retrieval | −.06 | −.36 | .00 |
Note:
p ≤.001
p ≤.01;
p ≤.05. (n= 75)
Fp1/T7 and Fp2/T8
The same predictors were entered into this regression equation using Fp1/T7 and Fp2/T8 coherence pairs. Step 1 accounted for 11% of the variance in EM performance, with PPVT (8%) contributing unique variance. Step 2 accounted for an additional 8% of the variance in EM performance, with PPVT (6%) and Fp1/T7 retrieval (7%) contributing unique variance (see Table 4).
Table 4.
Hierarchical Regression Analyses of Fpl/T7 and Fp2/T8 EEG Coherence Predicting Episodic Memory Performance
| R | R2 | R2Δ | FΔ | F | β | t | sr2 | |
|---|---|---|---|---|---|---|---|---|
| Step 1. | .34 | .11 | 2.93* | |||||
| Verbal IQ Proxy | .28 | 2.48* | .08 | |||||
| Fpl/T7 Encoding | −.16 | −1.14 | .02 | |||||
| Fp2/T8 Encoding | .22 | 1.55 | .03 | |||||
| Step 2: | .43 | .19 | .08 | 3.10* | 3.10* | |||
| Verbal IQ Proxy | .25 | 2.29* | .06 | |||||
| Fpl/T7 Encoding | −.20 | −1.25 | .02 | |||||
| Fp2/T8 Encoding | .29 | 1.82 | .04 | |||||
| Fpl/T7 Retrieval | .41 | 2.37* | .07 | |||||
| Fp2/T8 Retrieval | −.38 | −1.91 | .04 | |||||
Note:
p ≤.001
p ≤ 01;
p ≤ 05. (n= 73)
Executive Functions Predicting Episodic Memory Performance
PPVT percentile rank was entered into the first step of the regression equation. Inhibitory control, working memory, and cognitive flexibility were entered into the second step of the equation. Step 1 accounted for 9% of the variance in EM performance. The EF variables in Step 2 collectively accounted for an additional 19% of the variance, with PPVT (5%) and cognitive flexibility (9%) contributing unique variance to EM performance (see Table 3).
Discussion
We examined functional connectivity (i.e., EEG coherence) and EF (i.e., working memory, inhibitory control, cognitive flexibility) contributions to children’s performance on an EM task. Neuroscience research has demonstrated that neural networks exist between frontal and temporal regions and these networks may be associated with memory performance (Takahashi et al., 2007). Thus, our first research question focused on frontotemporal functional connectivity (assessed via EEG coherence) during encoding and retrieval contributions to EM performance during middle childhood. Indeed, frontotemporal functional connectivity during encoding predicted EM performance. This may be a result of short-term binding. Binding would have been necessary during the EM task, because children would need to connect the border color to the line drawing (Chalfonte & Johnson, 1996).
An effect for frontotemporal functional connectivity using EEG coherence measures was present during both encoding (F4/T8) and retrieval (Fp1/T7). These effects differed depending on the frontal electrode pair. It is possible that due to the distance between electrodes and the scalp locations of the frontotemporal pairs, they each represent different neural networks. The scalp electrodes in the F4/T8 pair are separated by 8.5 cm and are thought to overlie right hemisphere dorsolateral frontal and medial temporal regions. Despite the relatively poor spatial resolution of the EEG signal, we have confidence in this effect during encoding because of the type of stimuli we used (i.e., images). Previous research suggests that visual images are more likely to elicit activation within the right rather than left hemisphere (Kelley et al., 1998).
The electrodes in the Fp1/T7 pair are separated by 11.5 cm and EEG coherence at this location during retrieval predicted EM performance. This pair of electrodes is thought to overlie frontal pole and medial temporal regions. Frontal pole activation has been associated with EM retrieval (Lepage, Ghaffar, Nyberg, & Tulving, 2000). The left hemisphere effect may be due to the nature of the retrieval phase of our task. Children were prompted to name (verbally recall) the images they had seen during encoding. Left hemisphere activation is often observed during tasks requiring language (Ojemann & Whitaker, 1978). More research is required with this task to replicate these EEG coherence hemispheric effects during encoding and retrieval.
Children performed well on our task, with a mean of 16 out of a total possible score of 32 potentially misleading. After accuracy for correct border color is considered in relation to overall recall, participants displayed 89% accuracy on the task. This means that when they did recall an item, they linked it to the correct color border most of the time. It may be that our task activated other functional connections, or perhaps only temporal areas were activated. This latter possibility would not be observable with our EEG coherence analyses.
In addition to work associating neural networks with memory, the developmental literature strongly suggests a connection between composite EFs and EM performance (Mantyla, Carelli, & Forman, 2007; Rajan & Bell, 2014). Thus, our second research question focused on unique contributions of the core EFs. In our study, cognitive flexibility was the only EF to contribute unique variance to EM performance. Information regarding the influence of cognitive flexibility on memory is limited, but a connection has been found previously with adult participants (McCabe et al., 2010). The ability to flexibly switch attention from one stimulus to another may be necessary for memory tasks involving multiple stimuli. Cognitive flexibility would be essential for successfully disengaging attention from one stimulus pair (item and color) to another. In fact, children with attention deficit hyperactivity disorder (ADHD) have demonstrated impaired EM performance (Rhodes, Park, Seth, & Coghill, 2012). The connection between ADHD and EM performance is critical because ADHD impairs cognitive flexibility ability (for meta-analysis see Boonstra, Oosterlaan, Sergeant, & Buitelaar, 2005). Such findings support the importance of cognitive flexibility to EM performance. Given the lack of attention paid to cognitive flexibility in the EM literature, our results provide important information regarding EM performance during middle childhood.
Inhibitory control did not contribute to EM performance. This is contrary to the literature (Ruffman et al., 2001), but may be a result of task design. The blocks within the memory task contained different color borders and categories. The blocks were designed to aid encoding and limit interference. Because inhibitory control has been associated with suppression of memory interference, this may be why inhibitory control did not contribute to performance on our EM task. Our task provided the necessary supports and inhibitory control was not critical to task performance. Working memory was expected to be associated with EM performance based on prior research suggesting a connection (e.g., Gallo & Wheeler, 2012; Picard et al., 2012). However, in our study working memory did not contribute to EM performance, but the p-value was .06. Given that working memory aids in the process of encoding through short term contextual and item binding (i.e., source binding; Ruffman, Rustin, Garnham, & Parkin, 2001), our findings are perplexing. The lack of significance may be attributed to the large amount of variance explained by the other variables in the regression, including verbal IQ proxy and cognitive flexibility. Another possible explanation may be the stimuli used in the working memory task. Because the EM task used images, an image-based working memory task (rather than our verbally-based backward digit span) may have resulted in a stronger relation between working memory and EM. Furthermore, the backwards digit span task did not require binding of information. It is possible that a working memory task involving feature binding may be more likely to contribute to EM performance. Future studies should examine the contribution of working memory to our EM task using an image-based design incorporating binding (e.g., N-back task).
Results of our study provide insights into how individual EFs and frontotemporal functional connectivity (measured via EEG coherence) contribute to EM performance. However, there were some unexpected findings that suggest future research is needed. First, as mentioned previously, our inhibitory control and working memory tasks did not contribute to EM. This was unexpected and may be due to the tasks’ reliance on letters and or numbers rather than images. Future work should utilize imaged-based tasks, as our EM task was image based. Second, frontotemporal coherence differentially contributed to EM performance depending on the particular frontal locations with which the medial temporal electrodes were paired. We expected to see similar contributions from frontotemporal functional connectivity during encoding and retrieval regardless of the pairing. Indeed, we had made no hypotheses regarding specific frontotemporal pairs. Future studies should further explore frontotemporal functional connectivity between multiple electrode pairs during EM encoding and retrieval using multiple memory tasks in order to increase our understanding of the possible contributions of EEG coherence to each memory process.
In addition to these potential design influences, the generalizability of our study may be limited. The participants used were predominantly Caucasian and from upper middle class families. This being said, the participants were accurate reflections of the community where our research lab is located. Work with more diverse samples would be highly informative.
Limited information exists on how individual EFs contribute to EM performance. Our study provides evidence suggesting that cognitive flexibility should be considered when exploring EM. Furthermore, we are one of the first studies to take a developmental perspective when examining frontotemporal connectivity during EM. Our study demonstrated that EEG coherence associated with different frontotemporal electrode pairs during encoding contribute differentially to EM performance. Collectively, our findings provide insights on both cognitive and electrophysiology contributions to EM performance during middle childhood.
Table 5.
Hierarchical Regression Analyses of EFs Predicting Episodic Memory Performance
| R | R2 | R2Δ | FΔ | F | β | t | sr2 | |
|---|---|---|---|---|---|---|---|---|
| Step 1. | .30 | .09 | 6.66* | |||||
| Verbal IQ Proxy | .30 | 2.58* | .09 | |||||
| Step 2: | .28 | .28 | .15 | 6.11*** | 6.61*** | |||
| Verbal IQ Proxy | .22 | 2.09* | .05 | |||||
| Cognitive Flexibility | −.31 | −2.82** | .09 | |||||
| Inhibitory Control | −.15 | −1.28 | .02 | |||||
| Working Memory | .17 | 1.57 | .03 | |||||
Note:
p ≤.001
p ≤ .01;
p ≤.05. (n= 72)
Acknowledgments
This research was supported by grant HD049878 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. We are grateful to the families who participated in our study. We gratefully acknowledge the assistance of Leslie Patton and Allie Nancarrow with scheduling, data collection, and coding.
Contributor Information
Tashauna L. Blankenship, Department of Psychology, Virginia Tech
Martha Ann Bell, Department of Psychology, Virginia Tech.
References
- Baddeley A. The episodic buffer: a new component of working memory? Trends in Cognitive Sciences. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Boonstra MA, Oosterkaan J, Sergeant JA, Buitelaar JK. Executive functioning in adult ADHD: a meta-analytic review. Psychological Medicine. 2005;35:1097–1108. doi: 10.1017/s003329170500499x. [DOI] [PubMed] [Google Scholar]
- Bjorklund DF, Jacobs JW., III Associative and categorical processes in children’s memory: The role of automaticity in the development of organization in free recall. Journal of Experimental Child Psychology. 1985;39:599–617. [Google Scholar]
- Broek P. Integrating memory-based and constructionist processes in accounts of reading comprehension. Discourse Processes. 2005;39:299–316. [Google Scholar]
- Buckner RL, Kelley WM, Peterson SE. Frontal cortex contributes to human memory formation. Nature Neuroscience. 1999;2:311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Memory & Cognition. 1996;24:403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Chiu CP, Schmithorst VJ, Brown RD, Holland SK, Dunn S. Making memories: a cross-sectional investigation of episodic memory encoding in childhood using FMRI. Developmental Neuropsycholgy. 2006;29:321–340. doi: 10.1207/s15326942dn2902_3. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Constructing validity: Basic issues in objective scale development. Psychological Assessment. 1995;7:309–319. doi: 10.1037/pas0000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaster DM, Ghetti S. Developmental differences in hippocampal and cortical contributions to episodic retrieval. Cortex. 2013;49:1482–1493. doi: 10.1016/j.cortex.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. In: Stuss Donald T, Knight Robert T, editors. Principles of frontal lobe function. New York, NY, US: Oxford University Press; 2002. pp. 466–503. [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test, (PPVT™ - 4) Johannesburg: Pearson Education Inc. 2012 [Google Scholar]
- Fuster JM. The prefrontal cortex-an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Wheeler ME. Episodic Memory. In: Reisburg, editor. Oxford handbook of cognitive psychology. New York, NY: Oxford University Press; 2013. pp. 189–205. [Google Scholar]
- Ghetti S, Alexander KW. “If it happened, I would remember it”: strategic use od event memorability in the rejection of false autobiographical events. Child Development. 2004;75:542–561. doi: 10.1111/j.1467-8624.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Bulge SA. Neural changes underlying the development of episodic memory during middle childhood. Developmental Cognitive Neuroscience. 2012;2:381–395. doi: 10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, DeMaster DM, Yonelinas AP, Bunge SA. Developmental differences in medial temporal lobe function during memory encoding. The Journal of Neuroscience. 2010;30:9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme C, Thomson N, Muir C, Lawrence A. Speech rate and the development of short-term memory span. Journal of Experimental Child Psychology. 1984;38:241–253. doi: 10.1006/jecp.1993.1043. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Yonelinas A, Kroll NEA, Lazzara M, Rohm D, Gruber W. Theta synchronization during episodic retrieval: neural correlates of conscious awareness. Cognitive Brain Research. 2001;12:33–38. doi: 10.1016/s0926-6410(01)00024-6. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-Temporal circuitry for episodic encoding and subsequent memory. The Journal of Neuroscience. 2000;20:6173–6160. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Ghaffar O, Nyberg L, Tulving E. Prefrontal cortex and episodic memory retrieval mode. Proceedings of the National Academy of Sciences. 2000;97(1):506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Mantyla T, Carelli MG, Forman H. Time monitoring and executive functioning in children and adults. Journal of Experimental Child Psychology. 2007;96:1–19. doi: 10.1016/j.jecp.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak CD, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JE, Beaman PC, Hughes RW, Jones DM. Inhibitory control in memory: evidence for negative priming in free recall. Journal of Experimental Psychology. 2012;38:1377–1388. doi: 10.1037/a0027849. [DOI] [PubMed] [Google Scholar]
- Marshall DH, Drummey AB, Fox NA, Newcombe NS. An event-related potential study of item recognition memory in children and adults. Journal of Cognition and Development. 2002;3:201–224. [Google Scholar]
- McCabe DP, Roediger HL, McDaniel MA, Balota DA, Hambrick DZ. The relationship between working memory capacity and executive functioning: evidence for a common executive attention construct. Neuropsycology. 2010;24:222–243. doi: 10.1037/a0017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirandola C, Del Prete F, Ghetti S, Cornoldi C. Recollection but not familiarity differentiates memory for text in students with and without learning difficulties. Learning and Individual Differences. 2011;21:206–209. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex frontal lobe tasks: a latent variable analysis. Cognitive Psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Miyake A, Shah P. Toward unified theories of working memory: Emerging general consensus, unresolved theoretical issues, and future research directions. In: Miyake A, Shah P, editors. Models of working memory: Mechanisms of active maintenance and executive control. New York, NY: Cambridge University Press; 1999. pp. 442–481. [Google Scholar]
- Nunez PL. Electric Fields of the Brain: The Neurophysics of EEG. New York: Oxford University Press; 1981. p. 484pp. [Google Scholar]
- Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson KM, Ingvar M. Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia. 2003;41:371–377. doi: 10.1016/s0028-3932(02)00168-9. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Mclntosh AR, Houle S, Nilsson LG, Tulving E. Activation of medial temporal structures during episodic memory retrieval. Nature. 1996;380:715–717. doi: 10.1038/380715a0. [DOI] [PubMed] [Google Scholar]
- Ofen N, Koa Y, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JDE. Development of the declarative memory system in the human brain. Nature Neuroscience. 2007;10:1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Whitaker HA. Language localization and variability. Brain and language. 1978;6:239–260. doi: 10.1016/0093-934x(78)90061-5. [DOI] [PubMed] [Google Scholar]
- Ornstein PA, Baker-Ward L, Gordon BN, Pelphrey KA, Tyler CS, Gramzow E. The influence of prior knowledge and repeated questioning on children’s long-term retention of the details of a pedi-atric examination. Developmental Psychology. 2006;42:332–344. doi: 10.1037/0012-1649.42.2.332. [DOI] [PubMed] [Google Scholar]
- Passolunghi MC, Sigel LS. Short-term memory, working memory, and inhibitory control in children with difficulties in arithmetic problem solving. Journal of Experimental Child Psychology. 2001;80(1):44–57. doi: 10.1006/jecp.2000.2626. [DOI] [PubMed] [Google Scholar]
- Picard L, Cousin S, Cuillery-Girard B, Eustavche F, Piolino P. How do the different components of episodic memory develop? Role of executive functions and short-term feature-binding abilities. Child Development. 2012;83:1037–1050. doi: 10.1111/j.1467-8624.2012.01736.x. [DOI] [PubMed] [Google Scholar]
- Raj V, Bell MA. Cognitive processes supporting episodic memory formation in childhood: The role of source memory, binding, and executive functioning. Developmental Review. 2010;30:384–402. [Google Scholar]
- Rajan V, Bell MA. Episodic memory development in childhood: Contributions from brain electrical activity and executive functions. Developmental Cognitive Neuroscience. 2014 doi: 10.1016/j.dcn.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SM, Park J, Seth S, Coghill DR. A comprehensive investigation of memory impairment in attentional deficit hyperactivity disorder and oppositional defiant disorder. Journal of Child Psychology and Psychiatry. 2012;53:128–137. doi: 10.1111/j.1469-7610.2011.02436.x. [DOI] [PubMed] [Google Scholar]
- Riggins T, Miller NC, Bauer PJ, Georgieff MK, Nelson CA. Electrophysiological indices of memory for temporal order in early childhood: Implication for the development of recollection. Developmental Science. 2009;12:209–219. doi: 10.1111/j.1467-7687.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffman T, Rustin C, Garnham W, Parkin AJ. Source monitoring and false memories in children. Relation to certainty and executive functioning. Journal of Experimental Child Psychology. 2001;80:95–111. doi: 10.1006/jecp.2001.2632. [DOI] [PubMed] [Google Scholar]
- Saltzberg B, Burton DB, Burch NR, Flecther J, Michaels R. Electrophysiological measures of regional neural interactive coupling: Linear and non-linear dependence relationships among multiple channel electroencephalographic recordings. International Journal of Bio-Medical Computing. 1986;18:77–87. doi: 10.1016/0020-7101(86)90050-4. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Simons JA, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Stevenson HW, Newman RS. Long-term prediction of achievement and attitudes in mathematics and reading. Child Development. 1986;57:646–659. [PubMed] [Google Scholar]
- Szekely A, D’Amico S, Devescovi A, Federmeier K, Herron D, Iyer G, Jacobsen T, Bates E. Timed picture naming: extended norms and validation against previous studies. Behavioral Research Methods, Instruments, and Computers. 2003 doi: 10.3758/bf03195542. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Ohki K, Kim D. Diffusion tensor studies dissociated two fronto-temporal pathways in the human memory system. Neuroimage. 2008;34:827–838. doi: 10.1016/j.neuroimage.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher RW. Cyclic cortical reorganization: origins of human cognitive development. In: Dawson G, Fischer KW, editors. Human behavior and the developing brain. New York, NY: Guilford Press; 1994. pp. 232–266. [Google Scholar]
- Thatcher RW, Krause PJ, Hrybyk M. Cortico-cortisol associations and EEG coherence: a two-compartmental model. Electroencephalography Clinical Neurophysiology. 1986;64:123–143. doi: 10.1016/0013-4694(86)90107-0. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, North DM, Biver CJ. Development of cortical connections as measured by EEG coherence and phase delays. Human Brain Mapping. 2008;29:1400–1415. doi: 10.1002/hbm.20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature. 1999;401:699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic Press; 1972. pp. 381–402. [Google Scholar]
- Weiss S, Muller HM, Rappelsberger P. Theta synchronization predicts efficient memory encoding of concrete and abstract nouns. Neruoreport. 2000;11:2357–2361. doi: 10.1097/00001756-200008030-00005. [DOI] [PubMed] [Google Scholar]