Abstract
Continuous monitoring of glucose levels in human physiology is important for the long-term management of diabetes. New signaling methods/probes may provide an improved technology to monitor glucose and other physiologically important analytes. The glucose sensing probes, BMQBAs, fabricated using the 6-methylquinolinium moiety as a fluorescent indicator, and boronic acid as a chelating group, may have versatile applications in glucose sensing because of their unique properties. In this paper we discuss the design logic, synthesis, characterization and spectral properties of three new isomeric glucose sensors (BMQBAs), and a control compound (BMQ) in the presence and absence of sugars. The sensing ability of the new probes is based on a charge neutralization and stabilization mechanism upon sugar binding. The new probes have attractive fluorescence quantum yields, are highly water-soluble, and have spectral characteristics compatible with cheap and portable LEDs and LDs. One of the probes, o-BMQBA, has a sugar bound pKa of 6.1, and a dissociation constant KD of 100 mM glucose. These probes have been designed specifically to respond to tear glucose in a contact lens polymer for ophthalmic glucose monitoring, where the reduced sugar bound pKa affords for sensing, in a lens environment that we have previously shown to be mildly acidic.
Keywords: Ophthalmic diagnostics, Fluorescent probes, Glucose sensing, Contact lens, Continuous and non-invasive glucose monitoring
1. Introduction
Diabetes results in long-term health disorders including cardiovascular disease, blindness and cancer [1,2]. To date, a wide variety of methods for glucose analysis have been reported in the research literature, including electrochemistry [3,4], near infrared spectroscopy [5,6], optical rotation [7,8], colorimetric [9,10] and fluorescence detection [11–15]. The most commonly used technology for blood glucose determination is an enzyme-based method [16], which requires frequent blood sampling and therefore drawing. Although frequent “finger pricking” with a small needle to obtain the blood sample is a relatively painless process, this method does suffer from a few practical problems. The first one is inconvenience and the required compliance by patients, while the second is that this is not a continuous monitoring method, with patients tolerating only a few glucose checks a day. Thus, there is a growing interest in the development of continuous non-invasive glucose sensing technologies. To this end, our laboratories have recently made significant progress towards the development of a non-invasive and continuous glucose sensing method using a daily disposable, plastic contact lens embedded with intelligent glucose sensitive boronic acid probes [17,18]. This new technology promises to alleviate many of the current problems associated with continuous glucose monitoring and the current invasive methods employed for glucose sensing. Subsequently in this paper, we report the design rationale, new signaling mechanism and synthesis of these new contact lens fluorescent probes.
The boronic acid group has been long known to have high affinity for diol-containing compounds such as carbohydrates [19–21], where the strong complexation has been used for the construction of carbohydrate sensors [22–29], transporters [30], and chromatographic materials [31]. Naturally, boronic acid compounds have been considered as a chelating group for the synthesis of glucose sensors [32–39], where we note the work of Shinkai [32,33], Norrild [34], Lakowicz [35–39] and Drueckhammer [25]. However, the published probes developed for solution (blood/serum)-based measurements are not compatible for glucose sensing within a contact lens, because of the different microenvironment within the lens, in particular, the local pH and polarity [17]. Based on our recent contact lens findings, the pH inside a contact lens is relatively acidic (≈6.0) and the local polarity of the lens is not indifferent than that of methanol. Subsequently, published boronic acid probes embedded within a contact lens typically show a significantly reduced response towards glucose [17]. Hence there is a need to develop suitable fluorescent probe molecules for use in the contact lens. In addition to the environmental parameters and constraints such as pH and polarity, the probes have to be additionally sensitive to the very low concentrations of tear glucose, ≈500 μM, recalling that the blood glucose levels for a healthy person are ≈10-fold higher.
To address the environmental constraints imposed by the contact lens for glucose sensing, we considered lowering the pKa of the probe. The pKa of phenyl boronic acid is known to be tunable with the appropriate substituents [39], for example, an electron withdrawing group reduces the pKa while an electron donating group increases the pKa of the sugar bound form. We therefore considered the interaction between the quaternary nitrogen of the 6-methylquinolinium moiety, and the boronic acid group, which reduces the pKa of the probe. In this regard we have synthesized three isomeric boronic acid containing probes, o-, m- and p-BMQBA, where the spacing between the interacting moieties, quaternary nitrogen of the 6-methylquinolinium and boronic acid groups, enables an understanding of the sensing mechanism to be realized. Also, a control compound (BMQ), which does not contain the boronic acid moiety, and is therefore insensitive towards sugar, has been synthesized to understand the spectral properties of the probes (Fig. 1). A detailed photophysical aqueous study of the probes in the presence and in the absence of sugars is discussed in this paper; their response towards glucose within a contact lens is to be presented in a full paper elsewhere.
Fig. 1.
Molecular structure of ortho, meta and para-BMQBA probes and the control compound BMQ. BMQBA: N-(boronobenzyl)-6-methoxyquinolinium bromide, BMQ: N-benzyl-6-methoxyquinolinium bromide.
2. Experimental
2.1. Materials
All chemicals were purchased from Aldrich.
2.2. Methods
All steady-state fluorescence measurements were undertaken in 4 cm × 1 cm × 1 cm fluorometric plastic cuvettes (Sigma), using a Varian Cary Eclipse fluorometer, and all absorption measurements were performed using a Varian UV/VIS 50 spectrophotometer.
Time-resolved intensity decays were measured using reverse start–stop time-correlated single-photon timing (TC-SPC), with a Becker and Hickl Gmbh 630 SPC PC card and unamplified MCP-PMT. Vertically polarized excitation at ≈372 nm was obtained using a pulsed LED source (1 MHz repetition rate) and a dichroic sheet polarizer. The instrumental response function was ≈1.1 ns fwhm. The emission was collected at the magic angle (54.7°), using a long pass filter (Edmund Scientific) which cut-off the excitation wavelengths.
2.3. Data analysis
Titration curves with pH were determined in buffer solution: pH 3 and 4 acetate buffer; pH 5–9 phosphate buffer and pH 10 and 11 carbonate buffer. Titration curves were fitted and pKa (pKa = –log10 Ka) values were obtained using the relation:
| (1) |
where Iacid and Ibase are the intensity limits in the acid and base regions, respectively.
Stability (KS) and dissociation (KD) constants were obtained by fitting the titration curves, with sugar, using the relation:
| (2) |
where Imin and Imax are the initial (no sugar) and final (plateau) fluorescence intensities of the titration curves, where KD = (1/KS).
The fluorescence intensity decays were analyzed in terms of the multi-exponential model:
| (3) |
where αi are the amplitudes and τi the decay times, . The fractional contribution of each component to the steady-state intensity is given by:
| (4) |
The mean lifetime of the excited state is given by:
| (5) |
and the amplitude-weighted lifetime is given by:
| (6) |
The values of αi and τi were determined by non-linear least squares impulse reconvolution with a goodness-of-fit criterion.
2.4. Synthesis
The boronic acid containing fluorescent probes o-, m- and p-BMQBA and a control compound BMQ, were conveniently prepared using the following generic one step synthetic procedure, described below for the control compound BMQ. The corresponding o-, m-, or p-bromomethyl-phenyl boronic acid are employed instead of benzyl bromide to obtain the isomeric boronic acid derivatives o-, m- and p-BMQBA, respectively, Fig. 1. Equimolar amounts of 6-methylquinoline and benzylbromide were dissolved in 10 mL dry acetonitrile in a 25 mL round bottomed flask equipped with a magnetic stirrer. The reaction mixture was allowed to stir under an inert atmosphere for 24 h at room temperature. During this time a quantitative amount of quaternized salt was precipitated as a colorless solid. The solid product was recovered by filtration, washed several times with dry acetonitrile, and then dried under vacuum for 12 h.
2.4.1. Spectral data for compound BMQ
1H NMR (D2O) δ (ppm) 2.5 (s, 3H), 6.2 (s, 2H), 7.2–7.5 (m, 5H), 7.8 (d, 1H), 8.0 (m, 2H), 8.15 (d, 1H), 9.0 (d, 1H) and 9.3 (d, 1H). HRMS (FAB+, H2O) m/e calculated: 234.1283 (M+), found: 234.1291 (M+).
2.4.2. Spectral data for compound o-BMQBA
1H NMR (D2O) δ (ppm) 2.7 (s, 3H), 6.5 (s, 2H), 7.1 (s, 1H), 7.4–7.5 (m, 2H), 8.0–8.3 (m, 4H), 8.5 (d, 1H), 8.95 (d, 1H) and 9.2 (d, 1H). HRMS (FAB+, H2O) m/e calculated: 346.1978 (M+), found: 346.1960 (M+).
2.4.3. Spectral data for compound m-BMQBA
1H NMR (D2O) δ (ppm) 2.5 (s, 3H), 6.2 (s, 2H), 7.3–7.5 (m, 2H), 7.6 (s, 1H), 7.7 (d, 1H), 7.9 (d, 1H), 8.0 (m, 2H), 8.2 (d, 1H), 9.0 (d, 1H) and 9.25 (d, 1H). HRMS (FAB+, H2O) m/e calculated: 346.1978 (M+), found: 346.1988 (M+).
2.4.4. Spectral data for compound p-BMQBA
1H NMR (D2O) δ (ppm) 2.55 (s, 3H), 6.2 (s, 2H), 7.25 (d, 2H), 7.7 (d, 2H), 7.9 (t, 1H), 8.0–8.2 (m, 3H), 9.0 (d, 1H) and 9.25 (d, 1H). HRMS (FAB+, H2O) m/e calculated: 346.1978 (M+), found: 346.1960 (M+).
3. Results and discussion
3.1. Synthesis of the new glucose probes
Boronic acid probes (o-BMQBA–N-(2-boronobenzyl)-6-methylquinolinium bromide, m-BMQBA–N-(3-boronobenzyl)-6-methylquinolinium bromide, p-BMQBA–N-(4-boronobenzyl)-6-methylquinolinium bromide) and the control compound (BMQ–N-benzyl-6-methylquinolinium bromide), were conveniently prepared as described in Section 2.4. The NMR and HRMS data obtained for all four compounds is consistence with the structure of the probes.
3.2. Photophysical characterization, pH dependence and response to glucose
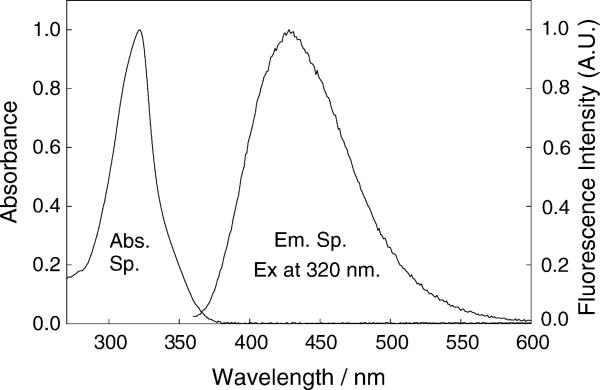
A representative absorption and emission spectra for o-BMQBA in water is shown in Fig. 2, which is characteristic of all three isomers and indeed the control compound. The spectral properties of the probes in water are summarized in Table 1. Typical absorption and emission band maxima of the dyes are ≈320 and 427 nm, respectively, with a large Stokes-shift of about 100 nm, ideal for fluorescence sensing. Table 1 also shows the quantum yield values for the probes in water obtained from a spectral comparison with N-(3-sulfopropyl)-6-methoxyquinolinium [(SPQ) (ϕf = 0.53 in water)] [40]. Another reference compound, N-methyl-6-methylquinolinium bromide (MMQ) previously published by the authors [41] exhibits very similar spectral properties, except for a noticeable quantum yield and mean lifetime difference, approximately 10-fold higher. This indicates interaction between the phenyl ring and quinolinium moiety for the new BMQBA and BMQ probes, Table 1. Hence we attribute the relatively shorter lifetime and quantum yields of the new probes and control compound to a photo-induced electron transfer mechanism, where the phenyl ring is the donor, and the quaternary nitrogen heterocyclic center is the acceptor.
Fig. 2.
Absorption and emission spectra of o-BMQBA in water. λex = 320 nm.
Table 1.
Spectral properties in water, pKa in the presence and absence of 100 mM sugar and dissociation constants of the probes in pH 7.5 phosphate buffer with glucose and fructose
| o-BMQBA | m-BMQBA | p-BMQBA | BMQ | MMQ | |
|---|---|---|---|---|---|
| λabs(max)/nm | 319 | 322 | 322 | 322 | 320a |
| λem(max)/nm | 427 | 427 | 427 | 427 | 420a |
| ϕ f | 0.043 | 0.025 | 0.023 | 0.045 | 0.500a |
| τf/nsb | 4.01 | 3.72 | 2.10 | 2.59 | 18.03 |
| pKa (buffer) | 6.70 | 7.75 | 7.80 | – | – |
| pKa (bulfer + glucose) | 6.10 | 6.85 | 6.95 | – | – |
| pKa (buffer + fructose) | 5.00 | 5.05 | 5.45 | – | – |
| KD/mM (glucose) | 100 | 476 | 370 | – | – |
| KD/mM (fructose) | 4.7 | 13.2 | 13.8 | – | – |
From Ref. [41].
Mean lifetime.
In addition to the quantum yield and fluorescence lifetime differences between the phenyl ring containing BMQBA and BMQ probes, and the MMQ probe, we can also see lifetime differences between the isomers themselves. We have attributed these changes due to the changes in electron donating ability of the different phenyl isomers, and additionally to their different through-space/through-bond interactions [42,43] with the positively charged quaternary nitrogen center, noting that some B−(OH)3 is likely to be present at neutral pH.
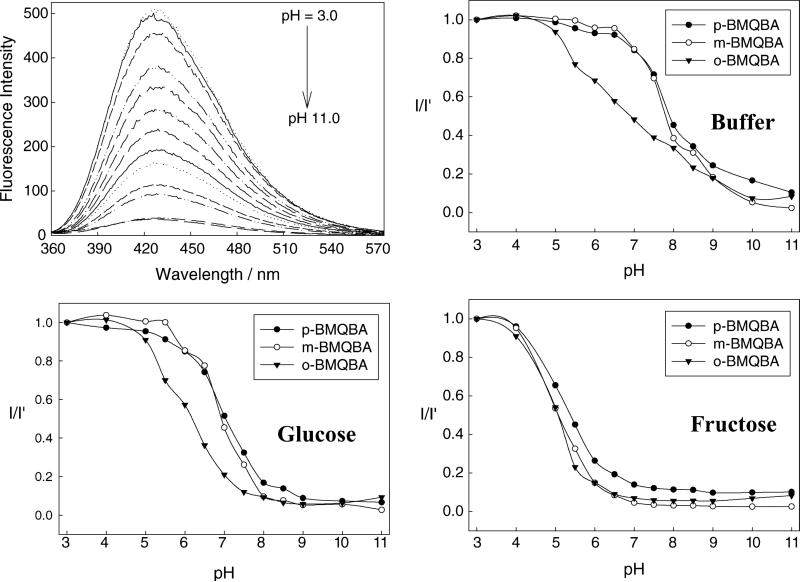
The emission spectra of o-BMQBA in different pH media are shown in Fig. 3. As the pH increases from 3 to 11, a steady decrease in fluorescence intensity of the boronic acid probes can be observed, whereas BMQ, having no boronic acid group, shows no change in fluorescence intensity (data not shown). The corresponding titration curves in the presence and absence of 100 mM glucose and fructose, obtained by plotting the normalized intensities at band maximum versus pH, are also shown in Fig. 3. The boronic acid group is an electron-deficient Lewis acid having an sp2-hybridized boron atom with a trigonal planar conformation. The anionic form of the boronic acid, formed in high pH solutions, is characterized by a more electron rich sp3-hybridized boron atom with a tetrahedral geometry. The change in the electronic properties and the geometry at the boron atom induces the fluorescence spectral changes of the probes. It is well-known that the quinine/quinoline compounds exhibit high quantum yields in acidic media, from the corresponding quaternized salt [40,41]. Similarly here, the boronic acid probes are more fluorescent in acidic solutions. However, when the pH of the medium is increased the electron density on the boron atom is increased, facilitating the partial neutralization of the positively charged quaternary nitrogen of the quinolinium moiety. We have termed this interaction as a charge neutralization–stabilization mechanism, and a schematic representation of this mechanism with regard to glucose binding/sensing is illustrated in Fig. 4, noting that the mechanism shown in Fig. 4 is one of charge interaction and not covalent bond formation. In any event, the addition of glucose and subsequent binding of glucose to the boronic acid moiety, leads to a quantifiable reduction in fluorescence intensity of these three new probes, the control compound BMQ being unperturbed.
Fig. 3.
Fluorescence spectra of o-BMQBA in buffer media (top left). λex = 320 nm. Emission intensity at 427 nm I, divided by the initial emission intensity, I′, as a function of pH (top right) and with 100 mM glucose (bottom left) and 100 mM fructose (bottom right).
Fig. 4.
A schematic representation of the charge neutralization–stabilization mechanism with regard to glucose sensing. The fluorescence off-state depicts the glucose-bound probe form. It should be noted that the figure reflects the increased B–N charge interaction in the presence of glucose, and not covalent bond formation.
The pKa values obtained from the titration curves shown in Fig. 3 are presented in Table 1. To the best of our knowledge, these pKa values are amongst the lowest reported for a phenylboronic acid derivative. As was mentioned earlier, the pKa of the phenylboronic acid can be tuned with suitable substituents, and hence the observed pKa values are not totally unexpected. The quaternary nitrogen of the quinolinium moiety not only reduces the pKa of the probes, but also stabilizes the boronatediester formed upon sugar complexation, which we believe facilitates sugar affinity. Typically, a lower pKa is observed because of an increased Lewis acidity of the boronic acid–sugar complex in the presence of sugars. This large decrease in the pKa of the probe–sugar complex, in comparison with that of the uncomplexed boronic acid group, allows the detection of the sugar at a near-neutral pH, where the maximum optical changes are observed. For our glucose sensing contact lens application, then this is most attractive, as the internal lens pH has been estimated to be ≈6, c.f. the pKa (buffer + glucose) values in Table 1.
Glucose induced spectral changes of the probes are shown in Fig. 5. In an analogous manner to that described for increasing pH previously, we observed a systematic decrease in fluorescence intensity of o-BMQBA in pH 7.5 phosphate buffer with increasing glucose concentrations. The other two isomers, m- and p-BMQBA show a very similar response towards glucose and indeed other monosaccharides. The corresponding titration curves obtained by plotting I′ divided by I, where I′ and I are fluorescence intensities at 427 nm in the absence and presence of sugar respectively, versus glucose concentration, are also shown in Fig. 5. A 2.4 → 3.0-fold decrease in fluorescence intensity with 60 mM glucose can be observed with these probes. Interestingly, these probes show an ≈12–20% intensity change in the presence of 2 mM glucose, noting that tear glucose levels can change from ≈500 μM to 5 mM glucose for diabetics [44,45]. As was expected, these monoboronic acid probes show a higher affinity towards fructose over glucose [32–39], hence a greater response towards fructose was observed, Fig. 6. From Fig. 6 one can see an ≈18-fold decrease in fluorescence intensity with 60 mM fructose, and a four-fold change with only 2 mM fructose. The dissociation constants of the probes with glucose and fructose in pH 7.5 phosphate buffer are presented in Table 1, calculated as described previously by the authors and others [32–39]. As mentioned above, a higher affinity for fructose is a general observation for monophenyl boronic acid derivatives, but it should be noted that the concentration of fructose in tears is substantially lower than for glucose [17,18], and therefore does not pose an interference in the measurement of glucose.
Fig. 5.
Emission spectra of o-BMQBA in pH 7.5 phosphate buffer with increasing glucose concentrations (top), the respective 427 nm intensity ratio for all three isomers in the absence I′, and presence I, of glucose, respectively (middle) and in the low concentration range of glucose, shown to correlate with tear glucose levels (bottom).
Fig. 6.
The 427 nm emission intensity ratio for the BMQBA probes in the absence I′, and presence I, of fructose (top) and in the low concentration range of fructose (bottom).
4. Conclusions
We have developed a range of new boronic acid containing fluorophores for the detection of monosaccharides, with a potential application to ocular fluid sensing. Unlike other boronic acid probes studied [32–39], these probes are moderately fluorescent and highly water-soluble. The binding affinities towards glucose and fructose are most attractive for ophthalmic glucose monitoring, in part due to the charge stabilization of the negatively charged boronatediester by the positively charged quaternary nitrogen. The new probes typically show up to a 13% change in fluorescence intensity for glucose concentrations <1 mM, Fig. 5 – bottom, potentially enabling the onset of both hyper- and hypoglycemic conditions to be monitored. In this paper, we have indeed shown how one can readily tune the pKa of probes to address the sensing constraints imposed by the microenvironment of a contact lens polymer. The actual response of these new probes in a contact lens, with regard to ophthalmic glucose determination, will be reported in due course.
Acknowledgements
This work was supported by the NIH National Center for Research Resource, RR-08119. The Authors also thank UMBI for financial support to CDG and JRL.
Abbreviations
- BA
boronic acid
- BAFs
boronic acid containing fluorophores
- BMQ
N-benzyl-6-methylquinolinium bromide
- BMQBA
N-(boronobenzyl)-6-methylquinolinium bromide
- LD
laser diode
- LED
light emitting diode
References
- 1.Diabetes. 1997;48:271–286. [Google Scholar]
- 2.Engl N. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Claremont DJ, Sambrook IE, Penton C, Pickup JC. Diabetologa. 1986;29:817. doi: 10.1007/BF00873223. [DOI] [PubMed] [Google Scholar]
- 4.Yokowama K, Sode K, Tamiya E, Karube I. Anal. Chim. Acta. 1989;218:137. [Google Scholar]
- 5.Robinson MR, Eaton RP, Haaland DM, Koepp GW, Thomas EV, Stallard BR, Robinson PL. Clin. Chem. 1992;38:1618–1622. [PubMed] [Google Scholar]
- 6.Heise HM, Marbach R, Koschinsky TH, Gries FA. Ann. Occup. Hyg. 1994;18:439–447. doi: 10.1111/j.1525-1594.1994.tb02230.x. [DOI] [PubMed] [Google Scholar]
- 7.March WF, Rabinovitch B, Adams R, Wise JR, Melton M. Trans. Am. Soc. Artif. Intern. Organs. 1982;28:232–235. [PubMed] [Google Scholar]
- 8.Rabinovitch B, March WF, Adams RL. Diabetes Care. 1982;5:254–258. doi: 10.2337/diacare.5.3.254. [DOI] [PubMed] [Google Scholar]
- 9.Schier GM, Moses RG, Gan IET, Blair SC. Diabetes Res. Clin. Pract. 1988;4:177–181. doi: 10.1016/s0168-8227(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 10.Clarke W, Becker DJ, Cox D, Santiago JV, White NH, Betschart J, Eckenrode K, Levandoski LA, Prusinki EA, Simineiro LM, Snyder AL, Tideman AM, Yaegar T. Diabetes Res. Clin. Pract. 1988;4:209–214. doi: 10.1016/s0168-8227(88)80020-2. [DOI] [PubMed] [Google Scholar]
- 11.Trettnak W, Wolfbeis OS. Anal. Chim. Acta. 1989;221:195–203. [Google Scholar]
- 12.Meadows D, Schultz JS. Talanta. 1988;35:145–150. doi: 10.1016/0039-9140(88)80053-5. [DOI] [PubMed] [Google Scholar]
- 13.Tolosa L, Malak H, Rao G, Lakowicz JR. Sens. Actuat. B, Chem. 1997;45:93–99. doi: 10.1016/S0925-4005(97)00275-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolosa L, Gryczynski I, Eichorn LR, Dattelbaum JD, Castellano FN, Rao G, Lakowicz JR. Anal. Biochem. 1999;267:114–120. doi: 10.1006/abio.1998.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Auria S, Dicesare N, Gryczynski Z, Gryczynski I, Rossi M, Lakowicz JR. Biochem. Biophys. Res. Commun. 2000;274:727–731. doi: 10.1006/bbrc.2000.3172. [DOI] [PubMed] [Google Scholar]
- 16.Kenneth ER, Ernest KJ. Diabetes Technol. Ther. 1999;1:3. [Google Scholar]
- 17.Badugu R, Lakowicz JR, Geddes CD. Anal. Chem. 2004;76:610–618. doi: 10.1021/ac0303721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badugu R, Lakowicz JR, Geddes CD. J. Fluoresc. 2003;13:371–374. doi: 10.1023/A:1026103804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugihara JM, Bowman CM. J. Am. Chem. Soc. 1958;80:2443. [Google Scholar]
- 20.Lorand JP, Edwards JO. J. Org. Chem. 1959;24:769. [Google Scholar]
- 21.Spingsteen G, Wang B. Tetrahedron. 2002;38:5291. [Google Scholar]
- 22.James TD, Sandanayake KRAS, Shinkai S. Nature. 1995;374:345. [Google Scholar]
- 23.Norrild JC, Eggert H. J. Am. Chem. Soc. 1995;117:1479. [Google Scholar]
- 24.Eggert H, Frederiksen J, Morin C, Norrild JC. J. Org. Chem. 1999;64:3846. [Google Scholar]
- 25.Yang W, He H, Drueckhammer DG. Angew. Chem. Int. Ed. 2001;40:1714. [PubMed] [Google Scholar]
- 26.Wang W, Gao S, Wang B. Org. Lett. 1999;1:1209. doi: 10.1021/ol9908732. [DOI] [PubMed] [Google Scholar]
- 27.Gao S, Wang W, Wang B. Bioorg. Chem. 2001;29:308. doi: 10.1006/bioo.2001.1219. [DOI] [PubMed] [Google Scholar]
- 28.Lavigne JJ, Anslyn EV. Angew. Chem. Int. Ed. 1999;38:3666. [PubMed] [Google Scholar]
- 29.Yoon J, Czarnik AW. J. Am. Chem. Soc. 1992;114:5874. [Google Scholar]
- 30.Smith BD, Gardiner SJ, Munro TA, Paugam MF, Riggs JA. J. Incl. Phenom. Mol. Recogn. Chem. 1998;32:121. [Google Scholar]
- 31.Soundararajan S, Badawi M, Kohlrust CM, Hagerman JH. Anal. Biochem. 1989;178:125. doi: 10.1016/0003-2697(89)90367-9. [DOI] [PubMed] [Google Scholar]
- 32.James TD, Sandenayake KRAS, Shinkai S. Angew, Chem. Int. Ed. Engl. 1994;33:2207. [Google Scholar]
- 33.James TD, Sandanayake KRAS, Iguchi R, Shinkai S. J. Am. Chem. Soc. 1995;117:8982. [Google Scholar]
- 34.Bielecki M, Eggert H, Norrild JC. J. Chem. Soc. Perkin Trans. 1999;2:449. [Google Scholar]
- 35.Dicesare N, Lakowicz JR. Anal. Biochem. 2001;294:154–160. doi: 10.1006/abio.2001.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dicesare N, Lakowicz JR. J. Photochem. Photobiol. A: Chem. 2001;143:39–47. doi: 10.1016/S1010-6030(01)00471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dicesare N, Lakowicz JR. Org. Lett. 2001;3(24):3891–3893. doi: 10.1021/ol016813p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dicesare N, Lakowicz JR. Tet. Lett. 2002;43:2615–2618. doi: 10.1016/s0040-4039(02)00312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dicesare N, Lakowicz JR. J. Phys. Chem. A. 2001;105:6834–6840. doi: 10.1021/jp010076x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfbeis OS, Urbano E, Heterocyclic Chem J. 1982;19:841–843. [Google Scholar]
- 41.Geddes CD, Apperson K, Karolin J, Birch DJS. Dyes Pigments. 2001;48:227–231. [Google Scholar]
- 42.Fox MA, Chanon M, editors. Photoinduced Electron Transfer. Parts A–D, Elsevier; New York: 1998. [Google Scholar]
- 43.Kavarnos GJ. Fundamentals of Photoinduced Electron Transfer. VCH; New York: 1993. [Google Scholar]
- 44.Gasser AR, Braverman LE, Fleming MC, Arky RA, Alter BR. Am. J. Ophthalmol. 1968;65(3):414–420. doi: 10.1016/0002-9394(68)93093-6. [DOI] [PubMed] [Google Scholar]
- 45.Das BN, Sengupta S, Das BK, Goswami NR. J. Indian Med. Assoc. 1995;93(4):127–128. [PubMed] [Google Scholar]