Abstract
Objective
The near epidemic rise of the incidence of human papillomavirus (HPV)-related oropharyngeal squamous cell carcinomas (OPSCC) presents the practitioner with a “new” head and neck cancer patient, vastly different from those with the traditional risk factors who formed the basis of most practitioners’ training experience. Accordingly, a thorough and disease-specific evaluation process is necessitated. This article will review the evaluation of the HPV-related cancer patient, including a review of the HPV-positive oropharyngeal cancer epidemic from the surgeon’s perspective, evaluation of the primary lesion, evaluation of the neck mass, and role of imaging, to provide a framework for addressing the challenging questions patients may ask.
Data Sources
Available peer-reviewed literature and practice guidelines.
Review Methods
Assessment of selected specific topics by authors solicited from the Head and Neck Surgery and Oncology Committee of the American Academy of Otolaryngology—Head and Neck Surgery Foundation and the American Head and Neck Society.
Conclusions and Implications for Practice
The dramatic rise in OPSSC related to HPV is characterized by a “new” cancer patient who is younger and lacks traditional risk factors. Today’s caregiver must be prepared to appropriately evaluate, counsel, and treat these patients with HPV-positive disease with the expectation that traditional treatment algorithms will evolve to maintain or improve current excellent cure rates while lessening treatment related side effects.
Keywords: oropharyngeal cancer, human papillomavirus (HPV), unknown primary, management neck mass
Introduction
While the incidence of squamous cell carcinoma (SCC)—the most common malignancy in the head and neck region—demonstrated a small and steady decrease in nearly all subsites over the past 3 decades, the incidence of oropharyngeal squamous cell cancers (OPSCC) markedly increased.1 Specifically, OPSCC associated with the human papillomavirus (HPV) increased 225% from 1988 to 2004 and will surpass the incidence of cervical cancer, the primary malignancy traditionally associated with HPV, by 2020.1 This epidemiologic shift manifests as a “new” head and neck cancer patient for the otolaryngology–head and neck surgery practitioner. The “new” HPV-positive OPSCC patients differ in several ways from the “traditional” head and neck cancer patients, who were older, had significant tobacco and alcohol exposure, and had potential tumors throughout the upper aerodigestive tract. Currently, the most commonly presenting head and neck cancer patients are younger, primarily male, and have no or relatively minimal exposures to tobacco and alcohol. Yet, they often have sexual histories notable for increased numbers of sexual encounters, specifically oral sex.2 The physical presentation of OPSCC is likewise shifting as HPV-positive patients often present with more advanced neck disease and with relatively small primary tumors.
As noted, the etiology of oropharyngeal squamous cell carcinoma has dramatically shifted over the past 3 to 4 decades, from carcinogen-exposed mucosal transformation to malignancy, to a predominantly virally mediated cancer. Review of recent randomized studies offers significant insight. RTOG 0129 represented the largest prospective, homogenously treated cohort of OPSCC patients and demonstrated the prognostic value of human papillomavirus infection as an etiologic agent in OPSCC. HPV infection directly determined by HPV DNA in situ hybridization (ISH) or indirectly determined through p16 overexpression using immunohistochemistry (IHC) conferred approximately 25% improvement in progression free survival (PFS) and overall survival (OS). RTOG 0129 found that OPSCC patients with HPV-positive tumors had a survival rate of 82.4% versus 57.1% for patients with HPV-negative tumors.3 The researchers also segregated the subsets with a history of tobacco smoking, demonstrating that tobacco exposure was independently associated with survival for both groups of patients (HPV positive and HPV negative). The risk of death in cancer progression increased by 1% for each pack-year of tobacco smoking. Ang and Gillison reported that the biological behavior of HPV-positive tumors may be altered by tobacco use. One potential mechanism for this change is genetic alteration induced by tobacco-associated carcinogens rendering HPV-positive tumors less responsive to nonsurgical cancer therapy.4 As pack-years of tobacco smoking increase, survival decreased.
The gradual shift in disease etiology to that of a predominantly HPV-positive process directly manifests in the current clinical presentation of OPSCC patients in the caregiver’s office. Although HPV-positive OPSCC demonstrates an increasing incidence that is alarming, this is balanced by a significant responsiveness to treatment regardless of advanced stage.3 For these reasons, the evaluating physician—the otolaryngologist–head and neck surgeon—must maintain a consistency and thoughtfulness that is current in the evaluation of the patient suspected of having HPV-positive OPSCC.
Methods
Critical aspects of the evaluation of patients with HPV-positive OPSCC are highlighted in this overview. Through analysis of the available peer-reviewed literature and published practice guidelines, selected authors solicited from the Head and Neck Surgery and Oncology Committee of the American Academy of Otolaryngology—Head and Neck Surgery and the American Head and Neck Society discuss presentation and history, evaluation of the primary site, evaluation and management of the neck, appropriate radiological evaluation, and appropriate consideration of ultimate therapeutic options.
Discussion
Presentation and History
Patients with HPV-positive OPSCC have 2 common presentations: (1) a symptomatic mass of the tonsil or base of tongue, with or without accompanying lymphadenopathy, and (2) an asymptomatic neck mass without a symptomatic primary site.5 The average age of presentation is in the fifth and sixth decades. Although most patients do not have histories of significant tobacco or alcohol use, patients should still be questioned for these risk factors, as they affect prognosis when coexisting with HPV-positive disease. The ubiquity of HPV infection is important, as up to 85% of adults may have an HPV infection at some point from any of the over 120 subtypes.6 Yet, only a small percentage develops malignancy, and these are primarily related to the HPV-16 subtype. Risk factors for HPV-positive OPSCC include specific sexual behaviors related to numbers of oral (>5) and vaginal (>25) sexual partners.2 Researchers also noted an increased risk of OPSCC in husbands of women with cervical cancer and in situ cancer.6
Primary Site Identification
Although HPV-positive OPSCC will present with symptoms related to primary tumors of the base of tongue or tonsils such as pain, globus sensation, hemoptysis, or voice and swallowing dysfunction, the majority of patients will seek attention for an asymptomatic neck mass. The thorough head and neck exam will identify the primary tumor in most cases. Although cervical lymph node metastasis from an unknown primary site is traditionally felt to be an uncommon presentation—usually reported as <5% of all head and neck malignancies7—the unknown primary remains a challenging clinical scenario that may be more frequent with increased incidence of HPV-positive OPSCC. An exhaustive search for the primary site is critical in that its identification allows radiation sparing of traditionally included sites, such as the nasopharynx and hypopharynx, thus mitigating radiation treatment intensity and long-term toxicity such as xerostomia and dysphagia. Similarly, truly local treatment may also have a survival advantage.
The first step in assessing the adult patient presenting with a neck mass is a meticulous head and neck examination. This includes palpation of the neck to determine the location and extent of the involved node(s), visualization and palpation of the oral cavity and oropharynx, and visualization of the pharynx (naso-, oro-, and hypo-) and larynx with a flexible scope. Evaluation of the upper aerodigestive tract with endoscopy (including pharyngoscopy, laryngoscopy, esophagoscopy, and bronchoscopy) and biopsies is essential in the diagnostic work-up. If no suspicious lesions are present on examination under anesthesia, a palatine tonsillectomy is recommended. This has been shown to have 10-fold greater yield in identifying a primary site over deep tonsil biopsies alone.8 Furthermore, the contralateral palatine tonsil may be involved in 10% of cases.9 For this reason, as well as to facilitate posttreatment surveillance, many authors advocate for bilateral tonsillectomy in this setting. Recently, there has been growing enthusiasm for complete lingual tonsillectomy in those patients who remain with unidentified primaries after the aforementioned work-up. This can be performed with either transoral laser microsurgery or transoral robotic surgery. Identification rates have been reported to be greater than 70% with either technique.10-14 The majority of primary sites will be identified at some point along this work-up, and those not identified will remain true unknown primary carcinomas of the head and neck.
Tissues biopsied during evaluation under anesthesia, whether from an obvious tumor or from suspected tissues, should not only undergo traditional H&E analysis but should also be sent for immunohistochemical analysis of both HPV and P16, as a surrogate marker for HPV infection. Findings on standard H&E pathology suspicious for HPV-related malignancy include an infiltrative, poorly differentiated pattern, with non-keratinizing squamous cells showing a basaloid appearance. These findings do not obviate the importance of immunohistochemical testing, such as P16, which should be completed on all malignant tissue samples.
Evaluation of the Neck Mass
A guiding principle in the ongoing HPV epidemic is to keep squamous cell carcinoma as the presumptive diagnosis in those young patients with a neck mass and no history of tobacco and/or alcohol use.1 Because of the richly supplied lymphatic drainage, T1 tumors of the oropharynx present with cervical lymphadenopathy in up to 70% of cases.15 Safely obtaining the diagnosis of a patient presenting with a neck mass requires careful thought and an unwavering suspicion for metastatic SCC. Fine needle aspiration biopsy (FNAB), when available, should be pursued in most cases. FNAB is a very safe procedure. Using a small needle, often a 25 gauge, there is little risk of inducing bleeding of any significance if a blood vessel is pierced. Prior to initiating FNAB, the clinician must always ensure that the “mass” is not pulsatile as may be the case in a mass emanating from, or closely associated with, the carotid artery. FNAB can be performed quickly in the outpatient clinic. This attribute allows the clinician to avoid the need for a surgical procedure in the operating room. Finally, if a cytologist is available to perform a preliminary analysis, the quality of the specimen can be assured, avoiding nondiagnostic samples. Cytologists are often able to render a preliminary diagnosis that may aid the subsequent work-up. With a preliminary diagnosis, the clinician can counsel the patient accordingly regarding the nature of the diagnosis and the possible treatment pathways.
In those situations in which the FNAB returns a result that is benign or nondiagnostic, further testing in the form of P16 staining can be performed to lessen the chance of false negatives. Given that HPV is a culprit in the majority of newly presenting oropharyngeal carcinomas, identification of P16 staining in a neck aspirate provides further evidence of a malignant etiology. Efficacy studies show that a positive P16 result is a reliable indicator of metastatic HPV-positive OPSCC.16 While false positives can occur (ie, a metastasis from an HPV negative tumor that stains positive for P16), this is uncommon. Furthermore, branchial cleft cysts will consistently be P16 negative on FNAB.17 Ultrasound guided FNAB may increase accuracy as sampling can be guided to the cyst wall, rather than the often acellular cystic fluid.
Despite the utility of FNAB, certain scenarios will arise in which this modality is unable to provide a diagnosis in a patient with a neck mass. In these cases, surgical intervention is required for diagnosis and, possibly, therapy. It is critical that the treating surgeon not perform neck mass excision without a contingency plan in case metastatic squamous cell carcinoma is identified. Two possible approaches are advocated. One option is to counsel patients that excisional biopsy of the neck mass will be performed with immediate frozen section analysis.18 In the event that metastatic SCC is identified during surgery, the surgeon is well positioned to complete a selective neck dissection of levels 2 and 3 or 2 through 4. This approach requires thoughtful incision planning such that a neck dissection can be readily accomplished by extension of the initial incision. The second option is to proceed with immediate selective neck dissection.19 This approach is perhaps the simplest and safest in that it does not rely on frozen section. Also, the compartmental approach essentially removes the risk of tumor “spillage” that could occur when dissecting along the wall of a cystic mass during excisional biopsy. The morbidity of selective neck dissection is relatively low given preservation of all the critical structures.20 This approach does create the possibility of neck dissection performed for a branchial cleft cyst when examined retrospectively. However, it can be argued that this is far more acceptable than the alternate scenario in which the patient receives incomplete clearance of the neck for what is eventually diagnosed as metastatic squamous cell carcinoma.
Imaging the HPV-positive Head and Neck Cancer Patient
The goals for imaging of the HPV-positive head and neck cancer patient are the same as for imaging of any cancer patient. As an adjunctive measure to clinical examination, imaging allows more accurate assessment of the extent and size of the tumor, involvement of regional lymph nodes, vascular invasion, and perineural spread. The most frequently utilized current imaging modalities include computed tomography (CT), magnetic resonance imaging (MR), and positron emission testing with computed tomography (PET/CT.)
CT scanning is the most readily available modality for imaging HNC patients with advantages over MR, including reduced cost and time. CT is particularly useful for oropharyngeal, hypopharyngeal, and laryngeal cancers, specifically the analysis of bony or cartilage invasion.21 There is also less artifact from breathing and motion. Use of contrast allows precise visualization of vascular structures and their relationship to a primary tumor and cervical nodes. Disadvantages of CT scanning include distortion from dental artifact, the potential for adverse allergic reaction, and renal toxicity due to iodine contrast.
MR imaging is particularly useful for primary tumors of the nasopharynx, sinonasal region, and salivary glands.21 Evidence of perineural spread is well seen with gadolinium enhancement and is useful in delineating cranial nerve involvement specifically at the skull base.22 Specific to OPSCC, MR can identify subtle asymmetries in Waldeyer’s ring, which may assist in the location unknown primary tumors. However, patients must lie still in a closed space for a long examination, and MR may not be an option for those with claustrophobia or anxiety disorders. Patient motion, fibrosis, osteonecrosis, and recent dental extractions may also decrease the quality of the images.
Fluorodeoxyglucose (FDG) PET/CT scans are increasingly utilized for baseline imaging, detection of unknown primary cancers, and evaluation of cervical and metastatic disease, as well as posttreatment surveillance.21 However, PET/CT is limited by spatial resolution and can only detect disease that is 5 mm or greater in size. There is normal FDG avidity in Waldeyer’s ring, which can limit usefulness in detecting malignancies in those regions. Figure 1 demonstrates normal FDG activity in the lingual tonsils but increased activity in a positive cervical node.
Figure 1.
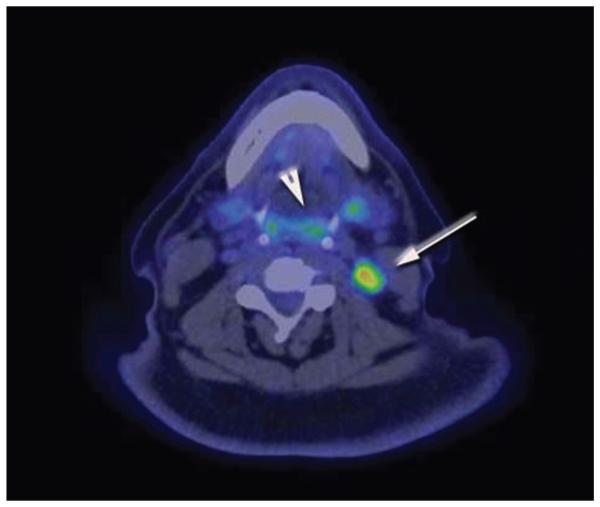
Positron emission testing with computed tomography (PET/CT) scan demonstrating normal fluorodeoxyglucose (FDG) activity in the lingual tonsils and Waldeyer’s ring (arrowhead). However, there is increased FDG activity in a positive metastatic lymph node (arrow).
In contrast to presentation of classic HNCs, primary HPV-positive OPSCC tend to be smaller, with prominent cervical nodal metastases. The patient may also present initially as an unknown primary with cervical metastases.23
HPV-positive metastases frequently present as cystic nodes. Goldenberg et al first described the high incidence of HPV-positive DNA in cystic nodal metastases.24 While 87% of cystic nodal metastases contained HPV-positive DNA, none of the solid nodal metastases did. Cantrell retrospectively reviewed 136 paired HPV+ and HPV− oropharyngeal cancer patients, matched for T stage, tumor subsite, and smoking status, and also found that HPV-positive cancers were more likely to have cystic nodal metastases (36% vs 10%, P = .002).25 Figure 2 demonstrates an example of an HPV-positive HNC with cystic cervical nodal metastases and involvement of multiple levels of nodes. In contrast, the CT scan in Figure 3 demonstrates cervical adenopathy with a necrotic center, rather than a cystic architecture, in a patient with an HPV primary cancer. Other imaging characteristics of HPV-positive cancers are decreased invasion of adjacent muscle and increased incidence of enhancing, exophytic, well-defined borders.25 However, these distinctive imaging characteristics are not absolute, as demonstrated in Figure 4, in which an HPV-positive tongue cancer exhibits infiltrative borders and invasion of muscle. Careful clinical acumen is required in the evaluation and work-up of OPSCC patients, particularly in the era of increasing HPV positivity.
Figure 2.
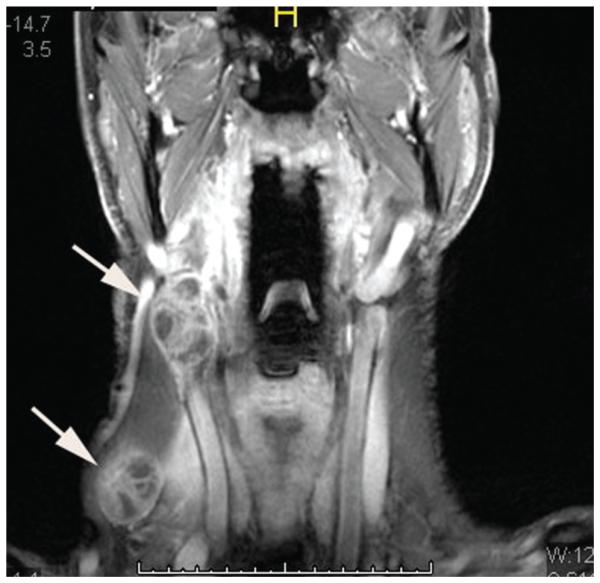
Magnetic resonance imaging scan of a patient with an HPV1 oropharyngeal cancer, demonstrating multiple cystic cervical lymph nodes (arrows).
Figure 3.
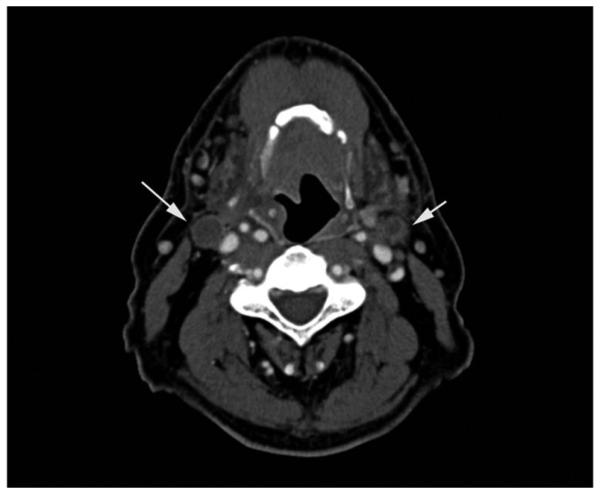
Computed tomography scan of a patient with an HPV− oropharyngeal cancer, demonstrating bilateral cervical adenopathy with necrotic centers (arrows), in contrast to the cystic lymph nodes seen in Figure 2.
Figure 4.
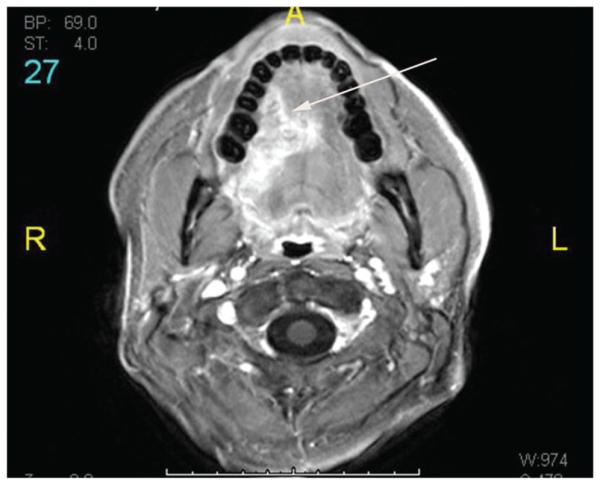
Magnetic resonance imaging scan of a patient with an HPV+ lateral tongue cancer (arrow). Although many HPV+ cancers exhibit exophytic, well-defined borders, in this case the tumor demonstrates infiltrative borders and invasion of intrinsic muscles of the tongue.
Implications for Practice Specific to HPV-positive OPSCC
As noted previously, numerous findings—whether clinical, laboratory, or radiographic—will indicate a high likelihood of a patient presenting to one’s office with an HPV-positive OPSCC.
Such patients are younger, predominantly male, and have limited tobacco and alcohol exposure histories. They present with advanced stage disease by AJCC criteria, often with large adenopathy and small primary tumors of the oropharynx. Radiographically, primary tumors may be quite small or undetectable, and cervical adenopathy may be predominantly cystic—giving the misimpression of a branchial cleft cyst. Work-up involves a disciplined approach to the primary site and the neck. Consideration of lingual tonsillectomy may assist in defining previous nonlocalized primary disease, and selective neck dissection should be employed when tissue diagnosis in the neck is nondiagnostic. Ultimately, specifically HPV requested immunohistochemical analysis (HPV or p16) of tissue specimens from primary site biopsy or FNAB of neck metastases will confirm the presence of an HPV-positive OPSCC.
Once confirmed, HPV-positive status carries powerful prognostic implications with cure rates significantly higher, even for advanced stages disease, than traditional non–HPV-related OPSCC. At the most basic level, such information is of paramount importance to the patients facing this life-altering diagnosis. At present, the treatment of OPSCC should follow National Comprehensive Cancer Network (NCCN) guidelines26 and not be altered on the basis of HPV status unless patients are specifically enrolled in Institutional Review Board–approved investigational research protocols such as ECOG 3311 (Phase II Randomized Trial of Transoral Surgical Resection followed by Low-dose or Standard-dose IMRT in Resectable p16+ Locally Advanced Oropharynx Cancer) and RTOG 1121 (Randomized Phase II Trial of Transoral Endoscopic Head and Neck Surgery followed by Risk-based IMRT and Weekly Cisplatin versus IMRT and Weekly Cisplatin for HPV Negative Oropharynx Cancer). Yet, the overall excellent cure rates for HPV-positive OPSCC allow for consideration and formulation of treatment deintensification protocols designed to maintain these high cures while mitigating treatment-related side effects—short and long term.
Finally, patients are more educated than ever and will readily access information on their own related to their HPV-positive OPSCC. This brings rise to many questions practitioners may not have faced in the past yet should be prepared to encounter and answer.
Is this the cancer Michael Douglas had? Yes and No
Yes Michael Douglas also had an HPV-related OPSCC and has done very well. Yet, everyone’s cancer is slightly different and a personalized plan for the treatment of your specific cancer will be put together to achieve the highest cure rate with the least morbidity.
Is this a sexually transmitted disease? Yes
Human papilloma virus infection is a sexually transmitted disease.
Am I contagious? Can I give this cancer to someone? No and No
Your exposure to HPV was likely long ago. The cancer cannot be transmitted to another person.
When was I infected? Many years ago
It was likely asymptomatic. Infection is quite common and addressed by the body’s immune system.
Should I get vaccinated? No
The vaccine has no role for someone who has already been infected with HPV and has no current role in cancer treatment.
Should my spouse get vaccinated? No
Vaccination is not recommended for the adults who have already been sexually active for a number of years.
Should my children get vaccinated? Yes
Vaccination is recommended for all adolescents and young adults (not only children of patients with OPSCC), boys and girls—ages 9 to 25, ideally prior to onset of sexual activity. This commonly utilized vaccine provides protection against the 4 strains of HPV most associated with cervical and oropharyngeal SCCa, including HPV-16.
Summary
We are seeing “new” cancer patients with increasing frequency in our practices. As reviewed, there is a near epidemic rise in OPSCC related to HPV, and today’s caregiver must be prepared to appropriately evaluate, counsel, and treat these patients with the expectation that traditional treatment algorithms will evolve to maintain or improve current excellent cure rates while lessening treatment related side effects.
Footnotes
Author Contributions
Daniel G. Deschler, design, authorship, editorial, substantial contributions to the conception and design of work; Jeremy D. Richmon, authorship, review, substantial contributions to the conception and design of work; Samir S. Khariwala, authorship, review, substantial contributions to the conception and design of work; Robert L. Ferris, authorship, review, substantial contributions to the conception and design of work; Marilene B. Wang, authorship, review, substantial contributions to the conception and design of work.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
This article was presented as a miniseminar at the 2013 AAO-HNSF Annual Meeting & OTO EXPO; September 29–October 3, 2013; Vancouver, British Columbia, Canada, by the Head and Neck Surgery & Oncology Committee of the AAO-HNS and The American Head and Neck Society (AHNS).
No sponsorships or competing interests have been disclosed for this article.
References
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Souza G, Kreimer AR, Viscidi R, et al. Case-controlled study of human papilloma virus and oropharyngeal cancer. N Engl J Med. 2010;33:1683–1694. [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30:2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen CT, Lewis JS, El-Mofty S, Haughey BH, Nussenbaum B. Human papilloma virus and oropharyngeal cancer: biology, detection and clinical implications. Laryngoscope. 2010;120:1756–1772. doi: 10.1002/lary.20936. [DOI] [PubMed] [Google Scholar]
- 6.Hemminki K, Dong C, Frisch M. Tonsillar and other upper aerodigestive tract cancers among cervical cancer patients and their husbands. Eur J Cancer Prev. 2000;9:433–437. doi: 10.1097/00008469-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Strojan P, Ferlito A, Medina JE, et al. Contemporary management of lymph node metastases from an unknown primary to the neck: I. A review of diagnostic approaches. Head Neck. 2013;35:286–293. doi: 10.1002/hed.21898. [DOI] [PubMed] [Google Scholar]
- 8.Waltonen JD, Ozer E, Schuller DE, Agrawal A. Tonsillectomy vs. deep tonsil biopsies in detecting occult tonsil tumors. Laryngoscope. 2009;119:102–106. doi: 10.1002/lary.20017. [DOI] [PubMed] [Google Scholar]
- 9.Koch WM, Bhatti N, Williams MF, Eisele DW. Oncologic rationale for bilateral tonsillectomy in head and neck squamous cell carcinoma of unknown primary source. Otolaryngol Head Neck Surg. 2001;124:331–333. doi: 10.1067/mhn.2001.114309. [DOI] [PubMed] [Google Scholar]
- 10.Durmus K, Rangarajan SV, Old MO, Agrawal A, Teknos TN, Ozer E. Transoral robotic approach to carcinoma of unknown primary. Head Neck. 2014;36(6):848–852. doi: 10.1002/hed.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagel TH, Hinni ML, Hayden RE, Lott DG. Transoral laser microsurgery for the unknown primary: A role for lingual tonsillectomy [published online April 25, 2013] Head Neck. doi: 10.1002/hed.23372. doi: 10.1002/hed.23372. [DOI] [PubMed] [Google Scholar]
- 12.Mehta V, Johnson P, Tassler A, et al. A new paradigm for the diagnosis and management of unknown primary tumors of the head and neck: a role for transoral robotic surgery. Laryngoscope. 2013;123:146–151. doi: 10.1002/lary.23562. [DOI] [PubMed] [Google Scholar]
- 13.Karni RJ, Rich JT, Sinha P, Haughey BH. Transoral laser microsurgery: a new approach for unknown primaries of the head and neck. Laryngoscope. 2011;121:1194–1201. doi: 10.1002/lary.21743. [DOI] [PubMed] [Google Scholar]
- 14.Patel SA, Magnuson JS, Holsinger FC, et al. Role of robotic surgery for an unknown primary head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2013;139:1203–1211. doi: 10.1001/jamaoto.2013.5189. [DOI] [PubMed] [Google Scholar]
- 15.Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446–1449. doi: 10.1002/1097-0142(197206)29:6<1446::aid-cncr2820290604>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 16.Jakscha J, Zlobec I, Storck C, et al. The clinical impact of p16 status in fine-needle aspirates of cervical lymph node metastasis of head and neck squamous cell carcinomas. Eur Arch Otorhinolaryngol. 2013;270:661–667. doi: 10.1007/s00405-012-2039-y. [DOI] [PubMed] [Google Scholar]
- 17.Begum S, Gillison ML, Nicol TL, Westra WH. Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:1186–1191. doi: 10.1158/1078-0432.CCR-06-1690. [DOI] [PubMed] [Google Scholar]
- 18.Gourin CG, Johnson JT. Incidence of unsuspected metastases in lateral cervical cysts. Laryngoscope. 2000;110:1637–1641. doi: 10.1097/00005537-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Andrews PJ, Giddings CE, Su AP. Management of lateral cystic swellings of the neck, in the over 40s’ age group. J Laryngol Otol. 2003;117:318–320. doi: 10.1258/00222150360600977. [DOI] [PubMed] [Google Scholar]
- 20.Chepeha DB, Taylor RJ, Chepeha JC, et al. Functional assessment using Constant’s Shoulder Scale after modified radical and selective neck dissection. Head Neck. 2002;24:432–436. doi: 10.1002/hed.10067. [DOI] [PubMed] [Google Scholar]
- 21.Rumboldt Z, Gordon L, Bonsall R, Ackermann S. Imaging in head and neck cancer. Curr Treat Options Oncol. 2006;7:23–34. doi: 10.1007/s11864-006-0029-2. [DOI] [PubMed] [Google Scholar]
- 22.Ginsberg LE. MR imaging of perineural tumor spread. Neuroimaging Clin N Am. 2004;14:663–677. doi: 10.1016/j.nic.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Corey AS, Hudgins PA. Radiographic imaging of human papillomavirus related carcinomas of the oropharynx. Head Neck Pathol. 2012;6:S25–S40. doi: 10.1007/s12105-012-0374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldenberg D, Begum S, Westra WH, et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30:898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 25.Cantrell SC, Peck BW, Li Q, Wei EM, Sturgis EM, Ginsberg LE. Differences in imaging characteristics of HPV-positive and HPV-negative oropharyngeal cancers: a blinded matched-pair analysis. AJNR Am J Neuroradiol. 2013;34:2005–2009. doi: 10.3174/ajnr.A3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network NCCN Practical Guidelines in Oncology (NCCN Guideliens) Head and Neck Cancers (Version 2.2013) http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed March 19, 2014. [Google Scholar]


