SUMMARY
Background
Chronic ulcerative colitis (CUC) and colonic Crohn’s disease (CD) increase colorectal neoplasia (CRN) risk. While sessile serrated polyp (SSP) is a known cancer precursor, serrated epithelial changes (SEC) are of uncertain prevalence and neoplastic risk.
Aim
To assess the serrated lesion detection rates in CUC and CD and documented incidence of subsequent CRN in a retrospective, single-centre cohort study.
Methods
Patients were identified by a central diagnostic index and pathology review confirmed SEC, SSP, CUC and CD diagnoses from 2006–12. Matched controls were identified from among all CUC and CD patients having colonoscopy during the second half of the time period. All were followed for incident CRN, estimated by the Kaplan–Meier method.
Results
Between 2006 and 2012, 79 SEC and 10 SSP cases were identified. Detection rates were estimated to be 10/1000 and 2/1000 patients, for SEC and SSP respectively, among 4208 unique CUC or CD patients having colonoscopy from 2010–12. With only 10 cases, SSP patients were not further analysed. Cumulative incidence of subsequent CRN at 1 and 3 years was 12% (95% CI, 0–30%) and 30% (3–57%), respectively, in SEC patients compared to 4% (0–12%) and 9% (0–23%), respectively, in CUC or CD controls (P = 0.047, log-rank). However, this statistical difference was not significant after patients were stratified for history of prior or synchronous dysplasia (P = 0.09).
Conclusions
Serrated epithelial changes and sessile serrated polyps are uncommonly detected by colonoscopy in chronic ulcerative colitis and Crohn’s disease patients. Histology with changes of serrated epithelium may be associated with risk of subsequent colorectal neoplasia, however further studies are needed to explore this relationship.
INTRODUCTION
Patients with both chronic ulcerative colitis (CUC) and Crohn’s disease (CD) of the colon are at a two to three fold increased risk for development of colorectal cancer (CRC) in comparison to the general population.1–3 Risk factors for the development of CRN in patients with inflammatory bowel diseases (IBD) include increased disease duration, extent, and age, along with comorbid primary sclerosing cholangitis (PSC).4–13 By expert consensus, aggressive surveillance with white light colonoscopy or dye-enhanced chromoendoscopy with random colonic mucosal biopsies is recommended in patients with long-standing (8 years or greater) CUC and CD involving more than 1/3 of the colon.11, 14–18
It is widely accepted that the finding of dysplasia in IBD patients, whether in focal lesions or on random biopsy, warrants either heightened surveillance or prophylactic colectomy.9–11, 14, 18–20 The same cannot be said about the finding of serrated lesions, as little is known about either the frequency or significance of such lesions in IBD. Lesions with serrated histology are frequently seen in the general population;21–23 while this heterogenous group includes indolent distal hyperplastic polyps, other serrated lesions such as sessile serrated polyps (SSP, formerly sessile serrated adenoma) and traditional serrated adenomas (TSA) may be precursors in up to 30% of all sporadic CRC.21, 24–28 Therefore, we are concerned that serrated lesions may play a greater role in IBD carcinogenesis than previously appreciated.
Serrated dysplastic lesions have been reported in mucosa adjacent to CRC in IBD patients.29 Additionally, DNA markers including mutant BRAF and microsatellite instability, typical of the sessile serrated pathway, have been observed in CRCs of IBD patients, even without serrated histological changes.26 Recently described in IBD,30 serrated epithelial change (SEC) is a histological finding in which epithelial serrations are characteristically seen in the upper half of the crypt, as is typical of benign hyperplastic polyps.24, 31 Early observations on the natural history of SEC in IBD are conflicting in their estimates of subsequent CRN risk. Parian, et al., reported that nearly a third of patients with SEC developed subsequent CRN, including CRC;30 however, Atwaibi and colleagues found no increase in neoplasia risk after adjusting for other clinical predictors.31
We measured the cross-sectional rate of diagnosis of serrated lesions in IBD patients, and then conducted a retrospective cohort study to measure rates of subsequent neoplasia in IBD patients with serrated lesions. We hypothesised that although these lesions are reported with increasing frequency, the outcomes of IBD patients with SEC may be influenced by known confounding risk factors including prior or synchronous adenomas.
METHODS
Patients
After Institutional Review Board approval, codes from the Systematised Nomenclature of Medicine Clinical Terms (SNOMED CT) were used to search a centralised pathology database at Mayo Clinic (Rochester, MN) between 2006 and 2012. Using the terms ‘colon’, ‘active chronic colitis’, ‘inactive chronic colitis’, and ‘serrated change’, potential cases of SEC and SSP were identified. Electronic records were reviewed by a single examiner to confirm CUC or CD, and to confirm the clinical pathologic diagnosis of SEC or SSP as the index lesion, excluding ‘hyperplastic’ polyps. While ileal resection or ileo-cecectomy for CD was permitted, patients with prior colonic surgery for dysplasia, CRC, or refractory CUC were excluded. Pathology slides from the index diagnosis from all of the remaining patients were all reviewed by a single expert GI pathologist (T. C. S.) to confirm SEC or SSP arising in chronic colitis.
To estimate the frequency of detection for serrated lesions in IBD, the total number of unique patients with CUC and CD undergoing colonoscopy from 2010 to 2012 was enumerated using American Medical Association Current Procedural Terminology (45378, 45380, 45381, 45382, 45384, 45385 and 45386) and International Classification of Diseases (9th Edition) codes (555, 555.1, 555.2, 555.9, 556, 556.1, 556.2, 556.3, 556.4, 556.5, 556.6, 556.8, 556.9). Because all SEC or SSP diagnoses were made by colonoscopy, and all were adults, IBD patients who underwent only flexible sigmoidoscopy were excluded, along with any patient who was not age 18 years or older at the time of endoscopy.
Data abstraction
A single individual extracted demographical data from the electronic medical record including gender, vital status, age at index and smoking history (current, former or past). IBD characteristics including disease type (CUC or CD), duration (in years from diagnosis), and disease extent (proximal or distal to splenic flexure) at index and at historical maximum, and the presence or absence of primary sclerosing cholangitis (PSC), were also abstracted. Colonoscopic data included type of index endoscopy (white light or chromoendoscopy), biopsy method (targeted or random) and the number of subsequent colonoscopies after index. Proximity of serrated lesions to adenomatous dysplasia was classified as ‘adjacent’ if it was found in the same of pathology specimen bottle. The date of the last colonoscopy or colectomy was used to determine length of follow-up for outcome analyses. The pathology record for each patient was also reviewed for prior adenomatous CRN, presence of synchronous adenomas at the time of SEC or SSP index diagnosis and the, development of subsequent CRN or CRC. To determine if SEC was more likely to segregate with clinical risk factors for CRN and to manually confirm the accuracy of search criteria, clinical variables were also abstracted on 50 IBD patients without SEC or SSP, randomly selected from the list of those undergoing colonoscopy between 2010 and 2012. SEC patients from 2006 to 2012 were frequency matched to 100 control IBD patients on disease type (CD vs. UC), disease extent (left-sided vs. extensive), disease duration (in years) and co-morbid primary sclerosing cholangitis. SEC and control groups were stratified by the presence of previous or synchronous CRN (yes/no) and followed for development of subsequent CRN, CRC, or colectomy. End-points occurring within 3 months of the index diagnosis were excluded from the analysis to avoid misclassifying an index diagnosis as an end-point. All data were entered into a secure database by the same abstracter (D.H.J).
Statistical analysis
For patients in the outcome analysis, differences in baseline characteristics were assessed by the Wilcoxon rank-sum test for continuous variables; proportions were assessed by the chi-square or Fisher’s exact test, where appropriate. Cumulative incidence of subsequent CRN, CRC or colectomy was estimated using the Kaplan–Meier method and reported with 95% confidence intervals (CI).
RESULTS
Detection rate and baseline characteristics
Figure 1 diagrams the flow of patients identified, excluded, re-classified and diagnosed. A total of 79 patients with SEC and 10 with SSP were identified and the number of cases was reported by year (Figure S1). A total of 4208 unique IBD patients underwent colonoscopy between 2010 and 2012. During the same time period, a total of 43 cases of SEC and 9 cases of SSP were found, for an estimated detection rate of 10 per 1000 (95% CI, 8–14) for SEC and 2 per 1000 (1–4) for SSP, respectively.
Figure 1.
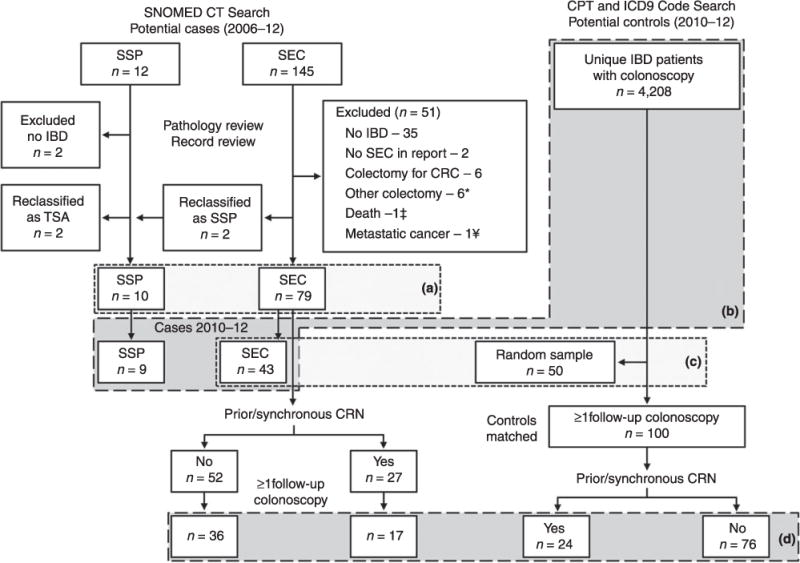
Flow diagram of patient selection: (a) Characteristics of chronic colitis patients with serrated epithelial changes (SEC) or sessile serrated polyp (SSP), 2006–12 (Table 1); (b) patients included in detection rate calculation; (c) characteristics of serrated epithelial change (SEC) patients and randomly selected control IBD patients undergoing colonoscopy between 2010–12 (Table 2); (d) patients included in time to event analysis (Tables 3, 4 and Figure 2); SNO-Med, systematised nomenclature of medicine; CPT, current procedural terminology (American Medical Association); ICD-9, international classification of diseases (9th Edition); SEC, serrated epithelial change; SSP, sessile serrated polyp; IBD, inflammatory bowel disease; TSA, traditional serrated adenoma. * Non-dysplastic indications; † Immediately after index - Lymphoma; ¥ Ovarian cancer metastatic to the colon.
Median ages at index were 57 years [interquartile range, (IQR) 48–65] and 48 years (IQR 42–57) for the SEC and SSP groups respectively (Table 1). CUC was the predominant form of IBD in SEC (84%) but was less common among SSP cases (40%) (P = 0.005). Median disease duration was longer in the SEC group compared to the SSP group at 18 (IQR 10.5–29) and 9.5 (IQR 1.5–22.5) years, respectively. Overall, the median age at IBD diagnosis was 34 years (IQR 25.5–46.5) in SEC and 34.5 years (31–44.75) in SSP. Proportions of patients with extensive disease were similar, 77% in SEC and 70% in SSP. PSC was found in 23 of SEC patients (29%), but was not seen in the SSP cohort. CRN was diagnosed prior to (n = 19) or synchronous with (n = 8) index lesions in 27 of SEC patients (34%). Six of 27 (22%) had both prior and synchronous dysplasia at index. SEC occurred in the same side of the colon (proximal or distal to splenic flexure) as prior dysplasia in 8 of 19 (42%), and in 11 of 14 (79%) of those with synchronous lesions. In SSP patients, CRN was diagnosed prior to (n = 2) or synchronous with (n = 4) in 6 of the 10 patients (60%). All SSP lesions were visible and therefore targeted at index colonoscopy; however, only 38% of SEC patients were diagnosed with targeted biopsies, despite similar use of chromoendoscopy (20%) at index in each group. Because only 10 SSP patients were found during the entire study period, outcome analyses were not performed on this group.
Table 1.
Characteristics of chronic colitis patients with serrated epithelial change (SEC) or sessile serrated polyp (SSP), 2006–12
| SEC | SSP | |
|---|---|---|
| N | 79 | 10 |
| Median age, years (IQR) | 57 (48–64.75) | 48 (41.5–57.25) |
| Males (%) | 49 (62%) | 7 (70%) |
| CUC (%) | 66 (84%) | 4 (40%) |
| Median IBD duration at index, years (IQR) | 18 (10.5–29) | 9.5 (1.5–22.5) |
| Extensive disease (%) | 61 (77%) | 7 (70%) |
| PSC (%) | 23 (29%) | 0 (0%) |
| Prior or synchronous CRN (%) | 27 (34%) | 6 (60%) |
| Targeted index biopsy (%) | 30 (38%) | 10 (100%) |
| Chromoendoscopy at index (%) | 16 (20%) | 2 (20%) |
CUC, chronic ulcerative colitis; PSC, primary sclerosing cholangitis; CRN, colorectal neoplasia.
The sub-set of IBD patients diagnosed with SEC between 2010 and 2012 was compared to a random sample of IBD patients who had colonoscopy during the same time period (Figure 1c, Table 2). A total of 52 records were reviewed to obtain 50 control patients, demonstrating high accuracy of the search criteria (kappa = 0.96). SEC patients were more likely to have clinical risk factors for CRN. They were older and had longer disease duration. PSC was also more common in SEC patients (28%, n = 12/43) compared to 6% (3/50) controls (P = 0.005). A history of prior CRN, the development of subsequent CRN and rate of colectomy were not significantly different between the SEC group and controls.
Table 2.
Characteristics of serrated epithelial change (SEC) patients and control IBD patients undergoing colonoscopy between 2010–12
| SEC | Control | P-value | |
|---|---|---|---|
| N | 43 | 50 | – |
| Median age years (IQR) | 58 (47–64) | 42 (27.25–60.5) | 0.0014 |
| Males (%) | 22 (51%) | 23 (46%) | 0.68 |
| CUC (%) | 34 (79%) | 28 (56%) | 0.03 |
| Median IBD duration at index, years (IQR) | 20 (12–29.5) | 7 (3.25–15.75) | <0.001 |
| Extensive disease (%) | 34 (79%) | 37 (74%) | 0.63 |
| Prior CRN (%) | 15 (35%) | 9 (18%) | 0.10 |
| Subsequent CRN (%) | 7 (16%) | 4 (8%) | 0.33 |
| Colectomy (%) | 4 (9%) | 2 (4%) | 0.41 |
| PSC (%) | 12 (28%) | 3 (6%) | 0.005 |
| Smoking (%) | |||
| Current | 2 (5%) | 7 (14%) | 0.17 |
| Past | 17 (40%) | 10 (20%) | 0.04 |
| Never | 23 (53%) | 30 (60%) | 0.54 |
| Unknown | 1 (2%) | 3 (6%) | 0.62 |
IBD, inflammatory bowel disease; CUC, chronic ulcerative colitis; PSC, primary sclerosing cholangitis; CRN, colorectal neoplasia. Bold values emphasize statistically significant P values.
Incidence of subsequent CRN
The full SEC cohort was then stratified into two groups: those with either prior or synchronous adenomatous dysplasia at index (n = 27) or none (n = 52). Further chart review excluded 26 patients from analysis of ‘to time of subsequent CRN’; 19 were for lack of subsequent endoscopy (3 with prior or synchronous CRN and 16 with none) and 7 developed subsequent CRN within 3 months of index diagnosis (7 with prior or synchronous CRN and 0 with none), leaving a total of 53 SEC patients for analysis of incident CRN (Figure 1d). Control patients with at least one follow-up colonoscopy were matched and stratified by colorectal neoplasia history. CUC was the predominant IBD type. There were no significant differences in median age, sex distribution, IBD subtype, IBD extent, the duration of IBD, or the presence of PSC. Rates of past and current smoking were also similar with the majority of patients in each group being never smokers. Median follow-up to last colonoscopy or colectomy was 24 months (IQR, 16–47) among SEC patients and 31 months (IQR, 19–58) among controls (P = 0.7); there were no significant differences when patients were stratified by prior or synchronous CRN history (Table 3).
Table 3.
Characteristics of patients included in incidence of subsequent colorectal neoplasia (CRN) analysis
| History of prior or synchronous CRN | ||||||
|---|---|---|---|---|---|---|
| Without | With | |||||
| SEC | Control | P-value | SEC | Control | P-value | |
| N | 36 | 76 | – | 17 | 24 | – |
| Median age years (IQR) | 58 (49–64) | 54 (51–58) | 0.2 | 59 (51–69) | 60 (55–62) | 0.9 |
| Males (%) | 22 (61%) | 40 (53%) | 0.4 | 11 (65%) | 10 (42%) | 0.2 |
| CUC (%) | 31 (86%) | 57 (75%) | 0.2 | 15 (88%) | 18 (75%) | 0.4 |
| Median IBD duration at index, years (IQR) | 19 (14–30) | 20.5 (11–29) | 0.7 | 14 (10–26) | 17.5 (9–22) | 0.7 |
| Extensive disease (%) | 30 (83%) | 63 (83%) | 1.0 | 12 (71%) | 21 (88%) | 0.2 |
| Colectomy (%) | 2 (6%) | 4 (5%) | 1.0 | 3 (18%) | 1 (4%) | 0.3 |
| PSC (%) | 11 (31%) | 16 (21%) | 0.3 | 4 (24%) | 3 (13%) | 0.4 |
| Smoking (%) | ||||||
| Current | 3 (8%) | 4 (5%) | 0.7 | 1 (6%) | 1 (4%) | 1.0 |
| Past | 12 (33%) | 17 (22%) | 0.3 | 5 (29%) | 12 (50%) | 0.2 |
| Never | 21 (58%) | 53 (70%) | 0.3 | 10 (59%) | 11 (46%) | 0.5 |
| Unknown | 0 (0%) | 2 (3%) | 0.6 | 1 (6%) | 0 (0%) | 0.4 |
| Median follow-up, months (IQR) | 31 (19–57) | 29 (14–37) | 0.2 | 20 (12–29) | 32 (7–37) | 0.07 |
SEC, serrated epithelial change; IQR, interquartile range; CUC, chronic ulcerative colitis; IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis.
Fifteen of 53 (28%) SEC patients and 11 of 100 (11%) control patients developed subsequent CRN (Table 4). Among SEC patients, subsequent neoplasia arose in the form of unifocal LGD (n = 8), multifocal LGD (n = 4), SSA (n = 1), traditional serrated adenoma (n = 1) and HGD (n = 1). No cancers arose in patients with SEC. Ten subsequent lesions were endoscopically visible (91%). Among SEC patients, subsequent CRN occurred on the same side of the colon (proximal or distal to splenic flexure) as the index SEC lesion in only 5 of 11 (45%) cases. Among controls, the subsequent neoplastic lesions included unifocal LGD (n = 6), multifocal LGD (n = 2), SSA (n = 1), HGD (n = 1) and CRC (n = 1). The single control patient who developed cancer had no history of prior or synchronous CRN.
Table 4.
Subsequent colorectal neoplasia events
| Neoplasia history | Subsequent CRN | Synchronous lesion | Prior lesion* |
|---|---|---|---|
| SEC | |||
| Prior or synchronous CRN | |||
| -Prior only | N = 5 | ||
| Unifocal LGD (3) | – | Unifocal LGD (3) | |
| Multifocal (LGD and SSP) | – | Unifocal LGD | |
| Multifocal LGD | – | Multifocal LGD | |
| -Prior and synchronous | N = 2 | ||
| Unifocal LGD | Unifocal LGD | Unifocal LGD | |
| Multifocal (HGD and LGD) | Multifocal indefinite | Multifocal indefinite | |
| -Synchronous only | N = 1 | ||
| Multifocal LGD | Multifocal LGD | – | |
| None | N = 7 | ||
| Unifocal LGD (4) | – | – | |
| Unifocal TSA | – | – | |
| Unifocal SSP | – | – | |
| Control | |||
| -Prior CRN | N = 8 | ||
| Unifocal LGD (5) | – | Unifocal LGD (5) | |
| Multifocal LGD (2) | – | Multifocal LGD (2) | |
| Unifocal HGD | – | Unifocal LGD TVA | |
| None | N = 3 | ||
| Unifocal LGD | – | – | |
| SSP | – | – | |
| CRC | – | – |
SEC, serrated epithelial changes; CRN, colorectal neoplasia; LGD, low grade dysplasia; SSP, sessile serrated polyp; HGD, high grade dysplasia; TSA, traditional serrated adenoma; TVA, tubulovillous adenoma; CRC, colorectal carcinoma.
In patients with multiple prior lesions, most recent is reported.
The cumulative incidence of subsequent CRN at 1 and 3 years was 12% (95% CI, 0–30%) and 30% (3–57%), respectively, in SEC patients was 4% (0–12%) and 9% (0–23%), respectively, in controls (P = 0.047, log-rank). However, this difference was not statistically significant following stratification for prior/synchronous CRN history. Thereafter, the cumulative incidence of subsequent CRN at 1 and 3 years was 25% (0–68%) and 60% (5–100%), respectively, in SEC patients with prior/synchronous CRN, and 17% (0–47%) and 31% (0–74%) respectively in controls with prior/synchronous CRN (P = 0.09). For those without prior/synchronous CRN, the 1 and 3 year incidence of subsequent CRN was 6% (0–21%) and 17% (0–44%), respectively, in SEC patients compared to 0% (0–0%) and 2% (0–8%), respectively in controls (P = 0.1) (Figure 2).
Figure 2.
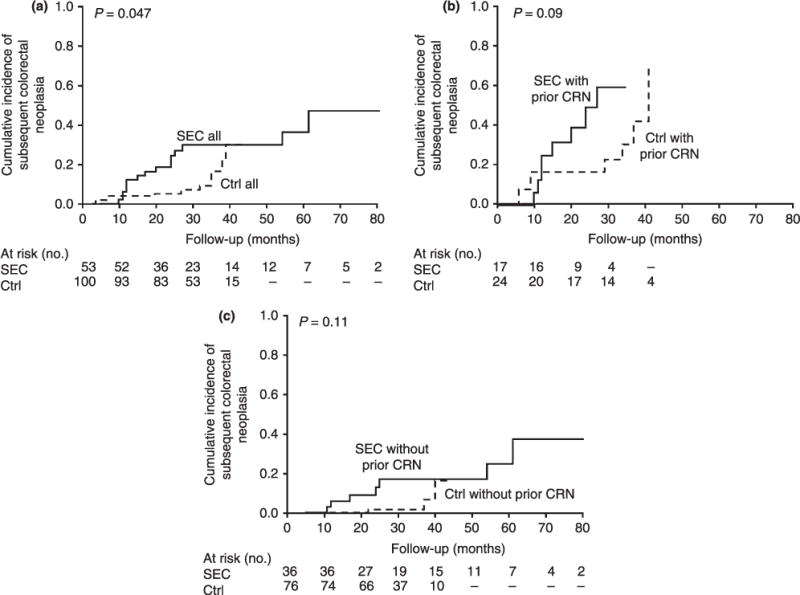
Cumulative incidence of subsequent colorectal neoplasia (CRN) for: (a) All serrated epithelial change patients (—SEC all) compared to IBD controls (- - Ctrl all); (b) serrated epithelial change patients with a history of prior or synchronous CRN (— SEC with prior CRN) compared to IBD controls with prior CRN (- - Ctrl with prior CRN); and (c) serrated epithelial change patients without history of prior or synchronous CRN (— SEC without prior CRN) compared to IBD controls without prior colorectal neoplasia (- - Ctrl without prior CRN).
DISCUSSION
With recognition of the serrated pathway in CRC pathogenesis, accurate diagnosis and prognostic assessment of serrated lesions in IBD patients has potentially important clinical relevance. To the best of our knowledge, this is the first full-length report of detection rate and neoplastic risk in IBD patients with SEC. We found a low but increasing prevalence of lesions with serrated morphology. When compared to control patients from our IBD population, patients in the SEC group were significantly more likely to have conventional risk factors for CRN, including long-standing IBD and PSC. A history of prior or synchronous CRN was associated with a significantly greater cumulative incidence of subsequent CRN. After stratifying for CRN history, the impact of SEC on subsequent neoplasia risk was not statistically significant.
Excluding hyperplastic polyps, serrated lesions now account for up to 13–19% of all sporadic polyps removed at screening colonoscopy, and are encountered more often in the proximal colon and in association with increasing age.23 Based on diagnostic records at our single centre, the number of serrated lesions diagnosed in IBD patients appears to have risen since 2009 (Figure S1), but the detection rate for these lesions appears to be substantially lower than in the sporadic population at our institution.23 Because of small numbers in the IBD cohort, this trend should be viewed with caution. Historically, serrated lesions were missed or misclassified in the sporadic setting.23 As SSPs are reported with increasing frequency, there has been a concordant decrease in the frequency of hyperplastic polyps, suggesting that reclassification may contribute in part to this trend.23 Based on our findings, serrated lesions are reported less frequently in IBD patients than in sporadic patients at our institution. It is not clear whether serrated lesions in IBD are truly less common or have been historically missed by colonoscopy or by pathology.
Serrated epithelial changes lesions were most frequently found in patients with other conventional risk factors for CRN. This suggests that SEC may represent mucosal injury response, identify patients with significant cumulative inflammatory burden or serve as a marker of future dysplasia. Forty per cent of SEC was identified by chromoendoscopy and targeted biopsies, commonly ordered at our institution for surveillance of IBD patients with prior CRN. While chromoendoscopy may be valuable in detecting flat lesions in IBD32 it may also result in over-diagnosis of indolent lesions. Because most lesions appear to have been endoscopically visible, at least by enhanced techniques, we have avoided use of the term ‘flat serrated change’ coined by previous authors.
Limited data are available for comparison. The current literature contains only two case series, published in abstract form, which differ in the assessment of neoplastic risk attributable to SEC.30, 31 Atwaibi, et al., did not find increased development of subsequent CRN in IBD patients with SEC compared to IBD controls31, but also did not report on neoplasia history of study patients. Parian, et al., in contrast, found that SEC was significantly associated with both concomitant and subsequent dysplasia.30 Subsequent dysplasia frequently occurred in the same anatomical location as SEC,30 a finding we did not confirm. The full reports of these observations have not yet been published, limiting comparisons among study methodology. Future studies of serrated lesions in IBD patients will benefit from a full pathologic review to avoid potential misclassification, a significant problem in serrated lesion diagnosis.33 Our data are internally consistent; the cumulative incidence of CRN among patients with a history of adenomatous dysplasia in the present study is similar to rates observed in patients with polypoid adenomatous dysplasia history in a prior study from our institution.34
We acknowledge several important limitations to this study. The number of patients studied was relatively small but was assembled from all available cases after a rigorous selection process. The sample size may have been underpowered to detect significant effects in multivariate comparisons. Additionally, the detection rate of serrated lesions in IBD patients was estimated from coding and billing records. Because of this, it is possible that we may have missed potential cases. However, all potential case patients were confirmed after review by a senior GI pathologist to ensure appropriate classification. It is also possible that neoplasia history or endpoints were missed or misclassified. To avoid this, we were conservative to include indefinite dysplasia among prior CRN, despite the potential to weaken the differences among groups. Additionally, any CRN found within 3 months of index was not considered for analysis. This minimised the likelihood of counting the index as an endpoint, or including a lesion that was missed at the index colonoscopy. We were also careful to exclude patients with prior history of HGD or CRC from analysis as these patients already met an endpoint that would mandate a significant change in management. We accounted for the variable duration of follow-up among patients by use of the Kaplan–Meier method to measure our pre-defined study endpoint; longer term follow-up is critical to validate these observations. There also appear to be limitations to histology as a stand-alone surveillance tool among high-risk individuals; a control patient developed CRC without any prior dysplasia history. Therefore, new tools, such as molecular markers, should be systematically studied for incremental value in neoplastic risk stratification of SEC lesions in IBD patients.24, 35
In summary, these early observations suggest that patients with SEC appear to have other high-risk clinical features, including extensive disease, long-standing disease duration and PSC. As a group, these patients are thus at high risk of subsequent adenomatous CRN; however, much of this risk may be influenced by other risk factors, especially a history of prior adenomatous dysplasia, or adenomatous dysplasia which is synchronous to SEC. Examination of larger studies will be needed to better estimate the effect size of SEC histology on risk of subsequent CRN to inform surveillance guidelines. Additionally, we found low rates of detection of SSP lesions among patients with IBD. Therefore, these results should serve as a catalyst to improve endoscopic recognition of adenomas and SSP, and improve phenotypic and molecular risk stratification of IBD patients at risk for CRN.
Supplementary Material
Figure S1. Serrated epithelial change (SEC) and sessile serrated polyp (SSP) diagnoses by year, 2006–2012.
Acknowledgments
Declaration of personal interests: Edward V. Loftus, MD, and employee of Mayo Clinic (Rochester, Minnesota, USA), has served as a consultant and advisory board member for AbbVie (Chicago, Illinois, USA), Janssen (Titusville, New Jersey, USA), Takeda (Osaka, Japan), Immune Pharmaceuticals (Tarrytown, New York, USA), and UCB (Anderlecht, Belgium). He has received research funding from AbbVie, Janssen, Takeda, UCB, Shire (Dublin, Ireland), GlaxcoSmithKline (Brentford, Middlesex, UK), Bristol-Myers Squibb (New York, New York, USA), Pfizer (New York, New York, USA), Genentech (San Francisco, California, USA), Santarus (San Diego, California, USA), Amgen (Thousand Oaks, California, USA), and Robarts Clinical Trials (London, Ontario, Canada), which is not relevant to content of the article. No stock or patents. Douglas W. Mahoney, an employee of Mayo Clinic (Rochester, Minnesota, USA) has developed intellectual property at Mayo Clinic, eligible for license to Exact Sciences (Madison, Wisconsin, USA), which is not relevant to content of the article. David A. Ahlquist, MD, and employee of Mayo Clinic (Rochester, Minnesota, USA), has served as a scientific advisor to and has received research funding from Exact Sciences (Madison, Wisconsin, USA). He owns stocks and shares in Exact Sciences. He is an inventor on technology licensed to Exact Sciences by his employer Mayo Clinic, and receives inventor share of royalties, which is not relevant to content of the article. John B. Kisiel, MD, an employee of Mayo Clinic (Rochester, Minnesota, USA) has developed intellectual property at Mayo Clinic, eligible for license to Exact Sciences (Madison, Wisconsin, USA), which is not relevant to content of the article.
Declaration of funding interests: This research was supported by a grant from the Maxine and Jack Zarrow Family Foundation of Tulsa Oklahoma (to J. B. K.) and by NCI CA90628 (to J. B. K.).
Footnotes
Author contributions: Johnson: concept and design, data collection, data analysis and manuscript draft. Khanna: concept and design, data collection, critical revision. Smyrk: concept and design, data collection, pathologic review and critical revision. Loftus: concept and design, and critical revision. Anderson and Mahoney: data analysis/interpretation and critical revision. Ahlquist: concept and design, data analysis/interpretation, critical revision and obtained funding. Kisiel: concept and design, data analysis, critical revision, obtained funding and study supervision. All authors approved the final version of the manuscript.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
References
- 1.Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(Suppl 2):1–5. doi: 10.1046/j.1365-2036.18.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 2.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639–45. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Herrinton LJ, Liu L, Levin TR, Allison JE, Lewis JD, Velayos F. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143:382–9. doi: 10.1053/j.gastro.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 4.Farmer RG, Hawk WA, Turnbull RB., Jr Carcinoma associated with mucosal ulcerative colitis, and with transmural colitis and enteritis (Crohn’s disease) Cancer. 1971;28:289–92. doi: 10.1002/1097-0142(197108)28:2<289::aid-cncr2820280205>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Sugita A, Sachar DB, Bodian C, Ribeiro MB, Aufses AH, Jr, Greenstein AJ. Colorectal cancer in ulcerative colitis. Influence of anatomical extent and age at onset on colitis-cancer interval. Gut. 1991;32:167–9. doi: 10.1136/gut.32.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torres J, Pineton de Chambrun G, Itzkowitz S, Sachar DB, Colombel JF. Review article: colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:497–508. doi: 10.1111/j.1365-2036.2011.04753.x. [DOI] [PubMed] [Google Scholar]
- 7.Jess T, Loftus EV, Jr, Velayos FS, et al. Risk factors for colorectal neoplasia in inflammatory bowel disease: a nested case-control study from Copenhagen county, Denmark and Olmsted county, Minnesota. Am J Gastroenterol. 2007;102:829–36. doi: 10.1111/j.1572-0241.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–105. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potack J, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease. Gut Liver. 2008;2:61–73. doi: 10.5009/gnl.2008.2.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–48. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Eaden JA, Mayberry JF. Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut. 2002;51(Suppl 5):V10–2. doi: 10.1136/gut.51.suppl_5.v10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butt JH, Lennard-Jones JE. A practical approach to the risk of cancer in inflammatory bowel disease. Med Clin North Am. 1980;64:1203–20. doi: 10.1016/s0025-7125(16)31564-4. [DOI] [PubMed] [Google Scholar]
- 13.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746–74. e1, 4. doi: 10.1053/j.gastro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 15.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–23. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 16.Ullman T, Odze R, Farraye FA. Diagnosis and management of dysplasia in patients with ulcerative colitis and Crohn’s disease of the colon. Inflamm Bowel Dis. 2009;15:630–8. doi: 10.1002/ibd.20766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011;4:53–61. [PMC free article] [PubMed] [Google Scholar]
- 18.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–89. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 19.Dyson JK, Rutter MD. Colorectal cancer in inflammatory bowel disease: what is the real magnitude of the risk? World J Gastroenterol. 2012;18:3839–48. doi: 10.3748/wjg.v18.i29.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itzkowitz SH, Present DH. Consensus conference: colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314–21. doi: 10.1097/01.mib.0000160811.76729.d5. [DOI] [PubMed] [Google Scholar]
- 21.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–29. doi: 10.1038/ajg.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA) Am J Surg Pathol. 2008;32:21–9. doi: 10.1097/PAS.0b013e318157f002. [DOI] [PubMed] [Google Scholar]
- 23.Anderson B, Smyrk TC, Sweetser SR, et al. Emerging prominence of sessile serrated polyps in a large colonoscopy practice: distribution trends by year, anatomic site, age, and sex. Am J Gastroenterol. 2013;108:S647. [Google Scholar]
- 24.Tadros M, Anderson JC. Serrated polyps: clinical implications and future directions. Curr Gastroenterol Rep. 2013;15:342. doi: 10.1007/s11894-013-0342-4. [DOI] [PubMed] [Google Scholar]
- 25.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1–10. doi: 10.1016/j.humpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Bossard C, Denis MG, Bezieau S, et al. Involvement of the serrated neoplasia pathway in inflammatory bowel disease-related colorectal oncogenesis. Oncol Rep. 2007;18:1093–7. [PubMed] [Google Scholar]
- 27.Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088–100. doi: 10.1053/j.gastro.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 28.Bauer VP, Papaconstantinou HT. Management of serrated adenomas and hyperplastic polyps. Clin Colon Rectal Surg. 2008;21:273–9. doi: 10.1055/s-0028-1089942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubio CA, Befrits R, Jaramillo E, Nesi G, Amorosi A. Villous and serrated adenomatous growth bordering carcinomas in inflammatory bowel disease. Anticancer Res. 2000;20:4761–4. [PubMed] [Google Scholar]
- 30.Parian AM, Koh JM, Badamas J, Giardiello FM, Montgomery EA, Lazarev M. Serrated epithelial changes are associated with colorectal dysplasia in inflammatory bowel disease. Gastroenterology. 2013;144:S–11. [Google Scholar]
- 31.Atwaibi M, Batts KP, Weinberg DI, McCabe RP. Flat serrated change: does it predict the development of colonic mucosal dysplasia in inflammatory bowel disease? Gastroenterology. 2012;1:S–665. [Google Scholar]
- 32.Jaramillo E, Watanabe M, Befrits R, Ponce de Leon E, Rubio C, Slezak P. Small, flat colorectal neoplasias in long-standing ulcerative colitis detected by high-resolution electronic video endoscopy. Gastrointest Endosc. 1996;44:15–22. doi: 10.1016/s0016-5107(96)70223-7. [DOI] [PubMed] [Google Scholar]
- 33.Singh H, Bay D, Ip S, et al. Pathological reassessment of hyperplastic colon polyps in a city-wide pathology practice: implications for polyp surveillance recommendations. Gastrointest Endosc. 2012;76:1003–8. doi: 10.1016/j.gie.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 34.Kisiel JB, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. Outcome of sporadic adenomas and adenoma-like dysplasia in patients with ulcerative colitis undergoing polypectomy. Inflamm Bowel Dis. 2012;18:226–35. doi: 10.1002/ibd.21687. [DOI] [PubMed] [Google Scholar]
- 35.Drini M, Young JP. Molecular change that distinguishes traditional serrated adenomas from sessile serrated adenomas. J Gastroenterol Hepatol. 2011;26:1472–4. doi: 10.1111/j.1440-1746.2011.06862.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Serrated epithelial change (SEC) and sessile serrated polyp (SSP) diagnoses by year, 2006–2012.


