Summary
ERK signaling requires RAS-induced RAF dimerization and is limited by feedback. Activated BRAF mutants evade feedback inhibition of RAS by either of two mechanisms. BRAF V600 mutants are activated monomers when RAS activity is low; all other activating BRAF mutants function as constitutive RAS-independent dimers. RAF inhibitors effectively inhibit mutant monomers, but not dimers; their binding to one site in the dimer significantly reduces their affinity for the second. Tumors with non-V600E BRAF mutants are insensitive to these drugs and increased expression of BRAF V600E dimers causes acquired resistance. A compound that equally inhibits both sites of mutant RAF dimers inhibits tumors driven by either class of mutants or those BRAF V600E tumors with dimer-dependent acquired resistance to monomer-specific inhibitors.
Introduction
The oncogenic activation of ERK signaling output is characteristic of many cancers. Physiologic activation of the pathway occurs when upstream signals stimulate the binding of RAS to GTP (Luo et al., 1996; Rajakulendran et al., 2009). Activated RAS binds to RAF family members and causes their homo- and heterodimerization and activation (Freeman et al., 2013). This, in turn, initiates the MEK/ERK kinase cascade and phosphorylation of effectors of the pathway by ERK. Activation of ERK also causes an array of negative regulatory events that serve to inhibit the pathway. ERK phosphorylates and inhibits receptors (Avraham and Yarden, 2011), the RAS GDP-GTP exchange factor SOS (Dong et al., 1996), and WT CRAF and BRAF (Dougherty et al., 2005). It also increases the expression of members of the Sprouty and DUSP families of proteins that inhibit the pathway (Pratilas et al., 2009). The former inhibit RTK activation of RAS whereas the latter are MAPK phosphatases (Lang et al., 2006). Thus, negative feedback limits the amplitude and duration of the ERK signal.
Oncogenic mutations of NRAS, KRAS, BRAF, MEK1, and NF1 drive the ERK dependent growth of many human cancers. These mutations activate both downstream signaling and potent negative feedback, as evidenced by reactivation of upstream and parallel components of the pathway in cells exposed to MEK or RAF inhibitors (Corcoran et al., 2012; Lito et al., 2012; Montero-Conde et al., 2013). We hypothesize that the elevated signaling output necessary for transformation requires selection of oncoproteins with decreased sensitivity to negative feedback. Mutations and translocations of RAF family genes are common in human tumors (Wan et al., 2004; Davies et al., 2002; Palanisamy et al., 2010). V600E is the most common BRAF mutation, but non-V600E mutations account for more than 50% of the RAF mutations in lung cancers (Paik et al., 2011) and occur in many other tumors. The kinase activity of many of these mutants has been shown to be activated compared to wild type, but some BRAF mutations are kinase-dead or have lower activity than WT BRAF (Wan et al., 2004). RAF fusions and truncations in which part of the aminoterminal domain of RAF is usually deleted also occur rarely in many tumors and in a high percentage of pilocytic astrocytomas (Berghoff and Preusser, 2014). Here we ask how activating mutations of RAF hyperactivate signaling in the setting of ERK dependent feedback inhibition of RAS.
Results
Activating mutants of BRAF hyperactivate ERK signaling and suppress RAS activation
RAS-GTP is suppressed in BRAF V600E tumor cells by ERK-dependent feedback (Lito et al., 2012). We asked whether this is a general property of tumor cells with activated mutant BRAFs. Levels of RAS-GTP and CRAF phosphorylation at serine 338 (pCRAF S338, a marker of CRAF activation) (Mason et al., 1999) were much lower in tumor cells with activating BRAF V600E, K601N, L597V, L597R or G469A mutations than in those with WT RAF (Figure S1A). In contrast, levels of phosphorylated MEK (p-MEK) are highly elevated in RAF mutant cells even compared to those with mutant RAS. These results suggest that MEK is activated but RAS and CRAF are feedback inhibited in tumor cells with activating BRAF mutants.
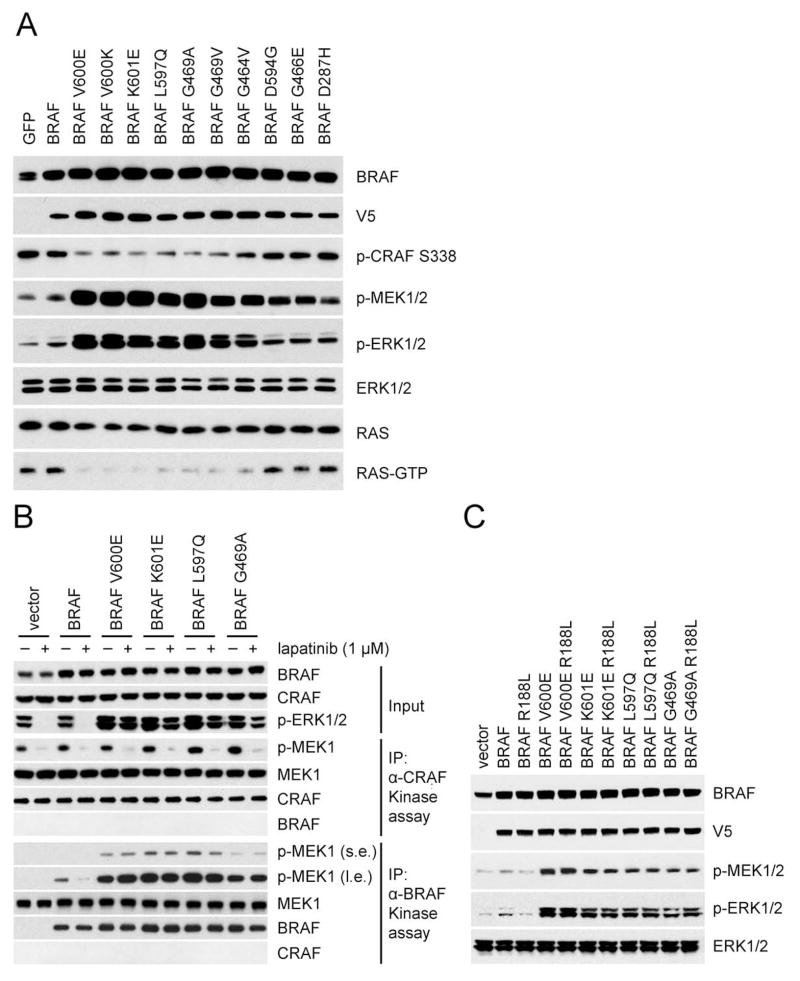
To determine whether suppression of RAS activity is a general property of these mutants, we expressed mutant BRAFs in an inducible fashion in NIH3T3 cells (Figure. 1A). Inductions of the expression of BRAF V600E, V600K, K601E, L597Q, G469A, G469V, or G464V at levels comparable to those of endogenous BRAF caused significant induction of p-MEK and p-ERK and marked inhibition of RAS-GTP and pCRAF S338. Induction of WT BRAF caused minor increases in p-MEK and p-ERK and had no effect on RAS-GTP or pCRAF S338. Thus, activated RAF mutants significantly increase ERK signaling despite causing feedback inhibition of RAS activity to almost undetectable levels. By contrast, kinase-dead BRAF D594G, and low activity BRAF G466E and D287H neither activated ERK signaling nor inhibited RAS function. These mutants may function in a fundamentally different way than the activating mutants, as previously suggested (Heidorn et al., 2010). This paper will focus on activating mutants of BRAF and how they function in cells in which RAS is feedback inhibited.
Figure 1. Activating BRAF mutants signal in a RAS-independent manner.
(A) NIH3T3 cells stably transduced with retrovirus carrying doxycycline-inducible wild-type BRAF or the indicated mutants were treated with doxycycline (30 ng/ml) for 24 hr. Expression and/or phosphorylation of the indicated proteins were assayed by Western blot. Cellular RAS-GTP levels were determined using the active RAS pull-down assay.
(B) SKBR3 cells transiently expressing V5 tagged WT BRAF or the indicated mutants were treated with lapatinib (1 μM) or DMSO for 1 hr. Cell lysate from each sample was divided into two portions for immunoprecipitation with either anti-V5 antibody or anti-CRAF antibody followed by an in vitro kinase assay with 0.5 μg K97R MEK1 protein. 5% of the whole cell extracts were used for immunoblot (Input panels). s.e.: short exposure. l.e.: longer exposure.
(C) Ectopic expression of V5 tagged wild-type BRAF or the indicated mutants were expressed in SKBR3 cells followed by lapatinib treatment (1 μM, 1 hr). ERK signaling was assessed by Western blot as in Figure 1A.
See also Figure S1.
Activation of signaling by hyperactivated RAF mutants is RAS-independent
Our findings imply that activated BRAF mutants signal in a RAS-independent manner and are thus insensitive to upstream feedback. To test this hypothesis, we used the ERBB2 amplified breast cancer cell SKBR3 in which RAS activity and ERK signaling are potently suppressed after 1 hr exposure to the HER2 inhibitor lapatinib (Figure S1B). This system allows us to assess the RAS dependence of exogenously expressed RAF mutants. lapatinib inhibited ERK signaling in SKBR3 cells overexpressing WT BRAF, but failed to do so in those that express activating BRAF mutants (Figure. 1B and S1C). Moreover, when CRAF or BRAF were immunoprecipitated from these cells, BRAF associated kinase activity was only sensitive to lapatinib in cells expressing WT BRAF, not in those expressing mutant BRAF (Figure. 1B and S1C). In contrast, CRAF associated kinase activity was sensitive to lapatinib in all cells. Thus, the kinase activity of activating BRAF mutants is RAS-independent. To exclude the possibility that their activity requires low levels of residual RAS-GTP, the R188L mutation that disrupts the RAS-BRAF interaction (Fabian et al., 1994) was introduced into the activating BRAF mutants and did not affect their ability to drive signaling (Figure. 1C). To further confirm that these activating BRAF mutants signal in a RAS-independent manner, we utilized conditional RAS-less mouse embryo fibroblasts (MEFs) (Drosten et al., 2014). Knockout of Kras, the only RAS gene in these cells, causes their proliferative arrest and abrogates ERK signaling, but they remain viable. All of the activating BRAF mutants rescue MEK/ERK phosphorylation in these cells, but WT BRAF does not (Figure S1D). We have tested 16 activating BRAF mutants (Table 1) and all signal in a RAS-independent manner.
Table 1.
Properties of WT and mutant BRAF alleles
| BRAF alleles | Feedback inhibition of RAS-GTP | RAS dependency of kinase activation | Sensitivity of R509H mediated inhibition | Sensitivity To vemurafenib | RAS dependency of Dimerization with CRAF | RAS dependency of homodimerformation |
|---|---|---|---|---|---|---|
| WT | N | Y | Y | N | Y | Y |
| V600E | Y | N | N | Y | Y | Y |
| V600K | Y | N | N | Y | Y | Y |
| V600D | Y | N | N | Y | Y | Y |
| V600R | Y | N | N | Y | Y | Y |
| V600M | Y | N | N | Y | Y | Y |
| K601E | Y | N | Y | N | Y | N |
| K601N | Y | N | Y | N | Y | N |
| K601T | Y | N | Y | N | Y | N |
| L597Q | Y | N | Y | N | Y | N |
| L597V | Y | N | Y | N | Y | N |
| G469A | Y | N | Y | N | Y | N |
| G469V | Y | N | Y | N | Y | N |
| G469R | Y | N | Y | N | Y | N |
| G464V | Y | N | Y | N | Y | N |
| G464E | Y | N | Y | N | Y | N |
| KIAA1549- BRAF | Y | N | Y | N | Y | N |
| p61 WT | Y | N | Y | N | Y | N |
| p61 V600E | Y | N | N | N | Y | N |
RAS-independent BRAF mutants fall in two classes: active constitutive dimers and mutants that are active as monomers in cells with low RAS activity
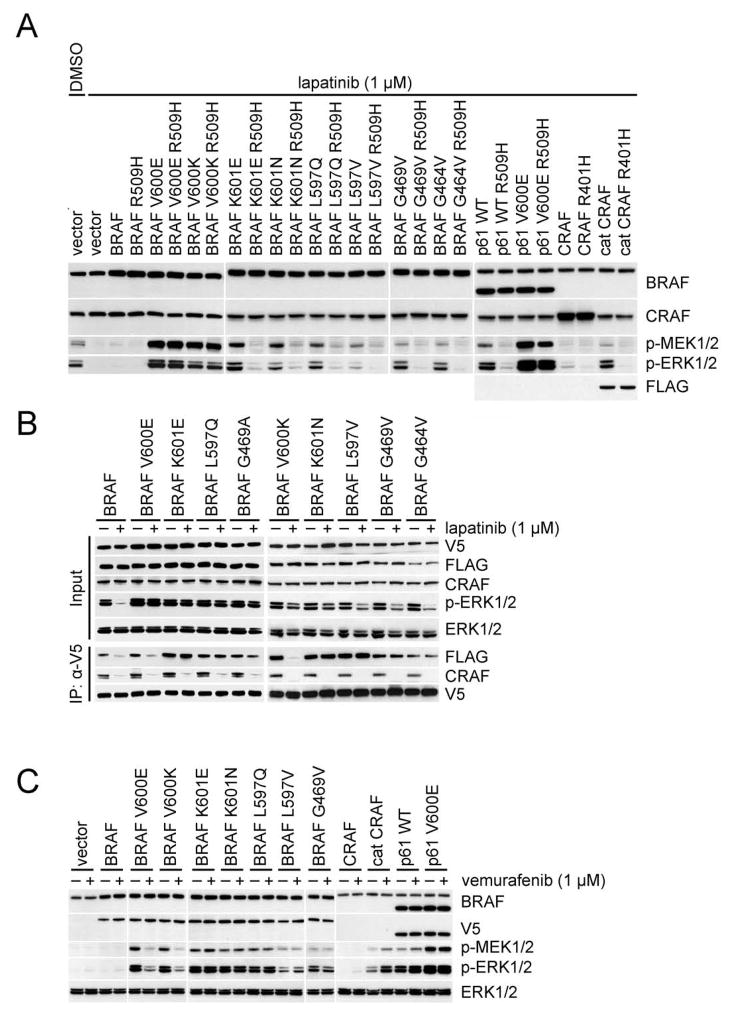
Previous data suggested that V600E BRAF can signal as a monomer and is thus RAS independent (Poulikakos et al., 2011). We asked whether this is the general mechanism that underlies the RAS-independence of activating RAF mutants. To address this question, we utilized the R509H and R401H/A mutations that impair dimerization of BRAF and CRAF, respectively, and eliminate their kinase activity (Poulikakos et al., 2010). R509H or R401H were introduced into BRAF or CRAF and multiple BRAF and CRAF mutants or truncations and expressed in SKBR3 cells. (Figure. 2A) ERK signaling in SKBR3 cells expressing these mutants was assessed after lapatinib treatment (low RAS-GTP state). ERK signaling was undetectable in cells when WT BRAF was overexpressed. Low levels of p-ERK were detected in cells overexpressing CRAF, but not in cells with CRAF 401H. The effects of R509H on ERK signaling were tested in 15 different activated BRAF mutants that were identified in human tumors (Figure. 2A; Table 1). ERK signaling driven by 11 of the 15 was reduced to very low levels. The 4 mutants unaffected by the R509H were all V600 mutants (V600E/K/D/R). In contrast, the activities of K601 mutants, including K601E, are abrogated by the R509H mutation.
Figure 2. Activated RAF proteins that signal as dimers are resistant to vemurafenib.
(A) SKBR3 cells were transfected with the indicated plasmids. After 24 hr, the cells were treated with 1 μM lapatinib for 1 hr. Cell lysates were then analyzed by Western blot.
(B) SKBR3 cells transiently co-expressing Flag-tagged and V5-tagged wild-type or mutant BRAF proteins were treated with lapatinib (1 μM, 1hr). BRAF dimerization was determined by immunoprecipitation.
(C) SKBR3 cells were transfected with the indicated plasmids. After 24 hr, the cells were treated with 1 μM lapatinib for 1 hr followed by 1 μM vemurafenib for 1 hr. The indicated endogenous or ectopic proteins were assayed by Western blot.
See also Figure S2.
The results suggest that, in cells with low RAS activity, BRAF V600 mutants signal as active monomers, whereas all other BRAF activating mutants signal as constitutive, RAS-independent dimers. We confirmed this conclusion in the RAS-deficient MEFs. BRAF V600 R509H mutants restore ERK signaling in these cells, R509H mutants of non-V600 BRAF mutants do not (Figure S2A).
We asked whether the non-V600 BRAF activating mutants function as BRAF homodimers or BRAF/CRAF heterodimers. When these mutants were expressed in Raf1, which encodes CRAF, knockout (KO) MEFs, they all potently activate ERK signaling and remain sensitive to R509H (Figure S2B). Thus, CRAF is not required for activation of ERK signaling by BRAF mutants that signal as constitutively active dimers. We evaluated the ability of these BRAF mutants to homodimerize and to heterodimerize with CRAF as a function of cellular RAS activity. We co-expressed V5- and FLAG-tagged BRAF mutants in SKBR3 cells, and assessed co-precipitation of V5 with either FLAG (mutant BRAF homodimers) or CRAF (mutant BRAF/WT CRAF heterodimers) (Figure. 2B). In control cells with adequate RAS-GTP levels, either wild type BRAF or BRAF mutants form both BRAF homodimers and BRAF/CRAF heterodimers. One hour after inhibition of RAS activity with lapatinib, BRAF/CRAF heterodimers were lost from all cells. BRAF homodimers were lost in cells overexpressing WT BRAF or BRAF V600 mutant alleles, but ERK phosphorylation was abrogated only in the former (Figure. 2B). Thus formation of BRAF V600E/K homodimers is RAS-dependent, but both BRAF V600E/K dimers and monomers can activate ERK signaling. In contrast, dimerization and activity of non-V600 BRAF mutants are RAS-independent (Figure. 2B; Table 1). We conclude that activating BRAF mutants evade feedback by either of two mechanisms: The dimerization of V600 mutants remains RAS-dependent, but their activity is not dependent on dimerization. Monomers of all of non-V600 BRAF activating mutants are inactive, and they all signal as active RAS-independent homodimers.
In cancer, RAF kinase is also activated by a variety of translocations and aberrant splice forms that encode fusion or truncated proteins in which an N-terminal domain containing the RAS-binding site is almost always deleted (Figure. S2C). Engineered deletion of this domain has been shown to result in constitutive dimerization and activation (Cutler et al., 1998), so it seems likely that these fusions are also activated in this way. Cat C, an engineered N-terminal deletion of CRAF, and p61 WT BRAF, an engineered N-terminal deletion of BRAF, are activated constitutive dimers whose activity is abrogated by the R401H and R509H mutants respectively (Figure. 2A). Similarly, ESRP1-CRAF and KIAA1549-BRAF are tumor-derived fusion proteins in which the N terminal domain of CRAF or BRAF has been replaced by the fusion partner (Figure. S2C). They also activate ERK signaling in a RAS-independent manner and their activity is abrogated by the R401A and R509H mutations respectively (Figure. S2D). Thus, fusions and truncated RAFs that lack the N-terminal domain are RAS independent kinases whose activity is dependent on their constitutive dimerization. A truncated p61 BRAF V600E was found to be responsible for the acquired resistance of some melanomas to RAF inhibitors. (Poulikakos et al., 2011) This truncated BRAF V600E is a RAS independent dimer, but its activity is not abrogated by R509H (Figure. 2A). The N-terminal truncation of BRAF V600E causes it to dimerize in a RAS-independent manner, but the V600E mutation causes it to be active as a monomer or dimer. Thus, with the singular exception of BRAF mutants with V600 missense mutations, so far all tested activating mutations, translocations, and fusions of RAF bypass ERK dependent feedback by constitutively dimerizing in a RAS-independent manner. Uniquely, the V600 mutants do so by functioning as active monomers when RAS-GTP is low.
RAF mutants that act as constitutive dimers are resistant to RAF inhibitors
The RAF inhibitor vemurafenib inhibits ERK signaling in tumors in which it is driven by BRAF V600E and not in those in which it is driven by RAS (Poulikakos et al., 2010). We used the SKBR3, low RAS-GTP (lapatinib treated) system to determine the sensitivity to vemurafenib of activating RAF mutants that signal as constitutive dimers. All such mutants were resistant, including non-V600 BRAF mutants, the truncated CRAF (Cat C) and BRAF (p61 WT) proteins (Figure. 2C; Table 1) and the two tested fusion proteins (Figure. S2D). Vemurafenib transactivates truncated WT dimers, whereas activated BRAF mutants that constitutively dimerize are resistant to the drug but not activated further (Figure. 2C). The only BRAF mutants that are sensitive to this drug are the four V600 mutants we tested (Figure. 2C; Table 1). Similar results were observed in Raf1 KO MEFs (Figure. S2E). So far there is an absolute correlation between the ability of a mutant to signal as a monomer and its sensitivity to vemurafenib.
Expression of BRAF V600E dimers causes acquired resistance to vemurafenib
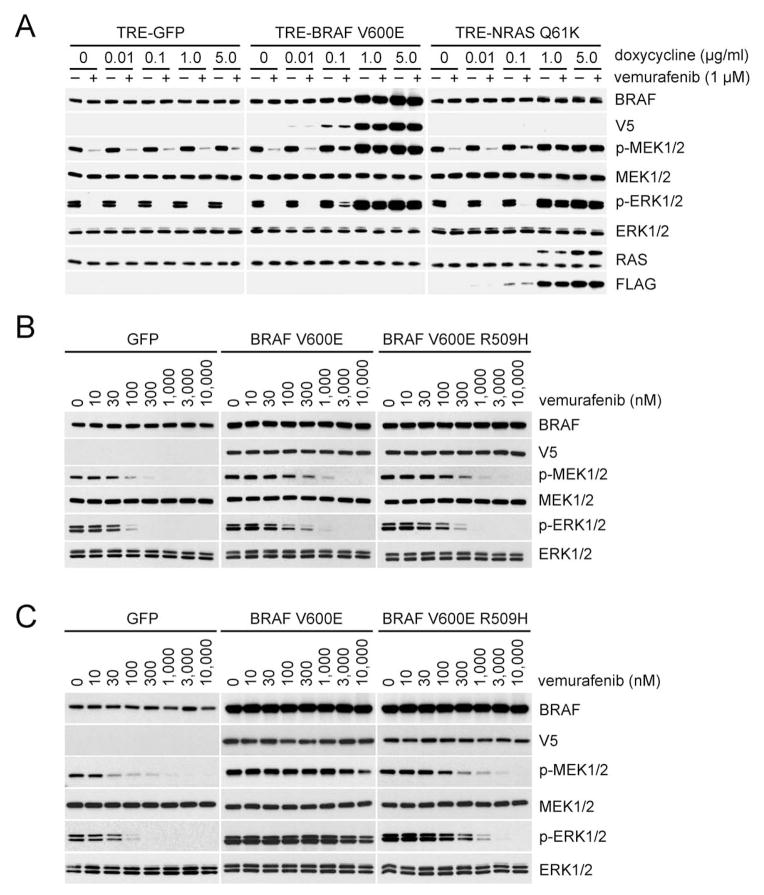
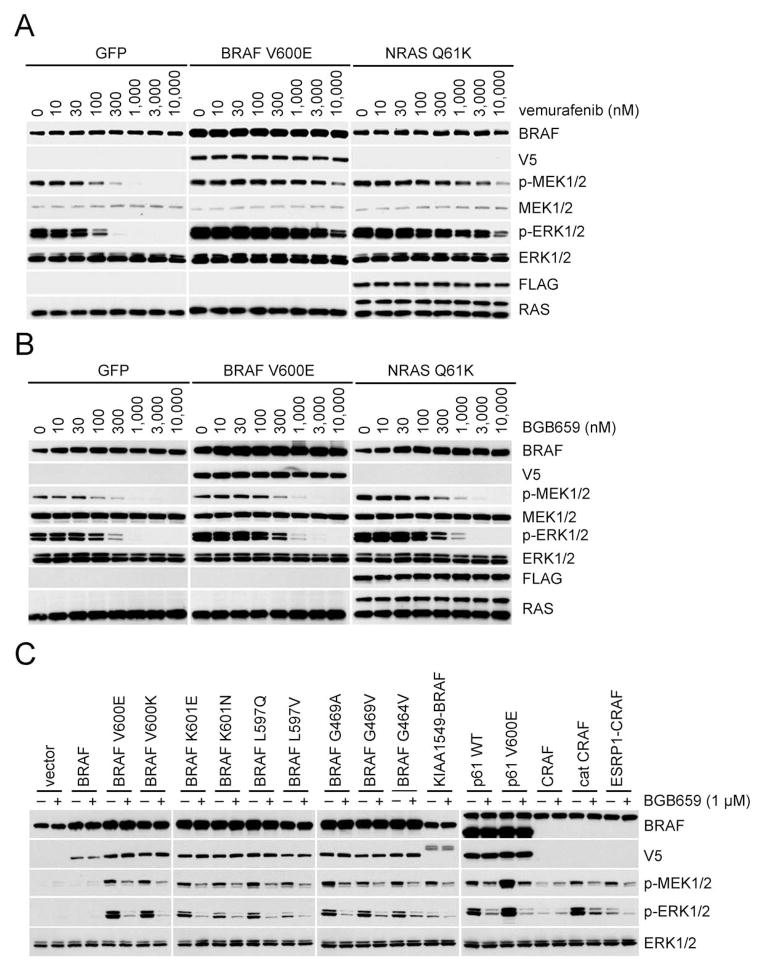
These results suggest that increased expression of activated RAF dimers will cause resistance to RAF inhibitors. Expression of p61 BRAF V600E or mutant NRAS and BRAF V600E amplification are common causes of acquired resistance to these drugs (Nazarian et al., 2010; Poulikakos et al., 2011; Shi et al., 2012). p61 BRAF V600E-dependent acquired resistance depends on its dimerization (Poulikakos et al., 2011) and mutant NRAS promotes the dimerization of BRAF V600E with CRAF (Figure. S3A). However, the mechanism whereby BRAF V600E amplification induces resistance remains unclear. We used A375, a melanoma cell line homozygous for BRAF V600E, to generate stable clones that expressed Tet-regulated BRAF V600E, BRAF V600E R509H or NRAS Q61K. Increased expression of either BRAF V600E, which is used to model BRAF V600E amplification, or mutant NRAS caused increasing resistance to inhibition of ERK signaling by vemurafenib (Figure. 3A). Expression of mutant NRAS was associated with an elevation of cellular RAS-GTP levels (data not shown) and induction of BRAF V600E-CRAF heterodimers (Figure. S3A. It is likely that induction of these RAS driven dimers is responsible for drug resistance).
Figure 3. Expression of BRAF V600E dimers causes resistance to vemurafenib.
(A) A375 cells expressing inducible GFP, V5-tagged BRAF V600E or FLAG-tagged NRAS Q61K were treated with the indicated concentrations of doxycycline for 24 hr, followed by treatment with vemurafenib (1 μM, 1 hr).
(B) and (C). A375 cells expressing inducible GFP, V5-tagged BRAF V600E, or V5-tagged BRAF V600E R509H were treated with either 0.02 μg/ml (B) or 0.2 μg/ml (C) doxycycline for 24 hr. Cells were then treated with vemurafenib at the indicated doses for 1 hr. Expression of the indicated proteins was assessed by Western blot.
See also Figure S3.
By contrast, in cells in which BRAF V600E was overexpressed, significant BRAF V600E homodimerization occurs. Increasing amount of plasmids encoding FLAG tagged BRAF V600E were transfected into A375 cells that expressed V5-tagged BRAF V600E. As shown in Figure. S3B, homodimers were detected when either tag was immunoprecipitated. Homodimer levels increased in direct proportion to the levels of expression of FLAG-tagged BRAF V600E. Under these conditions, neither V600E BRAF/CRAF heterodimerization nor induction of RAS-GTP occurs. These results suggest that BRAF V600E amplification causes acquired resistance to RAF inhibitors because it increases BRAF V600E homodimerization. To test this idea, we compared the effects of overexpressing BRAF V600E with those induced by overexpressing the dimerization impaired BRAF V600E R509H. As shown in Figure. 3B, cells expressing modest levels of BRAF V600E or BRAF V600E R509H are similarly sensitive to vemurafenib (IC50s ~100–300 nM). However, at higher levels of expression (Figure. 3C), cells with BRAF V600E were significantly less sensitive than those expressing the R509H mutant. (IC50> 10,000 nM vs ~300 nM) Thus, these data suggest that BRAF V600E amplification causes acquired resistance by increasing levels of BRAF V600E homodimers.
Binding of RAF inhibitors to one site in the dimer reduces their affinity for the other
Binding of inhibitors to CRAF or BRAF induces both RAF dimerization and the allosteric transactivation of the unbound protomer of the dimer (Hatzivassiliou et al., 2010; Poulikakos et al., 2010). However, neither mechanism explains why ATP-competitive inhibitors don’t bind to both sites and inhibit the dimer. Allosteric regulation of multimeric protein complexes is often associated with cooperative effects on ligand binding. We took advantage of the availability of systems in which the ability of drug to inhibit active monomeric or dimeric mutant BRAF can be compared to assess the possibility that drug binding to one protomer of the dimer reduces its affinity for the other. p61 BRAF V600E signals as a constitutive dimer, whereas p61 BRAF V600E R509H signals as a monomer (Poulikakos et al., 2011). p61 BRAF V600E and p61 BRAF V600E R509H were each expressed in SKBR3, and the effects of RAF inhibitors on ERK signaling were assessed in these cells under low RAS conditions. Vemurafenib inhibits ERK signaling in SKBR3 cells expressing p61 BRAF V600E R509H or BRAF V600E at similar concentrations (IC50 ~ 100–300 nM) (Figure. S4A). We take this as the concentration required to inhibit the monomer. Binding of drug to one protomer of truncated WT dimers causes transactivation of the other. We find that the 50% induction of p61 WT BRAF-driven ERK signaling by vemurafenib is also approximately 100–300 nM (Figure. S4B and S4C). This is thus the concentration required to bind to monomers and to the first site of the dimer.
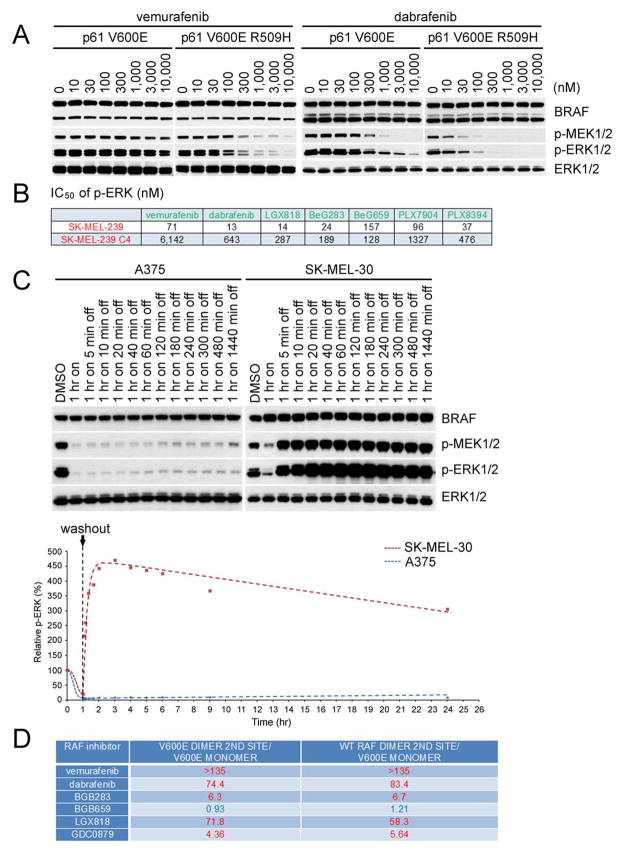
By contrast, more than 30-fold higher concentrations of vemurafenib were required to inhibit ERK driven by p61 V600E dimers than by p61 V600E R509H monomers (Figure. 4A) The same relative difference was observed with another, more potent, RAF inhibitor dabrafenib (Figure 4A). We take the concentration required to inhibit ERK signaling in the p61 BRAF V600E cells as the concentration required inhibiting both sites in the dimer. These data therefore imply that the relative affinity of vemurafenib for the first binding site in a BRAF homodimer is 30-fold higher than that for the second site when the first site is occupied by drug. In isogenic p61 BRAF experiments, the RAF mutants were expressed in a heterologous cellular system—SKBR3. To interrogate a tumor system in which activated BRAF V600E mutant monomers or dimers are expressed we used SK-MEL239, a BRAF V600E melanoma cell line, and SK-MEL239 C4, a vemurafenib resistant clone of SK-MEL239 (Poulikakos et al., 2011) that expresses p61 V600E dimers (Figure. S4D). 30-fold higher concentrations of dabrafenib were required to inhibit ERK signaling in the latter compared to the former (Figure. 4B). The data suggests that binding of the drug to the first site in the dimer reduces its affinity for the second and that this explains why monomer-driven ERK signaling is so much more sensitive to these drugs than that driven by dimers.
Figure 4. Identification of an equipotent inhibiter of mutant BRAF monomers and dimers.
(A) SKBR3 cells expressing p61 V600E or p61 V600E R509H were treated with increasing concentrations of vemurafenib or dabrafenib for 1 hr. Expression of phosphorylated MEK and phosphorylated ERK were assessed by Western blot.
(B) IC50s of p-ERK inhibition for a panel of compounds in SK-MEL-239 parental cells (BRAF V600E) or the C4 clone (which expresses p61 BRAF V600E) were calculated based on densitometry analysis of Western blot results (n=3) as shown in Figure S4D. Mean values are listed in the table.
(C) A375 and SK-MEL-30 cells were treated with DMSO or 3 μM LGX818 for 1hr. Cells were then washed 3 times with PBS and then placed in drug free media for the indicated times. p-ERK was assessed by Western blot (top) and quantitated by densitometry to generate the dose response curves using Prism6 (bottom); p-ERK levels relative to those from DMSO-treated cells as a function of time post treatment are shown in the accompanying graph.
(D) A375, SK-MEL-239 C4 and SK-MEL-30 cells were treated with 1 μM LGX818 for 1hr followed by drug washout and treatment with the indicated compounds for an additional 1hr. The indicated fold changes of p-ERK IC50 values were calculated based on the curves as shown in Figure S4H–d.
Identification of a compound that inhibits RAF monomers and dimers at similar concentrations
To identify compounds that inhibit monomer and dimers with similar potency, we screened 22 known RAF inhibitors against SK-MEL-239 and SK-MEL-239 C4. Results for six of these compounds are shown in Figure. S4D and their chemical structures are shown in Table S1. The concentrations required for five of these six to inhibit ERK signaling driven by the dimer were significantly higher (5 to 85-fold) than those that inhibit monomer driven signaling (Figure 4B and Figure. S4D). LGX818 inhibited monomer driven signaling at 14 nM, and dimer signaling at 287 nM, despite its high potency and low off-rate (see below.). By contrast, BGB659, a type II, ATP-competitive RAF inhibitor (compound 27 from Gould et al., 2011) inhibited ERK signaling driven by p61 BRAF V600E dimers and BRAF V600E monomers at similar doses (Figure 4B and Figure. S4D). Its inhibition of ERK signaling is mediated by its binding to BRAF; the T529 BRAF gatekeeper mutation (Heidorn et al., 2010) confers resistance to the drug (Figure. S4E). In cells that express either BRAF V600E T529N or p61 BRAF V600E T529N, ERK signaling is resistant to both vemurafenib and BGB659 but not to the MEK inhibitor trametinib.
BGB659 could inhibit BRAF V600E dimers by a variety of mechanisms. We show below (Figure. 6B) that, like most RAF inhibitors, BGB659 induces RAF dimerization, so it is not a ‘dimer breaker’. We have not been able to recreate the resistance of RAF dimers to inhibitors in defined in vitro systems. We therefore developed a cellular system in which only one site of the RAF dimer is occupied by a RAF inhibitor and used it to determine the concentration of drug required to bind to and inhibit the second site when the first is already bound. The RAF inhibitor LGX818, which has a very slow off-rate, was utilized. After 1 hr exposure of BRAF V600E tumor cells to vemurafenib or LGX818, ERK signaling is inhibited (Figure. S4F). 1 hr after washout of the drug, ERK phosphorylation returned to pre-exposure levels in the vemurafenib treated cells, but remains inhibited in the LGX818 treated cells. Thus, after 1 hr of wash out, the BRAF V600E monomer remains bound to LGX818.
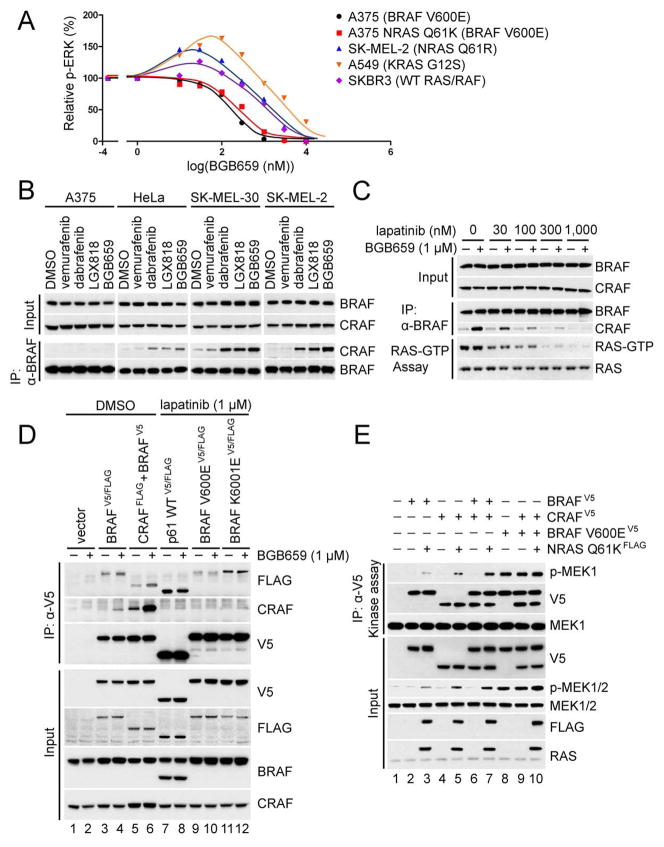
Figure 6. BGB659 preferentially inhibits signaling driven by mutant BRAF dimers.
(A) The indicated cell lines were treated with 0,10,30,100, 300, 1000, 3000 and 10,000 nM BGB659 for 1 hr. Whole cell lysates were assayed by Western blot with anti-p-ERK antibody. The p-ERK level of each sample was quantitated by densitometry and then normalized to the p-ERK level in untreated cells. The p-ERK response curves were generated using Prism6.
(B) A375, HeLa, SK-MEL-30 and SK-MEL-2 cells were treated with the indicated compounds for 1 hr. Endogenous BRAF was immunoprecipitated with anti-BRAF antibody. The input and isolated protein complexes were assayed by Western blot as indicated.
(C) SKBR3 cells were pre-treated with lapatinib at the indicated concentrations for 1 hr followed by treatment with 1 μM BGB659 or vehicle. Cell lysates were then subjected to immunoprecipitation with anti-BRAF antibody or the RAS-GTP pull-down assay. Binding of CRAF to BRAF and RAS-GTP levels were determined by Western blot. Expression levels of BRAF and CRAF were determined using whole cell lysates.
(D) The indicated RAF proteins were expressed in SKBR3 cells for 24 hr. Cells were then treated with either 1 μM lapatinib or the equivalent volume of DMSO for 1 hr, followed by treatment with 1 μM BGB659 or DMSO for an additional 1 hr. All cell lysates were collected and subjected to immunoprecipitation with an anti-V5 antibody. The isolated protein complexes and input were analyzed by Western blot.
(E) BRAF-V5, CRAF-V5, BRAF V600E-V5 and FLAG tagged NRAS Q61K were transiently expressed in SKBR3 cells followed by 1hr treatment with 1 μM lapatinib. The cells were then collected and lysed, and the ectopically expressed RAF proteins were isolated with anti-V5 beads followed by elution with V5 peptide. The kinase activity of isolated RAF kinases was determined by in vitro kinase assay with K97R MEK1 as substrate. The indicated proteins from both input and kinase assays were assayed by Western blot.
See also Figure S6.
SK-MEL-30 is a cell line with mutant NRAS and WT RAF. As expected, vemurafenib activates ERK signaling in these cells by binding to one protomer of WT RAF dimers and transactivating the other. (Figure. S4G) 1 hr after washout of vemurafenib, p-ERK returned to baseline, likely due to dissociation of drug from the first site. LGX818 also induced p-ERK at low concentrations, but, in contrast to Vemuraftenib, induction persisted after 1 hr of washout of the drug, consistent with persistent binding to the first site due to its low off-rate (Figure. S4G). Peak induction of ERK occurred at 100 nM LGX818 and actually increased 1 hr after the drug was washed out. At higher concentrations (300–3000 nM), LGX818 caused a concentration-dependent inhibition of ERK signaling. This is consistent with its binding to and inhibiting both sites in the dimer. At these concentrations, wash out for 1 hr hyperactivates ERK signaling compared to untreated controls. This result suggests that the dissociation of drug from the second site is more rapid than that from the first site. The hyper-activation after drug wash out is consistent with dissociation of drug from the second site, resulting in accumulation of the half-bound, transactivated RAF dimer.
This was confirmed when A375 (BRAF V600E) and SK-MEL-30 (NRAS Q61K) cells were treated with 3 μM LGX818 for 1 hr followed by washout of drug and incubation in normal media. (Figure. 4C) p-ERK was potently inhibited in both cell lines. After wash out, p-ERK remained inhibited in A375 for up to 24 hr, with a slight increase at that time, consistent with the very slow off rate from the BRAF V600E monomer. After drug is withdrawn from SK-MEL-30, which contains WT RAF dimers, p-ERK rises rapidly, reaching a maximum approximately 1 hr after washout with a half-time of 10 minutes. This is consistent with a rapid off-rate of drug bound to the second site in the dimer. In these cells, the maximum p-ERK is about five times higher than basal. After reaching this peak, p-ERK falls slowly, consistent with the slow off-rate of this drug from the first site in the dimer and is still three fold elevated compared to basal 24 hr after washout (Figure. 4C).
Thus, after exposure of cells to high concentrations of LGX818, washout of the drug for 1hr leads to accumulation of activated half-bound dimers. We reasoned that the concentration of other RAF inhibitors that is required to inhibit ERK signaling in these cells 1 hr after LGX818 washout reflects the relative affinity of the drug for the second (unoccupied) site. We used this system to determine the concentrations of six RAF inhibitors that is required to bind the second site of the dimer when the first is occupied by drug. For each inhibitor, this value was compared with the concentration at which it inhibits BRAF V600E monomers. 5 of the 6 compounds inhibited ERK phosphorylation in A375 BRAF V600E melanoma cells at concentrations ranging for 10–100 nM (Figure. S4H–a). This is the concentration required to inhibit the monomer (or the first site in the dimer.) To determine the concentration of these drugs required inhibit the second site when the first is bound to drug, SK-MEL-30 NRAS mutant cells were exposed to 1 μM LGX818 for 1 hr after which it was washed out for 1 hr. Other RAF inhibitors were then added for 1 hr to assess the concentration at which they inhibit ERK phosphorylation. All the drugs were able to inhibit pERK, but, for 5 of the 6, at concentrations much greater than those at which they inhibit the monomer (0.3–10 μM) (Figure. S4H–b vs Figure. S4H–a). Only BGB659 inhibited the monomer and the second site of the WT RAF dimer at approximately the same concentrations. Very similar results were obtained when the same experiment was done in SK-MEL-239 C4 with BRAF V600E dimers (p61 BRAF V600E) (Figure. S4H–c). These results are shown graphically (Figure. S4H–d) and as ratios of concentrations required to inhibit the second site compared to those that inhibit the monomer (Figure. 4D).
These data suggest that binding of most RAF inhibitors to one site in WT or mutant RAF dimers substantially reduces their affinity for the second site and that this accounts for the resistance of dimer driven ERK-signaling to these drugs. However, the binding of BGB659 is unaffected by occupancy of the first site and it inhibits monomers and the second site of dimers with similar potency (100–300 nM). Our model suggests that such a drug would be effective in treating tumors in which oncogenic ERK signaling is driven by RAF dimers.
BGB659 effectively inhibits ERK signaling driven by oncogenic BRAF dimers in tumor cells
In A375, expression of mutant NRAS or overexpression of BRAF V600E caused ERK signaling to become much less sensitive to RAF monomer selective inhibitors (Figure. 5A and S5A). By contrast, the concentration at which BGB659 inhibits ERK signaling was affected only marginally by BRAF V600E overexpression or mutant NRAS expression (Figure. 5B) and it inhibits ERK signaling in BRAF V600E melanoma SK-MEL-239 and its resistant counterpart SK-MEL-239 C4 at similar concentrations (Figure. 4B). The effects of these drugs on cell proliferation are closely correlated with their effects on ERK signaling. The concentrations of BGB659 that cause inhibition of the proliferation of A375, A375 expressing mutant NRAS or A375 overexpressing BRAF V600E are very similar. (Figure. S5B) Significantly higher concentrations of the other tested inhibitors were required to inhibit the proliferation of melanoma cells with any of the three mechanisms of dimer-dependent resistance. (Figure. S5B and S5C) Thus, BGB659 effectively inhibits the growth of melanoma cells in which acquired resistance to vemurafenib is driven by truncated or full length BRAF V600E dimers.
Figure 5. BGB659 effectively inhibits vemurafenib-resistant ERK signaling.
(A, B). A375 cells expressing inducible GFP(control), BRAF V600E or NRAS Q61K were treated with doxycycline (2 μg/ml, 24 hr) followed by treatment with vemurafenib (A) or BGB659 (B) at the indicated concentrations. Cell lysates were then analyzed by Western blot using the antibodies indicated.
(C) SKBR3 cells were transfected with plasmids encoding the indicated wild type or mutant proteins. After 24 hr, cells were treated with BGB659 (1 μM, 1 hr). Expression and/or phosphorylation of the indicated proteins were assayed by Western blot
See also Figure S5.
BGB659 also inhibited ERK signaling driven by RAF mutants that constitutively dimerize. All such mutants are resistant to inhibition by 1 μM vemurafenib (Figure 2C and Table 1) but, sensitive to 1 μM BGB659, (seven of eleven mutants tested are shown in Figure. 5C). BGB659 also inhibits ERK signaling driven by activated RAF fusion proteins (KIA1549-BRAF and ESRP1-CRAF) and truncations (p61 or Cat C) that constitutively dimerize (Figure. 5C). BGB659 effectively inhibits ERK signaling and the proliferation of JVM-3, a BRAF K601N CLL cell line that is resistant to vemurafenib (Figure. S5D and S5E). Thus, BGB659 inhibits ERK signaling driven by both oncogenic RAF monomers and dimers and inhibits the proliferation of tumor cells harboring these RAF mutants.
BGB659 preferentially inhibits mutant RAF-driven ERK signaling
An inhibitor of mutant and WT RAF dimers would inhibit ERK in normal cells and would have a narrow therapeutic index, as do MEK inhibitors. We compared the effects of BGB659 on signaling driven by WT dimers and mutant RAF dimers in NIH3T3 cells in which a variety of RAS or RAF were expressed in an inducible manner. The IC50s for BGB659 inhibition of ERK signaling driven by BRAF V600E monomers or three different mutant BRAF constitutive dimers were very similar and more than an order of magnitude lower than those required to inhibit signaling in cells in which WT BRAF or NRAS or NRAS Q61K were overexpressed (Figure. S6A). The data suggest that BGB659 does not inhibit all RAF dimers equally; BRAF mutant monomers and dimers are more sensitive than RAS driven WT dimers. In contrast, after 1 hr of exposure, the MEK inhibitor, trametinib inhibits ERK phosphorylation at similar doses whether it is driven by WT RAF or mutant BRAF (Figure. S6B)
This was also the case in tumor cell lines in which ERK is activated by different upstream mechanisms (Figure 6A), including mutant BRAF V600E (A375 melanoma), A375 in which NRAS Q61K is expressed, mutant NRAS Q61R (SK-MEL-2 melanoma), mutant KRAS G12S (A549 lung cancer), and HER2-activated wild type RAS (SKBR3 breast cancer). BGB659 inhibits ERK signaling at almost identical concentrations in A375 and in A375 in which mutant NRAS has been overexpressed (IC50s ~ 100–300 nM, almost complete inhibition at 1 μM). In contrast, in the other three cell lines, ERK phosphorylation increased after exposure to relatively low doses of the drug, with maxima occurring at 50–100 nM and declined at higher concentrations. In SKBR3 and SK-MEL-2, ERK phosphorylation declined 40% with 1 μM BGB659 and only approached complete inhibition at 10 μM. In A549 mutant KRAS cells, ERK declined to pretreatment levels at 1 μM drug and some residual activity remained at 10 μM. Thus, tumor cells with mutant RAF, with or without coexistent RAS mutation are most sensitive to BGB659. In cells in which ERK is driven by WT RAF (SK-MEL-2, A549, SKBR3), the effect of the drug varied with concentration in a biphasic manner, with ERK phosphorylation enhanced at low doses and inhibited at higher doses. The effects of BGB659 on tumor cell proliferation correlated with its effects on signaling.(Figure S6C) All 15 tumor cell lines with mutant BRAF were sensitive to the drug (9 with BRAF V600E, one with BRAF V600K, four with RAF mutants that constitutively dimerize, and one with p61 BRAF V600E) with IC50s from 100 nM to 600 nM. By contrast, the ten tumor cell lines with RAS mutation and fourteen with WT RAS and RAF were 10- to more than 50-fold less sensitive than the mutant RAF tumors.
A biphasic dose response is also observed when normal or tumor cells with activated RAS are treated with previously reported RAF inhibitors (Poulikakos et al., 2010). This phenomenon has been shown to result from both induction of RAF dimerization by the drug and its transactivation of RAF dimers (Hatzivassiliou et al., 2010; Lavoie et al., 2013; Poulikakos et al., 2010). BGB659 inhibits the second site of activated RAF dimers in cells with mutant NRAS with the same potency as it inhibits BRAF V600E monomers (Figure. S4H–a and -b). We therefore asked whether its differential effects on mutant and WT RAF dimers are due to differential induction of dimerization. BGB659 and other RAF inhibitors caused marked, dose-dependent induction of BRAF/CRAF heterodimers in HeLa and in two melanoma cell lines with mutant NRAS, but not in A375 (BRAF V600E) (Figure 6B). Induction by BGB659, and the RAF monomer inhibitors LGX 818 and dabrafenib was equivalent and much greater than that caused by vemurafenib (Figure. 6B). Since physiologic dimerization of RAF is RAS dependent and RAS-GTP levels are inhibited by feedback in A375, we asked whether induction of dimerization was RAS dependent. In SKBR3 cells, RAS-GTP levels and induction of BRAF/CRAF heterodimers by BGB659 were both inhibited in a dose dependent fashion by lapatinib and correlated closely with each other (Figure 6C.) These data suggest that induction of dimerization is RAS dependent and does not occur in cells with activating RAF mutations because their RAS-GTP levels are low.
In support of this model, we examined the ability of BGB659 to induce dimerization of WT RAF in cells with active RAS-GTP (Figure 6D lanes 1–6). In SKBR3, BGB659 induces WT BRAF homodimers and BRAF/CRAF heterodimers (Figure 6D lane 4 vs 3, 6 vs 5). Thus, in cells in which WT RAFs are activated by active RAS, BGB659 both induces the formation of active RAF dimers and inhibits their activity.
By contrast, in cells that express activated RAF mutants, RAS is feedback inhibited and ERK signaling is RAS-independent. Accordingly, we assessed the effects of BGB659 on RAF dimerization in lapatinib treated SKBR3 cells engineered to express different RAF mutants. (Figure 6D, lanes7–12) In BRAF mutant expressing cells, no mutant BRAF/WT CRAF heterodimers are detected. Significant levels of p61 BRAF (Figure 6D lanes 7 and 8) and BRAF K601E (Figure 6D lanes 11 and 12) homodimers are expressed in cells in which either mutant is expressed. In cells in which BRAF V600E is expressed, a low level of mutant homodimers is observed, (Figure 6D lanes 9 and 10). BGB659 did not induce mutant RAF homo- or heterodimerization in any of these cells. Thus, BGB659 induces RAS-dependent dimerization of WT RAF, but, in tumors with mutant RAF, RAS-GTP levels are too low to support induction of dimerization. Taken together, these data support the following model. BGB659 inhibits both sites of RAF dimers and also induces RAS-dependent RAF dimerization. In cells with adequate levels of RAS activation, this accounts for the biphasic response to increasing concentrations of BGB659. In contrast, in cells with activating RAF mutants, RAS-GTP is low and the drug inhibits the activity of RAF dimers without inducing their formation. Tumors with RAF mutants are thus more sensitive to this drug than those with mutant RAS or normal cells.
The exceptional case that complicates this model are BRAF V600E tumors with acquired resistance due to mutant NRAS. These tumors are as sensitive to BGB659 as those with BRAF V600E alone. We asked whether BGB659 affects RAF dimerization in cells with coexpression of BRAF V600E and mutant NRAS (Figure. S6D). BRAF homodimers and BRAF/CRAF heterodimers are barely detectable in SKBR3 treated with lapatinib. Co-expression of mutant NRAS with WT RAF significantly increases levels of homo- and heterodimers and BGB659 further enhances expression of these dimers (Figure. S6D lanes 2–4). When BRAF V600E and WT CRAF are overexpressed in SKBR3, BRAF V600E homodimers and heterodimers were barely detectable. These dimers were markedly enhanced when mutant NRAS was coexpressed and further enhanced by BGB659 (Figure. S6D lanes 6–8). Thus, the drug can induce BRAF V600E dimerization in tumors that co-express mutant RAS. However, in this case, induction of BRAF V600E dimerization is not associated with a significant induction of RAF kinase activity (Figure. 6E). Coexpression of mutant NRAS induces BRAF V600E dimerization (Figure. S6D) it has almost no effect on elevated RAF kinase activity (Figure. 6E lanes 8–10). This is consistent with our data that RAF inhibitors do not paradoxically activate p-ERK in such tumors. By contrast, co-expression of WT BRAF or CRAF with mutant NRAS is associated with increase RAF kinase activity (Figure 6E lanes 2–6). Thus, BGB659 effectively inhibits ERK signaling in tumors that coexpress BRAF V600E and mutant RAS because in these cells, it induces BRAF V600E dimerization, but not kinase activation. BGB659 works less well in cells with active RAS and WT RAF because it increases RAF kinase activity by inducing dimerization.
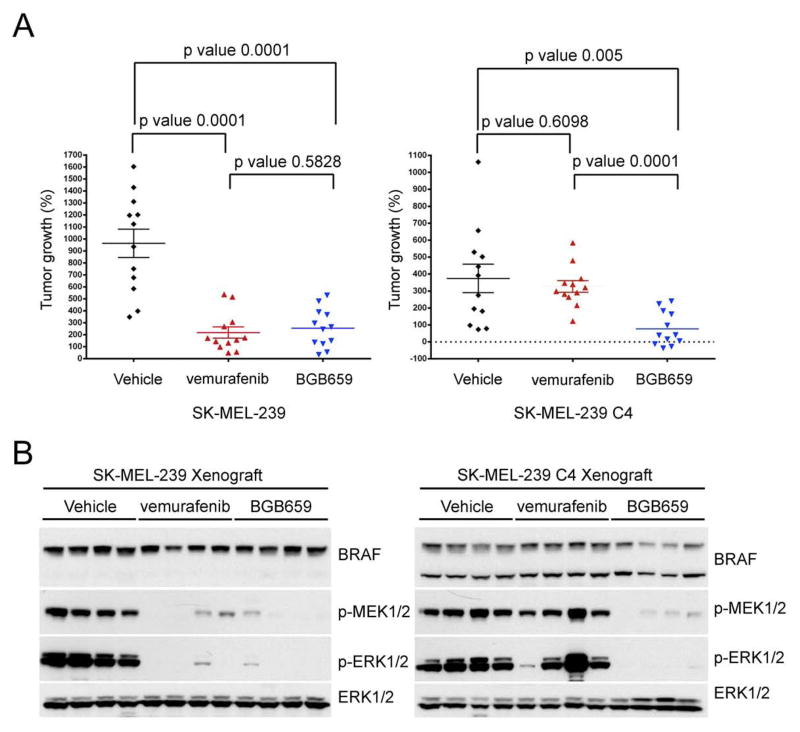
BGB659 inhibits the in vivo growth of tumors driven by mutant RAF monomer or dimers
Figure 6 and S6 suggest that BGB659 will inhibit ERK signaling in tumors driven by activating RAF mutants and fusion proteins more effectively than in normal cells, and could therefore be useful clinically. We tested whether BGB659 could inhibit the in vivo growth of SK-MEL-239 C4, cells in which acquired resistance to vemurafenib is mediated by the p61 BRAF V600E dimer. This model and the vemurafenib-sensitive parental SK-MEL-239 cell line were grown as subcutaneous murine xenografts. 100 mg/kg BGB659 given daily was unassociated with weight loss or other obvious toxicity. Mice carrying 100 mm3 tumors were treated daily for 32 days with vehicle, 75 mg/kg vemurafenib or 100 mg/kg BGB659 and then analyzed. Vemurafenib and BGB659 both effectively inhibited the growth of SK-MEL-239 tumors, but only the latter had activity against SK-MEL-239 C4 tumors (Figure 7A). Consistently, vemurafenib and BGB659 both potently inhibited ERK signaling in SK-MEL-239 tumors but only BGB659 inhibited ERK signaling in SK-MEL-239 C4 tumors (Figure 7B). These results support the possibility that drugs of this class could be effective in tumors driven by either mutant RAF monomers or dimers, including those that mediate acquired resistance to current RAF inhibitors.
Figure 7. BGB659 inhibits the in vivo growth of BRAF V600E tumors with acquired resistance to vemurafenib.
(A) and (B) SK-MEL-239 parental or C4 clone (p61 V600E) cells were injected subcutaneously into the opposite flanks of nude mice (1.5 ×107 cells per injection). After 10 days, all 12 tumors from each group were 100–150 mm3 in size and the indicated drug treatments were started The graph shows the size of each tumor after 32 days of daily treatment (A, n=12, Error bars: mean ± SEM, p values were calculated using the unpaired t test). Protein extracts from 4 random selected tumors from each group were analyzed by Western blot using the antibodies indicated (B).
Discussion
Almost two hundred BRAF mutants and many RAF translocations have been identified in human cancer and many of those have increased catalytic activity (Wan et al., 2004; Palanisamy et al., 2010). Our work divides the activating mutants into two functional classes based on the mechanism whereby they achieve RAS-independence. The dimerization of BRAF V600 mutants remains RAS dependent, but they also signal as RAS-independent monomers in tumor cells in which RAS is inhibited by feedback. All of the other tested RAF mutants and fusion proteins signal as RAS-independent constitutive dimers. These data support the idea that insensitivity to physiologic feedback is a common property of oncoproteins that is required for their hyperactivation of signaling output.
None of the RAF mutants that signal as constitutive dimers are sensitive to previously reported RAF inhibitor vemurafenib. They only inhibit active RAF monomers, which comprise all four of the BRAF V600 mutant alleles found in patients. These mutants are capable of signaling as monomers or RAS-dependent dimers; the feedback inhibition of RAS by ERK causes BRAF V600 mutants to exist as drug sensitive monomers in these tumors. Moreover, the three most common causes of acquired resistance of BRAF V600E melanomas to RAF inhibitors—NRAS mutation, splicing of BRAF V600E that produce a truncated BRAF kinase, and BRAF V600E overexpression due to gene amplification—all cause resistance by causing dimerization of BRAF V600E.
The mechanisms underlying the characteristic effects of RAF inhibitors, inhibition of BRAF V600E monomers, resistance of RAF dimers, and activation of ERK signaling in cells with active RAS and WT RAF remain controversial. The designation of these drugs as selective inhibitors of BRAF persists in the medical literature despite evidence to the contrary. They have been shown to inhibit the kinase activities of all three RAF family members in vitro and to activate ERK signaling in BRAF knockout cells with active RAS (Poulikakos et al., 2010). We show here that BRAF mutants that constitutively dimerize are resistant to these drugs as are V600E dimers in cells with coexistent NRAS mutation or overexpressing V600E. These drugs would be better described as inhibitors of BRAF monomers. However why these drugs are poor inhibitors of dimers is poorly understood. Binding of RAF inhibitors to one protomer in a WT CRAF dimer causes the allosteric transactivation of the unbound protomer. Most inhibitors also cause RAF to dimerize. Both of these effects play a role in transactivation, but cannot, by themselves, explain paradoxical activation of WT dimers or the insensitivity of mutant dimers, since, even if binding of the inhibitor to one protomer causes the other to adapt an active conformation, the drug ought to bind to the second site in the dimer with similar potency.
The obvious hypothesis is that binding of the drug to one site in the dimer causes an allosteric effect that reduces its affinity for the second site. We now provide evidence that this is the case. In isogenic models the concentrations of RAF inhibitor required to inhibit the monomer were much lower than those required to inhibit the dimer. We take these findings as reflecting the difference in concentration required to inhibit the first and second sites of the dimer and therefore demonstrating that negative cooperativity is induced on occupancy of the first site by the drug. This idea was supported by experiments in which the off-rate of a RAF inhibitor from the first and second sites of an active WT dimer were measured in cells and the latter was found to be considerable faster than the former. This system was used to measure the concentrations at which several RAF inhibitors inhibit the second site when the first was occupied by the low off-rate drug. In all cases, the concentration at which the standard RAF inhibitors block signaling driven by the second site is considerably higher than the concentration required for them to inhibit BRAF V600E monomers. Taken together, these data suggest that significantly higher concentrations of RAF inhibitors are required to inhibit dimers than monomers because occupancy of the first site in the dimer by the drug reduces its affinity for the second site.
Our data suggest that an inhibitor of RAF dimers could be useful for the treatment for many types of ERK dependent tumor. We identified BGB659 that inhibits BRAF dimers at about the same concentration as monomers. Moreover, binding of BGB659 to RAF dimers is unaffected by induction of negative cooperativity by drug occupancy of the first site. As predicted, BGB659 inhibits ERK signaling driven by both active monomers and constitutively activated dimers. It also inhibits ERK signaling in models in which acquired resistance to standard RAF inhibitors is mediated by mutant BRAF dimers. However, because it also induces the formation of activated WT RAF dimers in cells with active RAS, it is a much less potent inhibitor of ERK signaling in normal cells and in tumors with RAS mutation.
This work has several important clinical implications. First, it suggests that current RAF inhibitors will be effective in tumors driven by BRAF V600 mutants and not in those driven by any of the other, constitutively dimerizing, BRAF mutants or translocations. This must be a tentative conclusion since we do not understand the structural basis whereby all of these mutants (including K601E) form constitutive RAS-independent dimers and only BRAF V600 mutants can signal as either dimers or monomers. Crystal structures have not shed light on this question, perhaps because they all lack the amino terminal portion of RAF proteins that is critical in regulating dimerization. We are therefore engaged in characterizing the RAS- and dimerization dependence of all mutant RAF alleles found in tumors and determining their sensitivity to inhibitors. Second, we describe herein an algorithm for assessing the mechanism of action and drug sensitivity of uncharacterized or newly identified mutations and translocations. Third, BGB659 inhibits ERK signaling driven by mutant RAF monomers and dimers at doses at which it does not inhibit signaling in normal cells. These data suggest that this type of drug will have a wide therapeutic index and could be effective in tumors in which current RAF inhibitors are inactive--tumors driven by non-V600E BRAF mutants, activating RAF dimers encoded by gene translocations, and BRAF V600E tumors in which acquired resistance to RAF inhibitor is due to dimerization. It is also possible that such a drug will be superior to currently available RAF inhibitors as an initial treatment to the tumors driven by BRAF V600E, since they will not be subject to many of the most common mechanisms of acquired resistance to those drugs. With the widespread sequencing of human tumor tissue many such fusion and non-BRAF V600 activating mutants are being discovered and such drugs ought to have wide clinical utility.
Experimental Procedures
Compounds
BGB283 and BGB659 were obtained from Beigene. vemurafenib, PLX7904, PLX8394 from Plexxikon. lapatinib, trametinib, dabrafenib from GlaxoSmithKline. LGX818 is from Novartis. LY3009120 purchased from Active Biochem, Doxycycline from Sigma Aldrich, Puromycin, Hygromycin stock solution from Invitrogene., other drugs from Selleckchem. Drugs were dissolved in DMSO to yield 10mM stock and stored at −20°C.
Cell culture
All cell lines were obtained from either MSKCC cell collection or American Type Culture Collection except the conditional RAS knockout cell line which was provided by Mariano Barbacid. 22RV1, H1395, OCI/AML3, U266, JVM-3 and SIG-M5 were cultured in RPMI+10%FBS. Keratinocytes were maintained in the Defined K-SFM medium from Gibco. All other cell lines were grown in DMEM medium with glutamine, antibiotics and 10%FBS. The inducible expression cells were maintained in the medium with 50 μg/ml Hygromycin and 0.2 μg/ml Puromycin.
Antibodies
Western blot, immunoprecipitation and in vitro kinase assays were performed as described (Poulikakos et al., 2011). The following antibodies were used: anti-p217/p221-MEK1/2 (p-MEK1/2), anti-p202/p204-ERK1/2 (p-ERK1/2), anti-MEK1/2, anti-ERK1/2 from Cell Signaling, anti-V5 from Invitrogen, anti-BRAF from Santa Cruz Biotechnology, anti-FLAG from Sigma, anti-CRAF from BD Transduction Laboratories, anti-CRAF-S338 from Millipore. For immunoprecipitations of tagged proteins, the following reagents were used: anti-V5 agarose affinity gel (Invitrogen), anti-Flag M2 affinity gel (Sigma), protein G agarose gel (Invitrogene).
Plasmids
The pcDNA3-BRAF-V5/FLAG/myc, pcDNA3-CRAF-V5/FLAG/myc, pcDNA3-catC-FLAG and pcDNA3-p61/p61 R509H were constructed as previously described (Poulikakos et al., 2011; Poulikakos et al., 2010). The ESRP1-RAF1 and KIAA1549-BRAF fusion genes were sub-cloned into pcDNA3 with FLAG or V5 tag. Plasmids for retroviral based inducible expression system were provided by Scott Lowe lab in MSKCC. The BRAF and NRAS genes were sub-cloned into TTIGFP-MLUEX vector harboring tet-regulated promoter. Mutations were introduced by using the site-directed Mutagenesis Kit (Stratagene).
Animal model studies
Nu/nu athymic mice were obtained from Harlan Laboratories and maintained in compliance with IACUC guidelines. Subcutaneous xenografts and tumor measurements were performed as described. All studies were performed in compliance with institutional guidelines under an IACUC approved protocol.
Supplementary Material
Significance.
We show here that a fundamental property of activating BRAF mutants is their RAS independence. The mechanism whereby this occurs determines their sensitivity to current RAF inhibitors. These drugs potently inhibit signaling driven by active RAF monomers (BRAF V600) but not dimers. These findings can be use to characterize BRAF mutants detected in tumors and guide their treatment. A compound that is unaffected by induction of negative cooperativity inhibits signaling driven by activated mutant BRAF dimers or monomers and at doses lower than those required to inhibit wild-type RAF signaling. Such drugs may be useful in treating any tumor driven by an activating BRAF mutation, and could supplant current ‘BRAF-monomer’ inhibitors which are limited by dimer-driven acquired resistance.
Acknowledgments
The authors are grateful to Sarat Chandarlapaty, Piro Lito, and Rona Yaeger for useful discussion. We would like to thank Scott Lowe for providing the vectors of the retrovirus-based inducible expression system, Mariano Barbacid for providing the conditional RAS knockout cells, Manuela Baccarini for the RAF1 knockout MEFs, BeiGene (Beijing) Co., Ltd. for synthesis of the compounds BGB659 and BGB283. This research was supported by grants from the National institutes of Health ((P01CA129243—primary funder and R01CA169351), theSU2C-Melanoma Research Alliance (#SU2C-AACR-DT0612), and the Geoffery Beene Cancer Research Center. This study supported by the NIH/NCI Cancer Center Support Grant P30 CA008748. We would also like to acknowledge the continued support of the Arlene and Joseph Taub Foundation, without which this work would not have been possible. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author contributions
Z. Y. and N. R. conceived the hypotheses, designed and analyzed the experiments and wrote the manuscript. Z. Y., N. M. T., A. T., Y. G. Q. L. and E.S. performed and analyzed the experiments. O. A., L.L., D. B. S and P. P. contributed to experimental design and data analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avraham R, Yarden Y. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat Rev Mol Cell Biol. 2011;12:104–117. doi: 10.1038/nrm3048. [DOI] [PubMed] [Google Scholar]
- Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27:689–696. doi: 10.1097/WCO.0000000000000146. [DOI] [PubMed] [Google Scholar]
- Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, Brown RD, Della Pelle P, Dias-Santagata D, Hung KE, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–235. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RE, Jr, Stephens RM, Saracino MR, Morrison DK. Autoregulation of the Raf-1 serine/threonine kinase. Proc Natl Acad Sci U S A. 1998;95:9214–9219. doi: 10.1073/pnas.95.16.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Dong C, Waters SB, Holt KH, Pessin JE. SOS phosphorylation and disassociation of the Grb2-SOS complex by the ERK and JNK signaling pathways. J Biol Chem. 1996;271:6328–6332. doi: 10.1074/jbc.271.11.6328. [DOI] [PubMed] [Google Scholar]
- Dougherty MK, Muller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD, Conrads TP, Veenstra TD, Lu KP, Morrison DK. Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell. 2005;17:215–224. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Drosten M, Sum EY, Lechuga CG, Simon-Carrasco L, Jacob HK, Garcia-Medina R, Huang S, Beijersbergen RL, Bernards R, Barbacid M. Loss of p53 induces cell proliferation via Ras-independent activation of the Raf/Mek/Erk signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:15155–15160. doi: 10.1073/pnas.1417549111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian JR, Vojtek AB, Cooper JA, Morrison DK. A single amino acid change in Raf-1 inhibits Ras binding and alters Raf-1 function. Proc Natl Acad Sci U S A. 1994;91:5982–5986. doi: 10.1073/pnas.91.13.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AK, Ritt DA, Morrison DK. Effects of Raf dimerization and its inhibition on normal and disease-associated Raf signaling. Mol Cell. 2013;49:751–758. doi: 10.1016/j.molcel.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould AE, Adams R, Adhikari S, Aertgeerts K, Afroze R, Blackburn C, Calderwood EF, Chau R, Chouitar J, Duffey MO, et al. Design and optimization of potent and orally bioavailable tetrahydronaphthalene Raf inhibitors. J Med Chem. 2011;54:1836–1846. doi: 10.1021/jm101479y. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor SL, Vigers G, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer CJ, Pritchard C, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R, Hammer M, Mages J. DUSP meet immunology: dual specificity MAPK phosphatases in control of the inflammatory response. J Immunol. 2006;177:7497–7504. doi: 10.4049/jimmunol.177.11.7497. [DOI] [PubMed] [Google Scholar]
- Lavoie H, Thevakumaran N, Gavory G, Li JJ, Padeganeh A, Guiral S, Duchaine J, Mao DY, Bouvier M, Sicheri F, et al. Inhibitors that stabilize a closed RAF kinase domain conformation induce dimerization. Nat Chem Biol. 2013;9:428–436. doi: 10.1038/nchembio.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lito P, Pratilas CA, Joseph EW, Tadi M, Halilovic E, Zubrowski M, Huang A, Wong WL, Callahan MK, Merghoub T, et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell. 2012;22:668–682. doi: 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Tzivion G, Belshaw PJ, Vavvas D, Marshall M, Avruch J. Oligomerization activates c-Raf-1 through a Ras-dependent mechanism. Nature. 1996;383:181–185. doi: 10.1038/383181a0. [DOI] [PubMed] [Google Scholar]
- Mason CS, Springer CJ, Cooper RG, Superti-Furga G, Marshall CJ, Marais R. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 1999;18:2137–2148. doi: 10.1093/emboj/18.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, Ryder M, Ghossein RA, Rosen N, Fagin JA. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer discovery. 2013:520–533. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Arita T, Tsuchiya S, Donelan J, Chouitar J, Carideo E, Galvin K, Okaniwa M, Ishikawa T, Yoshida S. Antitumor activity of the selective pan-RAF inhibitor TAK-632 in BRAF inhibitor-resistant melanoma. Cancer research. 2013;73:7043–7055. doi: 10.1158/0008-5472.CAN-13-1825. [DOI] [PubMed] [Google Scholar]
- Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik PK, Arcila ME, Fara M, Sima CS, Miller VA, Kris MG, Ladanyi M, Riely GJ. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29:2046–2051. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanisamy N, Ateeq B, Kalyana-Sundaram S, Pflueger D, Ramnarayanan K, Shankar S, Han B, Cao Q, Cao X, Suleman K, et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat Med. 2010;16:793–798. doi: 10.1038/nm.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, Shi H, Atefi M, Titz B, Gabay MT, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratilas CA, Taylor BS, Ye Q, Viale A, Sander C, Solit DB, Rosen N. (V600E)BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci U S A. 2009;106:4519–4524. doi: 10.1073/pnas.0900780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakulendran T, Sahmi M, Lefrancois M, Sicheri F, Therrien M. A dimerization-dependent mechanism drives RAF catalytic activation. Nature. 2009;461:542–545. doi: 10.1038/nature08314. [DOI] [PubMed] [Google Scholar]
- Shi H, Moriceau G, Kong X, Lee MK, Lee H, Koya RC, Ng C, Chodon T, Scolyer RA, Dahlman KB, et al. Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun. 2012;3:724. doi: 10.1038/ncomms1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.