Abstract
Epicardial fat, a metabolically active tissue, has emerged as a risk factor and active player in metabolic and cardiovascular diseases. We investigated epicardial fat thickness in patients who had sustained an acute ischemic stroke, and we evaluated the relationship of epicardial fat thickness with other prognostic factors.
We enrolled 61 consecutive patients (age, ≥18 yr) who had sustained a first acute ischemic stroke and had been admitted to our hospital within 24 hours of the onset of stroke symptoms. The control group comprised 82 consecutive sex- and age-matched patients free of past or current stroke who had been admitted to our cardiology clinics. Blood samples were taken for measurement of N-terminal pro-brain natriuretic peptide (NT-proBNP) levels at admission. Aortic stiffness indices and epicardial fat thickness were measured by means of transthoracic echocardiography within the first 48 hours.
In comparison with the control group, the patients with acute ischemic stroke had significantly higher epicardial fat thickness (4.8 ± 0.9 vs 3.8 ± 0.7 mm; P <0.001), lower aortic distensibility (2.5 ± 0.8 vs 3.4 ± 0.9 cm2·dyn−1; P <0.001) and lower aortic strain (5.5% ± 1.9% vs 6.4% ± 1.8%; P=0.003).
We found a significant association between epicardial fat thickness, NT-proBNP levels, and arterial dysfunction in patients who had sustained acute ischemic stroke. Increased epicardial fat thickness might be a novel risk factor and might enable evaluation of subclinical target-organ damage in these patients.
Keywords: Adipose tissue/pathology/ultrasonography; brain ischemia/physiopathology; cerebral infarction/physiopathology; echocardiography; natriuretic peptide, brain/blood; pericardium/ultrasonography; predictive value of tests; prospective studies; risk factors; vascular stiffness/physiology
Cerebrovascular diseases cause death and disability.1 Each year, approximately 800,000 people have a stroke in the United States; 82% to 92% of these strokes are ischemic.2 Investigators have associated some laboratory and echocardiographic values with acute ischemic stroke (AIS). Elevated serum levels of N-terminal pro-brain natriuretic peptide (NT-proBNP), a powerful predictor of outcomes in patients who have cardiovascular disease, have been associated with impaired function and death after AIS3 and can also differentiate cardioembolic stroke from noncardioembolic causes.3 Arterial stiffness, a significant predictor of cardiovascular morbidity and death, has emerged as another risk marker in patients with AIS.4
Epicardial fat, found between the heart and pericardium,5 is a visceral fat deposit and a metabolically active tissue. It is a local source of inflammatory mediators associated with the development of atherosclerosis, metabolic syndrome, and cardiovascular disease.6 Akil and colleagues7 have shown that echocardiographically measured epicardial fat thickness (EFT) is higher in patients with ischemic stroke than in age- and sex-matched control patients, and that EFT is an independent predictor of ischemic stroke. However, the relationship of echocardiographically measured EFT and real-world AIS is unexplored. Therefore, we evaluated echocardiographically whether EFT, an index of cardiac adiposity, is related to NT-proBNP levels and arterial function in patients with AIS.
Patients and Methods
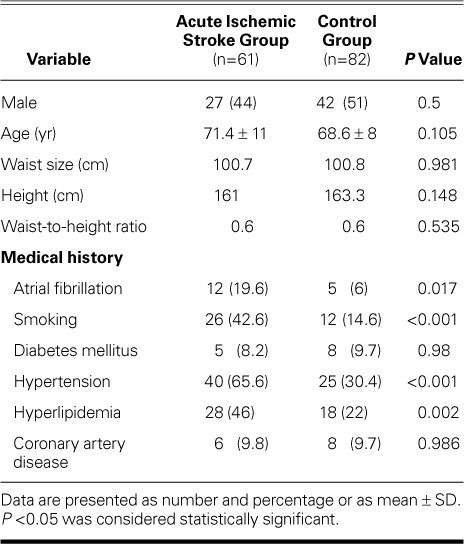
We enrolled all patients ≥18 years of age who had sustained a first-time AIS and who were admitted consecutively to our tertiary-care hospital from August 2014 through March 2015. Ischemic stroke was defined as clinical signs of focal disturbance of cerebral function, of presumed ischemic origin, lasting more than 24 hours.8 We excluded transient ischemic attacks in which the neurologic deficit cleared completely in less than 24 hours. Patients were excluded if they had had a previous ischemic or hemorrhagic stroke, if the time between the onset of neurologic symptoms and emergency-department presentation was more than 24 hours, or if evidence of acute hemorrhagic stroke was detected by means of computed tomography (CT). However, we did include patients with AIS who had CT evidence of previous infarcts that were clinically silent. Our AIS group comprised 61 patients (mean age, 71.4 ± 11 yr; 44% male); the control group consisted of 82 consecutive sex- and age-matched patients (mean age, 68.6 ± 8 yr; 51% male), free of past or current stroke, who were admitted to the cardiology clinics in our hospital. The study protocol was approved by the regional ethics committee, and all participants gave written informed consent.
Baseline demographic, clinical, and electrocardiographic data were obtained (Table I). The prevalence of hypertension, hyperlipidemia, atrial fibrillation, and smoking was higher in the AIS group than in the control group. Complete blood count, routine biochemical values, and NT-proBNP and troponin I concentrations were measured within the first 24 hours. All the patients in this study had at least one brain image taken: a brain CT scan was performed in 100%, and a brain magnetic resonance imaging scan in 84%. The National Institute of Health Stroke Scale (NIHSS) score at presentation was used as an index of stroke severity.9 Stroke subtype was determined in accordance with a modified Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification system.10
TABLE I.
Baseline Characteristics of the Study Groups
Standard transthoracic echocardiography (TTE) was performed in all patients with use of a Philips EPIQ 7 ultrasonography system (Koninklijke Philips N.V.; Best, The Netherlands) within 48 hours of admission. Standard views, including from the left lateral decubitus and supine positions, were obtained. The M-mode traces were recorded at a speed of 50 mm/s, and Doppler signals at a speed of 100 mm/s. M-mode echocardiographic measurements were obtained in accordance with the standards of the American Society of Echocardiography.11 The diameter of the ascending aorta was measured from the same view on the M-mode tracing at 3 cm above the aortic valve. The systolic diameter was measured at the maximal anterior motion of the aorta; the diastolic diameter was measured at the peak of the QRS complex on the simultaneously recorded electrocardiogram. Five consecutive cardiac beats were measured routinely and were averaged. Blood pressure was measured with use of an external sphygmomanometer. The aortic stiffness index, aortic distensibility, and aortic strain were determined as aortic elasticity properties. The formulas used in the calculation of these values were as follows12,13:
 |
The intra- and interobserver mean percentage errors (absolute difference between 2 observations divided by the mean) were determined for the aortic dimensions in 15 randomly selected subjects. Errors were 2.5% and 3.8% for the systolic dimensions and 2.9% and 3.9% for the diastolic.
Epicardial fat was defined as the relatively echo-free space between the outer wall of the myocardium and the visceral layer of the pericardium. Epicardial fat thickness was measured in end-diastole on the free wall of the right ventricle in the parasternal long- and short-axis views, as previously described.14 The maximal values at any site were measured, and the average value was considered. The intraobserver correlation coefficient was 0.95.
Statistical Analysis
Before data collection, we performed a power analysis to determine the sample sizes necessary to yield adequately precise estimates. In the analysis, we assumed that the size of the stroke and control groups were equal because the study was matched. We calculated the sample size as did Akil and colleagues7 and estimated that at least 50 patients per group were required for 80% power. Data were analyzed with use of SPSS 20.0 (IBM Corporation; Endicott, NY). Continuous variables were expressed as mean ± SD and were compared between groups by means of the 2-tailed Student t test. Nonparametric tests were used when necessary (Mann-Whitney U test). The χ2 test was used to compare categorical variables. Correlation analyses were performed with use of the Pearson test. Univariate and multivariate logistic regression analyses were applied to determine predictors of AIS. All variables with P <0.1 in the univariate analysis were included in the multivariate conditional logistic regression analysis model, and the respective odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. The usefulness of the NT-proBNP and EFT values in predicting the presence of AIS were analyzed by means of receiver operating characteristics (ROC) curve analyses. When a significant cutoff value was observed, the sensitivity and specificity values were presented. A 2-tailed P value of <0.05 was considered statistically significant.
Results
Among the 61 patients with first AIS, 14 (23%) were diagnosed with cardioembolic stroke, 6 (10%) with large-vessel disease, 17 (28%) with small-artery occlusion, and 24 (39%) with undetermined cause of stroke. Anterior-circulation ischemic stroke accounted for 45 cases (74%) and posterior-circulation ischemic stroke for 16 (26%). The mean NIHSS score in our AIS group was 6.5 ± 5.2, and the median value of the NIHSS stroke scale was 5 (interquartile range, 2.5–10).
Table II shows the comparative baseline laboratory, echocardiographic, and aortic stiffness values, including aortic elasticity indices, left ventricular ejection fractions, and EFT of the AIS and control patients. The AIS patients had significantly higher NT-proBNP, albumin, sedimentation, and mean platelet volume levels. The AIS patients had substantially higher troponin I levels than did the control patients (29 ± 89 vs 9 ± 7 ng/mL), although the difference did not reach statistical significance (P=0.054).
TABLE II.
Comparative Laboratory, Echocardiographic, and Aortic Stiffness Values between Groups
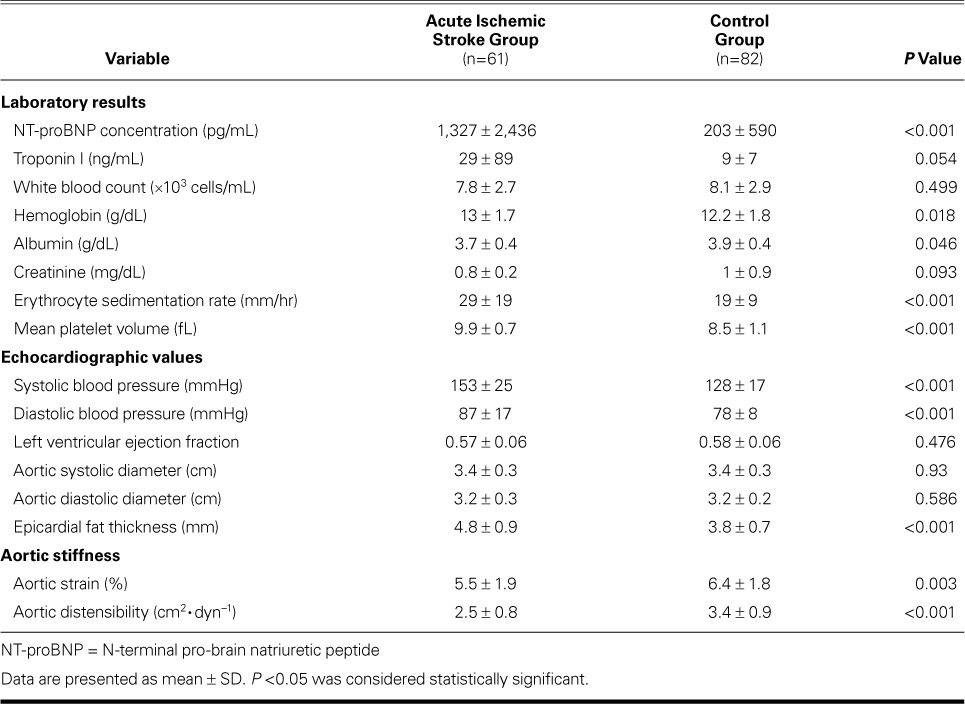
The AIS patients had significantly higher EFT than did the control patients (4.8 ± 0.9 vs 3.8 ± 0.7 mm; P <0.001), lower aortic distensibility (2.5 ± 0.8 vs 3.4 ± 0.9 cm2·dyn−1; P <0.001), and lower aortic strain (5.5% ± 1.9% vs 6.4% ± 1.8%; P=0.003). Left ventricular ejection fractions were similar between the groups.
Correlation between Epicardial Fat Thickness and Other Variables
Figure 1 shows correlation analyses among EFT and other variables. We found a significant positive correlation between EFT and NT-proBNP concentration (r=0.204, P=0.015) (Fig. 1A), erythrocyte sedimentation rate (r=0.277, P=0.001) (Fig. 1B), and mean platelet volume (r=0.338, P <0.001) (Fig. 1C). We found a significant negative correlation between EFT and aortic strain (r= −0.296, P <0.001) (Fig. 1D).
Fig. 1.
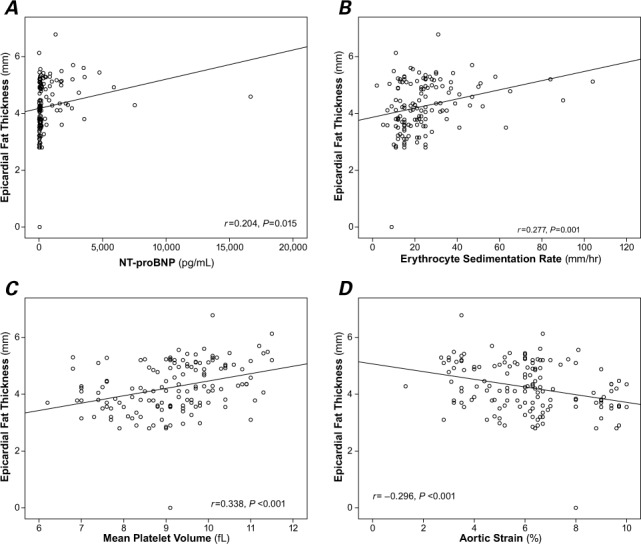
Scatter plots show significant positive correlations between epicardial fat thickness and A) N-terminal pro-brain natriuretic peptide (NT-proBNP), B) erythrocyte sedimentation rate, C) mean platelet volume, and D) a significant negative correlation with aortic strain. P <0.05 was considered statistically significant.
Predictors of Acute Ischemic Stroke
Upon our univariate analysis, we found associations between AIS and atrial fibrillation, smoking, hypertension, hyperlipidemia, NT-proBNP, troponin, hemoglobin, albumin, EFT, aortic distensibility, and aortic strain. Upon multivariate conditional logistic regression analysis, NT-proBNP concentration (OR=1.001; 95% CI, 1.001–1.002; P=0.054), EFT (OR=3.178; 95% CI, 1.404–7.195; P=0.006), mean platelet volume (OR=3.852; 95% CI, 1.928–7.694; P <0.001), and aortic strain (OR=0.68; 95% CI, 0.477–0.97; P =0.033) remained as significant variables associated with AIS (Table III). The optimal cutoff level, sensitivity, and specificity of NT-proBNP levels to distinguish the AIS group from the control group were 135 pg/mL, 80%, and 81%, respectively (ROC area under the curve, 0.819; 95% CI, 0.742–0.896; P <0.001). A cutoff EFT value of 4.28 mm was determined to predict AIS with 81% sensitivity and 81% specificity (ROC area under the curve, 0.84; 95% CI, 0.772–0.908; P <0.001) (Fig. 2).
TABLE III.
Multivariate Analysis of Acute Ischemic Stroke Risk Factors
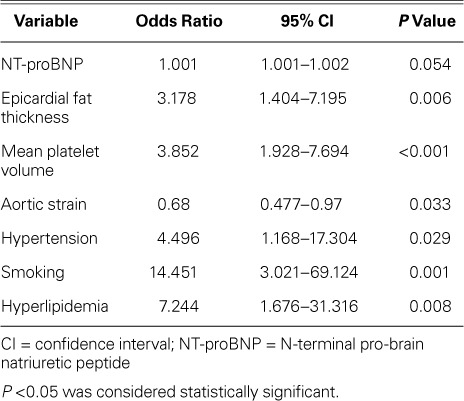
Fig. 2.
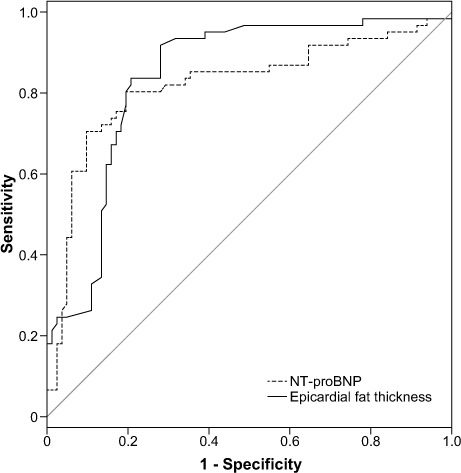
Receiver operator characteristic curve analysis shows the sensitivity and specificity of N-terminal pro-brain natriuretic peptide (NT-proBNP) and epicardial fat thickness in the prediction of acute ischemic stroke.
Discussion
This study provides the first evidence, in a prospective study of real-world AIS patients, that EFT is increased and correlates with aortic stiffness, NT-proBNP concentration, mean platelet volume, and erythrocyte sedimentation rate.
Epicardial fat—a relatively neglected component of the heart—is a source of several endocrine and inflammatory mediators and might play a central role in the pathogenesis of cardiovascular diseases.15–17 Investigators have reported that epicardial fat is associated with endothelial dysfunction,18 insulin resistance,19 and inflammatory markers.20 According to further study results, EFT is related to subclinical target-organ damage such as aortic stiffness,21 carotid intima-media thickness, and carotid plaque.22
However, the association between EFT and AIS has never been studied. In 2014, Akil and colleagues7 reported that EFT and the neutrophil-to-lymphocyte ratio might be novel predictors of ischemic stroke. The major drawback of this 2014 study was that the authors evaluated a very select AIS group23: only patients with large-artery atherosclerotic lesions were included, and patients with cardioembolic and lacunar strokes and comorbidities such as hypertension, diabetes mellitus, and obesity were excluded. Although Akil and colleagues7 did not encompass the whole spectrum of high-risk patients with AIS, and thus did not model real-world outcomes in all-comer high-risk patients, the authors found that EFT and the neutrophil–lymphocyte ratio are higher among patients with AIS in relation to age- and sex-matched control patients, and that EFT ≥5.35 mm predicts the presence of AIS.
In contrast to that study, our study included a patient population that resembles that of a clinical practice. We found that patients with AIS had significantly higher EFT and NT-proBNP levels than did control patients, and we found a significant positive correlation between EFT and NT-proBNP concentration. Increased vascular risk might also result from tobacco use, hypertension, and other risk factors in AIS patients; however, we found that EFT was an independent predictor of AIS.
N-terminal pro-brain natriuretic peptide is a diagnostic prognostic marker in patients with AIS.3,24 In 2015, it was shown that NT-proBNP predicts death in patients with first AIS and differentiates cardioembolic stroke from noncardioembolic stroke. An NT-proBNP level >235 pg/mL predicted 1-year death, and >155 pg/mL differentiated cardioembolic from noncardioembolic stroke.3 Aortic-stiffness indices have also been proposed as prognostic markers in stroke patients: increased aortic stiffness was associated with a greater risk of stroke in patients with essential hypertension25 and in the general population.26 It has also been shown that aortic stiffness, as evaluated by means of 2-dimensional TTE, predicts death beyond the prediction yielded by classic risk factors in patients with first AIS.3 However, associations between EFT and arterial function in normal populations or AIS patients have not been well studied, and the mechanisms involved in the relationship between EFT and arterial function are not known. Several pathologic mechanisms can be proposed. Epicardial fat thickness is associated with insulin resistance, which might be responsible for impaired arterial function.19 Increased expression of secretory type II phospholipase A2 in epicardial adipose tissue might be responsible for endothelial dysfunction and increased arterial stiffness.27 Kim and colleagues,21 who consecutively enrolled 655 subjects who underwent echocardiography and ankle–brachial pulse-wave velocity measurement, found that individuals with increased velocity and EFT had unfavorable metabolic and lipid profiles, and that EFT was significantly associated with ankle–brachial pulse-wave velocity.
Dogan and colleagues28 showed that in patients with newly diagnosed hypertension, increased EFT was significantly linked to impaired aortic elastic properties. Despite our different study population and approach to measuring arterial stiffness, our study shows that patients with AIS had lower aortic distensibility and aortic strain than did a control group. Moreover, there was a significant negative correlation between EFT and aortic-stiffness indices.
Study Limitations
Our study has some limitations. Although there was a correlation between EFT and NT-proBNP, mean platelet volume, aortic strain, and erythrocyte sedimentation rates, the r values were low, which might affect the power of relationship. This study was performed at a single center, and patients with a history of prior stroke were excluded. Therefore, our results cannot be directly applied to all patients with AIS. Although echocardiographic EFT is more accurate, easier, more readily available, and less expensive than that obtained with use of magnetic resonance imaging and CT, it might not reveal the total epicardial fat volume.
Conclusions
We found significant associations between EFT, NT-proBNP concentration, and arterial dysfunction in patients with AIS. Our findings suggest that echocardiographic EFT and NT-proBNP measurements can provide information on arterial function in patients with AIS.
These findings suggest that increased EFT is a novel risk factor and that echocardiographic EFT measurements might aid the evaluation of subclinical target-organ damage. If these results are confirmed in future studies, patients with higher EFT should receive more medical attention, to reduce unfavorable outcomes. Larger and prospective studies are needed to ascertain whether EFT is a causal factor in AIS and is the result of higher NT-proBNP concentration and arterial stiffness.
Footnotes
From: Departments of Cardiology (Drs. Akin, Altun, Basaran, and Biteker) and Neurology (Drs. Emir, Kutlu, and Unal), Faculty of Medicine, Mugla Sitki Kocman University, 48000 Mugla, Turkey
This research was financially supported by the department of Scientific Research Projects, Mugla Sitki Kocman University.
References
- 1.Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42(8):2351–5. doi: 10.1161/STROKEAHA.111.621904. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association [published erratum appears in Circulation 2012;125(22):e1002] Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biteker M, Ozden T, Dayan A, Tekkesin AI, Misirli CH. Aortic stiffness and plasma brain natriuretic peptide predicts mortality in acute ischemic stroke. Int J Stroke. 2015;10(5):679–85. doi: 10.1111/ijs.12049. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Cha MJ, Lee DH, Lee HS, Nam CM, Nam HS et al. The association between cerebral atherosclerosis and arterial stiffness in acute ischemic stroke. Atherosclerosis. 2011;219(2):887–91. doi: 10.1016/j.atherosclerosis.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Bertaso AG, Bertol D, Duncan BB, Foppa M. Epicardial fat: definition, measurements and systematic review of main outcomes [in Portuguese] Arq Bras Cardiol. 2013;101(1):e18–28. doi: 10.5935/abc.20130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med. 2010;14(9):2223–34. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akil E, Akil MA, Varol S, Ozdemir HH, Yucel Y, Arslan D et al. Echocardiographic epicardial fat thickness and neutrophil to lymphocyte ratio are novel inflammatory predictors of cerebral ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23(9):2328–34. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ. 1976;54(5):541–53. [PMC free article] [PubMed] [Google Scholar]
- 9.Brott T, Marler JR, Olinger CP, Adams HP, Jr, Tomsick T, Barsan WG et al. Measurements of acute cerebral infarction: lesion size by computed tomography. Stroke. 1989;20(7):871–5. doi: 10.1161/01.str.20.7.871. [DOI] [PubMed] [Google Scholar]
- 10.Lee LJ, Kidwell CS, Alger J, Starkman S, Saver JL. Impact on stroke subtype diagnosis of early diffusion-weighted magnetic resonance imaging and magnetic resonance angiography. Stroke. 2000;31(5):1081–9. doi: 10.1161/01.str.31.5.1081. [DOI] [PubMed] [Google Scholar]
- 11.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58(6):1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 12.Stefanadis C, Stratos C, Boudoulas H, Kourouklis C, Toutouzas P. Distensibility of the ascending aorta: comparison of invasive and non-invasive techniques in healthy men and in men with coronary artery disease. Eur Heart J. 1990;11(11):990–6. doi: 10.1093/oxfordjournals.eurheartj.a059639. [DOI] [PubMed] [Google Scholar]
- 13.Lacombe F, Dart A, Dewar E, Jennings G, Cameron J, Laufer E. Arterial elastic properties in man: a comparison of echo-Doppler indices of aortic stiffness. Eur Heart J. 1992;13(8):1040–5. doi: 10.1093/oxfordjournals.eurheartj.a060311. [DOI] [PubMed] [Google Scholar]
- 14.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22(12):1311–9. doi: 10.1016/j.echo.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Iacobellis G, Lonn E, Lamy A, Singh N, Sharma AM. Epicardial fat thickness and coronary artery disease correlate independently of obesity. Int J Cardiol. 2011;146(3):452–4. doi: 10.1016/j.ijcard.2010.10.117. [DOI] [PubMed] [Google Scholar]
- 16.Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22(11):450–7. doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson MR, Mookadam F, Thota V, Emani U, Al Harthi M, Lester SJ et al. Epicardial fat: an additional measurement for subclinical atherosclerosis and cardiovascular risk stratification? J Am Soc Echocardiogr. 2011;24(3):339–45. doi: 10.1016/j.echo.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Kaya H, Ertas F, Oylumlu M, Bilik MZ, Yildiz A, Yuksel M et al. Relation of epicardial fat thickness and brachial flow-mediated vasodilation with coronary artery disease. J Cardiol. 2013;62(6):343–7. doi: 10.1016/j.jjcc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Liang KW, Tsai IC, Lee WJ, Lin SY, Lee WL, Lee IT et al. Correlation between reduction of superior interventricular groove epicardial fat thickness and improvement of insulin resistance after weight loss in obese men. Diabetol Metab Syndr. 2014;6(1):115. doi: 10.1186/1758-5996-6-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 21.Kim BJ, Kim BS, Kang JH. Echocardiographic epicardial fat thickness is associated with arterial stiffness. Int J Cardiol. 2013;167(5):2234–8. doi: 10.1016/j.ijcard.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Cabrera-Rego JO, Iacobellis G, Castillo-Herrera JA, Valiente-Mustelier J, Gandarilla-Sarmientos JC, Marin-Julia SM, Navarrete-Cabrera J. Epicardial fat thickness correlates with carotid intima-media thickness, arterial stiffness, and cardiac geometry in children and adolescents. Pediatr Cardiol. 2014;35(3):450–6. doi: 10.1007/s00246-013-0799-9. [DOI] [PubMed] [Google Scholar]
- 23.Altun I, Akin F, Biteker M. The role of epicardial fat thickness and neutrophil-to-lymphocyte ratio are needed to be studied in real-world stroke patients. J Stroke Cerebrovasc Dis. 2015;24(5):1100. doi: 10.1016/j.jstrokecerebrovasdis.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Llombart V, Antolin-Fontes A, Bustamante A, Giralt D, Rost NS, Furie K et al. B-type natriuretic peptides help in cardioembolic stroke diagnosis: pooled data meta-analysis. Stroke. 2015;46(5):1187–95. doi: 10.1161/STROKEAHA.114.008311. [DOI] [PubMed] [Google Scholar]
- 25.Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34(5):1203–6. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 26.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113(5):657–63. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 27.Dutour A, Achard V, Sell H, Naour N, Collart F, Gaborit B et al. Secretory type II phospholipase A2 is produced and secreted by epicardial adipose tissue and overexpressed in patients with coronary artery disease. J Clin Endocrinol Metab. 2010;95(2):963–7. doi: 10.1210/jc.2009-1222. [DOI] [PubMed] [Google Scholar]
- 28.Dogan M, Turak O, Akyel A, Grbovic E, Mendi MA, Oksuz F et al. Increased epicardial adipose tissue thickness is linked to aortic stiffness in patients with primary hypertension. Blood Press. 2014;23(4):222–7. doi: 10.3109/08037051.2013.863991. [DOI] [PubMed] [Google Scholar]


