Abstract
Multiple reports of toxic myocarditis from inhalant abuse have been reported. We now report the case of a 23-year-old man found to have toxic myocarditis from inhalation of a hydrocarbon. The diagnosis was made by means of cardiac magnetic resonance imaging with delayed enhancement. The use of cardiac magnetic resonance to diagnose myocarditis has become increasingly common in clinical medicine, although there is not a universally accepted criterion for diagnosis. We appear to be the first to document a case of toxic myocarditis diagnosed by cardiac magnetic resonance. In patients with a history of drug abuse who present with clinical findings that suggest myocarditis or pericarditis, cardiac magnetic resonance can be considered to support the diagnosis.
Keywords: Aerosol propellants/poisoning; arrhythmias, cardiac/chemically induced; cardiac magnetic resonance imaging; cardiomyopathies/chemically induced; hydrocarbons, fluorinated/adverse effects; inhalant abuse; myocarditis/diagnosis/etiology; 1,1-difluoroethane
Myocarditis is an inflammatory disease of the cardiac muscle that can be caused by infections, autoimmune disorders, hypersensitivity reactions, and cardiotoxins.1 Although the exact prevalence of myocarditis is unknown, various studies of sudden death in adults younger than 40 years of age, athletes, and U.S. Air Force recruits found that 20% had died of myocarditis.1 Cardiotoxins causing myocarditis include catecholamines, cyclophosphamide, antipsychotic agents, cocaine, alcohol, and hydrocarbons. Hydrocarbons are propellants or refrigerants common in many household products and have been shown in multiple case reports to cause myocarditis, heart failure, and sudden death.2–5 Hydrocarbon exposure is typically from the intentional inhalation of fumes, known as huffing, sniffing, or dusting.2 We present an illustrative case and discuss the detection of toxic myocarditis by means of cardiac magnetic resonance (CMR) imaging with delayed enhancement.
Case Report
In December 2013, a 23-year-old man presented at our hospital's emergency department with a one-day history of palpitations and substernal chest pressure associated with dyspnea and diaphoresis. His chest pressure improved in the upright position and was not associated with exertion. His medical history was significant for substance abuse, bipolar disorder, and pseudo-seizures. The patient's last admitted use of cocaine was 2 years before admission, and he reported no current recreational drug use. Upon admission, the patient had a temperature of 97.9 °F, a heart rate of 76 beats/min, a blood pressure of 144/74 mmHg, and an oxygen saturation level of 97% on room air. The patient's examination was notable for his appearance as a large, athletic, and apparently healthy white male, with dilated pupils that were equally round and reactive to light. A cardiopulmonary examination revealed clear lung sounds and no chest-wall tenderness, murmurs, rubs, or gallops.
His electrocardiogram (ECG) showed sinus rhythm at a rate of 79 beats/min and incomplete right bundle branch block, with no ST-segment or T-wave abnormalities. His complete blood count, basic metabolic panel, liver function tests, D-dimer level, and urine toxicology screen (notably negative for cocaine) revealed nothing of concern, but his cardiac enzyme levels, save for normal total creatinine kinase (CK), were abnormal: a peak CK-MB fraction (CK-MB) of 6.5 μg/L and a peak troponin T level of 0.13 μg/L.
Cardiac magnetic resonance with delayed enhancement revealed subepicardial and midwall delayed enhancement, with surrounding edema consistent with myopericarditis. There was biventricular dilation with preserved right ventricular (RV) and left ventricular (LV) systolic function. Evaluation for the cause of the myopericarditis revealed negative antinuclear antibody, myeloperoxidase ab, and proteinase 3, together with negative titers for Coxsackie virus, hepatitis C virus, human immunodeficiency virus, Lyme immunoglobulin G, immunoglobulin M, and Trypanosoma cruzi.
The patient was discharged from the hospital on high-dose ibuprofen for treatment of his myopericarditis, but he returned to the emergency department 4 months later with a similar presentation. On his 2nd admission, the patient's cardiac enzyme levels were slightly higher: a peak troponin I of 0.8 μg/L and a peak CK-MB of 20 μg/L. The patient's urine toxicology testing was again negative for cocaine, benzodiazepines, barbiturates, marijuana, and opiates. Repeated CMR with delayed enhancement now showed mildly depressed RV and LV systolic function, with an RV ejection fraction (EF) decrease from 0.55 to 0.47, an LVEF decrease from 0.58 to 0.51, and edema on T2-weighted images—with hyperenhancement noted in the base and mid lateral wall and in the mid anterior wall (Fig. 1). On the 2nd hospital day, the patient was found huffing from a Dust-Off ® canister of 1,1-difluoroethane (DFE) (Falcon Safety Products Inc.; Branchburg, NJ) with several empty canisters in his bag. Further, he admitted to inhaling DFE before his first admission. We made a final diagnosis of toxic myopericarditis, probably secondary to inhaled halogenated hydrocarbons. The patient never followed up in clinic and has not returned to our hospital's emergency department.
Fig. 1.
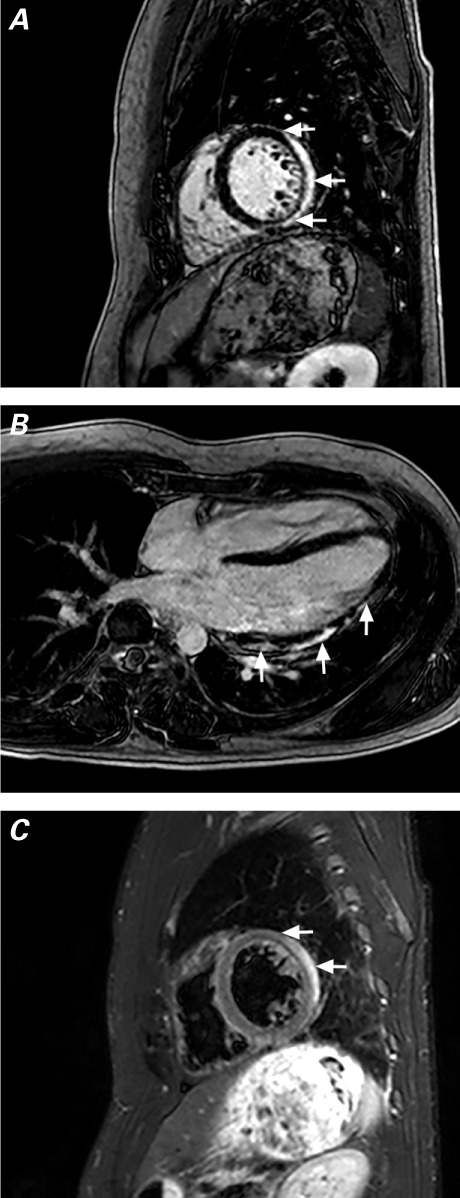
T1-weighted cardiac magnetic resonance images acquired during the patient's 2nd hospitalization show A) delayed enhancement (arrows) of the posterolateral wall, sparing the endocardium, and B) heterogeneous delayed enhancement (arrows) of the lateral wall from the midmyocardium to the subepicardium. C) T2-weighted image (corresponding to slice A) shows bright areas of edema (arrows) and a small pericardial effusion.
Discussion
Solvent abuse among youth has gained popularity since the 1960s.6 Historically, substances such as glue, paint thinners, and dry-cleaning fluids had been the products popularly misused. Cardiomyopathies arising from this type of abuse have been documented since 1971.6 Initial observational studies of asymptomatic industrial exposure to trichloroethylene have revealed a significant number of ECG abnormalities that suggest chronic microvascular damage.7 During the past 2 decades, inhaling DFE for recreational drug use has become more mainstream, resulting in such severe health consequences as encephalopathy, cerebellar degeneration, peripheral neuropathy, and neuropsychiatric disorders.8
Inhalant abuse as a cause of cardiomyopathy should be considered in patients who present with cardiac dysfunction of unknown cause, particularly in teenagers and young adults with histories of substance abuse. There have been prior reports of “huffing” as a cause of cardiomyopathy. In 2012, a case similar to our own was presented, but with transthoracic echocardiograms that showed a severe cardiomyopathy thought to be due to DFE spray.2 Three days into the hospitalization, the patient's cardiac enzyme levels started trending down, and repeat echocardiography showed normalization of LV function.2
A colorless gas, 1,1-difluoroethane is used in various commercial products as a propellant or refrigerant. The intentional inhalation of DFE and other halogenated hydrocarbons has been associated with several cardiac toxicities.
Cardiac Manifestations
There have been reported cases of chronic cardiac toxicity from solvent abuse, resulting in irreversible myocardial damage.5,9 Myocardial degenerative changes like interfibrillary edema, swollen and ruptured fibrils, congestive heart failure from chronic myocarditis, and fibrosis have been seen in chronic inhalant-abusers.5,9 The most common cause of “sudden sniffing death” is cardiac arrhythmia, usually associated with the use of toluene and 1,1,1-trichloroethane.9,10 Ventricular fibrillation, sinus bradycardia, and hypoxia-induced heart block have also been implicated as probable mechanisms.2,4,11
Toxic Myocarditis and the Role of Magnetic Resonance Imaging
Although myocardial biopsy is the gold standard for the diagnosis of myocarditis because of its high specificity, the sensitivity of such biopsy is thought to be too low for routine clinical use.10 Cardiovascular magnetic resonance is being used with increasing frequency as a diagnostic test in cases of suspected acute myocarditis.12 The International Consensus Group on CMR in Myocarditis states that a CMR study should be performed in symptomatic patients in whom the CMR results would affect the management of clinically suspected myocarditis.13,14 According to the Lake Louise Criteria,13 when 2 or more of the following 3 criteria are met, myocarditis can be diagnosed with an accuracy of 78%: 1) regional or global myocardial signal intensity in T2-weighted images, 2) an increased early gadolinium enhancement ratio between myocardium and skeletal muscle in gadolinium-enhanced T1-weighted images, and 3) one focal lesion with nonischemic regional distribution in late-gadolinium-enhancement imaging. The pattern of enhancement in the case presented here (Fig. 1) could also be consistent with sarcoidosis, but toxic myocarditis is more likely in a young white male found huffing.15 The use of CMR to diagnose myocarditis secondary to cardiotoxins is not well documented. The case presented here clinically suggests acute myocarditis from inhalant abuse, and the CMR findings are consistent with myopericarditis.
Although we are not the first to report inhalant abuse as a cause of myocarditis, this to our knowledge is the first documented use of CMR as a diagnostic tool for toxic myocarditis. Even when urinary toxicology screening is negative, inhaled solvents are not picked up on routine drug-screenings; use of these agents should be part of the patient's history if there is suspicion of solvent abuse leading to cardiomyopathy. Cardiac magnetic resonance is a useful tool in the diagnosis of myocarditis and, if available, should be used when a diagnosis of symptomatic myocarditis is suspected. This case shows that myocarditis probably secondary to inhalant abuse can be detected on CMR with gadolinium delayed-enhancement imaging.
Footnotes
From: Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, Texas 75390
References
- 1.Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343(19):1388–98. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 2.Samson R, Kado H, Chapman D. Huffing-induced cardiomyopathy: a case report. Cardiovasc Toxicol. 2012;12(1):90–2. doi: 10.1007/s12012-011-9143-x. [DOI] [PubMed] [Google Scholar]
- 3.Brown C, Budhram G. Evaluation of left ventricular function by bedside ultrasound in acute toxic myocarditis. J Emerg Med. 2013;45(4):588–91. doi: 10.1016/j.jemermed.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 4.Avella J, Wilson JC, Lehrer M. Fatal cardiac arrhythmia after repeated exposure to 1,1-difluoroethane (DFE) Am J Forensic Med Pathol. 2006;27(1):58–60. doi: 10.1097/01.paf.0000202715.71009.0e. [DOI] [PubMed] [Google Scholar]
- 5.Xiong Z, Avella J, Wetli CV. Sudden death caused by 1,1-difluoroethane inhalation. J Forensic Sci. 2004;49(3):627–9. [PubMed] [Google Scholar]
- 6.Mee AS, Wright PL. Congestive (dilated) cardiomyopathy in association with solvent abuse. J R Soc Med. 1980;73(9):671–2. doi: 10.1177/014107688007300916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaultier M, Efthymiou ML, Efthymiou T, Pebay-Peyroula P. Cardiac manifestations of trichloroethylene poisoning [in French] Ann Cardiol Angeiol (Paris) 1971;20(3):185–90. [PubMed] [Google Scholar]
- 8.Sisk MS, Hickey CN, Mycyk MB. Dusting right under our nose: difluoroethane abuse in the emergency department. Eur J Emerg Med. 2012;19(2):130. doi: 10.1097/MEJ.0b013e32834a29cb. [DOI] [PubMed] [Google Scholar]
- 9.Wiseman MN, Banim S. “Glue sniffer's” heart? Br Med J (Clin Res Ed) 1987;294(6574):739. doi: 10.1136/bmj.294.6574.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu PP, Yan AT. Cardiovascular magnetic resonance for the diagnosis of acute myocarditis: prospects for detecting myocardial inflammation. J Am Coll Cardiol. 2005;45(11):1823–5. doi: 10.1016/j.jacc.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Anderson CE, Loomis GA. Recognition and prevention of inhalant abuse. Am Fam Physician. 2003;68(5):869–74. [PubMed] [Google Scholar]
- 12.Cooper LT., Jr. Myocarditis. N Engl J Med. 2009;360(15):1526–38. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53(17):1475–87. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis. 2010;52(4):274–88. doi: 10.1016/j.pcad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120(20):1969–77. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]


