Supplemental Digital Content is available in the text.
Key Words: pain, sleep, intrathecal, morphine, naloxone
Abstract
Introduction:
This randomized, cross-over, double-blind, controlled study of continuous intrathecal morphine administration in patients with severe, long-term pain addresses whether the supplementation of low doses of naloxone in this setting is associated with beneficial clinical effects.
Methods:
All of the study subjects (n=11) provided informed consent and were recruited from a subset of patients who were already undergoing long-term treatment with continuous intrathecal morphine because of difficult-to-treat pain. The patients were (in a randomized order) also given intrathecal naloxone (40 ng/24 h or 400 ng/24 h). As control, the patients’ ordinary dose of morphine without any additions was used. The pain (Numeric Rating Scale, NRS) during activity, perceived quality of sleep, level of activity, and quality of life as well as the levels of several proinflammatory and anti-inflammatory cytokines in the blood were assessed. The prestudy pain (NRS during activity) in the study group ranged from 3 to 10.
Results:
A total of 64% of the subjects reported improved quality of sleep during treatment with naloxone at a dose of 40 ng per 24 hours as compared with 9% with sham treatment (P=0.024). Although not statistically significant, pain was reduced by 2 NRS steps or more during supplemental treatment with naloxone in 36% of subjects when using the 40 ng per 24 hours dose and in 18% of the subjects when using naloxone 400 ng per 24 hours dose. The corresponding percentage among patients receiving unaltered treatment was 27%.
Conclusions:
To conclude, the addition of an ultralow dose of intrathecal naloxone (40 ng/24 h) to intrathecal morphine infusion in patients with severe, persistent pain improved perceived quality of sleep. We were not able to show any statistically significant effects of naloxone on pain relief, level of activity, or quality of life.
Conventional pain therapies are insufficient for some patients with severe long-term pain. Pain treatment with indwelling spinal catheters and implantable pumps for noncancer pain syndromes has been shown to yield good results in these patients1,2 and to be safe.3–6 Even with this invasive method, acceptable pain relief is still not achieved for certain patients, and the identification of additional therapies to improve pain relief is essential. This study focused on the potential beneficial effects achieved by supplementing intrathecal (IT) continuous morphine infusion with concurrent and similarly administered naloxone (NAL).
Previous studies with combinations of intravenous morphine and NAL have shown no pain reduction compared with morphine alone, but an opioid sparing effect has been observed7,8 and a reduction in opioid-induced side effects.9 Only single case reports have suggested that the analgesic effects of IT morphine may be increased with the combined administration of NAL.10,11 The possible analgesic action of IT combinations of morphine and NAL has thus remained unclear. Of particular interest is the finding that the reduction of opioid-induced side effects attained with NAL are dose dependent.9,12 In the present study protocol, we used NAL at 2 dose levels, 40 ng/24 h (NAL40) and 400 ng/24 h (NAL400). Morphine activates the μ-opioid receptor, which in turn activates the Gi/o protein. The complex of µ-opioid receptor and Gi/o functions by multiple mechanisms to inhibit neural pain impulses, thereby decreasing pain sensations.13,14 In chronic pain states and also after long-term morphine treatment,15,16 the μ-opioid receptor shifts its coupling from the inhibitory Gi/o protein to the excitatory Gs protein.14,17–20 This switch causes diminished pain relief and increased morphine tolerance. NAL at ultralow concentrations has the ability to block µ-opioid receptor coupling to the excitatory Gs protein and causes the µ-opioid receptor to again increase its coupling to the inhibitory Gi/o protein,21–23 thereby improving pain relief.24,25
Long-term pain is associated with persistent low-grade inflammation.26–30 There is evidence indicating that treatment with IT morphine induces proinflammatory cytokines that oppose morphine-induced analgesia.31,32 NAL seems to have anti-inflammatory properties, with the capacity to increase the amount of anti-inflammatory and neuroprotective cytokines, glial-derived neurotrophic factor (GDNF) and interleukin (IL)-10,33 and inhibit the release of the proinflammatory cytokine IL-1β.34
This study was performed in a patient group with a history of long-term, difficult-to-treat pain and uninterrupted IT morphine therapy. The objective was to investigate whether the addition of simultaneous IT administration of NAL would confer improved analgesia, improved quality of sleep, alter patients’ quality of life, and/or change the levels of proinflammatory and anti-inflammatory cytokines in the blood.
METHODS
Inclusion of Patients
This was a randomized, cross-over, double-blind, controlled study in patients with a history of severe long-term pain treated by continuous IT morphine. The study was performed in a Swedish teaching hospital and was approved by the Gothenburg University Ethical Review Board and the Swedish Medical Product Agency. The study adhered to all applicable laws and regulations, Good Clinical Practice, and the Declaration of Helsinki. The study was conducted in accordance with the Consort checklist, Supplemental Digital Content 1, http://links.lww.com/CJP/A151.
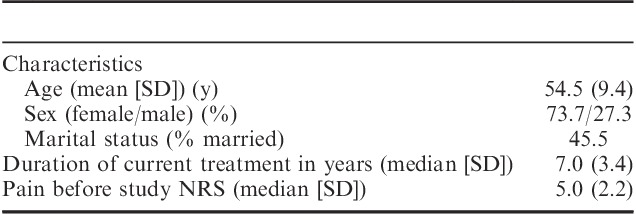
The study included male and female outpatients 18 years and above who had been receiving long term (≥2 y) continuous IT morphine infusion for severe pain and were still being treated this way. The exclusion criteria included disease that complicated assessments of pain status and functional capacity, inability to provide informed consent for the study, or pain NRS<3 during daily activities at the time of screening. At the time of screening for the study, 22 patients treated at the Pain Unit at Sahlgrenska University Hospital (Gothenburg, Sweden) with subcutaneous pumps and indwelling catheters fulfilled these criteria. Among these 22 patients, 12 gave informed written consent to participate in the study (Table 1). Of the 10 patients who declined to participate, 5 did so because the pain clinic was too far away or because it was too tiring to travel to the pain clinic every third week for 9 weeks. Two patients had planned for a trip abroad, and 3 did not want to risk a worsening of their pain.
TABLE 1.
Patient Characteristics
Pain History
The 12 study patients were 8 women and 4 men, aged 39 to 70 years (median, 52 y; mean, 54.5 y), who had had their IT systems installed for 2 to 13 years. This group of patients had a pain intensity score at the beginning of the study that ranged from 3 to 10 on the Numeric Rating Scale (NRS) in daily activities, such as walking or doing light housework. Nine patients claimed that their pain was due to failed back surgery. Two patients reported multiple abdominal surgeries and 1 patient reported trauma as the cause of chronic pain. All of the study participants had undergone multiple surgeries, and all had some neurological deficits such as hyperesthesia, numbness, tingling sensations, or buzzing. The type of pain was mixed neuropathic and nociceptive. Two patients worked part time patient worked part time. Two patients were retired, and 8 patients were on disability retirement. Regarding other medications, 5 of the study patients also used paracetamol (0.5 to 4 g/d) and/or diclofenac (50 to 150 mg/d); however, none of the patients used opioids apart from the IT morphine present at inclusion.
Background IT Morphine Medication
The IT infusion system consisted of an implanted subcutaneous pump incorporating a silicon membrane that could be punctured from the outside, allowing the pump to be filled with morphine alone or, in addition, also filled with other medications. The tip of the IT infusion catheter was at a high lumbar level. The catheter had been tunneled subcutaneously to the anterior side of the body, where it was connected to the subcutaneous pump (Synchro Med or Iso Med, Medtronic Corp., Minneapolis, MN).35 Each subcutaneous pump coupled to the IT catheter was set to deliver morphine continuously at an individually set dose and the patients could not alter the infusion rate. The patients in this study used morphine IT in the range of 0.6 to 4 mg/24 h, a dose that the treating physician considered maximal in relation to the observed and/or patient-reported negative side effects. The individual morphine infusion doses were left unchanged for at least 6 months before the study. During the study,patients experiencing severe self reported pain would receive oral oxycodone as needed.
Study Protocol
The study consisted of 3 consecutive 3-week periods, during which individual background treatment with IT morphine was kept unaltered. No wash-out periods were interpositioned, thus the total duration of the study was 9 weeks. Interventions, each performed one by one, comprised addition of NAL to the IT pump system at 2 respective dose levels, NAL40 and NAL400, and a sham treatment procedure (SHAM). At the start of the initial 3-week period, the patients were randomized in a nonstratified manner to 3 parallel groups based on the order of their first treatment (Fig. 1). Blinding was maintained throughout all treatment periods via strict routines at the pain clinic. An assisting nurse otherwise not directly involved in the study prepared the study medications for each patient arriving to reception according to the randomized protocol. Other staff and study patients were blinded to the randomized order of the procedures, and the infusion system particulars were coded but were otherwise similar in appearance. The subcutaneous pumps were individually refilled with the patients’ ordinary background morphine solution, of which 1 mL was immediately withdrawn. The latter allotment was either replaced by an equal volume of prepared NAL diluted in the morphine or, as SHAM, simply returned into the pump system.
FIGURE 1.
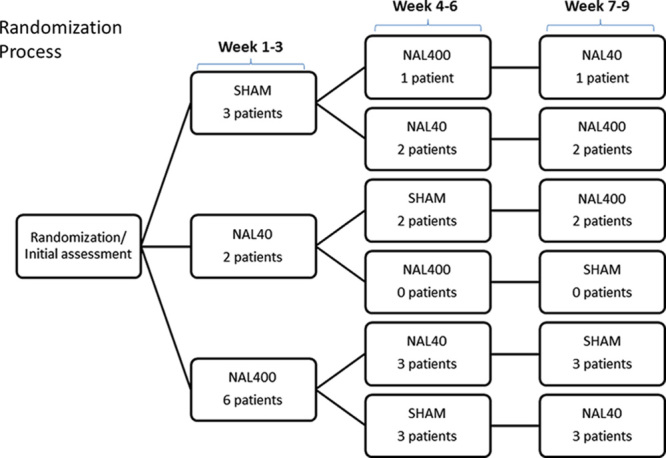
The patients included in this study were treated with individually established doses of IT morphine (0.6 to 4 mg/24 h) in addition to NAL40, NAL400, or SHAM in a randomized order. Each intervention was performed for 3 weeks.
Data Collection
Before the study intervention and after each 3-week period of treatment, the patients rated the pain intensity that they experienced during activities over the last 3 weeks. Activities were defined as walking or doing light housework. After study completion, the patients returned to their prestudy IT morphine regimen (ie, without NAL).
Self-reported NRS was used for the assessment of pain. The NRS is a well-validated scale that has been used in several human pain trials during the last years. The NRS is an 11-step scale in which 0 indicates no pain and 10 indicate the worst possible pain. The NRS scale has demonstrated a statistically significant correlation with the Visual Analogue Scale.36 The NRS is also recommended by the “Change Pain Advisory Board” for clinical trials.37 According to Cepeda et al,38 a 1.3-step change or a reduction of 30% in the NRS is experienced as a meaningful change by the patient. Similarly, in the present study, a 2-step change in the NRS scale in either direction was considered an improvement or a worsening of the pain. Safety assessments included occurrences of treatment-emergent adverse effects (TEAEs) and serious adverse events, which were defined as any event resulting in prolonged hospitalization or death, a life-threatening experience, severe or permanent disability, or relevant alterations in vital signs.
Assessments of perceived sleep quality and perceived level of activity were performed at the end of each 3-week study period. The study patients were asked (by one of the investigators, L.B.) how they rated their sleep quality during the last 3 weeks compared with their sleep quality before the study. According to the study protocol, “Improved,” “Unchanged,” or “Worse” was documented. After each treatment, the patients filled out a Short Form 36 (SF-36) quality of life assessment. The “Swedish standard version” was used.39–41
Peripheral venous blood was collected before the study and after each 3-week study period. The serum samples were analyzed with a colorimetric enzyme-linked immunosorbent assay using commercially available kits. Assays specific for human brain-derived neurotrophic factor (BDNF) (DY248; detection limit 23 pg/mL), human GDNF (DY212; 31 pg/mL), human IL-1β (DY201; 0.2 pg/mL), human IL-8 (DY208; 2.7 pg/mL), human IL-10 (DY217B; 0.9 pg/mL), and human tumor necrosis factor-alpha (TNF-α) (DY210; 0.5 pg/mL) were purchased from R&D Systems (Minneapolis, MN). All assays were run according to the recommendations of the manufacturers, and the color intensity was measured on a Spectramax 340 from Molecular Devices (Sunnyvale, CA).
Statistics
A power analysis was performed using a web-based statistical tool (http://www.quantitativeskills.com). To detect a 30% change in NRS with a SD for the difference of 30% (paired design), 10 patients were needed at a power of 80% with a significance level of 0.05. To compensate for missing values, 12 patients were included.
To compare the NRS scores, we used a nonparametric 2-related samples Wilcoxon signed-rank test. The scores achieved after treatment with NAL40 and NAL400 were compared. Comparisons between the 2 NAL treatments were also performed. The scores attained after SHAM were compared with the scores attained before the study. The scores from SF-36 were also assessed using the Wilcoxon signed-rank test. To analyze the quality of sleep and level of activity, the Fisher exact test was performed to compare the number of patients who found that their sleep improved with the number of patients who stated that their sleep was the same or worse. The Mann-Whitney U test was used to analyze the differences in cytokine levels between the groups.
RESULTS
Pain
At the initial patient assessment (ie, before any changes in analgesic treatment), the median NRS of the study group was 5.00, whereas the range of NRS was considerable (NRS 3-10, Fig. 2). None of the subsequent study interventions were associated with statistically significant changes in pain. The numerical findings were as follows.
FIGURE 2.
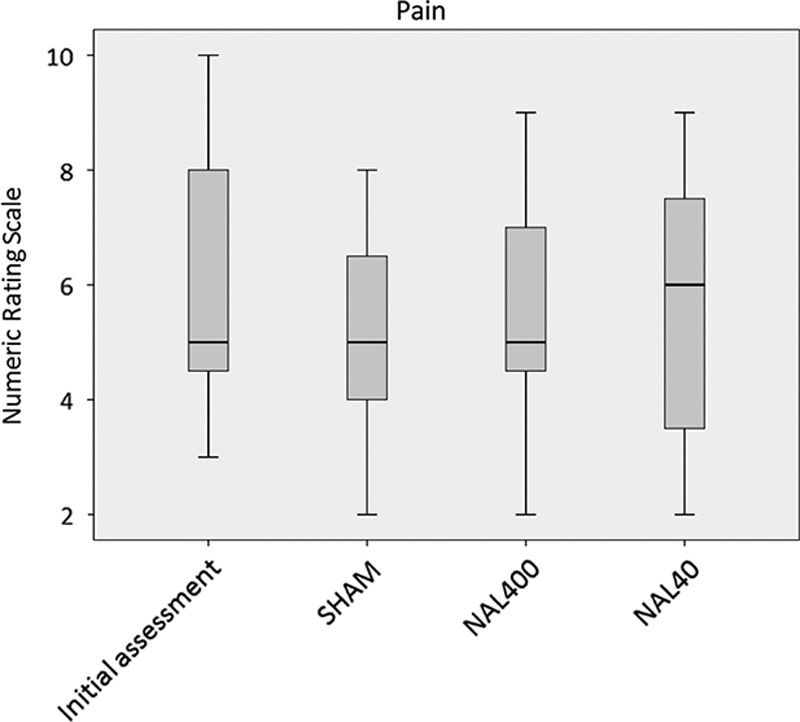
Pain (numerical rating scale during activity) at the initial assessment, with SHAM, with NAL400, or with NAL40. The figure shows the median, interquartile range, and outliers (n=11).
In this study, we considered an NRS change of 2 steps or more in either direction as clinically relevant; such a change was considered either an “improvement” or a “worsening” (see Methods section). Consequently, an NRS change of <2 steps was considered as not clinically important. Assessments concerning treatment with SHAM were compared with the initial assessments. In total, 27% of the patients reported improvement, 55% reported no change, and 18% reported worsening with SHAM. None of the patients needed rescue medication (Fig. 3).
FIGURE 3.

Effects of the interventions on pain (percent of patients) expressed as “Improved” (black), “Unchanged” (shaded), or “Worse” (striped) (n=11).
The findings for treatment with adjuvant NAL400 were compared with the findings obtained with SHAM. In total, 18% of the patients reported improvement, 36% reported no change, and 45% reported worsening. One patient was prescribed 5 doses of rescue medication (per oral oxycodone 10 mg) during treatment with NAL400 due to worsening in pain status.
The data obtained with adjuvant NAL40 were also compared with the data obtained with SHAM. A total of 36% of the patients reported improvement, 36% reported no change, and 27% reported worsening.
Quality of Sleep
The perceived quality of sleep (vs. initial assessment) was improved by NAL40 in 64% of the patients (P=0.024), but not significantly changed by NAL400 or SHAM (Fig. 4). There were no significant differences between the SHAM treatment and either dose of NAL regarding the level of activity (Fig. 5). However, a total of 36% of the patients reported an increased level of activity after receiving adjuvant NAL40 and all of these patients also reported improved perceived quality of sleep.
FIGURE 4.
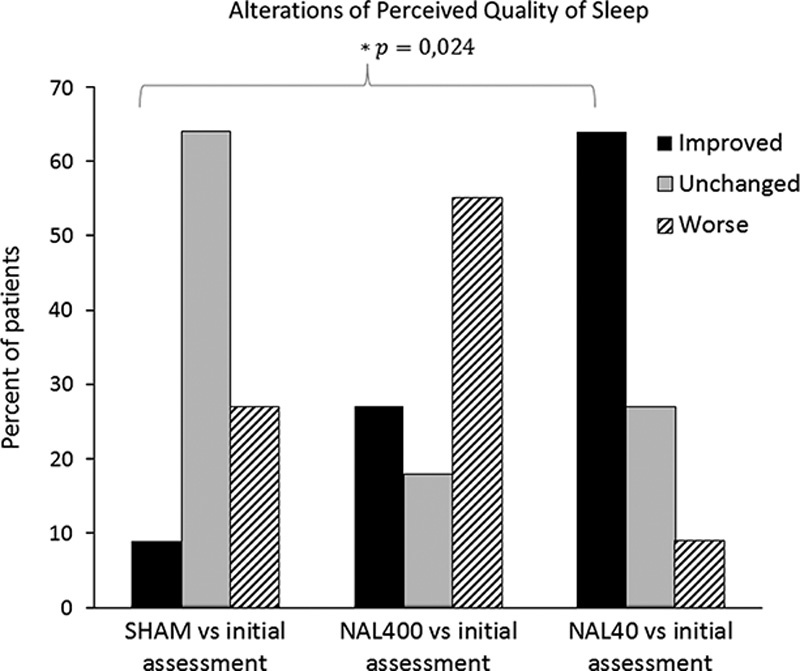
Effects of the interventions on perceived quality of sleep (percent of patients), expressed as “Improved” (black), “Unchanged” (shaded), or “Worse” (striped). P indicates a comparison between SHAM and NAL40 (n=11).
FIGURE 5.
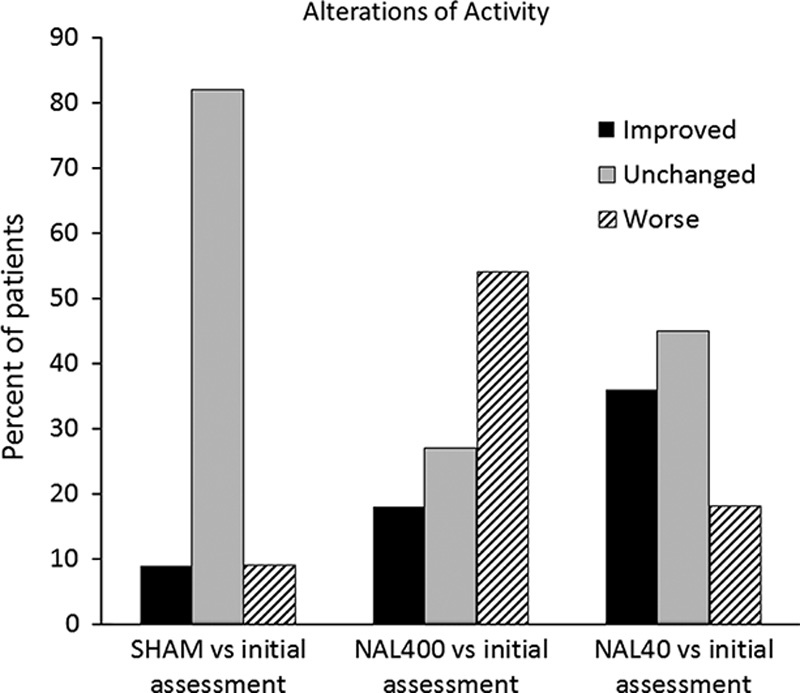
Effects of interventions on patient activity (percent of patients) expressed as “Improved” (black), “Unchanged” (shaded), or “Worse” (striped) (n=11).
Quality of Life
The SF-36 questionnaires demonstrated a markedly low quality of life among the study patients compared with the age-matched controls or chronic disease/handicap groups (Sullivan et al41) (Fig. 6). Compared with SHAM, treatment with adjuvant NAL400 was associated with lower SF-36 scores for the domains of Mental Health (P=0.028).
FIGURE 6.
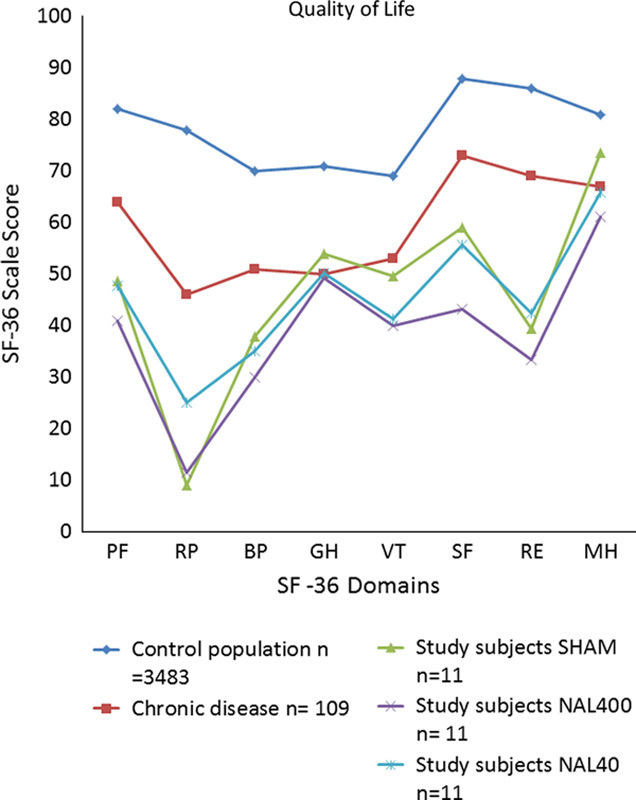
Composite illustrations of SF-36 scores depicting data from an age-matched healthy control population (blue), an age-matched population with chronic disease/handicap (red), and the present study cohort patients during treatment with SHAM (green), NAL400 (purple) or NAL40 (turquoise). Note that the study group had a comparatively low quality of life irrespective of the intervention. The SF-36 domains (x-axis) refer to Physical Function (PF), Role-Physical (RP), Body Pain (BP), General Health (GH), Vitality (VT), Social Function (SF), Role-Emotional (RE), and Mental Health (MH). Adapted with permission from Institute of Health and Care Sciences, Sahlgrenska Academy, University of Gothenburg. Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Blood Samples
There were no statistically significant correlations between the serum levels of the analyzed cytokines (GDNF, BDNF, IL-8, TNF-α, IL-10, and IL-1-β) and either given dose of adjuvant NAL or SHAM.
Safety and Side Effects
Safety assessments included occurrences of TEAEs and serious adverse.
Side effects during treatment with NAL were common but minor and did not differ in severity, frequency, or character from those that were observed with SHAM. However, 1 patient developed a severe headache requiring hospital admission after the first intervention with NAL40. This patient was concomitantly (a different concurrent study protocol) exposed to cerebrospinal fluid sampling through the IT catheter device, which may have caused meningeal irritation. However, it is possible that her symptoms were not related to the study intervention as such. Regardless of the underlying mechanism, we decided to decode the randomization protocol, to exclude the patient from the study and to report the case as a TEAE. The patient recovered uneventfully within a few days, and there were no demonstrable sequelae at follow-ups. One other patient experienced transient opioid withdrawal symptoms requiring hospital admission. This event was associated with a temporary unintended discontinuation of the subcutaneous pump infusion and could easily be corrected by restarting the pump. None of the patients who experienced increased pain during the study remained in this state, and they rapidly returned to their normal pain status at the end of the study.
DISCUSSION
This study addresses new developments of the use of morphine and NAL for pain therapy and associated changes in perceived sleep quality, level of activity, and quality of life in a particularly difficult-to-treat cohort of patients. We exclusively recruited study patients who had current experience with both long-term severe pain and continuous IT opioid administration. A noteworthy feature among the study patients was that despite this rather complex treatment, they were still significantly affected by pain that disturbed their quality of life.
The key finding in the study was that addition of NAL40 to patients with persistent noncancer pain who were being treated with continuous IT infusion of morphine was associated with improvements in self-reported sleep. This is a new finding, which illustrates a potential beneficial role for ultralow doses of NAL as an adjuvant to IT morphine. Importantly, the effects of NAL40 were significantly better than SHAM. Actually, all study patients who reported an increased level of activity also reported improved quality of sleep. This agrees with the previous reports that patients with chronic pain often have sleep disturbances.42,43 In particular, it seems that chronic pain leads to insomnia and more frequent awakenings.44,45 Reciprocally, there is a strong connection between sleep disturbances and exacerbation of pain in chronic pain patients.46,47 This suggests a pathophysiological correlation between the occurrence of impaired sleep quality and long-term nonmalignant pain. Possible underlying mechanisms for such interactions include disturbances of serotonergic pain-inhibitory pathways.48 However, it must be underscored that these proposed interdependences are yet not well understood and associations between long-term pain and sleep quality might simply be coincidental. Further research in larger well-defined patient cohorts, focusing on hither unknown mechanisms for pain versus sleep interactions is needed. Such studies would ideally use sleep laboratory methodology, avoided for ethical reasons in the present study.
The findings of this study do not statistically support that IT NAL given as an adjuvant to IT morphine would improve pain relief. However, 3 study patients who did not show reduced pain by SHAM experienced marked pain relief by both NAL40 and NAL400. It is noteworthy, that the pain relief attained by one of these 3 patients after having participated in the study was so pronounced that she wished to completely discontinue her IT treatment. She was consequently weaned from her IT morphine over the duration of 2 months and subsequently had her IT system removed. The other 2 improved patients achieved good pain relief that lasted for several weeks after the study.
The SF-36 data highlighted that the study patients generally had a low quality of life. In this respect, there were no significant differences between SHAM and adjuvant NAL40. However, during treatment with NAL400 there was a statistically significant deterioration in scores for the Mental Health, Physical Function, and Social Function domains. These results may indicate that NAL given in the latter higher dose range can worsen pain experienced by certain patients. Levels of the investigated cytokines in the present study were generally low, which could indicate a problem related to the detection levels, even with state-of-the-art cytokine analyses (see Methods section).
A central and highly relevant matter is the choice of NAL doses in this study. We set out to investigate effects of NAL over a fairly wide dose range. Our choice of the ultralow dose (NAL40) was based on a comparable report by Hamann et al in 200811 in which 1 patient experienced significant pain reduction during treatment with continuous IT infusion of morphine at a dose of 5 mg/24 h in combination with 50 ng NAL/24 hours. Concerning our choice of NAL400, the rational was to use a dose 10 times higher than NAL40. On the basis of the previous findings, we deemed NAL400 to still be a markedly lower dose than required to antagonize the effects of morphine.49–51
Our intention was to block the coupling of the µ-opioid receptor to the Gs protein and enhance its coupling to the Gi/o protein while avoiding doses high enough to block the μ-opioid receptor itself and thereby increase pain. Because 45% of the patients experienced increased pain during adjuvant treatment by NAL400, this dose may actually have been too high for some patients. One noteworthy observation was that the patients who experienced improved pain relief with any dose of NAL had a daily mean morphine dose of 1.13 mg IT. However, patients who did not have any improvement in pain relief had a daily mean morphine dose of 2.84 mg IT. There may be an optimal ratio between morphine and NAL to achieve the desired reaction. This speculation requires further investigation.
The underlying mechanisms of the clinical findings in this study are complex and not fully understood. To achieve improved pain relief with ultralow doses of NAL in settings with long-term morphine administration, the coupling between the µ-opioid receptor and the Gs protein is inhibited by the NAL.17,22–23 The reason for the variable pain relief achieved by patients given NAL is that the optimal dose ratio between morphine and NAL is individually variable due to the varying sensitivity of the Gs protein to NAL.
We previously investigated inflammatory-activated astrocytes and demonstrated that in the absence of a working astrocyte network, interactions between astrocytes and neurons are problematic; this disturbed communication may be a contributing factor to persistent pain.52–56 The inflammation-induced cellular alterations in astrocytes can be reversed using a combination of a μ-opioid receptor agonist, a μ-opioid receptor antagonist at an ultralow concentration and an antiepileptic agent that reduces IL-1β release in astrocyte cultures.57,58 In the present clinical study, we used the combination of morphine and ultralow doses of NAL without any statistically significant benefits with respect to pain relief. It may be necessary to use an adjuvant antiepileptic agent to achieve more pronounced effects.
There are some limitations to this study. Of particular relevance is, as a function of the inclusion criteria, that the patients’ pain histories were generally long-standing, whereas the duration of the study was relatively short. We acknowledge that this has implications for the generalizability of our findings. However, we argue that the inclusion strategy enabled us to focus on patients with well-documented, severe pain. In addition, we suggest that a longer study period could, through spontaneous individual variations in pain status, have endangered the interpretation of the study findings. We also acknowledge that assessments of the different dimensions of sleep are complex. In the interest of simplicity, we chose a robust technique when gauging alterations in sleep quality, that is a 3-alternative ordinal query. Furthermore, to maintain blinding throughout the study, we had to accept a nonstratified randomization format. Finally, more females than males were recruited for the study. However, this reflects the overrepresentation of females among patients with chronic pain.
To conclude, this study performed in patients with difficult-to-treat long-term pain, suggests that NAL in an ultralow dose range, given as an adjuvant to continuous IT morphine infusion, improves perceived quality of sleep, but fails to significantly alter pain, level of activity, quality of life, or systemic levels of proinflammatory and anti-inflammatory cytokines. Further research in larger well-defined patient cohorts, aiming at systematic exploration of mechanisms for pain versus sleep quality interactions, are needed.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the nurses at the Pain Unit at Sahlgrenska University Hospital, with special thanks to study nurse Yvonne Kratz, Specialistnurse, Sahlgrenska University Hospital, Gothenburg, Sweden; for patient care, to biomedical assistant Marita Ahlquist, Biomedical assistant, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; for help with the sampling procedures, and to Dr Lucas Lannemyr MD, Sahlgrenska University Hospital, Gothenburg, Sweden; for informing the patients about the study.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.clinicalpain.com.
Supported by grants from the University of Gothenburg, Gothenburg Medical Society, Sahlgrenska University Hospital, Lena and Per Sjöbergs Research Fund, and the Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, Gothenburg, Sweden. Naloxone, morphine, and other equipment were provided by institutional grants. This study was also supported by Edit Jacobbsons Foundation. Drs C.L. and P.D. declare that they have received payment for educational lectures about pain from Mundipharma AB, Gothenburg, Sweden. The remaining authors declare no conflict of interest.
REFERENCES
- 1.Nitescu P, Appelgren L, Hultman E, et al. Long-term, open catheterization of the spinal subarachnoid space for continuous infusion of narcotic and bupivacaine in patients with “refractory” cancer pain. A technique of catheterization and its problems and complications. Clin J Pain. 1991;7:143–161. [DOI] [PubMed] [Google Scholar]
- 2.Nitescu P, Dahm P, Appelgren L, et al. Continuous infusion of opioid and bupivacaine by externalized intrathecal catheters in long-term treatment of “refractory” nonmalignant pain. Clin J Pain. 1998;14:17–28. [DOI] [PubMed] [Google Scholar]
- 3.Dahm P, Nitescu P, Appelgren L, et al. Efficacy and technical complications of long-term continuous intraspinal infusions of opioid and/or bupivacaine in refractory nonmalignant pain: a comparison between the epidural and the intrathecal approach with externalized or implanted catheters and infusion pumps. Clin J Pain. 1998;14:4–16. [DOI] [PubMed] [Google Scholar]
- 4.Dahm PO, Nitescu PV, Appelgren LK, et al. Six years of continuous intrathecal infusion of opioid and bupivacaine in the treatment of refractory pain due to intrapelvic extrusion of bone cement after total hip arthroplasty. Reg Anesth Pain Med. 1998;23:315–319. [DOI] [PubMed] [Google Scholar]
- 5.Lundborg C, Dahm P, Nitescu P, et al. Clinical experience using intrathecal (IT) bupivacaine infusion in three patients with complex regional pain syndrome type I (CRPS-I). Acta Anaesthesiol Scand. 1999;43:667–678. [DOI] [PubMed] [Google Scholar]
- 6.Willis KD, Doleys DM. The effects of long-term intraspinal infusion therapy with noncancer pain patients: evaluation of patient, significant-other, and clinic staff appraisals. Neuromodulation. 1999;2:241–253. [DOI] [PubMed] [Google Scholar]
- 7.Gan TJ, Ginsberg B, Glass PS, et al. Opioid-sparing effects of a low-dose infusion of naloxone in patient-administered morphine sulfate. Anesthesiology. 1997;87:1075–1081. [DOI] [PubMed] [Google Scholar]
- 8.Movafegh A, Shoeibi G, Ansari M, et al. Naloxone infusion and post-hysterectomy morphine consumption: a double-blind, controlled study. Acta Anaesthesiol Scand. 2012;56:1241–1249. [DOI] [PubMed] [Google Scholar]
- 9.Cepeda MS, Alvarez H, Morales O, et al. Addition of ultralow dose naloxone to postoperative morphine PCA: unchanged analgesia and opioid requirement but decreased incidence of opioid side effects. Pain. 2004;107:41–46. [DOI] [PubMed] [Google Scholar]
- 10.Hamann S, Sloan P. Oral naltrexone to enhance analgesia in patients receiving continuous intrathecal morphine for chronic pain: a randomized, double-blind, prospective pilot study. J Opioid Manag. 2007;3:137–144. [DOI] [PubMed] [Google Scholar]
- 11.Hamann S, Sloan PA, Witt W. Low-dose intrathecal naloxone to enhance intrathecal morphine analgesia: a case report. J Opioid Manag. 2008;4:251–254. [DOI] [PubMed] [Google Scholar]
- 12.Cepeda MS, Africano JM, Manrique AM, et al. The combination of low dose of naloxone and morphine in PCA does not decrease opioid requirements in the postoperative period. Pain. 2002;96:73–79. [DOI] [PubMed] [Google Scholar]
- 13.Connor M, Christie MD. Opioid receptor signalling mechanisms. Clin Exp Pharmacol Physiol. 1999;26:493–499. [DOI] [PubMed] [Google Scholar]
- 14.Crain SM, Shen KF. Modulation of opioid analgesia, tolerance and dependence by Gs-coupled, GM1 ganglioside-regulated opioid receptor functions. Trends Pharmacol Sci. 1998;19:358–365. [DOI] [PubMed] [Google Scholar]
- 15.Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watkins LR, Hutchinson MR, Johnston IN, et al. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–669. [DOI] [PubMed] [Google Scholar]
- 17.Tsai RY, Tai YH, Tzeng JI, et al. Ultra-low dose naloxone restores the antinociceptive effect of morphine in pertussis toxin-treated rats by reversing the coupling of mu-opioid receptors from Gs-protein to coupling to Gi-protein. Neuroscience. 2009;164:435–443. [DOI] [PubMed] [Google Scholar]
- 18.Wang HY, Friedman E, Olmstead MC, et al. Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and Gbetagamma signaling. Neuroscience. 2005;135:247–261. [DOI] [PubMed] [Google Scholar]
- 19.Wang HY, Frankfurt M, Burns LH. High-affinity naloxone binding to filamin a prevents mu opioid receptor-Gs coupling underlying opioid tolerance and dependence. PLoS One. 2008;3:e1554. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Wang HY, Burns LH. Naloxone’s pentapeptide binding site on filamin A blocks Mu opioid receptor-Gs coupling and CREB activation of acute morphine. PLoS One. 2009;4:e4282. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Block L, Forshammar J, Westerlund A, et al. Naloxone in ultralow concentration restores endomorphin-1-evoked Ca2+ signaling in lipopolysaccharide pretreated astrocytes. Neuroscience. 2012;205:1–9. [DOI] [PubMed] [Google Scholar]
- 22.Crain SM, Shen KF. Ultra-low concentrations of naloxone selectively antagonize excitatory effects of morphine on sensory neurons, thereby increasing its antinociceptive potency and attenuate tolerance/dependence during chronic cotreatment. Proc Natl Acad Sci U S A. 1995;92:10540–10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crain SM, Shen KF. Antagonists of excitatory opioid receptor functions enhance morphine’s analgesic potency and attenuate opioid tolerance/dependence liability. Pain. 2000;84:121–131. [DOI] [PubMed] [Google Scholar]
- 24.Shen KF, Crain SM. Antagonists at excitatory opioid receptors on sensory neurons in culture increase potency and specificity of opiate analgesics and attenuate development of tolerance/dependence. Brain Res. 1994;636:286–297. [DOI] [PubMed] [Google Scholar]
- 25.Shen KF, Crain SM. Ultra-low doses of naltrexone or etorphine increase morphine’s antinociceptive potency and attenuate tolerance/dependence in mice. Brain Res. 1997;757:176–190. [DOI] [PubMed] [Google Scholar]
- 26.Hansson E. Long-term pain, neuroinflammation and glial activation. Scand J Pain. 2010;1:67–72. [DOI] [PubMed] [Google Scholar]
- 27.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. [DOI] [PubMed] [Google Scholar]
- 28.Calvo M, Dawes JM, Bennett DL. The role of the immune system in the generation of neuropathic pain. Lancet neurology. 2012;11:629–642. [DOI] [PubMed] [Google Scholar]
- 29.Vallejo R, Tilley DM, Vogel L, et al. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10:167–184. [DOI] [PubMed] [Google Scholar]
- 30.Ellis A, Bennett DL. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth. 2013;111:26–37. [DOI] [PubMed] [Google Scholar]
- 31.Hutchinson MR, Coats BD, Lewis SS, et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008;22:1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis SS, Hutchinson MR, Rezvani N, et al. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience. 2010;165:569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin SL, Tsai RY, Tai YH, et al. Ultra-low dose naloxone upregulates interleukin-10 expression and suppresses neuroinflammation in morphine-tolerant rat spinal cords. Behav Brain Res. 2010;207:30–36. [DOI] [PubMed] [Google Scholar]
- 34.Watkins LR, Hansen MK, Nguyen KT, et al. Dynamic regulation of the proinflammatory cytokine, interleukin-1beta: molecular biology for non-molecular biologists. Life Sci. 1999;65:449–481. [DOI] [PubMed] [Google Scholar]
- 35.Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040–4049. [DOI] [PubMed] [Google Scholar]
- 36.Paice JA, Cohen FL. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs. 1997;20:88–93. [DOI] [PubMed] [Google Scholar]
- 37.Muller-Schwefe G, Jaksch W, Morlion B, et al. Make a CHANGE: optimising communication and pain management decisions. Curr Med Res Opin. 2011;27:481–488. [DOI] [PubMed] [Google Scholar]
- 38.Cepeda MS, Africano JM, Polo R, et al. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105:151–157. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan M, Karlsson J, Ware JE., Jr The Swedish SF-36 Health Survey—I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med. 1995;41:1349–1358. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan M, Karlsson J. The Swedish SF-36 Health Survey III. Evaluation of criterion-based validity: results from normative population. J Clin Epidemiol. 1998;51:1105–1113. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan M, Karlsson J, Taft C. SF-36 Swedish Manual and Interpretation Guide. 2002:2nd edGothenburg, Sweden: Sahlgrenska University Hospital. [Google Scholar]
- 42.Moldofsky H. Sleep and pain. Sleep Med Rev. 2001;5:385–396. [DOI] [PubMed] [Google Scholar]
- 43.Menefee LA, Frank ED, Doghramji K, et al. Self-reported sleep quality and quality of life for individuals with chronic pain conditions. Clin J Pain. 2000;16:290–297. [DOI] [PubMed] [Google Scholar]
- 44.Morin CM, Gibson D, Wade J. Self-reported sleep and mood disturbance in chronic pain patients. Clin J Pain. 1998;14:311–314. [DOI] [PubMed] [Google Scholar]
- 45.Ohayon MM. Relationship between chronic painful physical condition and insomnia. J Psychiatr Res. 2005;39:151–159. [DOI] [PubMed] [Google Scholar]
- 46.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–369. [DOI] [PubMed] [Google Scholar]
- 48.Foo H, Mason P. Brainstem modulation of pain during sleep and waking. Sleep Med Rev. 2003;7:145–154. [DOI] [PubMed] [Google Scholar]
- 49.Meissner W, Leyendecker P, Mueller-Lissner S, et al. A randomised controlled trial with prolonged-release oral oxycodone and naloxone to prevent and reverse opioid-induced constipation. Eur J Pain. 2009;13:56–64. [DOI] [PubMed] [Google Scholar]
- 50.Yang CP, Cherng CH, Wu CT, et al. Intrathecal ultra-low dose naloxone enhances the antihyperalgesic effects of morphine and attenuates tumor necrosis factor-alpha and tumor necrosis factor-alpha receptor 1 expression in the dorsal horn of rats with partial sciatic nerve transaction. Anesth Analg. 2013;117:1493–1502. [DOI] [PubMed] [Google Scholar]
- 51.Maxwell LG, Kaufmann SC, Bitzer S, et al. The effects of a small-dose naloxone infusion on opioid-induced side effects and analgesia in children and adolescents treated with intravenous patient-controlled analgesia: a double-blind, prospective, randomized, controlled study. Anesth Analg. 2005;100:953–958. [DOI] [PubMed] [Google Scholar]
- 52.Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochem Int. 2004;45:389–395. [DOI] [PubMed] [Google Scholar]
- 53.Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forshammar J, Block L, Lundborg C, et al. Naloxone and ouabain in ultralow concentrations restore Na+/K+-ATPase and cytoskeleton in lipopolysaccharide-treated astrocytes. J Biol Chem. 2011;286:31586–31597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154suppl 1S10–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haghikia A, Ladage K, Hinkerohe D, et al. Implications of antiinflammatory properties of the anticonvulsant drug levetiracetam in astrocytes. J Neurosci Res. 2008;86:1781–1788. [DOI] [PubMed] [Google Scholar]
- 58.Block L, Bjorklund U, Westerlund A, et al. A new concept affecting restoration of inflammation-reactive astrocytes. Neuroscience. 2013;250:536–545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


