Article first published online 6 August 2015.
Supplemental Digital Content is Available in the Text.
Key Words: PNPLA3, inflammatory bowel disease, liver steatosis, ALT, controlled attenuation parameter
Abstract
Background:
Inflammatory bowel diseases (IBD) are characterized by chronic relapsing inflammation of the gastrointestinal tract and encompass Crohn's disease and ulcerative colitis. IBD are often associated with extraintestinal manifestations affecting multiple organs including the liver. Increased levels of serum aminotransferases, possibly related to nonalcoholic fatty liver disease, constitute one of the most frequently described IBD-related liver diseases. The PNPLA3 I148M substitution is a major common genetic determinant of hepatic fat content and progression to chronic liver disease. The aim of this study was to investigate whether carriers of PNPLA3 148M allele with IBD have higher risk of liver steatosis and increase in transaminases levels.
Methods:
The PNPLA3 I148M (rs738409) genotype was performed by Taqman assays in 158 individuals from Southern Italy (namely, Catanzaro cohort) and in 207 individuals from Northern Italy (namely, Milan cohort) with a definite diagnosis of IBD. Demographic and clinical data and also alanine transaminase levels were collected for both cohorts. The Catanzaro cohort underwent liver evaluation by sonography and liver stiffness and controlled attenuation parameter measurements by transient elastography.
Results:
Here, we show for the first time that carriers of the PNPLA3 148M allele with IBD have a greater risk of hepatic steatosis (odds ratio, 2.9, and confidence interval, 1.1–7.8), higher controlled attenuation parameter values (P = 0.029), and increased circulating alanine transaminase (P = 0.035) in the Catanzaro cohort. We further confirm the higher alanine transaminase levels in the Milan cohort (P < 0.001).
Conclusions:
Our results show that PNPLA3 148M carriers with IBD have higher susceptibility to hepatic steatosis and liver damage.
Inflammatory bowel diseases (IBD) are immune-mediated disorders characterized by remittent and relapsing progressive inflammation of the gastrointestinal tract. There are 2 major clinical forms of IBD: Crohn's disease (CD) and ulcerative colitis (UC).1 IBD are often associated with extraintestinal manifestations involving various organs. Extraintestinal manifestations of the skin, eyes, and peripheral joints usually follow the disease activity, whereas extraintestinal manifestations involving the hepatobiliary and pulmonary systems typically do not parallel the disease activity.2–4 Increased circulating levels of aminotransferases represent one of the most frequently described IBD-related liver diseases; this is usually ascribed to nonalcoholic fatty liver disease.4 Indeed, the prevalence of hepatic steatosis ranges from 13% to 100% in individuals with IBD5 and with no differences between CD and UC.5,6 Several possible mechanisms underlying IBD-related hepatic damage have been already described, including malnutrition, protein loss, and medications.5 Despite a large number of studies on liver abnormalities, the pathogenesis of hepatic steatosis and liver damage in individuals with IBD is not completely understood, and it is not known whether IBD per se represent an independent risk factor for this condition.
The patatin-like phospholipase domain-containing 3 (PNPLA3) protein is a triglyceride and retinyl esterase lipase7–9 highly expressed in human hepatocytes and hepatic stellate cells. The PNPLA3 rs738409 genetic variant is a guanine to cytosine substitution at position 617 of the gene, and it encodes an isoleucine to methionine substitution at position 148 (I148M) of the PNPLA3 protein. This substitution results in a loss of function of the protein enzymatic activity.10–13 The PNPLA3 I148M substitution is a major common genetic determinant of hepatic fat content13 and progression to chronic liver disease under a large variety of harmful stimuli for the liver including severe obesity,14 visceral adiposity,15 diet,16,17 viruses,11,18 iron overload,19 and excess alcohol consumption.20 However, it is not yet known whether PNPLA3 148M carriers with IBD are more susceptible to chronic liver disease.
The aim of this study was to investigate whether carriers of PNPLA3 148M allele with IBD have higher risk of liver steatosis and increase in transaminases levels. Here, we show for the first time that carriers of the PNPLA3 148M allele with IBD have a greater risk of hepatic steatosis and increased circulating alanine transaminase (ALT) in a cohort of 158 patients from Southern Italy. We further confirm the higher ALT levels in an independent cohort of 207 individuals with IBD from Northern Italy.
MATERIALS AND METHODS
Study Cohorts
Study Cohort Recruitment
A total of 158 consecutive outpatients from the Gastroenterology Unit (University Hospital Magna Græcia of Catanzaro) were recruited between June 2014 and October 2014, namely, Catanzaro cohort. Patients of both sexes, aged more than 18 years with a diagnosis of IBD based on established clinical, endoscopic, radiological, and histological criteria were included in the study. Demographic and clinical data and also ALT levels were collected at the time of enrollment. Body mass index (BMI) was calculated on self-reported height and weight. The data collected include previous ileocecal resection for CD. Based on Mayo score< 2 (UC)21 and Harvey Bradshaw index< 5 (CD),22 patients have been classified as having active or inactive (remission) disease.
A total of 207 consecutive outpatients of the Gastroenterology clinic at the “Ospedale Policlinico Milano” were recruited between 2004 and 2005, namely, Milan cohort. Patients of both sexes, aged more than 18 years with a definite diagnosis of IBD were included in the study. ALT levels were measured on frozen serum samples. Disease activity and localization have been determined as described above.
No individuals with at risk alcohol intake (>30/20 g/d in M/F) were included in the study cohorts.
Liver Evaluation
Sonography
In the Catanzaro cohort, a total of 109 out of 158 patients underwent sonographic liver evaluation. Abdominal sonography was performed from the same experience operator with a gray-scale scanner devices (Toshiba Nemio, Shimoishigami, Japan) using a 3.5-MHz convex transducer. Individuals were at least 4 hours fasting before the procedure. Before the procedure, individuals followed a fiber free diet and took 80 mg simethicone three times per day for 3 days. Hepatic steatosis was graded as mild (steatosis grade 1 or S1), moderate (steatosis grade 2 or S2), or severe (steatosis grade 3 or S3).
The features of the mild liver steatosis (S1) were defined as a slight increase in liver echogenicity with a slight exaggeration of liver and kidney echo discrepancy. Moderate liver steatosis (S2) features were defined as increase in liver echogenicity and loss of echoes from the wall of the portal vein with a greater posterior beam attenuation and greater discrepancy between hepatic and renal echoes. The features of severe liver steatosis (S3) were defined as a greater reduction in beam penetration, loss of echoes from most of the portal vein wall, and an even larger discrepancy between hepatic and renal echoes.23,24 Hepatic steatosis was defined as a steatosis grade ≥S1.
Transient Elastography
In the Catanzaro cohort, a total of 109 out of 158 patients underwent liver stiffness and controlled attenuation parameter (CAP) measurements by transient elastography using FibroScan 502 touch (Echosens, Paris, France). All measurements were performed using the 3.5 MHz standard M probe. Transient elastographic liver evaluation was performed the same day of sonography from the same operator (which was different from the one performing the sonography). Measurements were performed according to manufacture's instructions.25 Briefly, patient was lying in the dorsal decubitus position with the right arm in maximal abduction; the tip of the transducer probe was covered with coupling gel and placed on the skin between the ribs at level of the right lobe of the liver. Only results from subjects with 10 valid shots and interquartile range/median liver stiffness ratio ≤30% were included in the study according to manufacturer26 (n = 106). The final liver stiffness result was expressed in kPa and was the median value of 10 measurements. Liver stiffness and CAP were obtained simultaneously and in the same volume of liver parenchyma. CAP was computed only when the associated liver stiffness measurement was valid. The final CAP value result was expressed in dB/m and was the median value of 10 measurements.
Hepatic steatosis was graded as mild (steatosis grade 1 or S1), moderate (steatosis grade 2 or S2), or severe (steatosis grade 3 or S3) based on previous reported cutoff.27 Briefly, mild liver steatosis (S1) was defined as CAP values between 216 and 252 dB/m; moderate liver steatosis (S2) was defined as CAP values between 253 and 296 dB/m; severe liver steatosis (S3) was defined as CAP > 296 dB/m. Hepatic steatosis was defined as a steatosis grade ≥S1.
PNPLA3 rs738409 Genotyping
For the Catanzaro cohort, DNA was extracted from saliva by NucleoSpin technology (Macherey-Nagel GmbH & Co., Düren, Germany) or from buccal brush by Puregene Buccal Cell Core Kit (Qiagen, Venlo, the Netherlands), and the rs738409 polymorphism (encoding for PNPLA3 I148M) determined by 5'-nuclease Taqman assays (Life Technologies, Carlsbad, CA) as described before.7 For the Milan cohort, DNA was extracted from peripheral blood samples by the phenol-chloroform method, and the rs738409 polymorphism determined using the Taqman assay as described before.28
Statistical Analyses
Genotype and allele frequencies and also categorical variables' distribution across genotypes were compared by χ2 test or by Fisher's exact test. In the Catanzaro cohort, the P values for continuous variables were calculated using linear regression analysis under an additive model after adjusting for age, sex, BMI, and mesalamine use when appropriate; the P values for steatosis presence were calculated by binary logistic regression analysis under an additive model after adjusting for age, sex, BMI, and mesalamine use. In the Milan cohort, the P value for ALT levels was calculated using linear regression analysis under an additive model after adjusting for age, sex, and mesalamine use. ALT levels among PNPLA3 I148M genotypes in subjects with CD and in subjects with UC were analyzed using linear regression analysis under an additive model after adjusting for age, sex, and mesalamine use (Catanzaro and Milan cohorts) or for age, sex, mesalamine use, and recruitment center (Catanzaro plus Milan cohort). Weighted-mean and weighted-SD were calculated to show ALT levels in the combined Catanzaro plus Milan cohort.
All statistical analyses were performed using the Statistical Package for Social Sciences (version 19.0; SPSS, Inc., Chicago, IL). P values < 0.05 were considered as statistically significant.
ETHICAL CONSIDERATIONS
The study was approved by ethics committee of University Magna Graecia (protocol number 2014/49) and by the Institutional Review Board of the Fondazione IRCCS Ca' Granda Ospedale Policlinico, Milano. All patients participated in this study in confirmation with the principles outlined in the Declaration of Helsinki. Informed written consent was obtained from each participating patient.
RESULTS
PNPLA3 148M Carriers with IBD Have Higher Risk of Hepatic Steatosis and Higher Circulating ALT in the Catanzaro Cohort
A total of 158 European individuals (mean age 45 ± 13 yr) with IBD from Southern Italy (namely, Catanzaro cohort) were examined. Characteristics of the Catanzaro cohort are shown in Table 1. Most individuals from this cohort were normal weight, approximately one-third had CD, whereas the remaining had UC. The mean disease duration was of 8 years, and approximately 40% of individuals were in remission. Among individuals with CD, most had ileal or ileocolonic disease location and 25% underwent ileocecal resection. Among individuals with UC, most (60%) had pancolitis disease location. Hepatic steatosis estimated by elastography (CAP ≥ 216 db/m) was present in approximately 70% of the individuals.
TABLE 1.
Anthropometric, Clinical, and Biochemical Characteristics of Individuals with CD or UC from Catanzaro
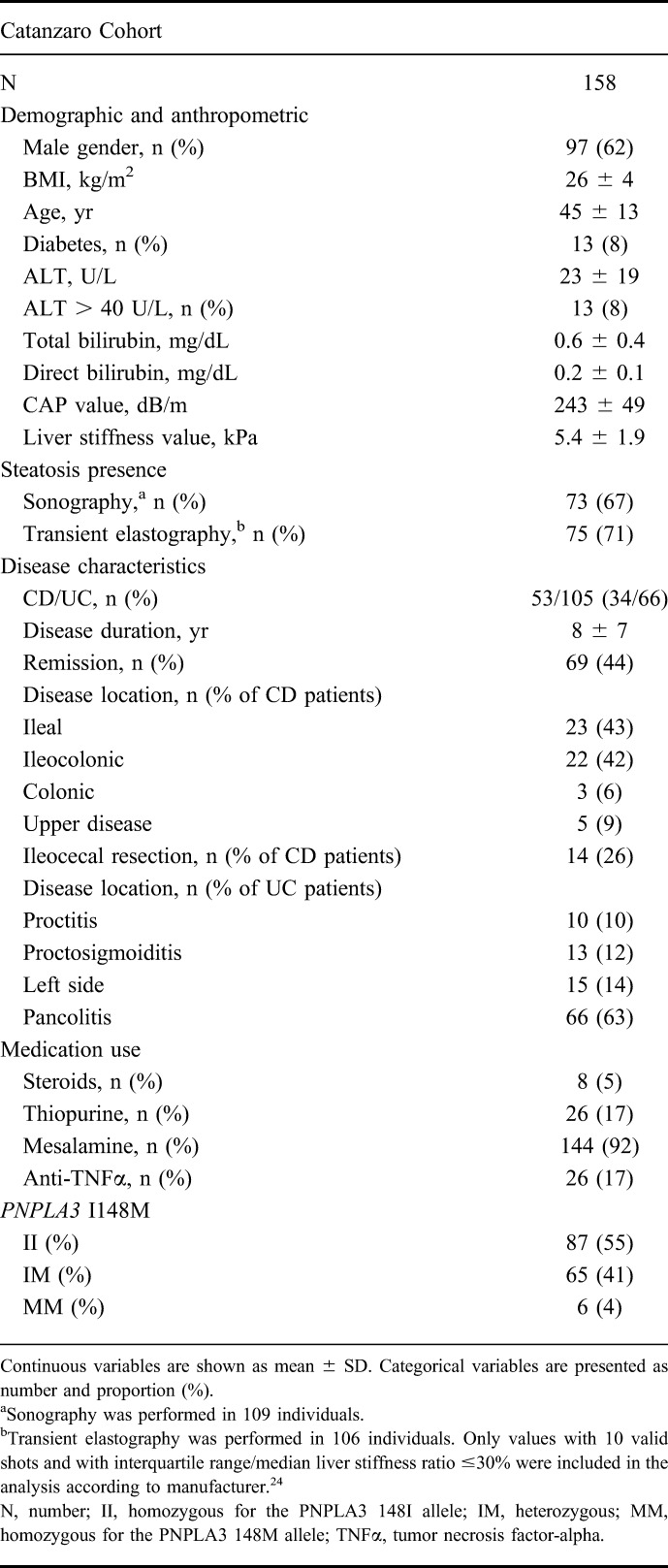
We found a positive correlation between the presence of hepatic steatosis assessed by sonography and by elastography (r = 0.414 and P < 0.001). ALT levels were progressively higher in individuals with increasing degree of hepatic fat content measured by elastography (Fig. 1).
FIGURE 1.
ALT levels stratified by steatosis grade measured by transient elastography. ALT levels are progressively higher in individuals with increasing degree of hepatic steatosis assessed by transient elastography. Hepatic steatosis was graded as absent (S0, CAP ≤ 215 dB/m), mild (S1, CAP values between 216 and 252 dB/m), moderate (S2, CAP values between 253 and 296 dB/m), or severe (S3, CAP > 296 dB/m). The difference in ALT levels among steatosis grades was examined using linear regression analysis. Values were log-transformed before entering the model. Data are shown as mean; numbers in brackets above the bars represent the SDs.
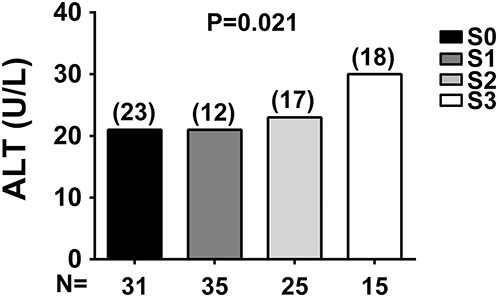
The PNPLA3 I148M genotypes and allele frequencies were in Hardy–Weinberg equilibrium (P = 0.144) with a minor allele frequency of 0.24. Carriers of the PNPLA3 148M allele had higher CAP values (P = 0.029) after adjusting for age, sex, BMI, and mesalamine use or after adjusting for age, sex, BMI, and treatment (P = 0.046) (see Tables, Supplemental Digital Content 1 and 2, http://links.lww.com/IBD/B85 and http://links.lww.com/IBD/B86), suggesting a higher content of fat in the liver. P value for CAP values remained significant also after adjusting for age, sex, BMI, mesalamine use, and disease duration (P = 0.033). Carriers of the 148M allele have higher prevalence of hepatic steatosis defined as CAP ≥ 216 db/m (P = 0.036 after adjusting for age, sex, BMI, and mesalamine use and P = 0.035 after adjusting also for disease duration, see Table, Supplemental Digital Content 1, http://links.lww.com/IBD/B85) and increased risk for this condition (odds ratio, 2.9, and confidence interval, 1.1–7.8) independently from other risk factors. After adjusting for age, sex, BMI, and treatment, P = 0.061 for hepatic steatosis (see Table, Supplemental Digital Content 3, http://links.lww.com/IBD/B87). In patients with CD, steatosis presence measured by transient elastography did not correlate with ileocecal resection status (r = −0.167 and P = 0.309) or with disease location (r = 0.221 and P = 0.176). PNPLA3 148M carriers had also higher ALT levels (P = 0.035, Fig. 2A and see Table, Supplemental Digital Content 1, http://links.lww.com/IBD/B85) after adjusting for age, sex, BMI, and mesalamine use or after adjusting for age, sex, BMI, and treatment (P = 0.021, see Table, Supplemental Digital Content 4, http://links.lww.com/IBD/B88). P value for ALT levels remained significant also after adjusting for age, sex, BMI, mesalamine use, and disease duration (P = 0.034). The frequency of individuals with ALT > 40 U/L was higher in carriers of the M allele (P = 0.004). No differences in the disease activity or disease location were detected across PNPLA3 genotypes.
FIGURE 2.
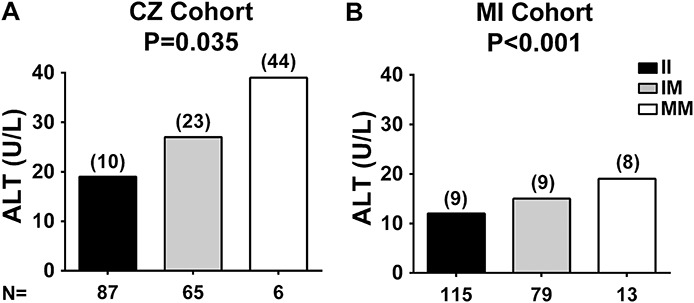
ALT levels stratified by PNPLA3 I148M genotype. In both Catanzaro (CZ) and Milan (MI) cohorts, ALT levels are higher in PNPLA3 148M allele carriers with CD or UC. The difference in ALT levels among PNPLA3 I148M genotypes in the (A) CZ cohort and (B) MI cohort was examined using linear regression analyses under an additive model after adjusting for age, sex, BMI, and mesalamine use (CZ cohort and MI cohort). Values were log-transformed before entering the model. Data are shown as mean; numbers in brackets above the bars represent the SDs. N, number of individuals; II, individuals with two 148I alleles; IM, heterozygotes; MM, individuals with two 148M alleles.
PNPLA3 148M Carriers with IBD Have Higher Serum Aminotransferase Activities in the Milan Cohort
To confirm our data, we examined the differences in ALT levels among PNPLA3 I148M genotypes in an independent cohort of individuals with IBD from Milan (Milan cohort, Table 2). In this cohort, the proportion of subjects with CD was higher and individuals were older (CD 34% versus 45% and P = 0.025; mean age 45 ± 13 versus 49 ± 15 yr and P = 0.012, in Catanzaro and Milan cohort, respectively). The proportion of individuals in remission was higher (44% versus 56% and P = 0.023). Furthermore, possibly because of the different clinical characteristics, overall ALT levels were lower in subjects from the Milan cohort compared with the Catanzaro cohort (14 ± 9 versus 23 ± 19 U/L, P < 0.001). Among individuals with CD from the Milan cohort, most had ileal or ileocolonic disease location, whereas among individuals with UC, most (44%) had proctosigmoiditis.
TABLE 2.
Anthropometric, Clinical, and Biochemical Characteristics of Individuals with CD or UC from Milan

The PNPLA3 I148M genotypes in the Milan cohort were in Hardy–Weinberg equilibrium (P = 0.908) with a minor allele frequency of 0.25. Consistent with the findings in the Catanzaro cohort, ALT levels were higher in carriers of the 148M allele from the Milan cohort (P < 0.001) after adjusting for age, sex, and mesalamine use (Fig. 2B). No differences in the frequency of individuals with ALT > 40 U/L, in the disease activity or disease location across PNPLA3 genotypes, were detected in the Milan cohort (see Table, Supplemental Digital Content 5, http://links.lww.com/IBD/B89).
PNPLA3 148M Carriers with CD and UC Have Higher Liver Enzymes
To investigate whether the increased ALT levels found in carriers of the PNPLA3 148M allele were disease specific, we examined ALT levels among genotypes separately in individuals with CD and UC after pooling the Catanzaro and Milan cohorts. Carriers of the PNPLA3 148M allele had higher ALT levels in both CD and UC subgroups (P = 0.002 and P < 0.001, respectively) after adjusting for age, sex, mesalamine use, and center of recruitment (Table 3 and Fig. 3). In a sensitivity analysis, we examined the impact of PNPLA3 148M on ALT separately in the 2 cohorts. ALT was higher in PNPLA3 148M carriers with CD and UC in both cohorts, although statistical significance was observed only in the Milan cohort (P = 0.002 and P = 0.001, respectively) (Table 3).
TABLE 3.
ALT Levels in Individuals with CD and in Those with UC Stratified by PNPLA3 I148M
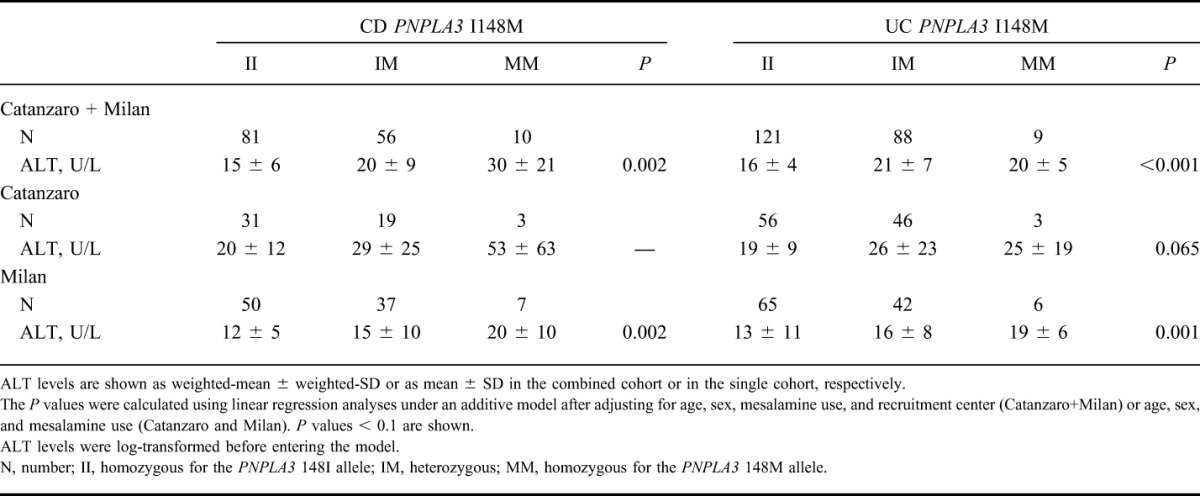
FIGURE 3.
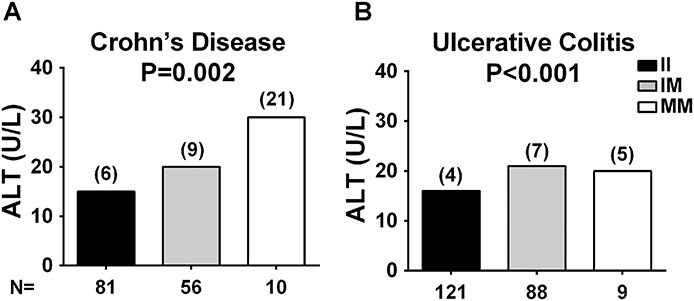
ALT levels stratified by PNPLA3 I148M genotype in subjects with CD or UC from the combined Catanzaro and Milan cohort. ALT levels are higher in PNPLA3 148M allele carriers with CD or UC. The difference in ALT levels among PNPLA3 I148M genotypes in individuals with (A) CD or (B) UC from the combined Catanzaro and Milan cohort was examined using linear regression analyses under an additive model after adjusting for age, sex, mesalamine use, and recruitment center. ALT values were log-transformed before entering the model. Data are shown as weighted-mean; numbers in brackets above the bars represent the weighted-SD. N, number of individuals; II, individuals with two 148I alleles; IM, heterozygotes; MM, individuals with two 148M alleles.
DISCUSSION
In this study, we report for the first time that PNPLA3 148M carriers with IBD have higher risk of hepatic steatosis and higher biomarker of liver damage. Specifically, we showed increased hepatic steatosis and higher fat content measured by transient elastography in PNPLA3 148M carriers with IBD from Southern Italy. We also showed that PNPLA3 148M carriers have higher hepatic damage measured by ALT levels in 2 independent cohorts from Italy. Among all medication treatment, mesalamine use was different across PNPLA3 genotypes. Therefore, at multivariate analysis, we adjusted the association of PNPLA3 genotype with liver damage for this confounder.
Sonography is the most used noninvasive method to assess the presence of hepatic steatosis. Transient elastography is a method used to estimate the severity of liver fibrosis and recently hepatic fat content. We found a strong correlation between hepatic steatosis assessed by sonography and the CAP evaluated by elastography, indicating that elastography allows detecting hepatic steatosis. ALT levels were progressively higher with increasing degree of hepatic fat content assessed by transient elastography. These data show that increased hepatic fat content is associated with higher ALT levels in individuals with IBD. We also found an increased frequency of individuals with ALT > 40 U/L in carriers of the M allele from the Catanzaro cohort. We did not detect the same difference in individuals from the Milan cohort. The lack of association in the Milan cohort is possibly explained by the lower overall ALT levels and different clinical profiles of this cohort.
IBD encompass CD and UC, 2 distinct diseases with peculiar features. The association between IBD and increased hepatic fat has been previously reported.5,6 To test whether the susceptibility to higher hepatic damage conferred by the PNPLA3 I148M variant was disease specific, we examined the ALT levels separately in the 2 diseases. We found carriers of the PNPLA3 148M allele to have higher liver damage in both CD and UC. These data suggest a common mechanism responsible for the susceptibility to hepatic steatosis in the 2 diseases.
It has been reported that hepatic steatosis is not related to IBD site, duration, disease activity, or medical treatment,6 although drug-induced liver damage has been described.4,29,30 Abnormal composition of the gut microbiota and increased intestinal permeability are a hallmark of IBD.1 Several studies show a relationship among the gut microbiota, intestinal permeability, and hepatic steatosis and damage.31–33 Inflamed gut mucosa is enriched in bacteria metabolizing lipids.34 PNPLA3 has a triglyceride hydrolase activity,8,9 but the mutant 148M protein is a loss of function. We, therefore, speculate that differences in the hepatic fat content may be due to a failure in the hydrolysis of triglycerides in carriers of the mutant protein. Additionally, when the bowel integrity is compromised the disruption of the enterohepatic circulation of bile salts, the altered composition of the gut microbiota and intestinal immunity may also be important underlying mechanisms.35 Indeed, increased intestinal permeability and subsequent exposure of the liver to cytokines and bacterial products may contribute in the pathogenesis of increased liver fat.
PNPLA3 has been studied in hepatocyte19 and hepatic stellate cells.7 Genetic studies on primary sclerosing cholangitis and on primary biliary cirrhosis suggest that this protein may have a role also in the cholangiocyte.36,37 Indeed, the I148M variant has been associated with worse prognosis in patients with severe primary sclerosing cholangitis, a disease of biliary tree strongly associated with IBD.37 We and others have shown that in a variety of conditions including obesity,14 viral infections,11,18 and excess alcohol intake,20 PNPLA3 148M carriers have higher hepatic fat content and liver damage. Here, we expand the interaction of PNPLA3 genotype also to the IBD.
In conclusion, our results show that PNPLA3 148M carriers with IBD have higher susceptibility to hepatic steatosis and liver damage. Future studies are warranted to understand the molecular mechanism behind this association.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.ibdjournal.org).
Supported by the Swedish Research Council (V.R., 254439006), the Swedish Heart Lung Foundation (244439007), the Swedish federal government funding under the LUA/ALF agreement (76290), the Novonordisk Foundation Grant for Excellence in Endocrinology (244439012), the Swedish Diabetes Foundation [DIA 2014-052] (S.R.), and from Ricerca Corrente Fondazione Ca' Granda IRCCS Policlinico of Milan, Associazione Malattie Metaboliche del Fegato ONLUS (L.V.). V.L. is supported by a cofinanced grant from the European Commission, the European Social Found, and Calabria Region.
The authors have no conflict of interest to disclose.
R. M. Mancina and R. Spagnuolo contributed equally. F. Caprioli, L. Valenti, and S. Romeo contributed equally as senior authors.
REFERENCES
- 1.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricart E, Panaccione R, Loftus EV, et al. Autoimmune disorders and extraintestinal manifestations in first-degree familial and sporadic inflammatory bowel disease: a case-control study. Inflamm Bowel Dis. 2004;10:207–214. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827–836. [DOI] [PubMed] [Google Scholar]
- 4.Vo HD, Xu J, Rabinowitz SS, et al. The liver in pediatric gastrointestinal disease. J Pediatr Gastroenterol Nutr. 2014;59:288–299. [DOI] [PubMed] [Google Scholar]
- 5.Navaneethan U, Shen B. Hepatopancreatobiliary manifestations and complications associated with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1598–1619. [DOI] [PubMed] [Google Scholar]
- 6.Bargiggia S, Maconi G, Elli M, et al. Sonographic prevalence of liver steatosis and biliary tract stones in patients with inflammatory bowel disease: study of 511 subjects at a single center. J Clin Gastroenterol. 2003;36:417–420. [DOI] [PubMed] [Google Scholar]
- 7.Pirazzi C, Valenti L, Motta BM, et al. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Hum Mol Genet. 2014;23:4077–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pingitore P, Pirazzi C, Mancina RM, et al. Recombinant PNPLA3 protein shows triglyceride hydrolase activity and its I148M mutation results in loss of function. Biochim Biophys Acta. 2013;1841:574–580. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Cohen JC, Hobbs HH. Expression and characterization of a PNPLA3 isoform (I148M) associated with nonalcoholic fatty liver disease. J Biol Chem. 2011;286:37085–37093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burza MA, Pirazzi C, Maglio C, et al. PNPLA3 I148M (rs738409) genetic variant is associated with hepatocellular carcinoma in obese individuals. Dig Liver Dis. 2012;44:1037–1041. [DOI] [PubMed] [Google Scholar]
- 11.Valenti L, Rumi M, Galmozzi E, et al. Patatin-Like phospholipase domain-containing 3 I148M polymorphism, steatosis, and liver damage in chronic hepatitis C. Hepatology. 2011;53:791–799. [DOI] [PubMed] [Google Scholar]
- 12.Corradini SG, Burza MA, Molinaro A, et al. Patatin-like phospholipase domain containing 3 sequence variant and hepatocellular carcinoma. Hepatology. 2011;53:1776; author reply 1777. [DOI] [PubMed] [Google Scholar]
- 13.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romeo S, Sentinelli F, Dash S, et al. Morbid obesity exposes the association between PNPLA3 I148M (rs738409) and indices of hepatic injury in individuals of European descent. Int J Obes (Lond). 2010;34:190–194. [DOI] [PubMed] [Google Scholar]
- 15.Miraglia Del Giudice E, Grandone A, Cirillo G, et al. The association of PNPLA3 variants with liver enzymes in childhood obesity is driven by the interaction with abdominal fat. PLoS One. 2011;6:e27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis JN, Le KA, Walker RW, et al. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am J Clin Nutr. 2011;92:1522–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santoro N, Savoye M, Kim G, et al. Hepatic fat accumulation is modulated by the interaction between the rs738409 variant in the PNPLA3 gene and the dietary omega6/omega3 PUFA intake. PLoS One. 2012;7:e37827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vigano M, Valenti L, Lampertico P, et al. PNPLA3 I148M affects liver steatosis in patients with chronic hepatitis B. Hepatology. 2013;58:1245–1252. [DOI] [PubMed] [Google Scholar]
- 19.Dongiovanni P, Donati B, Fares R, et al. PNPLA3 I148M polymorphism and progressive liver disease. World J Gastroenterol. 2013;19:6969–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burza MA, Molinaro A, Attilia ML, et al. PNPLA3 I148M (rs738409) genetic variant and age at onset of at-risk alcohol consumption are independent risk factors for alcoholic cirrhosis. Liver Int. 2013;34:514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. [DOI] [PubMed] [Google Scholar]
- 22.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 23.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed). 1986;292:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Needleman L, Kurtz AB, Rifkin MD, et al. Sonography of diffuse benign liver disease: accuracy of pattern recognition and grading. AJR Am J Roentgenol. 1986;146:1011–1015. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz Y, Ergelen R, Akin H, et al. Noninvasive detection of hepatic steatosis in patients without ultrasonographic evidence of fatty liver using the controlled attenuation parameter evaluated with transient elastography. Eur J Gastroenterol Hepatol. 2013;25:1330–1334. [DOI] [PubMed] [Google Scholar]
- 26.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. [DOI] [PubMed] [Google Scholar]
- 27.de Lédinghen V, Vergniol J, Foucher J, et al. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911–918. [DOI] [PubMed] [Google Scholar]
- 28.Valenti L, Al-Serri A, Daly AK, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209–1217. [DOI] [PubMed] [Google Scholar]
- 29.Gisbert JP, Luna M, González-Lama Y, et al. Liver injury in inflammatory bowel disease: long-term follow-up study of 786 patients. Inflamm Bowel Dis. 2007;13:1106–1114. [DOI] [PubMed] [Google Scholar]
- 30.Cappello M, Randazzo C, Bravatà I, et al. Liver function test abnormalities in patients with inflammatory bowel diseases: a hospital-based survey. Clin Med Insights Gastroenterol. 2014;7:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gangarapu V, Yıldız K, Ince AT, et al. Role of gut microbiota: obesity and NAFLD. Turk J Gastroenterol. 2014;25:133–140. [DOI] [PubMed] [Google Scholar]
- 32.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. [DOI] [PubMed] [Google Scholar]
- 33.Yang SQ, Lin HZ, Lane MD, et al. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94:2557–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davenport M, Poles J, Leung JM, et al. Metabolic alterations to the mucosal microbiota in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visschers RG, Luyer MD, Schaap FG, et al. The gut-liver axis. Curr Opin Clin Nutr Metab Care. 2013;16:576–581. [DOI] [PubMed] [Google Scholar]
- 36.Krawczyk M, Milkiewicz M, Marschall HU, et al. Variant adiponutrin confers genetic protection against cholestatic itch. Sci Rep. 2014;4:6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedrich K, Rupp C, Hov JR, et al. A frequent PNPLA3 variant is a sex specific disease modifier in PSC patients with bile duct stenosis. PLoS One. 2013;8:e58734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


