Abstract:
Initial investigations reported GATA3 to be a sensitive and relatively specific marker for mammary and urothelial carcinomas. Recently, GATA3 expression has been described in several other epithelial tumors. However, there has been only limited investigation of GATA3 expression in cutaneous epithelial tumors. The objective of this study was to examine the immunohistochemical expression of GATA3 in a wide variety of cutaneous epithelial neoplasms. GATA3 expression was evaluated in 99 benign and 63 malignant cutaneous epithelial tumors. GATA3 was consistently and usually strongly expressed in clear cell acanthoma, trichofolliculoma, trichoepithelioma, trichilemmoma, sebaceous adenoma, sebaceoma, apocrine hidrocystoma, apocrine tubular papillary adenoma, hidradenoma papilliferum, and syringocystadenoma papilliferum. Hidradenomas exhibited variable positive staining. Most poromas, syringomas, chondroid syringomas, cylindromas, and spiradenomas were negative or only focally and weakly positive. Focal staining was present in all pilomatrixomas. Thirteen of 14 basal cell carcinomas, 21 of 24 squamous carcinomas, and all 6 sebaceous carcinomas exhibited positive staining. The 1 apocrine carcinoma, both mucinous carcinomas, and 2 of 3 microcystic adnexal carcinomas also exhibited positive staining, whereas the 1 eccrine porocarcinoma and the 1 adenoid cystic carcinoma were negative. One of 11 Merkel cell carcinomas exhibited focal weak staining. Our findings demonstrate that GATA3 is expressed in a wide variety of benign and malignant cutaneous epithelial neoplasms. In addition to carcinomas of breast and urothelial origin and other more recently described GATA3-positive tumors, the differential diagnosis of a metastatic tumor of unknown primary origin that expresses GATA3 should also include a carcinoma of cutaneous epithelial origin.
Key Words: cutaneous adnexal neoplasms, epidermal neoplasms, GATA3, immunohistochemistry, skin
INTRODUCTION
GATA3 is a member of the GATA family of zinc finger nuclear transcription factors, which bind to G-A-T-A nucleotide sequences in the promoter regions of target genes, thereby activating or suppressing the function of these genes.1,2 GATA3 is involved in the development and differentiation of many cell types, such as the luminal glandular epithelium of the breast,3,4 T-lymphocytes,5–7 parathyroid gland,8 kidney,9 sympathetic nervous system,10 and hair follicles of the skin.11–13
Initial studies on the immunohistochemical expression of GATA3 in tumors suggested that GATA3 is relatively sensitive and specific for tumors of breast or urothelial origin,14,15 and GATA3 has therefore been advocated as a useful marker for identifying tumors of breast or urothelial origin in the setting of a metastatic carcinoma of unknown primary origin.15 Several very recent publications, however, have broadened the spectrum of tumors with reported positive immunoreactivity for GATA3, including transitional cell proliferations in the female genital tract,16 certain salivary gland tumors,17 and parathyroid tumors,18,19 as well as malignant mesotheliomas, pancreatic adenocarcinomas, oncocytomas and chromophobe carcinomas of the kidney, choriocarcinomas, and endodermal sinus tumors.20 Immunoreactivity for GATA3 has also been described in several nonepithelial neoplasms, including pheochromocytomas, paragangliomas, neuroblastomas, ganglioneuroblastomas, ganglioneuromas, and the epithelial component of biphasic synovial sarcomas.19–21
Although GATA3 is known to play a role in epithelial differentiation in the epidermis and hair follicles, the distribution of immunoreactivity for GATA3 in normal skin has not been fully described, and there is only limited published information on the expression of GATA3 in cutaneous epithelial neoplasms.20,22–25 The objectives of this study were therefore to examine the distribution of immunoreactivity for GATA3 in normal skin and to investigate the expression of GATA3 in a wide variety of benign and malignant neoplasms of the epidermis and skin adnexal structures.
MATERIALS AND METHODS
Study Design
Institutional review board approval was obtained for the study. One hundred sixty-four cases of benign and malignant epidermal and cutaneous adnexal neoplasms were retrieved from the surgical pathology archives of Cedars-Sinai Medical Center from 1990 to 2013. Hematoxylin and eosin slides were reviewed, and the neoplasms were classified independently by 2 experienced dermatopathologists (B.L.B. and D.P.F.) according to the WHO classification of skin tumors26; initial discrepancies in classification were resolved by subsequent review of slides using a double-headed microscope. Two cases (1 benign and 1 malignant) for which a consensus diagnosis could not be reached were excluded from the study. Of the remaining 162 neoplasms that were included in the study, 99 were benign tumors and 63 were malignant tumors. Of the 14 basal cell carcinomas, 6 exhibited a superficial pattern, 4 exhibited a nodular pattern, and 4 exhibited a mixed nodular and infiltrative pattern; 2 of the basal cell carcinomas were pigmented (1 with a superficial pattern and 1 with a nodular pattern). Of the 24 squamous carcinomas, 7 were well differentiated, 9 were moderately differentiated, and 8 were poorly differentiated (including 2 poorly differentiated tumors with spindle-cell features).
Immunohistochemistry
Immunohistochemical detection of GATA3 was performed on 4-μm tissue sections using Biocare Medical monoclonal antibody (clone L50-823; Concord, CA) at 1:600 dilution. Staining was performed on the Ventana BenchMark Ultra (Tucson, AZ) automated slide stainer using onboard heat-induced epitope retrieval method in high pH CC1 buffer, and staining was demonstrated using the Ventana ultraView DAB detection system. The slides were subsequently counterstained with Mayer's hematoxylin. Only nuclear staining for GATA3 was scored; cytoplasmic staining was generally absent or minimal/weak. In those cases in which nonneoplastic epithelium was also present in the histological sections, the intensity of staining of nonneoplastic epithelium was graded as weak, moderate, or strong. The extent of staining of the neoplastic cells for GATA3 was graded as 0 (negative), 1+ (≤10%), 2+ (11%–50%), or 3+ (>50%). The intensity of staining of tumor cells was graded as weak (1+) or strong (2+ or 3+). The extent and intensity of staining of the tumor were scored independently by 2 pathologists (M.N.P.V. and R.B.M.), and initial discrepancies in scoring were resolved by subsequent review of stains using a double-headed microscope.
RESULTS
GATA3 Expression in Normal Epidermis and Cutaneous Adnexal Structures
GATA3 expression in normal epidermis and cutaneous adnexal structures is illustrated in Figure 1. Normal epidermis exhibited positive staining for GATA3; the staining was generally of moderate to strong intensity and was patchy to diffuse in the basal layer and diffuse in the spinous layer. The granular layer was consistently negative for GATA3. Staining for GATA3 was often reduced and sometimes absent in the epidermis directly overlying tumors. Hair follicles demonstrated positive staining for GATA3, with the intensity of staining varying in different regions of the follicle. The strongest staining for GATA3 in all epithelial components of the skin was observed in certain layers of the inner root sheath of the hair follicles, which consistently demonstrated intense strongly positive staining, whereas the matrix area of the hair bulb was negative for GATA3. The outer root sheath layers typically exhibited weak to moderately strong staining for GATA3. Weak to moderately strong staining for GATA3 was also observed in most cells of sebaceous glands, with stronger staining observed in the peripheral germinative cells and weaker staining in the differentiated vacuolated cells in the central portions of the glands; some mature sebocytes in the central portions of the glands were negative for GATA3. Within apocrine sweat glands, weakly to strongly positive staining for GATA3 was observed in the majority of the inner layer of cells within the secretory coils and excretory ducts, whereas the outer cell layer of both the secretory coils and excretory ducts appeared to be mostly negative for GATA3. In contrast, both the secretory coils and excretory ducts of eccrine glands were typically negative for GATA3, although infrequently, a few positive cells were seen within the secretory coil, and weak staining of epithelial cells lining the excretory ducts was also rarely observed. Nuclear staining for GATA3 was also observed in some background lymphocytes in cases in which chronic inflammation was present.
FIGURE 1.
GATA3 expression in normal skin. Epidermis (×200): positive staining for GATA3 is present in the basal and spinous layers of the epidermis but is absent in the granular cell layer. Pilosebaceous unit (×100): positive staining for GATA3 is seen in both the outer root sheath of the hair follicle and in the sebaceous glands. Hair bulb and root sheath (×100): strong positive staining for GATA3 is present in some layers of the inner root sheath, with less intense staining also present in the outer root sheath, whereas the matrix cells of the hair bulb are negative for GATA3. Sweat glands (×200): apocrine glands (left) exhibit positive staining for GATA3, whereas eccrine glands (right) are negative for GATA3.
GATA3 Expression in Benign Epidermal and Cutaneous Adnexal Neoplasms
The results of GATA3 staining in the 99 benign epidermal and cutaneous adnexal neoplasms are summarized in Table 1, and representative examples are illustrated in Figure 2. GATA3 was consistently expressed in clear cell acanthomas (3+, strong). Among tumors of hair follicle origin, extensive staining for GATA3 was observed in trichofolliculomas (3+, strong), trichoepitheliomas (3+, strong), and trichilemmomas (3+, weak to strong), whereas only focal (1+ or 2+) weak to strong staining for GATA3 was present in pilomatrixomas. Most constituent cells of pilomatrixomas in fact were negative for GATA3, although a very characteristic feature of all pilomatrixomas was the presence of very focal aggregates of cells with intensely positive nuclear staining. All sebaceous neoplasms were positive for GATA3, with 3+, weak to strong staining seen in sebaceous adenomas and 3+, strong staining seen in the 1 case of sebaceoma. Among sweat gland tumors, GATA3 was expressed in all cases of apocrine hidrocystoma, hidradenoma papilliferum, and syringocystadenoma papilliferum, with most cases showing 2+ or 3+, strong staining, although 3+, weak staining was observed in 1 case each of apocrine hidrocystoma and syringocystadenoma papilliferum and in the single case of apocrine tubular papillary adenoma. In these sweat gland tumors, in which the neoplastic epithelium is characteristically bilayered, positive staining was generally limited to the inner layer of epithelial cells. Hidradenomas were consistently positive for GATA3 but exhibited a wide range of staining extent and intensity, ranging from 1+ to 3+ in extent and weak to strong in intensity. Most cases of poroma, syringoma, chondroid syringoma, cylindroma, and spiradenoma were negative or only focally (1+) and weakly positive for GATA3.
TABLE 1.
Immunoreactivity for GATA3 in Benign Cutaneous Epithelial Neoplasms
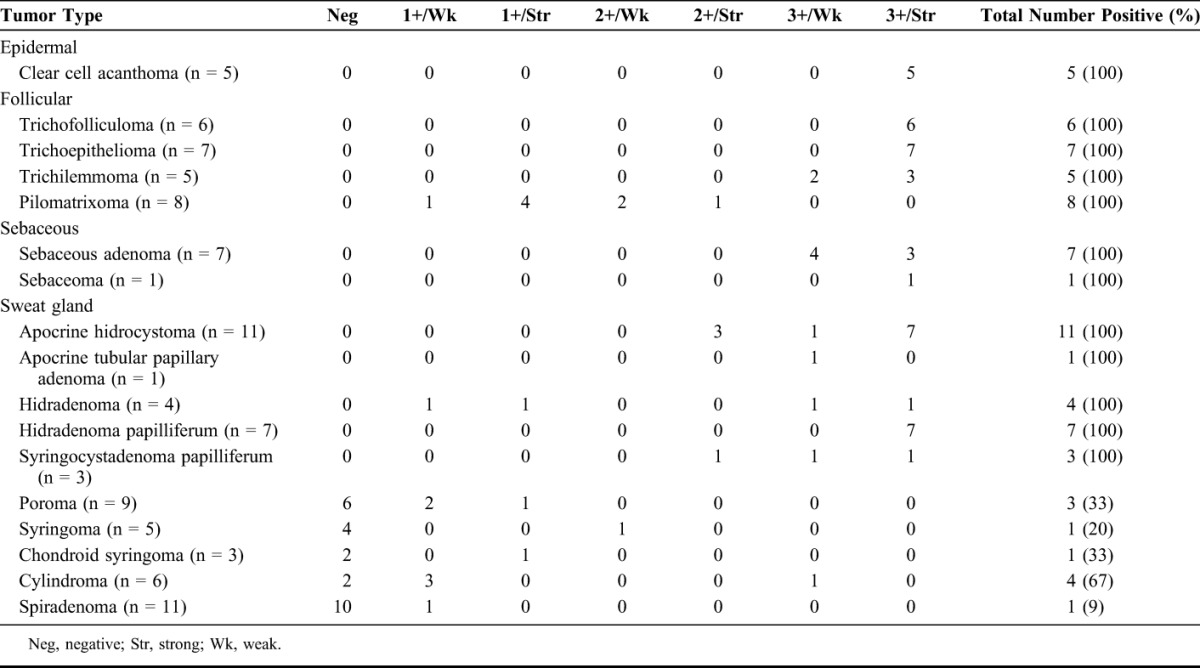
FIGURE 2.
GATA3 expression in benign cutaneous epithelial neoplasms. Positive staining for GATA3 is demonstrated in these examples of trichofolliculoma (×200), sebaceous adenoma (×200), and hidradenoma papilliferum (×200), whereas the spiradenoma (×200) is negative for GATA3.
GATA3 Expression in Malignant Epidermal and Cutaneous Adnexal Neoplasms
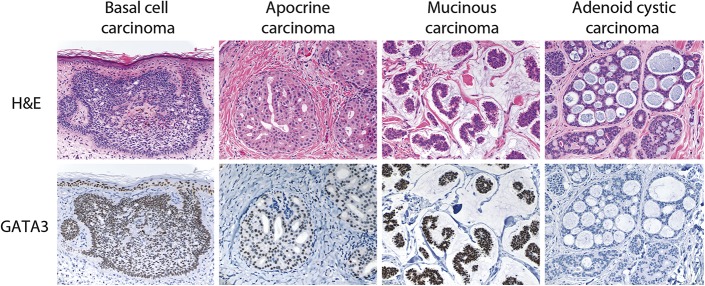
The results of GATA3 staining in the 63 malignant epidermal and cutaneous adnexal neoplasms are summarized in Table 2, and representative examples are illustrated in Figure 3. The majority of both basal cell carcinomas (13/14) and squamous carcinomas (21/24) were positive for GATA3. All positively stained basal cell carcinomas exhibited 3+, strong staining. The 1 negative basal cell carcinoma exhibited a mixed nodular and infiltrative growth pattern. In contrast, the positively stained squamous carcinomas exhibited a wider range of staining extent and intensity, ranging from 1+ to 3+ in extent and weak to strong in intensity. Staining for GATA3 was diffuse (3+) and strong in only 4 of the squamous carcinomas, and 8 of the squamous carcinomas exhibited only focal (1+) and weak staining for GATA3. The 3 negative squamous carcinomas included 1 well differentiated tumor, 1 moderately differentiated tumor, and 1 poorly differentiated tumor with spindle-cell features. The staining intensity was weak in all positively stained well differentiated squamous carcinomas but was weak or strong in the positively stained moderately differentiated and poorly differentiated squamous carcinomas. In the 1 case of basal cell carcinoma and the 3 cases of squamous carcinoma that were negative for GATA3, intact positive staining for GATA3 was observed in benign epidermis, hair follicles, and (when present) sebaceous glands, which served as internal positive controls in these cases. All 6 sebaceous carcinomas exhibited positive staining for GATA3, ranging from 1+ to 3+ in extent and usually weak in intensity, although 1 tumor exhibited 3+, strong staining. Among the small number of malignant tumors of sweat gland origin included in the study, the single apocrine carcinoma and both mucinous carcinomas exhibited 3+, strong staining for GATA3 and 2 of 3 microcystic adnexal carcinomas were also positive for GATA3 (2+, weak or strong staining), whereas the single adenoid cystic carcinoma and the single eccrine porocarcinoma were both negative for GATA3. One of 11 Merkel cell carcinomas exhibited 1+, weak staining for GATA3, whereas the remainder were negative.
TABLE 2.
Immunoreactivity for GATA3 in Malignant Cutaneous Epithelial Neoplasms

FIGURE 3.
GATA3 expression in malignant cutaneous epithelial neoplasms. Positive staining for GATA3 is demonstrated in these examples of basal cell carcinoma (×200), apocrine carcinoma (×200), and mucinous carcinoma (×200), whereas the adenoid cystic carcinoma (×200) is negative for GATA3.
DISCUSSION
Initial investigations on the distribution of GATA3 in tumors indicated that GATA3 is a sensitive and relatively specific marker for carcinomas of breast and urothelial origin,14,15 but more recent studies have demonstrated that GATA3 is also expressed in several other epithelial tumors, such as certain salivary gland tumors17 and parathyroid tumors.18,19 Although GATA3 has been known for some time to play a role in epithelial differentiation in the skin, there are few published reports on the expression of GATA3 in cutaneous epithelial neoplasms.20,22–25 Our study represents the largest published series of cutaneous epithelial neoplasms studied for the expression of GATA3 and demonstrates that GATA3 is expressed in a wide variety of benign and malignant epidermal and cutaneous adnexal neoplasms, as well as in normal epidermis and most skin adnexal structures.
The expression of GATA3 in normal skin and cutaneous adnexal structures has not been fully described in the literature, with most reports focusing only on the expression of GATA3 within the epidermis and/or hair follicles. In a recently published limited description of the expression of GATA3 in normal adult skin, Miettinen et al20 reported that GATA3 was expressed in epidermis, sebaceous epithelia, and hair shafts, with extensive positive staining in apocrine glands and focal positive staining in eccrine sweat glands. Our observations in normal skin adjacent to tumors are generally similar to those of Miettinen et al20 but are described in greater detail. In the epidermis, staining for GATA3 was limited to the basal and spinous layers, whereas the granular cell layer was consistently negative. This distribution of GATA3 expression within the epidermis has previously been described by some authors,27 whereas others have claimed that GATA3 is expressed only in the spinous layer13 or is found in all layers of the epidermis.28 In our study, the various components of the hair follicle exhibited differential staining for GATA3; intensely positive staining for GATA3 was observed in certain layers of the inner root sheath, whereas the matrix region of the hair bulb was negative for GATA3, and the outer root sheath exhibited weak to moderately strong staining for GATA3. Our finding of strong staining of the inner root sheath is consistent with previously reported studies on the distribution of GATA3 in hair follicles and its role in folliculogenesis. In elegant studies using immunofluorescence to examine differentiation of hair follicles in mouse embryos, Kaufman et al11 demonstrated that expression of GATA3 in hair follicles is restricted to the Huxley and cuticle layers of the inner root sheath, whereas the Henley layer of the inner root sheath is negative for GATA3. Localization of GATA3 to the inner root sheath of hair follicles was also confirmed by immunofluorescence studies performed by Chikh et al27 on human skin. Using immunoperoxidase studies on human skin, Sellheyer and Krahl13 also demonstrated that GATA3 expression in hair follicles is confined to the Huxley layer and inner root sheath cuticle. Expression of GATA3 in the outer root sheath (which we observed in this study) was not described in these 3 prior studies but had previously been described by Kurek et al12 in mouse embryos during anagen phase of the hair growth cycle. It is perhaps not surprising that GATA3 would be expressed in the outer root sheath, as the outer root sheath is contiguous with and biochemically similar to the basal layer of the epidermis from which it is derived and which in our study also expressed GATA3 in a diffuse or patchy fashion. The expression of GATA3 in normal sebaceous glands and sweat glands has received little attention in the literature. Like Miettinen et al,20 we observed positive staining for GATA3 in sebaceous glands and in apocrine glands. However, whereas Miettinen et al20 described eccrine sweat glands as being focally positive for GATA3 and Lee et al22 reported that staining for GATA3 was seen in eccrine glands (in addition to basal keratinocytes and sebocytes), we found the majority of eccrine sweat glands to be entirely negative for GATA3; these results are similar to those of Deftereos et al24 who reported that normal eccrine adnexal structures were negative for GATA3.
Our observation that expression of GATA3 was often decreased or sometimes even absent in the epidermis overlying tumors suggests downregulation of GATA3 expression in reactive epidermal squamous epithelium; this hypothesis is supported by similar observations of reduced GATA3 expression in psoriatic skin and in conditions of epidermal regeneration during wound healing.28
There are only limited published reports on the expression of GATA3 in benign tumors of the epidermis and skin adnexal structures. In the study by Miettinen et al,20 immunoreactivity for GATA3 was reportedly identified in trichoepitheliomas, basaloid cells of pilomatrixomas, epithelia of pilar cysts, and seborrheic keratoses (4 cases of each studied), and focal positivity was seen in mixed tumors of the skin, cylindromas, eccrine spiradenomas, and hidradenomas/eccrine acrospiromas (2 cases of each studied). In the herein reported much larger series of benign cutaneous epithelial neoplasms, we also observed positive staining in cases of trichoepithelioma, pilomatrixoma (focal), and hidradenoma, whereas all 10 cases of spiradenoma, 2 of 3 cases of chondroid syringoma (cutaneous mixed tumor), and 2 of 6 cases of cylindroma were negative for GATA3. The reasons for these discrepancies between our observations and those of Miettinen et al20 are unclear. We also observed positive staining for GATA3 in all benign sebaceous gland tumors, which were not studied by Miettinen et al.20 In abstract form, Tilson et al25 have recently reported that nearly all skin adnexal tumors of follicular origin and sebaceous origin exhibited positive immunoreactivity for GATA3, whereas staining for GATA3 was variable in sweat gland tumors, most of which were negative for GATA3 or exhibited only focal staining; the authors indicate that most of the cases included in their study were benign skin adnexal neoplasms. With regard to the staining of pilomatrixomas for GATA3, we found that most cells of pilomatrixomas were negative for GATA3, similar to cells of the matrix region of the normal hair follicle. However, a characteristic feature of all pilomatrixomas was the presence of very focal aggregates of cells with intensely positive nuclear staining, reminiscent of the strong staining seen in some layers of the inner root sheath of the normal hair follicle.
Initial investigations that concluded that GATA3 is a relatively specific marker for carcinomas of breast or urothelial origin largely neglected malignant cutaneous epithelial neoplasms in their study design. In the study by Liu et al,15 which surveyed the expression of GATA3 in 1110 tumors of various organs and histological types, 100 melanomas were examined (all of which were negative for GATA3), but no malignant cutaneous epithelial neoplasms were included in the study. Likewise, in the study by Higgins et al,14 which examined the expression of GATA3 in 1211 tissue cores from a variety of tumor sites (concentrating on tumors of the bladder, kidney, and prostate), cutaneous epithelial neoplasms were not specifically mentioned as having been included in the study; melanomas were reportedly negative for GATA3, and squamous carcinomas of unspecified site of origin were reportedly negative for GATA3, but it is uncertain whether any of these squamous carcinomas were of cutaneous origin.
In the recently published large survey of the expression of GATA3 in 2500 epithelial and nonepithelial tumors, Miettinen et al20 described positive immunoreactivity in 61 of 62 basal cell carcinomas of the skin and in 25 of 31 squamous carcinomas of the skin, but no malignant skin adnexal tumors were studied; the 4 Merkel cell carcinomas were all negative for GATA3. Positive staining of the majority of cutaneous basal cell carcinomas and squamous carcinomas for GATA3 has also been recently reported in abstract form by Deftereos et al,24 and absence of staining of Merkel cell tumors for GATA3 has been previously noted by Nonaka et al19 and Deftereos et al.24 Reports of the expression of GATA3 in malignant sweat gland tumors22,23,25 and sebaceous carcinomas25 have only very recently appeared in the literature.
Our study confirms the expression of GATA3 in the vast majority of basal cell carcinomas and in the majority of cutaneous squamous carcinomas, though whereas the staining was generally diffuse and strong in basal cell carcinomas, a wide range of staining extent and intensity was observed in squamous carcinomas. With the exception of 1 case, which exhibited focal weak staining for GATA3, all cases of Merkel cell carcinoma in our study were negative for GATA3, similar to the results of Miettinen et al,20 Nonaka et al,19 and Deftereos et al.24 Our study also demonstrated the presence of GATA3 in a subset of malignant skin adnexal tumors. Positive staining of variable extent and intensity was observed in all 6 sebaceous carcinomas, similar to the results recently reported in abstract form by Tilson et al.25 Only small numbers of malignant sweat gland tumors were included in our study, reflecting the scarcity of such tumors in clinical practice and precluding any definitive statements regarding GATA3 expression in any particular type of malignant sweat gland tumor. However, 5 of 8 malignant sweat gland tumors were positive for GATA3, including 1 apocrine carcinoma, both mucinous carcinomas, and 2 of 3 microcystic adnexal carcinomas. The 1 adenoid cystic carcinoma, the 1 eccrine porocarcinoma, and 1 of the 3 microcystic adnexal carcinomas included in the study were negative for GATA3. Our results are generally similar to and extend the observations of Lee et al,22 who have recently published the largest series of malignant sweat gland carcinomas studied for GATA3 expression to date. In that study, 4 of 11 eccrine carcinomas, all 9 apocrine carcinomas, 2 of 7 hidradenocarcinomas, 3 of 15 porocarcinomas, 5 of 12 microcystic adnexal carcinomas, all 3 trichilemmal carcinomas, and both mucinous carcinomas were positive for GATA3; no adenoid cystic carcinomas were examined for GATA3 expression in their study.
The histogenesis of particular types of sweat gland tumors is frequently a matter of debate, with uncertainty in the literature as to whether the tumors are of eccrine or apocrine origin.26 The distinctly different expression of GATA3 in normal eccrine glands and apocrine glands raises the possibility that GATA3 may be a useful marker of apocrine origin or differentiation in sweat gland tumors. Among benign sweat gland tumors, it is interesting to note that tumors considered to be of unequivocal apocrine origin or differentiation (apocrine hidrocystoma, apocrine tubular papillary neoplasm, and hidradenoma papilliferum) were all consistently positive for GATA3 (analogous to the positive staining observed in normal apocrine glands), whereas syringomas (generally considered to be tumors of eccrine origin) were usually negative for GATA3, akin to normal eccrine glands. There is less unanimity in the literature regarding the histogenesis of other benign sweat gland tumors, including hidradenoma, syringocystadenoma papilliferum, poroma, chondroid syringoma, cylindroma, and spiradenoma. The presence or absence of GATA3 expression in these tumors may provide an immunohistochemical clue to their histogenesis. For example, the positive staining for GATA3 observed in our cases of syringocystadenoma papilliferum is more supportive of apocrine differentiation than eccrine differentiation. On the other hand, poromas, chondroid syringomas, cylindromas, and spiradenomas were all either negative or only focally and weakly positive for GATA3, suggesting that these tumors (like syringomas) are more likely of eccrine than apocrine origin or differentiation. Hidradenomas exhibited quite variable staining for GATA3, raising the possibility that some may be of eccrine and others of apocrine origin or differentiation. Similar observations and conclusions may also be applicable to malignant sweat gland tumors, but the numbers of malignant sweat gland tumors included in our study were too small to draw any definitive conclusions in this regard. Study of GATA3 expression in a larger series of benign and malignant sweat gland tumors will be required to more clearly determine the utility of GATA3 expression as a marker of apocrine differentiation in sweat gland tumors.
In summary, our findings expand on previously published studies on the distribution of immunoreactivity for GATA3 in normal skin and on the spectrum of expression of GATA3 in cutaneous epithelial neoplasms. These results confirm that carcinomas of cutaneous epithelial origin should be included in the differential diagnosis of a metastatic carcinoma of unknown primary origin which is positive for GATA3. This is of particular importance in a woman with metastatic carcinoma of unknown primary origin in an axillary lymph node; in this setting, immunoreactivity of the tumor for GATA3 should not be construed as proof of mammary origin of the metastatic tumor.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical assistance of Fai Chung in the performance of immunohistochemical studies and Drs. Jinping Lai and Brent Larson in the preparation of the figures.
Footnotes
The authors declare no conflicts of interest.
Presented in part at the 103rd Annual Meeting of the United States and Canadian Academy of Pathology, March 1–7, 2014, San Diego, CA.
REFERENCES
- 1.Chou J, Provot S, Werb Z. GATA3 in development and cancer differentiation: cells GATA have it! J Cell Physiol. 2010;222:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng R, Blobel GA. GATA transcription factors and cancer. Genes Cancer. 2010;1:1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asselin-Labat ML, Sutherland KD, Barker H, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. [DOI] [PubMed] [Google Scholar]
- 4.Kouros-Mehr H, Slorach EM, Sternlicht MD, et al. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosoya T, Maillard I, Engel JD. From the cradle to the grave: activities of GATA-3 throughout T-cell development and differentiation. Immunol Rev. 2010;238:110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yagi R, Zhu J, Paul WE. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int Immunol. 2011;23:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oosterwegel M, Timmerman J, Leiden J, et al. Expression of GATA-3 during lymphocyte differentiation and mouse embryogenesis. Dev Immunol. 1992:3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grigorieva IV, Thakker RV. Transcription factors in parathyroid development: lessons from hypoparathyroid disorders. Ann N Y Acad Sci. 2011;1237:24–38. [DOI] [PubMed] [Google Scholar]
- 9.Grote D, Souabni A, Busslinger M, et al. Pax2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development. 2006;133:53–61. [DOI] [PubMed] [Google Scholar]
- 10.Tsarovina K, Pattyn A, Stubbusch J, et al. Essential role of Gata transcription factors in sympathetic neuron development. Development. 2004;131:4775–4786. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman CK, Zhou P, Pasolli HA, et al. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003;17:2108–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurek D, Garinis GA, van Doorninck JH, et al. Transcriptome and phenotypic analysis reveals Gata3-dependent signaling pathways in murine hair follicles. Development. 2007;134:261–272. [DOI] [PubMed] [Google Scholar]
- 13.Sellheyer K, Krahl D. Expression pattern of GATA-3 in embryonic and fetal human skin suggests a role in epidermal and follicular morphogenesis. J Cutan Pathol. 2010;37:357–361. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Kaygusuz G, Wang L, et al. Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol. 2007;31:673–680. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Shi J, Wilkerson ML, et al. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol. 2012;138:57–64. [DOI] [PubMed] [Google Scholar]
- 16.Esheba GE, Longacre TA, Atkins KA, et al. Expression of the urothelial differentiation markers GATA3 and placental S100 (S100P) in female genital tract transitional cell proliferations. Am J Surg Pathol. 2009;33:347–353. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz LE, Begum S, Westra WH, et al. GATA3 immunohistochemical expression in salivary gland neoplasms. Head Neck Pathol. 2013;7:311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ordonez NG. Value of GATA3 immunostaining in tumor diagnosis: a review. Adv Anat Pathol. 2013;20:352–360. [DOI] [PubMed] [Google Scholar]
- 19.Nonaka D, Wang BY, Edmondson D, et al. A study of Gata3 and Phox2b expression in tumors of the autonomic nervous system. Am J Surg Pathol. 2013;37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 20.Miettinen M, McCue PA, Sarlomo-Rikala M, et al. GATA3: a multispecific but potentially useful marker in surgical pathology. A systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol. 2014;38:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.So JS, Epstein JI. GATA3 expression in paragangliomas: a pitfall potentially leading to misdiagnosis of urothelial carcinoma. Mod Pathol. 2013;26:1365–1370. [DOI] [PubMed] [Google Scholar]
- 22.Lee JJ, Mochel MC, Piris A, et al. p40 exhibits better specificity than p63 in distinguishing primary skin adnexal carcinomas from cutaneous metastases. Hum Pathol. 2014;45:1078–1083. [DOI] [PubMed] [Google Scholar]
- 23.Piris A, Peng Y, Boussahmain C, et al. Cutaneous and mammary apocrine carcinomas have different immunoprofiles. Hum Pathol. 2014;45:320–326. [DOI] [PubMed] [Google Scholar]
- 24.Deftereos G, Uchin JM, Silverman JF, et al. GATA3 immunohistochemistry expression in primary cutaneous tumors and breast carcinomas metastatic to the skin. Mod Pathol. 2014;27(suppl 2):133A. [Google Scholar]
- 25.Tilson MP, Illei PB, Argani P, et al. The utility of GATA3 in distinguishing breast ductal carcinoma from skin adnexal neoplasms. Mod Pathol. 2014;27(suppl 2):143A. [Google Scholar]
- 26.LeBoit PE, Burg G, Weedon D, et al., eds. World Health Organization Classification of Tumours. Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006. [Google Scholar]
- 27.Chikh A, Sayan E, Thibaut S, et al. Expression of GATA-3 in epidermis and hair follicle: relationship to p63. Biochem Biophys Res Commun. 2007;361:1–6. [DOI] [PubMed] [Google Scholar]
- 28.Rácz E, Kurek D, Kant M, et al. GATA3 expression is decreased in psoriasis and during epidermal regeneration; induction by narrow-band UVB and IL-4. PLoS One. 2011;6:e19806. [DOI] [PMC free article] [PubMed] [Google Scholar]