Abstract:
KIT (CD117, c-kit) is a receptor tyrosine kinase involved in the tumorigenesis of several neoplasms. KIT is expressed by the secretory cells of normal sweat glands. We studied the KIT expression and KIT mutational status in various benign and malignant tumors of eccrine and apocrine glands. We included a total of 108 cases comprising 10 benign and 6 malignant sweat gland tumors, and KIT expression was immunohistochemically detected (positive rate): 10 syringomas (0%), 8 poromas (25%), 20 mixed tumors (40%), 21 spiradenomas (43%), 1 cylindroma (0%), 5 hidradenomas (40%), 7 syringocystadenoma papilliferum cases (0%), 1 papillary hidradenoma (100%), 2 tubulopapillary hidradenomas (50%), 8 hidrocystomas (29%), 2 adenoid cystic carcinomas (100%), 5 porocarcinomas (20%), 6 apocrine carcinomas (33%), 10 extramammary Paget diseases (30%), 1 spiradenocarcinoma (100%), and 1 syringocystadenocarcinoma papilliferum (0%). Most KIT-positive cells were luminal cells, arising from glandular structures. We performed polymerase chain reaction–single-strand conformation polymorphism for detecting KIT mutational status. All cases showed no mutations at hot spots for KIT (exons 9, 11, 13, and 17). KIT mutation does not seem to be mechanism for KIT expression, but the expression may be from native sweat glands.
Key Words: sweat gland tumor, KIT (CD117), immunohistochemistry, mutation, tumorigenesis
INTRODUCTION
The proto-oncogene KIT (CD117) is located on the long arm of chromosome 4 (4q11–12), and it is a member of the transmembrane receptor tyrosine kinase family.1 After its ligand binds to the receptor, it stimulates KIT phosphorylation, which begins a signaling cascade and contributes to the regulation of cell proliferation.1 In healthy individuals, KIT expression is observed in breast, salivary gland cells, urinary bladder cells, skin cells, central nervous system, the interstitial cells of Cajal from the gastrointestinal tract, and mast cells.2 In normal skin, KIT is expressed in the cytoplasm of melanocytes and the secretory cells of sweat glands.2
It has been reported that KIT can be expressed in a wide variety of malignant tumors, such as chronic myeloid leukemia, gastrointestinal stromal tumor, malignant melanoma, seminoma, and adenoid cystic carcinoma of the salivary gland.3 The drug imatinib, which targets KIT, is routinely used as an effective chemotherapy for chronic myeloid leukemia and gastrointestinal stromal tumor. The KIT-positive rate in malignant melanoma is 36%; therefore, imatinib can occasionally be used as a treatment for malignant melanoma.3–5 In malignant melanoma, the patients with KIT gene mutation were sensitive to imatinib,5 but even without c-kit protein, the expression remained sensitive to imatinib.
As mentioned above, the secretory cells of normal sweat glands express KIT. Some sweat gland tumors are expected to be KIT positive, but there have been few reports about KIT expression in sweat gland tumors.6 Therefore, we comprehensively examined KIT expression and KIT mutations in various benign and malignant tumors of sweat gland origin.
MATERIALS AND METHODS
Case Selection
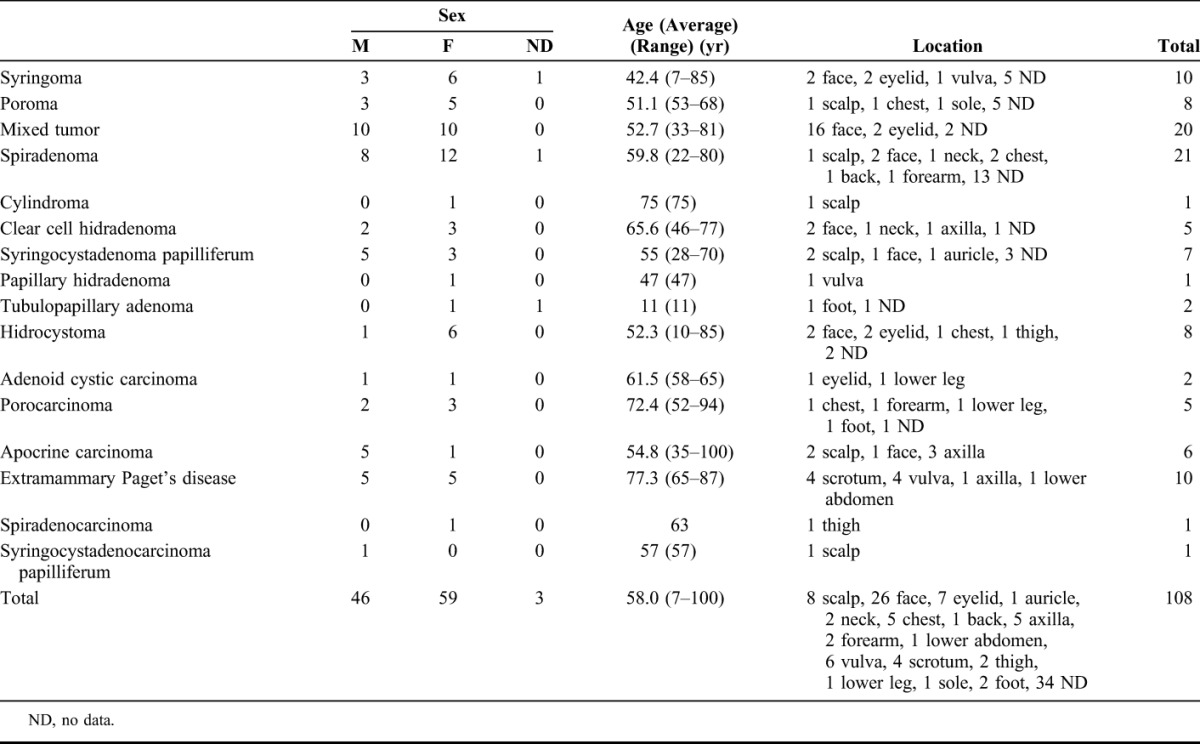
Specimens of a total of 108 cases, comprising 10 types of benign and 6 types of malignant sweat gland tumors, were retrieved from the archive of the Laboratory Division of the Oita University Hospital and Oita Prefectural Hospital; this selection was based on the availability of hematoxylin-eosin–stained glass slides that had been prepared from formalin-fixed, paraffin-embedded tissue blocks. These cases consisted of 10 syringomas, 8 poromas, 20 mixed tumors (of the skin), 21 spiradenomas, 1 cylindroma, 5 (clear cell) hidradenomas, 7 syringocystadenoma papilliferum, 1 papillary hidradenomas, 2 tubulopapillary hidradenomas (1 papillary eccrine adenoma and 1 tubular apocrine adenoma), 8 hidrocystomas, 2 adenoid cystic carcinomas, 5 porocarcinomas, 6 apocrine carcinomas, 10 extramammary Paget disease, 1 spiradenocarcinoma, and 1 syringocystadenocarcinoma papilliferum. As control, the sections of the normal tissue in the samples of wide resection of the other malignant cutaneous tumors were used for the evaluation of normal eccrine and apocrine glands. The clinicopathological data of each tumor have been summarized in Table 1.
TABLE 1.
Clinicopathological Profile of Sweat Gland Tumors Included in this Study
Immunohistochemical Staining and Analysis
For KIT immunostaining, we performed the standard streptavidin–biotin complex method (SAB-PO kit; Nichirei, Corporation, Tokyo, Japan) and the heat-induced antigen retrieval method. The tissue blocks were sectioned at a thickness of 3–4 μm, and sections were placed on glass slides. The sections were deparaffinized, rehydrated, and heated in a citric acid buffer (pH, 6.0) at 95°C for 40 minutes for antigen retrieval. After blocking, the sections were immersed with anti-KIT antibody (rabbit polyclonal, 1:50; DAKO, Carpinteria, CA) at room temperature for 30 minutes; they were then treated with biotin-labeled antirabbit antibody and streptavidin peroxidase, and visualized with diaminobenzidine. Each specimen was evaluated by 2 of the authors (H.N. and S.Y.). The intensity grade was classified as follows: strongly positive (2+, as well as mast cell staining), positive (1+), faintly positive (+/−), and negative (−) (Fig. 1). Positive cases were defined as the presence of ≥10% positive cells with 1+ or 2+ intensity among the tumor cells, and all other cases were regarded as negative.7
FIGURE 1.
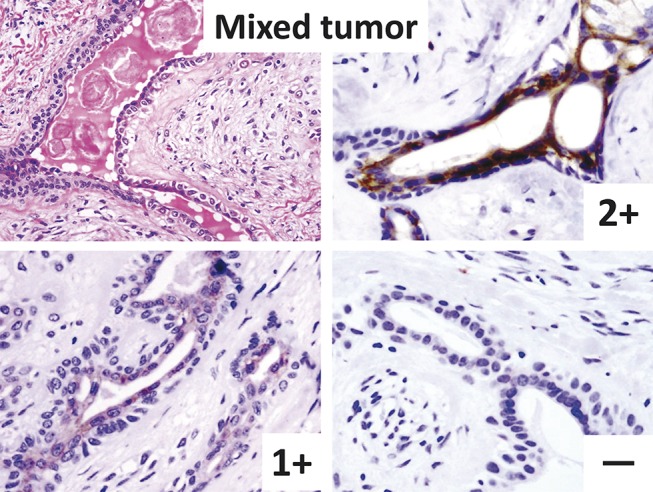
Positive and negative cases of mixed tumor. Top left (hematoxylin–eosin staining): tumor has 2-layered ductal structures within chondromyxoid stroma. Top right to bottom right (immunostaining): intensity grade 2+, 1+, and − are shown. In the positive case, only luminal cells are KIT positive.
Analysis of KIT Mutation
All tumors described above were analyzed for KIT mutations. For this purpose, 3–7 sections were made at a thickness of 10 μm from each tissue block and placed in a 1.5-mL microtube. DNA was extracted from these specimens using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Hotspot mutation sites (exons 9, 11, 13, and 17) were amplified by polymerase chain reaction (PCR) using the extracted DNA (Table 2). In this process, the reaction was performed with the GeneAmp PCR System 9700 thermal cycler (Applied Biosystems, Foster city, CA) as follows: 10 minutes at 95°C, followed by 35 cycles of 30 seconds each at 95°C, at 57.5°C, and at 72°C, and finally 7 minutes at 72°C. After performing PCR, single-strand conformation polymorphism analysis was performed using the Pharmacia Biotech Gene Phor system (Amersham Biosciences, GE Healthcare, Freiburg, Germany). For the screening of the mutation, these products were electrophoresed after heating a polyacrylamide gel (GeneGel Excel 12.5/24 kit; Amersham Biosciences, GE Healthcare) to 100°C in formamide and then silver stained using the Hoefer Processor Plus (Amersham Biosciences, GE Healthcare).
TABLE 2.
The Primers Used in This Study

RESULTS
KIT Expression by Immunohistochemical Staining
In the normal eccrine (47/59, 80%) and apocrine (25/46, 54%) glands, only the secretory cells in the secretory portion of the glands were positive; however, the myoepithelial cells of the secretory portion and the inner cuticular cells and outer basaloid/poroid cells of the ductal portion were negative (Fig. 2). The results of immunohistochemical staining have been summarized in Table 3 (Figs. 1, 3–9). Most positive cells were the luminal cells from the glandular structures, although some positive cells were observed in a sheet-like pattern in the poroma/porocarcinomas, (clear cell) hidradenomas, porocarcinomas, and apocrine carcinomas (Figs. 3, 5). In each case of spiradenocarcinoma and syringocystadenocarcinoma papilliferum, although the benign and malignant parts were mixed, both sections showed the same results. Even in most positive cases, the positive cells were generally small in number; however, >70% positive cells were observed in 4 mixed tumors, 4 spiradenomas, and 2 adenoid cystic carcinomas.
FIGURE 2.
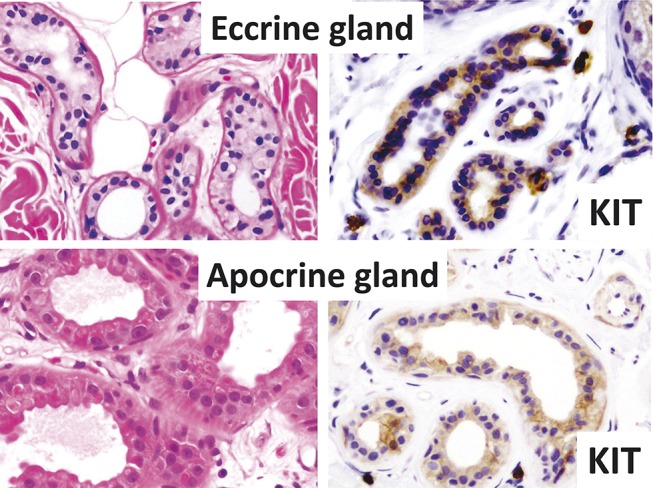
Normal sweat glands (top: eccrine gland and bottom: apocrine gland; hematoxylin–eosin staining and immunostaining). Secretory cells of both glands are KIT positive (right).
TABLE 3.
Results of Immunohistochemical Staining
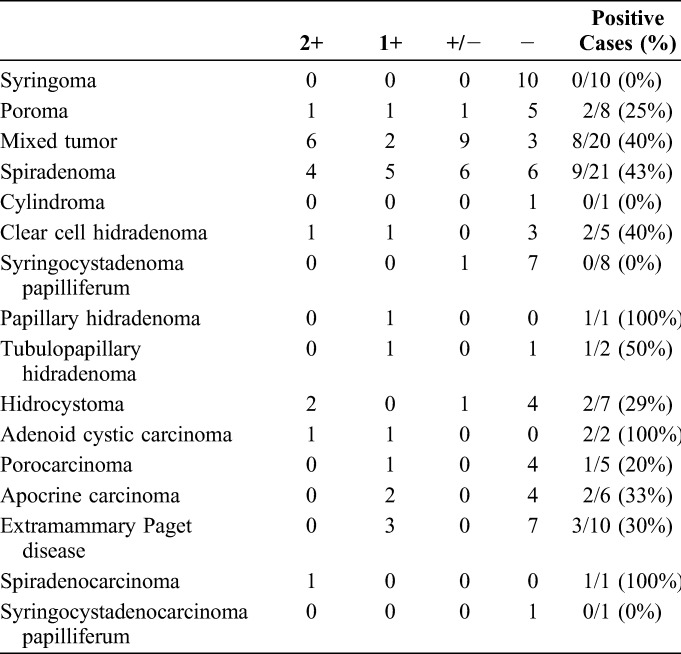
FIGURE 3.
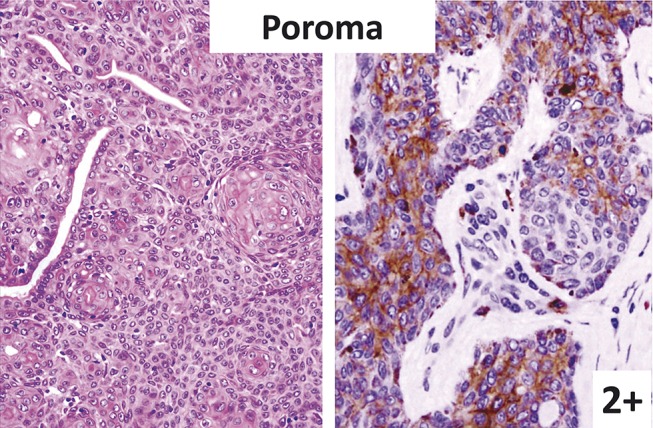
Poroma (left: hematoxylin–eosin staining; right: immunostaining). Tumor cells (poroid cells) in sheet pattern are KIT positive.
FIGURE 9.
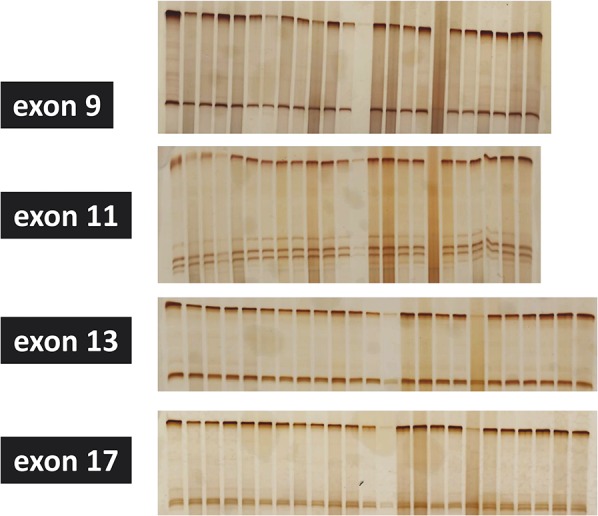
Mutational states of 24 tumors (from left to right: 10 mixed tumors, 10 spiradenomas, 4 syringomas). No mutation is detected in exons 9, 11, 13, and 17 of KIT.
FIGURE 5.
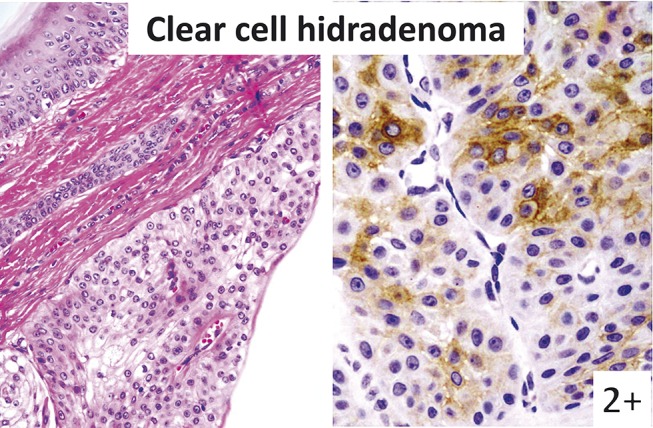
Clear cell hidradenoma (left: hematoxylin–eosin staining; right: immunostaining). Tumor cells without glandular structure are KIT positive.
FIGURE 4.
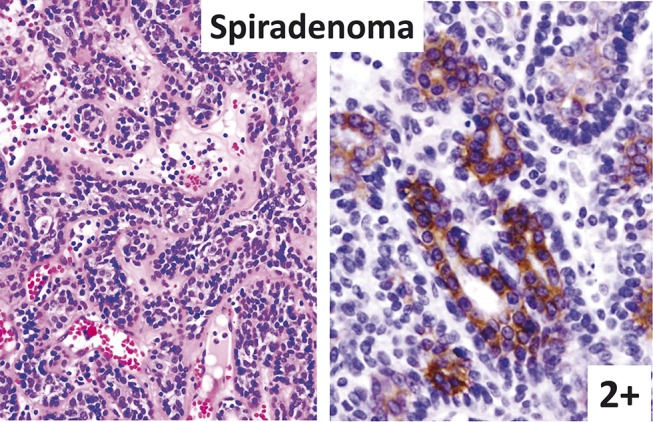
Spiradenoma (left: hematoxylin–eosin staining; right: immunostaining). Inner liminal cells are strongly KIT positive.
FIGURE 6.
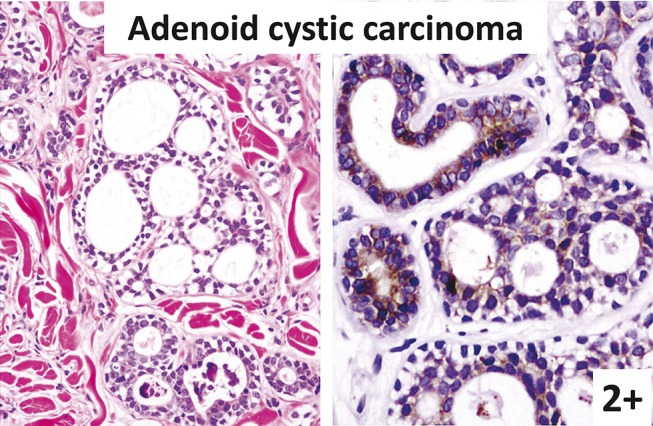
Adenoid cystic carcinoma (left: hematoxylin–eosin staining; right: immunostaining). Most tumor cells surrounding the lumens are strongly KIT positive.
FIGURE 7.
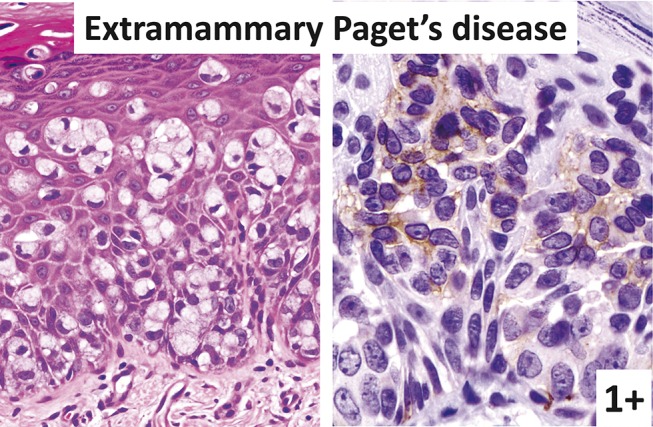
Extramammary Paget disease (left: hematoxylin–eosin staining; right: immunostaining). Some Paget cells are KIT positive.
FIGURE 8.
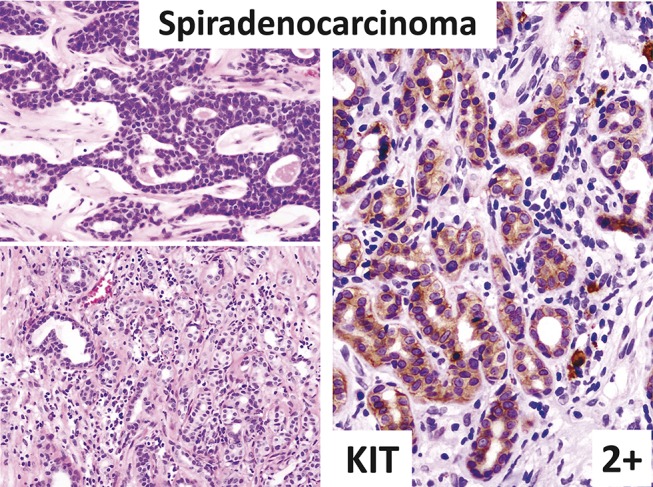
Spiradenocarcinoma (left: hematoxylin–eosin staining; right: immunostaining). Tumor is composed of spiradenoma (top left) and invasive spiradenocarcinoma (bottom left). Spiradenocarcinoma is strongly KIT positive (right).
KIT Mutation
We analyzed the mutations of exons 9, 11, 13, and 17 of KIT in a total of 98 cases from which DNA was successfully extracted, including 7 syringomas, 8 poromas, 18 mixed tumors, 18 spiradenomas, 1 cylindroma, 3 (clear cell) hidradenomas, 8 syringocystadenoma papilliferum cases, 1 papillary hidradenoma, 2 tubulopapillary hidradenomas, 7 hidrocystomas, 2 adenoid cystic carcinomas, 5 porocarcinomas, 6 apocrine carcinomas, 10 extramammary Paget disease cases, 1 spiradenocarcinoma, and 1 syringocystadenocarcinoma papilliferum. However mutations in any of the cases were not detected (Fig. 9).
DISCUSSION
Normal sweat glands are classified into eccrine and apocrine types. Eccrine glands are distributed over the entire skin surface and open to the epidermis, whereas apocrine glands are confined to certain areas, such as axilla, mons pubis, and perianal and periareolar regions, and open to the follicular infundibulum. Sweat glands are composed of 3 segments: the intraepidermal (eccrine)/intrainfundibular (apocrine) duct, the intradermal duct, and the secretory portion. The ducts of both the eccrine and apocrine glands show the same structure consisting of eosinophilic inner cuticular cells and basophilic outer basal cells/poroid cells. The secretory portion of eccrine glands has a narrow tubular structure composed of pale inner secretory cells and outer myoepithelial cells, whereas apocrine glands shows large tubules composed of eosinophilic secretory cells with decapitation secretion and peripheral myoepithelial cells (Fig. 2, left). In this study, we did not determine if the cells were of eccrine or apocrine origin, and the line of differentiation of a tumor does not necessarily reflect its cell of origin because the classification of the sweat gland tumors as either eccrine or apocrine origin is controversial. But we established that tumors with decapitation secretion originated at the secretory portion of apocrine glands. A few sweat gland tumors, such as syringomas and poromas, apparently seem to differentiate to ductal portion, whereas most tumors are considered to differentiate to secretory cells or both ductal and secretory cells.
In normal sweat glands, KIT was positive only on the secretory cells but was negative on the ductal and myoepithelial cells. Among a total of 108 cases comprising 16 types of benign and malignant sweat gland tumors, 34 cases of 12 different types were KIT positive, including poromas (2/8), mixed tumors (8/20), spiradenomas (9/21), (clear cell) hidradenomas (2/5), papillary hidradenomas (1/1), tubulopapillary hidradenomas (1/2), hidrocystomas (2/7), adenoid cystic carcinomas (2/2), porocarcinomas (1/5), apocrine carcinomas (2/6), extramammary Paget disease cases (3/10), and spiradenocarcinomas (1/1) (Table 3). The positive cells were mainly located in the areas with luminal cells except for in the poroma/porocarcinomas, apocrine carcinomas, and extramammary Paget disease. Most positive cases belonged to tumors that differentiated to secretory cells or to both ductal and secretory cells. As an exception, a few cases of poroma/porocarcinoma of ductal cell origin were positive, and all cases of syringocystadenoma/syringocystadenocarcinoma papilliferum with apparent decapitation secretion were negative. Decapitation secretion was observed in mixed tumors, syringocystadenoma/syringocystadenocarcinoma papilliferum cases, papillary hidradenomas, hidrocystomas, and apocrine carcinomas; however, KIT expression seemed to be inconsistent even in the apocrine tumors. One case of spiradenocarcinoma was composed of benign and malignant sections; in this case, the benign tumor had signs of malignant transformation, and both the benign and malignant parts showed the same staining pattern. This result may lead to the conclusion that KIT had no relationship to the malignant transformation, although KIT-positive cells were small in number. KIT expression was observed in the majority of the benign and malignant types of sweat gland tumors with some degree. Although KIT expression in adenoid cystic carcinoma is well known, it does not seem to have diagnostic value in this spectrum of tumors. It is unknown why the KIT expression was so variable in benign and malignant sweat gland tumors and in normal sweat glands. It might be related to secretory activity of luminal cells.
Even in cases that were immunohistochemically KIT positive, there was no mutation in the examined exons of KIT. KIT might be activated by other mechanisms than gene mutation, such as gene fusion, amplification, autocrine and paracrine receptor stimulation by its ligand, loss of phosphatase activity, cross-activation by another kinases, or promoter activation/inactivation via methylation/demethylation.8 However, because KIT expression on secretory cells is a normal event, the native sweat gland KIT expression is most likely unrelated to the tumorigenesis of sweat gland tumors.
Some types of sweat gland and salivary gland tumors, for example, mixed tumor and pleomorphic adenoma, spiradenoma/cylindroma and basal cell adenoma, apocrine carcinoma and salivary duct carcinoma, and adenoid cystic carcinoma of the salivary glands and that of skin, share morphological characteristics. In normal salivary glands, weak KIT expression is observed on the ductal cells but not on the acinar cells.9,10 A minority of pleomorphic and basal cell adenomas of the salivary gland have been reported as being KIT positive similar to the mixed tumor and spiradenoma of the skin.9 Recent reports also stress that adenoid cystic carcinoma of the salivary gland,10,11 breast,12 and skin6 characteristically expressed KIT. Moreover, some adenoid cystic carcinoma of the salivary gland showed KIT mutations.13 Among the 25 cases of 6 types of malignant tumors, as shown in Table 3, 9 cases were KIT positive without KIT mutations. Even in KIT-positive cases, the population of KIT-positive cells was generally so small that imatinib will probably be not be useful in malignant sweat gland tumors, except for adenoid cystic carcinoma. However, KIT positive rate of adenoid cystic carcinoma in any organ was high. KIT mutation was also reported in adenoid cystic carcinoma of the salivary gland, and some cases responded to the imatinib.13,14 Imatinib treatment might be efficacious in cases of adenoid cystic carcinoma of the skin similarly, but only 2 adenoid cystic carcinomas were examined in the present study. It will be necessary to examine more cases in the future.
CONCLUSIONS
In this study, we found that KIT expression was detected in 7 of 10 types of the benign sweat gland tumors and 4 of 6 types of the malignant ones. Despite KIT expression, there was no KIT mutation in the examined exons of KIT. KIT is likely to be expressed by other mechanisms than KIT mutation, but the expression may be from native sweat glands.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Ni S, Huang D, Chen X, et al. c-kit gene mutation and CD117 expression in human anorectal melanomas. Hum Pathol. 2012;43:801–807. [DOI] [PubMed] [Google Scholar]
- 2.Lammie A, Drobnjak M, Gerald W, et al. Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem. 1994;42:1417–1425. [DOI] [PubMed] [Google Scholar]
- 3.Went PT, Dirnhofer S, Bundi M, et al. Prevalence of KIT expression in human tumors. J Clin Oncol. 2004;22:4514–4522. [DOI] [PubMed] [Google Scholar]
- 4.Johnson DB, Sosman JA. Update on the targeted therapy of melanoma. Curr Treat Options Oncol. 2013;14:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown MC, Casasola RJ. Complete response in a melanoma patient treated with imatinib. J Laryngol Otol. 2012;126:638–640. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishnan R, Chaudhry IH, Ramdial P, et al. Primary cutaneous adenoid cystic carcinoma: a clinicopathologic and immunohistochemical study of 27 cases. Am J Surg Pathol. 2013;37:1603–1611. [DOI] [PubMed] [Google Scholar]
- 7.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. [DOI] [PubMed] [Google Scholar]
- 8.Sihto H, Sarlomo-Rikala M, Tynninen O, et al. KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol. 2005;23:49–57. [DOI] [PubMed] [Google Scholar]
- 9.Mino M, Pilch BZ, Faquin WC. Expression of KIT (CD117) in neoplasms of the head and neck: an ancillary marker for adenoid cystic carcinoma. Mod Pathol. 2003;16:1224–1231. [DOI] [PubMed] [Google Scholar]
- 10.Jeng YM, Lin CY, Hsu HC. Expression of the c-kit protein is associated with certain subtypes of salivary gland carcinoma. Cancer Lett. 2000;154:107–111. [DOI] [PubMed] [Google Scholar]
- 11.Freier K, Flechtenmacher C, Walch A, et al. Differential KIT expression in histological subtypes of adenoid cystic carcinoma (ACC) of the salivary gland. Oral Oncol. 2005;41:934–939. [DOI] [PubMed] [Google Scholar]
- 12.Crisi GM, Marconi SA, Makari-Judson G, et al. Expression of c-kit in adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;124:733–739. [DOI] [PubMed] [Google Scholar]
- 13.Vila L, Liu H, Al-Quran SZ, et al. Identification of c-kit gene mutations in primary adenoid cystic carcinoma of the salivary gland. Mod Pathol. 2009;22:1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faivre S, Raymond E, Casiraghi O, et al. Imatinib mesylate can induce objective response in progressing, highly expressing KIT adenoid cystic carcinoma of the salivary glands. J Clin Oncol. 2005;23:6271–6273. [DOI] [PubMed] [Google Scholar]


