Supplemental Digital Content is available in the text.
Keywords: angiogenesis, gremlin, integrins, lipid rafts, VEGFR2
Abstract
Objective—
During neovessel formation, angiogenic growth factors associate with the extracellular matrix. These immobilized factors represent a persistent stimulus for the otherwise quiescent endothelial cells (ECs), driving directional EC migration and proliferation and leading to new blood vessel growth. Vascular endothelial growth factor receptor 2 (VEGFR2) is the main mediator of angiogenesis. Although VEGFR2 signaling has been deeply characterized, little is known about its subcellular localization during neovessel formation. Aim of this study was the characterization and molecular determinants of activated VEGFR2 localization in ECs during neovessel formation in response to matrix-immobilized ligand.
Approach and Results—
Here we demonstrate that ECs stimulated by extracellular matrix–associated gremlin, a noncanonical VEGFR2 ligand, are polarized and relocate the receptor in close contact with the angiogenic factor–enriched matrix both in vitro and in vivo. GM1 (monosialotetrahexosylganglioside)-positive planar lipid rafts, β3 integrin receptors, and the intracellular signaling transducers focal adhesion kinase and RhoA (Ras homolog gene family, member A) cooperate to promote VEGFR2 long-term polarization and activation.
Conclusions—
A ligand anchored to the extracellular matrix induces VEGFR2 polarization in ECs. Long-lasting VEGFR2 relocation is closely dependent on lipid raft integrity and activation of β3 integrin pathway. The study of the endothelial responses to immobilized growth factors may offer insights into the angiogenic process in physiological and pathological conditions, including cancer, and for a better engineering of synthetic tissue scaffolds to blend with the host vasculature.
Tumor neovascularization and angiogenesis-dependent diseases are characterized by the uncontrolled release of angiogenic growth factors, leading to endothelial cell (EC) activation.1 Numerous angiogenesis inducers, including vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) family members, are produced by tumor or inflammatory cells, accumulate in extracellular matrix (ECM), and interact with tyrosine kinase (TK) receptors expressed on EC surface.2 In most of the in vitro studies, the characterization of angiogenic growth factors is performed after their addition to EC culture medium, implying their interaction with the luminal aspect of endothelium. However, angiogenic growth factors bind different ECM components, including heparan-sulfate proteoglycans (HSPGs), leading to the formation of immobilized ECM-bound complexes. Accordingly, they are found associated with endothelial ECM in vitro and basal membranes in vivo.2,3 Even though various proteases and glycosidases may mobilize ECM-anchored angiogenic mediators,2,4 substratum-immobilized growth factors induce EC activation by interacting with the basal aspect of the endothelium, leading to long-term EC stimulation.3,5,6 This implies that angiogenic growth factor signaling is likely to be driven by abluminal rather than luminal receptors in ECs.7 Nevertheless, the fate and subcellular localization of angiogenic TK receptors during neovessel formation triggered by ECM-associated growth factors have not been described yet.
Gremlin is a bone morphogenic protein antagonist produced by human tumors8 and expressed by FGF2-activated ECs in vitro and tumor endothelium in vivo.9 Similar to other heparin-binding angiogenic factors, gremlin binds HSPGs and accumulates on cell surface and ECM rather than in culture medium.10 Gremlin stimulates EC intracellular signaling, sprouting, migration, and 3-dimensional gel invasion in vitro, leading to a potent angiogenic response in vivo.9,11 This is because of the capacity of gremlin to bind and activate VEGF receptor 2 (VEGFR2),12,13 as well as HSPGs that act as functional co-receptors.10
Although gremlin does not interact with neuropilin 1,10 it exerts a VEGFR2-dependent activation of ECs, leading to a tight VEGFR2/αvβ3 integrin crosstalk with mechanisms that are, at least in part, similar to VEGFs.14
These bases prompted us to assess the capacity of ECM-associated gremlin to induce a proangiogenic, VEGFR2-dependent response in ECs and to investigate the subcellular fate of VEGFR2 during this process.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Matrix-Bound Gremlin Recruits VEGFR2 at the Basal Aspect of ECs
Gremlin is a proangiogenic, heparin-binding VEGFR2 ligand produced by human tumors.8 It accumulates on cell surface– and ECM-associated HSPGs10 of producing cells in vitro and in the perivascular stroma of human tumor grafts in vivo (Figure IA in the online-only Data Supplement). This supports the notion that angiogenic growth factors released by tumor or inflammatory cells accumulate in ECM, thus attracting ECs from preexisting blood vessels to generate a new vascular network. It is therefore possible to hypothesize that these factors interact with their receptors at the basal aspect of ECs. However, limited experimental evidences are available to support this assumption.
To this aim, the subcellular localization of endothelial VEGFR2 was analyzed during in vivo neovascularization induced by ECM-bound gremlin in a murine Matrigel matrix plug angiogenesis assay.15 Matrigel is a tumor-derived basement membrane matrix composed of a variety of ECM proteins16 able to retain and slowly release ECM-binding growth factors, mimicking what occurs in tumor stroma. Moreover, at variance with tumor models, the Matrigel plug assay allows the analysis of the effect of a single, selected angiogenic factor. On this basis, gremlin (1.0 mg/mL) was dissolved in Matrigel, and plugs were implanted subcutaneously in C57BL/6 mice. In parallel, VEGF-A and FGF2 plugs were used as a further VEGFR2-dependent and VEGFR2-independent stimulus, respectively. After 7 days, plugs were harvested, and VEGFR2 localization was investigated by immunohistochemical analysis of newly formed blood vessels.
Independent from the angiogenic stimulus, ECs of functional vessels grown in Matrigel plugs were polarized as demonstrated by luminal podocalyxin staining (Figure 1A). In polarized gremlin-induced vessels, VEGFR2 localized at the basal side of ECs, whereas the luminal side was characterized by CD31 and podocalyxin immunoreactivity only (Figure 1A). At variance, EC sprouts invading the gremlin-enriched Matrigel plug showed a homogeneous distribution of VEGFR2. Similar results were obtained for VEGF-A–induced vessels (data not shown). Specificity of VEGFR2 polarization was supported by the homogeneous, nonpolarized distribution of the receptor in FGF2-induced vessels (Figure II in the online-only Data Supplement). These observations were confirmed by image-based quantification of VEGFR2 fluorescence at the basal aspect of acquired vessels (Figure 1B).
Figure 1.
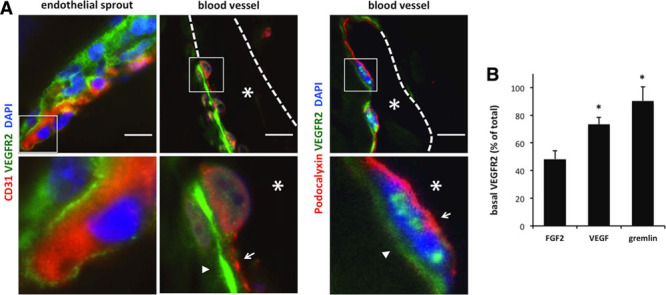
Vascular endothelial growth factor receptor 2 (VEGFR2) relocate at the basal aspect of endothelial cells (ECs) during in vivo vessel formation induced by extracellular matrix (ECM)–bound gremlin. Matrigel plugs containing 1.0 μg/mL of gremlin, vascular endothelial growth factor A, or fibroblast growth factor 2 (FGF2) were implanted subcutaneously in C57BL/6 mice. After 1 week, plugs were stained for CD31 or podocalyxin (red), VEGFR2 (green), and nuclei (blue) and analyzed. Images of 0.3 μm sections were collected using a Zeiss Axiovert 200 mol/L epifluorescence microscope equipped with a Plan-Apochromat 63×/1.4 NA oil objective and ApoTome system. A, VEGFR2 (arrowheads) and CD31 or podocalyxin (arrows) immunolocalization and DAPI staining in gremlin induced EC sprout or neovessel in gremlin-enriched Matrigel plugs. Asterisks indicate vessel lumens (Bar, 10 μm). B, Percentage of VEGFR2 staining, respect to the total VEGFR2 positivity, at the basal aspect of ECs in gremlin-, VEGF-A– or FGF2-stimulated lumen-equipped neovessels (mean±SD, n=4 with 2 vessels per experiment; *P<0.001, Student t test).
In a second set of experiments, a haptotactic migration assay was performed to confirm the ability of ECM-associated gremlin to induce VEGFR2 relocation in ECs co-cultured with gremlin-expressing cells. To this aim, mock and gremlin-overexpressing VEGF−/− fibroblasts were seeded on the lower face of 8-μm-pore filters and allowed to deposit their own ECM for the next 48 hours (Figure III in the online-only Data Supplement).10 Then filters were inserted into a Boyden chamber, and GM7373 (transformed fetal bovine aortic endothelial GM7373 cells) ECs overexpressing the enhanced yellow fluorescent protein (EYFP)–tagged extracellular domain (ECD) of VEGFR2 (ECD-VEGFR2-EYFP GM7373 cells) were seeded on the upper face of the filter and allowed to migrate for 1 hour through the membrane pores in response to the haptotactic stimulus (Figure 2A). Digital cross-sections showed the recruitment of the ECD-VEGFR2-EYFP receptor at the basal side and in cytoplasmic protrusions extending into the filter pores of ECs haptotactically attracted by the ECM deposited by gremlin-overexpressing VEGF−/− fibroblasts (Figure 2B). In contrast, despite a similar cell adhesion to the filter (data not shown), little amount of VEGFR2 was at the basal side and in the protrusions of ECs seeded on filters conditioned by mock VEGF−/− fibroblasts (Figure 2B–2D). β3 integrin staining was used to unequivocally stain cells and migrative protrusions. Of note, ECD-VEGFR2-EYFP expression did not vary under the different experimental conditions tested (Figure 2E).
Figure 2.
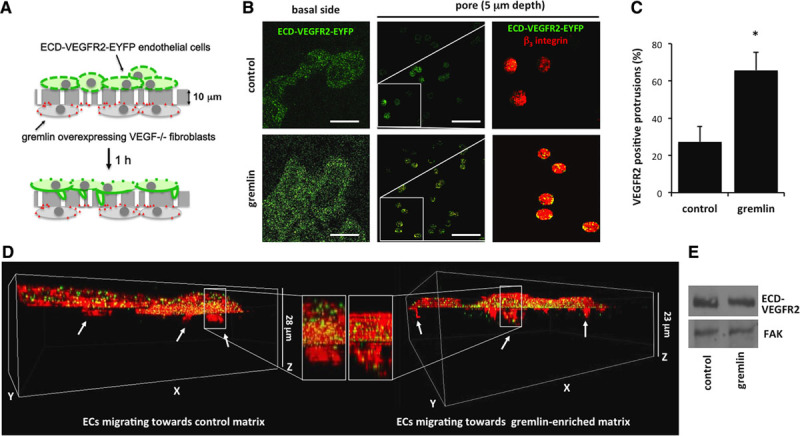
Substrate-bound gremlin recruits vascular endothelial growth factor receptor 2 (VEGFR2) at the basal aspect and the leading protrusions of migrating endothelial cells (ECs). Mock and gremlin-overexpressing vascular endothelial growth factor (VEGF)−/− fibroblasts were seeded on the lower face of 8-μm-pore chemotaxis filters and incubated for 48 hours to allow them to deposit their own ECM containing gremlin or not. Filters were inserted in a Boyden chamber and enhanced yellow fluorescent protein (EYFP)–tagged extracellular domain (ECD) of VEGFR2 (ECD-VEGFR2-EYFP) GM7373 (transformed fetal bovine aortic endothelial GM7373 cells) cells were seeded at 1.0×106 cells/mL in the upper compartment. After 1 hour of migration, cells were fixed, stained for β3 integrin, and analyzed for ECD-VEGFR2-EYFP and β3 integrin localization using an LSM510 Meta confocal microscope equipped with Plan-Apochromat 63×/1.4 NA oil objective (Bar, 20 μm). A, The assay for gremlin-overexpressing VEGF−/− fibroblasts. B, ECD-VEGFR2-EYFP in Z-stack sections of basal portion and in pores protrusions of cells hapotatically migrating toward the ECM deposited by mock or gremlin-overexpressing VEGF−/− fibroblasts. Higher magnifications (white squares) show the recruitment of ECD-VEGFR2-EYFP (green) and β3 integrin (red) in pore protrusions of human umbilical vein endothelial cells (HUVECs) haptotactically attracted by ECM-anchored gremlin. C, Fifty cell protrusions extending into pores were counted, and their positivity for VEGFR2 at the depth of 5 μm was quantified. Data are expressed as percentage of VEGFR2-positive protrusions ±SD (*P<0.001, Student t test). D, 3D reconstruction of ECD-VEGFR2-EYFP (green) and β3 integrin (red) in cells migrating to mock and gremlin-overexpressing VEGF−/− fibroblasts with their magnifications. E, After 1 hour of migration, cell lysates were prepared and probed by EYFP Western blotting. Uniform loading was confirmed by focal adhesion kinase (FAK) Western blotting.
We next assessed the capacity of VEGFR2 to relocate at the basal aspect of human umbilical vein endothelial cells (HUVECs) when challenged by gremlin bound to the substratum. To this purpose, HUVECs were seeded on tissue culture plastic irreversibly adsorbed with gremlin and fibrinogen (Figure IV in the online-only Data Supplement), used as a negative control here. No differences in cell adhesion were observed on the 2 substrata (Figure 3A). As expected, confocal Z-stack imaging of adherent cells showed a significant polarization of VEGFR2 at the basal aspect of cells 4 hours after seeding on immobilized gremlin. This was paralleled by the decrease of VEGFR2 immunoreactivity at the apical side of adherent cells. No VEGFR2 polarization was observed in ECs seeded on fibrinogen (Figure 3A).
Figure 3.
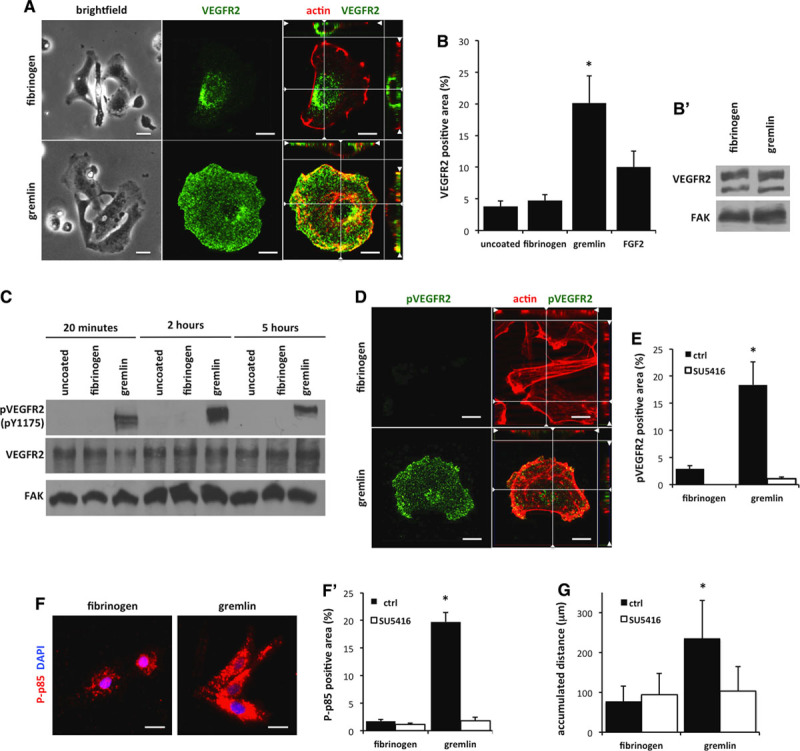
Vascular endothelial growth factor receptor 2 (VEGFR2) is recruited and activated at the basal portion of endothelial cells (ECs) on immobilized gremlin. A, Human umbilical vein endothelial cells (HUVECs) seeded on substrate-bound fibrinogen (FG) or gremlin show, after 4 hours, a similar adhesive capacity (brightfield images; Bar, 20 μm). Adherent ECs were stained for VEGFR2 (green) and actin (red) and analyzed using a LSM510 Meta confocal microscope equipped with Plan-Apochromat 63×/1.4 NA oil objective. Images show the basal portion of adherent cells with the orthogonal z reconstruction of the whole cell (Bar, 10 μm). B, Ventral plasma membranes (VPMs) from VEGFR2-overexpressing GM7373 (transformed fetal bovine aortic endothelial GM7373 cells) ECs (VEGFR2-GM7373) adherent on immobilized FG, gremlin, fibroblast growth factor 2 (FGF2), or uncoated coverslips for 4 hours were stained for VEGFR2, actin, and nuclei. Samples were analyzed with epifluorescence microscope equipped with Plan-Apochromat 63×/1.4 NA oil objective (Bar, 10 μm). Total VEGFR2 was quantified in 30 cells/sample using Image-Pro Plus software. Data are expressed as percentage±SEM of VEGFR2-positive area in respect to the total VPM area, as defined by actin staining (*P<0.01, Student t test). B′, After 4 hours of adhesion, VEGFR2-GM7373 cells seeded on immobilized FG or gremlin were lysed, and lysates were probed by VEGFR2 Western blotting analysis. Uniform loading was confirmed by focal adhesion kinase (FAK) Western blotting. C, 50 μg of cell extracts of HUVECs seeded for the indicated time on substrate-bound FG or gremlin or on uncoated cell culture plates were probed by phospho-VEGFR2 (pVEGFR2 [phospho-vacular endothelial growth factor receptor 2]; pTyr 1175) or total VEGFR2 Western blotting. Uniform loading was confirmed by FAK Western blotting. D, HUVECs were left adhered on substrate-bound FG or gremlin for 4 hours and stained for pVEGFR2 (pTyr 1175; green) and actin (red) and analyzed using a LSM510 Meta confocal microscope equipped with Plan-Apochromat 63×/1.4 NA oil objective. Images show the basal portion of adherent cells with the orthogonal z reconstruction of the whole cell (Bar, 10 μm). E, VPMs from VEGFR2-GM7373 cells left adhered on immobilized FG or gremlin for 4 hours in the absence or in the presence of SU5416 (Sugen5416) were stained for VEGFR2, actin, and nuclei. Samples were analyzed with epifluorescence microscope equipped with Plan-Apochromat 63×/1.4 NA oil objective (Bar, 10 μm). pVEGFR2 was quantified in 30 cells/sample using Image-Pro Plus software. Data are expressed as percentage±SEM of pVEGFR2-positive area in respect to the total VPM area, as defined by actin staining (*P<0.01, Student t test). F, VEGFR2-GM7373 cells adherent on immobilized gremlin or FG for 4 hours were stained for phospho-p85 (P-p85; red) and nuclei (blue) and analyzed with an epifluorescence microscope equipped with Plan-Apochromat 63×/1.4 NA oil objective (Bar, 10 μm). F′, Cells were left adhered in the absence or presence of SU5416. Phospho-p85 was quantified in 30 cells/sample using Image-Pro Plus software. Data are expressed as percentage±SEM of phospho-p85–positive area in respect to the total cell area (*P<0.001, Student t test). G, HUVECs were treated or not with SU5416 and seeded on immobilized gremlin or FG. Cell motility was assessed by time-lapse video microscopy using an inverted microscope (Zeiss Axiovert 200 mol/L). Phase-contrast snap photographs (one frame every 10 minutes) were digitally recorded for 8 hours. Cell paths (40–50 cells per experimental point) were generated from centroid positions, and migration parameters were analyzed with the Chemotaxis and Migration Tool of ImageJ Software (http://rsbweb.nih.gov/ij). Graph shows the accumulated distances (in μm) of HUVECs seeded on immobilized gremlin or fibrinogen in the absence or presence of SU5416 (*P<0.05, Student t test).
Finally, VEGFR2-overexpressing GM7373 ECs10 were seeded on immobilized gremlin, fibrinogen, or uncoated coverslips for 4 hours. Ventral plasma membranes (VPMs) were prepared from adherent cells by osmotic shock17 and decorated for the presence of immunoreactive VEGFR2. In all the experiments, the absence of DAPI (4’,6-diamidino-2-phenylindole) staining and the persistence of actin filaments were used to unequivocally identify the VPM remnants bound to the substratum. As shown in Figure VA in the online-only Data Supplement and Figure 3B, VEGFR2 was specifically recruited in VPMs of cells seeded on immobilized gremlin but not in cells seeded on fibrinogen or uncoated coverslips. This occurred in the absence of any change in VEGFR2 expression (Figure 3B′).
Taken together, these data provide in vivo and in vitro experimental evidences about a directional relocation of VEGFR2 after receptor engagement by its substratum-associated ligand.
Matrix-Bound Gremlin Induces VEGFR2 Activation at the Basal Aspect of ECs
To assess the ability of substratum-immobilized gremlin to exert a directional stimulation of ECs through a productive interaction with VEGFR2, VEGFR2 phosphorylation was analyzed by Western blotting performed on the total cell lysates of HUVECs adherent to immobilized gremlin at 20 minutes, 2 hours, and 5 hours after seeding. Immobilized gremlin induces a rapid and long-lasting phosphorylation of VEGFR2, whereas no receptor activation was detectable in HUVECs seeded on fibrinogen or uncoated wells. Of note, total VEGFR2 expression did not vary during the timing of the experiment (Figure 3C).
Immunofluorescence analysis of HUVECs seeded on immobilized gremlin confirmed that VEGFR2 recruited at the basal aspect was phosphorylated (Figure 3D). Similarly, VEGFR2 was activated in VPMs of VEGFR2-overexpressing GM7373 ECs seeded on immobilized gremlin (Figure VB in the online-only Data Supplement and Figure 3E). We also observed a significant increase in the phosphorylation of the PI3-kinase regulatory subunit p8518 (Figure 3F). VEGFR2 and p85 activations were both inhibited by the VEGFR2 TK inhibitor SU5416 (Sugen5416; Figure 3E and 3F′).
Finally, we analyzed EC motility as a biological response to VEGFR2 activation. As shown in Figure 3G, time-lapse video microscopy of individual cells demonstrates that immobilized gremlin induces an increase of cell migration when compared with fibrinogen. As anticipated, SU5416 reduced the cellular response to immobilized gremlin.
Matrix-Bound Gremlin Induces VEGFR2/β3 Integrin Complex Formation in Planar Lipid Rafts
VEGFR2/β3 integrin complex formation plays a pivotal role in mediating the EC response to free gremlin.14 On this basis, we assessed the capacity of immobilized gremlin to induce a direct VEGFR2–β3 integrin interaction at the basal portion of ECs. To this purpose, β3-enhanced cyan fluorescent protein/ECD-VEGFR2-EYFP co-transfected GM7373 cells were allowed to adhere on immobilized gremlin or fibrinogen. A cytoplasmic cyan fluorescent protein–yellow fluorescent protein fusion was used as a control of fluorescence resonance energy transfer efficiency.19 After 4 hours of adhesion, fluorescence resonance energy transfer acceptor photobleaching analysis showed a direct interaction between VEGFR2 and β3 integrin in VPMs of ECs seeded on gremlin but not in cells adherent to fibrinogen (Figure 4A and 4A′).
Figure 4.
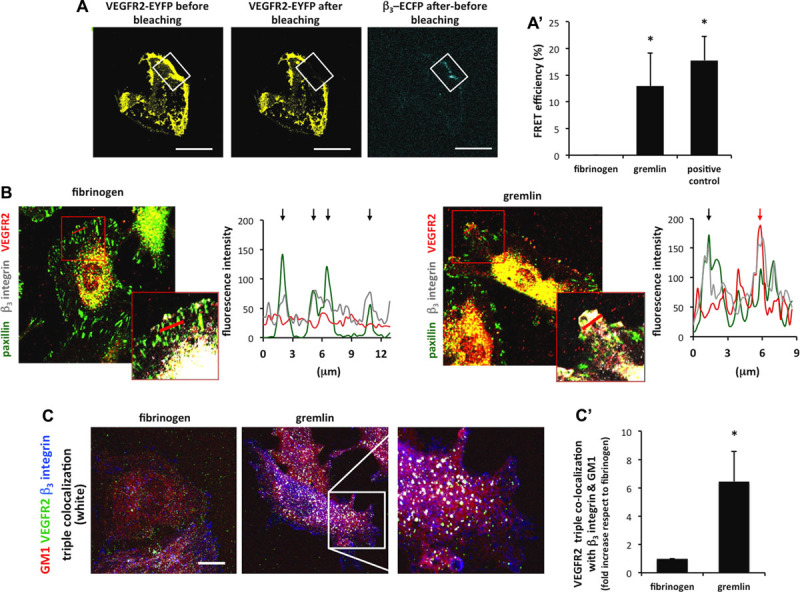
Vascular endothelial growth factor receptor 2 (VEGFR2) interacts with β3 integrin and associates with lipid rafts. A, Ventral plasma membranes (VPMs) from β3 enhanced cyan fluorescent protein (ECFP)-GM7373 (transformed fetal bovine aortic endothelial GM7373 cells) cells transiently expressing enhanced yellow fluorescent protein (EYFP)–tagged extracellular domain (ECD) of VEGFR2 (ECD-VEGFR2-EYFP) and left adhered for 4 hours on substrate-bound gremlin or fibrinogen (FG) were subjected to fluorescence resonance energy transfer (FRET) analysis. A region of interest (white square) was selected and photobleached by applying 100% intensity of 514 nm laser. FRET efficiency was calculated by using the formula: FRET=(Dpost−Dpre)/Dpost, where Dpost and Dpre represent the donor (ECFP) emission intensities before and after the photobleaching. FRET efficiency was also measured in a nonphotobleached region of the same cell as an in situ control. Cells transfected with an ECFP-EYFP fusion protein were a FRET-positive control (Bar, 10 μm). Data are expressed as percentage±SD of FRET efficiency (A′; *P<0.005, Student t test). B, Basal aspects of human umbilical vein endothelial cells (HUVECs) left adhered for 4 hours on immobilized FG or gremlin, stained for VEGFR2 (red), paxillin (green), and β3 integrin (white, showed only in selections), and analyzed using a LSM510 Meta confocal microscope equipped with Plan-Apochromat 63×/1.4 NA oil objective. Fluorescence profiles for paxillin (green), VEGFR2 (red), and β3 integrin (grey) where evaluated on red line–marked cell sections; black arrows mark paxillin/β3 integrin co-localization spikes, and red arrow marks VEGFR2/β3 integrin co-localization spike. C, HUVECs were left adhered on immobilized FG or gremlin for 4 hours; stained for VEGFR2 (green), β3 integrin (blue), and GM1 (monosialotetrahexosylganglioside) ganglioside (red); and acquired using a LSM510 Meta confocal microscope equipped with Plan-Apochromat 63×/1.4 NA oil objective. Images show the basal portion of adherent ECs (Bar, 10 μm). Pictures were analyzed using BlobProb ImageJ plugin20: white blobs represent areas in which VEGFR2, β3 integrin, and GM1 ganglioside co-localize. Triple co-localization was quantified in 10 cells/sample (n=3; see Figure VII in the online-only Data Supplement), and data are expressed as increase of co-localization-positive area compared with FG (C′; *P<0.001, Student t test).
Engagement by ECM proteins causes the recruitment of integrin receptors into paxillin+ focal adhesions.19 Accordingly, immobilized fibrinogen induced the enrichment of β3 integrin in paxillin+ focal adhesions at the basal aspect of β3-EGFP–overexpressing GM7373 cells and HUVECs (Figure VIA in the online-only Data Supplement). At variance, ECs seeded on immobilized gremlin showed small paxillin+ focal adhesions and a widespread distribution of β3 integrin at the basal side of the cell (Figure VIB in the online-only Data Supplement). Indeed, triple co-localization experiments demonstrated that 2 β3 integrin subpopulations exist at the basal aspect of HUVECs seeded on immobilized gremlin: one subpopulation involved in the formation of small paxillin+ focal adhesions and a second VEGFR2-associated subpopulation recruited in distinct paxillin-negative cell membrane structure(s) (Figure 4B).
Lipid rafts are cholesterol- and sphingolipid-rich plasma membrane microdomains in which multiple signaling molecules and receptors are assembled to provide the molecular proximity for activation of downstream signaling.21 To identify the structure(s) involved in VEGFR2/β3 integrin complex formation at the basal aspect of cells seeded on immobilized gremlin, we compared the immunolocalization of VEGFR2 and β3 integrin to the distribution of GM1 (monosialotetrahexosylganglioside) ganglioside, a lipid raft marker,22 highlighted by fluorescently labeled cholera toxin B subunit.19 Triple co-localization analysis20,23 at the basal portion of HUVECs seeded on immobilized gremlin revealed that VEGFR2 co-localizes with β3 integrin and GM1 in paxillin-negative structures with a frequency 6× higher than that on fibrinogen (Figure 4C and Figure VII in the online-only Data Supplement). Of note, VEGFR2 polarized at the basal aspect of ECs is not recruited in caveolae, as ruled out by VEGFR2 and Caveolin 1 double-immunostaining, thus suggesting its presence in planar lipid raft structures24 (Figure VIII in the online-only Data Supplement). Importantly, GM1-associated VEGFR2 is activated, as shown by the immunolocalization of pVEGFR2 (phospho-vascular endothelial growth factor receptor 2) in GM1-positive membrane microdomains (Figure 5A). As expected, no pVEGFR2 is associated with GM1 ganglioside at the apical side of cells adhered to immobilized gremlin (Figure 5A). On these bases, to assess the role of lipid rafts in VEGFR2 activation by immobilized gremlin, HUVECs were treated with the lipid raft–disrupting agent 2,6-di-O-methyl-β-cyclodextrin (MβCD).19 Pretreatment with MβCD prevented VEGFR2 relocation and phosphorylation at the basal aspect of ECs seeded on immobilized gremlin, and its effect was fully reverted by cholesterol repletion of MβCD-treated cells (Figure 5A and 5B). Accordingly, MβCD treatment caused a significant reduction in the motogenic response of HUVECs to immobilized gremlin, fully reverted by cholesterol repletion (Figure 5C).
Figure 5.
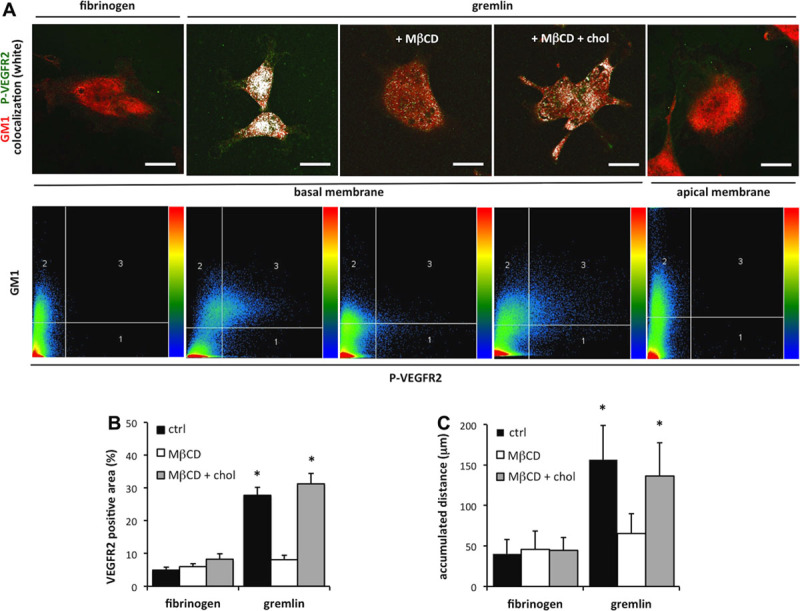
Lipid raft integrity is essential for vascular endothelial growth factor receptor 2 (VEGFR2) activation and relocation on immobilized gremlin. A, Human umbilical vein endothelial cells (HUVECs) were treated with 10 mmol/L 2,6-di-O-methyl-β-cyclodextrin (MβCD) followed or not by 400 mg/mL of cholesterol, left adhered on immobilized fibrinogen (FG) or gremlin, and stained for phospho-VEGFR2 (pTyr 1175) (green) and GM1 (monosialotetrahexosylganglioside) ganglioside (red). Samples were analyzed using a LSM510 Meta confocal microscope equipped with Plan-Apochromat 63×/1.4 NA oil objective. Images of the basal and apical aspect of cells were then analyzed for fluorescence signal co-localization (white dots; Bar, 10 μm). All image pixels are displayed in the scattergrams below; co-localized pixels are represented in the upper right quadrant. B, Ventral plasma membranes (VPMs) of VEGFR2-GM7373 (transformed fetal bovine aortic endothelial GM7373 cells) cells treated with 10 mmol/L MβCD followed or not by 400 mg/mL of cholesterol and left adhered on immobilized gremlin or FG for 4 hours were stained for VEGFR2 and actin. Stained VPMs were photographed under an epifluorescence microscope equipped with Plan-Apochromat 63×/1.4 NA oil objective (Bar, 10 μm). Total VEGFR2 was quantified in 30 cells/sample using Image-Pro Plus software. Data are expressed as percentage±SEM of VEGFR2-positive area in respect to the total VPM area, as defined by actin staining (*P<0.01, Student t test). C, HUVECs were treated or not with 10 mmol/L MβCD followed or not by 400 mg/mL of cholesterol and seeded on immobilized gremlin or FG. Cell motility was assessed by time-lapse video microscopy using an inverted microscope (Zeiss Axiovert 200 mol/L). Phase-contrast snap photographs (one frame every 10 minutes) were digitally recorded for 8 hours. Cell paths (40–50 cells per experimental point) were generated from centroid positions, and migration parameters were analyzed with the Chemotaxis and Migration Tool of ImageJ Software (http://rsbweb.nih.gov/ij). Graph shows the accumulated distances (in μm) of HUVECs seeded on immobilized gremlin or fibrinogen in the absence or presence of MβCD, cholesterol, or MβCD plus cholesterol (*P<0.05, Student t test).
Long-Lasting Retention of VEGFR2 at the Basal Aspect of ECs Requires β3 Integrin Signaling
The data above prompted us to assess the role, if any, of β3 integrin in VEGFR2 recruitment at the basal aspect of ECs. To this purpose, β3-enhanced cyan fluorescent protein and ECD-VEGFR2-EYFP GM7373 cells were cultured on glass coverslips that were flipped upside-down on gremlin- or fibrinogen-coated microslides (Figure 6A). Time-lapse analysis of Z-stack sections was performed to follow the recruitment of VEGFR2 and β3 integrin at the basal side of cells during cell adhesion to the substratum. As shown in Figure 6A′ and 6A″, VEGFR2 rapidly moved to the membrane portion in close contact with immobilized gremlin but not with fibrinogen. VEGFR2 recruitment was already detectable 6 to 8 minutes after EC/gremlin interaction and preceded the slow relocation of β3 integrin that occurred 60 to 120 minutes thereafter. In contrast, higher levels of β3 integrin were rapidly recruited in cells in contact with fibrinogen in the absence of any VEGFR2 polarization (Figure 6A″).
Figure 6.
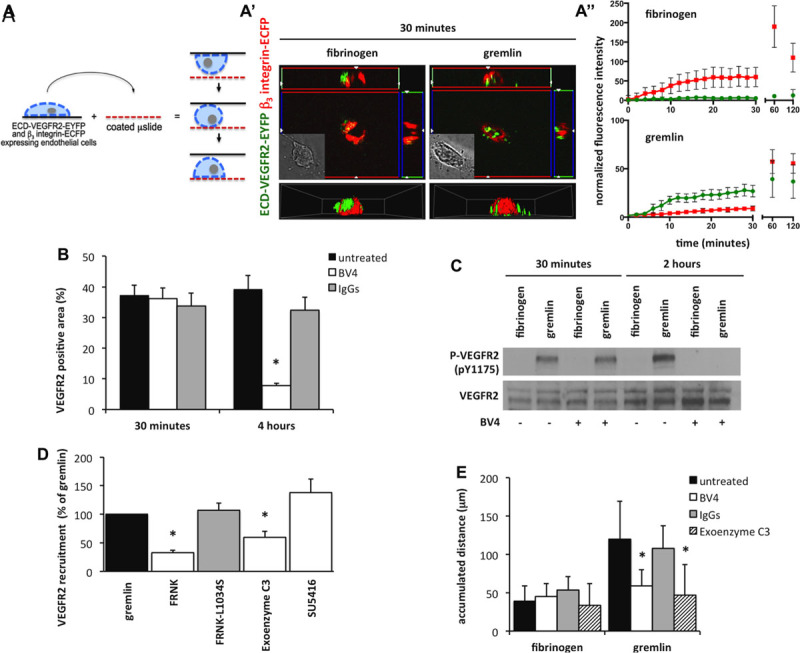
β3 integrin signaling mediates long-lasting vascular endothelial growth factor receptor 2 (VEGFR2) activation and retention by immobilized gremlin. A, β3 enhanced cyan fluorescent protein (ECFP) and enhanced yellow fluorescent protein (EYFP)–tagged extracellular domain (ECD) of VEGFR2 (ECD-VEGFR2-EYFP) co-expressing GM7373 (transformed fetal bovine aortic endothelial GM7373 cells) cells were cultured on coverslips. Coverslips were then flipped on gremlin or fibrinogen (FG)-coated microslides and analyzed for 120 minutes. Z-stack images in time lapse were recorded using a Zeiss Axiovert 200 mol/L epifluorescence microscope equipped with a Plan-Apochromat 63×/1.4 NA oil objective and ApoTome system. A′, Images show ECD-VEGFR2-EYFP (green) and β3-ECFP (red) distribution at the basal portion of cells in contact with the FG- or gremlin-coated surface at 30 minutes with their respective brightfield images, cell orthogonal z reconstructions, and a 3D reconstructions. A″, Quantification of normalized fluorescence of ECD-VEGFR2-EYFP (green lines) and β3-ECFP (red lines) on FG- or gremlin-coated surfaces during cell adhesion (mean±SEM, n=6). B, Ventral plasma membranes (VPMs) of VEGFR2-GM7373 cells treated or not with 10 μg/mL of BV4 or irrelevant IgGs and seeded on immobilized gremlin for 30 minutes or 4 hours were stained for VEGFR2 and actin. Stained VPMs were photographed under an epifluorescence microscope equipped with Plan-Apochromat 63×/1.4 NA oil objective (Bar, 10 μm). Total VEGFR2 was quantified in 30 cells/sample using Image-Pro Plus software. Data are expressed as percentage±SEM of VEGFR2-positive area in respect to the total VPM area, as defined by actin staining (*P<0.01, Student t test). C, 50 μg of cell extracts of human umbilical vein endothelial cells (HUVECs) seeded for 30 minutes or 2 hours on substrate-bound FG or gremlin in absence or presence of 10 μg/mL of BV4 antibody were probed by phospho-VEGFR2 (pTyr 1175) Western blotting. Uniform loading was confirmed by total VEGFR2 Western blotting. D, VEGFR2-GM7373 cells were alternatively treated with 1.0 μg/mL exoenzyme C3, 5.0 μmol/L SU5416 (Sugen5416), or transiently transfected with focal adhesion kinase-related nonkinase (FRNK) or FRNK S1034L and seeded on substrate-bound gremlin. VPMs were prepared, stained for VEGFR2, actin, and nuclei, and photographed under an epifluorescence microscope equipped with Plan-Apochromat 63×/1.4 NA oil objective. Total VEGFR2 was quantified in 30 cells/sample using Image-Pro Plus software. Data are expressed as fold change of the percentage±SEM of VEGFR2-positive area in respect to the total VPM area (defined by actin staining) compared with gremlin sample (*P<0.05, Student t test). E, HUVECs were seeded on immobilized FG or gremlin in the absence or presence of 10 μg/mL of BV4 antibody, irrelevant IgG, or 1 μg/mL of exoenzyme C3, and cell motility was assessed by time-lapse video microscopy using an inverted photomicroscope (Zeiss Axiovert 200 mol/L). Phase-contrast snap photographs (one frame every 10 minutes) were digitally recorded for 8 hours. Cell paths (40–50 cells per experimental point) were generated from centroid positions, and migration parameters were analyzed with the Chemotaxis and Migration Tool of ImageJ Software (http://rsbweb.nih.gov/ij). Data are expressed as accumulated distances (in μm; *P<0.01, Student t test).
Next, the effect of the neutralizing anti–β3 BV4 antibody on VEGFR2 recruitment and phosphorylation induced by immobilized gremlin was assessed at 30 minutes and at 2 to 4 hours after seeding. Of note, the BV4 antibody is known to inhibit β3 integrin at the dose tested without affecting EC adhesion.14,18 As shown in Figure 6B and 6C, VEGFR2 ventral localization and phosphorylation observed at 2 and 4 hours after seeding of ECs on immobilized gremlin was reduced by the BV4 antibody, whereas no effect was observed at 30 minutes. Together, these data point to a nonredundant role of β3 integrin in the long-lasting phosphorylation of VEGFR2 and in its retention at the basal side of ECs engaged by immobilized gremlin, being instead dispensable during the early phases of receptor relocalization and activation.
Phosphorylation (pTyr759 [phospho-tyrosine 759]) of β3 integrin is a crucial event for its direct interaction with VEGFR2 after stimulation by free VEGF-A.25 We found that immobilized gremlin induces a transient phosphorylation of β3 integrin with a peak at 30 minutes after EC seeding (Figure IX in the online-only Data Supplement). At variance, in agreement with previous observations,25 only a limited β3 integrin Tyr759 phosphorylation was observed in ECs adherent to fibrinogen. On this basis, to assess whether focal adhesion kinase (FAK)/Ras homolog gene family, member A (RhoA) β3 integrin downstream signaling26 may mediate VEGFR2 long-lasting retention at the basal membrane, VEGFR2-overexpressing GM7373 ECs were stably transfected with the cDNA encoding for the FAK C-terminal domain (focal adhesion kinase-related nonkinase [FRNK]) or with the FRNK L1034S mutant.26 FRNK exerts a dominant negative effect on FAK activation, promoting its dephosphorylation at Tyr397, effect that is abolished by the L1034S point mutation preventing FRNK localization to focal contact sites.27 As shown in Figure 6D, FRNK overexpression inhibited VEGFR2 retention, whereas FRNK L1034S was ineffective. In addition, similar to BV4 antibody treatment or FRNK overexpression, VEGFR2 polarization driven by immobilized gremlin was prevented also by the Rho inhibitor exoenzyme C3 (Figure 6D). At variance, SU5416, although inhibiting the phosphorylation of recruited VEGFR2 (Figure 3E), did not affect the amount of total VEGFR2 detected in VPMs of ECs stimulated by immobilized gremlin (Figure 6D). This is in keeping with the observation that VEGFR2 was able to rearrange on the plasma membrane and complex with β3 integrin in response to gremlin stimulation also when devoid of its intracellular TK moiety (Figure 2 and 4A). Finally, we investigated the effect of BV4 antibody and exoenzyme C3 on the motility of ECs seeded on immobilized gremlin. Time-lapse video microscopy of individual cells showed that the increase of cell motility induced by immobilized gremlin was significantly reduced by treatment with BV4 antibody or exoenzyme C3 (Figure 6E).
Discussion
The anchorage of growth factors to ECM represents an important event of the angiogenic switch that drives neovessel formation. Even though proteases and glycosidases may mediate the spatial distribution of these factors by generating freely diffusible forms,2,4 in vitro and in vivo evidences show that ECM-immobilized angiogenic growth factors are bioactive and retain the capacity to engage their signaling receptors.
Gremlin is a heparin-binding factor produced by tumors8 and retained in the stromal ECM9 surrounding CD31-positive vessels; this is in keeping with the observation that the concentration of heparin-binding VEGF in tumor interstitium is ≈10× higher than that in the plasma of tumor-bearing patients.28 This implies that VEGFR signaling in ECs is likely to be driven by abluminal rather than luminal events. Moreover, recent observations have shown a different distribution of VEGFR1 and VEGFR2 between the apical and basal sides of established brain and retina vasculatures, reflecting the presence of different signaling subcellular compartments in ECs.7 Here we demonstrate that Matrigel-anchored gremlin induces VEGFR2 relocation at the basal side of gremlin-induced neovessels in which lumen formation and EC polarization have occurred. Of note, endothelial sprouts are characterized by a diffuse distribution of VEGFR2 on the EC surface, in keeping with a nondirectional localization of the protein embedded in the matrix surrounding the whole sprout. Moreover, no recruitment of VEGFR2 at the basal aspect of polarized ECs was observed in neovessels induced by a VEGFR2-independent stimulus, that is, FGF2. Thus, VEGFR2 redistribution represents a nonredundant consequence of EC exposure to a specific directional stimulus represented by ECM-anchored gremlin.
VEGF electrostatically or covalently bound to the substratum induces a prolonged activation of VEGFR2, leading to the proliferation of adherent ECs.29 Similar results were obtained for VEGF bound to collagen gel,6 substratum-immobilized FGF2,5 and other covalently immobilized cytokines/growth factors.30–32 Also, VEGF isoforms result in different cellular behaviors depending on their affinity for ECM-associated HSPGs,3 whereas angiopoietin-1 triggers different EC responses depending on Tie2 receptor engagement at cell–substratum versus cell–cell contacts.33 Here we demonstrate that ECs stimulated by immobilized gremlin relocate VEGFR2 at the basal side of the cell, leading to a sustained receptor phosphorylation. This is paralleled by the formation of bioactive VEGFR2/β3 integrin complexes that localize in lipid raft domains. In agreement with our previous findings14 and the observation that VEGFR2/β3 integrin interaction is extracellular,34 the formation of VEGFR2/β3 integrin complexes does not require the TK activity of the receptor, being observed in ECs expressing only the ECD moiety of the receptor. Finally, as already observed for other TK receptor/integrin couples, such as platelet-derived growth factor receptor/αvβ3 integrin,35 VEGFR2/β3 integrin interaction occurs in the absence of any direct binding of gremlin to β3 integrins.14 Further experiments will be required to define the molecular bases of gremlin-mediated VEGFR2/β3 integrin interaction.
Under our experimental conditions, VEGFR2/β3 integrin complexes are localized in paxillin- and Caveolin 1–negative cell membrane structures identified as GM1-positive lipid rafts. Lipid rafts are cholesterol- and sphingolipid-rich plasma membrane microdomains that favor the interaction between transmembrane receptors and the recruitment/activation of downstream second messengers.21 Accordingly, EC pretreatment with the lipid raft–disrupting agent MβCD prevents VEGFR2 phosphorylation and recruitment and inhibits the motogenic response of ECs to immobilized gremlin, both rescued by cholesterol repletion. These data are in keeping with the role of lipid rafts in mediating VEGFR2/β3 integrin interaction by immobilized HIV-Tat (Human Immunodeficiency Virus trans-activator) protein19 and VEGFR2 activation by free VEGF.36 Of note, ECs exposed to collagen-bound VEGF organize VEGFR2/β1 integrin complexes.6 This suggests that integrin partnership and receptor complex localization after VEGFR2 engagement are contextual and may depend on the ECM microenvironment surrounding angiogenic vessels.
Our observations demonstrate that the kinetics of recruitment by immobilized gremlin of VEGFR2 and β3 integrin at the basal aspect of ECs differs between the 2 receptors. Indeed, VEGFR2 relocation largely precedes β3 integrin activation and its interaction with VEGFR2. Accordingly, the neutralizing anti–β3 integrin antibody BV4 has no effect on the early recruitment of VEGFR2 and its phosphorylation. At variance, this antibody hampers the long-lasting phosphorylation and basal membrane retention of VEGFR2 and inhibits the motogenic activity exerted by the substratum-bound angiogenic factor on adherent ECs.
The role of β3 integrin in the long-term relocation and activation of VEGFR2 by immobilized gremlin is further supported by the capacity of FAK and Rho inhibitors to suppress these responses in adherent ECs. The FAK residue Leu1034 acts as a docking site for p190RhoGEF, a Rho-specific GDP/GTP exchange factor,37 and abrogation of FAK activity hampers the capacity of ECs to activate RhoA in response to immobilized Tat.26 RhoA is a monomeric GTPase protein known to regulate actin polymerization in controlling cell shape, polarity, and locomotion. A tight crosstalk exists between actin cytoskeleton and lipid rafts: rafts control actin cytoskeleton, regulating focal adhesion turnover in migrating cells38; on the contrary, an intact actin cytoskeleton is required for several structural and functional properties of lipid rafts, such as the formation of large rafts macrodomains39 and the co-clustering of raft-associated proteins through the condensation of the plasma membrane.40 Here we demonstrate that the downregulation of FAK activity by FRNK overexpression and the inhibition of Rho by C3 exoenzyme efficiently blocks VEGFR2 retention at the basal aspect of ECs and cell motility induced by immobilized gremlin, highlighting the importance of integrin signaling in the long-lasting functionality of lipid rafts–associated VEGFR2.
Overall, these results indicate that immobilized gremlin retains its biological activity, representing a long-lasting stimulus that induces VEGFR2 polarization at the basal aspect of ECs in vivo and in vitro. Recruited VEGFR2 forms complexes with β3 integrin in EC lipid rafts, and this organization is essential for long-lasting VEGFR2 retention, phosphorylation, and activation of biological responses in ECs.
Our results highlight the notion that accumulation of angiogenic growth factors in ECM may represent a receptor-polarizing stimulus for the otherwise quiescent endothelium, leading to directional migration and proliferation and, eventually, neovessel formation. The study of the EC responses to immobilized growth factors may offer insights into the angiogenic process in physiological and pathological conditions, including cancer, and for a better engineering of synthetic tissue scaffolds to blend with the host vasculature.
Acknowledgments
We thank K. Ballmer-Hofer and his collaborators (PSI, Villigen, Switzerland) for the ECD-VEGFR2 pcDNA3/EYFP expression vector; D.D. Schlaepfer for FRNK and FRNK S1034L expression vectors; C. Urbinati and M. Rusnati for helpful advice; and D. Parazzoli and M. Garrè for imaging technical support and advice.
Sources of Funding
This work was supported by grants from Ministero dell’Istruzione, Università e Ricerca (FIRB project RBAP11H2R9 2011) to M. Presta and from Associazione Italiana per la Ricerca sul Cancro to M. Presta (AIRC grant no 14395) and S. Mitola (MFAG no 9161). C. Ravelli was supported by a fellowship from Fondazione Italiana per la Ricerca sul Cancro (FIRC), from Ministero dell’Istruzione, Università e Ricerca (FIRB project RBAP11H2R9 2011), and by New Opportunities and Ways towards ERC - NOW ERC grant from Cariplo Foundation.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronysms
- EC
- endothelial cell
- ECD
- extracellular domain
- ECM
- extracellular matrix
- EYFP
- enhanced yellow fluorescent protein
- FAK
- focal adhesion kinase
- FGF2
- fibroblast growth factor 2
- HSPG
- Heparan-sulfate proteoglycan
- HUVEC
- human umbilical vein endothelial cell
- MβCD
- 2,6-di-O-methyl-β-cyclodextrin
- TK
- tyrosine kinase
- VEGF
- vascular endothelial growth factor
- VEGFR2
- vascular endothelial growth factor receptor 2
- VPM,
- ventral plasma membrane
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.115.306230/-/DC1.
Significance
Engagement by extracellular matrix–associated angiogenic factors drives cognate receptors to the basal aspect of neovascular endothelium.
References
- 1.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Rusnati M, Presta M. Extracellular angiogenic growth factor interactions: an angiogenesis interactome survey. Endothelium. 2006;13:93–111. doi: 10.1080/10623320600698011. doi: 10.1080/10623320600698011. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol Biol Cell. 2010;21:687–690. doi: 10.1091/mbc.E09-07-0590. doi: 10.1091/mbc.E09-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy R, Zhang B, Moses MA. Making the cut: protease-mediated regulation of angiogenesis. Exp Cell Res. 2006;312:608–622. doi: 10.1016/j.yexcr.2005.11.022. doi: 10.1016/j.yexcr.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Tanghetti E, Ria R, Dell’Era P, Urbinati C, Rusnati M, Ennas MG, Presta M. Biological activity of substrate-bound basic fibroblast growth factor (FGF2): recruitment of FGF receptor-1 in endothelial cell adhesion contacts. Oncogene. 2002;21:3889–3897. doi: 10.1038/sj.onc.1205407. doi: 10.1038/sj.onc.1205407. [DOI] [PubMed] [Google Scholar]
- 6.Chen TT, Luque A, Lee S, Anderson SM, Segura T, Iruela-Arispe ML. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J Cell Biol. 2010;188:595–609. doi: 10.1083/jcb.200906044. doi: 10.1083/jcb.200906044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson N, Powner MB, Sarker MH, Burgoyne T, Campbell M, Ockrim ZK, Martinelli R, Futter CE, Grant MB, Fraser PA, Shima DT, Greenwood J, Turowski P. Differential apicobasal VEGF signaling at vascular blood-neural barriers. Dev Cell. 2014;30:541–552. doi: 10.1016/j.devcel.2014.06.027. doi: 10.1016/j.devcel.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sneddon JB, Zhen HH, Montgomery K, van de Rijn M, Tward AD, West R, Gladstone H, Chang HY, Morganroth GS, Oro AE, Brown PO. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci U S A. 2006;103:14842–14847. doi: 10.1073/pnas.0606857103. doi: 10.1073/pnas.0606857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stabile H, Mitola S, Moroni E, Belleri M, Nicoli S, Coltrini D, Peri F, Pessi A, Orsatti L, Talamo F, Castronovo V, Waltregny D, Cotelli F, Ribatti D, Presta M. Bone morphogenic protein antagonist Drm/gremlin is a novel proangiogenic factor. Blood. 2007;109:1834–1840. doi: 10.1182/blood-2006-06-032276. doi: 10.1182/blood-2006-06-032276. [DOI] [PubMed] [Google Scholar]
- 10.Chiodelli P, Mitola S, Ravelli C, Oreste P, Rusnati M, Presta M. Heparan sulfate proteoglycans mediate the angiogenic activity of the vascular endothelial growth factor receptor-2 agonist gremlin. Arterioscler Thromb Vasc Biol. 2011;31:e116–e127. doi: 10.1161/ATVBAHA.111.235184. doi: 10.1161/ATVBAHA.111.235184. [DOI] [PubMed] [Google Scholar]
- 11.Mitola S, Moroni E, Ravelli C, Andres G, Belleri M, Presta M. Angiopoietin-1 mediates the proangiogenic activity of the bone morphogenic protein antagonist Drm. Blood. 2008;112:1154–1157. doi: 10.1182/blood-2007-09-111450. doi: 10.1182/blood-2007-09-111450. [DOI] [PubMed] [Google Scholar]
- 12.Mitola S, Ravelli C, Moroni E, Salvi V, Leali D, Ballmer-Hofer K, Zammataro L, Presta M. Gremlin is a novel agonist of the major proangiogenic receptor VEGFR2. Blood. 2010;116:3677–3680. doi: 10.1182/blood-2010-06-291930. doi: 10.1182/blood-2010-06-291930. [DOI] [PubMed] [Google Scholar]
- 13.Maiolo D, Mitola S, Leali D, Oliviero G, Ravelli C, Bugatti A, Depero LE, Presta M, Bergese P. Role of nanomechanics in canonical and noncanonical pro-angiogenic ligand/VEGF receptor-2 activation. J Am Chem Soc. 2012;134:14573–14579. doi: 10.1021/ja305816p. doi: 10.1021/ja305816p. [DOI] [PubMed] [Google Scholar]
- 14.Ravelli C, Mitola S, Corsini M, Presta M. Involvement of αvβ3 integrin in gremlin-induced angiogenesis. Angiogenesis. 2013;16:235–243. doi: 10.1007/s10456-012-9309-6. doi: 10.1007/s10456-012-9309-6. [DOI] [PubMed] [Google Scholar]
- 15.Corsini M, Moroni E, Ravelli C, Andrés G, Grillo E, Ali IH, Brazil DP, Presta M, Mitola S. Cyclic adenosine monophosphate-response element-binding protein mediates the proangiogenic or proinflammatory activity of gremlin. Arterioscler Thromb Vasc Biol. 2014;34:136–145. doi: 10.1161/ATVBAHA.113.302517. doi: 10.1161/ATVBAHA.113.302517. [DOI] [PubMed] [Google Scholar]
- 16.Hughes CS, Radan L, Betts D, Postovit LM, Lajoie GA. Proteomic analysis of extracellular matrices used in stem cell culture. Proteomics. 2011;11:3983–3991. doi: 10.1002/pmic.201100030. doi: 10.1002/pmic.201100030. [DOI] [PubMed] [Google Scholar]
- 17.Cattelino A, Albertinazzi C, Bossi M, Critchley DR, de Curtis I. A cell-free system to study regulation of focal adhesions and of the connected actin cytoskeleton. Mol Biol Cell. 1999;10:373–391. doi: 10.1091/mbc.10.2.373. doi: 10.1091/mbc.10.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urbinati C, Ravelli C, Tanghetti E, Belleri M, Giacopuzzi E, Monti E, Presta M, Rusnati M. Substrate-immobilized HIV-1 Tat drives VEGFR2/α(v)β(3)-integrin complex formation and polarization in endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:e25–e34. doi: 10.1161/ATVBAHA.111.242396. doi: 10.1161/ATVBAHA.111.242396. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher PA, Scriven DR, Schulson MN, Moore ED. Multi-image colocalization and its statistical significance. Biophys J. 2010;99:1996–2005. doi: 10.1016/j.bpj.2010.07.006. doi: 10.1016/j.bpj.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Insel PA, Patel HH. Membrane rafts and caveolae in cardiovascular signaling. Curr Opin Nephrol Hypertens. 2009;18:50–56. doi: 10.1097/MNH.0b013e3283186f82. doi: 10.1097/MNH.0b013e3283186f82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Echarri A, Muriel O, Del Pozo MA. Intracellular trafficking of raft/caveolae domains: insights from integrin signaling. Semin Cell Dev Biol. 2007;18:627–637. doi: 10.1016/j.semcdb.2007.08.004. doi: 10.1016/j.semcdb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Schulson MN, Scriven DR, Fletcher P, Moore ED. Couplons in rat atria form distinct subgroups defined by their molecular partners. J Cell Sci. 2011;124(pt 7):1167–1174. doi: 10.1242/jcs.080929. doi: 10.1242/jcs.080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- 25.Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res. 2007;101:570–580. doi: 10.1161/CIRCRESAHA.107.155655. doi: 10.1161/CIRCRESAHA.107.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbinati C, Bugatti A, Giacca M, Schlaepfer D, Presta M, Rusnati M. alpha(v)beta3-integrin-dependent activation of focal adhesion kinase mediates NF-kappaB activation and motogenic activity by HIV-1 Tat in endothelial cells. J Cell Sci. 2005;118(pt 17):3949–3958. doi: 10.1242/jcs.02518. doi: 10.1242/jcs.02518. [DOI] [PubMed] [Google Scholar]
- 27.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112(pt 16):2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 28.Finley SD, Popel AS. Effect of tumor microenvironment on tumor VEGF during anti-VEGF treatment: systems biology predictions. J Natl Cancer Inst. 2013;105:802–811. doi: 10.1093/jnci/djt093. doi: 10.1093/jnci/djt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson SM, Chen TT, Iruela-Arispe ML, Segura T. The phosphorylation of vascular endothelial growth factor receptor-2 (VEGFR-2) by engineered surfaces with electrostatically or covalently immobilized VEGF. Biomaterials. 2009;30:4618–4628. doi: 10.1016/j.biomaterials.2009.05.030. doi: 10.1016/j.biomaterials.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu LM, Wosnick JH, Shoichet MS. Miniaturized system of neurotrophin patterning for guided regeneration. J Neurosci Methods. 2008;171:253–263. doi: 10.1016/j.jneumeth.2008.03.023. doi: 10.1016/j.jneumeth.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Fan VH, Tamama K, Au A, Littrell R, Richardson LB, Wright JW, Wells A, Griffith LG. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25:1241–1251. doi: 10.1634/stemcells.2006-0320. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 32.Leclerc C, Brose C, Nouzé C, Leonard F, Majlessi L, Becker S, von Briesen H, Lo-Man R. Immobilized cytokines as biomaterials for manufacturing immune cell based vaccines. J Biomed Mater Res A. 2008;86:1033–1040. doi: 10.1002/jbm.a.31751. doi: 10.1002/jbm.a.31751. [DOI] [PubMed] [Google Scholar]
- 33.Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- 34.Borges E, Jan Y, Ruoslahti E. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J Biol Chem. 2000;275:39867–39873. doi: 10.1074/jbc.M007040200. doi: 10.1074/jbc.M007040200. [DOI] [PubMed] [Google Scholar]
- 35.Amano H, Ikeda W, Kawano S, Kajita M, Tamaru Y, Inoue N, Minami Y, Yamada A, Takai Y. Interaction and localization of Necl-5 and PDGF receptor beta at the leading edges of moving NIH3T3 cells: Implications for directional cell movement. Genes Cells. 2008;13:269–284. doi: 10.1111/j.1365-2443.2008.01167.x. doi: 10.1111/j.1365-2443.2008.01167.x. [DOI] [PubMed] [Google Scholar]
- 36.Labrecque L, Royal I, Surprenant DS, Patterson C, Gingras D, Béliveau R. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol Biol Cell. 2003;14:334–347. doi: 10.1091/mbc.E02-07-0379. doi: 10.1091/mbc.E02-07-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhai J, Lin H, Nie Z, Wu J, Cañete-Soler R, Schlaepfer WW, Schlaepfer DD. Direct interaction of focal adhesion kinase with p190RhoGEF. J Biol Chem. 2003;278:24865–24873. doi: 10.1074/jbc.M302381200. doi: 10.1074/jbc.M302381200. [DOI] [PubMed] [Google Scholar]
- 38.Wang R, Bi J, Ampah KK, Ba X, Liu W, Zeng X. Lipid rafts control human melanoma cell migration by regulating focal adhesion disassembly. Biochim Biophys Acta. 2013;1833:3195–3205. doi: 10.1016/j.bbamcr.2013.09.007. doi: 10.1016/j.bbamcr.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Chichili GR, Rodgers W. Cytoskeleton-membrane interactions in membrane raft structure. Cell Mol Life Sci. 2009;66:2319–2328. doi: 10.1007/s00018-009-0022-6. doi: 10.1007/s00018-009-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaus K, Chklovskaia E, Fazekas de St Groth B, Jessup W, Harder T. Condensation of the plasma membrane at the site of T lymphocyte activation. J Cell Biol. 2005;171:121–131. doi: 10.1083/jcb.200505047. doi: 10.1083/jcb.200505047. [DOI] [PMC free article] [PubMed] [Google Scholar]


