Abstract
Aims
The purpose of this study was to determine the effect of a 12-week pelvic floor muscle (PFM) training program on urethral morphology and mobility in women with stress urinary incontinence (SUI).
Methods
Forty women with SUI were randomly assigned to one of two groups: the treatment group received 12 weekly physiotherapy sessions during which they learned how to properly contract their pelvic floor muscles (PFMs) and a home exercise program was prescribed, reviewed, and progressed; the control group received no treatment. Before and after the 12-week study period, ultrasound imaging was used to evaluate bladder neck position and mobility during coughing and Valsalva maneuver in supine and in standing, as well as urethral morphology. Secondary outcome measures included a 3-day bladder diary, 30-min pad test, the Incontinence Impact Questionnaire (IIQ-7) and the Urogenital Distress Inventory (UDI-6).
Results
The women in the treatment group demonstrated reduced bladder neck mobility during coughing and increased cross-sectional area of their urethra after as compared to before the training. These changes were not evident in the control group. No differences in the resting position of the bladder neck or in bladder neck excursion during Valsalva maneuver were noted in either group. Concomitantly the women in the treatment group demonstrated significant improvements in the 3-day bladder diary and IIQ-7 after the PFM training and improved significantly more than the control group.
Conclusion
Physiotherapist-supervised PFM training reduces bladder neck motion during coughing, and results in hypertrophy of the urethral sphincter in women who present with SUI.
Keywords: pelvic floor, physiotherapy, exercise, stress urinary incontinence, ultrasound, urethra
INTRODUCTION
Stress urinary incontinence (SUI) appears to result from multiple failures in the continence mechanism, where defects in sphincteric function,1 the PFMs,2,3 connective tissues4 or neural structures5,6 may all play a role. Physical therapy, mainly involving strength training of the PFMs and training motor control strategies to prevent urine loss,7–9 has shown positive results in women with SUI. As such, physical therapy has been recommended by the International Continence Society as the first-line treatment for women whose primary complaint is SUI.10 Published results demonstrate reductions of up to 80% in the frequency of incontinence episodes11 and cure rates in as many as 50% of women.8 In one study many women chose not to continue with medical treatment based on the success of this conservative therapy.11 That said, physical therapy does not offer a complete cure in half of women who undergo treatment, and the mechanisms through which PFM training improves symptoms of incontinence are not clear.
Using ultrasound imaging, a recent study showed that PFM training reduced the dimensions of the levator hiatus, elevated the resting position of the bladder and rectum, and reduced symptom severity in women with pelvic organ prolapse.12 Using magnetic resonance imaging (MRI), Dumoulin et al.13 found that in a small (n = 5) sample of women who underwent a PFM training program, the PFM surface area at rest decreased relative to that before the training program, as did movement of the coccyx during PFM contraction. They attributed these changes to an increase in passive stiffness in the levator ani.
The PFMs are also active contributors to the continence mechanism.4 During tasks that challenge the continence system, such as coughing, sneezing or laughing, increases in intra-abdominal pressure are transmitted to the urethra to enhance closure only if the urethra remains in position between the pubic symphysis (PS) (or anterior vaginal wall) and the pelvic floor. Measurements of urethral trajectory and acceleration during functional tasks such as coughing14–16 have shown that women with SUI demonstrate a larger excursion of the urethra and/or anorectal angle (ARA) during coughing maneuvers than their continent counterparts. These results suggest that the urethra is not effectively held in place behind the PS to ensure that it is compressed against the pelvic floor when there is an increase in intra-abdominal pressure, which in turn suggests that women with SUI have ineffective endopelvic fascia (pubourethral ligaments) and/or PFMs that are slow or ineffective in offering support to the urethra so that it can be pressed closed. To date, no one has investigated the impact of PFM training on urethral structure, support, or mobility.
The purpose of this study was to investigate the impact of a physiotherapist-supervised 12-week PFM training program for women with SUI on resting bladder neck position, bladder neck mobility during coughing and Valsalva tasks, and on urethral morphology.
MATERIALS AND METHODS
This study was approved by the Queen’s University and Affiliated Hospitals Health Sciences Research Ethics Board, and all women provided written informed consent prior to participating.
Participants
No studies were found in the literature from which sample size estimates could be determined for the key measures employed in this study (i.e., changes in urethral cross-sectional area induced by exercise therapy). Results from a related study were therefore extrapolated to estimate the sample size required to generate a power of 0.8 at an alpha level of 0.05 on this measure. In Digesu et al.,1 rhabdosphincter volumes were measured preoperatively in 91 women who underwent Burch colposuspension surgery, where 74 were objectively cured and 17 were not. Based on a mean rhabdosphincter volume difference of 2.66 cm3 between the surgical successes and failures and a pooled standard deviation of 2.8 determined from the data presented, 19 subjects in each group were deemed sufficient to detect differences in rhabdosphincter volume induced by treatment. A randomized controlled trial by Balmforth et al.17 was used to determine the sample size required to study the effect of PFM training on pad weight after a standardized pad test. Ninety-seven women were randomized to either a 14-week PFM training program or no therapy, and the treatment group showed a marked reduction in pad weight (6.76 g) after therapy whereas the control group did not. With standard deviation estimated conservatively at 2 g, a sample size of 20 women per group was determined to be adequate for the current study for a power of 0.8 at alpha = 0.05. As such, it was determined that a sample size of 40 women (20 per group) would be required to achieve a power of 0.8 at an alpha level of 0.05 in this study.
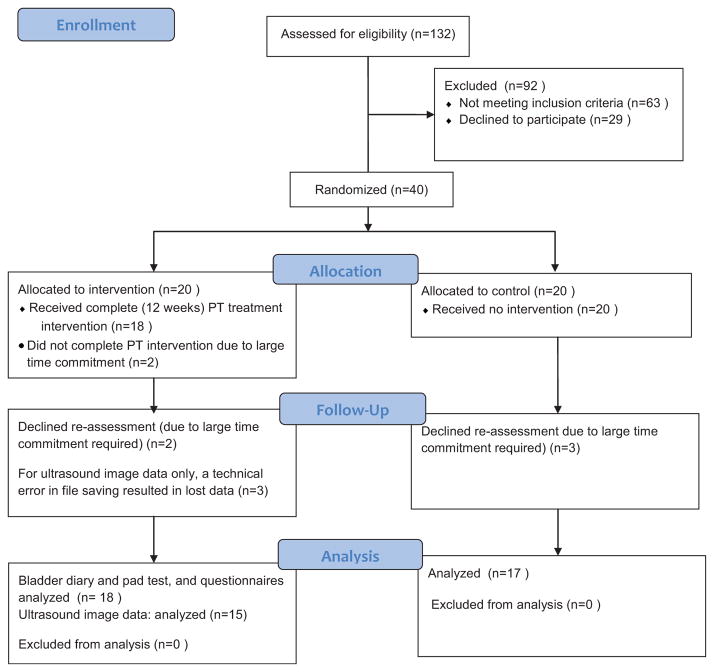
A summary of the recruitment and participation statistics are summarized in the CONSORT flow diagram presented in Figure 1. Women who were on the waiting lists to see one of three local urogynecologists due to complaints of SUI were recruited to participate in this study. Women were included if they were 18 years or older, had symptoms of SUI with or without urge incontinence, nocturia or anterior compartment prolapse, but not if they had fecal incontinence. Women with fecal incontinence were specifically excluded because our experience is that women who lose feces during the collection of ultrasound image data during the study tasks results in suboptimal performance of these tasks. Potential volunteers were also excluded if they were on medications known to increase or alleviate incontinence,18 if they had known neurological impairments involving the central nervous system or the sacral nerves19 or known connective tissue disorders.20
Fig. 1.
CONSORT flow diagram.
Volunteers who met the screening criteria above underwent urodynamic studies, and those with detrusor instability were excluded from participation. Next, the women underwent a clinical urogynecological exam including palpation, reflex and sensory testing, and the POP-Q,21 and those with evidence of neurological defects, pelvic mass or prolapse greater than stage two were excluded. Recruitment continued until 40 women who met all inclusion and exclusion criteria agreed to participate, 20 of these were randomly allocated to the intervention cohort and 20 were allocated to the control cohort using a custom automated computer algorithm.
Data Collection
In the week prior to the first data collection visit, all participants completed a 3-day bladder diary22 recording voiding frequency and number of episodes of urine leakage, and the activities that provoked the leakage over three representative days (2 week days, 1 week-end day). At the first data collection visit, the research physical therapist reviewed the bladder diary results to ensure that the participant predominantly recorded episodes of SUI as opposed to urge urinary incontinence (UUI) and if it did predominantly report UUI episodes, they were excluded from the study at that point. Volunteers were asked to avoid more than one caffeinated beverage on the day of their appointment, to empty their bladder 2 hr prior to their appointment, and then to drink 500 ml of water at that time. On arrival, bladder volume was assessed using trans-abdominal ultrasound imaging. If the bladder volume was between 300 and 400 ml, then the subject began a standardized pad test.23,24 If the bladder volume was less than 300 ml, the volunteer was asked to drink 200 ml of water and waited an additional 20 min before bladder volume was measured again. This process was repeated until the required bladder volume was achieved. For the pad test, a pre-weighed incontinence pad was adhered to the participant’s undergarment. The participant performed a standardized circuit of walking and stair climbing for 20 min, then performed 10 forceful coughs, flexed forward at the waist as far as she could go five times, stood up and sat down 10 times, performed 10 jumping jacks and washed her hands under water for 1 min.10 The weight of the pad was measured again after these tasks and the difference in pad weight was recorded as the outcome of the test. The volunteer emptied her bladder immediately following the test and then completed the Urogenital Distress Inventory (UDI-6) questionnaire25,26 and the Incontinence Impact Questionnaire (IIQ-7).25
An ultrasound imaging evaluation followed. Two- and three-dimensional ultrasound imaging of the pelvic structures was performed using the GE Voluson-i ultrasound system operated by a trained research physical therapist. A 3D mechanical curvilinear probe (6.5–10 MHz) was covered in ultrasound gel, a latex free glove was stretched over the probe head, and more gel was applied. During the ultrasound examination, women were in the lithotomy position on a plinth with stirrups. Before each task, each woman was reminded that urine or gas leakage during the test is normal, and was instructed not to hold back if she felt leakage was about to occur.
2D ultrasound images (real time, B-Mode) in the sagittal plane were acquired transperineally while the participant remained relaxed, to identify the position of the PS, the bladder, the urethra, and the ARA. With these structures still in view, volunteers performed three repetitions of a maximal effort cough, and three repetitions of a maximal effort Valsalva maneuver as per Dietz and Lekskulchai,27 while receiving standardized verbal encouragement. The PS and ARA remained within the visual field of the ultrasound images during all tasks, and the cine-loops were stored for off-line data processing.
Next, a 7.5 MHz mechanical sector endoprobe (General Electric, http://www3.gehealthcare.ca) was covered in ultrasound gel, a condom, then more ultrasound gel, and the tip of the probe was placed at the external meatus of the urethra. 3D images of the full length of the urethra were captured while the participant kept her PFMs relaxed. The striated urethral sphincter was centered in the sagittal and axial planes during acquisition to ensure that the full structure would be visible in axial slices during post-processing.
The volunteer was then asked to move to the standing position and sagittal plane imaging using the curvinilinar probe was repeated to evaluate the position of the bladder neck relative to the levator hiatus while the participant stood quietly, and to evaluate the motion of the urethra relative to the PS and ARA in standing while the women repeated three maximal effort coughs and three maximal effort Valsalva maneuvers. Out of plane rotation of the transducer during activities has been found to have inconsequential effects on kinematic data of the ARA,15 however every effort was made to minimize rotation of the transducer during the ultrasound recordings. The entire data collection procedure took approximately 90 min.
Intervention
The women assigned to the PFM strength training group attended weekly private physiotherapy sessions. In the first session, participants learned to perform a proper PFM contraction using manual palpation and feedback to optimize PFM contraction quality,28,29 and in which they learned to contract their PFMs before tasks that increase intra-abdominal pressure including coughing, laughing, sneezing, and postural perturbations. These women were instructed to practice three sets of 12 PFM contractions daily until their next visit 1 week later.12 At subsequent weekly visits, the physiotherapist reviewed and reinforced the proper PFM contraction technique, evaluated PFM strength using a modified Oxford scale to provide feedback about progress, reviewed the technique of contracting the PFMs before coughing or postural perturbations, and encouraged the participant to continue with her home exercise program. Each session lasted approximately 30 min. Women in both groups returned to the laboratory after 12 weeks and repeated the 3-day bladder diary, the standardized pad test, the UDI and IIQ questionnaires and the ultrasound evaluation as described above.
Data Processing
Using Image-J software (National Institutes of Health; http://rsbweb.nih.gov/ij/), the sagittal plane images recorded while the women remained relaxed (rest) and when they reached maximal excursion of the bladder neck during the Valsalva maneuver had a reference line drawn from the most caudal and posterior point of the PS to the posterior aspect of the apex of the ARA. The perpendicular distance from the bladder neck to the reference line was measured for all conditions (rest trials and start and end of the Valsalva maneuver) and positions (supine and standing). For the Valsalva maneuver, bladder neck excursion was calculated as the difference in position of the bladder neck relative to the reference line on the first frame recorded before the Valsalva command was given and on the frame where the bladder neck was furthest away from its starting position while the Valsalva was performed. The bladder neck excursion was recorded for the coughing trials using the method described by Dietz and Lekskulchai,27 although only the excursion of the bladder neck is reported here.
3D volume images of the urethra were rendered in 4D View™ analysis software (General Electric). The length of the urethra was identified by points marked at the bladder neck and at the external meatus. The mid-point of the urethra was identified along a line tracing the urethra from the bladder neck to the external meatus. Tomographic images of the cross-section of the urethra were identified at the midpoint of the urethra, and at points 2.5 and 5 mm cranial and caudal to the midpoint. At each point, because the urethra did not follow a straight line, care was taken to rotate the sagittal plane image to ensure that the cross-sectional slice was perpendicular to the path of the urethra in both the sagittal and axial planes. The cross-sectional area of the urethral wall was determined by subtracting the area bordered by the external boundary of the hypoechoic urethral lumen (including lumen and submucosa) from the total area of the urethra indicated by the external boundary of the hyperechoic urethral wall.
Data Analysis
Data analysis was performed using Minitab™ version 16 (E-academy). All outcome variables were analyzed to ensure that they were normally distributed using the Kolmogorov–Smirnov test prior to using parametric statistics. For descriptive purposes, the treatment and control groups were compared in terms of demographic information (age, parity, BMI) and scores on the UDI and IIQ recorded at the first screening session to ensure that no group differences existed.
Two-way repeated measures analyses of variance (ANOVAs) were used to determine differences in the UDI, IIQ, pad test and bladder diary results between groups and sessions (pre vs. post-treatment). The interaction between group and session was included in these models.
To investigate the effect of the PFM training program on urethral support while participants remained relaxed, a three-way repeated measures ANOVA was used. The outcome measure was the perpendicular distance between the bladder neck and the reference line drawn between the PS and ARA, and the main effects were group (treatment vs. control), session (pre-treatment vs. post-treatment) and position (supine vs. standing). All two- and three-way interactions were included in the model. Similarly, to investigate the effects of the PFM training program on bladder neck excursion during Valsalva and during coughing, three-way repeated measures ANOVAs were used, and included group, session, position and all two- and three-way interactions. Finally, to investigate the effect of the PFM training program in urethral cross-sectional area, a two-way repeated measures multivariate ANOVA (MANOVA) was used, with the outcome variables being the cross-sectional area of the urethra at the five different points, and the effects being group and session. An alpha level of 0.05 was used for all ANOVAs and MANOVAs.
RESULTS
Of the 40 women recruited to participate, 17 women in the control group and 18 women in the treatment group completed the study (see Fig. 1). The mean age of participants was not significantly different between the groups (control group 54.0 ± 8.4 years, treatment group 49.5 ± 8.2 years); nor did parity (control group 2.2 ± 1.0 vaginal births, treatment group 2.6 ± 1.1 vaginal births) or body mass index (control group 28.6 ± 11.3 kg/m2, treatment group 27.0 ± 3.8 kg/m2).
There was no difference in bladder diary, pad test, IIQ-6 or UDI-7 scores between the groups at the start of the study (P > 0.05). The two-way repeated measures ANOVAs revealed significant interactions between group (treatment vs. control) and session (pre-treatment vs. post-treatment) for the number of leakage episodes over 3 days attributed to stress events on the bladder diary and for the IIQ-7 scores, but no significant interactions between these factors for the pad test or the UDI-6 scores. Table I summarizes the bladder diary, pad test and questionnaire results, with P-values reported indicating the P-value of the Tukey’s post hoc pairwise comparisons between sessions (pre-treatment vs. post-treatment) for each group.
TABLE I.
Patient-Oriented Outcomes
| Control
|
P-value | Treatment
|
P-value | |||
|---|---|---|---|---|---|---|
| Pre-Rx | Post-Rx | Pre-Rx | Post-Rx | |||
| Bladder diary (episodes) | 7.6 (4.2) | 4.9 (3.1) | P = 0.199 | 6.4 (4.8) | 2.5 (2.7) | P = 0.007 |
| Pad test (g) | 20.2 (24.7) | 11.4 (15.5) | P = 0.225 | 22.2 (25.9) | 9.7 (15.8) | P = 0.197 |
| IIQ-7 | 27.0 (19.3) | 29.2 (21.9) | P = 0.998 | 40.9 (22.0) | 9.5 (7.2) | P = 0.0003 |
| UDI-6 | 48.0 (20.4) | 49.2 (21.6) | P = 0.998 | 48.0 (21.0) | 33.2 (20.0) | P = 0.401 |
Mean (SD) values for the bladder diary, pad test, UDI and IIQ are presented. Rx denotes “treatment.” No significant differences in these measures were found between the groups at the start of the study (i.e., Pre-Rx). Significant differences between sessions (i.e., Pre-Rx vs. Post-Rx) were found for the bladder diary and the IIQ-7 in the treatment group only and are noted in bold.
Ultrasound image data were contaminated (technical errors during file saving caused files to be unreadable) for three of the 18 participants in the treatment group and therefore the ultrasound results reported include data for 15 participants in the treatment group and 17 participants in the control group.
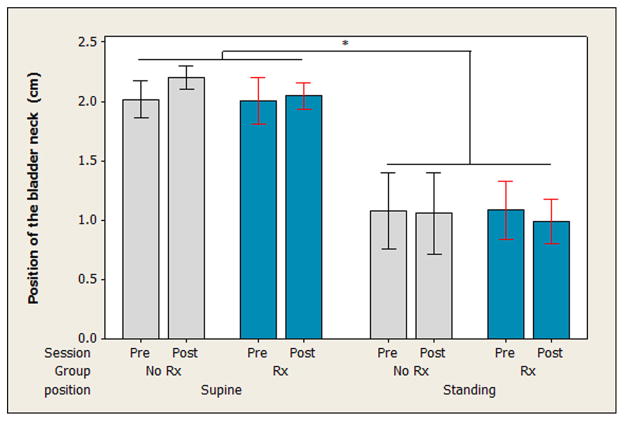
The three-way ANOVA on the resting position of the bladder neck revealed no significant two- or three-way interactions between group, session, and position. There was a main effect for position (F = 179.55, P < 0.0005), where the bladder neck sat closer to the levator plate in standing compared to supine (see Fig. 2).
Fig. 2.
Vertical position of the bladder neck relative to the levator plate at rest. The perpendicular distance between the bladder neck and a line drawn between the inferior aspect of the pubic symphysis and the anorectal angle is presented. Measures were taken in supine and in standing both before (Pre-Rx) and after (Post-Rx) the 12-week study period. Bars indicate mean distance and whiskers indicate 95% confidence intervals for the mean. The bladder neck was located significantly higher in standing than in supine, as indicated by “*.” No change in this distance was seen after the study period as compared to before the study period.
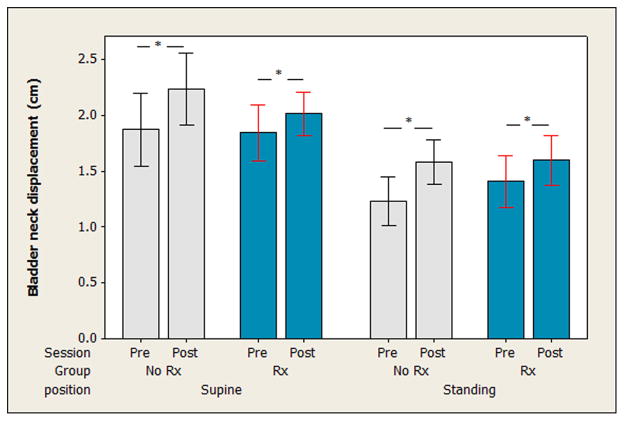
The three-way ANOVA on excursion of the bladder neck during the Valsalva maneuver revealed no significant two- or three-way interactions (P > 0.05). There was no main effect of group on the amount of descent of the bladder neck on Valsalva maneuver (F = 0.03, P = 0.81). There was a main effect for session (F = 8.98, P = 0.003), where, in both positions, both groups demonstrated more bladder neck descent on Valsalva after 12 weeks than they did before the 12-week period. There was also a main effect for position (F = 36.21, P < 0.0005) where there was more descent of the bladder neck during Valsalva when the maneuver was performed in standing as compared to in supine (see Fig. 3).
Fig. 3.
Displacement of the bladder neck during a maximal effort Valsalva maneuver. The change in perpendicular distance between the bladder neck and a line drawn between the inferior aspect of the pubic symphysis and the anorectal angle is presented for both groups before (Pre-Rx) and after (Post-Rx) the 12-week study period. Measures were taken in both the supine and standing positions. Bars indicate means and whiskers indicate the 95% confidence interval for the mean. In both groups and positions, there was more bladder neck displacement during the Valsalva noted at Post-Rx as compared to Pre-Rx, as indicated by “*.”
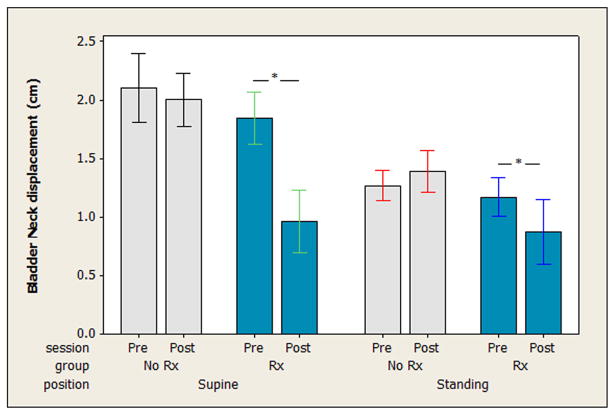
For bladder neck displacement during coughing, the three-way ANOVA revealed no significant three-way interaction (F = 1.05, P = 0.305). There was a significant two-way interaction between group and session (F = 11.84, P = 0.001). The Tukey’s post hoc analysis showed that the treatment group demonstrated a reduction in bladder neck displacement during the cough between the pre- and post-test sessions (P < 0.0005), whereas no difference in bladder neck displacement between the pre- and post-test sessions was observed in the control group (P = 0.9998). The treatment effect is evident in both positions but is more marked when the coughs were performed in supine than when they were performed in standing (see Fig. 4).
Fig. 4.
Bladder neck displacement during coughing. The excursion of the bladder neck measured in the sagittal plane during coughing is presented before (Pre-Rx) and after (Post-Rx) the 12-week study period for both groups (No Rx, Rx) in both the supine and standing positions. Bars in indicate means and whiskers indicate 95% confidence intervals for the mean. In the treatment group, in both supine and standing, there was a significant reduction in bladder neck displacement during coughing after the study period compared to before the study period, as indicated by “*.” The control group did not demonstrate this same effect.
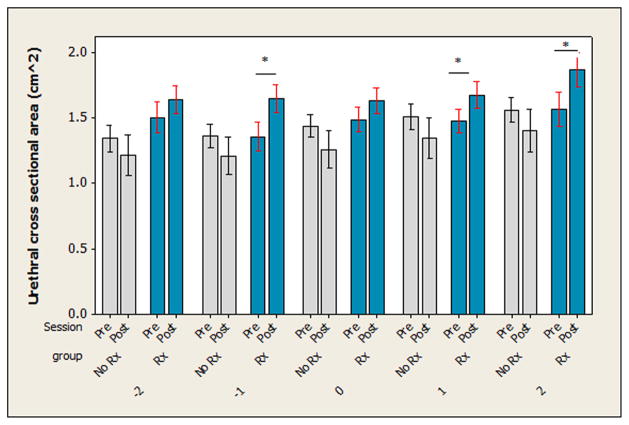
The MANOVA including the urethral cross-sectional area at all five measurement points indicated there was a group by session interaction (Wilks F = 3.615, P = 0.004). Examining Figure 5, the urethral cross-sectional area was larger after the 12-week treatment session in women in the treatment group but appeared to be smaller in the women in the control group. Tukey’s post hoc comparisons for the urethral cross-sectional area measured at each site indicated that urethral cross-sectional area was significantly larger in the treatment group after 12 weeks of exercise at one cm below and at one and two cm above the midpoint of the urethra. There was a trend where the urethral cross-sectional area was smaller in the control group after the 12-week period, but this effect was not significant at any of the individual measurement points when tested using post hoc analyses.
Fig. 5.
Urethral wall cross sectional area. The cross sectional area of the urethral wall was measured in both groups before (Pre-Rx) and after (Post-Rx) the study period. Overall the MANOVA revealed a significant (P = 0.004) difference between groups, where the treatment (Rx) group showed an increase in cross sectional area but the control group (No Rx) did not. Univariate ANOVA results are presented here, where “*” indicates significant differences between Pre- and Post-Rx for each group at each specific point that was measured along the length of the urethra.
DISCUSSION
This study demonstrates that supervised PFM training reduces urethral mobility during coughing and increases the cross-sectional area of the urethra, but does not reduce the extent of bladder neck excursion seen during maximal effort Valsalva maneuvers. This result was as expected. The action of the PFMs has been shown to hold the urethra in place such that it can be compressed between that anterior vaginal wall and the PS during tasks that increase intra-abdominal pressure.4,14–16 As such, a reduction of bladder neck excursion during coughing is an expected outcome. Conversely, a properly performed Valsalva maneuver involves complete relaxation of the PFMs,28,30 and therefore challenges the passive resistance of the PFMs and endopelvic fascia. Strengthening the PFMs is not expected to appreciably improve the integrity of the surrounding connective tissues and as such, it was not expected that the training program would reduce bladder neck excursion during a maximal effort Valsalva maneuver. Interestingly, the participants in both groups demonstrated more bladder neck descent after the 12-week period compared to their original excursion. This change is likely due to participants learning how to perform a true maximal Valsalva effort through the encouragement provided by the research assistant, or being more comfortable with the researchers and letting the PFMs completely relax during this maneuver, without worrying about the leakage of urine or gas.28
The finding that the resting position of the bladder neck relative to the levator plate did not change after PFM training is inconsistent with the findings of Hoff Braekken et al.12 who found the opposite in women with pelvic organ prolapse. This can be explained by differences between the cohorts recruited in the present study, where none of the participants had POP-Q grades greater than 2, and in the Hoff Braekken study, where women had larger degrees of prolapse, and therefore had more room for improvement in the active support offered by the PFMs.
The finding that urethral cross-sectional area increased in the cohort that performed supervised PFM exercises but not in the control cohort is particularly interesting. Although ultrasound measures of urethral cross-sectional area do not provide adequate resolution to determine whether an increase in diameter is related to increased connective tissue or sphincter muscle volume, it can be postulated that the increased diameter is the result of muscle hypertrophy. Using MRI, Delancey’s group29 recently showed that the mean striated urethral sphincter dimensions in women with SUI were smaller than those of a control group. Interestingly, PFM contraction strength on maximum voluntary contraction was positively associated with urethral sphincter length, cross-sectional area and a surrogate measure for volume. An early study by Bø and Stien32 showed that during a voluntary contraction of the PFMs, the striated urethral sphincter muscle contracts automatically. This result suggests that training the PFMs may cause improvements in striated urethral sphincter volume and function, which was further supported by Miller coworkers31 who showed that women without a visible pubococcygeus muscle on MRI could still increase their urethral closure pressures when performing a PFM contraction. Given the findings of Bo and Stein32 and Miller et al.,33 it is reasonable to postulate that the PFM exercises performed by the treatment group in this study resulted in hypertrophy of the urethral sphincters.
In terms of patient-oriented outcomes, the results of this study are interesting. The bladder diary results demonstrated a significant improvement in the pelvic floor exercise cohort but not in the control cohort, whereas the standardized pad test demonstrated no such effect. It therefore appears that the 3-day bladder diary is more sensitive to change induced by PFM training than a standardized pad test. This difference may be due to the application and interpretation of the measures. Since the intervention was designed to specifically target SUI, only incontinence events associated with stress tasks were counted from the bladder diary data in this study. If urge incontinence occurred during the 3-day log, it would not have been counted. Alternatively, the standardized pad test included tasks that would provoke both stress and urge incontinence episodes. Since the women in our sample had a clear diagnosis of SUI, but could also have symptoms of urge incontinence, the way in which these tests were applied and interpreted could have influenced the results. Perhaps separate pad tests should be designed such that one test evaluates stress incontinence-provoking tasks (e.g., coughing, jumping, sitting, standing, walking) while a separate test could evaluate tasks that normally induce urge incontinence (e.g., running water, manipulating keys, etc.).
Similarly, the IIQ-7 demonstrated significant improvements in women’s perceptions of the impact of their SUI after the 12-week PFM training program that were not present in the control group. The UDI-6 did not show any significant difference in either cohort. It is possible that interaction with the physical therapist, quite separately from the strengthening exercises, might have influenced the results of these questionnaires. Since there was a large drop in UDI-6 scores in the treatment group but not in the control group, it is clear that the UDI-6 results would have been significantly different between the groups if a larger sample had been recruited. A post hoc power analysis (power = 0.8, alpha = 0.05) for a single paired sample suggests that our sample size of 18 per group should have been adequate to detect this difference so had we not lost participants to follow-up, this result might have been different. Nevertheless, as a result of these findings, researchers should consider using the 3-day bladder diary coding stress incontinence events and the IIQ-7 when evaluating patient-oriented outcomes in future studies on the effectiveness of PFM exercises on SUI in women.
CONCLUSIONS
This study demonstrates that PFM training is effective in reducing urethral excursion and enhancing urethral sphincter cross-sectional area in women with SUI. Both of these effects are likely contribute to a reduction in the frequency of incontinence episodes in this population since our treatment group showed significant improvements in their bladder diary reports and on the IIQ. The finding that PFM training results in hypertrophy of the urethral sphincter is novel and of particular clinical relevance as it suggests a mechanism through which PFM training may be effective in treating SUI in women who do not have intact PFMs or in whom the PFMs are already strong but the urethral sphincters are damaged or weak.
Footnotes
Conflict of interest: none.
Mickey Karram led the peer-review process as the Associate Editor responsible for the paper.
References
- 1.Digesu GA, Robinson D, Cardozo L, et al. Three-dimensional ultrasound of the urethral sphincter predicts continence surgery outcome. Neurourol Urodyn. 2009;28:90–4. doi: 10.1002/nau.20566. [DOI] [PubMed] [Google Scholar]
- 2.Abrams P, Andersson KE, Birder L, et al., editors. Fourth international consultation on incontinence recommendations of the international scientific committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn. 2010;29:213–40. doi: 10.1002/nau.20870. [DOI] [PubMed] [Google Scholar]
- 3.Motswin J, Bourcier A, Habb F, et al. Pathophysiology of urinary incontinence: Fecal incontinence and pelvic organ prolapse. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 3rd International Consultation on Incontinence. West Caldwell: Health Publications Ltd; 2005. [Google Scholar]
- 4.DeLancey JOL, Gossling J, Creed K, et al. Gross anatomy and cell biology of the lower urinary tract. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 2nd International Consultation on Incontinence. Paris: Health Publications Ltd; 2002. pp. 17–82. [Google Scholar]
- 5.Snooks SJ, Swash M, Henry MM, et al. Risk factors in childbirth causing damage to the pelvic floor innervation. Int J Colorectal Dis. 1986;1:20–4. doi: 10.1007/BF01648831. [DOI] [PubMed] [Google Scholar]
- 6.Smith ARB, Hosker GL, Warrell DW. The role of pudendal nerve damage in the aetiology of genuine stress incontinence in women. Br J Obstet Gynecol. 1985;96:29. doi: 10.1111/j.1471-0528.1989.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 7.Grewar H, McLean L. The integrated continence mechanism: A manual therapy approach to the management of stress urinary incontinence. The integrated continence system: A manual therapy approach to the treatment of stress urinary incontinence. Manual Therapy. 2008;13:375–86. doi: 10.1016/j.math.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Bø K, Talseth T, Holme I. Single blind, randomised controlled trial of pelvic floor exercises, electrical stimulation, vaginal cones, and no treatment in management of genuine stress incontinence in women. Br Med J. 1999;318:487–93. doi: 10.1136/bmj.318.7182.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hay-Smith EJC, Dumoulin C. Pelvic floor muscle training versus no treatment or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858. Art. no: CD005654. [DOI] [PubMed] [Google Scholar]
- 10.Abrams P, Cardozo L, Khoury S, Wein K, editors. Incontinence: 2nd International Consultation on Incontinence. Paris: Health Publications Ltd; 2002. [Google Scholar]
- 11.Burgio K, Lochler JS, Goode PS, et al. Behavioral vs drug treatment for urge urinary incontinence in older women: A randomized controlled trial. J Am Med Assoc. 1998;280:1995–2000. doi: 10.1001/jama.280.23.1995. [DOI] [PubMed] [Google Scholar]
- 12.Hoff Braekken JI, Majida M, Engh ME, et al. Can pelvic floor muscle training reverse pelvic organ prolapse and reduce prolapse symptoms? An assessor-blinded, randomized, controlled trial. Am J Obstet Gynecol. 2010;203:170.e1–7. doi: 10.1016/j.ajog.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 13.Dumoulin C, Peng Q, Stodkilde-Jorgensen H, et al. Changes in levator ani anatomical configuration following physiotherapy in women with stress urinary incontinence. J Urol. 2007;178:970–7. doi: 10.1016/j.juro.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Cassado J, Pessarodoma A, Telleuda R, et al. Introital ultrasonography: A comparison of women with stress uncontinence due to urethral hypermoblity and continent women. BJU Int. 2006;98:822–8. doi: 10.1111/j.1464-410X.2006.06404.x. [DOI] [PubMed] [Google Scholar]
- 15.Peng Q, Jones R, Shishido K, et al. Spatial distribution of vaginal closure pressures of continent and stress urinary incontinent women. Physiol Meas. 2007;28:1429–50. doi: 10.1088/0967-3334/28/11/009. [DOI] [PubMed] [Google Scholar]
- 16.Shek KL, Dietz HP. The urethral motion profile: A novel method to evaluate urethral support and mobility. Aust N Z J Obstet Gynecol. 2008;48:337–42. doi: 10.1111/j.1479-828X.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- 17.Balmforth JR, Mantle J, Bidmead J, et al. A prospective observational trial of pelvic floor muscle training for female stress urinary incontinence. BJU Int. 2006;98:811–7. doi: 10.1111/j.1464-410X.2006.06393.x. [DOI] [PubMed] [Google Scholar]
- 18.Morrison J, et al. Neurophysiology and neuropharmacology. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 2nd International Consultation on Incontinence. Paris: Health Publications Ltd; 2002. pp. 83–163. [Google Scholar]
- 19.Nickell K, Boone TB. Peripheral neuropathy and peripheral nerve injury. Urol Clin N Am. 1996;23:491–500. doi: 10.1016/s0094-0143(05)70328-1. [DOI] [PubMed] [Google Scholar]
- 20.Norton PA. Pelvic floor disorders: The role of fascia and ligaments. Clin Obstet Gynecol. 1993;36:926–38. doi: 10.1097/00003081-199312000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 22.Richter HE, Burgio KL, Brubaker L, et al. Urinary incontinence treatment network: Factors associated with incontinence frequency in a surgical cohort of stress incontinent women. Am J Obstet Gynecol. 2005;193:2088–93. doi: 10.1016/j.ajog.2005.07.068. [DOI] [PubMed] [Google Scholar]
- 23.Turbaro A, Artibani W, Bartram C, et al. Imaging and other investigations. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 3rd Consultation on Incontinence. West Caldwell: Health Publications Ltd; 2005. [Google Scholar]
- 24.Constantini E, Lazzeri M, Bini V, et al. Sensitivity and specificity of one-hour pad test as a predictive value for female urinary incontinence. Urol Int. 2008;81:153–9. doi: 10.1159/000144053. [DOI] [PubMed] [Google Scholar]
- 25.Shumaker SA, Wyman JF, Uebersax JS, et al. Health-related quality of life measures for women with urinary incontinence: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Qual Life Res. 1994;3:291–306. doi: 10.1007/BF00451721. [DOI] [PubMed] [Google Scholar]
- 26.Yalcin I, Patrick DL, Summers K, et al. Minimal clinically important differences in Incontinence Quality-of-Life scores in stress urinary incontinence. Urology. 2006;67:1304–8. doi: 10.1016/j.urology.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Dietz HP, Lekskulchai O. Ultrasound assessment of pelvic organ prolapse: The relationship between prolapse severity and symptoms. Ultrasound Obstet Gynecol. 2007;29:688–69. doi: 10.1002/uog.4024. [DOI] [PubMed] [Google Scholar]
- 28.Bø K, Hagen RH, Kvarstein B, et al. Pelvic floor muscle exercise for the treatment of female stress urinary incontinence: II. Validity of vaginal pressure measurements of pelvic floor muscle strength and the necessity of supplementary methods for control of correct contraction. Neurourol Urodyn. 1990;9:479–87. [Google Scholar]
- 29.Bump RC, Hurt WG, Fantl JA, et al. Assessment of Kegel pelvic muscle exercise performance after brief verbal instruction. Am J Obstet Gynecol. 1991;165:322–9. doi: 10.1016/0002-9378(91)90085-6. [DOI] [PubMed] [Google Scholar]
- 30.Örnö1 AK, Dietz HP. Levator co-activation is a significant confounder of pelvic organ descent on Valsalva maneuver. Ultrasound Obstet Gynecol. 2007;30:346–50. doi: 10.1002/uog.4082. [DOI] [PubMed] [Google Scholar]
- 31.Delancey JO, Miller JM, Kearney R, et al. Vaginal birth and de novo stress incontinence—Relative contributions of urethral dysfunction and mobility. Obstet Gynecol. 2007;110:354–62. doi: 10.1097/01.AOG.0000270120.60522.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bo K, Stien R. Needle EMG registration of striated urethral wall and pelvic floor muscle activity patterns during cough, Valsalva, abdominal, hip adductor, and gluteal muscle contractions in nulliparous healthy females. Neurourol Urodyn. 1994;13:35–41. doi: 10.1002/nau.1930130106. [DOI] [PubMed] [Google Scholar]
- 33.Miller JM, Unik WH, Delancey JO, et al. Can women without visible pubococcygeal muscle in MR images still increase uretheral closure pressures? Am J Obstet Gynecol. 2004;1:171–5. doi: 10.1016/j.ajog.2004.03.082. [DOI] [PubMed] [Google Scholar]