Abstract
Background
Mechanical ventilation can induce organ injury associated with overwhelming inflammatory responses. Excessive activation of poly(adenosine diphosphate-ribose) polymerase enzyme following massive DNA damage may aggravate inflammatory responses. We thus hypothesized that the pharmacological inhibition of poly(adenosine diphosphate-ribose) polymerase by PJ-34 will attenuate ventilator-induced lung injury.
Methods
Anesthetized rats were subjected to intratracheal instillation of lipopolysaccharide at a dose of 6 mg/kg. The animals were then randomized to receive mechanical ventilation at either low tidal volume (6 mL/kg) with 5 cmH2O positive end-expiratory pressure or high tidal volume (15 mL/kg) with zero positive end-expiratory pressure, in the presence and absence of intravenous administration of PJ-34.
Results
The high tidal volume ventilation resulted in an increase in poly (adenosine diphosphate-ribose) polymerase activity in the lung. The treatment with PJ-34 maintained a greater oxygenation and a lower airway plateau pressure than the vehicle control group. This was associated with a decreased level of interleukin-6, active plasminogen activator inhibitor-1 in the lung, attenuated leukocyte lung transmigration and reduced pulmonary edema and apoptosis. The administration of PJ-34 also decreased the systemic levels of tumor necrosis factor-α and interleukin-6, and attenuated the degree of apoptosis in the kidney.
Conclusion
The pharmacological inhibition of poly(adenosine diphosphate-ribose) polymerase reduces ventilator-induced lung injury and protects kidney function.
Introduction
Injurious mechanical ventilation can lead to the development of an overwhelming inflammatory response and multiple organ dysfunction syndrome 1–5. Acute renal failure is the most prevalent form of distal organ dysfunction associated with endothelial and epithelial cell death in patients with ventilator-induced lung injury (VILI) 2,6–8.
The clinical importance of VILI has been highlighted in a multicenter clinical trial demonstrating that mechanical ventilation with low tidalvolumes (Vt) significantly decreased cytokine responses, multiple organ dysfunction syndrome, and mortality rate compared to a high Vt in patients with acute respiratory distress syndrome (ARDS) 9;10. However, in situations where a fully lung protective strategy is not possible, it would be necessary to use pharmacological therapies to mitigate the consequences of VILI and multiple organ dysfunction syndrome.
Poly(adenosine diphosphate-ribose) polymerase (PARP) -1 is the most abundant member of PARP family 11, whose primary role is to sense DNA damage, repair DNA and maintain genomic stability 12. However, when severe DNA injury occurs in response to oxidative stress, excessive upregulation of PARP may be detrimental by depleting cellular adenosine-triphosphate stores, resulting in cell dysfunction and death 13–16. This cellular suicide mechanism has been implicated in the pathophysiology of acute lung injury 17, acute renal failure secondary to ischemia-reperfusion 18 and sepsis 19. It has been reported that PARP-1 can directly interact with both subunits of p65 and p50 and synergistically coactivates NF-kappaB (NF-κB) 20–23. The potent PARP inhibitor PJ-34 can decrease PARP-1 activity thus NF-κB activation in animal models of endotoxic and hemorrhagic shock 17–19;24–27.
In the present study, we tested the hypothesis that inhibition of PARP by PJ-34 would attenuate VILI and preserve kidney function by its anti-inflammatory property. We demonstrated that high Vt ventilation induced an increase in PARP activity in the lung associated with an enhanced inflammatory response. The treatment with PJ-34 attenuated the mechanical ventilation-induced cytokine responses, decreased the level of active plasminogen activator inhibitor (PAI)-1 in the lung, and reduced leukocyte infiltration and pulmonary edema. Furthermore, inhibition of PARP resulted in fewer kidney apoptosis thus preserved renal function during high Vt ventilation.
Materials and Methods
Animal preparation
The protocol was approved by the Insitutional Animal Care Committee at the St. Michael’s Hospital. Thirty-six male Sprague Dawley rats (Charles Rivers, St Constan, QC, Canada) weighing 290 ± 10 g were anesthetized with intraperitoneal injection of xylazine 10 mg/kg (Bayer, Toronto, ON, Canada) and ketamine 100 mg/kg (Bimeda-MTC, Cambridge, ON, Canada). Anesthesia was maintained with xylazine 1 mg/kg/h, ketamine 20 mg/kg/h via a jugular vein; muscle relaxation was achieved by intravenous administration of pancuronium bromide 0.6 mg/kg/h (Sabex Inc, QC, Canada). Rats were placed on a heating pad to maintain core temperature at 37°C. A tracheostomy was performed for intratracheal cannulation (14 gauge). The right carotid artery was catheterized for blood sampling and continuous arterial blood pressure measurements. The bladder was catheterized and sutured using a transabdominal approach for urine sampling.
Experimental protocol
The rats were initially ventilated at Vt 6 mL/kg and positive end-expiratory pressure (PEEP) of 5 cm H2O (Servo 300 ventilator, Siemens, Munich, Germany). After a baseline arterial blood gas measurement (Corning 248 blood gas analyser -Ciba Corning, Medfield, USA) to confirm similar gas-exchange conditions in all animals, lipopolysaccharide (LPS, 055:B5, Sigma-Aldrich, ST. Louis, MO) at a dose of 6 mg/kg in 0.5 ml normal saline was administered by using an intratracheal aerosolizer (PennCentury Inc, Philadelphia, PA). Five min later, a recruitment manoeuvre was performed by increasing PEEP level to 25 cm H2O for five breaths, followed by 15 min of stabilization under the ventilator settings described above. The rats were then randomly allocated into four groups (n = 9 each) and ventilated for 4 h: Group 1 (low Vt+PJ-34): Vt of 6 mL/kg, PEEP 5 cm H2O with infusion of PJ-34 (Alexis Biochemicals, Lausen, Switzerland); Group 2 (low Vt+Vehicle): Vt of 6 mL/kg, PEEP 5 cm H2O with the vehicle solution (normal saline); Group 3 (high Vt+PJ-34): Vt of 15 mL/kg, no PEEP with infusion of PJ-34; and Group 4 (high Vt+Vehicle): Vt of 15 mL/kg, no PEEP with vehicle solution. Immediately after the randomization, PJ-34 was administered intravenously as a loading dose of 10 mg/kg over 30 min, followed by continuous infusion at 2 mg/kg/h for the remainder of the experiments 28. PaCO2 was maintained at 40 ± 5 mmHg by adjusting respiratory rate. Inspiration to expiration ratio was set to 1:2 and the fraction of inspired oxygen was 0.45.
Measurements
Arterial blood gases were analyzed 30 min after randomization and hourly thereafter. Urine samples were collected during the last hour after emptying the urine tube. Upon completion of the mechanical ventilation, whole blood was collected for measurements of cytokines and creatinine, and the animals were sacrificed with an overdose of anaesthesia. Lungs and kidneys were harvested for histological examination. Plasma and urine were stored at −80°C until assayed.
PARP activity assay
PARP activity (PARP Universal Colorimetric Assay Kit, R&D Systems, Inc., Minneapolis, MN) was determined in lung homogenates by following the manufacturer’s instruction, and the results were expressed as units of PARP per gram protein.
Bronchoalveolar Lavage and Wet to Dry ratio
The left upper lobe was excised for histologic examination. The right middle lobe was used to estimate wet/dry weight ratio and the right lower lobe was snap frozen for cytokine measurements. The left lower and the right upper lobes were lavaged by intratracheal instillation of 2 mL of cold phosphatate-buffered saline (Sigma-Aldrich, ST. Louis, MO, USA). After 5 seconds, the bronchoalveolar lavage fluid was obtained. This procedure was repeated twice.
After centrifugation, the bronchoalveolar lavage fluid was frozen at −80°C until further analysis. The cell pellet was resuspended in 1 mL phosphatate-buffered saline for cell differentiation by using the Hemacolor Stain Set (EM Diagnostic System, Gibbstown, NJ).
Measurements of Cytokines, PAI-1 activity and tissue factor activity
Analysis of TNF-α and IL-6 in plasma, lung and kidney homogenates was performed in a blinded fashion using rat-specific ELISA kits (BioSource International, Camarillo, CA) at 450 nm (Multiskan Asscent microplate photometer, Thermo Lab systems, Helsinki, Finland). PAI-1 activity (Innovative Research, Inc, Southfield, MI) and tissue factor activity (American Diagnostica Inc, Stamford, CT) were determined in plasma and lung homogenates. The tissue factor activity kit is specific for human but crossly reacts with rat tissue factor 29. Total protein concentration in lung and kidney homogenates was determined by a Bradford assay (Bio-Rad Laboratories, Inc, Hercules, CA) using bovine serum albumin to construct a standard curve.
Lung and kidney apoptosis
Apoptosis was quantified from paraffin sections of lung and kidney by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay. Hematoxylin staining for nucleus was also performed to identify individual cell. Twelve fields randomly chosen in each section were read in a blinded fashion. An apoptotic index was calculated as [100% × (TUNEL positive cells)/(total cells)].
Caspase 3 enzymatic activity
Caspase 3 activity was determined in lung and kidney homogenates (Caspase-3 Colorimetric Assay kit; R&D Systems, Inc.). Recombinant human caspase-3 enzyme was used to construct a standard curve (R&D Systems, Inc., Minneapolis, MN). Results were normalized to protein levels.
Lactate Dehydrogenase Assay
The lactate dehydrogenase assay (Cytotoxicity Detection Kit, Roche Applied Science, Mannheim, Germany) was performed at 492 nm.
Histology
The lung injury scores including alveolar collapse, perivascular hemorrhage, alveolar hemorrhage, perivascular edema, vascular congestion, alveolar polymorhponuclear leukocytes, membranes, alveolar edema, macrophages, bronchial epithelial lesions were performed by a pathologist who was unaware of the experimental groups. Five regions from each specimen were examined, and an injury score of 0 to 3 (0, normal; 1, mild; 2, moderate; 3, severe) was assigned and then calculated for a total score of lung injury.
Creatinine Clearance
Creatinine clearance was calculated over the last hour of experiments using the formula CC=UCrxV/PCr, where UCr represents the creatinine concentration in urine (mmol/L), V the urine flow (ml/min), and PCr the creatinine concentration in plasma (mmol/L).
Statistics
Results are reported as mean ± SEM. Data were analyzed in non-parametric tests by using Prism Graphpad 4.0 software package (Prism, San Diego, CA). Comparison among groups was performed using Kruskal-Wallis test. When an overall p < 0.05, a Dunn’s multiple-comparison post hoc analysis was conducted. A p value < 0.05 was considered statistically significant.
Results
Effects of PJ-34 on hemodynamics, gas exchange and respiratory mechanics
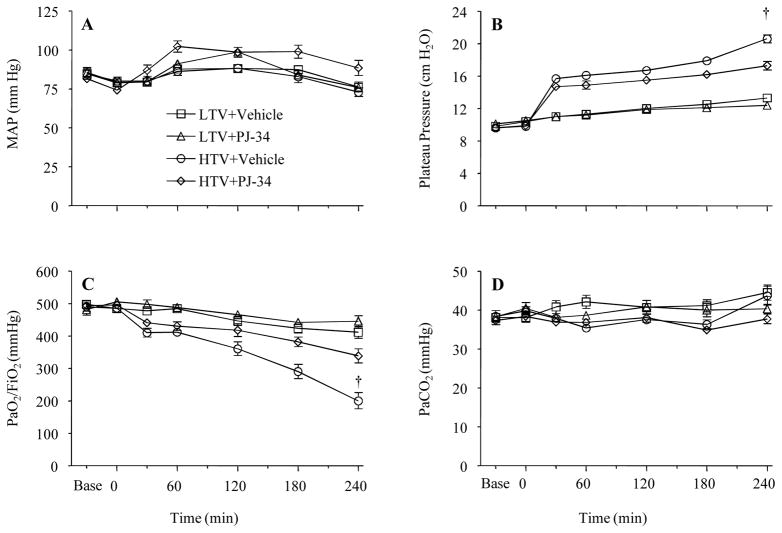
Mean arterial pressures were similar at baseline and during the experiments among groups (Figure 1A) as was fluid administration (low Vt+Vehicle: 1.4 ± 0.1 mL/h; low Vt+PJ-34: 1.5 ± 0.1 mL/h; high Vt+Vehicle: 1.7 ± 0.1 mL/h; high Vt+PJ-34: 1.4 ± 0.1 mL/h, p = NS). Airway plateau pressure was higher in the high Vt groups which was attenuated by the treatment with PJ-34 (Figure 1B). Mean values of PaO2/FiO2 ratio were similar in all animals until the 2nd h of mechanical ventilation when the PaO2/FiO2 ratio decreased in the high Vt group without PJ-34 treatment compared to the other groups (Figure 1C). There were no differences in the levels of PaCO2 (Figure 1D), pH and HCO3− among groups (data not shown).
Figure 1.
Effect of PJ-34 on PARP activity
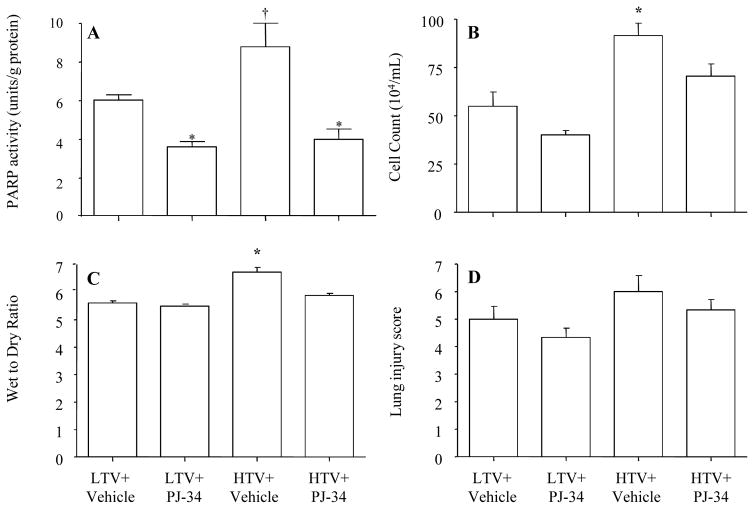
PARP activity was increased in the high Vt group compared to the low Vt group. The treatment with PJ-34 decreased the PARP activity (Figure 2A).
Figure 2.
Effect of PJ-34 on leukocyte migration and lung injury
The leukocyte count in bronchoalveolar lavage fluid and the mean value of the lung wet/dry ratio were greater in the high Vt than in the low Vt group, and the treatment with PJ-34 attenuated the leukocyte migration in the lung and lung edema (Figure 2B–C). Although the lung injury score had a similar pattern as the wet/dry ratio, the differences did not statistical reach significance (Figure 2D).
Effect of PJ-34 on the production of cytokines and coagulation variables
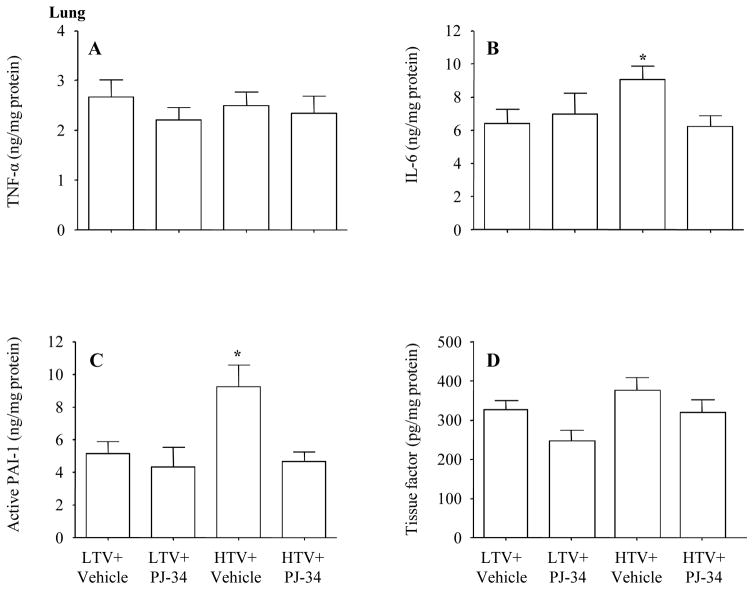
1. Lung tissue
TNF-α is an early and central cytokine in response to tissue injury 30;31. IL-6 has been used to guide therapeutic intervention in clinical trials 32;33. We found no differences in TNF-α among groups, but IL-6 levels were higher in the high Vt group than other groups, and the treatment with PJ-34 decreased IL-6 level to control levels (Figure 3A–B).
Figure 3.
Previous studies demonstrated that ARDS was associated with increased coagulation and decreased fibrinolysis 34;35. PAI-1 is a main component in the anti-fibrinolytic system, and tissue factor may initiate the extrinsic coagulation pathway. We observed that the PAI-1 activity of the lung increased in the high Vt group than other groups, and the treatment with PJ-34 normalized PAI-1 levels at a control level (Figure 3C). There was no significant difference in tissue factor activity among the groups (Figure 3D).
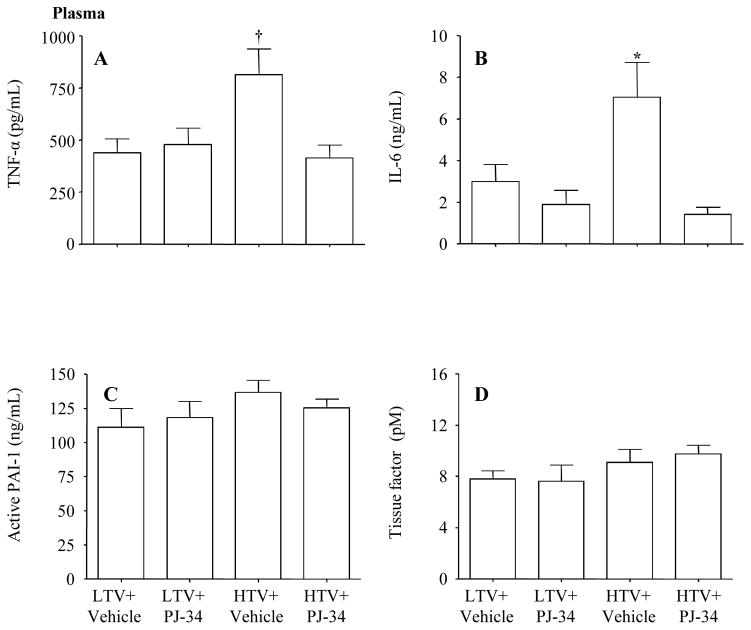
2. Plasma
Plasma levels of TNF-α and IL-6 increased in the high Vt group compared to the low Vt group which was blunted by the administration of PJ-34 (Figure 4A–B). The expression of PAI-1 and tissue factor activity was similar in all groups (Figure 4C–D).
Figure 4.
3. Kidney tissue
There were no significant differences in the levels of TNF-α, IL-6, PAI-1 and tissue factor activity between the high Vt and low Vt groups irrespective of PJ-34 treatment (data not shown).
Organ apoptosis
1. Lung tissue
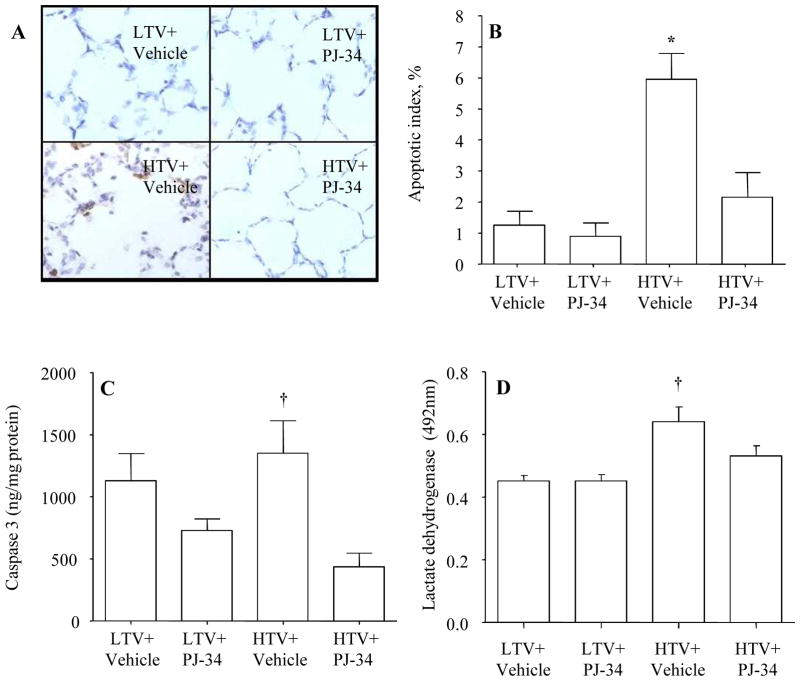
Figure 5A shows a representative image of the TUNEL staining to detect apoptosis. The apoptotic index (defined as percentage of TUNEL positive cells divided by the total cells) was higher in the high Vt group than other groups and the treatment with PJ-34 reduced the apoptotic index (Figure 5B). This observation was in agreement with a decreased caspase 3 activity in the high Vt group treated with PJ-34 (Figure 5C). This observation was further confirmed by an increased level of lactate dehydrogenase activity as an index of cell death in the high Vt compared to the low Vt group, and treatment of PJ-34 lowered lactate dehydrogenase activity (Figure 5D).
Figure 5.
2. Kidney tissue
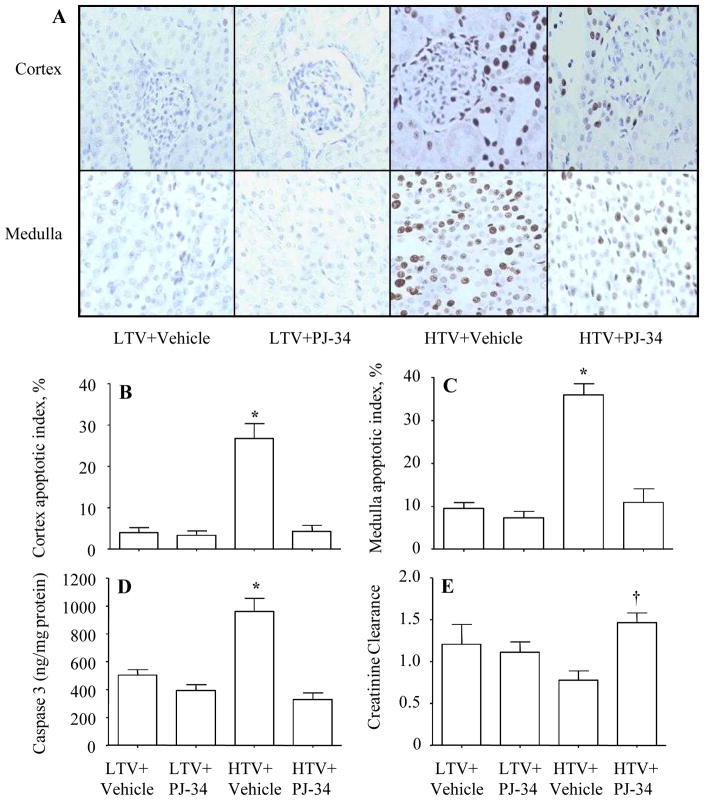
The degree of apoptosis was greater in the high Vt group than other groups, and there appeared to be more apoptotic cells in the medulla compared to the cortex (Figure 6A–C). The greater number of apoptotic cells was associated with higher levels of caspase 3 activity (Figure 6D). The administration of PJ-34 reduced the apoptotic index as well as the caspase 3 activity (Figure 6A–D). The decreased apoptotic index was associated with an increased creatinine clearance (Figure 6E).
Figure 6.
Discussion
The present study provides evidence that PARP activation plays an important role in the development of VILI and inflammatory responses during mechanical ventilation following lipopolysaccharide priming. Inhibition of PARP with PJ-34 reduced lung injury, inflammatory responses and preserved kidney function.
Sepsis-associated ARDS shows the highest mortality rate in ARDS population 36;37; mortality is lower when ARDS occurs following gastric aspiration, trauma, or fat embolism 38. A higher incidence of ARDS is present in patients with sepsis where overwhelming inflammatory responses have taken place 36;37. To portray this clinical situation we employed a two-hit model combining an initial lipopolysaccharide instillation to induce pulmonary inflammation, followed by mechanical ventilation. The choice of Vt was based on certain clinical applications, i.e., a Vt of 6 mL/kg has been suggested to ventilate patients with ARDS 9, and a Vt of 15 mL/kg is reportedly used in patients without previous lung injury subjecting to a short term mechanical ventilation 39. Similar to other two-hit models such as acid aspiration and ischemia reperfusion followed by high Vt ventilation 2;6, we observed an increased plateau pressure, a lower PaO2/FiO2 ratio and an enhanced pulmonary and systemic inflammatory response, and distal organ dysfunction. Since ARDS is implicated with inflammatory responses we thus believe that the results observed in the present two-hit model may also apply to a single hit of ARDS resulted from pulmonary source.
We demonstrated in the present model an increased PARP activity in the lung of the animals ventilated with high Vt compared to the low Vt group. PARP inhibition by PJ-34 attenuated inflammatory responses and protected lung and kidney function. We believe that the mechanisms by which PJ-34 exerted beneficial effects in our model are through inhibition of both PARP and NF-κB activity. It has been shown that pharmacological inhibition of PARP attenuated the DNA binding capacity and subsequent reduction of NF-κB transcriptional activity 40–43. The expression of NF-κB dependent pro-inflammatory mediators was decreased in PARP-1 deficiency mice 20;23. It has been suggested that PARP binds NF-κB after the translocation of the κB heterodimer in the nucleus at the stage of the formation of the transcription complex, altering DNA binding affinity to NF-κB 23;44. These studies suggest that NF-κB could be a downstream pathway of PARP-1. Interestingly, other studies reported that neither enzymatic activity nor the DNA-binding activity of PARP-1 was required for NF-kB dependent transcriptional activation 21. We did not measure NF-κB activation in the present study but we and others have previously shown that mechanical ventilation resulted in NF-κB translocation in the lung of animal models of acute lung injury and ARDS 45;46. It has been demonstrated that inhibition of NF-κB translocation resulted in a reduction in VILI by using other pharmacological interventions such as phosphoinositide 3-OH kinase inhibitor and genistein 46–49.
Pharmacological inhibition of PARP has been investigated in a variety of experimental conditions of acute lung injury and lipopolysaccharide-induced organ injury17;28;50–52. When mice were subjected to intratracheal injection of lipopolysaccharide for 24 h, the treatment with PJ-34 attenuated lung injury by reducing leukocytes extravasation and pulmonary inflammation 50. In a ovine pneumonia model, the treatment with PARP inhibitor INO-1001 preserved lung histology after intrabronchial injection of P. aeruginosa bacteria associated with an increased oxygenation and a better respiratory mechanic 51. The administration of the PARP inhibitor 3-aminobenzamide protected against endothelial dysfunction in a rat model of endotoxic shock 52. Moreover, it has been reported that PJ-34 improved survival rate and cardiovascular function in a pig model of sepsis induced by E. coli 28. Our results are in accord with the previous studies to support the concept that PARP plays an important role in the development of inflammation. We further expand the previous studies by demonstrating that inhibition of PARP can attenuate mechanical ventilation associated biotrauma in the context of VILI.
It has been shown that lung parenchymal cells produce proinflammatory cytokines in response to tissue stretch contributing to VILI 53. Damage to the alveolar–capillary barrier in combination with release of inflammatory cytokines is thought to be a major contributor to the development of MODS and death 54. Our data demonstrate that PARP inhibition can attenuate IL-6 release in the lung, and attenuate concentrations of both TNF-α and IL-6 in the circulation. These results are consistent with previous reports demonstrating that inhibition of PARP resulted in a down-regulation of chemokines and cytokines in several animal models of lung injury 50;55;56. A decreased level of IL-6 might have led to an attenuated expression of PAI-1 in the lungs following PJ-34 treatment 57.
Leukocyte transmigration is an important feature of diffused alveolar damage characterizing VILI 58. We find that PARP inhibition reduced leukocyte infiltration in the lung, decreased permeability, improved oxygenation and respiratory mechanics. Other studies have reported a role of PARP in the inhibition of leukocyte trafficking in conditions such as inflammation, shock and ischemia reperfusion injury 50;59;60.
We have previously observed some degree of lung epithelial apoptosis with dominant expression of necrosis in an acid-induced acute lung injury model in rabbits undergoing ventilation with a high Vt 2. In the present study, our results show higher levels of apoptosis than necrosis in the lungs. We also noted that in the kidneys, the baseline apoptosis rate was about 10%, and increased to 30–40% with high Vt, which is higher than that observed in the acid aspiration model in rabbits 7. The differences are likely due to the different priming stimuli as acid aspiration resulted in a more severe and direct lung injury while lipopolysaccharide induce more systemic effects. Also, the ventilatory strategies were somewhat different where higher PEEP levels were used in the low Vt group and some PEEP level was applied in the high Vt group in the previous study 7, compared to the present study. Finally, the species difference might have a role with respect to organ sensitivity in response to mechanical ventilation. Of interest, we observed that the administration of PJ-34 reduced apoptosis in the kidney. The exact mechanisms remain to be elucidated but PARP-deficient mice are protectedagainst ischemic renal injury 18;61.
In conclusion, we demonstrated that mechanical ventilation can induce PARP activation, the pharmacological inhibition of PARP reduced inflammatory responses, VILI and preserved kidney function in the rat model of lipopolysaccharide priming followed by mechanical ventilation.
Acknowledgments
Supported by the Canadian Institutes of Health Research (Ottawa, ON, Canada) to HZ and AS. RV was supported by the Regione Piemonte (Turin, Italy). JWK was supported by the Ter Meulen Fund of the Royal Netherlands Academy of Arts and Science (Amsterdam, Netherlands).
References
- 1.Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:109–16. doi: 10.1164/ajrccm.160.1.9803046. [DOI] [PubMed] [Google Scholar]
- 2.Imai Y, Parodo J, Kajikawa O, de PM, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, Marshall JC, Ranieri VM, Slutsky AS. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104–12. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- 3.Slutsky AS. Ventilator-induced lung injury: from barotrauma to biotrauma. Respir Care. 2005;50:646–59. [PubMed] [Google Scholar]
- 4.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–52. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med. 2006;32:24–33. doi: 10.1007/s00134-005-2817-8. [DOI] [PubMed] [Google Scholar]
- 6.Crimi E, Zhang H, Han RN, Sorbo LD, Ranieri VM, Slutsky AS. Ischemia and reperfusion increases susceptibility to ventilator-induced lung injury in rats. Am J Respir Crit Care Med. 2006;174:178–86. doi: 10.1164/rccm.200507-1178OC. [DOI] [PubMed] [Google Scholar]
- 7.Kuiper JW, Groeneveld AB, Slutsky AS, Plotz FB. Mechanical ventilation and acute renal failure. Crit Care Med. 2005;33:1408–15. doi: 10.1097/01.ccm.0000165808.30416.ef. [DOI] [PubMed] [Google Scholar]
- 8.Ranieri VM, Giunta F, Suter PM, Slutsky AS. Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA. 2000;284:43–4. doi: 10.1001/jama.284.1.43. [DOI] [PubMed] [Google Scholar]
- 9.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 10.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. [DOI] [PubMed] [Google Scholar]
- 11.Kraus WL, Lis JT. PARP goes transcription. Cell. 2003;113:677–83. doi: 10.1016/s0092-8674(03)00433-1. [DOI] [PubMed] [Google Scholar]
- 12.Herceg Z, Wang ZQ. Functions of poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat Res. 2001;477:97–110. doi: 10.1016/s0027-5107(01)00111-7. [DOI] [PubMed] [Google Scholar]
- 13.Berger NA. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res. 1985;101:4–15. [PubMed] [Google Scholar]
- 14.D’Amours D, Sallmann FR, Dixit VM, Poirier GG. Gain-of-function of poly(ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: implications for apoptosis. J Cell Sci. 2001;114:3771–8. doi: 10.1242/jcs.114.20.3771. [DOI] [PubMed] [Google Scholar]
- 15.Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci USA. 1999;96:13978–82. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 17.Kiefmann R, Heckel K, Doerger M, Schenkat S, Kupatt C, Stoeckelhuber M, Wesierska-Gadek J, Goetz AE. Role of PARP on iNOS pathway during endotoxin-induced acute lung injury. Intensive Care Med. 2004;30:1421–31. doi: 10.1007/s00134-004-2301-x. [DOI] [PubMed] [Google Scholar]
- 18.Zheng J, valaraja-Narashimha K, Singaravelu K, Padanilam BJ. Poly(ADP-ribose) polymerase-1 gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol. 2005;288:F387–F398. doi: 10.1152/ajprenal.00436.2003. [DOI] [PubMed] [Google Scholar]
- 19.Jagtap P, Soriano FG, Virag L, Liaudet L, Mabley J, Szabo E, Hasko G, Marton A, Lorigados CB, Gallyas F, Jr, Sumegi B, Hoyt DG, Baloglu E, VanDuzer J, Salzman AL, Southan GJ, Szabo C. Novel phenanthridinone inhibitors of poly (adenosine 5′-diphosphate-ribose) synthetase: potent cytoprotective and antishock agents. Crit Care Med. 2002;30:1071–82. doi: 10.1097/00003246-200205000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Hassa PO, Hottiger MO. A role of poly (ADP-ribose) polymerase in NF-kappaB transcriptional activation. Biol Chem. 1999;380:953–9. doi: 10.1515/BC.1999.118. [DOI] [PubMed] [Google Scholar]
- 21.Hassa PO, Covic M, Hasan S, Imhof R, Hottiger MO. The enzymatic and DNA binding activity of PARP-1 are not required for NF-kappa B coactivator function. J Biol Chem. 2001;276:45588–97. doi: 10.1074/jbc.M106528200. [DOI] [PubMed] [Google Scholar]
- 22.Hassa PO, Haenni SS, Buerki C, Meier NI, Lane WS, Owen H, Gersbach M, Imhof R, Hottiger MO. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. J Biol Chem. 2005;280:40450–64. doi: 10.1074/jbc.M507553200. [DOI] [PubMed] [Google Scholar]
- 23.Oliver FJ, Menissier-de MJ, Nacci C, Decker P, Andriantsitohaina R, Muller S, de la RG, Stoclet JC, de MG. Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly (ADP-ribose) polymerase-1 deficient mice. EMBO J. 1999;18:4446–54. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goebel DJ, Winkler BS. Blockade of PARP activity attenuates poly(ADP-ribosyl)ation but offers only partial neuroprotection against NMDA-induced cell death in the rat retina. J Neurochem. 2006;98:1732–45. doi: 10.1111/j.1471-4159.2006.04065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roesner JP, Vagts DA, Iber T, Eipel C, Vollmar B, Noldge-Schomburg GF. Protective effects of PARP inhibition on liver microcirculation and function after haemorrhagic shock and resuscitation in male rats. Intensive Care Med. 2006;32:1649–57. doi: 10.1007/s00134-006-0335-y. [DOI] [PubMed] [Google Scholar]
- 26.Szabo G, Bahrle S, Stumpf N, Sonnenberg K, Szabo EE, Pacher P, Csont T, Schulz R, Dengler TJ, Liaudet L, Jagtap PG, Southan GJ, Vahl CF, Hagl S, Szabo C. Poly(ADP-Ribose) polymerase inhibition reduces reperfusion injury after heart transplantation. Circ Res. 2002;90:100–6. doi: 10.1161/hh0102.102657. [DOI] [PubMed] [Google Scholar]
- 27.Veres B, Gallyas F, Jr, Varbiro G, Berente Z, Osz E, Szekeres G, Szabo C, Sumegi B. Decrease of the inflammatory response and induction of the Akt/protein kinase B pathway by poly-(ADP-ribose) polymerase 1 inhibitor in endotoxin-induced septic shock. Biochem Pharmacol. 2003;65:1373–82. doi: 10.1016/s0006-2952(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 28.Goldfarb RD, Marton A, Szabo E, Virag L, Salzman AL, Glock D, Akhter I, McCarthy R, Parrillo JE, Szabo C. Protective effect of a novel, potent inhibitor of poly(adenosine 5′-diphosphate-ribose) synthetase in a porcine model of severe bacterial sepsis. Crit Care Med. 2002;30:974–80. doi: 10.1097/00003246-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Kubo-Inoue M, Egashira K, Usui M, Takemoto M, Ohtani K, Katoh M, Shimokawa H, Takeshita A. Long-term inhibition of nitric oxide synthesis increases arterial thrombogenecity in rat carotid artery. Am J Physiol Heart Circ Physiol. 2002;282:H1478–H1484. doi: 10.1152/ajpheart.00739.2001. [DOI] [PubMed] [Google Scholar]
- 30.Waage A. Production and clearance of tumor necrosis factor in rats exposed to endotoxin and dexamethasone. Clin Immunol Immunopathol. 1987;45:348–55. doi: 10.1016/0090-1229(87)90087-0. [DOI] [PubMed] [Google Scholar]
- 31.Waage A, Halstensen A, Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987;1:355–7. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- 32.Miura E, Procianoy RS, Bittar C, Miura CS, Miura MS, Mello C, Christensen RD. A randomized, double-masked, placebo-controlled trial of recombinant granulocyte colony-stimulating factor administration to preterm infants with the clinical diagnosis of early-onset sepsis. Pediatrics. 2001;107:30–5. doi: 10.1542/peds.107.1.30. [DOI] [PubMed] [Google Scholar]
- 33.Sack U, Biereder B, Elouahidi T, Bauer K, Keller T, Trobs RB. Diagnostic value of blood inflammatory markers for detection of acute appendicitis in children. BMC Surg. 2006;6:15–22. doi: 10.1186/1471-2482-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Idell S, James KK, Levin EG, Schwartz BS, Manchanda N, Maunder RJ, Martin TR, McLarty J, Fair DS. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J Clin Invest. 1989;84:695–705. doi: 10.1172/JCI114217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welty-Wolf KE, Carraway MS, Miller DL, Ortel TL, Ezban M, Ghio AJ, Idell S, Piantadosi CA. Coagulation blockade prevents sepsis-induced respiratory and renal failure in baboons. Am J Respir Crit Care Med. 2001;164:1988–96. doi: 10.1164/ajrccm.164.10.2105027. [DOI] [PubMed] [Google Scholar]
- 36.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 37.Hudson LD, Steinberg KP. Epidemiology of acute lung injury and ARDS. Chest. 1999;116:74S–82S. doi: 10.1378/chest.116.suppl_1.74s-a. [DOI] [PubMed] [Google Scholar]
- 38.Estenssoro E, Dubin A, Laffaire E, Canales H, Saenz G, Moseinco M, Pozo M, Gomez A, Baredes N, Jannello G, Osatnik J. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30:2450–6. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Wrigge H, Zinserling J, Stuber F, von ST, Hering R, Wetegrove S, Hoeft A, Putensen C. Effects of mechanical ventilation on release of cytokines into systemic circulation in patients with normal pulmonary function. Anesthesiology. 2000;93:1413–7. doi: 10.1097/00000542-200012000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Chang WJ, varez-Gonzalez R. The sequence-specific DNA binding of NF-kappa B is reversibly regulated by the automodification reaction of poly (ADP-ribose) polymerase 1. J Biol Chem. 2001;276:47664–70. doi: 10.1074/jbc.M104666200. [DOI] [PubMed] [Google Scholar]
- 41.Chiarugi A, Moskowitz MA. Poly(ADP-ribose) polymerase-1 activity promotes NF-kappaB-driven transcription and microglial activation: implication for neurodegenerative disorders. J Neurochem. 2003;85:306–17. doi: 10.1046/j.1471-4159.2003.01684.x. [DOI] [PubMed] [Google Scholar]
- 42.Genovese T, Mazzon E, Muia C, Patel NS, Threadgill MD, Bramanti P, De SA, Thiemermann C, Cuzzocrea S. Inhibitors of poly(ADP-ribose) polymerase modulate signal transduction pathways and secondary damage in experimental spinal cord trauma. J Pharmacol Exp Ther. 2005;312:449–57. doi: 10.1124/jpet.104.076711. [DOI] [PubMed] [Google Scholar]
- 43.Oumouna-Benachour K, Hans CP, Suzuki Y, Naura A, Datta R, Belmadani S, Fallon K, Woods C, Boulares AH. Poly(ADP-ribose) polymerase inhibition reduces atherosclerotic plaque size and promotes factors of plaque stability in apolipoprotein E-deficient mice: effects on macrophage recruitment, nuclear factor-kappaB nuclear translocation, and foam cell death. Circulation. 2007;115:2442–50. doi: 10.1161/CIRCULATIONAHA.106.668756. [DOI] [PubMed] [Google Scholar]
- 44.Hassa PO, Hottiger MO. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell Mol Life Sci. 2002;59:1534–53. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Held HD, Boettcher S, Hamann L, Uhlig S. Ventilation-induced chemokine and cytokine release is associated with activation of nuclear factor-kappaB and is blocked by steroids. Am J Respir Crit Care Med. 2001;163:711–6. doi: 10.1164/ajrccm.163.3.2003001. [DOI] [PubMed] [Google Scholar]
- 46.Uhlig U, Fehrenbach H, Lachmann RA, Goldmann T, Lachmann B, Vollmer E, Uhlig S. Phosphoinositide 3-OH kinase inhibition prevents ventilation-induced lung cell activation. Am J Respir Crit Care Med. 2004;169:201–8. doi: 10.1164/rccm.200303-343OC. [DOI] [PubMed] [Google Scholar]
- 47.Jafari B, Ouyang B, Li LF, Hales CA, Quinn DA. Intracellular glutathione in stretch-induced cytokine release from alveolar type-2 like cells. Respirology. 2004;9:43–53. doi: 10.1111/j.1440-1843.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 48.Jerng JS, Hsu YC, Wu HD, Pan HZ, Wang HC, Shun CT, Yu CJ, Yang PC. Role of the renin-angiotensin system in ventilator-induced lung injury: an in vivo study in a rat model. Thorax. 2007;62:527–35. doi: 10.1136/thx.2006.061945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parker JC, Ivey CL, Tucker A. Phosphotyrosine phosphatase and tyrosine kinase inhibition modulate airway pressure-induced lung injury. J Appl Physiol. 1998;85:1753–61. doi: 10.1152/jappl.1998.85.5.1753. [DOI] [PubMed] [Google Scholar]
- 50.Liaudet L, Pacher P, Mabley JG, Virag L, Soriano FG, Hasko G, Szabo C. Activation of poly(ADP-Ribose) polymerase-1 is a central mechanism of lipopolysaccharide-induced acute lung inflammation. Am J Respir Crit Care Med. 2002;165:372–7. doi: 10.1164/ajrccm.165.3.2106050. [DOI] [PubMed] [Google Scholar]
- 51.Murakami K, Enkhbaatar P, Shimoda K, Cox RA, Burke AS, Hawkins HK, Traber LD, Schmalstieg FC, Salzman AL, Mabley JG, Komjati K, Pacher P, Zsengeller Z, Szabo C, Traber DL. Inhibition of poly (ADP-ribose) polymerase attenuates acute lung injury in an ovine model of sepsis. Shock. 2004;21:126–33. doi: 10.1097/01.shk.0000108397.56565.4a. [DOI] [PubMed] [Google Scholar]
- 52.Szabo C, Cuzzocrea S, Zingarelli B, O’Connor M, Salzman AL. Endothelial dysfunction in a rat model of endotoxic shock. Importance of the activation of poly (ADP-ribose) synthetase by peroxynitrite. J Clin Invest. 1997;100:723–35. doi: 10.1172/JCI119585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tremblay LN, Miatto D, Hamid Q, Govindarajan A, Slutsky AS. Injurious ventilation induces widespread pulmonary epithelial expression of tumor necrosis factor-alpha and interleukin-6 messenger RNA. Crit Care Med. 2002;30:1693–700. doi: 10.1097/00003246-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157:1721–5. doi: 10.1164/ajrccm.157.6.9709092. [DOI] [PubMed] [Google Scholar]
- 55.Farivar AS, Woolley SM, Fraga CH, Thomas R, Salzman AL, Szabo C, Mulligan MS. Intratracheal poly (ADP) ribose synthetase inhibition ameliorates lung ischemia reperfusion injury. Ann Thorac Surg. 2004;77:1938–43. doi: 10.1016/j.athoracsur.2003.10.120. [DOI] [PubMed] [Google Scholar]
- 56.Woolley SM, Farivar AS, Naidu BV, Salzman A, Szabo C, Thomas R, Fraga C, Mulligan MS. Role of poly (ADP) ribose synthetase in lung ischemia-reperfusion injury. J Heart Lung Transplant. 2004;23:1290–6. doi: 10.1016/j.healun.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 57.Samad F, Bergtrom G, Amrani DL. Regulation of plasminogen activation by interleukin-6 in human lung fibroblasts. Biochim Biophys Acta. 1994;1221:307–14. doi: 10.1016/0167-4889(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 58.Choudhury S, Wilson MR, Goddard ME, O’Dea KP, Takata M. Mechanisms of early pulmonary neutrophil sequestration in ventilator-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L902–L910. doi: 10.1152/ajplung.00187.2004. [DOI] [PubMed] [Google Scholar]
- 59.Liaudet L, Soriano FG, Szabo E, Virag L, Mabley JG, Salzman AL, Szabo C. Protection against hemorrhagic shock in mice genetically deficient in poly(ADP-ribose)polymerase. Proc Natl Acad Sci USA. 2000;97:10203–8. doi: 10.1073/pnas.170226797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zingarelli B, Szabo C, Salzman AL. Blockade of Poly(ADP-ribose) synthetase inhibits neutrophil recruitment, oxidant generation, and mucosal injury in murine colitis. Gastroenterology. 1999;116:335–45. doi: 10.1016/s0016-5085(99)70130-7. [DOI] [PubMed] [Google Scholar]
- 61.Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109:665–78. doi: 10.1016/s0002-9343(00)00612-4. [DOI] [PubMed] [Google Scholar]