Abstract
Cornelia de Lange syndrome (CdLS) is a dominant multisystem disorder caused by a disruption of cohesin function. The cohesin ring complex is composed of four protein subunits and more than 25 additional proteins involved in its regulation. The discovery that this complex also has a fundamental role in long-range regulation of transcription in Drosophila has shed light on the mechanism likely responsible for its role in development. In addition to the three cohesin proteins involved in CdLS, a second multisystem, recessively inherited, developmental disorder, Roberts-SC phocomelia, is caused by mutations in another regulator of the cohesin complex, ESCO2. Here we review the phenotypes of these disorders, collectively termed cohesinopathies, as well as the mechanism by which cohesin disruption likely causes these diseases.
Keywords: Cornelia de Lange syndrome, Roberts-SC phocomelia, NIPBL, SMC1A, SMC3, ESCO2
CORNELIA DE LANGE SYNDROME
Cornelia de Lange syndrome (CdLS) (OMIM #122470 and #300590) was first described in 1849 by Vrolik, who reported a severe example of oligodactyly (72). In 1916, Brachmann (10) provided a detailed account of an individual with symmetrical monodactyly, antecubital webbing, dwarfism, cervical ribs, and hirsutism (64). In the 1930s Cornelia de Lange, a Dutch pediatrician, reported two unrelated girls with strikingly similar features and named the condition after the city in which she worked: degeneration typus amstelodamensis (17, 18). Although some literature refers to the disorder as Brachmann-de Lange syndrome (BDLS), the disorder is widely referred to as Cornelia de Lange syndrome in honor of Dr. d e Lange’s contributions to the formal characterization of this disorder.
CdLS is a dominantly inherited, genetically heterogeneous, multisystem developmental disorder. The phenotype consists of characteristic facial features (including synophrys, long eyelashes, depressed nasal root with an up-tilted tip of the nose and anteverted nares, long philtrum, thin upper lip, small widely spaced teeth, small brachycephalic head, and low-set, posteriorly angulated ears), hirsutism, various ophthalmologic abnormalities, abnormalities of the upper extremities that range from small hands with single palmar creases and subtle changes in the phalanges and metacarpal bones to severe forms of oligodactyly and truncation of the forearms that primarily involves the ulnar structures, gastroesophageal dysfunction, growth retardation, and neurodevelopmental delay (42, 48) (Figure 1). Other frequently seen findings include ptosis, myopia, intestinal malrotation, cryptorchidism, hypospadias, pyloric stenosis, congenital diaphragmatic hernias, cardiac septal defects, seizures, and hearing loss. The mental retardation seen in CdLS is severe; IQs range from less than 30 to 102 with an average of 53. Many patients also demonstrate autistic behavior, including self-destructive tendencies, and may avoid or reject social interactions and physical contact (48). The prevalence of this syndrome has been estimated to be approximately 1 in 10,000 (73), although this may be an underestimate because gene testing has allowed for an even broader expansion of our clinical understanding of this disorder; the mildest end of the spectrum approaches apparent isolated mild mental retardation (19).
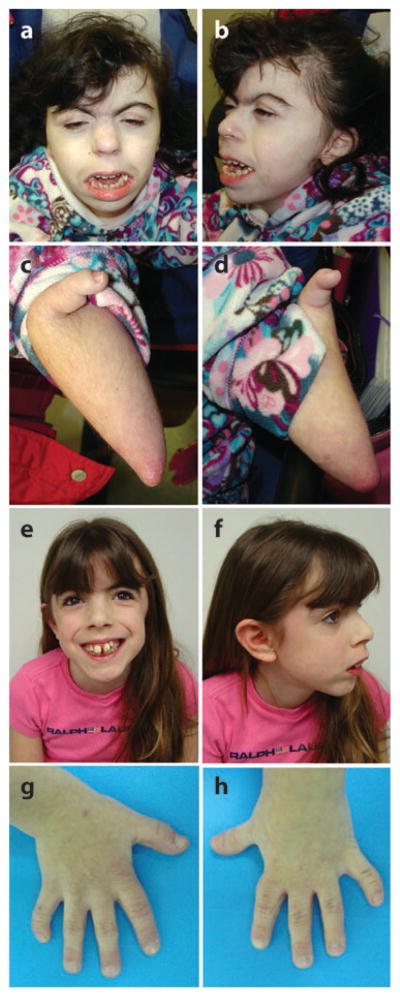
Figure 1.
Typical phenotypic characteristics of Cornelia de Lange syndrome (CdLS). (a–d ) Manifestations in a severely affected 17-year-old girl. (a–b) Note characteristic facial features such as arched eyebrows, synophrys, long eyelashes, ptosis, short nose, long philtrum, thin upper lip, and posteriorly angulated ears. (c–d ) Note severe oligodactyly with single radial digit, hypoplasia of ulnar structures, and pterygia of antecubital region bilaterally. (e–h) The characteristic mild phenotype in an 8-year-old girl. (e–f ) Note the characteristic facial features described above (without the ptosis) but with much milder expression. ( g–h) Note the small hands with fifth finger clinodactyly and proximally placed thumbs bilaterally.
The facial features seen in individuals with classical CdLS are striking and easily recognizable, and may be one of the most useful diagnostic signs. However, marked variability exists and a milder phenotype has been consistently described (1, 42, 83, 89, 101) (Figure 1). In fact, even the first descriptions of CdLS by Brachmann in 1916 (10) and de Lange in 1933 (17, 18) were discrepant in the lack of major limb abnormalities in de Lange’s cases. With increasing recognition of a milder CdLS phenotype, both isolated and familial cases have been diagnosed and reported (14, 32, 82). The mild phenotype is distinguished by less significant psychomotor and growth retardation, a lower incidence of major malformations, and milder limb anomalies as compared with the more severe phenotype (1). The milder phenotype has been estimated to account for approximately 20–30% of the CdLS population (1). Again, this is most likely an underrepresentation because many of these children are unlikely to be diagnosed with CdLS. Standard growth charts for height, weight, and head circumference as well as specific psychomotor developmental milestone charts have been developed for CdLS (51, 52).
GENE IDENTIFICATION
Most individuals with CdLS have normal chromosomes; however, a number of chromosomal rearrangements in individuals with CdLS have been reported over the years (21, 54). In some of the earlier described cases the diagnosis is now considered insecure and in several cases the same type of abnormality has been reported in unaffected individuals. However, i n some CdLS individuals the reported chromosomal abnormality may be specific. Researchers have reported three affected sporadic children with CdLS with apparently balanced de novo translocations: t(14;21)(q32;q11), t(X;8)(p11.2;q24.3), and t(3;17)(q26.3;q23.1) (26, 43, 110). Recently a 0.6-Mb de novo 9p duplication was found in a Swedish boy with a CdLS-like phenotype after array comparative genomic hybridization analysis (84). The 3q26.3 breakpoint had been considered to be of particular interest because of phenotypic overlap between CdLS individuals and individuals with duplication 3q syndrome. A critical region has been defined at 3q26.3-q27 (3, 44, 78). The dup 3q syndrome has long been considered to be a phenocopy of CdLS because of the clinical overlap (39, 63, 65, 87, 109), but the two entities can be differentiated easily on clinical exam.
Several genes that map to these previously reported candidate regions were screened as potential CdLS disease genes, including CHRD and SOX2 (3q27) (93), SHOT (3q25-q26) (7, 74), GSC (14q32) (93), NAALADL2 (3q26.3) [found to be disrupted by the t(3;17) (98)], and NLGN1, a gene involved in synaptogenesis in the central nervous system, the gene dosage of which has been implicated in the mental retardation associated with the dup(3q) syndrome (65). Unfortunately, none of these genes were found to be mutated among other CdLS patients and the CDL1 locus at 3q26.3 did not segregate in at least half of familial cases studied, questioning the veracity of this region as a CdLS locus (56).
A genome-wide linkage exclusion analysis performed on 12 families was used to narrow candidate gene loci to five genomic regions. One region at 5p13.1 corresponded to an additional patient with a d e novo balanced t(5;13)(p13.1;q11.2). Candidate gene screening was used to identify a novel gene named Nipped-B-Like (NIPBL) after its Drosophila homolog, nipped-b; heterozygous mutations in NIPBL were found to cause CdLS (55). NIPBL is a regulator of the cohesin complex. This finding was the first implication of the cohesin complex and its regulators with a developmental disorder. Shortly afterwards mutations in other cohesin components, structural maintenance of chromosomes 1A and 3 (SMC1A and SMC3), were also found to cause CdLS (19, 69).
THE COHESIN COMPLEX AND ITS REGULATORS
During mitosis and meiosis, sister chromatid cohesion is established when the DNA replication products are held together by a multi-subunit complex called cohesin. In yeast, the cohesin complex consists of an Smc1 and Smc3 heterodimer and at least two non-SMC subunits [Scc1/Mcd1/RAD21 and Scc3/Stromalins (SAs)] (Figure 2). The Smc1 and Smc3 subunits are rod-shaped molecules with globular ATPase domains at one end and dimerization domains at the other end. The ATPase head domains of Smc1 and Smc3 are formed by their N and C termini, and the intervening sequence forms antiparallel coiled-coil structures that fold back on themselves in between. Smc1 and Smc3 interact between their hinge regions and form a V-shaped heterodimer. The two ATPase head domains further interact with the N and C termini of Scc1/Rad21, respectively, creating a triangular structure that could trap sister chromatids (2, 33) (Figure 2). During interphase, cohesin binds along chromosome arms roughly every 10 or 20 kb in yeast or higher organisms (25, 31). Centromeres recruit more cohesin than other chromosome sites do; protein complexes constructed by cohesin and heterochromatin proteins appear to regulate several cellular functions (4, 76). Most cohesin bound to chromosome arms is removed at mitotic prophase (97, 106). Centromere-associated cohesin is regulated differently and involves the shugosin protein (107). Cohesin dissociates completely from chromosomes at anaphase owing to separase cleavage of Scc1/Rad21 and sister chromatid disjunction is triggered afterwards (70) (Figure 3). Cohesin starts to be loaded onto chromosomes in telophase, but in Saccharomyces cerevisiae the loading starts at the G1/S phase transition (66). The mechanisms by which sister chromatid cohesion is established remain unclear, although it appears that the cohesin loading (or adherin) complex Scc2/Scc4 and the acetyl-transferase Eco1/Ctf 7 (chromosome transmission fidelity) are essential for this process (see 71 and 38 for reviews). Mutations in the human homolog of Eco1/Ctf 7, establishment of cohesion 1 homolog 2 (ESCO2) result in Roberts syndrome (see below). During or after DNA replication, replication factor C and DNA helicase are also required to establish cohesion (see 91 for a review).
Figure 2.
Schematic representation of a single open cohesin molecule on a DNA strand. Structural maintenance of chromosomes 1A and 3 (SMC1A and SMC3), mutations in which have been identified in Cornelia de Lange syndrome (CdLS), are attached at their hinge domains. Coiled-coil arms connect the hinge domain to the head domain. The head domains contain ATP-binding cassettes crucial in the dynamic opening and closing of the ring structure, which is mediated by the RAD21 and stromalin proteins. Also shown are the cohesin regulatory proteins Nipped-B-Like (NIPBL) (and interacting protein MAU-2) and establishment of cohesion 1 homolog 2 (ESCO2) (and putative interacting factor PDS5), involved in CdLS and Roberts/SC phocomelia syndrome (RBS-SC), respectively.
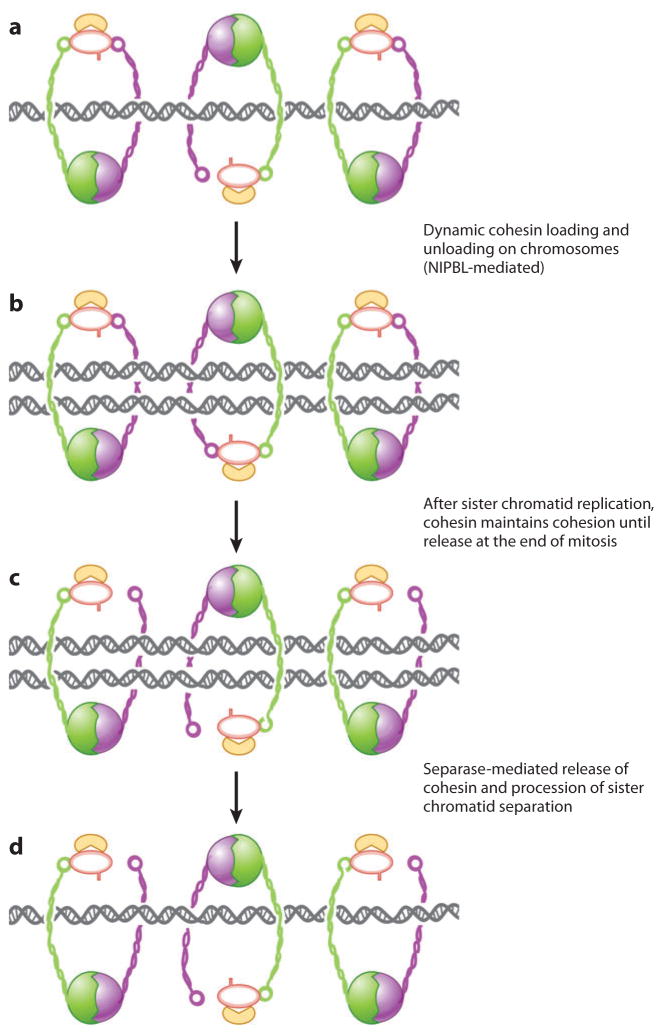
Figure 3.
The sister chromatid “embracing” model of cohesin function, which depicts the dynamic way in which cohesin is opened and closed and loaded on and off of chromosomes at precise times during the cell cycle to correctly release sister chromatids for appropriate segregation to daughter cells after mitosis. NIPBL, Nipped-B-Like.
Four topological models of sister chromatid cohesion have been proposed, differing in whether one ring (embracing model) or two rings act in concert to secure the chromatids, and whether cohesins bind directly to chromatin or not (61). In most eukaryotic cells, the cohesin cleaved by separase at the onset of anaphase composes only a small portion of the cohesin population (106), whereas the majority of cohesin dissociates from chromosome arms owing to separase-independent mechanisms during prophase (28, 57). Therefore, a large amount of intact and free cohesin is available to reassociate with chromosomes near the beginning of the G1 phase in the following cell cycle. Hence, cohesin steadily associates with chromosomes for most of the cell division cycle and has expected functions linked to replication. Many recent lines of evidence implicate cohesin as a key regulator of gene expression. Because sister chromatid cohesion seems to be minimally affected or not affected at all by dosage changes of cohesin and its associated proteins (as evidenced by the rare presence of sister chromatid cohesion defects), the clinical phenotype of CdLS is likely the result of cohesin-mediated gene dysregulation during embryonic development (reviewed in 24). Defects in the establishment or maintenance of sister chromatid cohesion may contribute to the phenotypic manifestations in CdLS because a mild effect on sister chromatids, named precocious sister chromatid separation (PSCS), has been identified in CdLS probands with heterozygous NIPBL mutations (49).
HETEROZYGOUS MUTATIONS OF THE COHESIN LOADING PROTEIN NIPBL AND COHESIN SUBUNITS SMC1A AND SMC3 LEAD TO CORNELIA DE LANGE SYNDROME
Human NIPBL is located on chromosome 5q13 and the full-length transcript is approximately 10 kb in length. Various smaller alternative transcripts exist, and two protein isoforms are conserved in vertebrates (55, 99). The NIPBL protein is also referred to as delangin. SMC1A is located on Xp11.22-p11.21 and escapes X inactivation in humans (12). SMC3 is located on 10q25. NIPBL transcripts are ubiquitously expressed in humans but obvious tissue diversities exist, with the highest expression found in heart and skeletal tissue (55, 99). In situ hybridization studies on whole-mount mouse embryos showed NIPBL expression in developing limbs, craniofacial bones and muscles, the craniofacial mesenchyme surrounding the cochlear canal, the respiratory and gastrointestinal systems, heart, renal tubules, and genitourinary system, concordant with the clinically involved systems in CdLS (55). Mouse Nipbl is robustly expressed in limb buds, branchial arch, and craniofacial mesenchyme, although other tissue and organs also show expression at various levels (99). Xenopus tropicalis delangin is found throughout the ectoderm and in the mesoderm during gastrulation (95). UniGene cluster and expressed sequence tag (EST) studies showed that both SMC1A and SMC3 are universally expressed and there is some increased expression in certain tissues (http://source.stanford.edu/cgi-bin/source/sourceSearch).
NIPBL MUTATION SCREENING AND GENOTYPE-PHENOTYPE CORRELATIONS IN CORNELIA DE LANGE SYNDROME
Mutational analysis by sequencing of NIPBL exons and flanking intronic regions has been performed among various ethnic groups (5, 29, 84, 90, 111), as has SMC1A mutation analysis (8, 19). A wide variety of pathogenic mutations have been identified. Overall only approximately 50% of CdLS probands are found to carry an NIPBL mutation, most of which are point mutations or small insertions and deletions in coding regions or splice junctions. These mutations are assumed to produce either malfunctioning full-length or truncated NIPBL proteins, consistent with haploinsufficiency (29). In rare cases, researchers have found large genomic rearrangements as well as alterations in the upstream noncoding region of the gene. For example, one affected girl and her mildly affected father have a heterozygous deletion-insertion mutation in the NIPBL 5′-untranslated region (UTR) (9), and in another patient multiplex ligation-dependent probe amplification (MLPA) revealed a 5.2-kb deletion that encompasses exons 41–42 of NIPBL (6). Among NIPBL mutation–negative CdLS probands approximately 8% (4% of the total CdLS population) carry an SMC1A mutation (19). One mildly affected adult male with CdLS was found to carry a de novo SMC3 mutation. At least 40% of clinically diagnosed patients with CdLS do not carry a mutation in any of these genes, suggesting the existence of other CdLS genes or that potential alternative mechanisms of alteration in these known genes may be involved.
Genotype-phenotype correlations are beginning to emerge but mostly involve gross observations that individuals with missense mutations or no identifiable mutations tend to present a milder phenotype than those with truncating mutations; individuals with SMC1A mutations also tend to have a milder phenotype and rarely, i f ever, manifest structural organ or limb defects (19, 29). At the cellular level, B-lymphoblastoid cells from CdLS patients also manifest some phenotypic features: (a) 41% of metaphase spreads from NIPBL mutation–positive probands show PSCS, whereas this phenotype appears in only 9% of control samples (49); and (b) both fibroblast and B-lymphoblastoid cells derived from CdLS patients, either with or without detectable NIPBL mutations, are more sensitive to DNA crosslinker mitomycin C (MMC) than wild type and demonstrate a decreased ability to repair double-strand DNA breaks at G2 phase after X-ray exposure (105). Rare CdLS-associated malignant cases such as Wilm’s tumor have been reported, but in general cancer is not a predominant manifestation (13).
COHESIN BINDING IMPACTS GENE EXPRESSION
In both yeast and Xenopus the loading of cohesin onto chromatin in G1 phase is functionally separable from the establishment of sister chromatid cohesion at S phase (62, 100). In S. cerevisiae, cohesin loading requires a protein complex formed by Scc2 and Scc4 (15) and cohesion establishment and maintenance require Pds5 (36, 75). Scc2, Scc4, and Pds5 all contain HEAT (Huntington, elongation factor 3, PR65/A, TOR) domains.
It is becoming increasingly clear that different factors manage the binding and dissociation of cohesin for different chromosomal regions [e.g., arms, centromeres, telomeres, ribosomal DNA (rDNA)], raising the possibility that different chromosomal regions use different pathways for cohesin-mediated sister chromatid cohesion. In addition, not all cohesion is due to cohesin. Other mechanisms independent of cohesin, such as phosphatase Cdc14- and condensin-mediated cohesion, have also been proposed in budding yeast (16, 96). The presence of cohesin in chromatin may serve functions other than cohesion, e.g., the establishment of silencing or regulation of transcription by blocking the communication between enhancers and promoters (24).
The cohesin apparatus is composed of a ring-like structure that contains four subunits: Smc1, Smc3, Scc1/Rad21, and Scc3/SA (Figure 2). During the mitotic cell cycle, co-hesin is loaded onto chromosomes at telophase and removed from the arms at the subsequent prophase. Several mechanisms have been proposed for the formation of the ring structure and the corresponding chromatid encircling models (38, 45, 46). Nipped-B, the homolog of cohesin loading protein Scc2, was identified in a genetic screen in Drosophila that measured the activation of cut and Ultrabithorax gene expression. Nipped-B alleviates the gypsy insulator function by assisting long distance promoter-enhancer interactions (81). Cut regulates Drosophila wing and limb development. Nipped-B null mutations are lethal to the fly, whereas heterozygous mutations in Nipped-B result in lower expression of cut protein than wild type and notch wing phenotypes (81). In Drosophila, heterozygous Nipped-B mutants do not show cohesion defects, whereas homozygous Nipped-B mutants only show the defects right before death at the second instar stage, indicating sister chromatid cohesion is independent from cohesin-regulated gene expression. Cohesin binds to chromosomes throughout interphase, when gene expression also takes place; cohesin binds to the cut regulatory sequences in cultured cells and to the cut locus in Drosophila salivary gland chromosomes (25). Reducing cohesin levels boosts cut expression, whereas reducing Nipped-B levels diminishes cut expression in the emergent wing margin (25, 80). Cohesin binding may interfere with enhancer-promoter communication at the cut gene locus to inhibit gene expression. Nipped-B is able to reverse these suppression effects by dynamically removing the bound cohesin (23).
The results of genome-wide mapping of the cohesin and cohesin loading protein binding sites in yeast and Drosophila are conflicting. Cohesin binds to intergenic regions between convergent transcription units almost exclusively and does not reside on the same sequences as Scc2 in S. cerevisiae, suggesting that Scc2 loads cohesin first, and then other factors, such as RNA polymerase, push cohesin to its binding places (31, 59). In Drosophila, on the contrary, salivary polytene chromosome immunostaining and the cut locus chromatin immunoprecipitation (ChIP) assay have demonstrated the colocalization of Nipped-B and cohesin. Misulovin and coworkers (67) reported the mapping of Nipped-B and cohesin binding sites by ChIP/microarray (ChIP-on-chip) assays in the entire nonrepetitive Drosophila genome and found that not only do Nipped-B and cohesin colocalize, but they also both bind preferentially to active transcription units. The reason for this discrepancy between yeast and Drosophila is unknown, but colocalization of Nipped-B with cohesin may be critical for Nipped-B to relieve cohesin from blocking the long-range communication between promoters and enhancers, thus permitting gene activation. Because gene regulation through distant DNA elements is not a major regulatory mechanism in yeast, higher organisms may possess additional mechanisms for cohesin to bind in the genome and to regulate gene expression (67).
Scc4 (mau-2 in other species) interacts with Scc2 and is also essential for sister chromatid cohesion. In Drosophila, Nipped-B interacts with Mau-2 (30, 88). In Caenorhabditis elegans cells with RNAi knockdown of mau-2 have abnormal axonal migration without sister chromatid cohesion defects, whereas in human cultured cells knockdown of human MAU-2 by RNAi does cause cohesion defects (88). Mau-2 mutations in C. elegans may have a similar effect as heterozygous Nipped-B mutations in Drosophila, which may reduce gene expression and activity without obvious effect on cohesion (80, 81). Compared with Nipped-B, Mau-2 expression changes seem less critical because partial knockdown of Mau-2 by RNAi in Drosophila has no effects on either cut gene expression or sister chromatid cohesion (80, 88). These observations suggest that the sister chromatid cohesion apparatus can influence both gene expression and cohesion during development, but the latter may require more dramatic protein changes. Thus, gene expression appears to be more sensitive to subtle dosage alterations of the cohesin apparatus. Another cohesion factor, the Pds5B gene, is conserved from fungi to man, dynamically interacts with cohesin, and is involved in establishment and/or maintenance of sister chromatid cohesion (25). A single pds5 gene exists in Drosophila, and heterozygous pds5 mutations alter cut gene expression without effects on cohesion. Homozygous mutants show both gene expression and cohesion defects (25). Mammals have two Pds5 proteins. Homozygous Pds5B knockout mice show CdLS-like developmental abnormalities without cohesion defects, suggesting that changes in gene expression likely also underlie Pds5B function in mouse development (112).
In Drosophila, Nipped-B might facilitate the interaction between promoter and remote enhancers for target genes such as cut and Ultrabithorax, possibly by regulating chromosome structure (81). Nipped-B may operate at various regulating levels and directly control the expression of target genes. In humans, CdLS is caused by heterozygous loss-of-function mutations in the Nipped-B-Like (NIPBL) ortholog of Nipped-B and, in fewer cases, by mutations that all presumably maintain the open reading frame in the SMC1A or SMC3 cohesin subunit genes (19, 55, 69, 99). The maintenance of the open reading frame and production of a protein product with residual, albeit decreased, function would be critical for SMC1A mutant viability. Because this gene is on the X chromosome and escapes inactivation in humans, loss-of-function mutations would likely be incompatible with life in males because they would have no functional SMC1A protein and therefore no functional cohesin. In females, loss-of-function mutations would likely result in no phenotype as long as the alternate allele was normal. Given the constellation of developmental abnormalities present in individuals with CdLS, and that the majority show no significant evidence of a cohesion defect (49, 105), the alterations of cohesin regulation and structure seen in these individuals most likely results in gene expression dysregulation similar to what has been observed in Drosophila.
IMPLICATION OF COHESIN APPARATUS–MEDIATED GENE REGULATION THROUGH LONG-RANGE REGULATORY ELEMENT-PROMOTER INTERACTIONS
The Nipped-B protein, an Scc2 ortholog, was identified in Drosophila through a genetic screen for proteins that facilitated expression of the cut homeobox gene in the developing wing margin. Expression of cut is driven by a distant transcriptional enhancer located more than 80 kb upstream of the transcription start site. Nipped-B is an essential protein; homozygous Nipped-B mutants die as second instar larvae (upon depletion of maternal Nipped-B protein). Heterozygous mutants reduce the ability of the enhancer to overcome the gypsy insulator inserted between the distal enhancer and the cut gene promoter and thereby enhance the phenotype caused by the gypsy insertion (34, 81). Nipped-B is highly dosage sensitive: Heterozygous Nipped-B mutations reduce Nipped-B messenger RNA (mRNA) levels by only 25% and a 50% reduction induced by RNAi is lethal to the fly (80).
Therefore, the critical means by which NIPBL and cohesin affect transcriptional control appears to be through long-range enhancer-promoter interactions (Figure 4). For most genes the region immediately upstream of the minimal promoter contains the requisite transcription factor binding sites to regulate expression of the gene. However, for many genes multiple cis-acting distal elements [most commonly enhancers, but also silencers, insulators, and locus control regions (LCRs)] are required for correct spatiotemporal expression (50). These elements may be located upstream, downstream, or within introns and can reside greater than 1 Mb from the target gene (104). Disruption of these long-range regulatory interactions can result in human disease phenotypes either through global or partial tissue-specific loss or gain of expression (reviewed in 50). The majority of the genes responsible for these disorders when disrupted are transcription factors that in turn regulate the expression of tissue-specific targets critical for normal morphogenesis of the organ systems involved. An additional layer of complexity in understanding the role of long-range enhancers in human disease is that the phenotype that results from disruption of a regulatory element can be quite different from that caused by a mutation in the coding region of the gene. This phenomenon is likely due to disruption in a subset of tissues or during a specific developmental window that results from a regulatory mutation versus an effect on all tissues and developmental stages where the gene product is affected by a coding mutation. An example of this is the varying phenotypes caused by mutations in the coding region versus the regulatory components of the sonic hedgehog (SHH ) gene. Mutations in the coding region of SHH cause holoprosencephaly (HPE3), a defect of the midline structures of the face and brain (79), whereas disruption of a regulatory element 1 M b upstream of theSHH promoter results in the limb-specific phenotype of preaxial polydactyly (60). This regulatory element mutation results in loss of the normal restriction of SHH expression to the posterior margin of the anterior limb bud, thereby allowing development of additional digits on the opposite axis.
Figure 4.
Putative model by which cohesin can hinder and relieve transcriptional control (in this case transcriptional repression) of genes regulated by distal elements. (a) Cohesin loaded on the chromosome inhibits the propagation of a transcriptional control signal from a distal enhancer to increase transcription via the target promoter. (b) With release of cohesin from the chromosome, the transcription activation signal from the distal enhancer is free to propagate to the promoter and increase transcription of the target gene.
This finding demonstrates that dysregulation of distal regulatory elements can result in not only loss of function of the target gene in a spatiotemporal manner but also gains of function when the normal suppression of expression is lost. In the study that first identified Nipped-B in Drosophila, researchers noted that the alteration of this gene and the resulting disruption of the long-range enhancer-promoter interaction affected the regulation of the homeobox gene Ultrabithorax (81). Homeobox gene clusters in humans, such as the HOX genes, that play a key role in body patterning are also regulated by distal enhancers that are capable of controlling their sequential activation. The identification of a global control region approximately 240 kb upstream of the HOXD cluster that regulates downstream expression of at least six genes in a tissue- and temporal-specific manner makes this an attractive candidate for some of the structural defects seen in CdLS (20, 37, 94).
The results of genome-wide ChIP-on-chip assays designed to examine the binding sites of Nipped-B, RNA polymerase II (Pol II), and cohesin subunit SMC1A in three different Drosophila cell lines showed that Nipped-B colocalizes with cohesin, which supports the idea that Nipped-B dynamically regulates cohesin binding (67). The preferential association of cohesin with transcribed regions suggests additional mechanisms by which cohesin binding might affect transcription, and vice versa. Results from the same study have also suggested that cohesin could interfere with both transcriptional activation and elongation because cohesin binds to the active Abd-B gene in Sg4 cells and some cohesin and Pol II peaks overlap within both the Abd-B transcriptional unit and the regulatory region. Genes with distant regulatory elements, such as cut and Ubx, may be more sensitive to Nipped-B dosage because of the combined effects on activation and elongation.
Other studies suggest that cohesin might have positive effects on gene expression as well. Inhibiting Rad21 expression results in reduced runx gene expression during early zebrafish development, although it is unknown if the effect is direct (40). Possible ways in which cohesin could directly facilitate gene expression include (a) preserving the targeted chromatin in an open state that is more accessible to transcription or (b) upholding chromatin boundaries, separating active and inactive chromatin regions, and preventing the distribution of silencing influence across domains. To support the second idea, in S. cerevisiae mutations in Smc1 cause the loss of the boundaries surrounding the HMR silent-mating-type loci and the spread of SIR (silent information regulator) complexes into neighboring regions (22); in Drosophila a cohesin/Nipped-B peak was found at the Fab-7 boundary element flanking the active Abd-B domain (67).
COHESIN AND CHROMOSOME REMODELING
Cohesin forms stable associations with chromatin remodeling complexes in vivo (35). A human ISWI (SNF2h)-containing complex was copurified with cohesin and NuRD. The hRAD21 subunit of the cohesin complex directly interacts with the ATPase subunit of SNF2h. Loading hRAD21 onto chromatin might involve ATPase activity of SNF2h (35). Alu sequences specifically bind to hRAD21, SNF2h, and Mi2, demonstrating that Alu repeats may also act as cohesin binding sites. Modifications of histone tails may be associated with the SNF2h/cohesin complex as well. Cohesin binds to AT-rich noncoding regions, which are the bases of chromosome loops attached by chromatin fibers and flanked by genes transcribed convergently in yeast (27). The yeast Sir2 protein recruits cohesin to multiple tandem ribosomal DNA arrays and suppresses chromatin recombination (53). Additional support for the role of NIPBL and cohesin in chromatin remodeling and epigenetic regulation came from the demonstration that NIPBL binds directly to the chromoshadow domain (CSD) of heterochromatin protein 1α (HP1α) with high affinity. The HP1α protein regulates epigenetic gene silencing by promoting and maintaining chromatin condensation. The HP1α chromodomain also binds to the methylated histone H3 (58). (See Supplemental Material. Follow the Supplemental Material link from the Annual Reviews home page at http://www.annualreviews.org.)
OTHER COHESINOPATHIES
Roberts Syndrome and SC Phocomelia
The identification of a developmental disorder caused by mutation in the cohesin regulator ESCO2, the Roberts-SC phocomelia syndrome (RBS) (OMIM #268300; SC OMIM #269000), further implicates this pathway as a critical regulator of normal human development (103). ESCO2 is homologous to yeast Eco1/Ctf7, which encodes an acetyltransferase that interacts with proliferating cell nuclear antigen (PCNA) and is essential for establishment of sister chromatid cohesion at S phase (68).
Despite the fact that the clinical presentations have some overlap and the molecular mechanisms are similar, these two congenital diseases are quite distinct. CdLS is a dominant disorder: 50% of probands carry heterozygous mutations in NIPBL, 5 % carry heterozygous mutations in SMC1A, and one proband carries an SMC3 mutation (19). RBS-SC phocomelia is an autosomal recessive disorder: All probands have either homozygous or compound heterozygous mutations of ESCO2. RBS-SC phocomelia is characterized by symmetric hypomelia, which varies from tetraphocomelia to a lesser degree of limb deficiency that is more severe in the upper limbs. Only ~25% of CdLS probands have limb reduction defects and these are usually asymmetric and involve the ulnar structures of upper extremities. RBS-SC phocomelia probands also have pre- and postnatal growth delay, as well as mild to severe mental retardation; both disorders have craniofacial anomalies, but each has distinct characteristic facial features (29, 102) (Figure 5). Cytogenetically, heterochromatin repulsion (HR) appears in 100% of RBS-SC phocomelia probands, is highly correlated with the phenotype and ESCO2 mutations (85), and has been used for prenatal diagnosis (86). Genotype-phenotype correlations exist among individuals with CdLS in that probands with protein-truncating mutations in NIPBL tend to be severely affected, whereas those with mis-sense mutations have milder phenotypes; all probands carrying an SMC1A mutation are more mildly affected and display almost no structural anomalies but do show mental retardation (19, 29). By contrast, there is no genotype-phenotype correlation among RBS-SC phocomelia patients (85). SC phocomelia syndrome is now known to be a milder form of RBS because it is also due to recessive mutations in ESCO2 (the same mutations cause both RBS and SC phocomelia) and all have HR (85).
Figure 5.
Phenotypic features in Roberts-SC phocomelia syndrome. Both patients have the same causative mutations in ESCO2 (establishment of cohesion 1 homolog 2). (a) Typical features of SC phocomelia, including upper limb long bone shortening (radial hypoplasia > ulnar hypoplasia) and contractures with relative sparing of lower extremities. (b) Roberts syndrome patient with more severe phenotype, including tetraphocomelia and craniofacial abnormalities. Photographs courtesy of Dr. Hugo Vega from the Mount Sinai School of Medicine and Universidad Nacional de Colombia.
ESCO2 maps to 8p21.1 and is the human homolog of the budding yeast gene Eco1/Ctf 7, which is involved in triggering cohesion initiation (92). Eco1 has a C2H2 zinc finger domain at the N terminus and an acetyltransferase domain at the C terminus; Eco1 is able to acetylate itself and cohesin subunits in vitro (47), but no in vivo enzyme activity has been demonstrated (11). The protein structure of Eco1/Ctf7 is highly conserved from yeast to human. Functional Eco1/Cft7 proteins are important in all studied organisms; for example, sister chromatid cohesion is disrupted at the centromere and chromosome alignment is delayed in Drosophila mutants, although cohesion along sister chromatid arms seems unaffected (108). In human cell lines, ESCO2 binds to chromatin, possesses acetyltransferase activity, and is required for stable sister chromatid cohesion. Defective chromosome congregation or segregation is induced by RNAi knockdown of ESCO2 protein expression (41). The replication protein PCNA bridges cohesion establishment with DNA replication, because direct physical interaction between Eco1 and PCNA is essential for the engagement of Eco1 to chromatin and the initiation of sister chromatid cohesion in both yeast and human cells (68). The understanding of the relationship between ESCO2 and other cohesin subunits or regulatory factors is still very limited at this time.
α-Thalassemia/Mental Retardation Syndrome, X-Linked
α-Thalassemia/mental retardation syndrome, X-linked (ATRX) (OMIM #301040), caused by mutations in the ATRX gene on the X chromosome, was recently found to also involve a chromosome cohesion defect (77). ATRX is a multisystem disorder of postnatal growth deficiency, mental retardation, microcephaly, dysmorphic craniofacial features (midface hypoplasia; small, low-set ears; hypertelorism; anteverted nares; full lips with protruding tongue), genital abnormalities in males, expressive speech absence, seizures, mild hypochromic microcytic anemia, and a mild form of hemoglobin H (Hb H) disease. The ATRX gene encodes a chromatin remodeling enzyme that is highly enriched at pericentromeric heterochromatin in mouse and human cells and associates with the chromoshadow domain of HP1α (as does NIPBL) (77). Defective sister chromatid cohesion, chromosome congression at the metaphase plate, and mitotic defects have recently been described in various mammalian cells (77). Defects in the ATRX gene that lead to the ATRX syndrome are postulated to be a result of perturbed cohesin targeting or loading/unloading; the phenotype is thought to be wholly or partially caused by the resultant gene dysregulation or mitotic defects.
SUMMARY POINTS.
Studies of mutations in structural components of cohesin [structural maintenance of chromosomes 1A and 3 (SMC1A and SMC3) i n Cornelia de Lange syndrome (CdLS)] and in regulators of the cohesin complex [Nipped-B-Like (NIPBL) i n CdLS and establishment of cohesion 1 homolog 2 (ESCO2) i n Roberts-SC phocomelia] directly implicate the role of this complex in normal human development.
Several other developmental disorders are likely due to a disruption of the cohesin complex and its regulation given that more than 25 genes have been primarily implicated in this cellular event. The exact manner by which cohesin mediates its control in developmental processes is unclear but is slowly being revealed and multiple molecular mechanisms are involved.
The regulation of gene expression through long-range regulatory elements, as well as possible roles in chromatin remodeling and epigenetic regulation of transcription, is likely the more critical underlying mechanism by which developmental control is regulated rather than by cohesin’s canonical role in sister chromatid cohesion and segregation.
FUTURE ISSUES.
Mammalian animal models, such as a CdLS mouse model, will be invaluable tools to resolve the conflicts currently being uncovered in nonmammalian species and to identify target genes regulated by cohesin at different embryonic stages and within each organ system involved in these disorders.
Genome-wide expression studies and the identification of cohesin and NIPBL binding sites in human cells will also be necessary to correlate cohesin binding with specific gene transcription.
Potential inter/intrachromosomal association mediated by NIPBL/cohesin and the corresponding impact on gene regulation need more attention.
Novel areas of transcriptional control (i.e., noncoding RNAs) may also be affected by cohesin dysfunction and these areas will need to be investigated on a genome-wide and spatiotemporal level in embryogenesis as well to appreciate their contribution to the phenotypes seen in the cohesinopathies.
Acknowledgments
The authors would like to acknowledge the support of the NICHD (PO1HD052860) (I.D.K.) and the CdLS Foundation Fellowship Grant ( J.L.).
Glossary
- CdLS
Cornelia de Lange syndrome
- NIPBL
nipped-B-like
- SMC1A
structural maintenance of chromosome 1A
- SMC3
structural maintenance of chromosome 3
- ESCO2
establishment of cohesion 1 homolog 2
- PCNA
proliferating cell nuclear antigen
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
Contributor Information
Jinglan Liu, Email: liujin@email.chop.edu.
Ian D. Krantz, Email: ian2@mail.med.upenn.edu.
LITERATURE CITED
- 1.Allanson JE, Hennekam RC, Ireland M. De Lange syndrome: subjective and objective comparison of the classical and mild phenotypes. J Med Genet. 1997;34:645–50. doi: 10.1136/jmg.34.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson DE, Losada A, Erickson HP, Hirano T. Condensin and cohesin display different arm conformations with characteristic hinge angles. J Cell Biol. 2002;156:419–24. doi: 10.1083/jcb.200111002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aqua MS, Rizzu P, Lindsay EA, Shaffer LG, Zackai EH, et al. Duplication 3q syndrome: molecular delineation of the critical region. Am J Med Genet. 1995;55:33–37. doi: 10.1002/ajmg.1320550111. [DOI] [PubMed] [Google Scholar]
- 4.Bernard P, Maure JF, Partridge JF, Genier S, Javerzat JP, Allshire RC. Requirement of heterochromatin for cohesion at centromeres. Science. 2001;294:2539–42. doi: 10.1126/science.1064027. [DOI] [PubMed] [Google Scholar]
- 5.Bhuiyan ZA, Klein M, Hammond P, van Haeringen A, Mannens MM, et al. Genotype-phenotype correlations of 39 patients with Cornelia De Lange syndrome: the Dutch experience. J Med Genet. 2006;43:568–75. doi: 10.1136/jmg.2005.038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhuiyan ZA, Stewart H, Redeker EJ, Mannens MM, Hennekam RC. Large genomic rearrangements in NIPBL are infrequent in Cornelia de Lange syndrome. Eur J Hum Genet. 2007;15:505–8. doi: 10.1038/sj.ejhg.5201776. [DOI] [PubMed] [Google Scholar]
- 7.Blaschke RJ, Monaghan AP, Schiller S, Schechinger B, Rao E, et al. SHOT, a SHOX-related homeobox gene, is implicated in craniofacial, brain, heart, and limb development. Proc Natl Acad Sci USA. 1998;95:2406–11. doi: 10.1073/pnas.95.5.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borck G, Zarhrate M, Bonnefont JP, Munnich A, Cormier-Daire V, Colleaux L. Incidence and clinical features of X-linked Cornelia de Lange syndrome due to SMC1L1 mutations. Hum Mutat. 2007;28:205–6. doi: 10.1002/humu.9478. [DOI] [PubMed] [Google Scholar]
- 9.Borck G, Zarhrate M, Cluzeau C, Bal E, Bonnefont JP, et al. Father-to-daughter transmission of Cornelia de Lange syndrome caused by a mutation in the 5′ untranslated region of the NIPBL gene. Hum Mutat. 2006;27:731–35. doi: 10.1002/humu.20380. [DOI] [PubMed] [Google Scholar]
- 10.Brachmann W. Ein fall von symmetrischer Monodactylie durch Ulnadefekt, mit symmetrischer flughautbildung in den Ellenbeugen, sowie anderen Abnormalitaten (Zwerghaftigkeit, Halsrippen, Behaarung) Jahrb Kinderheilkd. 1916;84:225–35. [Google Scholar]
- 11.Brands A, Skibbens RV. Ctf7p/Eco1p exhibits acetyltransferase activity–but does it matter? Curr Biol. 2005;15:R50–51. doi: 10.1016/j.cub.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 12.Brown CJ, Miller AP, Carrel L, Rupert JL, Davies KE, Willard HF. The DXS423E gene in Xp11.21 escapes X chromosome inactivation. Hum Mol Genet. 1995;4:251–55. doi: 10.1093/hmg/4.2.251. [DOI] [PubMed] [Google Scholar]
- 13.Charles AK, Porter HJ, Sams V, Lunt P. Nephrogenic rests and renal abnormalities in Brachmann-de Lange syndrome. Pediatr Pathol Lab Med. 1997;17:209–19. [PubMed] [Google Scholar]
- 14.Chodirker BN, Chudley AE. Male-to-male transmission of mild Brachmann-de Lange syndrome. Am J Med Genet. 1994;52:331–33. doi: 10.1002/ajmg.1320520315. [DOI] [PubMed] [Google Scholar]
- 15.Ciosk R, Shirayama M, Shevchenko A, Tanaka T, Toth A, et al. Cohesin’s binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Mol Cell. 2000;5:243–54. doi: 10.1016/s1097-2765(00)80420-7. [DOI] [PubMed] [Google Scholar]
- 16.D’Amours D, Stegmeier F, Amon A. Cdc14 and condensin control the dissolution of cohesin-independent chromosome linkages at repeated DNA. Cell. 2004;117:455–69. doi: 10.1016/s0092-8674(04)00413-1. [DOI] [PubMed] [Google Scholar]
- 17.de Knecht-van Eekelen A, Hennekam RC. Historical study: Cornelia C. de Lange (1871–1950)–a pioneer in clinical genetics. Am J Med Genet. 1994;52:257–66. doi: 10.1002/ajmg.1320520302. [DOI] [PubMed] [Google Scholar]
- 18.de Lange C. Sur un type nouveau de dégénération (typus Amstelodamensis) [On a new type of degeneration (type Amsterdam)] Arch Méd Enfants. 1933;36:715–19. [Google Scholar]
- 19.Deardorff MA, Kaur M, Yaeger D, Rampuria A, Korolev S, et al. Mutations in cohesin complex members SMC3 and SMC1A cause a mild variant of Cornelia de Lange syndrome with predominant mental retardation. Am J Hum Genet. 2007;80:485–94. doi: 10.1086/511888. Identification of SMC3 as a CdLS gene and genotype-phenotype c orrelation for SMC1A mutation-positive probands. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Campo M, Jones MC, Veraksa AN, Curry CJ, Jones KL, et al. Monodactylous limbs and abnormal genitalia are associated with hemizygosity for the human 2q31 region that includes the HOXD cluster. Am J Hum Genet. 1999;65:104–10. doi: 10.1086/302467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeScipio C, Kaur M, Yaeger D, Innis JW, Spinner NB, et al. Chromosome rearrangements in Cornelia de Lange syndrome (CdLS): report of a der(3)t(3;12)(p25.3;p13.3) in two half sibs with features of CdLS and review of reported CdLS cases with chromosome rearrangements. Am J Med Genet A. 2005;137:276–82. doi: 10.1002/ajmg.a.30857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donze D, Adams CR, Rine J, Kamakaka RT. The boundaries of the silenced HMR domain in Saccharomyces cerevisiae. Genes Dev. 1999;13:698–708. doi: 10.1101/gad.13.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorsett D. Adherin: key to the cohesin ring and Cornelia de Lange syndrome. Curr Biol. 2004;14:R834–36. doi: 10.1016/j.cub.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 24.Dorsett D. Roles of the sister chromatid cohesion apparatus in gene expression, development, and human syndromes. Chromosoma. 2007;116:1–13. doi: 10.1007/s00412-006-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorsett D, Eissenberg JC, Misulovin Z, Martens A, Redding B, McKim K. Effects of sister chromatid cohesion proteins on cut gene expression during wing development in Drosophila. Development. 2005;132:4743–53. doi: 10.1242/dev.02064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egemen A, Ulger Z, Ozkinay F, Gulen F, Cogulu O. A de novo t (X;8)(p11.2;q24.3) demonstrating Cornelia de Lange syndrome phenotype. Genet Couns. 2005;16:27–30. [PubMed] [Google Scholar]
- 27.Filipski J, Mucha M. Structure, function and DNA composition of Saccharomyces cerevisiae chromatin loops. Gene. 2002;300:63–68. doi: 10.1016/s0378-1119(02)00848-x. [DOI] [PubMed] [Google Scholar]
- 28.Gandhi R, Gillespie PJ, Hirano T. Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr Biol. 2006;16:2406–17. doi: 10.1016/j.cub.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillis LA, McCallum J, Kaur M, DeScipio C, Yaeger D, et al. NIPBL mutational analysis in 120 individuals with Cornelia de Lange syndrome and evaluation of genotype-phenotype correlations. Am J Hum Genet. 2004;75:610–23. doi: 10.1086/424698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giot L, Bader JS, Brouwer C, Chaudhuri A, Kuang B, et al. A protein interaction map of Drosophila melanogaster. Science. 2003;302:1727–36. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- 31.Glynn EF, Megee PC, Yu HG, Mistrot C, Unal E, et al. Genome-wide mapping of the cohesin complex in the yeast Saccharomyces cerevisiae. PLoS Biol. 2004;2:E259. doi: 10.1371/journal.pbio.0020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenberg F, Robinson LK. Mild Brachmann-de Lange syndrome: changes of phenotype with age. Am J Med Genet. 1989;32:90–92. doi: 10.1002/ajmg.1320320119. [DOI] [PubMed] [Google Scholar]
- 33.Gruber S, Haering CH, Nasmyth K. Chromosomal cohesin forms a ring. Cell. 2003;112:765–77. doi: 10.1016/s0092-8674(03)00162-4. [DOI] [PubMed] [Google Scholar]
- 34.Hagstrom KA, Meyer BJ. Condensin and cohesin: more than chromosome compactor and glue. Nat Rev Genet. 2003;4:520–34. doi: 10.1038/nrg1110. [DOI] [PubMed] [Google Scholar]
- 35.Hakimi MA, Bochar DA, Schmiesing JA, Dong Y, Barak OG, et al. A chromatin remodelling complex that loads cohesin onto human chromosomes. Nature. 2002;418:994–98. doi: 10.1038/nature01024. [DOI] [PubMed] [Google Scholar]
- 36.Hartman T, Stead K, Koshland D, Guacci V. Pds5p is an essential chromosomal protein required for both sister chromatid cohesion and condensation in Saccharomyces cerevisiae. J Cell Biol. 2000;151:613–26. doi: 10.1083/jcb.151.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herault Y, Fraudeau N, Zakany J, Duboule D. Ulnaless (Ul ), a regulatory mutation inducing both loss-of-function and gain-of-function of posterior Hoxd genes. Development. 1997;124:3493–500. doi: 10.1242/dev.124.18.3493. [DOI] [PubMed] [Google Scholar]
- 38.Hirano T. At the heart of the chromosome: SMC proteins in action. Nat Rev Mol Cell Biol. 2006;7:311–22. doi: 10.1038/nrm1909. [DOI] [PubMed] [Google Scholar]
- 39.Holder SE, Grimsley LM, Palmer RW, Butler LJ, Baraitser M. Partial trisomy 3q causing mild Cornelia de Lange phenotype. J Med Genet. 1994;31:150–52. doi: 10.1136/jmg.31.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horsfield JA, Anagnostou SH, Hu JK, Cho KH, Geisler R, et al. Cohesin-dependent regulation of Runx genes. Development. 2007;134:2639–49. doi: 10.1242/dev.002485. [DOI] [PubMed] [Google Scholar]
- 41.Hou F, Zou H. Two human orthologues of Eco1/Ctf7 acetyltransferases are both required for proper sister-chromatid cohesion. Mol Biol Cell. 2005;16:3908–18. doi: 10.1091/mbc.E04-12-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ireland M, Donnai D, Burn J. Brachmann-de Lange syndrome. Delineation of the clinical phenotype. Am J Med Genet. 1993;47:959–64. doi: 10.1002/ajmg.1320470705. [DOI] [PubMed] [Google Scholar]
- 43.Ireland M, English C, Cross I, Houlsby WT, Burn J. A d e novo translocation t(3;17)(q26.3;q23.1) in a child with Cornelia de Lange syndrome. J Med Genet. 1991;28:639–40. doi: 10.1136/jmg.28.9.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ireland M, English C, Cross I, Lindsay S, Strachan T. Partial trisomy 3q and the mild Cornelia de Lange syndrome phenotype. J Med Genet. 1995;32:837–38. doi: 10.1136/jmg.32.10.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ivanov D, Nasmyth K. A topological interaction between cohesin rings and a circular minichro-mosome. Cell. 2005;122:849–60. doi: 10.1016/j.cell.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Ivanov D, Nasmyth K. A physical assay for sister chromatid cohesion in vitro. Mol Cell. 2007;27:300–10. doi: 10.1016/j.molcel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Ivanov D, Schleiffer A, Eisenhaber F, Mechtler K, Haering CH, Nasmyth K. Eco1 is a novel acetyltransferase that can acetylate proteins involved in cohesion. Curr Biol. 2002;12:323–28. doi: 10.1016/s0960-9822(02)00681-4. [DOI] [PubMed] [Google Scholar]
- 48.Jackson L, Kline AD, Barr MA, Koch S. de Lange syndrome: a clinical review of 310 individuals. Am J Med Genet. 1993;47:940–46. doi: 10.1002/ajmg.1320470703. The first clinical review of a large c ohort of CdLS patients in the United States. [DOI] [PubMed] [Google Scholar]
- 49.Kaur M, DeScipio C, McCallum J, Yaeger D, Devoto M, et al. Precocious sister chromatid separation (PSCS) in Cornelia de Lange syndrome. Am J Med Genet A. 2005;138:27–31. doi: 10.1002/ajmg.a.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleinjan DA, van Heyningen V. Long-range control of gene expression: emerging mechanisms and disruption in disease. Am J Hum Genet. 2005;76:8–32. doi: 10.1086/426833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kline AD, Barr M, Jackson LG. Growth manifestations in the Brachmann-de Lange syndrome. Am J Med Genet. 1993;47:1042–49. doi: 10.1002/ajmg.1320470722. [DOI] [PubMed] [Google Scholar]
- 52.Kline AD, Stanley C, Belevich J, Brodsky K, Barr M, Jackson LG. Developmental data on individuals with the Brachmann-de Lange syndrome. Am J Med Genet. 1993;47:1053–58. doi: 10.1002/ajmg.1320470724. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi T, Horiuchi T, Tongaonkar P, Vu L, Nomura M. SIR2 regulates recombination between different rDNA repeats, but not recombination within individual rRNA genes in yeast. Cell. 2004;117:441–53. doi: 10.1016/s0092-8674(04)00414-3. [DOI] [PubMed] [Google Scholar]
- 54.Kousseff BG, Newkirk P, Root AW. Brachmann-de Lange syndrome. 1994 update. Arch Pediatr Adolesc Med. 1994;148:749–55. doi: 10.1001/archpedi.1994.02170070087016. [DOI] [PubMed] [Google Scholar]
- 55.Krantz ID, McCallum J, DeScipio C, Kaur M, Gillis LA, et al. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat Genet. 2004;36:631–35. doi: 10.1038/ng1364. Identification of NIPBL as CdLS gene. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krantz ID, Tonkin E, Smith M, Devoto M, Bottani A, et al. Exclusion of linkage to the CDL1 gene region on chromosome 3q26.3 in some familial cases of Cornelia de Lange syndrome. Am J Med Genet. 2001;101:120–29. [PMC free article] [PubMed] [Google Scholar]
- 57.Kueng S, Hegemann B, Peters BH, Lipp JJ, Schleiffer A, et al. Wapl controls the dynamic association of cohesin with chromatin. Cell. 2006;127:955–67. doi: 10.1016/j.cell.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 58.Lechner MS, Schultz DC, Negorev D, Maul GG, Rauscher FJ., 3rd The mammalian heterochromatin protein 1 binds diverse nuclear proteins through a common motif that targets the chromoshadow domain. Biochem Biophys Res Commun. 2005;331:929–37. doi: 10.1016/j.bbrc.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 59.Lengronne A, Katou Y, Mori S, Yokobayashi S, Kelly GP, et al. Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature. 2004;430:573–78. doi: 10.1038/nature02742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lettice LA, Heaney SJ, Purdie LA, Li L, de Beer P, et al. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet. 2003;12:1725–35. doi: 10.1093/hmg/ddg180. [DOI] [PubMed] [Google Scholar]
- 61.Losada A. Cohesin regulation: fashionable ways to wear a ring. Chromosoma. 2007;116:321–29. doi: 10.1007/s00412-007-0104-x. [DOI] [PubMed] [Google Scholar]
- 62.Losada A, Hirano M, Hirano T. Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev. 1998;12:1986–97. doi: 10.1101/gad.12.13.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McKenney RR, Elder FF, Garcia J, Northrup H. Brachmann-de Lange syndrome: autosomal dominant inheritance and male-to-male transmission. Am J Med Genet. 1996;66:449–52. doi: 10.1002/(SICI)1096-8628(19961230)66:4<449::AID-AJMG13>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 64.Meinecke P, Hayek H. Brief historical note on the Brachmann-de Lange syndrome: a patient closely resembling the case described by Brachmann in 1916. Am J Med Genet. 1990;35:449–50. doi: 10.1002/ajmg.1320350328. [DOI] [PubMed] [Google Scholar]
- 65.Meins M, Hagh JK, Gerresheim F, Einhoff E, Olschewski H, et al. Novel case of dup(3q) syndrome due to a de novo interstitial duplication 3q24–q26.31 with minimal overlap to the dup(3q) critical region. Am J Med Genet A. 2005;132:84–89. doi: 10.1002/ajmg.a.30384. [DOI] [PubMed] [Google Scholar]
- 66.Michaelis C, Ciosk R, Nasmyth K. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell. 1997;91:35–45. doi: 10.1016/s0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- 67.Misulovin Z, Schwartz YB, Li XY, Kahn TG, Gause M, et al. Association of cohesin and Nipped-B with transcriptionally active regions of the Drosophila melanogaster genome. Chromosoma. 2007;117:89–102. doi: 10.1007/s00412-007-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moldovan GL, Pfander B, Jentsch S. PCNA controls establishment of sister chromatid cohesion during S phase. Mol Cell. 2006;23:723–32. doi: 10.1016/j.molcel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 69.Musio A, Selicorni A, Focarelli ML, Gervasini C, Milani D, et al. X-linked Cornelia de Lange syndrome owing to SMC1L1 mutations. Nat Genet. 2006;38:528–30. doi: 10.1038/ng1779. Identification of SMC1A as a CdLS gene. [DOI] [PubMed] [Google Scholar]
- 70.Nakajima M, Kumada K, Hatakeyama K, Noda T, Peters JM, Hirota T. The complete removal of cohesin from chromosome arms depends on separase. J Cell Sci. 2007;120:4188–96. doi: 10.1242/jcs.011528. [DOI] [PubMed] [Google Scholar]
- 71.Nasmyth K, Haering CH. The structure and function of SMC and kleisin complexes. Annu Rev Biochem. 2005;74:595–648. doi: 10.1146/annurev.biochem.74.082803.133219. Athorough review of cohesin biology. [DOI] [PubMed] [Google Scholar]
- 72.Oostra RJ, Baljet B, Hennekam RC. Brachmann-de Lange syndrome “avant la lettre. Am J Med Genet. 1994;52:267–68. doi: 10.1002/ajmg.1320520303. [DOI] [PubMed] [Google Scholar]
- 73.Opitz JM. The Brachmann-de Lange syndrome. Am J Med Genet. 1985;22:89–102. doi: 10.1002/ajmg.1320220110. [DOI] [PubMed] [Google Scholar]
- 74.Ozkinay F, Cogulu O, Gunduz C, Levent E, Ozkinay C. A case of Brachman de Lange syndrome with cerebellar vermis hypoplasia. Clin Dysmorphol. 1998;7:303–5. doi: 10.1097/00019605-199810000-00013. [DOI] [PubMed] [Google Scholar]
- 75.Panizza S, Tanaka T, Hochwagen A, Eisenhaber F, Nasmyth K. Pds5 cooperates with cohesin in maintaining sister chromatid cohesion. Curr Biol. 2000;10:1557–64. doi: 10.1016/s0960-9822(00)00854-x. [DOI] [PubMed] [Google Scholar]
- 76.Partridge JF, Scott KS, Bannister AJ, Kouzarides T, Allshire RC. Cis-acting DNA from fission yeast centromeres mediates histone H3 methylation and recruitment of silencing factors and cohesin to an ectopic site. Curr Biol. 2002;12:1652–60. doi: 10.1016/s0960-9822(02)01177-6. [DOI] [PubMed] [Google Scholar]
- 77.Ritchie K, Seah C, Moulin J, Isaac C, Dick F, Bérubé NG. Loss of ATRX leads to chromosome cohesion and congression defects. J Cell Biol. 2008;180:315–24. doi: 10.1083/jcb.200706083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rizzu P, Haddad BR, Vallcorba I, Alonso A, Ferro MT, et al. Delineation of a duplication map of chromosome 3q: a new case confirms the exclusion of 3q25–q26.2 from the duplication 3q syndrome critical region. Am J Med Genet. 1997;68:428–32. [PubMed] [Google Scholar]
- 79.Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, et al. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet. 1996;14:357–60. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- 80.Rollins RA, Korom M, Aulner N, Martens A, Dorsett D. Drosophila nipped-B protein supports sister chromatid cohesion and opposes the stromalin/Scc3 cohesion factor to facilitate long-range activation of the cut gene. Mol Cell Biol. 2004;24:3100–11. doi: 10.1128/MCB.24.8.3100-3111.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rollins RA, Morcillo P, Dorsett D. Nipped-B, a Drosophila homologue of chromosomal adherins, participates in activation by remote enhancers in the cut and Ultrabithorax genes. Genetics. 1999;152:577–93. doi: 10.1093/genetics/152.2.577. Identification of Nipped-B and its role in long-range enhancer promoter interactions in Drosophila. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Russell KL, Ming JE, Patel K, Jukofsky L, Magnusson M, Krantz ID. Dominant paternal transmission of Cornelia de Lange syndrome: a new case and review of 25 previously reported familial recurrences. Am J Med Genet. 2001;104:267–76. doi: 10.1002/ajmg.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saul RA, Rogers RC, Phelan MC, Stevenson RE. Brachmann-de Lange syndrome: diagnostic difficulties posed by the mild phenotype. Am J Med Genet. 1993;47:999–1002. doi: 10.1002/ajmg.1320470712. [DOI] [PubMed] [Google Scholar]
- 84.Schoumans J, Wincent J, Barbaro M, Djureinovic T, Maguire P, et al. Comprehensive mutational analysis of a cohort of Swedish Cornelia de Lange syndrome patients. Eur J Hum Genet. 2007;15:143–49. doi: 10.1038/sj.ejhg.5201737. [DOI] [PubMed] [Google Scholar]
- 85.Schule B, Oviedo A, Johnston K, Pai S, Francke U. Inactivating mutations in ESCO2 cause SC phocomelia and Roberts syndrome: no phenotype-genotype correlation. Am J Hum Genet. 2005;77:1117–28. doi: 10.1086/498695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schulz S, Gerloff C, Ledig S, Langer D, Volleth M, et al. Prenatal diagnosis of Roberts syndrome and detection of an ESCO2 frameshift mutation in a Pakistani family. Prenat Diagn. 2008;28:42–45. doi: 10.1002/pd.1904. [DOI] [PubMed] [Google Scholar]
- 87.Sciorra LJ, Bahng K, Lee ML. Trisomy in the distal end of the long arm of chromosome 3. A condition clinically similar to the Cornelia de Lange syndrome. Am J Dis Child. 1979;133:727–30. doi: 10.1001/archpedi.1979.02130070063013. [DOI] [PubMed] [Google Scholar]
- 88.Seitan VC, Banks P, Laval S, Majid NA, Dorsett D, et al. Metazoan Scc4 homologs link sister chromatid cohesion to cell and axon migration guidance. PLoS Biol. 2006;4:e242. doi: 10.1371/journal.pbio.0040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Selicorni A, Lalatta F, Livini E, Briscioli V, Piguzzi T, et al. Variability of the Brachmann-de Lange syndrome. Am J Med Genet. 1993;47:977–82. doi: 10.1002/ajmg.1320470708. [DOI] [PubMed] [Google Scholar]
- 90.Selicorni A, Russo S, Gervasini C, Castronovo P, Milani D, et al. Clinical score of 62 Italian patients with Cornelia de Lange syndrome and correlations with the presence and type of NIPBL mutation. Clin Genet. 2007;72:98–108. doi: 10.1111/j.1399-0004.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- 91.Skibbens RV. Unzipped and loaded: the role of DNA helicases and RFC clamp-loading complexes in sister chromatid cohesion. J Cell Biol. 2005;169:841–46. doi: 10.1083/jcb.200503129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Skibbens RV, Corson LB, Koshland D, Hieter P. Ctf7p is essential for sister chromatid cohesion and links mitotic chromosome structure to the DNA replication machinery. Genes Dev. 1999;13:307–19. doi: 10.1101/gad.13.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith M, Herrell S, Lusher M, Lako L, Simpson C, et al. Genomic organisation of the human chordin gene and mutation screening of candidate Cornelia de Lange syndrome genes. Hum Genet. 1999;105:104–11. doi: 10.1007/s004399900068. [DOI] [PubMed] [Google Scholar]
- 94.Spitz F, Gonzalez F, Duboule D. A global control region defines a chromosomal regulatory landscape containing the HoxD cluster. Cell. 2003;113:405–17. doi: 10.1016/s0092-8674(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 95.Strachan T. Cornelia de Lange Syndrome and the link between chromosomal function, DNA repair and developmental gene regulation. Curr Opin Genet Dev. 2005;15:258–64. doi: 10.1016/j.gde.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 96.Sullivan M, Higuchi T, Katis VL, Uhlmann F. Cdc14 phosphatase induces rDNA condensation and resolves cohesin-independent cohesion during budding yeast anaphase. Cell. 2004;117:471–82. doi: 10.1016/s0092-8674(04)00415-5. [DOI] [PubMed] [Google Scholar]
- 97.Sumara I, Vorlaufer E, Gieffers C, Peters BH, Peters JM. Characterization of vertebrate cohesin complexes and their regulation in prophase. J Cell Biol. 2000;151:749–62. doi: 10.1083/jcb.151.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tonkin ET, Smith M, Eichhorn P, Jones S, Imamwerdi B, et al. A giant novel gene undergoing extensive alternative splicing is severed by a Cornelia de Lange-associated translocation breakpoint at 3q26.3. Hum Genet. 2004;115:139–48. doi: 10.1007/s00439-004-1134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tonkin ET, Wang TJ, Lisgo S, Bamshad MJ, Strachan T. NIPBL, encoding a homolog of fungal Scc2-type sister chromatid cohesion proteins and fly Nipped-B, is mutated in Cornelia de Lange syndrome. Nat Genet. 2004;36:636–41. doi: 10.1038/ng1363. An independent study that also identified NIPBL as the CdLS gene. [DOI] [PubMed] [Google Scholar]
- 100.Uhlmann F, Nasmyth K. Cohesion between sister chromatids must be established during DNA replication. Curr Biol. 1998;8:1095–101. doi: 10.1016/s0960-9822(98)70463-4. [DOI] [PubMed] [Google Scholar]
- 101.Van Allen MI, Filippi G, Siegel-Bartelt J, Yong SL, McGillivray B, et al. Clinical variability within Brachmann-de Lange syndrome: a proposed classification system. Am J Med Genet. 1993;47:947–58. doi: 10.1002/ajmg.1320470704. [DOI] [PubMed] [Google Scholar]
- 102.Van Den Berg DJ, Francke U. Roberts syndrome: a review of 100 cases and a new rating system for severity. Am J Med Genet. 1993;47:1104–23. doi: 10.1002/ajmg.1320470735. [DOI] [PubMed] [Google Scholar]
- 103.Vega H, Waisfisz Q, Gordillo M, Sakai N, Yanagihara I, et al. Roberts syndrome i s caused by mutations in ESCO2, a human homolog of yeast ECO1 that is essential for the establishment of sister chromatid cohesion. Nat Genet. 2005;37:468–70. doi: 10.1038/ng1548. Identification of ESCO2 as the Roberts-SC phocomelia gene. [DOI] [PubMed] [Google Scholar]
- 104.Velagaleti GV, Bien-Willner GA, Northup JK, Lockhart LH, Hawkins JC, et al. Position effects due to chromosome breakpoints that map approximately 900 kb upstream and approximately 1.3 Mb downstream of SOX9 in two patients with campomelic dysplasia. Am J Hum Genet. 2005;76:652–62. doi: 10.1086/429252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vrouwe MG, Elghalbzouri-Maghrani E, Meijers M, Schouten P, Godthelp BC, et al. Increased DNA damage sensitivity of Cornelia de Lange syndrome cells: evidence for impaired recombinational repair. Hum Mol Genet. 2007;16:1478–87. doi: 10.1093/hmg/ddm098. [DOI] [PubMed] [Google Scholar]
- 106.Waizenegger IC, Hauf S, Meinke A, Peters JM. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell. 2000;103:399–410. doi: 10.1016/s0092-8674(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 107.Watanabe Y, Kitajima TS. Shugoshin protects cohesin complexes at centromeres. Philos Trans R Soc London Ser B. 2005;360:515–21. doi: 10.1098/rstb.2004.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Williams BC, Garrett-Engele CM, Li Z, Williams EV, Rosenman ED, Goldberg ML. Two putative acetyltransferases, san and deco, are required for establishing sister chromatid cohesion in Drosophila. Curr Biol. 2003;13:2025–36. doi: 10.1016/j.cub.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 109.Wilson GN, Hieber VC, Schmickel RD. The association of chromosome 3 duplication and the Cornelia de Lange syndrome. J Pediatr. 1978;93:783–88. doi: 10.1016/s0022-3476(78)81077-4. [DOI] [PubMed] [Google Scholar]
- 110.Wilson WG, Kennaugh JM, Kugler JP, Wyandt HE. Reciprocal translocation 14q;21q in a patient with the Brachmann-de Lange syndrome. J Med Genet. 1983;20:469–71. doi: 10.1136/jmg.20.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yan J, Saifi GM, Wierzba TH, Withers M, Bien-Willner GA, et al. Mutational and genotype-phenotype correlation analyses in 28 Polish patients with Cornelia de Lange syndrome. Am J Med Genet A. 2006;140:1531–41. doi: 10.1002/ajmg.a.31305. [DOI] [PubMed] [Google Scholar]
- 112.Zhang B, Jain S, Song H, Fu M, Heuckeroth RO, et al. Mice lacking sister chromatid cohesion protein PDS5B exhibit developmental abnormalities reminiscent of Cornelia de Lange syndrome. Development. 2007;134:3191–201. doi: 10.1242/dev.005884. [DOI] [PubMed] [Google Scholar]