Abstract
OBJECTIVE
To examine the psychometric properties (test-retest reliability, concurrent validity, construct validity) of the Balance Rehabilitation Unit (BRU) during testing of sensory integration processes in healthy adults and individuals with vestibular disorders.
DESIGN
Experimental cross-sectional design.
SETTING
Vestibular disorders clinic.
PARTICIPANTS
Participants (N= 90); 30 subjects with vestibular disorders (18 to 85 years); 30 young healthy adults (18 to 50 years); and 30 older healthy adults (60 to 85 years).
INTERVENTIONS
Not applicable.
MAIN OUTCOME MEASURES: Participants were tested twice with the BRU and once with the Smart Equitest Sensory Organization Test (SOT)
The Center of Pressure (COP) was recorded in the anteroposterior (COPap) and mediolateral (COPml) directions. The COPap and COPml time series were used to estimate the COP area andvelocity.
RESULTS
The intra-class correlation coefficient (ICC) of the BRU COP area and velocity measures for all subjects were at least 0.76 in all sensory organization conditions (p<.001). Significant correlations were found between the BRU and SOT, ranging from 0.64 to 0.81 for COP area and from 0.44 to 0.76 for COP velocity. The older control group had significantly greater COP area and velocity compared with the younger controls for BRU and SOT. COP (area, velocity) was significantly higher for the younger vestibular group compared with the younger controls.
CONCLUSIONS
The reliability and validity of COP measurements obtained during testing of sensory integration processes were demonstrated using the BRU. Future work should examine the responsiveness of these measures when individuals with balance disorders participate in rehabilitation.
Keywords: Posturography, sensory organization, posture
Introduction
Individuals with vestibular disorders and older adults have a higher risk for falling compared with younger, healthy adults.1, 2 Many risk factors for falls are associated with decreased sensory function, including reduced visual, vestibular, and somatosensory function.3–6 Measuring the effect of sensory feedback on postural control during standing has been investigated using numerous clinical and experimental methods. Shumway-Cook and Horak (1986) described the use of a sensory conflict dome and foam to provide a head-fixed visual environment and inaccurate somatosensory feedback and called the test paradigm the Clinical Test of Sensory Integration on Balance (CTSIB).7 The foam provides destabilization in conditions 4, 5, and 6; the dome provides a head-fixed visual reference in conditions 3 and 6. Methods of testing the sensory control of balance have also been developed using computerized dynamic posturography such as the Equitest™ (Neurocom, Inc) Sensory Organization Test (SOT), which utilizes a tilting floor and moving walls to provide inaccurate somatosensory and visual sway-referenced feedback during standing. Tilting of the walls and floor provides sensory destabilization in the sagittal plane resulting in increased sway in the antero-posterior (AP) direction. The SOT has been used in many clinical and experimental investigations of balance control.8–11
The difference between the use of a foam pad versus the use of a tilting floor to provide inaccurate somatosensory feedback has been studied. El Kashlan et al. (1998) found that the total time spent in all stance conditions of the CTSIB was significantly correlated with the summed equilibrium scores of the SOT conditions.12 However, correlations for individual conditions were not reported. Allum et al (2002) compared the amount of sway associated with standing on a foam pad with eyes open and closed, with standing on a platform tilting in the anteroposterior (AP) or mediolateral (ML) direction with eyes open and closed.13 Greater trunk sway in the AP direction was induced by the Equitest platform when subjects had their eyes closed with AP sway-referencing compared with standing on foam. On the contrary, less trunk sway velocity in the ML direction was observed with AP sway-referencing compared with foam. Postural responses have greater AP and ML symmetry when using foam compared with the AP sway-referencing provided by the Equitest platform.
Virtual reality and gaming technologies are being used for balance assessment and rehabilitation.14–16 It is likely that these systems can be used for the evaluation of an individual’s use of sensory feedback for balance control. Rather than using a visual conflict dome for assessing the influence of visual feedback on balance, head mounted displays (HMD) can be used to provide head-fixed visual stimuli.17, 18 The Balance Rehabilitation Unit™ (BRU, Medicaa Balance for Life, Montevideo - Uruguay) is one such system that utilizes a head mounted display and foam in order to test the sensory contributions to balance.19–21 However, before systems such as this are used clinically, a thorough evaluation of the reliability and validity of their measurements should be conducted. The aims of this study include: 1) to examine the test-retest reliability of the BRU in young healthy, older healthy, and individuals with vestibular disorders, 2) to examine the concurrent validity of the BRU compared with the SOT, and 3) to examine the construct known groups validity by investigating the effects of age and disease on balance performance using the BRU.
Methods
Design
The study is an experimental, cross-sectional design. Each subject was tested twice using the BRU and one time using the SOT during the same visit. Subjects were given a 15 minute break after each test with the BRU and SOT. To minimize the “order effect” bias, the order of testing of the two systems was changed with every other subject (BRU 1- BRU2 - SOT, SOT – BRU 1 – BRU 2). For both systems, every subject performed three trials of six conditions, each lasting for 20 seconds.
Subjects
The study protocol was approved by the University of Pittsburgh Institutional Review Board and all subjects provided informed consent. The study included 90 subjects; 30 subjects with vestibular disorders between the ages of 18 to 85 years with diagnoses confirmed by a neurotologist; 30 young healthy controls between the ages of 18 to 50; and 30 older healthy controls between the ages of 60 to 85. The control subjects were recruited through local advertisements and from previous balance research studies. The sample size estimate was based on an effect size (Cohen’s d) of 0.75 between older and younger subjects from previously collected posturography data.22
Exclusion criteria for all subjects included known pregnancy and the use of assistive devices for standing. For the healthy control subjects, exclusion criteria included symptoms of inner ear disorders such as complaints of dizziness, vertigo, or balance problems. Healthy controls were screened before testing to confirm that subjects did not have vestibular disorders. Exclusion criteria for controls included the following: 1) observation of spontaneous nystagmus, 2) abnormal ocular motor function, 3) a positive Dix-Hallpike or roll test for benign paroxysmal positional vertigo, or 4) a positive horizontal head shake test or head thrust test.
Vestibular disorder diagnosis and vestibular laboratory test results (ocular motor testing, positional testing, caloric testing, rotational chair testing, and cervical vestibular evoked myogenic potentials (VEMPs)) were retrieved from the patients’ medical records. The demographic and clinical information of all subjects including age, gender, duration of symptoms, location of dysfunction, and laboratory tests for patients are presented in Table 1.
Table 1.
Characteristics of all subjects including age, gender, duration of symptoms, location of dysfunction, and laboratory test results for patients [ocular motor testing, positional testing, caloric testing, rotational chair testing, and vestibular-evoked myogenic potentials (VEMPs)] Subject groups include: Young Control (YC), Older Control (OC), Young Vestibular (YV), Older Vestibular (OV) (n = 90).
| YC | OC | YV | OV | |
|---|---|---|---|---|
|
| ||||
| N | 30 | 30 | 15 | 15 |
|
| ||||
| Age (years) | ||||
| Mean ± SD | 28 ± 6 | 77.2 ± 5 | 40 ± 11 | 66 ± 8 |
| Range | 19–45 | 68–85 | 25–55 | 56–81 |
|
| ||||
| Gender (male; %) | 17; 56% | 12; 40% | 5; 33% | 5; 33% |
|
| ||||
| Duration of symptoms (months) | N/A | N/A | ||
| Mean ± SD | 43 ± 78 | 31 ± 43 | ||
| Median | 7.5 | 12 | ||
| Range | 1–240 | 1–132 | ||
|
| ||||
| Location of dysfunction (n; %) | N/A | N/A | ||
| Peripheral | 7 (47%) | 9 (60%) | ||
| Central | 5 (33%) | 4 (27%) | ||
| Mixed | 3 (20%) | 2 (13%) | ||
|
| ||||
| Abnormal laboratory testing (n;%) | N/A | N/A | ||
| Ocular motor (abnormal timing of saccades) | 0 (0%) | 1 (7%) | ||
| Positional (slow component velocity > 6 deg/sec) | 5 (33%) | 7 (47%) | ||
| Caloric testing (reduced vestibular response > 24%) | 7 (47%) | 8 (53%) | ||
| Rotational testing (decreased gain or asymmetry) | 10 (67%) | 8 (53%) | ||
| Cervical VEMPs (asymmetry) | 5 (33%) | 8 (53%) | ||
N/A: Not applicable (control subjects)
Assessment systems
The BRU consists of a force platform, a head mounted display (HMD), an overhead safety harness, and a foam cushion. The BRU measures the area of a 95% confidence ellipse of the center of pressure (COP) excursion in the AP and ML directions and average velocity of the COP during six different conditions that assess the same sensory integration abilities as the SOT and CTSIB, including: Condition 1: standing on a firm surface with eyes open, without using the HMD; Condition 2: standing on a firm surface with eyes closed; Condition 3: standing on a firm surface viewing a stationary visual scene (basketball gym) displayed in the HMD; Condition 4: standing on foam with eyes open without using the HMD; Condition 5: standing on foam with eyes closed; and Condition 6: standing on foam viewing a stationary visual scene displayed in the HMD. In a similar fashion to the SOT conditions, in conditions 3 and 6 the head-fixed visual environment moves with the subject, and thus provides visual sway referencing. In conditions 4, 5, and 6, the foam surface distorts the normal reference to ground sensed by lower extremity somatosensation.
The Smart Equitest (Neurocom, Inc, in USA) consists of a movable platform that is surrounded on three sides by a visual enclosure that can rotate in the sagittal plane about the ankle. Testing conditions included: 1) standing on a firm surface with eyes open, looking forward, 2) standing on a firm surface with eyes closed, 3) standing on a firm surface with a sway-referenced visual surround, looking forward, 4) standing on a sway-referenced platform that pivots about the ankles with eyes open, looking forward, 5) standing on a sway-referenced platform with eyes closed, and 6) standing on a sway-referenced platform and sway-referenced visual surround, looking forward.
Outcome measures
The COP represents the location of the net vertical ground reaction force vector, and reflects the torque needed to control the movement of the center of mass.23 The COP was recorded in the anteroposterior (COPap) and mediolateral (COPml) directions at a sampling frequency of 50 Hz. The COPap and COPml time series were used to estimate the COP area and velocity.
Area was estimated by computing a 95 % confidence ellipse of the distribution of COPap and COPml coordinates. The statistical definition of this ellipse is that 95% of the points on the COP path would be enclosed inside the ellipse. The following standard formula was used to calculate the area24:
where F is the Fisher distribution at 95%, σ2ap is the variance of COP data in the AP direction, σ2ml is the variance of COP data in the ML direction, and σ2ap,ml is the covariance of COP data in the AP and ML directions. For the sample of 1000 data points (20 s * 50 Hz), F is equal to 3.
Velocity was computed using the following:
where T is the duration of the trial.
Statistical analysis
To investigate relative test-retest reliability, the intra-class correlation coefficient (ICC) was used for the entire group of 90 subjects and within each study group; the ICC considers both correlation and agreement and provides a single index to describe reliability.25 The ICC considers the differences between observed scores that are due to variations of the measurement system including factors related to the testing environment and subject conditions. The purpose and design of this study involved the use of the same rater representing the only raters of interest, with no intention of generalizing findings beyond the raters involved. The rater was considered a fixed factor and not randomly selected in this design. Consequently, ICC model (3, 1) was used to describe the level of agreement between test and retest. The average of 3 trials/condition was used. The COP Area and Velocity values were transformed using the natural logarithm to satisfy the assumption of a normal distribution of between-trial error.
For absolute reliability, the average mean difference between trials with confidence limits, the standard error of measurment (SEM), and the Bland and Altman method were used to describe the extent to which a balance score varied on test-retest measurements.27–33
Secondary analysis included evaluation of standard error of measurement proportion (SEM %), minimal detectable change (MDC95), and minimal detectable change proportion (MDC %), and were calculated using the following formulas:
The SEM is the standard deviation of measurement errors; the smaller the SEM, the smaller the deviation of measurement errors around the true mean and the more reliable the measure.34 SEM% also provides information about measurement error, and smaller SEM% means lower measurement error. MDC95 is a clinically useful measure for absolute reliability and estimates the true change versus the error change.34 It indicates how much change must occur in a measure with a given degree of random error (the SEM) and with 95% certainty, to conclude that change is due to true change, not error change. The MDC% provides information about measurement responsiveness, and the smaller the MDC% the higher the responsiveness.
To examine the concurrent validity of the BRU in the assessment of balance in young healthy, old healthy, and in persons with vestibular disorders, Spearman rank order correlation (rho) was computed between the BRU (time 1) and the SOT measures for each of the six conditions, using the entire subject sample to estimate the concurrent validity. The average of the three trials performed for each condition was used. Nonparametric statistics were used since assumptions of normality were not met. To examine group differences among sensory conditions to investigate age and disease effects, a nonparametric Mann-Whitney U test was used because assumptions of normality were not met (Komolgorov-Smirnov test). The False Discovery Rate method was used to adjust for multiple comparisons.36 This method first divides the family-wise level of significance (α) by the number of comparisons (m). Then the p-values of all the comparisons are rank ordered, and compared with the product of their rank and the adjusted level of significance (α/m).
Results
Reliability
The relative reliability (ICC) and absolute reliability (SEM) of COP Area and COP Velocity measures from the BRU (n= 90) are reported in Table 2. For all subjects, the ICCs for both outcome measures in all sensory organization conditions ranged from 0.76 to 0.90 (p < .001), with greater ICCs for the Velocity measures. Within the groups (YC, OC, and V), ICC varied from 0.61 to 0.93, with greater reliability values in the vestibular group and older controls compared with younger controls. The SEM was small for all groups indicating high reliability for the instrument. The smallest SEMs were found among the young control group. The greatest SEMs occurred for the vestibular group in all measures, and was at least twice as large as the SEMs for the older controls in some conditions.
Table 2.
Intraclass correlation coefficient (ICC) and standard error of the measurement (SEM) of COP Area and Velocity (Vel) measures from the Balance Rehabilitation Unit (BRU). Both Area and Velocity are measured for all 6 sensory conditions (C1 to C6) in the BRU. ICC and SEM are calculated for all subjects (ALL), young controls (YC), older controls (OC), and individuals with vestibular disorders (V). See text for explanation of sensory conditions.
| Sensory Condition | ICC* | SEM | ||||||
|---|---|---|---|---|---|---|---|---|
| ALL | YC | OC | V | ALL | YC | OC | V | |
| Area - C1 | 0.76 | 0.61 | 0.70 | 0.77 | 0.8 | 0.5 | 0.8 | 1.0 |
| Area - C2 | 0.84 | 0.74 | 0.72 | 0.83 | 2.2 | 0.4 | 1.2 | 3.8 |
| Area - C3 | 0.81 | 0.76 | 0.76 | 0.78 | 1.7 | 0.9 | 0.9 | 2.6 |
| Area - C4 | 0.86 | 0.78 | 0.85 | 0.84 | 1.8 | 0.5 | 1.6 | 2.6 |
| Area - C5 | 0.84 | 0.73 | 0.82 | 0.83 | 5.0 | 1.9 | 3.3 | 7.7 |
| Area - C6 | 0.85 | 0.76 | 0.78 | 0.87 | 6.4 | 1.6 | 3.5 | 5.3 |
| Vel - C1 | 0.85 | 0.67 | 0.82 | 0.85 | 0.3 | 0.1 | 0.2 | 0.4 |
| Vel - C2 | 0.88 | 0.62 | 0.91 | 0.79 | 0.6 | 0.1 | 0.4 | 1.0 |
| Vel - C3** | 0.87 | 0.78 | 0.86 | 0.83 | 0.5 | 0.1 | 0.2 | 0.8 |
| Vel - C4 | 0.89 | 0.78 | 0.78 | 0.89 | 0.4 | 0.1 | 0.4 | 0.6 |
| Vel - C5 | 0.89 | 0.77 | 0.87 | 0.86 | 0.6 | 0.4 | 0.4 | 1.0 |
| Vel - C6 | 0.90 | 0.86 | 0.73 | 0.93 | 0.4 | 0.2 | 0.5 | 0.5 |
Area and Velocity values were transformed to natural logarithm to satisfy the assumption of a normal distribution of between-trial error.
Failed to demonstrate normal distribution of error values after transformation.
Figure 1 (A–D) presents the Bland-Altman plots of intertrial difference versus mean values for the COP Area and Velocity for conditions 5 and 6 in healthy older and younger controls and subjects with vestibular disease. Intertrial variation is observed to increase with mean COP measure on each sensory condition. According to criteria described by Bland and Altman (1986), intertrial agreement is acceptable when 95% of the values are within 2 SD of the mean intertrial difference.33 According to this criterion, nearly all conditions show acceptable intertrial variation, with a minimum proportion of 93% of COP Area and Velocity values within 2 SD of the mean intertrial difference across all sensory conditions.
Figure 1.

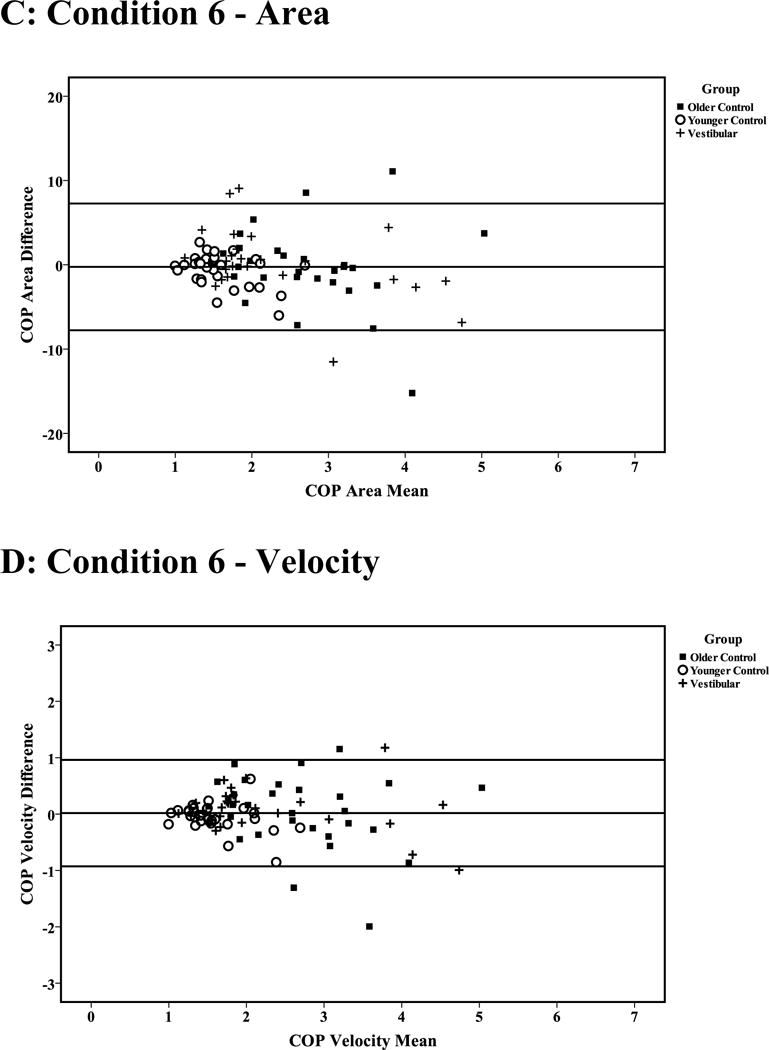
Bland-Altman plots of the intertrial difference versus mean COP area (cm2) and velocity (cm/s) during sensory conditions 5 (A and B) and 6 (C and D) for older and younger control subjects and individuals with vestibular disease. Horizontal lines indicate mean intertrial differences ± 2 SD.
The standard error of measurement proportion (SEM %), minimal detectable change (MDC95), and minimal detectable change proportion (MDC %) for both COP Area and Velocity are detailed in Table 3. The magnitude of measurement error associated with COP Area was at least 20% higher than the magnitude of measurement error associated with COP Velocity across the six sensory control conditions. Minimal detectable change values for COP Area revealed the highest MDC scores for the vestibular group, then older controls, then younger controls in all sensory conditions. Conditions 5 and 6 show the highest MDC for all groups, and Condition 1 shows the smallest MDC for all groups. The MDC% for COP velocity was smaller than the MDC% for COP Area for all conditions.
Table 3.
Standard error of measurement proportion (SEM %), minimal detectable change (MDC95), and minimal detectable change proportion (MDC%) for both COP Area and Velocity (Vel) measures for all 6 Balance Rehabilitation Unit (BRU) sensory conditions (C1 to C6). See text for explanation of sensory conditions.
| Sensory Condition | SEM% | MDC95 | MDC% | |||
|---|---|---|---|---|---|---|
| All | All | YC | OC | V | All | |
| Area - C1 | 56 | 2.2 | 1.2 | 2.2 | 2.9 | 157 |
| Area - C2 | 83 | 6.2 | 1.0 | 3.3 | 10.4 | 230 |
| Area - C3 | 70 | 4.6 | 2.5 | 3.0 | 7.2 | 193 |
| Area - C4 | 45 | 4.9 | 1.5 | 4.5 | 7.2 | 125 |
| Area - C5 | 48 | 13.9 | 5.4 | 9.3 | 21.5 | 133 |
| Area - C6 | 51 | 10.7 | 4.5 | 9.8 | 14.8 | 141 |
| Vel - C1 | 27 | 0.7 | 0.3 | 0.6 | 1.1 | 76 |
| Vel - C2 | 43 | 1.7 | 0.4 | 1.1 | 2.7 | 120 |
| Vel - C3 | 37 | 1.3 | 0.4 | 0.5 | 2.2 | 103 |
| Vel - C4 | 26 | 1.1 | 0.4 | 1.1 | 1.7 | 71 |
| Vel - C5 | 23 | 1.7 | 1.1 | 1.2 | 2.8 | 64 |
| Vel - C6 | 18 | 1.1 | 0.5 | 1.4 | 1.4 | 49 |
Concurrent validity
All 90 subjects were tested with the SOT on the same day as the BRU. Significant correlations (p<.001) were found between the BRU and the SOT in COP Area (Spearman’s rho ranged from 0.64 to 0.81), and COP Velocity (Spearman’s rho ranged from 0.44 to 0.76) (Table 4).
Table 4.
Concurrent validity (Spearman rank order correlation) of the Balance Rehabilitation Unit (BRU) with the Sensory Organization Test (SOT) in the assessment of balance in young healthy, old healthy, and in persons with vestibular disorders. See text for explanation of sensory conditions.
| C1 | C2 | C3 | C4 | C5 | C6 | p (for all 6 conditions) | |
|---|---|---|---|---|---|---|---|
| Area | 0.67 | 0.81 | 0.73 | 0.64 | 0.62 | 0.71 | p < .001 |
| Velocity | 0.47 | 0.67 | 0.56 | 0.69 | 0.76 | 0.78 | p < .001 |
Construct known groups validity
The older control group had significantly greater COP Area and Velocity compared with the younger control group in all conditions for both BRU and SOT (Table 5). To investigate the effect of vestibular disease on the COP measures, the younger control group was compared with the young vestibular group. For the BRU, the COP Area and Velocity for younger vestibular subjects was significantly higher than for the younger controls. For the SOT, the COP Area for younger vestibular subjects was significantly greater than for younger controls in all conditions except C1; also the COP Velocity was significantly greater for younger vestibular compared with younger controls in conditions 4, 5 and 6. No differences in COP Area or Velocity between older vestibular and older controls were observed, except for a lower COP Velocity in older vestibular subjects compared with older controls during conditions 1 and 2 of the SOT.
Table 5.
Mean (SD) COP Area and Velocity (Vel.) values during sensory conditions C1–C6 of both the Balance Rehabilitation Unit (BRU) and Sensory Organization Test (SOT). Subject groups were young controls (YC) (n= 30), older controls (OC) (n= 30), young vestibular (YV) (n= 15), and older vestibular (OV) (n= 15). See text for explanation of sensory conditions.
| Group | C1 | C2 | C3 | C4 | C5 | C6 | ||
|---|---|---|---|---|---|---|---|---|
| BRU | Area | YV | 2.5 (0.7)b | 5.8 (1.9)b | 3.9 (1.0)b | 6.6 (1.7)b | 17.5 (4.3)b | 16.0 (6.3)b |
| YC | 0.7 (0.1) | 1.0 (0.1) | 0.8 (0.1) | 2.3 (0.3) | 5.6 (0.6) | 3.9 (0.4) | ||
| OC | 1.4 (0.3)a | 2.1 (0.4)a | 1.7 (0.3)a | 4.4 (0.5)a | 10.3 (1.2)a | 7.7 (1.0)a | ||
| OV | 1.5 (0.3) | 3.2 (0.7) | 2.5 (0.6) | 4.5 (1.0) | 13.2 (2.8) | 8.9 (2.1) | ||
|
| ||||||||
| BRU | Vel. | YV | 1.3 (0.3)b | 2.0 (0.4)b | 1.7 (0.4)b | 2.0 (0.3)b | 3.4 (0.4)b | 2.8 (0.5)b |
| YC | 0.7 (0.03) | 0.9 (0.04) | 0.8 (0.04) | 1.2 (0.1) | 2.0 (0.1) | 1.6 (0.1) | ||
| OC | 1.1 (0.1)a | 1.6 (0.1)a | 1.4 (0.1)a | 2.1 (0.1)a | 3.4 (0.2)a | 2.7 (0.2)a | ||
| OV | 1.0 (0.1) | 1.5 (0.2) | 1.3 (0.1) | 1.9 (0.2) | 3.2 (0.4) | 2.6 (0.3) | ||
|
| ||||||||
| SOT | Area | YV | 1.3 (0.3) | 3.8 (1.2)b | 4.8 (1.7)b | 15.4 (2.9)b | 34.6 (8.1)b | 25.5 (3.8)b |
| YC | 0.7 (0.1) | 1.0 (0.1) | 1.1 (0.1) | 4.5 (0.6) | 10.2 (1.1) | 8.4 (0.9) | ||
| OC | 1.2 (0.1)a | 1.8 (0.2)a | 1.9 (0.3)a | 7.9 (0.8)a | 18.5 (1.5)a | 16.0 (1.4)a | ||
| OV | 1.1 (0.3) | 1.7 (0.3) | 2.7 (0.7) | 13.6 (2.9) | 29.0 (5.9) | 22.3 (4.5) | ||
|
| ||||||||
| SOT | Vel. | YV | 1.5 (0.1) | 2.1 (0.3) | 2.4 (0.5) | 3.7 (0.5)b | 6.5 (0.9)b | 5.8 (0.8)b |
| YC | 1.5 (0.04) | 1.6 (0.04) | 1.7 (0.04) | 2.4 (0.1) | 4.1 (0.2) | 3.3 (0.1) | ||
| OC | 1.7 (0.1)a | 2.2 (0.1)a | 2.2 (0.1)a | 4.0 (0.2)a | 6.5 (0.3)a | 6.0 (0.3)a | ||
| OV | 1.4 (0.1)c | 1.8 (0.1)c | 2.0 (0.2) | 4.0 (0.4) | 6.7 (0.8) | 5.4 (0.6) | ||
Significant difference between YC and OC using the False Discovery Rate method.36
Significant difference between YC and YV using the False Discovery Rate method.
Significant difference between OC and OV using the False Discovery Rate method.
Discussion
The current study investigated if a system that uses foam and a head mounted display provides a reliable and valid measure of sensory organization abilities for healthy persons and people with vestibular disorders. The BRU had reliability values greater than 0.76 in all of the COP measures for the six sensory conditions. Significant positive correlations were found between the BRU and SOT in all of the outcome measures, indicating concurrent validity with another standard clinical test of sensory organization. Furthermore, known groups validity was established by finding differences in the sway measures that depended on the age and health of the vestibular system.
We established the reliability of the BRU among healthy subjects and patients with vestibular disorders for different age groups. For all subjects, the relative reliability of the Area measure ranged between 0.76 to 0.86, and the reliability of the Velocity measures ranged from 0.85 to 0.90. With respect to specific subject groups, the relative reliability of the measures for the young control subjects ranged from 0.61 to 0.86, which compare favorably with the test-retest reliability of the SOT among young healthy subjects (range 0.35 to 0.79).37 Among older controls, there is much overlap in reliability between the current investigation of the BRU (range 0.70 to 0.91) and previous examinations of the SOT (range 0.73 to 0.94) The slightly greater reliability in older adults is probably due to greater score variance.38 The group with vestibular disorders demonstrated similar ICCs as the older control group.
The estimate of absolute reliability as indicated by the SEM was largest for the vestibular group followed by the older healthy group and smallest for the young healthy group. A smaller SEM indicates a more reliable measure. Within-subject variability is expected to be higher among patients and older healthy groups compared with the young healthy group. Another interpretation for SEM differences among groups can be inferred from the Bland and Altman plot that shows that most of the subjects had intertrial differences that depended on the magnitude of COP Area and Velocity (Figure 1).
The SEM was substantially higher during conditions 5 and 6 for all subgroups. Conditions 5 and 6 involve absent/inaccurate sensory information from both visual and/or somatosensory systems important for balance sensory control. Conditions 5 and 6 are the most difficult conditions in the test. Consequently, the amount of variability would be expected to be higher during these conditions.
Concurrent validity
Significant correlations were found between the BRU and the SOT measures for all study groups, indicating that both systems examine similar sensory organization constructs during standing. Relatively lower correlation values may be explained by smaller inter-subject variance (e.g. in condition 1) or differences in the way that the sensory modality was altered (e.g. foam vs. sway referenced platform in condition 4). Importantly, measurements of postural control for both the BRU and SOT were derived from the center of pressure obtained from force platform recordings using identical analysis routines. Although the psychometric properties of the SOT including the clinical efficacy, validity, and reliability were established using primarily equilibrium scores, these scores are derived from COP measures that are highly correlated with the COP area and velocity.8–10, 39 Consequently, by demonstrating concurrent validity of the BRU with the SOT using the COP-based measures, it is likely that the BRU would have similar clinical validity and efficacy. Likewise, the association between the BRU and SOT support the use of foam and HMD-based technologies as a clinical tool in the management of patients with balance disorders.
Construct known groups validity
The final objective was to examine group differences to investigate if the BRU test measurements discriminated between known effects of age and vestibular disease on balance. Significant differences in COP measures between young and older healthy subjects were found in both the SOT and the BRU suggesting that these measures captured the well-described physiological manifestations of aging on the balance system. For example, aging is associated with declines in the somatosensory, visual, and vestibular systems. Vibratory sensation, tactile sensation, and pressure sensation has been shown to be impaired with aging, especially in the lower extremities.40, 41 Rosenhall et al. reported that the vestibular system loses around 40% of the nerve and hair cells in people over the age of 60.42 Our study findings were consistent with previous studies that show that in conditions where visual or somatosensation information was inaccurate for orientation in space, COP measures were greater in older control groups compared with younger control groups.43, 44
To investigate the effect of vestibular disease during standing balance, we compared younger individuals with vestibular disease and younger control subjects (age 55 years and younger). The significant difference between the two groups suggests that the BRU tests are able to unmask the changes that have occurred to the vestibular system for subjects 55 and younger. On average, the younger subjects with vestibular disease were older than the younger control subjects. Although it is possible that this age difference may contribute to the differences in sway magnitude between the individuals with vestibular disease and controls, it is unlikely that it is a major effect. For one, sway magnitude does not vary greatly across adults less than 55 years of age (Peterka and Black, 1990). Secondly, the sway magnitude for younger adults with vestibular disease is greater than the magnitude measured in older controls, suggesting that indeed, vestibular disease was the primary factor.
For most conditions in the BRU and SOT, there was no significant difference in the COP measures between older controls and older persons with vestibular disease. The only significant difference between the older adult groups was for the COP velocity during SOT conditions 1 and 2. This result is consistent with the findings of Sparto et al., who found no difference in the amount of head sway associated with visual optic flow stimulation between older healthy controls and older individuals with unilateral vestibular loss.45 It is possible that the greater variability in sway within the populations of older controls and older individuals with vestibular disease resulted in overlapping distributions that are not statistically different.
One previous study investigated the efficacy of using the BRU to discriminate between patients with relapsing-remitting multiple sclerosis (MS) with peripheral vestibular disorders and healthy older adults.46 The MS group had significantly greater COP Area and Velocity from the control group in all conditions and the BRU was able to discriminate between the MS group and the control group.46
A minor limitation of the subject enrollment was the larger number of male subjects in the young control group compared with the other groups. However, for younger adult subjects, there usually is not a significant gender difference in the magnitude of sway. In addition, it is possible that performance of the balance tests within the same day may lead to inflated reliability values. However, we were concerned that the test performance of individuals with vestibular disease on different days would be adversely affected because of changes in symptoms, and thus lead to artificially lower reliability in this group.
Conclusion
The reliability and validity of center of pressure measurements obtained during testing of sensory integration processes using the BRU was demonstrated. The results demonstrated credence for using testing procedures that include a foam support surface and head mounted display technology to assess the contribution of visual, somatosensory, and vestibular feedback for balance. Future work should examine the responsiveness of these measures when individuals with balance disorders participate in rehabilitation.
List of abrevations
- BRU
Balance Rehabilitation Unit
- SOT
Sensory Organization Test
- COP
Center of Pressure
- ICC
Interclass Correlation Coefficient
References
- 1.CDC. 2006 – 2010, United States Unintentional Fall Nonfatal Injuries and Rates per 100,000. 2012 [cited 2012 12/19/2012]. Available from: URL: http://webappa.cdc.gov/sasweb/ncipc/nfirates2001.html.
- 2.Pothula VB, Chew F, Lesser TH, Sharma AK. Falls and vestibular impairment. Clin Otolaryngol Allied Sci. 2004;29(2):179–82. doi: 10.1111/j.0307-7772.2004.00785.x. [DOI] [PubMed] [Google Scholar]
- 3.Herdman SJ, Blatt P, Schubert MC, Tusa RJ. Falls in patients with vestibular deficits. Am J Otol. 2000;21(6):847–51. [PubMed] [Google Scholar]
- 4.Lord SR, Clark RD, Webster IW. Visual acuity and contrast sensitivity in relation to falls in an elderly population. Age & Ageing. 1991;20(3):175–81. doi: 10.1093/ageing/20.3.175. [DOI] [PubMed] [Google Scholar]
- 5.Lord SR, Ward JA, Williams P, Anstey KJ. Physiological factors associated with falls in older community-dwelling women. Journal of the American Geriatrics Society. 1994;42(10):1110–7. doi: 10.1111/j.1532-5415.1994.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 6.Koski K, Luukinen H, Laippala P, Kivela SL. Risk factors for major injurious falls among the home-dwelling elderly by functional abilities. A prospective population-based study. Gerontology. 1998;44(4):232–8. doi: 10.1159/000022017. [DOI] [PubMed] [Google Scholar]
- 7.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction on balance. Physical Therapy. 1986;66:1548–50. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 8.Badke MB, Shea TA, Miedaner JA, Grove CR. Outcomes after rehabilitation for adults with balance dysfunction. Arch Phys Med Rehabil. 2004;85(2):227–33. doi: 10.1016/j.apmr.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Ford-Smith CD, Wyman JF, Elswick RKJ, Fernandez T, Newton RA. Test-retest reliability of the sensory organization test in noninstitutionalized older adults. Archives of Physical Medicine & Rehabilitation. 1995;76(1):77–81. doi: 10.1016/s0003-9993(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 10.Perez N, Santandreu E, Benitez J, Rey-Martinez J. Improvement of postural control in patients with peripheral vestibulopathy. Eur Arch Otorhinolaryngol. 2006;263(5):414–20. doi: 10.1007/s00405-005-1027-x. [DOI] [PubMed] [Google Scholar]
- 11.Peterka RJ, Black FO. Age-related changes in human posture control: sensory organization tests. Journal of Vestibular Research. 1990;1(1):73–85. [PubMed] [Google Scholar]
- 12.El-Kashlan HK, Shepard NT, Asher AM, Smith-Wheelock M, Telian SA. Evaluation of clinical measures of equilibrium. Laryngoscope. 1998;108(3):311–9. doi: 10.1097/00005537-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Allum JH, Zamani F, Adkin AL, Ernst A. Differences between trunk sway characteristics on a foam support surface and on the Equitest ankle-sway-referenced support surface. Gait Posture. 2002;16(3):264–70. doi: 10.1016/s0966-6362(02)00011-5. [DOI] [PubMed] [Google Scholar]
- 14.Sparrer I, Duong Dinh TA, Ilgner J, Westhofen M. Vestibular rehabilitation using the Nintendo® Wii Balance Board - a user-friendly alternative for central nervous compensation. Acta Otolaryngologica. 2013;133(3):239–45. doi: 10.3109/00016489.2012.732707. [DOI] [PubMed] [Google Scholar]
- 15.Meldrum D, Glennon A, Herdman S, Murray D, McConn-Walsh R. Virtual reality rehabilitation of balance: assessment of the usability of the Nintendo Wii() Fit Plus. Disability & Rehabilitation Assistive Technology. 2012;7(3):205–10. doi: 10.3109/17483107.2011.616922. [DOI] [PubMed] [Google Scholar]
- 16.Clark RA, Bryant AL, Pua Y, McCrory P, Bennell K, Hunt M. Validity and reliability of the Nintendo Wii Balance Board for assessment of standing balance. Gait & Posture. 2010;31(3):307–10. doi: 10.1016/j.gaitpost.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Nyberg L, Lundin-Olsson L, Sondell B, Backman A, Holmlund K, Eriksson S, et al. Using a virtual reality system to study balance and walking in a virtual outdoor environment: a pilot study. Cyberpsychology & Behavior. 2006;9(4):388–95. doi: 10.1089/cpb.2006.9.388. [DOI] [PubMed] [Google Scholar]
- 18.Lott A, Bisson E, Lajoie Y, McComas J, Sveistrup H. The effect of two types of virtual reality on voluntary center of pressure displacement. Cyberpsychology & Behavior. 2003;6(5):477–85. doi: 10.1089/109493103769710505. [DOI] [PubMed] [Google Scholar]
- 19.Nogueira S, Ferreira E, Geisinger D, San Roman C, Suarez H. Model of postural control system applied in Parkinson’s disease patients. Conf Proc IEEE Eng Med Biol Soc. 2010:5452–5. doi: 10.1109/IEMBS.2010.5626509. [DOI] [PubMed] [Google Scholar]
- 20.Suarez H, Geisinger D, Ferreira ED, Nogueira S, Arocena S, Roman CS, et al. Balance in Parkinson’s disease patients changing the visual input. Braz J Otorhinolaryngol. 2011;77(5):651–5. doi: 10.1590/S1808-86942011000500019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suarez H, Suarez A, Lavinsky L. Postural adaptation in elderly patients with instability and risk of falling after balance training using a virtual-reality system. Int Tinnitus J. 2006;12(1):41–4. [PubMed] [Google Scholar]
- 22.Whitney SL, Roche JL, Marchetti GF, Lin CC, Steed DP, Furman GR, et al. A comparison of accelerometry and center of pressure measures during computerized dynamic posturography: a measure of balance. Gait & Posture. 2011;33(4):594–9. doi: 10.1016/j.gaitpost.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. Journal of Neurophysiology. 1996;75(6):2334–43. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]
- 24.Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM. Measures of postural steadiness: Differences between healthy young and elderly adults. IEEE Transactions on Biomedical Engineering. 1996;43:956–66. doi: 10.1109/10.532130. [DOI] [PubMed] [Google Scholar]
- 25.Shrout P, Fleiss JL. Intraclass correlation: uses in assessing rater reliability. Psychological Bulletin. 1979;86(2):420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 26.Fleiss JL. The design and analysis of clinical experiments. New York: Wiley and Son; 1986. [Google Scholar]
- 27.Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26(4):217–38. doi: 10.2165/00007256-199826040-00002. [DOI] [PubMed] [Google Scholar]
- 28.Baumgarter TA. Norm-referenced measurement: Reliability. In: Safrit MJ, Wood TM, editors. Norm-referenced measurement: Reliability. Champaign, IL: Human Kinetics; 1989. pp. 45–72. [Google Scholar]
- 29.Hopkins WG. Measures of reliability in sports medicine and science. Sports Med. 2000;30(1):1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 30.Liaw LJ, Hsieh CL, Lo SK, Chen HM, Lee S, Lin JH. The relative and absolute reliability of two balance performance measures in chronic stroke patients. Disabil Rehabil. 2008;30(9):656–61. doi: 10.1080/09638280701400698. [DOI] [PubMed] [Google Scholar]
- 31.Rankin G, Stokes M. Reliability of assessment tools in rehabilitation: an illustration of appropriate statistical analyses. Clin Rehabil. 1998;12(3):187–99. doi: 10.1191/026921598672178340. [DOI] [PubMed] [Google Scholar]
- 32.Tyson SF. Measurement error in functional balance and mobility tests for people with stroke: what are the sources of error and what is the best way to minimize error? Neurorehabil Neural Repair. 2007;21(1):46–50. doi: 10.1177/1545968306290662. [DOI] [PubMed] [Google Scholar]
- 33.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 34.Haley SM, Fragala-Pinkham MA. Interpreting change scores of tests and measures used in physical therapy. Phys Ther. 2006;86(5):735–43. [PubMed] [Google Scholar]
- 35.Portney LG, Watkins MP. Correlation. In: Cohen M, editor. Foundations of clinical research: application to practice. New Jersey: Pearson education; 2009. pp. 523–37. [Google Scholar]
- 36.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 37.Wrisley DM, Stephens MJ, Mosley S, Wojnowski A, Duffy J, Burkard R. Learning effects of repetitive administrations of the sensory organization test in healthy young adults. Arch Phys Med Rehabil. 2007;88(8):1049–54. doi: 10.1016/j.apmr.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Wigglesworth JK, Dayhoff NE, Suhrheinrich J. The reliability of our measures of postural control using the Smart Balance Master. J American Coll Sports Med. 1996;29 [Google Scholar]
- 39.Black FO. Clincial status of computerized dynamic posturography in neurotology Current opinion in otolaryngology and head and neck surgery. 2001;9:314–8. [Google Scholar]
- 40.Kenshalo DR. Aging effects on cutaneous and kinesthetic sensibilities. In: Hann SS, Coons DH, editors. Special senses in aging. Ann Arbor: University of Michigan; 1979. [Google Scholar]
- 41.Whanger AD, Wang HS. Clinical correlates of the vibratory sense in elderly psychiatric patients. J Gerontol. 1974;29(1):39–45. doi: 10.1093/geronj/29.1.39. [DOI] [PubMed] [Google Scholar]
- 42.Rosenhall U, Rubin W. Degenerative changes in the human vestibular sensory epithelia. Acta Otolaryngol. 1975;79(1–2):67–80. doi: 10.3109/00016487509124657. [DOI] [PubMed] [Google Scholar]
- 43.Sundermier L, Woollacott MH, Jensen JL, Moore S. Postural sensitivity to visual flow in aging adults with and without balance problems. J Gerontol A Biol Sci Med Sci. 1996;51(2):M45–52. doi: 10.1093/gerona/51a.2.m45. [DOI] [PubMed] [Google Scholar]
- 44.Wade MG, Lindquist R, Taylor JR, Treat-Jacobson D. Optical flow, spatial orientation, and the control of posture in the elderly. J Gerontol B Psychol Sci Soc Sci. 1995;50(1):51–8. doi: 10.1093/geronb/50b.1.p51. [DOI] [PubMed] [Google Scholar]
- 45.Sparto PJ, Furman JM, Redfern MS. Head sway response to optic flow: effect of age is more important than the presence of unilateral vestibular hypofunction. J Vestib Res. 2006;16(3):137–45. [PMC free article] [PubMed] [Google Scholar]
- 46.Kessler N, Gananca MM, Gananca CF, Gananca FF, Lopes SC, Serra AP, et al. Balance Rehabilitation Unit (BRU) posturography in relapsing-remitting multiple sclerosis. Arq Neuropsiquiatr. 2011;69(3):485–90. doi: 10.1590/s0004-282x2011000400015. [DOI] [PubMed] [Google Scholar]


