Abstract
Background
HIV antiretroviral therapy (ART) is being rapidly scaled up in Sub-Saharan Africa, including recently to patients with CD4+ T-cell counts >350 cells/uL. However, concerns persist about adherence and virologic suppression among these asymptomatic, high CD4+ count individuals.
Objective
To determine the virologic efficacy and safety of ART among asymptomatic HIV-positive Ugandan adults with high CD4+ counts ≥350 cells/uL via a streamlined model of care.
Design
Prospective non-randomized clinical study (EARLI Study: clinicaltrials.gov NCT#01479634).
Setting
Prototypic rural Ugandan HIV clinic.
Subjects/Participants
N=197 asymptomatic ART-naïve adults (age>18) with CD4+ ≥350, without pregnancy or WHO stage 3/4 illness.
Interventions
ART included tenofovir/emtricitabine/efavirenz, with ritonavir/lopinavir substitution for efavirenz available. Streamlined ART model included nurse-driven visits with physician backup, basic safety laboratory monitoring with HIV viral load (VL), clinician telephone contact, and defaulter tracking. No incentives were provided.
Outcomes
Undetectable VL (≤400 copies/mL) at 24 and 48 weeks (intention-to-treat [ITT]; missing=detectable), self-reported ART adherence, retention in care, and laboratory/clinical ART toxicities.
Results
Of 197 patients with CD4>350, median CD4 was 569 (IQR 451-716). Undetectable VL was achieved in 189/197 (95.9%, ITT) and 189/195 (96.9%, ITT) of participants at weeks 24 and 48, respectively. Self-reported adherence was 98% and 192/197 (97%) of patients were retained at week 48. Laboratory adverse events and hospitalizations were rare.
Conclusions
We demonstrate high virologic suppression, retention, and safety among asymptomatic individuals with CD4>350 in a prototypic Ugandan clinic. Our results challenge current concerns that high CD4+ count individuals lack motivation for ART, and may not achieve sustained virologic suppression.
Keywords: Antiretroviral therapy, high CD4+ count, viral load testing, streamlined care, ART scale-up, task-shifting
Introduction
HIV antiretroviral therapy (ART) access is being rapidly scaled up worldwide according to 2013 WHO guidelines [1, 2], with over 9.7 million individuals now receiving ART [3]. However, in Sub-Saharan Africa, ART is only recently being offered to patients with high CD4+ T cell counts (e.g., ≥350 cells/uL) [4]. The 2013 WHO guidelines on ART now recommend expanding ART eligibility to individuals with CD4+ counts 350-500/uL, as well as to several special focus groups regardless of CD4+ count including pregnant and breastfeeding women and individuals with HIV-negative (serodiscordant) sexual partners [1].
As countries adapt WHO recommendations into their existing treatment frameworks, one concern is that high CD4+ count individuals—who are healthier, often asymptomatic, and more frequently engaged in the workforce [5]— will have lower ART adherence, retention in care, and viral suppression. In a group of South African patients eligible for ART initiation, “feeling healthy”’ was associated with declination of therapy [6]. In a southwestern Ugandan clinic, virologic suppression following ART initiation was 80% in individuals with CD4+ 250-350, and 90% in individuals with CD4+ <250 [7], suggesting lower adherence in the higher CD4+ count patients.
Within Malawi's national “Option B+” program—ART initiation for pregnant women regardless of CD4+ count—a recent study reported higher rates of loss to follow-up of Option B+ pregnant women compared to non-pregnant women initiating ART due to having CD4+ <350 [8]. Additionally, in a qualitative study of serodiscordant couples enrolled in a pre-exposure prophylaxis (PrEP) trial, many HIV-positive index individuals expressed negativity towards ART due to a perception that it portended imminent mortality [9].
However, other data on specialized populations of high CD4+ count patients with CD4+ >350 present a distinctly different picture. HIV/TB co-infected patients with CD4+ >350 had 86% virologic suppression at 6 months in a Ugandan trial of early vs. delayed ART [10]. Furthermore, in sero-discordant couples in the HPTN 052 study, 886 HIV-positive individuals with CD4+ counts of 350-550 had >89% virologic suppression throughout the median 2.1 years of follow-up [11, 12].
Against this backdrop of emerging data, to increase understanding of ART outcomes in asymptomatic high CD4+ count adults, we investigated ART efficacy and safety within a streamlined care model in a prototypic rural Ugandan HIV clinic.
Methods
Research/Ethics Approvals
The study was approved by institutional review boards at Makerere University, Kampala, Uganda, the University of California, San Francisco, the Uganda National Council for Science and Technology, and the Ugandan National Drug Authority. All participants provided written informed consent for participation.
Study Design and Setting
The Early Antiretroviral Therapy in Resource Limited Settings in Patients with High CD4+ Counts Study (EARLI), is a prospective, two-arm, non-randomized study of open-label ART, evaluating the safety and efficacy of ART administration under a streamlined care delivery system. Participants were HIV-infected individuals with high CD4+ cell counts (CD4+ ≥250) in Mbarara District, Uganda. The study was conducted at Bwizibwera Health Center (a level-IV government clinic 25km from Mbarara town providing HIV care via the Makerere University Joint AIDS Program, a PEPFAR-sponsored implementing partner). This study is registered with ClinicalTrials.gov, #NCT01479634.
Participant Screening and Enrollment
Three methods were used for initial screening of participants. First, a database of registered clinic patients was searched for individuals meeting four criteria: (1) age ≥18, (2) not receiving ART, (3) latest CD4+ count ≥250 performed <6 months prior, and (4) residence <30km from the health center. Clinic charts were reviewed to verify ART-naïve status. Staff then invited qualifying patients by phone for screening. Second, HIV-positive individuals attending clinic visits during the screening period were invited for screening if they met the above criteria. Lastly, individuals referred to Bwizibwera Health Center after diagnosis with HIV during a community-wide HIV testing campaign in May 2011 [13] were also invited to screen if they met criteria as above.
Further inclusion criteria were (1) ability to give informed consent, (2) documentation of HIV-1 infection in the medical chart or community health campaign results, and (3) ability to swallow medications. Exclusion criteria were (1) intent to relocate >30km from the clinic during the subsequent three years, (2) receipt of ART for >7 days, except during pregnancy or for occupational exposure, (3) an active WHO stage 3 or 4 illness on physical examination, and (4) pregnancy by self-report or examination.
Eligible participants then underwent secondary laboratory screening; female participants also had a urine beta-HCG test for pregnancy. Laboratory exclusion criteria were: (1) CD4<250, (2) absolute neutrophil count ≤500 cells/uL, (3) hemoglobin ≤7.0 g/dL, (4) platelets≤50,000/uL, (5) ALT ≥5× upper normal range value, (6) eGFR≤60 mL/min by the Modification of Diet in Renal Disease (MDRD) formula, (7) positive Cepheid Xpert MTB/RIF assay result, and (8) positive urine pregnancy test. If eligibility criteria were met, consent for study participation was undertaken.
Antiretroviral Therapy
In participants with CD4+ >350, ART consisted of Truvada® (co-formulated tenofovir [TDF] 300mg/emtricitabine [FTC] 200mg, one tablet daily administered as a fixed dose combination) with efavirenz [EFV] 600mg (one tablet daily). Participants could initiate Aluvia (co-formulated ritonavir [RTV] 50mg/lopinavir [LPV] 200mg, two tablets twice per day administered as a fixed dose combination) instead of EFV if they had concerns about becoming pregnant while on EFV. For suspected toxicity related to Truvada, drug substitutions with another approved ART medication or medications were allowed at the discretion of study clinicians. TDF was discontinued if eGFR≤50 mL/min. Participants could continue FTC and/or EFV if desired, and could restart TDF or Truvada, at clinicians' discretion. For suspected Aluvia (RTV/LPV) toxicity, alternate ART medications were offered at clinicians' discretion. Participants with CD4+ 250-350 received standard first-line ART in line with 2009 Uganda national guidelines [14].
Streamlined Care
In our streamlined care model, nurses conducted rapid-throughput routine visits unless symptom screening indicated that physician evaluation was needed. Baseline, week 24 and week 48 viral load (VL) results were discussed with patients. Participants were asked if they had questions about their VL results, and were given encouragement when VL was undetectable. Phlebotomy was done on site, and 1-month ART refills (for visits at weeks 0, 4, and 8) or 3-month ART refills (for visits at weeks 12, 24, 36, and 48) were provided at an on-site pharmacy window. Participants were informed at enrollment that they could access the clinic medical officer by telephone or text messaging, and could present to the clinic outside of the regular visit schedule for any urgent care complaints. Participants were tracked if they missed visits. No incentives were provided for study participation.
Streamlined Care Assessments
Participants were assessed at week 0 (enrollment) and week 4 by a medical officer, and at weeks 8, 12, 24, 36 and 48 by a nurse. Upon enrollment, participants completed a multiple-choice questionnaire regarding their motivations for participation and expectations about the impact of ART.
Screening for toxicities and adverse events
At all visits, clinic staff screened participants for clinical symptoms or problems. At enrollment and week 4 visits, the medical officer evaluated patients for adverse events. During nurse visits (weeks 8-48), positive responses triggered conversion of the visit to the medical officer who evaluated participant for an adverse event. All grade 3 and 4 adverse events, as well as serious adverse events (as defined by the National Institutes of Health, Division of AIDS [DAIDS] scale) were tabulated and reported to review boards.
Adherence
At all visits, a 3-day recall test was performed, asking participants how many medication doses they had taken 1, 2, and 3 days prior. Visits where all ART medications were reported taken on all three days were considered adherent. Visits with any missed dose reported were considered non-adherent.
Laboratory evaluations
Sodium, potassium, chloride, bicarbonate and glucose were measured at enrollment and week 48. Blood urea nitrogen and creatinine were assessed at weeks 8 and 48. Complete blood count and liver function tests (AST, ALT, total and direct bilirubin, and alkaline phosphatase) were assessed at weeks 12 and 48. Viral load was assessed at enrollment and weeks 24 and 48. CD4+ count was assessed at enrollment and week 24.
Pregnancy testing
Female participants were asked each visit if they might have become pregnant since their prior visit. Positive responses triggered urine pregnancy testing. The date of last menses was also sought; participants reporting menses ≥6 weeks prior also underwent urine pregnancy testing.
Retention in care and participant tracking
Upon completing each visit, participants were given a target return date for their next visit. Visits were considered made “on schedule” if they occurred from 7 days prior to 7 days after the target date. If a participant failed to appear on the target date, study staff attempted to contact them via telephone. If a participant did not appear by one week after the target visit date, the visit was considered “missed”, and the tracker attempted to visit the participant at his/her residence to encourage them to return to clinic. The medical officer handled all visits following a missed visit. Participants were withdrawn from the study if they missed 3 consecutive visits, and transitioned to routine care at the health center without ART interruption.
Outcome Measures
The primary outcome was the proportion of participants with an undetectable plasma HIV-1 RNA level (VL ≤400 copies/mL) at 48 weeks. Secondary outcomes were: (1) proportion of participants with undetectable viral load at 24 weeks, (2) retention in care at 24 and 48 weeks, (3) self-reported rates of adherence at each study visit, and (4) prevalence of grade 3/4 toxicities and adverse events occurring during the first 48 weeks of ART.
Streamlined Care Measurements: Medical Officer Utilization
To assess the need for medical officer evaluations outside of the standard visit schedule (i.e., enrollment and week 4), we tabulated visits including: (1) visits converted by the nurse to the medical officer due to a suspected clinical issue or adverse event, (2) unscheduled “drop-in” visits (all of which were seen by medical officer), (3) follow-up visits that were scheduled by the medical officer (e.g., to re-evaluate a laboratory abnormality), (4) visits following a missed visit, or (5) other.
Participant Time and Motion Study
To assess the impact of our streamlined care intervention on study visit duration and waiting times, the lengths of participants' study visits were measured in a time and motion study spanning 3 months, consisting of clinic visit observations from week 8-48. During this period, staff were informed that visits were being timed. A tracker recorded participants' time of entry into the clinic waiting room, and staff noted the start and finish time for the portions of the visit conducted by the nurse, medical officer, phlebotomist, and pharmacist, respectively. Final exit time from the pharmacy window was also recorded. Total entry-to-exit time, as well as the fraction of total time spent waiting were calculated.
Results
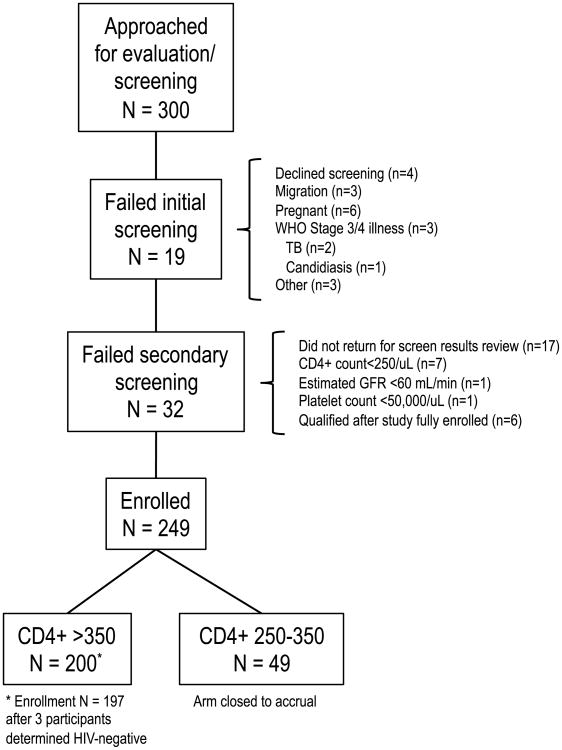
From October 2011—May 2012, 300 patients were screened for EARLI Study entry; 200 individuals enrolled in the CD4+ >350 arm and 49 enrolled in the CD4+ 250-350 arm (Figure 1). Enrolment in the CD4 250-350 arm was affected by the 2011 change in Uganda ART initiation guidelines from a CD4+ threshold of 250 to 350 [15]. Many patients with CD4+ counts 250-350 had ART initiated through standard clinic operations and were not available for recruitment; this study arm was thus closed to accrual, having insufficient follow-up for analyses.
Fig. 1. Paricipant Screening and Enrollment Characteristics.
Of 300 individuals approached and evaluated for screening and study participation, 249 enrolled: 200 in the CD4+ >350/uL arm, and 49 in the CD4+ 250-350/uL arm prior to close of accrual. TB, tuberculosis; GFR, glomerular filtration rate.
Reasons for Study Enrollment and ART Initiation
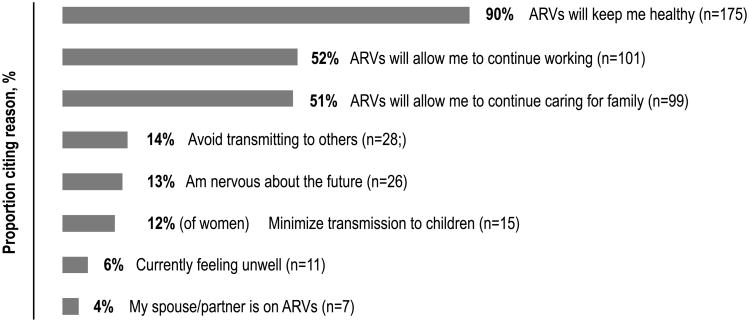
At enrollment, top reasons for participation cited by participants with CD4+ >350/uL included preserving individual health and work productivity, as well as minimizing HIV transmission to others (Figure 2). A small fraction reported being motivated by current illness.
Fig. 2. Reasons for Study Participation and ART Start Among Participants With CD4>350 (n=195).
Motivations for enrolling in study and initiating ART are displayed for 195 individuals with CD4+ T cell count >350/uL surveyed upon study entry. Participants could indicate one or more motivations.
Demographics, Baseline Clinical Characteristics, and Antiretroviral Therapy Regimens
Among participants with CD4+ >350, 65% were female, median age was 35 years (IQR, 29-41), and agriculture was the predominant occupation, consistent with previously reported demographics in southwestern Uganda (Table 1) [16, 17]. CD4+ counts were high: the median was 564, and 25% of participants had CD4+ >712. Median baseline VL was 22,400 copies/mL. In the CD4+ >350 arm, all participants initiated TDF/FTC + EFV, except for three participants who initiated TDF/FTC + RTV/LPV.
Table 1. Demographic and Baseline Characteristics.
| Variable | CD4+ >350/uL Participants (n=197) |
|---|---|
| Age, median (IQR) | 36 (29-41) |
| Sex, % female | 64.5% |
| CD4, median (IQR), cells/uL | 569 (451-716) |
| HIV RNA, median (IQR), c/mL | 23,200 (4,170-88,194) |
|
| |
| Occupation | |
| Agriculture - animal or farm | 136 |
| Trader / Shop worker | 16 |
| Business owner | 15 |
| Domestic / House work (maid, etc.) | 8 |
| Construction worker | 4 |
| Restaurant/bar worker | 3 |
| Other | 15a |
IQR, interquartile range.
carpenter/metal worker (n=4), driver/transportation (n=3), mechanic, civil servant (n=2 each), teacher, factory worker, nurse (n=1 each), N/A (n=1)
Retention in Care
Of 200 participants originally enrolled in the CD4+ >350 arm, 3 were withdrawn after baseline viral load testing showed an undetectable plasma RNA level, and subsequent testing confirmed HIV-negative serostatus. After 24 weeks, 194/197 participants remained in the study (98%). Two patients died between week 24-48; thus overall retention at week 48 was 192/197 (97%) (Table 2).
Table 2. Antiretroviral Treatment Outcomes in Participants with CD4+ >350/uL.
| Parameter | Study Visit | Outcome |
|---|---|---|
| Virologic Efficacy | Week 24 | 189/197 (96.4%, ITT) |
| Week 48 | 189/195 (96.9%, ITT) | |
|
| ||
| ART Adherence | 4 | 94.4% (186/197) |
| 8 | 97.8% (182/186) | |
| 12 | 99.4% (183/184) | |
| 24 | 98.9% (188/190) | |
| 36 | 98.3% (176/179) | |
| 48 | 98.9% (178/180) | |
| Total | 97.9% (1093/1116) | |
|
| ||
| Retention in Care | Week 24 | 194/197 (98%) |
| Week 48 | 192/197 (97%) | |
|
| ||
| Adverse Events | Grade 3 or 4 laboratory adverse event | 22a |
| Elevated creatinine | 2 | |
| Hospitalization | 8b (4%) | |
| Death | 2c | |
ITT, Intention to treat: considered missing viral load values detectable.
events occurred in 18 participants of 197 total. Events included neutropenia (n=11), creatinine elevation (n=2), thrombocytopenia (n=2), hyponatremia (n=2), and ALT elevation (n=1).
causes included: community acquired pneumonia (n=3), sepsis/renal failure, tuberculosis, altered mental status, malaria, and gastric carcinoma (n=1 each).
causes included : post-operative complications following cholecystectomy (n=1) and inoperable gastric carcinoma (n=1).
Virologic Efficacy of ART
In the CD4+ >350 arm, 189/197 (95.9%) of participants achieved an undetectable HIV-1 plasma RNA level at Week 24 by intention-to-treat analysis [ITT] that treated missing values as detectable. At week 48, 189/195 participants were undetectable (96.9%, ITT; Table 2).
ART Adherence
Self-reported adherence was very high by three-day recall interview: in the CD4+ >350 arm, participants reported taking ART on all three days prior at 97.9% of study visits (Table 2).
Adverse Events
The overall rate of adverse laboratory events, as well as hospitalizations and deaths was low (Table 2). Among CD4+ >350 patients, a total of 22 grade 3 or grade 4 laboratory adverse events occurred across 18 participants. The most common was asymptomatic neutropenia (n=11). Creatinine elevations occurred in two participants, both of whom interrupted Truvada but subsequently resumed it after creatinine normalization. Hospitalizations were rare, occurring in 4% (8/200) of participants overall. The most common reason was community-acquired pneumonia. Two patients died before week 48: one from post-operative complications following cholecystectomy, and one from inoperable gastric carcinoma. Both participants had an undetectable week 24 VL prior to their deaths.
Streamlined Care Measurements
Non-routine medical officer utilization outside of the standard visit schedule was modest (Table 3). In addition to the 1,743 routine study visits (249 participants × 7 visits), 384 non-routine medical officer visits occurred (average 1.5 visits/participant by week 48). Overall, 51% of these non-routine visits represented unscheduled drop-in visits; the majority were for minor dermatologic conditions, respiratory complaints (e.g., cough or rhinitis), or mild gastrointestinal symptoms (e.g., dyspepsia or diarrhea). Only 24% of non-routine visits were visit conversions from the nurse to the medical officer. Many of these occurred per study protocol, e.g. when pregnancy was suspected. Lastly, 17% of non-routine visits occurred because a participant had missed a prior visit; protocol mandated physician evaluation in such cases.
Table 3. Streamlined Care Outcomes: Physician Utilization and Mean Transit Time for Clinic Visits.
| Physician utilization outside standard visit schedule | |||||||
| Reason for Visit | Converted from RN | Unscheduled | Scheduled by MD | Missed last visit | Other | ||
|
|
|||||||
| No. of visits (% of total non-routine physician visits, N=384) | 92 (24%) | 193 (51%) | 28 (7%) | 65 (17%) | 6 (2%) | ||
|
|
|||||||
| Mean participant transit time during clinic visits | |||||||
| Visit Week | Week 8 | Week 12 | Week 24 | Week 36 | Week 48 | Unscheduled | |
|
|
|||||||
| Visit duration (min) | 35 | 32 | 35 | 25 | 54 | 30 | |
| Waiting time (min) | 15 | 14 | 19 | 13 | 28 | 11 | |
RN, study nurse; MD, study physician.
Transit Time through Clinic
Average start-to-finish visit durations ranged from 25-54 minutes, with most visits being conducted in approximately 30 minutes (Table 3). Average waiting time during visits ranged from 13-28 minutes, and for most visit types represented approximately one-half of the total visit duration.
Discussion
We report here virologic suppression rates exceeding 95%, excellent retention in care, and low toxicity during the first year of a clinical study of asymptomatic patients with CD4+ cell counts >350 cells/uL receiving ART and HIV care via a streamlined care delivery model in a prototypical HIV clinic in rural Uganda. Our results challenge current concerns that high CD4+ count individuals may not desire or adhere well to ART, or be able to achieve robust virologic suppression.
Prior literature has focused on individuals with CD4+ counts <350 [6, 7, 9], since this is the CD4+ threshold that has largely defined ART eligibility since the start of the ART scale-up in Sub-Saharan Africa. In addition, prior studies have focused on potential reasons why healthy asymptomatic patients may decline ART when eligible, or adhere suboptimally after initiating ART. However, it is possible that patient attitudes to ART in previous years were, in part, shaped by clinical messaging to high CD4+ count patients that ART was not indicated and its benefits uncertain.
By contrast, our results from high CD4+ count (>350) patients provide data on current motivations for ART among asymptomatic individuals. Our participants cited a desire to preserve health and productivity, as well as reduce the risk of transmission. These attitudes may reflect a growing knowledge among patients that ART initiation at higher CD4+ can reduce clinical events [12] and dramatically reduce transmission to partners [11]. These factors should continue to be incorporated into health education around HIV care and updated to share accumulating clinical evidence on the benefits of earlier ART.
Several additional factors may have contributed to our strong clinical results. First, the streamlined nurse-driven system of care may have helped patients complete study visits with less disruption to their work and other routines. Strong evidence demonstrates the effectiveness of nurse-driven care models [18-21]; our study expands this evidence base with time and motion data indicating that visits can be effectively shortened to 30 minutes or less while retaining good outcomes. Visit durations in our study were far shorter than mean visit lengths of 183-270 minutes that have been reported in other HIV clinics in Mbarara District [22, 23]; these previously reported longer times may have been partially driven by mandatory counseling and educations sessions offered at patient visits.
Our short study visits were primarily focused on simple screening for ART toxicities and clinical problems. Because toxicities were infrequent in these largely asymptomatic patients, screening was rapid, with participants directly proceeding to phlebotomy and/or to the pharmacy. Streamlined pharmacy visits dispensed a 3-month supply of ARV's rather than a more typical 2-month supply, a strategy that has been investigated previously in Uganda [24, 25]. Patients nonetheless still spent almost 50% of their visit duration waiting, suggesting that further streamlining of visits is possible.
A second likely factor contributing to our strong clinical results was the provision of a clinician mobile phone number to all patients in the study. While prior studies have demonstrated a positive impact of mobile phone reminders on ART adherence [26-28], and on staff members' ability to obtain clinical advice on HIV management from experts [29], the impact of patient access to clinicians via mobile phone is less well explored. The ability of our participants to contact the study clinician allowed for triage of complaints and action-oriented recommendations—for example, to present for an outpatient clinic evaluation, to proceed to the hospital for a more serious problem, or simply to present for the next scheduled visit. With ready telephone access, basic ART questions (e.g., on side effects or dosing schedules), as well as appointment dates, were easily resolved. These may have prevented missed ART doses, and helped to avert missed visits (which can lower the chance of exhaustion of an individual's ART supply). All of these factors may have helped promote eventual virologic suppression. Most importantly, the telephone link may have provided patients with a greater sense of connectedness, mutual respect and trust, fostering both adherence and retention [30].
A third possible explanation for our results was our performance of viral load testing at 24 and 48 weeks, and the in-person review of these results with participants at subsequent clinic visits. Apart from certain national programs, e.g., in South Africa, viral load testing is not currently widely performed, although it is recommended in the 2013 WHO guidelines on ART administration [1]. In our study, participants anecdotally demonstrated strong interest in their numerical viral load results at baseline, and at 24 and 48 weeks. We believe that the ability of staff to demonstrate an undetectable viral load allowed for reassurance about medication efficacy, encouraged patients to remain adherent, and afforded positive reinforcement of self-efficacy—namely, that patient effort was yielding positive results.
Our study has certain limitations. First, we did not randomize high CD4+ count individuals to our streamlined care intervention versus standard clinical care; as such, the precise impact of our streamlined care model is hard to separate from our patients' generally positive health. Second, participants were not selected purely at random from the overall population of high CD4+ count individuals in the clinic or in the geographic region. Some participants were already registered clinic patients; as such, they may have had a higher propensity to be retained in care due to their demonstrated retention in “pre-ART” care. However, we did recruit from amongst these individuals randomly, and simultaneously enrolled participants who were new registrants to the clinic, including newly diagnosed adults identified during mobile HIV testing at a large scale, community-wide health campaign [13]. Thus the overall impact of this phenomenon is unclear. Additionally, we did not specifically recruit participants from marginalized populations (e.g. sex workers, MSM, intravenous drug utilizing individuals); as such, our results may be less generalizable to these groups. Third, we utilized only one measure of self-reported adherence to ART, which is limited in capture of true adherence. Finally, our results encompass one year of observation; longer-term data we are collecting over the next two years will help to confirm the durability of the clinical success we report here.
In summary, we report excellent ART outcomes in Ugandan patients with CD4+ counts >350 cells/uL using a nurse-driven model of care that allows patients to spend a relatively short time conducting clinic visits. Current assumptions and concerns about low adherence and worse outcomes among healthier, higher CD4+ count patients may not fully account for patients' actual motivations and updated knowledge of the health and productivity-preserving benefits of ART, or for improvements in the tolerability of ART medications. Furthermore, measuring viral load and sharing results with patients in real-time (now universally recommended in Sub-Saharan Africa [1]), may add motivation and encouragement—benefits beyond the established HIV monitoring function it clearly fulfills. Further research will be needed to demonstrate the long-term durability of streamlined care models for high CD4+ count populations who are expected to preserve health and high CD4+ counts during long-term therapy.
Acknowledgments
Funding/Support: This work was supported by National Institute of Allergy and Infectious Diseases (NIAID) [National Institutes of Health (NIH)] U01 AI069502 to DH, P30 AI027763 to Paul Volberding, UCSF, and NIH UCSF-CTSI KL2TR000143 to VJ. This study also received material support in the form of donated HIV antiretroviral medications (Truvada) and funding via an investigator-initiated grant to VJ from Gilead Sciences, Inc.
Potential conflicts of interest. VJ receives research grant support from Gilead Sciences, Inc. and the U.S. National Institutes of Health.
Additional Contributions. We are grateful to the EARLI Study participants for their generous donation of time and effort. We appreciate and acknowledge the contributions of the EARLI Study staff, and Makerere University Joint AIDS Program (MJAP) staff members at Bwizibwera HC-IV. We are grateful to the Infectious Disease Research Collaboration (IDRC), Kampala, Uganda for administrative, logistic, and scientific support. V.J. thanks A.M. for feedback and editing.
Footnotes
Author Contributions: V.J. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Jain, Byonanebye, Amanyire, Kamya, Havlir
Acquisition of data: Jain, Byonanebye, Black, Amanyire
Analysis and interpretation of data: Jain, Byonanebye, Amanyire, Black, Kamya, Havlir
Drafting of the manuscript: Jain, Byonanebye, Kamya, Havlir
Critical revision of the manuscript for important intellectual content: all others
Obtained funding: Jain, Charlebois, Kamya, Havlir
Administrative, technical, or material support: Jain, Black, Clark, Amanyire
Study supervision: Jain, Byonanebye, Black, Clark, Amanyire, Kamya, Havlir
Prior Presentation: This work was presented at the 2014 Conference on Retroviruses and Opportunistic Infections (CROI), Boston, MA, March 4, 2014 (Abstract #1048).
References
- 1.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2013:1–272. [PubMed] [Google Scholar]
- 2.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach - 2010 rev. 2010:1–156. [PubMed] [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2013. pp. 1–198. [Google Scholar]
- 4.Joint United Nations Programme on HIV/AIDS. Access to Antiretroviral Therapy in Africa: Status Report on Progress Towards the 2015 Targets. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2013. [Google Scholar]
- 5.Thirumurthy H, Chamie G, Jain V, Kabami J, Kwarisiima D, Clark TD, et al. Improved employment and education outcomes in households of HIV-infected adults with high CD4 cell counts: evidence from a community health campaign in Uganda. AIDS. 2013;27:627–634. doi: 10.1097/QAD.0b013e32835c54d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz IT, Essien T, Marinda ET, Gray GE, Bangsberg DR, Martinson NA, et al. Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS. 2011;25:2177–2181. doi: 10.1097/QAD.0b013e32834b6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adakun SA, Siedner MJ, Muzoora C, Haberer JE, Tsai AC, Hunt PW, et al. Higher baseline CD4 cell count predicts treatment interruptions and persistent viremia in patients initiating ARVs in rural Uganda. J Acq Imm Def Syndr. 2013;62:317–321. doi: 10.1097/QAI.0b013e3182800daf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenthani L, Haas AD, Tweya H, Jahn A, van Oosterhout JJ, Chimbwandira F, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS. 2014;28:589–598. doi: 10.1097/QAD.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curran K, Ngure K, Shell-Duncan B, Vusha S, Mugo NR, Heffron R, et al. ‘If I am given antiretrovirals I will think I am nearing the grave’: Kenyan HIV serodiscordant couples' attitudes regarding early initiation of antiretroviral therapy. AIDS. 2014;28:227–233. doi: 10.1097/QAD.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanteza MW, Mayanja-Kizza H, Charlebois E, Srikantiah P, Lin R, Mupere E, et al. A randomized trial of punctuated antiretroviral therapy in Ugandan HIV-seropositive adults with pulmonary tuberculosis and CD4(+) T-cell counts of >/= 350 cells/muL. J Infect Dis. 2011;204:884–892. doi: 10.1093/infdis/jir503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14:281–290. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamie G, Kwarisiima D, Clark TD, Kabami J, Jain V, Geng E, et al. Leveraging Rapid Community-Based HIV Testing Campaigns for Non-Communicable Diseases in Rural Uganda. PLoS ONE. 2012;7:e43400. doi: 10.1371/journal.pone.0043400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uganda Ministry of Health. National Antiretroviral Treatment Guidelines for Adults, Adolescents, and Children. 3rd. 2009. [Google Scholar]
- 15.Uganda Ministry of Health. Uganda Integrated National Guidelines on Antiretroviral Therapy, Prevention of Mother to Child Transmission of HIV, and Infant & Young Child Feeding. 2012 [Google Scholar]
- 16.Chamie G, Kwarisiima D, Clark TD, Kabami J, Jain V, Geng E, et al. Uptake of community-based HIV testing during a multi-disease health campaign in rural Uganda. PLoS ONE. 2014;9:e84317. doi: 10.1371/journal.pone.0084317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain V, Byonanebye DM, Liegler T, Kwarisiima D, Chamie G, Kabami J, et al. Changes in population HIV RNA levels in Mbarara, Uganda, during scale-up of HIV antiretroviral therapy access. J Acq Imm Def Syndr. 2014;65:327–332. doi: 10.1097/QAI.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fairall L, Bachmann MO, Lombard C, Timmerman V, Uebel K, Zwarenstein M, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet. 2012;380:889–898. doi: 10.1016/S0140-6736(12)60730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rich ML, Miller AC, Niyigena P, Franke MF, Niyonzima JB, Socci A, et al. Excellent clinical outcomes and high retention in care among adults in a community-based HIV treatment program in rural Rwanda. J Acq Imm Def Syndr. 2012;59:e35–42. doi: 10.1097/QAI.0b013e31824476c4. [DOI] [PubMed] [Google Scholar]
- 20.Sanne I, Orrell C, Fox MP, Conradie F, Ive P, Zeinecker J, et al. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): a randomised non-inferiority trial. Lancet. 2010;376:33–40. doi: 10.1016/S0140-6736(10)60894-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shumbusho F, van Griensven J, Lowrance D, Turate I, Weaver MA, Price J, et al. Task shifting for scale-up of HIV care: evaluation of nurse-centered antiretroviral treatment at rural health centers in Rwanda. PLOS Med. 2009;6:e1000163. doi: 10.1371/journal.pmed.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wanyenze RK, Wagner G, Alamo S, Amanyire G, Ouma J, Kwarisima D, et al. Evaluation of the efficiency of patient flow at three HIV clinics in Uganda. AIDS Pat Care STDs. 2010;24:441–446. doi: 10.1089/apc.2009.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amanyire G, Wanyenze R, Alamo S, Kwarisiima D, Sunday P, Sebikaari G, et al. Client and provider perspectives of the efficiency and quality of care in the context of rapid scale-up of antiretroviral therapy. AIDS Pat Care STDs. 2010;24:719–727. doi: 10.1089/apc.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castelnuovo B, Babigumira J, Lamorde M, Muwanga A, Kambugu A, Colebunders R. Improvement of the patient flow in a large urban clinic with high HIV seroprevalence in Kampala, Uganda. Int J STD AIDS. 2009;20:123–124. doi: 10.1258/ijsa.2008.008174. [DOI] [PubMed] [Google Scholar]
- 25.Nakiwogga-Muwanga A, Katabira E, Sempa J, Kambugu A, Nakibuuka-Lubwama E, Lamorde M, et al. A Pharmacy-Only Refill Program at a Large HIV Clinic in Uganda: Experience and Satisfaction of Patients. J Int Assoc Provid AIDS. 2013 doi: 10.1177/2325957413488179. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence M, Mia LvdK, Richard TL, Harsha T, Cristian PE, Chenglin Y, et al. Mobile phone text messages for improving adherence to antiretroviral therapy (ART): an individual patient data meta-analysis of randomised trials. BMJ Open. 2013;3:e003950–e003950. doi: 10.1136/bmjopen-2013-003950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cristian PE, Harsha T, James PH, Joshua GZ, Markus PG, Damien de W, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011;25:825–834. doi: 10.1097/QAD.0b013e32834380c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunutsor S, Walley J, Katabira E, Muchuro S, Balidawa H, Namagala E, et al. Using mobile phones to improve clinic attendance amongst an antiretroviral treatment cohort in rural Uganda: a cross-sectional and prospective study. AIDS Behav. 2010;14:1347–1352. doi: 10.1007/s10461-010-9780-2. [DOI] [PubMed] [Google Scholar]
- 29.Chang LW, Kagaayi J, Nakigozi G, Galiwango R, Mulamba J, Ludigo J, et al. Telecommunications and health Care: an HIV/AIDS warmline for communication and consultation in Rakai, Uganda. J Int Assoc Provid AIDS. 2008;7:130–132. doi: 10.1177/1545109708318525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ware NC, Wyatt MA, Geng EH, Kaaya SF, Agbaji OO, Muyindike WR, et al. Toward an understanding of disengagement from HIV treatment and care in sub-Saharan Africa: a qualitative study. PLoS Med. 2013;10:e1001369–e1001369. doi: 10.1371/journal.pmed.1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]