SUMMARY
The present study tested the hypothesis that the scavenger receptor SR-A modulates granuloma formation in response to pulmonary infection with Mycobacterium tuberculosis (MTB). To test this hypothesis, we monitored survival and histopathology in WT and SR-A-deficient mice following aerosol infection with MTB Rv. SR-A-deficient (SR-A−/−) mice infected with MTB survived significantly longer than WT mice; the mean survival of SR-A−/− mice exceeded 430 days compared to 230 days for WT mice. Early granuloma formation was not impaired in SR-A−/− mice. The extended survival of SR-A−/− mice was associated with 13- and 3-fold higher number of CD4+ lymphocytes and antigen presenting cells in SR-A−/− lungs compared to WT mice 280 after infection. The histopathology of chronically infected SR-A−/− lungs, however, was marked by abundant cholesterol clefts in parenchymal lesions containing infection in multinucleated giant cells. The present study indicates SR-A as a candidate gene of the innate immune system influencing the chronic phase of M. tuberculosis infection.
Keywords: Scavenger receptor class A, Tuberculosis, Multinucleated giant cells, Granuloma
1. Introduction
Mycobacterium tuberculosis (MTB) is a highly successful pathogen having infected over a third of the global population. The current disease burden of tuberculosis is estimated at 8–10 million new cases of active disease and 2–3 million deaths annually.1 Initial infection of alveolar macrophages results in granuloma formation where cell-mediated immunity both arrests mycobacterial replication and confers long-term protection against active tuberculosis. Granulomatous cell-mediated immunity resolves primary infection in over 95% of MTB infected individuals. The prevailing notion is that MTB is not completely eradicated but remains in the host in a dormant or latent state in macrophages.2 Alternatively, the host may tolerate replication of a very small number of MTB organisms which continue to re-seed macrophages without causing overt tuberculosis.3,4 Advanced imaging techniques suggest that latent tuberculosis is a heterogeneous disease represented by a range of dormant and subclinical infections in different individuals.5 Susceptibility to primary tuberculosis reflects immune deficiencies or polymorphisms in non-redundant cytokine genes, e.g. TNFa, IFNγ, or IL-12, that regulate development of adaptive immunity.6 Post-primary tuberculosis resulting from either re-infection or activation of latent tuberculosis contributes 80% of active clinical disease.7 Secondary tuberculosis may represent failure of granulomas to curb replication of the resurgent pathogen.2,8 However, historical reassessment of human pathology and re-evaluation of mouse models indicate that post-primary tuberculosis manifests as a distinct non-granulomatous lipid pneumonia resulting from reactivation or re-infection of previously exposed individuals.7 Delayed type hypersensitivity reactions of antigen experienced immune cells that had previously controlled the primary infection assume a pathogenic role in formation of encapsulated caseous necrosis lesions, bronchial obstruction, and cavities that drain infectious organisms into conducting airways in secondary tuberculosis. Caseous necrosis forms from host lipids derived from adipose and lung tissue.9,10 Necrosis resembling human secondary tuberculosis has been observed in primary infection in genetically susceptible mice11,12 or in resistant mice after prior adjuvant stimulation of the immune system.7,13 Histological features similar to human pulmonary tuberculosis were also observed in monkeys infected with low aerosol dose of M. tuberculosis Erdman.14 In the mouse model, it was noted that the lungs of genetically susceptible mice infected with M. tuberculosis progress quickly to necrosis. Similarly, pre-sensitization of peripheral immunity with cord factor, the major adjuvant on the mycobacterial cell wall, primes the lungs of resistant mice for development of lung necrosis after reactivation of MTB by withdrawal of antibiotics. Post-primary tuberculosis may resolve spontaneously or fester for a long time until death. In the meantime, coughing expels a large number of infectious organisms spreading the infection to the community.7
Macrophage-derived foam cells characterize pulmonary lesions in chronic and post-primary tuberculosis in both mice and humans.3,7,15 Foam cells may determine the fate of MTB as a persistent or a resurgent pathogen during latent16,17 and post-primary tuberculosis,7 respectively. On the other hand, MTB plays an active role in foam cell formation by inducing accumulation of lipid bodies in macrophages.3 Lipid bodies may then fuse with phagolysosomes providing cholesterol and other host lipids as nutrients for resident MTB. Lipids and cholesterol are considered essential for both metabolism of the organism and process of the disease. For example, cholesterol serves as an important source of carbon and for the long-term survival of the organism in the host.17–19 The class A scavenger receptor SR-A contributes to macrophage foam cell differentiation and pathogenic sequestration of cholesterol having a role in clearance of oxidized lipoproteins.20 SR-A is involved in pathogenic sequestration of cholesterol, formation and fate of foam cells in prominent metabolic diseases including diabetes21 and atherosclerosis.20,22 SR-A is expressed at low levels in alveolar macrophages, but it is induced in foamy macrophages chronically infected with MTB.23 SR-A contributes to the clearance of oxidized surfactant phospholipids.24 Under normal conditions, expression of SR-A is held at low levels by the surfactant protein A receptor SP-R210,25 suggesting that SR-A function is influenced by the local microenvironment enriched in surfactant lipids. Given the role of SR-A in chronic diseases influenced by abnormalities in lipid metabolism, the present report assessed the role of SR-A in pulmonary tuberculosis.
2. Materials and methods
2.1. Animals
Male WT C57BL/6 mice were purchased from NCI (Fredericksburg, MD). A breeding pair of transgenic SR-A-deficient (SR-A−/−) mice26 in the C57BL/6 genetic background was kindly provided by Dr. Lester Kobzik, Harvard School of Public Health. Mice were maintained in the University of Texas Health Science Center animal facility under pathogen-free conditions. SR-A−/− mice were bred locally in a barrier facility within the UTHSCT vivarium. All mice were maintained in micro-isolator cages and provided sterile water and food ad libitum. Mice were fed Pico lab mouse diet 20 (PMI International, LabDiet Cat no: 5058). Mice were transferred into the UTHSCT BSL3 facility prior to infections. Animal experiments were approved by the Institutional Animal Care and Use Committee.
2.2. Bacteria
M. tuberculosis strain H37Rv (ATCC cat. #25618) colonies grown on 7H11 agar were sub-cultured in 7H9 broth and log phase organisms were harvested 8–10 days later. Bacteria were washed in 0.9% saline, sonicated at 5 W for 15 s to disperse organisms, and stored in aliquots at −80 °C in 0.9% saline. Thawed aliquots were diluted 10-fold in 0.9% saline and plated for cfu counts on 7H11 agar.27
2.3. Infections
Aerosol infections with H37Rv were performed in a University of Wisconsin Aerosol Chamber (Madison, WI) according to directions provided by Dr. D.N. McMurray (Department of Microbial and Molecular Pathogenesis, Texas A&M Health Science Center, College of Medicine, College Station, TX). A sonically dispersed suspension of H37Rv at 1 × 106 cfu/mL in saline was nebulized for 15 min, which implanted around 50–100 cfu/mouse lung. All mouse groups, 6–8 weeks old, were exposed to the H37Rv aerosol at the same time.
2.4. Histology
Lungs were fixed in 4% paraformaldehyde and embedded in paraffin. Sections were cut 4.5 μm thick and tissue pathology was visualized by hematoxylin and eosin staining.27 Images were captured using an Olympus BX41 upright microscope equipped with an Olympus DP11 digital camera (Olympus, Melville, NY) at 10X, 20X, or 40× magnification. Images were processed using Paint.Net software (http://www.getpaint.net) and imported into Microsoft® Word. To observe the presence of M. tuberculosis bacteria, tissue sections were processed for Ziehl–Nielsen acid fast staining performed according to a standard protocol in the Clinical Pathology Laboratory of The University of Texas Health Science Center at Tyler.
2.5. Generation of lung cell suspensions
Lung cell suspensions were obtained by a two-step digestion procedure using Dispase II supplemented with 0.1% collagenase A (Roche Diagnostics).28,29 Lungs were first instilled intratrachealy with 1–1.5 ml of 2.4 U/ml Dispase II supplemented with 0.1% collagenase A (Roche). The trachea was then tied with suture to retain the enzymatic solution in the lung. The lungs were then excised and minced on ice in a 30 mm Petri dish containing 3 ml of enzyme digestion solution supplemented with 80–100 U/ml of DNAase I and incubated at 37 °C for 30 min. The enzymatic reaction was then stopped by addition of 8 ml of PBS supplemented with 5 mM EDTA, pH 8.0 and 2% FBS and filtered through a 70 mm strainer (Falcon) to remove tissue fragments. The cells were collected by centrifugation, washed once in PBS containing 2% FBS, and then incubated 5–7 min at room temperature in 1 ml erythrocyte lysis buffer (0.54 M NH4Cl, 1 mM KHCO3. 0.09 mM EDTA, pH 7.4, filtered). Washed cells were blocked in 1 mL PBS supplemented with 2% normal goat serum and 0.5% FBS, and 10 mg/mL Fc block, washed once by centrifugation and re-suspended in 1 ml of blocking buffer. Cells were counted in a hema-cytometer and viability, assessed by trypan blue exclusion, was greater than 95%.
2.6. Flow cytometry
Lung cells were placed in blocking buffer for 1 h, and then dispersed in 100 ml aliquots and incubated with recommended concentrations of fluorescent FITC-CD4, PE/Cy5-CD11c and PE-MHC-II antibodies (eBioscience) for 30 min on ice. The cells were washed twice in 0.5 ml of blocking buffer, re-suspended in PBS and analyzed by flow cytometry using a BD FACS calibur flow analyzer (BD Pharmingen). Cells were separated according to forward and side-scatter properties and gated to eliminate events from cellular debris and dead cells. Voltage adjustment was applied on unstained cells to set autofluorescent cells as negative events. Quadratic or linear gating was used to determine the percent of cells expressing CD4, CD11c, or MHC-II compared to background staining with isotype control antibodies. Linear gating over fluorescent Gaussian histograms was used to obtain mean fluorescence. The percentage of positive cells was multiplied by the number of cells in suspension to calculate the number of lineage positive cells.
2.7. Statistics
Survival curves were generated by the Kaplan–Meier method and statistical comparison of survival curves was performed using the Gehan-Breslow-Wilcoxon test.
3. Results
3.1. Increased survival of SR-A−/− mice to pulmonary tuberculosis
To determine the role of SR-A in pulmonary tuberculosis, we monitored survival and histological presentation of pulmonary tuberculosis in C57BL/6 WT and SR-A-deficient (SR-A−/−) mice. Figure 1A shows that the mean survival of SR-A−/− mice was 430 days (14 months) significantly longer than 230 days (14 months) for WT mice. The mean survival of WT mice infected with MTB Rv via aerosol was similar to previous findings.30 Serial dilution of lung homogenates determined mycobacterial burden in four surviving WT mice and 3 SR-A−/− mice 281 days (9.4 months) after infection (Figure 1B). Mycobacterial CFU were approximately 1 log lower in SR-A−/− mice compared to WT mice but the number of mice was insufficient for calculating statistical differences.
Figure 1.
Increased resistance of SR-A−/− mice to pulmonary tuberculosis. A) Mouse survival after aerosol infection with 50–100 CFU of M. tuberculosis Rv. N = 30 mice per group, p < 0.0001. B) Mycobacterial CFU were evaluated in lung homogenates 281 days after infection with M. tuberculosis Rv. N = 4.
3.2. Cholesterol clefts and formation of multinucleated giant cells in lungs of SR-A−/− mice infected with MTB
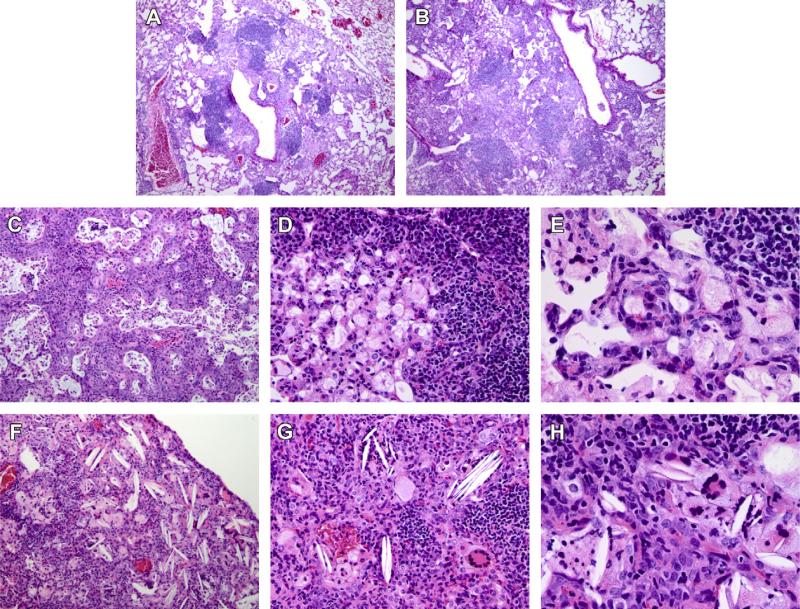
Histopathology was then evaluated at early (1 month) and late (9.4 months) stages of the infection. Lymphocytic nodules in distal and proximal airways and parenchymal expansion of epithelioid macrophages typified early murine tuberculosis in both WT (Figure 2A) and SR-A−/− mice (Figure 2B) at 4 weeks. However, distinct histo-pathological features were noted between WT and SR-A−/− mice 281 days after infection. Obstruction of alveolar and bronchial spaces with necrotic macrophages marked terminal tuberculosis in WT mice (Figure 2C). In addition, thickened parenchyma in obstructed areas of the lung resembled squamous cell metaplasia as reported recently31(Figure 2C). Adjoining lesions contained intense lymphocytic foci overlooking foamy infiltrates in distal airspaces (Figure 2D) and filling of the alveoli with foam cells (Figure 2E). In contrast, the pathology of SR-A−/− mice was marked by the presence of cholesterol clefts (Figure 2F–H) and multinucleated foamy cells (Figure 2G and H). Higher magnification (Figure 2H) indicates association of cholesterol clefts with multinucleated foamy macrophages in alveolar spaces (Figure 2H).
Figure 2.
Multinucleated giant cells and cholesterol clefts in the lungs of SR-A−/− mice. Lung histopathology was evaluated on H&E stained sections at 1 (A and B) and 9.4 months (C–H) after infection of WT (A, C–E) or SR-A−/− (F–H) mice with M. tuberculosis Rv. Images 10× (A, B, C, and F), 20× (D and H) or 40× magnification (E and F). Representative images from n = 2 mice per group are shown.
3.3. Localization of MTB in multinucleated cells and cholesterol clefts in SR-A−/− lungs
Acid fast staining was used to localize MTB in lungs of WT (Figure 3A–C) and SR-A−/− (3D-F) mice. The closed arrows of lung tissue sections from WT mice show MTB in epithelioid macrophages (Figure 3A), foamy macrophages (Figure 3B), and necrotic debris in adjacent alveolar and bronchial spaces in WT lungs (Figure 3C). In SR-A−/− mice, however, mycobacteria were identified in foamy macrophages (Figure 3D, closed arrows), multinucleated giant macrophages (Figure 3E, closed arrows), and in dense material around cholesterol clefts (Figure 3F, closed arrows). These multinucleated cells were characterized by foamy sub-membranous cytoplasm, crystalline vesicles (Figure 3E, open arrow), and central granular material. The latter had similar morphology with cholesterol cleft remnants containing mycobacteria (Figure 3F), indicating that cholesterol deposition and formations of multinucleated cells are related processes.
Figure 3.
Localization of M. tuberculosis in MGCs in lungs of SR-A−/− mice. M. tuberculosis bacteria were visualized by the Ziehls-Nielsen acid fast staining of lung tissue sections from WT (A–C) or SR-A−/− (D–F) mice. Images were captured at 40× magnification. Representative images from n = 2 mice per group are shown.
3.4. SR-A suppresses cell-mediated immunity in the chronic phase of MTB infection
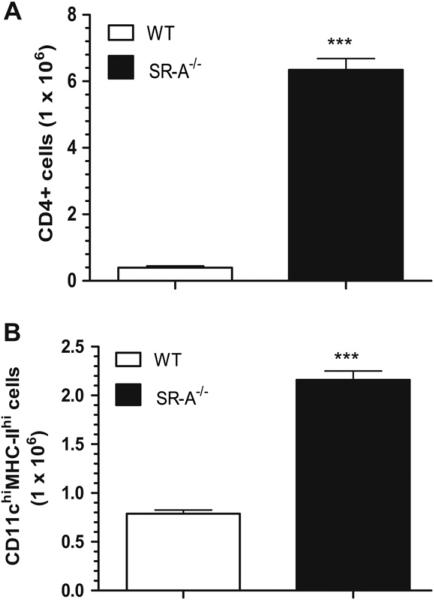
Flow cytometry analysis of lung cell suspensions revealed that the lungs of the resistant SR-A−/− mice contained, respectively, 13- and 3-fold more CD4+ lymphocytes (Figure 4A) and antigen presenting cells (Figure 4B) 281 days after MTB infection when most WT mice had already succumbed to the infection (Figure 1). These results indicate that SR-A is a negative regulator of pulmonary adaptive immunity during chronic MTB infection.
Figure 4.
Long-term retention of adaptive immune cells in the lungs of SR-A−/− mice infected with M. tuberculosis Rv. Lung cell suspensions were isolated 281 days after infection with M. tuberculosis Rv. The cells were incubated with FITC-conjugated CD4, PE-conjugated MHC-II, and PE/Cy5-conjugated CD11c rat anti-mouse antibodies and analyzed by flow cytometry. The percentages of lineage positive cells were compared to the total cell number to obtain the number of CD4T lymphocytes (A) and CCD11chiMHC-IIhi antigen presenting cells (B) in WT and SR-A−/− lungs as indicated. Data shown are means±SEM, n = 3 mice for WT and n = 4 mice for SR-A−/− mice. ***p < 0.001.
4. Discussion
The extended survival of SR-A−/− mice is associated with formation of multinucleated giant cells (MGC). Foamy MGCs distinguished the lung histopathology of SR-A−/− mice chronically infected with MTB (Figure 3). Previous studies have noted that MGCs are a characteristic feature in human but not mouse tuberculosis. MGCs contribute to host resistance against tuberculosis in humans having crucial roles in enhancing antigen presentation and killing of mycobacteria.32 Interestingly, MGCs were identified as the site of cholesterol cleft formation in SR-A−/− mice, consistent with the notion that SR-A suppresses formation of MGCs. A potential mechanism underlying this observation is the ability of SR-A to promote macrophage apoptosis in the context of cholesterol induced ER stress.22 In this regard, SR-A may promote cholesterol-mediated death of MTB infected macrophages contributing to airway obstruction and parenchymal spread of the infection instead of containment of the pathogen in MGCs.
Cholesterol is the most abundant neutral lipid and the second most abundant lipid in pulmonary surfactant.33 Histological evidence indicated pulmonary surfactant uptake in macrophages within granulomas of mice chronically infected with MTB.27 Pulmonary surfactant, a specialized lipoprotein containing unique proteins (SP-A, SP-B, SP-C, and SP-D), phospholipids and neutral lipids, is required for alveolar stability and local host defense.34 At least two surfactant components, SP-A and SP-D, are involved in macrophage phagocytosis and modulation of adaptive immunity to MTB.35,36 On the other hand, the mechanisms of surfactant uptake and degradation by alveolar macrophages have not been determined. SR-A, however, mediates clearance of oxidized surfactant phospholipids produced by environmental inflammation.24 Therefore, local modulation of SR-A may have a role in uptake of surfactant-derived cholesterol and oxidized lipids establishing chronic infection with M. tuberculosis.
On the other hand, the present findings indicate that SR-A suppresses cell-mediated immunity in the chronic phase of the disease. Apart from its role in metabolism of damaged proteins and lipids, SR-A augments innate immunity. SR-A enhances innate immunity as a non-opsonic phagocytic receptor of microorganisms including Mycobacterium bovis BCG and M. tuberculosis.37,38 SR-A regulates innate and adaptive immunity independent of its ligand binding activities. Thus, SR-A attenuates TLR-4 signaling in dendritic cells through interaction with TRAF-6, thereby inhibiting activation of NFkB by TLR-4.39 SR-A inhibits macrophage activation by M. tuberculosis cord factor.23 Furthermore, SR-A coordinates the timing and magnitude of inflammatory responses during clearance of SP-A-opsonized bacteria via the SP-A receptor SP-R210, limiting excessive inflammation and damage to the lung from acute bacterial infections.25 Negative regulation of innate immunity may have long-term consequenses; SR-A suppresses activation of CD8+ lymphocytes by antigen presenting cells.40 The present studies support the notion that SR-A silences adaptive immunity in the chronic phase of the disease.
In summary, the present study demonstrates that SR-A influences long term host survival to pulmonary tuberculosis. In particular, SR-A deficiency delayed progression to terminal tuberculosis rather than blocking early granuloma formation or the intermediate chronic phase of the disease. Lack of SR-A enhanced formation and apparently longer containment of the infection in MGCs. Unlike SR-A-deficient mice, formation of MGCs and cholesterol clefts are infrequent during the terminal phase of pulmonary tuberculosis in WT mice; decline in immunological protection accelerates progression to the terminal phase of the disease.41 In this manner, loss of SR-A function identifies a mouse model which reproduced at least one aspect of human pulmonary tuberculosis, supporting the beneficial role of MGCs in host resistance to M. tuberculosis. Earlier in vitro findings reported that inflammatory mediators suppress SR-A expression in human macrophages but enhance SR-A expression in mouse macrophages.42,43 suggesting species differences in regulation of SR-A in M. tuberculosis infection. Differences in sub-cellular trafficking of cholesterol in SR-A-deficient macrophages may play a role in fusion of MTB infected macrophages. It is reasonable to speculate that, in the absence of SR-A, MGCs may help prolong immunological protection through immune synapses that facilitate progression into latent infection in humans. A crucial question for further study is whether variation of SR-A gene regulation and polymorphisms in different individuals modulate progression to latent or post-primary tuberculosis in humans.
Acknowledgments
We thank Dr. Mark AL Atkinson, M.A. D.Phil. Director of Research for financial support, Dr. Lester Kobzik, Harvard School of Public Health for providing the SR-A−/− mice, Dr. Homayoun Shams, UTHSCT for aerosol infections, and Dr. Timothy Allen, Chair of the Department Pathology for facilitating processing and staining of tissue sections at the UTHSCT Clinical Histology Laboratory.
Funding: Financial support for this work was provided by the Potts Memorial Foundation to ZCC, a UTHSCT Research Council Award to ZSC, and funds provided by the University of Texas Health Science Center at Tyler Office of the Director of Research.
Abbreviations
- SR-A
Scavenger receptor class A
- SR-A−/−
SR-A deficient mice
- WT
Wild type
- MTB
Mycobacterium tuberculosis
- CFU
Colony forming units
- MGC
Multinucleated giant cells
Footnotes
Ethical approval: Human subjects: None.
Animals: All animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee, University of Texas Health Science Center at Tyler.
Competing interests: None.
References
- 1.WHO report 2006. Global tuberculosis control-surveillance, planning, financing. 2006 Anonymous WHO/HTM/TB/2006362. [Google Scholar]
- 2.Ulrichs T, Kaufmann SH. New insights into the function of granulomas in human tuberculosis. J Pathol. 2006;208:261–9. doi: 10.1002/path.1906. [DOI] [PubMed] [Google Scholar]
- 3.Caceres N, Tapia G, Ojanguren I, Altare F, Gil O, Pinto S, et al. Evolution of foamy macrophages in the pulmonary granulomas of experimental tuberculosis models. Tuberculosis (Edinb) 2009;89:175–82. doi: 10.1016/j.tube.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Cardona PJ. New insights on the nature of latent tuberculosis infection and its treatment. Inflamm Allergy Drug Targets. 2007;6:27–39. doi: 10.2174/187152807780077282. [DOI] [PubMed] [Google Scholar]
- 5.Barry CE, 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–55. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter RL, Jagannath C, Actor JK. Pathology of postprimary tuberculosis in humans and mice: contradiction of long-held beliefs. Tuberculosis (Edinb) 2007;87:267–78. doi: 10.1016/j.tube.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Russell DG. Who puts the tubercle in tuberculosis? Nat Rev Microbiol. 2007;5:39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- 9.Hunter RL, Olsen M, Jagannath C, Actor JK. Trehalose 6,6′-dimycolate and lipid in the pathogenesis of caseating granulomas of tuberculosis in mice. Am J Pathol. 2006;168:1249–61. doi: 10.2353/ajpath.2006.050848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter RL, Olsen MR, Jagannath C, Actor JK. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann Clin Lab Sci. 2006;36:371–86. [PubMed] [Google Scholar]
- 11.Kramnik I. Genetic dissection of host resistance to Mycobacterium tuberculosis: the sst1 locus and the Ipr1 gene. Curr Top Microbiol Immunol. 2008;321:123–48. doi: 10.1007/978-3-540-75203-5_6. [DOI] [PubMed] [Google Scholar]
- 12.Kramnik I, Dietrich WF, Demant P, Bloom BR. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2000;97:8560–5. doi: 10.1073/pnas.150227197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidry TV, Hunter RL, Jr, Actor JK. CD3+ cells transfer the hypersensitive granulomatous response to mycobacterial glycolipid trehalose 6,6′-dimycolate in mice. Microbiology. 2006;152:3765–75. doi: 10.1099/mic.0.29290-0. [DOI] [PubMed] [Google Scholar]
- 14.Capuano SV, 3rd, Croix DA, Pawar S, Zinovik A, Myers A, Lin PL, et al. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 2003;71:5831–44. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943–8. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peyron P, Vaubourgeix J, Poquet Y, Levillain F, Botanch C, Bardou F, et al. Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 2008;4:e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandey AK, Sassetti CM. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci USA. 2008;105:4376–80. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brzostek A, Dziadek B, Rumijowska-Galewicz A, Pawelczyk J, Dziadek J. Cholesterol oxidase is required for virulence of Mycobacterium tuberculosis. FEMS Microbiol Lett. 2007;275:106–12. doi: 10.1111/j.1574-6968.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 19.Av-Gay Y, Sobouti R. Cholesterol is accumulated by mycobacteria but its degradation is limited to non-pathogenic fast-growing mycobacteria. Can J Microbiol. 2000;46:826–31. [PubMed] [Google Scholar]
- 20.Ricci R, Sumara G, Sumara I, Rozenberg I, Kurrer M, Akhmedov A, et al. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science. 2004;306:1558–61. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- 21.Guest CB, Hartman ME, O'Connor JC, Chakour KS, Sovari AA, Freund GG. Phagocytosis of cholesteryl ester is amplified in diabetic mouse macrophages and is largely mediated by CD36 and SR-A. PLoS ONE. 2007;2:e511. doi: 10.1371/journal.pone.0000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, et al. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozeki Y, Tsutsui H, Kawada N, Suzuki H, Kataoka M, Kodama T, et al. Macrophage scavenger receptor down-regulates mycobacterial cord factor-induced proinflammatory cytokine production by alveolar and hepatic macrophages. Microb Pathog. 2006;40:171–6. doi: 10.1016/j.micpath.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Dahl M, Bauer AK, Arredouani M, Soininen R, Tryggvason K, Kleeberger SR, et al. Protection against inhaled oxidants through scavenging of oxidized lipids by macrophage receptors MARCO and SR-AI/II. J Clin Invest. 2007;117:757–64. doi: 10.1172/JCI29968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sever-Chroneos Z, Krupa A, Davis J, Hasan M, Yang CH, Szeliga J, et al. Surfactant protein A (SP-A)-mediated clearance of Staphylococcus aureus involves binding of SP-A to the staphylococcal adhesin eap and the macrophage receptors SP-A receptor 210 and scavenger receptor class A. J Biol Chem. 2011;286:4854–70. doi: 10.1074/jbc.M110.125567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H, Imrich A, Kobzik L. Characterization of immortalized MARCO and SRAI/II-deficient murine alveolar macrophage cell lines. Part Fibre Toxicol. 2008;5(7) doi: 10.1186/1743-8977-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chroneos ZC, Midde K, Sever-Chroneos Z, Jagannath C. Pulmonary surfactant and tuberculosis. Tuberculosis. 2009;89(Suppl. 1):S10–4. doi: 10.1016/S1472-9792(09)70005-8. [DOI] [PubMed] [Google Scholar]
- 28.Gurel O, Ikegami M, Chroneos ZC, Jobe AH. Macrophage and type II cell catabolism of SP-A and saturated phosphatidylcholine in mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1266–72. doi: 10.1152/ajplung.2001.280.6.L1266. [DOI] [PubMed] [Google Scholar]
- 29.Kotton DN, Summer RS, Sun X, Ma BY, Fine A. Stem cell antigen-1 expression in the pulmonary vascular endothelium. Am J Physiol Lung Cell Mol Physiol. 2003;284:L990–6. doi: 10.1152/ajplung.00415.2002. [DOI] [PubMed] [Google Scholar]
- 30.Medina E, North RJ. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology. 1998;93:270–4. doi: 10.1046/j.1365-2567.1998.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nalbandian A, Yan BS, Pichugin A, Bronson RT, Kramnik I. Lung carcinogenesis induced by chronic tuberculosis infection: the experimental model and genetic control. Oncogene. 2009;28:1928–38. doi: 10.1038/onc.2009.32. [DOI] [PubMed] [Google Scholar]
- 32.Lay G, Poquet Y, Salek-Peyron P, Puissegur MP, Botanch C, Bon H, et al. Langhans giant cells from M. tuberculosis-induced human granulomas cannot mediate mycobacterial uptake. J Pathol. 2007;211:76–85. doi: 10.1002/path.2092. [DOI] [PubMed] [Google Scholar]
- 33.Orgeig S, Daniels CB. The roles of cholesterol in pulmonary surfactant: insights from comparative and evolutionary studies. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:75–89. doi: 10.1016/s1095-6433(01)00307-5. [DOI] [PubMed] [Google Scholar]
- 34.Chroneos ZC, Sever-Chroneos Z, Shepherd VL. Pulmonary surfactant: an immunological perspective. Cell Physiol Biochem. 2010;25:13–26. doi: 10.1159/000272047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samten B, Townsend JC, Sever-Chroneos Z, Pasquinelli V, Barnes PF, Chroneos ZC. An antibody against the surfactant protein A (SP-A)-binding domain of the SP-A receptor inhibits T cell-mediated immune responses to Mycobacterium tuberculosis. J Leukoc Biol. 2008;84:115–23. doi: 10.1189/jlb.1207835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson JS, Martin JL, Azad AK, McCarthy TR, Kang PB, Voelker DR, et al. Surfactant protein D increases fusion of Mycobacterium tuberculosis-containing phagosomes with lysosomes in human macrophages. Infect Immun. 2006;74:7005–9. doi: 10.1128/IAI.01402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmerli S, Edwards S, Ernst JD. Selective receptor blockade during phagocytosis does not alter the survival and growth of Mycobacterium tuberculosis in human macrophages. Am J Resp Cell Mol Biol. 1996;15:760–70. doi: 10.1165/ajrcmb.15.6.8969271. [DOI] [PubMed] [Google Scholar]
- 38.Haworth R, Platt N, Keshav S, Hughes D, Darley E, Suzuki H, et al. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med. 1997;186:1431–9. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu X, Yi H, Guo C, Zuo D, Wang Y, Kim HL, et al. Pattern recognition scavenger receptor CD204 attenuates toll-like receptor 4-induced NF-{kappa}B activation by Directly inhibiting ubiquitination of tumor necrosis factor (TNF) receptor-associated factor 6. J Biol Chem. 2011;286:18795–806. doi: 10.1074/jbc.M111.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi H, Yu X, Gao P, Wang Y, Baek SH, Chen X, et al. Pattern recognition scavenger receptor SRA/CD204 down-regulates Toll-like receptor 4 signaling-dependent CD8 T-cell activation. Blood. 2009;113:5819–28. doi: 10.1182/blood-2008-11-190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhoades ER, Frank AA, Orme IM. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 42.Fitzgerald ML, Moore KJ, Freeman MW, Reed GL. Lipopolysaccharide induces scavenger receptor A expression in mouse macrophages: a divergent response relative to human THP-1 monocyte/macrophages. J Immunol. 2000;164:2692–700. doi: 10.4049/jimmunol.164.5.2692. doi: ji_v164n5p2692[pii] [DOI] [PubMed] [Google Scholar]
- 43.Van Lenten BJ, Fogelman AM, Seager J, Ribi E, Haberland ME, Edwards PA. Bacterial endotoxin selectively prevents the expression of scavenger-receptor activity on human monocyte-macrophages. J Immunol. 1985;134:3718–21. [PubMed] [Google Scholar]